Development of Flexible Ion-Selective Electrodes for Saliva Sodium Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrodes Fabrication
2.3. Small Sensor System
2.4. Characterization of Sensor
2.5. Sampling of Blood and Saliva
2.6. Measurement of Ion Levels
3. Results and Discussion
3.1. Sodium Ion Sensor System for Saliva Testing
3.2. Film Sensor Structure and Sensing Capability
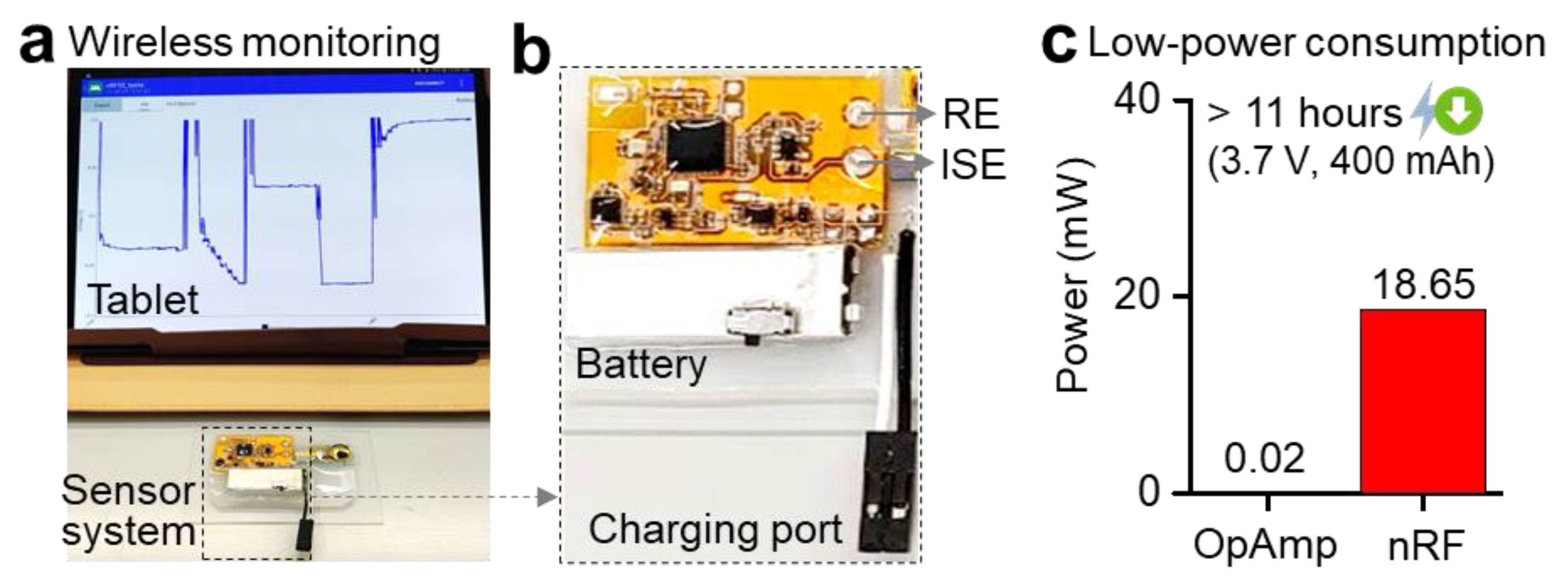
3.3. Low-Power Circuit for Wireless Continuous Data Transmission
3.4. Correlation between Blood and Saliva Ion Levels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, H.-R.; Kim, H.S.; Qazi, R.; Kwon, Y.-T.; Jeong, J.-W.; Yeo, W.-H. Advanced Soft Materials, Sensor Integrations, and Applications of Wearable Flexible Hybrid Electronics in Healthcare, Energy, and Environment. Adv. Mater. 2020, 32, 1901924. [Google Scholar] [CrossRef]
- Goldoni, R.; Farronato, M.; Connelly, S.T.; Tartaglia, G.M.; Yeo, W.-H. Recent advances in graphene-based nanobiosensors for salivary biomarker detection. Biosens. Bioelectron. 2020, 112723. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kwon, Y.-T.; Lim, H.-R.; Kim, J.-H.; Kim, Y.-S.; Yeo, W.-H. Recent Advances in Wearable Sensors and Integrated Functional Devices for Virtual and Augmented Reality Applications. Adv. Funct. Mater. 2020, 2005692. [Google Scholar] [CrossRef]
- Lee, Y.; Howe, C.; Mishra, S.; Lee, D.S.; Mahmood, M.; Piper, M.; Kim, Y.; Tieu, K.; Byun, H.-S.; Coffey, J.P.; et al. Wireless, intraoral hybrid electronics for real-time quantification of sodium intake toward hypertension management. Proc. Natl. Acad. Sci. USA 2018, 115, 5377. [Google Scholar] [CrossRef]
- Lim, H.-R.; Hillman, N.; Kwon, Y.-T.; Kim, Y.-S.; Choa, Y.-H.; Yeo, W.-H. Ultrathin, long-term stable, solid-state reference electrode enabled by enhanced interfacial adhesion and conformal coating of AgCl. Sens. Actuators B Chem. 2020, 309, 127761. [Google Scholar] [CrossRef]
- Buzalaf, M.A.R.; Ortiz, A.D.C.; Carvalho, T.S.; Fideles, S.O.M.; Araújo, T.T.; Moraes, S.M.; Buzalaf, N.R.; Reis, F.N. Saliva as a diagnostic tool for dental caries, periodontal disease and cancer: Is there a need for more biomarkers? Expert Rev. Mol. Diagn. 2020, 20, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Stein, A.; Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. TrAC Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- User Guid Manual by Thermo Scientific. Available online: https://pim-resources.coleparmer.com/instruction-manual/58824-56.pdf (accessed on 15 February 2021).
- Shea, A.M.; Hammill, B.G.; Curtis, L.H.; Szczech, L.A.; Schulman, K.A. Medical Costs of Abnormal Serum Sodium Levels. J. Am. Soc. Nephrol. 2008, 19, 764. [Google Scholar] [CrossRef]
- White, A.G.; Entmacher, P.S.; Rubin, G.; Leiter, L. Physiological and pharmacological regulation of human salivary electrolyte concentrations; with a discussion of electrolyte concentrations of some other exocrine secretions. J. Clin. Investig. 1955, 34, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Iyer, E.M.; Malik, H. Relative changes in salivary sodium and potassium in relation to expose to high G stress. Med. J. Armed Forces India 1994, 50, 261–265. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Marson, F.A.L.; Mendonça, R.M.H.; Bertuzzo, C.S.; Paschoal, I.A.; Ribeiro, J.D.; Ribeiro, A.F.; Levy, C.E. Chloride and sodium ion concentrations in saliva and sweat as a method to diagnose cystic fibrosis. J. Pediatr. 2019, 95, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Labat, C.; Thul, S.; Pirault, J.; Temmar, M.; Thornton, S.N.; Benetos, A.; Bäck, M. Differential Associations for Salivary Sodium, Potassium, Calcium, and Phosphate Levels with Carotid Intima Media Thickness, Heart Rate, and Arterial Stiffness. Dis. Markers 2018, 2018, 3152146. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Miyoshi, M.; Matsumoto, S.; Fujimoto, T.; Yamamoto, Y. Reflection of salt concentrations of blood upon those of saliva. Jpn. J. Physiol. 1963, 13, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Marson, F.A.; Mendonça, R.M.; Ribeiro, J.D.; Ribeiro, A.F.; Paschoal, I.A.; Levy, C.E. Saliva as a potential tool for cystic fibrosis diagnosis. Diagn. Pathol. 2013, 8, 46. [Google Scholar] [CrossRef]
- Kallapur, B.; Ramalingam, K.; Bastian, A.M.; Sarkar, A.; Sethuraman, S. Quantitative estimation of sodium, potassium and total protein in saliva of diabetic smokers and nonsmokers: A novel study. J. Nat. Sci. Biol. Med. 2013, 4, 341–345. [Google Scholar]
- Faragó, P.; Gălătuș, R.; Hintea, S.; Boșca, A.B.; Feurdean, C.N.; Ilea, A. An Intra-Oral Optical Sensor for the Real-Time Identification and Assessment of Wine Intake. Sensors 2019, 19, 4719. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-R.; Kim, Y.-S.; Kwon, S.; Mahmood, M.; Kwon, Y.-T.; Lee, Y.; Lee, S.M.; Yeo, W.-H. Wireless, Flexible, Ion-Selective Electrode System for Selective and Repeatable Detection of Sodium. Sensors 2020, 20, 3297. [Google Scholar] [CrossRef]
- Cuartero, M.; Parrilla, M.; Crespo, G.A. Wearable Potentiometric Sensors for Medical Applications. Sensors 2019, 19, 363. [Google Scholar] [CrossRef]
- Williamson, S.; Munro, C.; Pickler, R.; Grap, M.J.; Elswick, R.K. Comparison of Biomarkers in Blood and Saliva in Healthy Adults. Nurs. Res. Pract. 2012, 2012, 246178. [Google Scholar] [CrossRef] [PubMed]
- Jasim, H.; Olausson, P.; Hedenberg-Magnusson, B.; Ernberg, M.; Ghafouri, B. The proteomic profile of whole and glandular saliva in healthy pain-free subjects. Sci. Rep. 2016, 6, 39073. [Google Scholar] [CrossRef] [PubMed]
- Bellagambi, F.G.; Lomonaco, T.; Salvo, P.; Vivaldi, F.; Hangouët, M.; Ghimenti, S.; Biagini, D.; Di Francesco, F.; Fuoco, R.; Errachid, A. Saliva sampling: Methods and devices. An overview. TrAC Trends Anal. Chem. 2020, 124, 115781. [Google Scholar] [CrossRef]
- Aps, J.K.M.; Martens, L.C. Review: The physiology of saliva and transfer of drugs into saliva. Forensic Sci. Int. 2005, 150, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Zhang, Y.; Pan, J.; Li, S.; Sun, X.; Zhang, B.; Peng, H. Weaving sensing fibers into electrochemical fabric for real-time health monitoring. Adv. Funct. Mater. 2018, 28, 1804456. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Martin, A.; García-Carmona, L.; Barfidokht, A.; Kurniawan, J.F.; Moreto, J.R.; Tang, G.; Shin, A.; Liu, X.; Escarpa, A. Skin-worn Soft Microfluidic Potentiometric Detection System. Electroanalysis 2019, 31, 239–245. [Google Scholar] [CrossRef]
- Guinovart, T.; Crespo, G.A.; Rius, F.X.; Andrade, F.J. A reference electrode based on polyvinyl butyral (PVB) polymer for decentralized chemical measurements. Anal. Chim. Acta 2014, 821, 72–80. [Google Scholar] [CrossRef] [PubMed]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology; Blackwell Science Oxford: Oxford, UK, 1997; Volume 1669. [Google Scholar]
- Malamud, D. Saliva as a Diagnostic Fluid. Dent. Clin. N. Am. 2011, 55, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Priya, K.Y.; Prathibha, K.M. Methods of collection of saliva—A review. Int. J. Oral Health Dent. 2017, 3, 149–153. [Google Scholar]
- Guimerà, X.; Moya, A.; Dorado, D.A.; Illa, X.; Villa, R.; Gabriel, D.; Gamisans, X.; Gabriel, G. A Minimally Invasive Microsensor Specially Designed for Simultaneous Dissolved Oxygen and pH Biofilm Profiling. Sensors 2019, 19, 4747. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, Y.; Bühlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric selectivity coefficients of ion-selective electrodes. Part I. Inorganic cations (technical report). Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Bakker, E. Selectivity of liquid membrane ion-selective electrodes. Electroanalysis 1997, 9, 7–12. [Google Scholar] [CrossRef]
- Lim, H.-R.; Lee, Y.; Jones, K.A.; Kwon, Y.-T.; Kwon, S.; Mahmood, M.; Lee, S.M.; Yeo, W.-H. All-in-one, wireless, fully flexible sodium sensor system with integrated Au/CNT/Au nanocomposites. Sens. Actuators B Chem. 2021, 331, 129416. [Google Scholar] [CrossRef]
- Burtis, C.A.; Ashwood, E.R.; Bruns, D.E. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics—E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Kwon, Y.-T.; Kim, Y.-S.; Kwon, S.; Mahmood, M.; Lim, H.-R.; Park, S.-W.; Kang, S.-O.; Choi, J.J.; Herbert, R.; Jang, Y.C. All-printed nanomembrane wireless bioelectronics using a biocompatible solderable graphene for multimodal human-machine interfaces. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-T.; Kim, H.; Mahmood, M.; Kim, Y.-S.; Demolder, C.; Yeo, W.-H. Printed, Wireless, Soft Bioelectronics and Deep Learning Algorithm for Smart Human-Machine Interfaces. ACS Appl. Mater. Interfaces 2020, 12, 49398–49406. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, M.; Sadowska, K.; Pijanowska, D.G.; Pomećko, R.; Bocheńska, M. Potentiometric Solid-Contact Ion-Selective Electrode for Determination of Thiocyanate in Human Saliva. Sensors 2020, 20, 2817. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, M.; Jasiński, A.; Jasińska, M.; Drucis, K.; Ekman, M.; Szarmach, A.; Suchodolski, R.; Pomećko, R.; Bocheńska, M. Simultaneous Determination of Na+, K+, Ca2+, Mg2+ and Cl− in Unstimulated and Stimulated Human Saliva Using All Solid State Multisensor Platform. Electroanalysis 2017, 29, 2232–2238. [Google Scholar] [CrossRef]



| Reference | Design | Non-Invasive Sodium Sensing Performance | |||
|---|---|---|---|---|---|
| Sensor | Device Size | Wireless Monitoring | [Na+] (mEq/L) (1) | Correlation to Blood | |
| This work | Flexible SS-ISE | 4 × 5.5 cm2 | Yes | 13.69 ± 2.75 | Linear |
| [18] | Flexible SS-ISE (2) | 3 × 2 cm2 | Yes | 6.5–11.8 | - |
| [37] | SS-ISE | Table-top | No | 0.8 ± 0.1 | - |
| [38] | SS-ISE | Table-top | No | 1.97–10.32 | - |
| [12] | ISE | Table-top | No | 10–13 | - |
| [13] | ECL (3) | Table-top | No | 6–35 | - |
| [16] | Photometer | Table-top | No | 11.5–217.3 | Linear |
| [14] | Photodetector | Table-top | No | 10–30 | Linear |
| [15] | Radiometer | Table-top | No | 12.26 ± 4.31 | Linear |
| [12] | Radiometer | Table-top | No | 12.87 ± 5.85 | Linear |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.-R.; Lee, S.M.; Mahmood, M.; Kwon, S.; Kim, Y.-S.; Lee, Y.; Yeo, W.-H. Development of Flexible Ion-Selective Electrodes for Saliva Sodium Detection. Sensors 2021, 21, 1642. https://doi.org/10.3390/s21051642
Lim H-R, Lee SM, Mahmood M, Kwon S, Kim Y-S, Lee Y, Yeo W-H. Development of Flexible Ion-Selective Electrodes for Saliva Sodium Detection. Sensors. 2021; 21(5):1642. https://doi.org/10.3390/s21051642
Chicago/Turabian StyleLim, Hyo-Ryoung, Soon Min Lee, Musa Mahmood, Shinjae Kwon, Yun-Soung Kim, Yongkuk Lee, and Woon-Hong Yeo. 2021. "Development of Flexible Ion-Selective Electrodes for Saliva Sodium Detection" Sensors 21, no. 5: 1642. https://doi.org/10.3390/s21051642
APA StyleLim, H.-R., Lee, S. M., Mahmood, M., Kwon, S., Kim, Y.-S., Lee, Y., & Yeo, W.-H. (2021). Development of Flexible Ion-Selective Electrodes for Saliva Sodium Detection. Sensors, 21(5), 1642. https://doi.org/10.3390/s21051642









