A Robust Optical Sensor for Remote Multi-Species Detection Combining Frequency-Division Multiplexing and Normalized Wavelength Modulation Spectroscopy
Abstract
1. Introduction
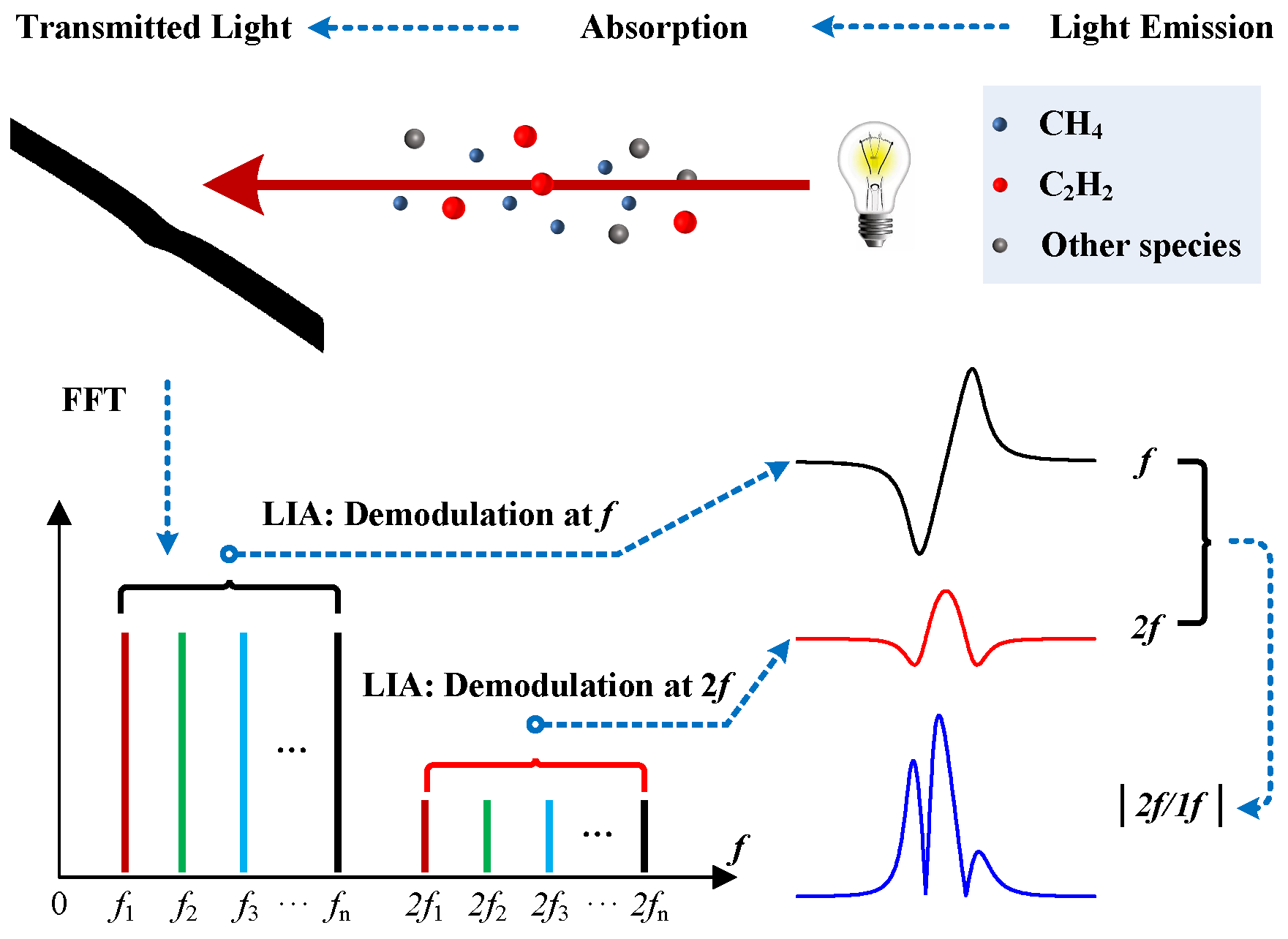
2. Principle
3. Instrumentation
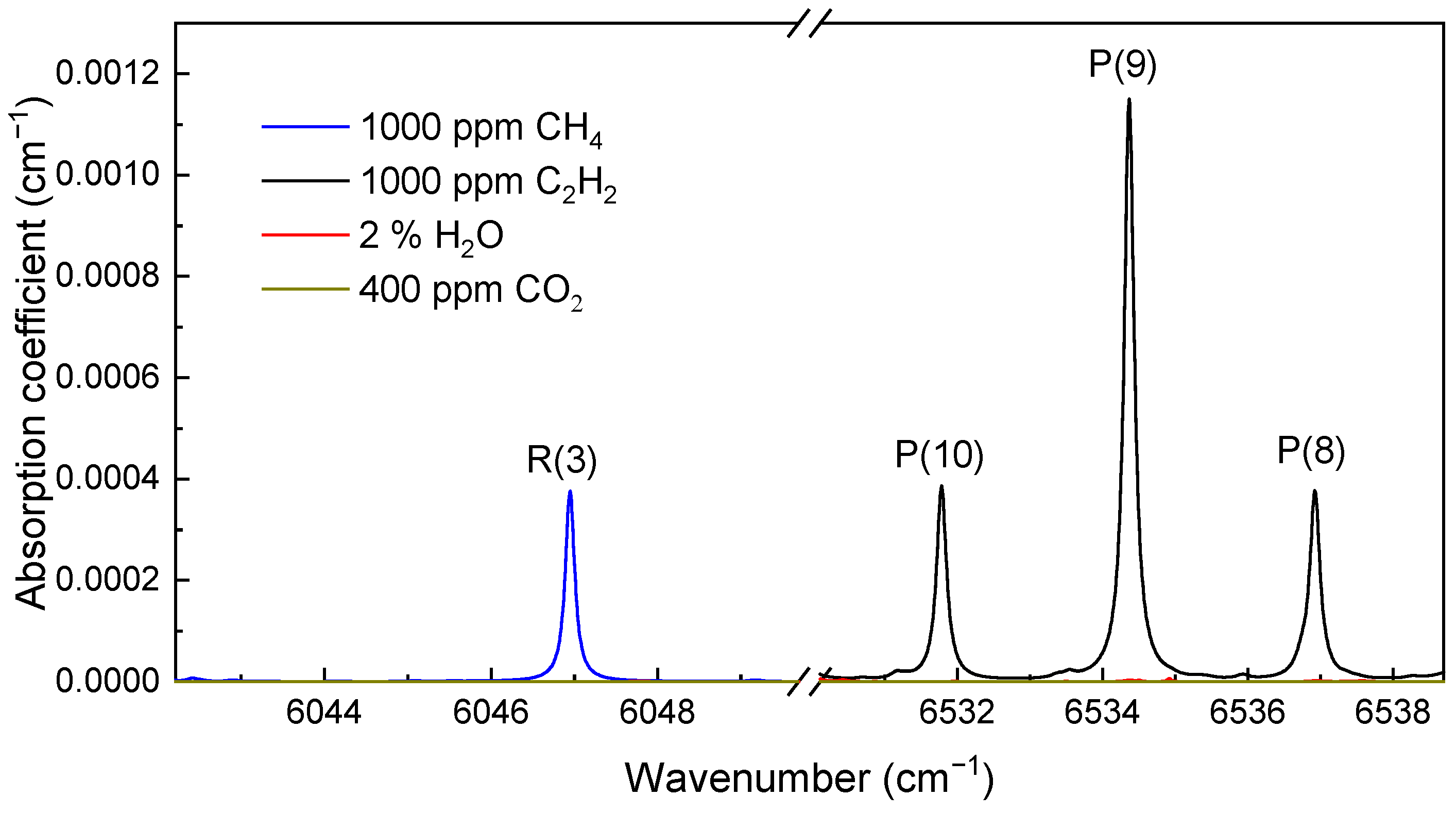
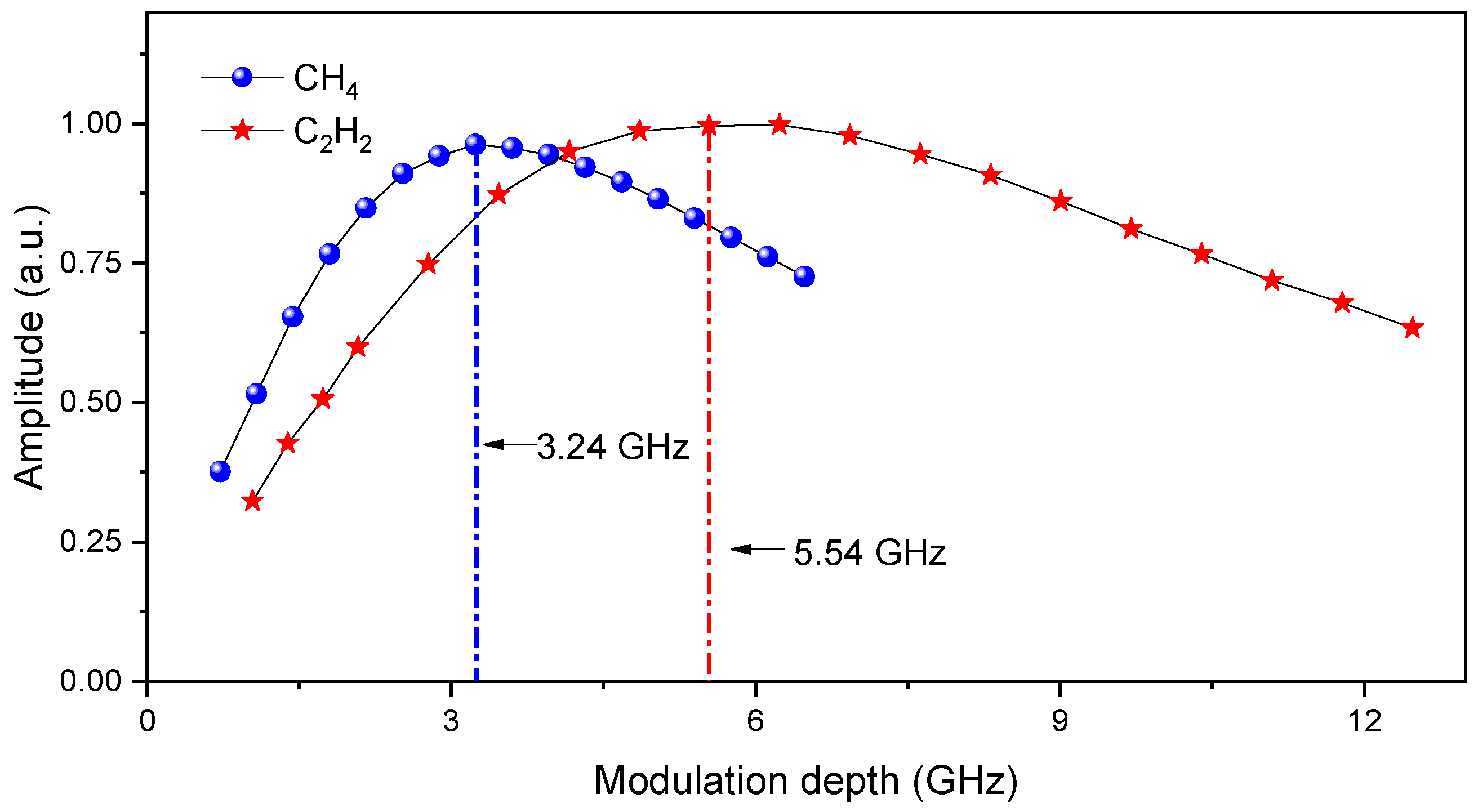
3.1. Absorption Transition Selection
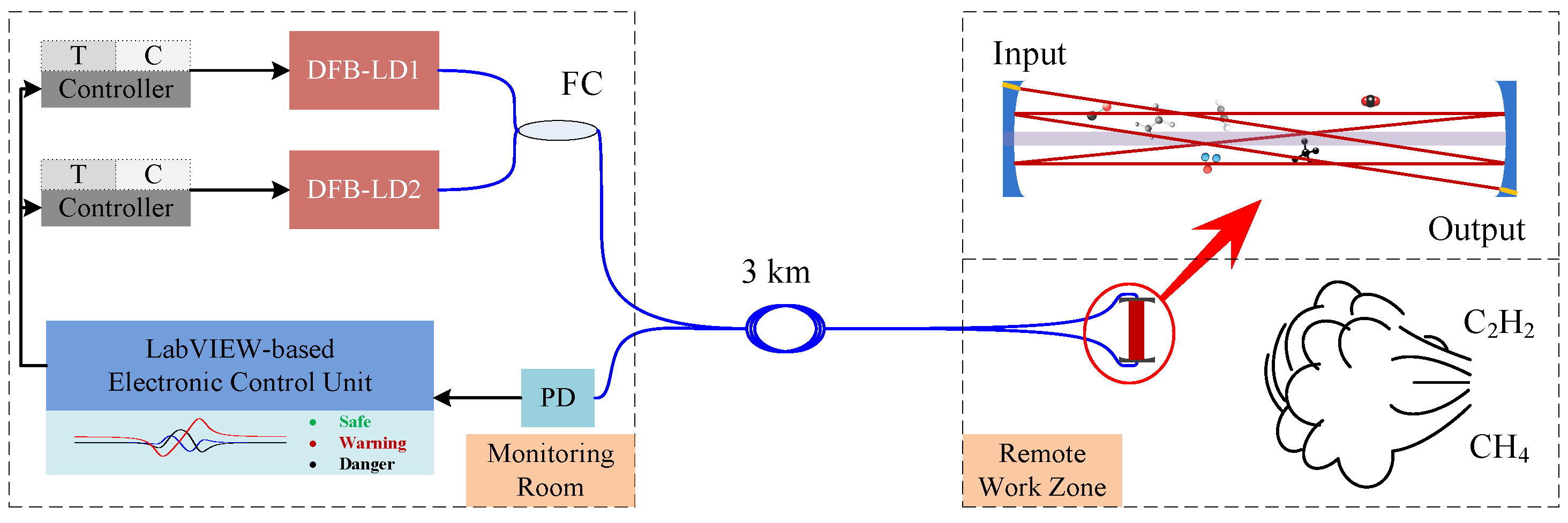
3.2. Experimental Apparatus
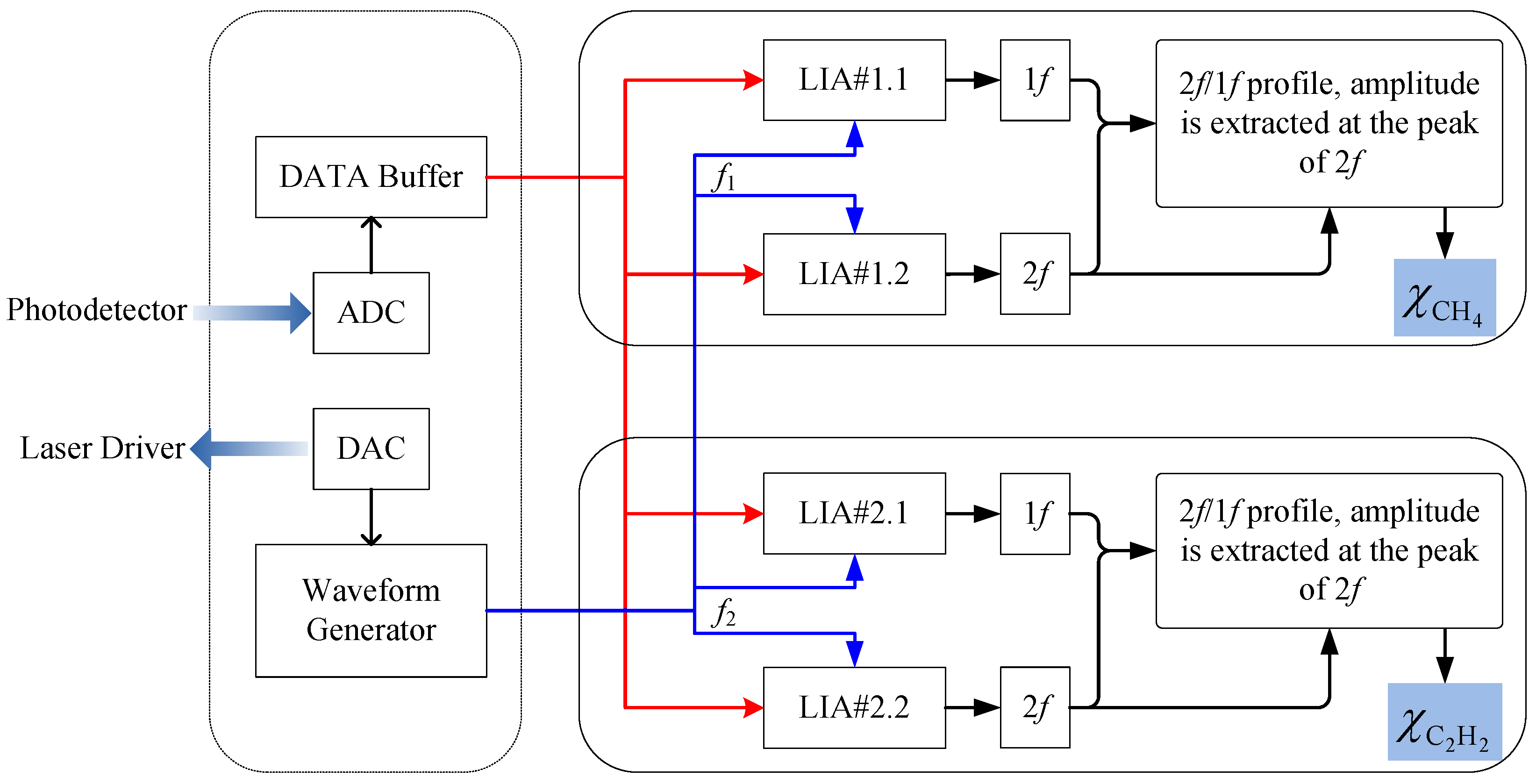
3.3. Flow Chart of the Data Retrieval with a LabVIEW-Based Platform
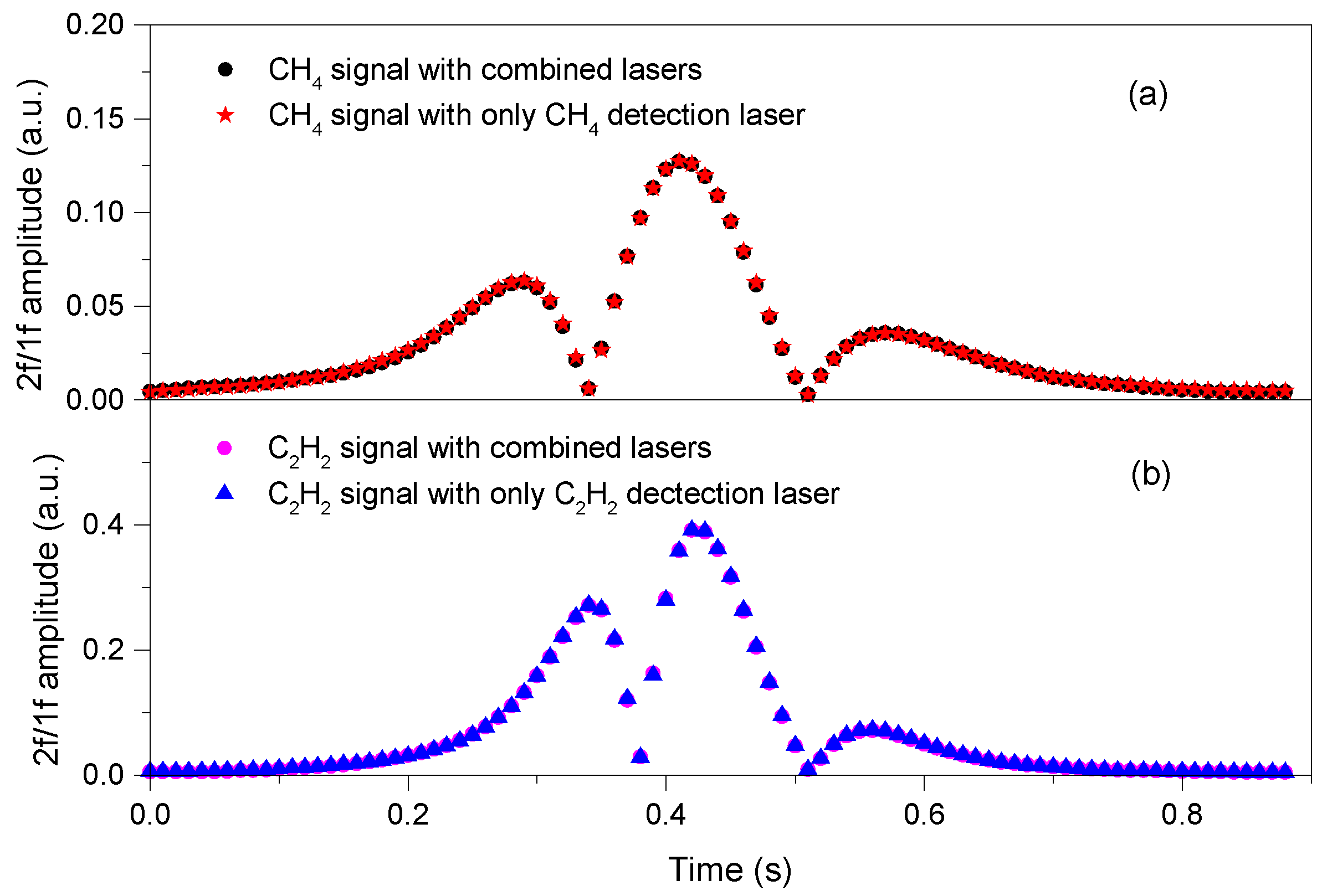
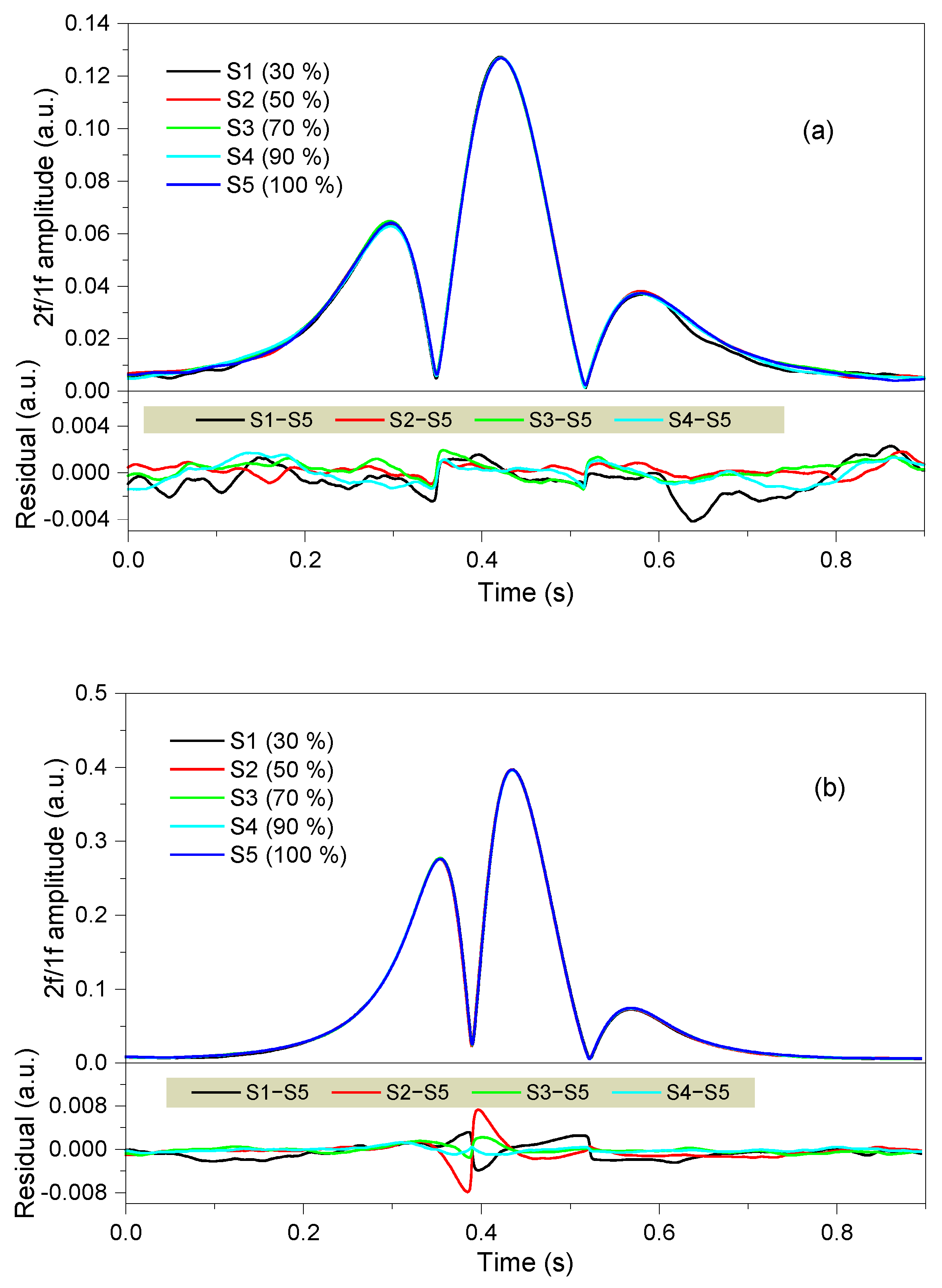
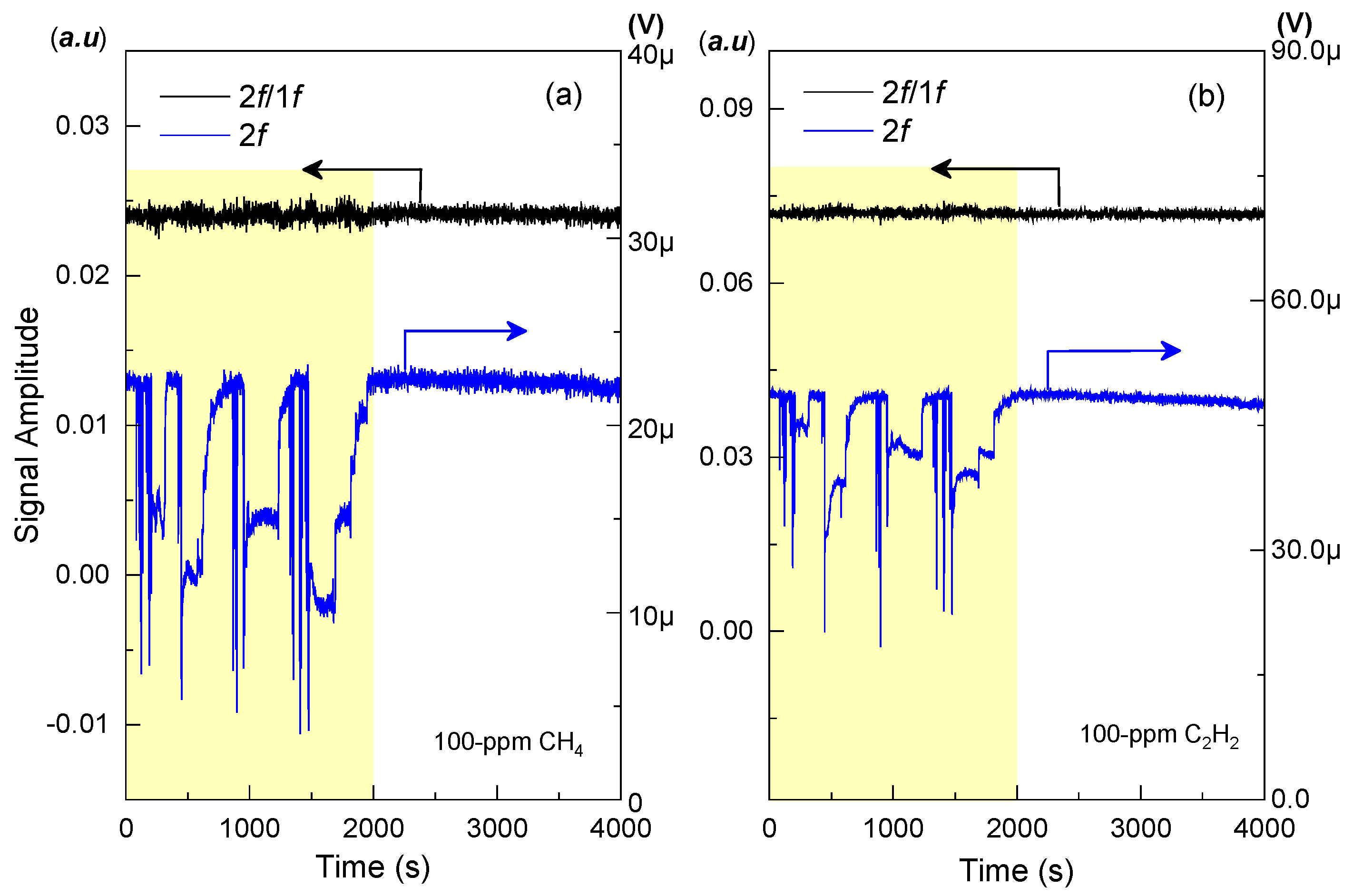
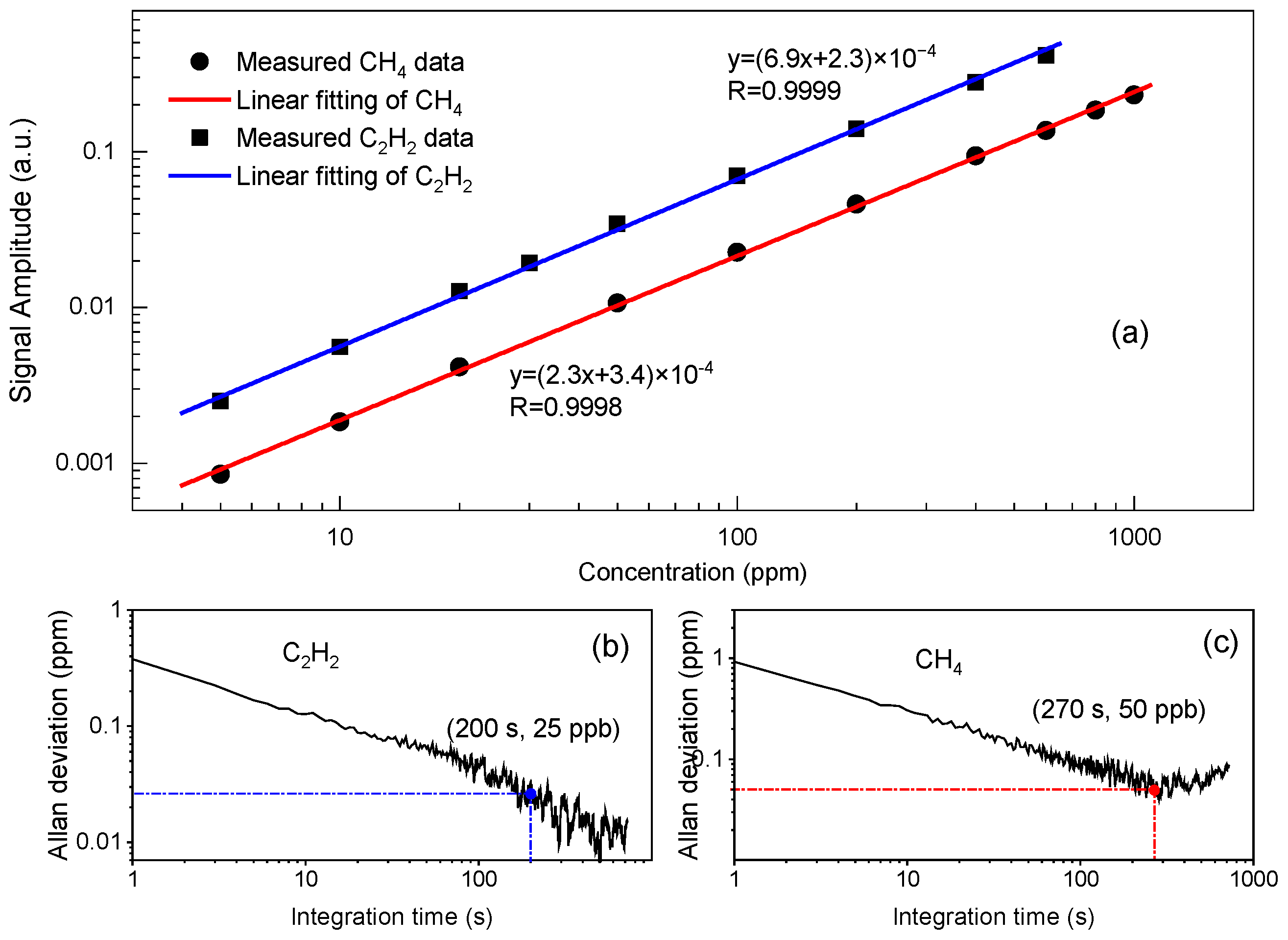
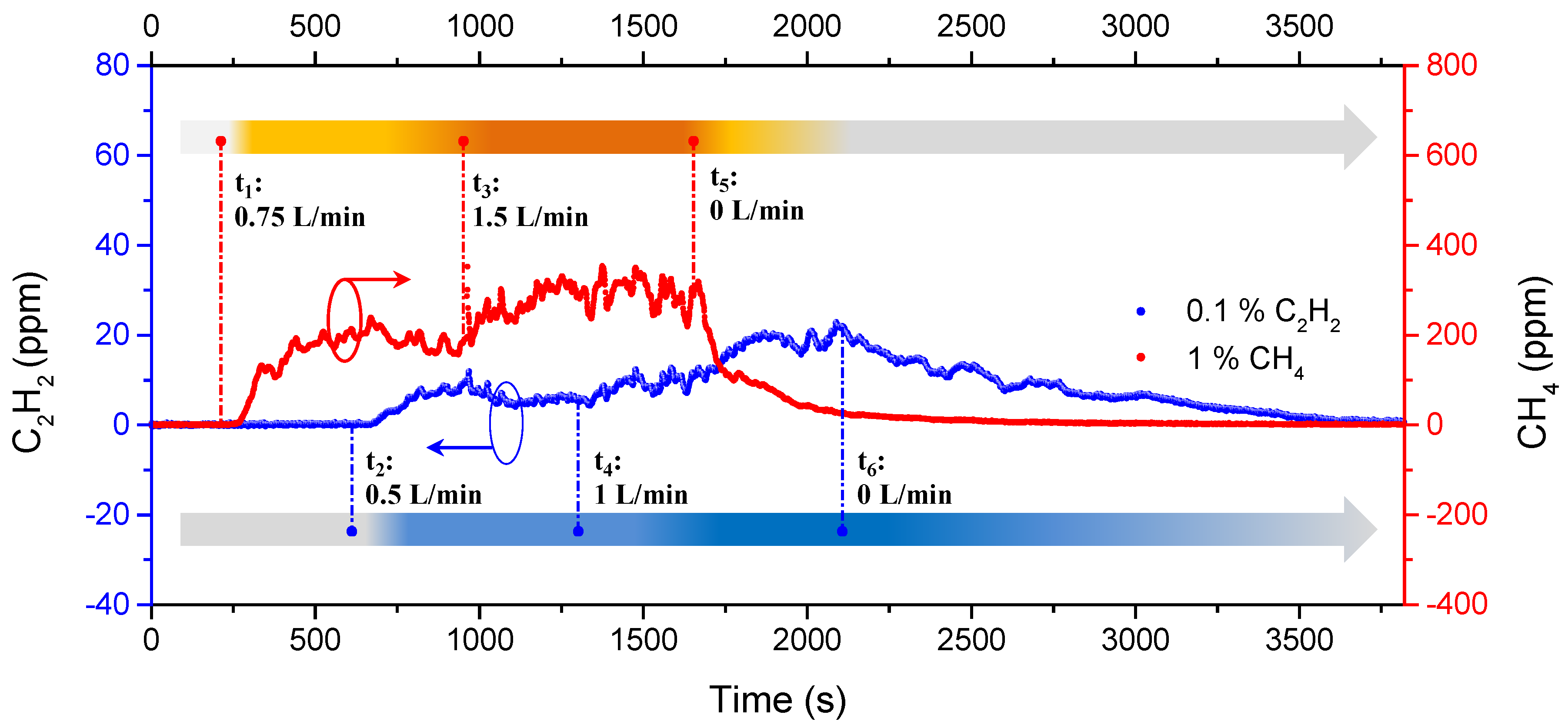
4. Experimental Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Wu, C.; Reniers, G.; Huang, L.; Kang, L.; Zhang, L. The future of hazardous chemical safety in China: Opportunities, problems, challenges and tasks. Sci. Total Environ. 2018, 643, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Yu, Y.; Wu, Y.; Feng, R.; Yu, N. Hierarchical leak detection and localization method in natural gas pipeline monitoring sensor networks. Sensors 2012, 12, 189–214. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Ching, J.Y.L.; Wu, J.C.Y.; Zhang, G.; Ren, W. A portable low-power QEPAS-based CO2 isotope sensor using a fiber-coupled interband cascade Lasers. Sensor. Actuators B. Chem. 2017, 246, 710–715. [Google Scholar] [CrossRef]
- Huang, Y.; Sinha, A.; Zhao, H.; Dang, X.; Zhang, Y.; Quan, X. Real time detection of hazardous hydroxyl radical using an electrochemical approach. Chem. Select. 2019, 4, 12507–12511. [Google Scholar] [CrossRef]
- Ma, X.; Zou, J.; Li, W.; He, J.J. Miniature spectrometer based on a Fourier transform spectrometer chip and a commercial photodetector array. Chin. Opt. Lett. 2019, 17, 123001. [Google Scholar] [CrossRef]
- Werle, P.; Slemr, F.; Maurer, K.; Kormann, R.; Mücke, R.; Jänker, B. Near-and mid-infrared laser-optical sensors for gas analysis. Opt. Lasers Eng. 2002, 37, 101–114. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Tatam, R.P. Optical gas sensing: A review. Meas. Sci. Technol. 2013, 24, 012004. [Google Scholar] [CrossRef]
- Wu, H.; Yin, X.; Dong, L.; Pei, K.; Sampaolo, A.; Patimisco, P.; Zheng, H.; Ma, W.; Zhang, L.; Yin, W. Simultaneous dual-gas QEPAS detection based on a fundamental and overtone combined vibration of quartz tuning fork. Appl. Phys. Lett. 2017, 110, 121104. [Google Scholar] [CrossRef]
- Liu, K.; Mei, J.; Zhang, W.; Chen, W.; Gao, X. Multi-resonator photoacoustic spectroscopy. Sensor. Actuators B. Chem. 2017, 251, 632–636. [Google Scholar] [CrossRef]
- Rieker, G.B.; Giorgetta, F.R.; Swann, W.C.; Kofler, J.; Zolot, A.M.; Sinclair, L.C.; Baumann, E.; Cromer, C.; Petron, G.; Sweeney, C.; et al. Frequency-comb-based remote sensing of greenhouse gases over kilometer air paths. Optica 2014, 1, 290. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Wang, G.; Zou, M.; Tan, T.; Liu, K.; Chen, W.; Gao, X. A fibered near-infrared laser heterodyne radiometer for simultaneous remote sensing of atmospheric CO2 and CH4. Opt. Lasers Eng. 2020, 129, 106083. [Google Scholar] [CrossRef]
- Tommasi, E.D.; Casa, G.; Gianfrani, L. An Intensity-Stabilized Diode-Laser Spectrometer for Sensitive Detection of NH3. IEEE Trans. Instrum. Meas. 2007, 56, 309–312. [Google Scholar] [CrossRef]
- Zhu, C.; Chang, J.; Wang, P.; Wei, W.; Wang, Q.; Wang, F.; Zhang, S. Continuously wavelength-tunable light source with constant-power output for elimination of residual amplitude modulation. IEEE Sens. J. 2015, 15, 316–321. [Google Scholar] [CrossRef]
- Wang, Q.; Chang, J.; Kong, D.L.; Liu, Y.N.; Wang, F.P.; Zhu, C.G.; Wei, W.; Liu, X.Z. Optical Measurement of Water Vapor Concentration and Gas Pressure. IEEE Sens. J. 2014, 14, 563–569. [Google Scholar] [CrossRef]
- Chang, H.; Chang, J.; Huang, Q.; Wang, Q.; Tian, C.; Wei, W.; Liu, Y. Immunity to laser power variation in a DFB diode laser based optical gas sensor using a division process. Sensors 2015, 15, 9582–9591. [Google Scholar] [CrossRef]
- Rieker, G.B.; Jeffries, J.B.; Hanson, R.K. Calibration-free wavelength-modulation spectroscopy for measurements of gas temperature and concentration in harsh environments. Appl. Opt. 2009, 48, 5546–5560. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Chao, X.; Sur, R.; Goldenstein, C.; Jeffries, J.; Hanson, R. Analysis of calibration-free wavelength-scanned wavelength modulation spectroscopy for practical gas sensing using tunable diode lasers. Meas. Sci. Technol. 2013, 24, 125203. [Google Scholar] [CrossRef]
- Spearrin, R.M.; Ren, W.; Jeffries, J.B.; Hanson, R.K. Multi-band infrared CO2 absorption sensor for sensitive temperature and species measurements in high-temperature gases. Appl. Phys. B Lasers 2014, 116, 855–865. [Google Scholar] [CrossRef]
- Li, H.; Rieker, G.B.; Liu, X.; Jeffries, J.B.; Hanson, R.K. Extension of wavelength-modulation spectroscopy to large modulation depth for diode laser absorption measurements in high-pressure gases. Appl. Opt. 2006, 45, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chang, J.; Lian, J.; Liu, Z.; Wang, Q.; Zhu, C. A time difference method for measurement of phase shift between distributed feedback laser diode (DFB-LD) output wavelength and intensity. Sensors 2015, 15, 16153–16161. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, G.; Zheng, Z.; Wang, R.; Tao, X.; Wang, P. A Method for Real-Time Monitoring of Inherent System Loss Designed for FLRDS-Based Gas Sensors. IEEE Photonics J. 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Friedlein, J.T.; Baumann, E.; Briggman, K.A.; Colacion, G.M.; Giorgetta, F.R.; Goldfain, A.M.; Herman, D.I.; Hoenig, E.V.; Hwang, J.; Newbury, N.R. Dual-comb photoacoustic spectroscopy. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Coddington, I.; Newbury, N.; Swann, W. Dual-comb spectroscopy. Optica 2016, 3, 414–426. [Google Scholar] [CrossRef]
- Cossel, K.C.; Waxman, E.M.; Giorgetta, F.R.; Cermak, M.; Coddington, I.R.; Hesselius, D.; Ruben, S.; Swann, W.C.; Truong, G.W.; Rieker, G.B. Open-path dual-comb spectroscopy to an airborne retroreflector. Optica 2017, 4, 724–728. [Google Scholar] [CrossRef]
- Chen, K.; Guo, M.; Liu, S.; Zhang, B.; Deng, H.; Zheng, Y.; Chen, Y.; Luo, C.; Tao, L.; Lou, M. Fiber-optic photoacoustic sensor for remote monitoring of gas micro-leakage. Opt. Express 2019, 27, 4648–4659. [Google Scholar] [CrossRef]
- Tombez, L.; Zhang, E.; Orcutt, J.; Kamlapurkar, S.; Green, W. Methane absorption spectroscopy on a silicon photonic chip. Optica 2017, 4, 1322–1325. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Vaittinen, O.; Metsälä, M.; Kraus, P.; Halonen, L. Direct detection of acetylene in air by continuous wave cavity ring-down spectroscopy. Appl. Phys. B Lasers 2010, 101, 671–682. [Google Scholar] [CrossRef]
- Gordon, I.E.; Rothman, L.S.; Hill, C.; Kochanov, R.V.; Tan, Y.; Bernath, P.F.; Birk, M.; Boudon, V.; Campargue, A.; Chance, K.V.; et al. The HITRAN2016 molecular spectroscopic database. J. Quant. Spectrosc. RA 2017, 203, 3–69. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, W.; Zhang, H.; Hu, M.; Hu, M.; Wei, Y.; Liang, J.; Kan, R.; Wang, Q. A Robust Optical Sensor for Remote Multi-Species Detection Combining Frequency-Division Multiplexing and Normalized Wavelength Modulation Spectroscopy. Sensors 2021, 21, 1073. https://doi.org/10.3390/s21041073
Jin W, Zhang H, Hu M, Hu M, Wei Y, Liang J, Kan R, Wang Q. A Robust Optical Sensor for Remote Multi-Species Detection Combining Frequency-Division Multiplexing and Normalized Wavelength Modulation Spectroscopy. Sensors. 2021; 21(4):1073. https://doi.org/10.3390/s21041073
Chicago/Turabian StyleJin, Wenling, Hui Zhang, Mai Hu, Mengpeng Hu, Yubin Wei, Jingqiu Liang, Ruifeng Kan, and Qiang Wang. 2021. "A Robust Optical Sensor for Remote Multi-Species Detection Combining Frequency-Division Multiplexing and Normalized Wavelength Modulation Spectroscopy" Sensors 21, no. 4: 1073. https://doi.org/10.3390/s21041073
APA StyleJin, W., Zhang, H., Hu, M., Hu, M., Wei, Y., Liang, J., Kan, R., & Wang, Q. (2021). A Robust Optical Sensor for Remote Multi-Species Detection Combining Frequency-Division Multiplexing and Normalized Wavelength Modulation Spectroscopy. Sensors, 21(4), 1073. https://doi.org/10.3390/s21041073






