Towards Clean and Safe Water: A Review on the Emerging Role of Imprinted Polymer-Based Electrochemical Sensors
Abstract
:1. Introduction
2. Scope of the Review
- Methods of synthesis of molecularly imprinted polymers
- Design of sensing electrodes
- Electrochemical characterization techniques
- Case studies of imprinted polymer-based electrochemical sensors of the target species. The focus is on imprinted vinylic, conjugated and sol-gel polymers.
3. Methods of Preparation of Imprinted Polymers and Electrode Materials
3.1. Monomers and Polymers
3.1.1. Vinylic Imprinted Polymers
3.1.2. Conductive Polymers
3.1.3. Sol-Gel Synthesis
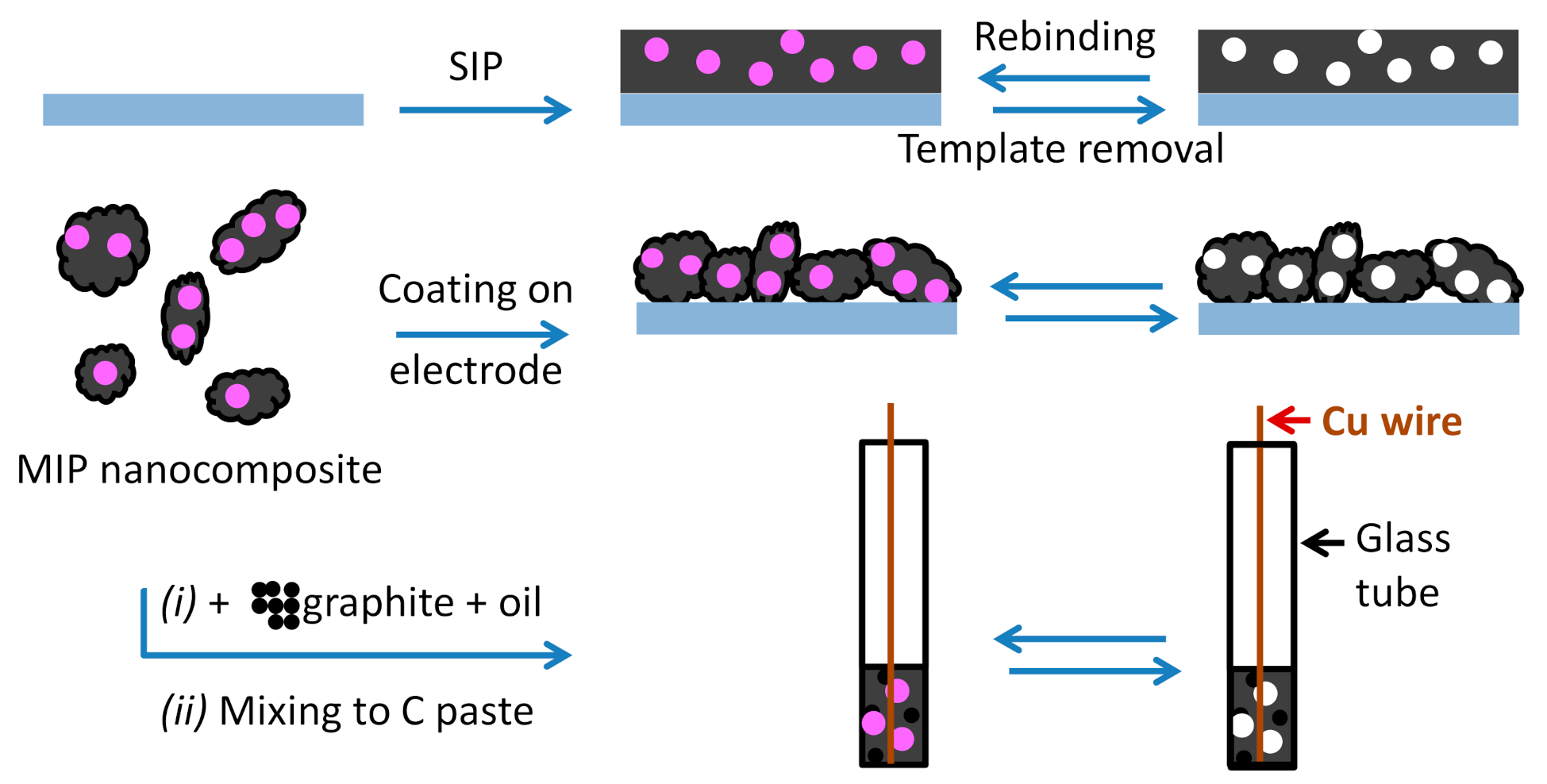
3.2. Electrode Material Preparation
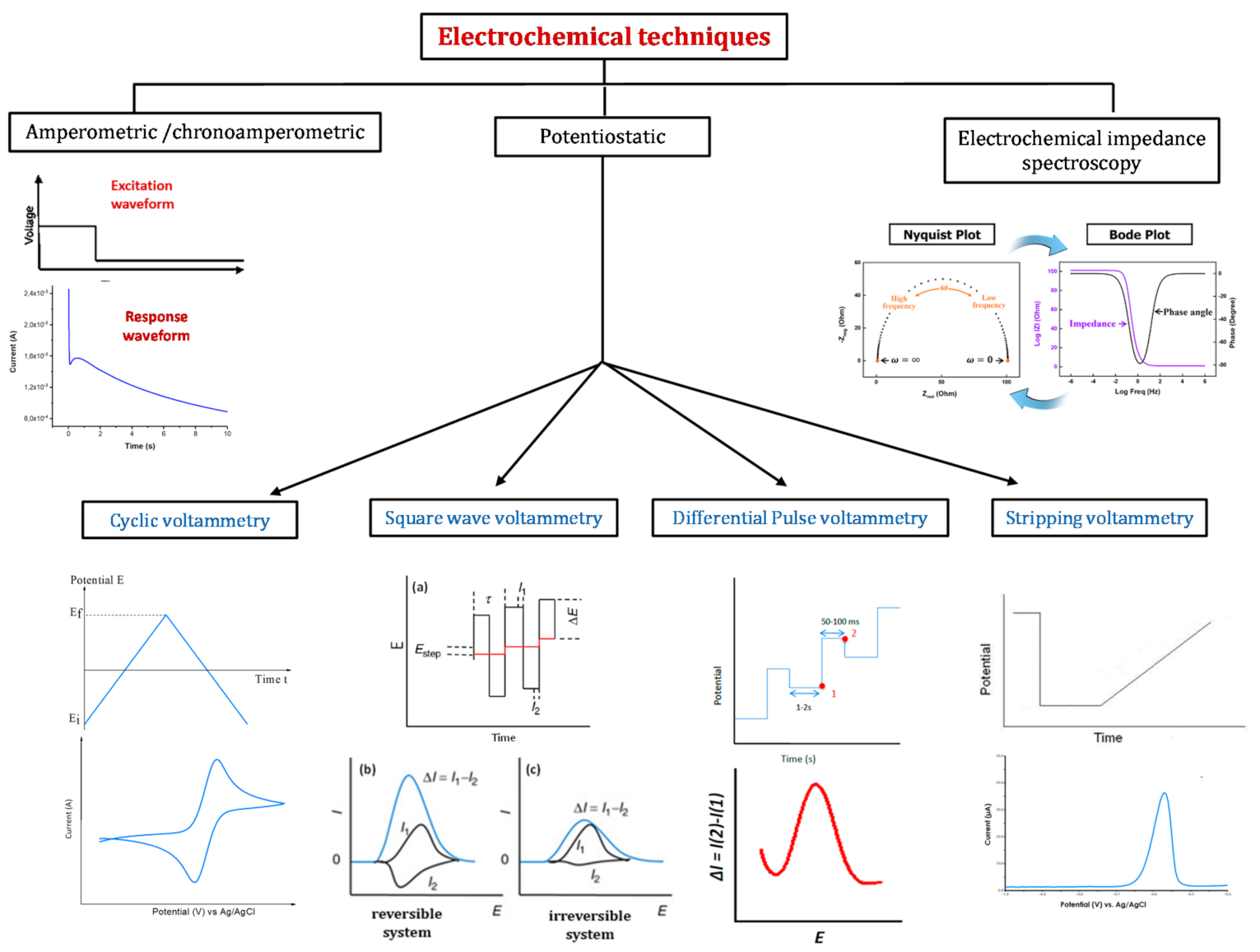
4. Detection Methods
4.1. Cyclic Voltammetry
4.2. Differential Pulse Voltammetry
4.3. Square Wave Voltammetry
4.4. Amperometric Methods
4.5. Stripping Voltammetry
4.6. Electrochemical Impedance Spectroscopy
4.7. Comparison of Electrochemical Techniques
5. Applications of Imprinted Polymer-Based Electrochemical Sensors
5.1. Tracking Pesticides with Molecularly Imprinted Polymers
5.1.1. Pesticide Imprinted Sol-Gels (PISGs)
5.1.2. Pesticide Imprinted Vinylic Polymers (PIVPs)
5.1.3. Pesticide Imprinted Conductive Polymers (PICPs)
5.1.4. Summary of Pesticide Imprinted Polymer-Based Electrochemical Sensors
5.2. Ion Imprinted Polymers
5.2.1. Ion Imprinted Vinylic Polymers (IIVPs)
5.2.2. Ion Imprinted Conductive Polymers (IICPs)
5.2.3. Ion Imprinted Sol-Gels (IISGs)
Detection of Copper Ions Cu(II)
Detection of Cadmium Ions Cd(II)
Detection of UO22+
Detection of Europium Eu3+
5.2.4. Summary of Experimental Conditions of Preparation and Performances of Ion Imprinted Polymers
5.3. Bacteria Imprinted Polymers
5.3.1. Whole Cell Imprinting
5.3.2. Bacterial Surface Imprinting
5.3.3. Bacterial Protein A Surface Imprinting
5.3.4. Imprinting of Bacterial Flagella Proteins
5.3.5. Bacterial Spore Imprinting
5.3.6. Imprinting Quorum Sensing Signaling Molecules
5.3.7. Summary of Bacteria Imprinted Polymers
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kümmerer, K.; Dionysiou, D.D.; Olsson, O.; Fatta-Kassinos, D. A path to clean water. Science 2018, 361, 222–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stradiotto, N.R.; Yamanaka, H.; Zanoni, M.V.B. Electrochemical sensors: A powerful tool in analytical chemistry. J. Braz. Chem. Soc. 2003, 14, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Zhou, A.Y.; Baruch, M.; Ajo-Franklin, C.M.; Maharbiz, M.M. A portable bioelectronic sensing system (BESSY) for environmental deployment incorporating differential microbial sensing in miniaturized reactors. PLoS ONE 2017, 12, e0184994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branger, C.; Meouche, W.; Margaillan, A. Recent advances on ion-imprinted polymers. React. Funct. Polym. 2013, 73, 859–875. [Google Scholar] [CrossRef]
- Li, Q.; Xia, Y.; Wan, X.; Yang, S.; Cai, Z.; Ye, Y.; Li, G. Morphology-dependent MnO2/nitrogen-doped graphene nanocomposites for simultaneous detection of trace dopamine and uric acid. Mater. Sci. Eng. C 2020, 109, 110615. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.-T.; Liu, Y.; Qi, X.-M.; Jin, H.-G.; Yang, C.; Liu, J.; Li, G.-L.; He, Q.-G. Recent Advances in Black Phosphorus-Based Electrochemical Sensors: A Review. Anal. Chim. Acta 2021, 1170, 338480. [Google Scholar] [CrossRef]
- Majdinasab, M.; Daneshi, M.; Marty, J.L. Recent developments in non-enzymatic (bio) sensors for detection of pesticide residues: Focusing on antibody, aptamer and molecularly imprinted polymer. Talanta 2021, 232, 122397. [Google Scholar] [CrossRef]
- Saylan, Y.; Akgönüllü, S.; Yavuz, H.; Ünal, S.; Denizli, A. Molecularly imprinted polymer based sensors for medical applications. Sensors 2019, 19, 1279. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Feng, T.; Xu, J.; Xue, C. Recent advances of molecularly imprinted polymer-based sensors in the detection of food safety hazard factors. Biosens. Bioelectron. 2019, 141, 111447. [Google Scholar] [CrossRef]
- Arreguin-Campos, R.; Jiménez-Monroy, K.L.; Diliën, H.; Cleij, T.J.; van Grinsven, B.; Eersels, K. Imprinted Polymers as Synthetic Receptors in Sensors for Food Safety. Biosensors 2021, 11, 46. [Google Scholar] [CrossRef]
- Waheed, A.; Mansha, M.; Ullah, N. Nanomaterials-based electrochemical detection of heavy metals in water: Current status, challenges and future direction. TrAC Trends Anal. Chem. 2018, 105, 37–51. [Google Scholar] [CrossRef]
- Beluomini, M.A.; da Silva, J.L.; de Sá, A.C.; Buffon, E.; Pereira, T.C.; Stradiotto, N.R. Electrochemical sensors based on molecularly imprinted polymer on nanostructured carbon materials: A review. J. Electroanal. Chem. 2019, 840, 343–366. [Google Scholar] [CrossRef]
- Mazouz, Z.; Rahali, S.; Fourati, N.; Zerrouki, C.; Aloui, N.; Seydou, M.; Yaakoubi, N.; Chehimi, M.M.; Othmane, A.; Kalfat, R. Highly selective polypyrrole MIP-based gravimetric and electrochemical sensors for picomolar detection of glyphosate. Sensors 2017, 17, 2586. [Google Scholar] [CrossRef] [Green Version]
- Ait-Touchente, Z.; Sakhraoui, H.E.E.Y.; Fourati, N.; Zerrouki, C.; Maouche, N.; Yaakoubi, N.; Touzani, R.; Chehimi, M.M. High performance zinc oxide nanorod-doped ion imprinted polypyrrole for the selective electrosensing of mercury II ions. Appl. Sci. 2020, 10, 7010. [Google Scholar] [CrossRef]
- Mrabet, B.; Mejbri, A.; Mahouche, S.; Gam-Derouich, S.; Turmine, M.; Mechouet, M.; Lang, P.; Bakala, H.; Ladjimi, M.; Bakhrouf, A. Controlled adhesion of Salmonella Typhimurium to poly (oligoethylene glycol methacrylate) grafts. Surf. Interface Anal. 2011, 43, 1436–1443. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, Z.; Li, J.; Ma, X.; Chen, L.; Yang, X. Molecular imprinting technology for microorganism analysis. TrAC Trends Anal. Chem. 2018, 106, 190–201. [Google Scholar] [CrossRef]
- Roushani, M.; Sarabaegi, M.; Rostamzad, A. Novel electrochemical sensor based on polydopamine molecularly imprinted polymer for sensitive and selective detection of Acinetobacter baumannii. J. Iran. Chem. Soc. 2020, 17, 2407–2413. [Google Scholar] [CrossRef]
- Hande, P.E.; Samui, A.B.; Kulkarni, P.S. Highly selective monitoring of metals by using ion-imprinted polymers. Environ. Sci. Pollut. Res. 2015, 22, 7375–7404. [Google Scholar] [CrossRef]
- Adumitrăchioaie, A.; Tertiș, M.; Cernat, A.; Săndulescu, R.; Cristea, C. Electrochemical methods based on molecularly imprinted polymers for drug detection. A review. Int. J. Electrochem. Sci 2018, 13, 2556–2576. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Amine, A. Recent advances in electrochemical sensors based on molecularly imprinted polymers and nanomaterials. Electroanalysis 2019, 31, 188–201. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Torbati, M.; Mousavi, M.-M.; de la Guardia, M.; Dolatabadi, J.E.N. Nanomaterial-based molecularly imprinted polymers for pesticides detection: Recent trends and future prospects. TrAC Trends Anal. Chem. 2020, 115943. [Google Scholar] [CrossRef]
- Karrat, A.; Lamaoui, A.; Amine, A.; Palacios-Santander, J.M.; Cubillana-Aguilera, L. Applications of Chitosan in Molecularly and Ion Imprinted Polymers. Chem. Afr. 2020, 3, 513–533. [Google Scholar] [CrossRef]
- Herrera-Chacón, A.; Cetó, X.; Del Valle, M. Molecularly imprinted polymers-towards electrochemical sensors and electronic tongues. Anal. Bioanal. Chem. 2021, 1–24. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.; Abd-Elaal, A.A.; Badr, E.A.; Abou Kana, M.T. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Wei, P.; Li, Z.; Zhao, X.; Song, R.; Zhu, Z. Fe3O4/SiO2/CS surface ion-imprinted polymer modified glassy carbon electrode for highly sensitivity and selectivity detection of toxic metal ions. J. Taiwan Inst. Chem. Eng. 2020, 113, 107–113. [Google Scholar] [CrossRef]
- Cheong, W.J.; Yang, S.H.; Ali, F. Molecular imprinted polymers for separation science: A review of reviews. J. Sep. Sci. 2013, 36, 609–628. [Google Scholar] [CrossRef]
- Ndunda, E.N. Molecularly imprinted polymers—A closer look at the control polymer used in determining the imprinting effect: A mini review. J. Mol. Recognit. 2020, 33, e2855. [Google Scholar] [CrossRef]
- Li, L.; Lin, Z.-Z.; Chen, X.-M.; Zhang, H.-Y.; Lin, Y.-D.; Lai, Z.-Z.; Huang, Z.-Y. Molecularly imprinted polymers for extraction of malachite green from fish samples prior to its determination by HPLC. Microchim. Acta 2015, 182, 1791–1796. [Google Scholar] [CrossRef]
- Piletsky, S.A.; Turner, A.P. Electrochemical sensors based on molecularly imprinted polymers. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2002, 14, 317–323. [Google Scholar] [CrossRef]
- Suryanarayanan, V.; Wu, C.T.; Ho, K.C. Molecularly imprinted electrochemical sensors. Electroanalysis 2010, 22, 1795–1811. [Google Scholar] [CrossRef]
- Naarmann, H. Strategies for synthesizing conducting polymers. Synth. Met. 1991, 41, 1–6. [Google Scholar] [CrossRef]
- Ansari, R. Polypyrrole conducting electroactive polymers: Synthesis and stability studies. E-J. Chem. 2006, 3, 186–201. [Google Scholar] [CrossRef] [Green Version]
- Dickey, F.H. The preparation of specific adsorbents. Proc. Natl. Acad. Sci. USA 1949, 35, 227. [Google Scholar] [CrossRef] [Green Version]
- Wuff, G.; Sarhan, A. The use of polymers with enzyme-analogous structures for the resolution of racemate. Angew. Chem. Int. Ed. 1972, 11, 341–345. [Google Scholar]
- Boitard, C.; Lamouri, A.; Ménager, C.; Griffete, N.b.w. Whole Protein Imprinting over Magnetic Nanoparticles Using Photopolymerization. ACS Appl. Polym. Mater. 2019, 1, 928–932. [Google Scholar] [CrossRef]
- Ahmad, R.; Félidj, N.; Boubekeur-Lecaque, L.; Lau-Truong, S.; Gam-Derouich, S.; Decorse, P.; Lamouri, A.; Mangeney, C. Water-soluble plasmonic nanosensors with synthetic receptors for label-free detection of folic acid. Chem. Commun. 2015, 51, 9678–9681. [Google Scholar] [CrossRef]
- Msaadi, R.; Yilmaz, G.; Allushi, A.; Hamadi, S.; Ammar, S.; Chehimi, M.M.; Yagci, Y. Highly selective copper ion imprinted clay/polymer nanocomposites prepared by visible light initiated radical photopolymerization. Polymers 2019, 11, 286. [Google Scholar] [CrossRef] [Green Version]
- Msaadi, R.; Ammar, S.; Chehimi, M.M.; Yagci, Y. Diazonium-based ion-imprinted polymer/clay nanocomposite for the selective extraction of lead (II) ions in aqueous media. Eur. Polym. J. 2017, 89, 367–380. [Google Scholar] [CrossRef]
- Bakas, I.; Salmi, Z.; Gam-Derouich, S.; Jouini, M.; Lépinay, S.; Carbonnier, B.; Khlifi, A.; Kalfat, R.; Geneste, F.; Yagci, Y. Molecularly imprinted polymeric sensings layers grafted from aryl diazonium-modified surfaces for electroanalytical applications. A mini review. Surf. Interface Anal. 2014, 46, 1014–1020. [Google Scholar] [CrossRef]
- Mousli, F.; Snoussi, Y.; Khalil, A.M.; Jlassi, K.; Mekki, A.; Chehimi, M.M. Surface Modification of Polymeric Substrates with Photo-and Sonochemically Designed Macromolecular Grafts. Surf. Modif. Polym. Methods Appl. 2019, 273–315. [Google Scholar]
- Ahmad, R.; Griffete, N.b.w.; Lamouri, A.; Felidj, N.; Chehimi, M.M.; Mangeney, C. Nanocomposites of gold nanoparticles@ molecularly imprinted polymers: Chemistry, processing, and applications in sensors. Chem. Mater. 2015, 27, 5464–5478. [Google Scholar] [CrossRef]
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Garcia-Cruz, A.; Piletsky, S.A. Molecularly imprinted polymers in electrochemical and optical sensors. Trends Biotechnol. 2019, 37, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Shoaie, I.S.; Khalilzadeh, M.A.; Asl, M.S.; Van Le, Q.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent developments in conducting polymers: Applications for electrochemistry. RSC Adv. 2020, 10, 37834–37856. [Google Scholar] [CrossRef]
- Choi, W.; An, T.; Lim, G. Fabrication of conducting polymer nanowires. In Nanowires-Implementations and Applications; InTech: Rijeka, Croatia, 2011; pp. 440–454. [Google Scholar]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.-B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, A.; Kaur, H. Review on nanomaterials/conducting polymer based nanocomposites for the development of biosensors and electrochemical sensors. Polym. Plast. Technol. Mater. 2020, 60, 504–521. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive polymers: Opportunities and challenges in biomedical applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Mandi, U.; Pramanik, M.; Roy, A.S.; Salam, N.; Bhaumik, A.; Islam, S.M. Chromium (VI) grafted mesoporous polyaniline as a reusable heterogeneous catalyst for oxidation reactions in aqueous medium. RSC Adv. 2014, 4, 15431–15440. [Google Scholar] [CrossRef]
- Peterson, D.S. Sol–Gel Technique. In Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer: Boston, MA, USA, 2013; pp. 1–7. [Google Scholar]
- Huang, J.; Zhang, X.; Lin, Q.; He, X.; Xing, X.; Huai, H.; Lian, W.; Zhu, H. Electrochemical sensor based on imprinted sol–gel and nanomaterials for sensitive determination of bisphenol A. Food Control 2011, 22, 786–791. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, Y.; Zhang, H.; Luo, L.; Yao, S. Layer-by-layer assembly sensitive electrochemical sensor for selectively probing l-histidine based on molecular imprinting sol–gel at functionalized indium tin oxide electrode. Biosens. Bioelectron. 2010, 26, 696–702. [Google Scholar] [CrossRef]
- Kia, S.; Fazilati, M.; Salavati, H.; Bohlooli, S. Preparation of a novel molecularly imprinted polymer by the sol–gel process for solid phase extraction of vitamin D3. RSC Adv. 2016, 6, 31906–31914. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef]
- Gam-Derouich, S.; Ngoc Nguyen, M.; Madani, A.; Maouche, N.; Lang, P.; Perruchot, C.; Chehimi, M.M. Aryl diazonium salt surface chemistry and ATRP for the preparation of molecularly imprinted polymer grafts on gold substrates. Surf. Interface Anal. 2010, 42, 1050–1056. [Google Scholar] [CrossRef]
- Mahouche-Chergui, S.; Gam-Derouich, S.; Mangeney, C.; Chehimi, M.M. Aryl diazonium salts: A new class of coupling agents for bonding polymers, biomacromolecules and nanoparticles to surfaces. Chem. Soc. Rev. 2011, 40, 4143–4166. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Dong, H.; Jakubowski, W.; Pietrasik, J.; Kusumo, A. Grafting from surfaces for “everyone”: ARGET ATRP in the presence of air. Langmuir 2007, 23, 4528–4531. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.-A. Surface-initiated controlled radical polymerization: State-of-the-art, opportunities, and challenges in surface and interface engineering with polymer brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef] [Green Version]
- Lo, M.; Seydou, M.; Bensghaïer, A.; Pires, R.; Gningue-Sall, D.; Aaron, J.-J.; Mekhalif, Z.; Delhalle, J.; Chehimi, M.M. Polypyrrole-wrapped carbon nanotube composite films coated on diazonium-modified flexible ITO sheets for the electroanalysis of heavy metal ions. Sensors 2020, 20, 580. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhou, G.; Li, H.; Liu, Q.; Zhang, S.; Kong, J. A novel molecularly imprinted sensor for selectively probing imipramine created on ITO electrodes modified by Au nanoparticles. Talanta 2009, 78, 26–32. [Google Scholar] [CrossRef]
- Sulitzky, C.; Rückert, B.; Hall, A.J.; Lanza, F.; Unger, K.; Sellergren, B. Grafting of molecularly imprinted polymer films on silica supports containing surface-bound free radical initiators. Macromolecules 2002, 35, 79–91. [Google Scholar] [CrossRef]
- Bakas, I.; Salmi, Z.; Jouini, M.; Geneste, F.; Mazerie, I.; Floner, D.; Carbonnier, B.; Yagci, Y.; Chehimi, M.M. Picomolar detection of melamine using molecularly imprinted polymer-based electrochemical sensors prepared by UV-graft photopolymerization. Electroanalysis 2015, 27, 429–439. [Google Scholar] [CrossRef]
- Iskierko, Z.; Sharma, P.S.; Bartold, K.; Pietrzyk-Le, A.; Noworyta, K.; Kutner, W. Molecularly imprinted polymers for separating and sensing of macromolecular compounds and microorganisms. Biotechnol. Adv. 2016, 34, 30–46. [Google Scholar] [CrossRef]
- Qi, P.; Wan, Y.; Zhang, D. Impedimetric biosensor based on cell-mediated bioimprinted films for bacterial detection. Biosens. Bioelectron. 2013, 39, 282–288. [Google Scholar] [CrossRef]
- Mazzotta, E.; Turco, A.; Chianella, I.; Guerreiro, A.; Piletsky, S.A.; Malitesta, C. Solid-phase synthesis of electroactive nanoparticles of molecularly imprinted polymers. A novel platform for indirect electrochemical sensing applications. Sens. Actuators B: Chem. 2016, 229, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Simões, F.R.; Xavier, M.G. Electrochemical sensors. In Nanoscience and Its Applications; Elsevier Inc.: Oxford, UK, 2017; pp. 155–178. [Google Scholar]
- Brett, C.; Oliveira Brett, A.M. Electrochemistry: Principles, Methods, and Applications; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Deroco, P.B.; de Fátima Giarola, J.; Júnior, D.W.; Lorga, G.A.; Kubota, L.T. based electrochemical sensing devices. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 89, pp. 91–137. [Google Scholar]
- Lo, M.; Ktari, N.; Gningue-Sall, D.; Madani, A.; Aaron, S.E.; Aaron, J.-J.; Mekhalif, Z.; Delhalle, J.; Chehimi, M.M. Polypyrrole: A reactive and functional conductive polymer for the selective electrochemical detection of heavy metals in water. Emergent Mater. 2020, 3, 815–839. [Google Scholar] [CrossRef]
- Margarit-Mattos, I. EIS and organic coatings performance: Revisiting some key points. Electrochim. Acta 2020, 354, 136725. [Google Scholar] [CrossRef]
- Kemp, N. A Tutorial on Electrochemical Impedance Spectroscopy and Nanogap Electrodes for Biosensing Applications. IEEE Sens. J. 2021. [Google Scholar] [CrossRef]
- Kanoun, O. Impedance Spectroscopy: Advanced Applications: Battery Research, Bioimpedance, System Design; De Gruyter: Berlin, Germany, 2019. [Google Scholar]
- Grysiński, T.; Moroń, Z. Planar sensors for local conductivity measurements in biological objects—Design, modelling, sensitivity maps. Sens. Actuators B Chem. 2011, 158, 190–198. [Google Scholar] [CrossRef]
- Hall, D.M.; Duffy, T.; Ziomek-Moroz, M.; Lvov, S.N. Electrochemical impedance spectroscopy and finite element analysis modeling of a 4-electrode humidity sensor for natural gas transportation pipelines. Rev. Sci. Instrum. 2019, 90, 015005. [Google Scholar] [CrossRef]
- Choi, W.; Shin, H.-C.; Kim, J.M.; Choi, J.-Y.; Yoon, W.-S. Modeling and applications of electrochemical impedance spectroscopy (EIS) for lithium-ion batteries. J. Electrochem. Sci. Technol. 2020, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Scully, J.R.; Silverman, D.C.; Kendig, M.W. Electrochemical Impedance: Analysis and Interpretation; ASTM International: West Conshohocken, PA, USA, 1993. [Google Scholar]
- Lasia, A. Semiconductors and Mott-Schottky Plots. In Electrochemical Impedance Spectroscopy and Its Applications; Springer: Berlin/Heidelberg, Germany, 2014; pp. 251–255. [Google Scholar]
- Gupta, R.; Raza, N.; Bhardwaj, S.K.; Vikrant, K.; Kim, K.-H.; Bhardwaj, N. Advances in nanomaterial-based electrochemical biosensors for the detection of microbial toxins, pathogenic bacteria in food matrices. J. Hazard. Mater. 2021, 401, 123379. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Du, X. Electrochemical biosensors for detection of foodborne pathogens. Micromachines 2019, 10, 222. [Google Scholar] [CrossRef] [Green Version]
- Zdrachek, E.; Bakker, E. Potentiometric sensing. Anal. Chem. 2020, 93, 72–102. [Google Scholar] [CrossRef]
- Cuartero, M.; Colozza, N.; Fernández-Pérez, B.M.; Crespo, G.A. Why ammonium detection is particularly challenging but insightful with ionophore-based potentiometric sensors–an overview of the progress in the last 20 years. Analyst 2020, 145, 3188–3210. [Google Scholar] [CrossRef] [Green Version]
- Stern, H.A.; Sadoway, D.R.; Tester, J.W. Copper sulfate reference electrode. J. Electroanal. Chem. 2011, 659, 143–150. [Google Scholar] [CrossRef]
- Iqbal, M.; Rangreez, T.; Mobin, R. Ion selective membrane electrodes as sensors for detection of heavy metal ions. Mater. Res. Found. 2017. [Google Scholar] [CrossRef]
- Hall, D.; Beck, J.; Lvov, S.; Ziomek-Moroz, M. Review of pH and reference electrodes for monitoring corrosion in HPHT extreme environments. In Proceedings of the NACE—International Corrosion Conference Series, Dallas, TX, USA, 15–19 March 2015. [Google Scholar]
- Orellana, G.; Cano-Raya, C.; López-Gejo, J.; Santos, A.R. 3.10—Online Monitoring Sensors. In Treatise on Water Science; Wilderer, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 221–261. [Google Scholar]
- Beduk, T.; Bihar, E.; Surya, S.G.; Castillo, A.N.; Inal, S.; Salama, K.N. A paper-based inkjet-printed PEDOT:PSS/ZnO sol-gel hydrazine sensor. Sens. Actuators B Chem. 2020, 306, 127539. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D. Nanomaterials in electrochemical biosensors for pesticide detection: Advances and challenges in food analysis. Microchim. Acta 2016, 183, 2063–2083. [Google Scholar] [CrossRef]
- Chapalamadugu, S.; Chaudhry, G.R. Microbiological and biotechnological aspects of metabolism of carbamates and organophosphates. Crit. Rev. Biotechnol. 1992, 12, 357–389. [Google Scholar] [CrossRef]
- Pundir, C.; Malik, A. Bio-sensing of organophosphorus pesticides: A review. Biosens. Bioelectron. 2019, 140, 111348. [Google Scholar] [CrossRef]
- Hu, H.; Wang, B.; Li, Y.; Wang, P.; Yang, L. Acetylcholinesterase Sensor with Patterned Structure for Detecting Organophosphorus Pesticides Based on Titanium Dioxide Sol-gel Carrier. Electroanalysis 2020, 32, 1834–1842. [Google Scholar] [CrossRef]
- Cui, H.-F.; Wu, W.-W.; Li, M.-M.; Song, X.; Lv, Y.; Zhang, T.-T. A highly stable acetylcholinesterase biosensor based on chitosan-TiO2-graphene nanocomposites for detection of organophosphate pesticides. Biosens. Bioelectron. 2018, 99, 223–229. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, B.; Li, Y.; Shu, W.; Hu, H.; Yang, L. An acetylcholinesterase biosensor with high stability and sensitivity based on silver nanowire–graphene–TiO 2 for the detection of organophosphate pesticides. RSC Adv. 2019, 9, 25248–25256. [Google Scholar] [CrossRef]
- Song, Y.; Chen, J.; Sun, M.; Gong, C.; Shen, Y.; Song, Y.; Wang, L. A simple electrochemical biosensor based on AuNPs/MPS/Au electrode sensing layer for monitoring carbamate pesticides in real samples. J. Hazard. Mater. 2016, 304, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Maulidiyah, M.; Azis, T.; Lindayani, L.; Wibowo, D.; Salim, L.O.A.; Aladin, A.; Nurdin, M. Sol-gel TiO2/Carbon Paste Electrode Nanocomposites for Electrochemical-assisted Sensing of Fipronil Pesticide. J. Electrochem. Sci. Technol. 2019, 10, 394–401. [Google Scholar] [CrossRef] [Green Version]
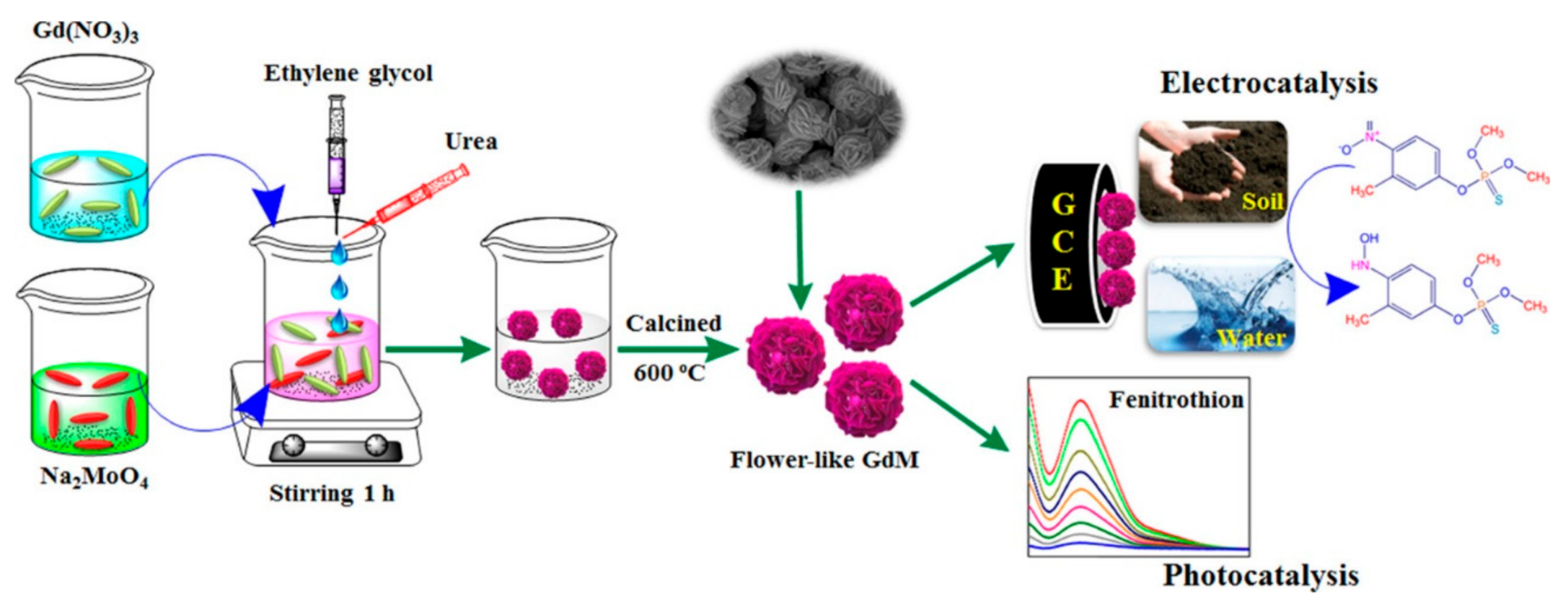
- Vinoth Kumar, J.; Karthik, R.; Chen, S.-M.; Natarajan, K.; Karuppiah, C.; Yang, C.-C.; Muthuraj, V. 3D flower-like gadolinium molybdate catalyst for efficient detection and degradation of organophosphate pesticide (fenitrothion). ACS Appl. Mater. Interfaces 2018, 10, 15652–15664. [Google Scholar] [CrossRef]
- Wei, X.-P.; Luo, Y.-L.; Xu, F.; Chen, Y.-S.; Yang, L.-H. In-situ non-covalent dressing of multi-walled carbon nanotubes@ titanium dioxides with carboxymethyl chitosan nanocomposite electrochemical sensors for detection of pesticide residues. Mater. Des. 2016, 111, 445–452. [Google Scholar] [CrossRef]
- Aman, S.; Bhuvnesh, Y.; Shipra, R.; Baljeet, Y. Cypermethrin toxicity: A review. J. Fors. Sci. Cri. Inves 2018, 9, 555767. [Google Scholar]
- Leepheng, P.; Limthin, D.; Homchan, W.; Suramitr, S.; Phromyothin, D. An experimental and theoretical study of molecularly imprinted electrode based on methyl methacrylate polymer for pesticide detection. Jpn. J. Appl. Phys. 2020, 59, SIIJ09. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Dang, Y.; Chen, Z.; Zhang, R.; Li, Y.; Ye, B.-C. A robust electrochemical sensing of molecularly imprinted polymer prepared by using bifunctional monomer and its application in detection of cypermethrin. Biosens. Bioelectron. 2019, 127, 207–214. [Google Scholar] [CrossRef]
- Zouaoui, F.; Bourouina-Bacha, S.; Bourouina, M.; Abroa-Nemeir, I.; Ben Halima, H.; Gallardo-Gonzalez, J.; El Hassani, N.E.A.; Alcacer, A.; Bausells, J.; Jaffrezic-Renault, N. Electrochemical impedance spectroscopy determination of glyphosate using a molecularly imprinted chitosan. Sens. Actuators B Chem. 2020, 309, 127753. [Google Scholar] [CrossRef]
- Aghoutane, Y.; Diouf, A.; Österlund, L.; Bouchikhi, B.; El Bari, N. Development of a molecularly imprinted polymer electrochemical sensor and its application for sensitive detection and determination of malathion in olive fruits and oils. Bioelectrochemistry 2020, 132, 107404. [Google Scholar] [CrossRef]
- Hassan, A.H.; Moura, S.L.; Ali, F.H.; Moselhy, W.A.; Sotomayor, M.d.P.T.; Pividori, M.I. Electrochemical sensing of methyl parathion on magnetic molecularly imprinted polymer. Biosens. Bioelectron. 2018, 118, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.-R.; Lee, G.-J.; Haridharan, N.; Wu, J.J. Electrochemical sensor using molecular imprinting polymerization modified electrodes to detect methyl parathion in environmental media. Electrocatalysis 2018, 9, 1–9. [Google Scholar] [CrossRef]
- He, B.; Mao, Y.-L.; Zhang, Y.; Yin, W.; Hou, C.-J.; Huo, D.-Q.; Fa, H.-B. A porphyrin molecularly imprinted biomimetic electrochemical sensor based on gold nanoparticles and carboxyl graphene composite for the highly efficient detection of methyl parathion. Nano 2017, 12, 1750046. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Zhang, J.; Sun, J.; Gan, T.; Liu, Y. A disposable molecularly imprinted electrochemical sensor for the ultra-trace detection of the organophosphorus insecticide phosalone employing monodisperse Pt-doped UiO-66 for signal amplification. Analyst 2020, 145, 3245–3256. [Google Scholar] [CrossRef]
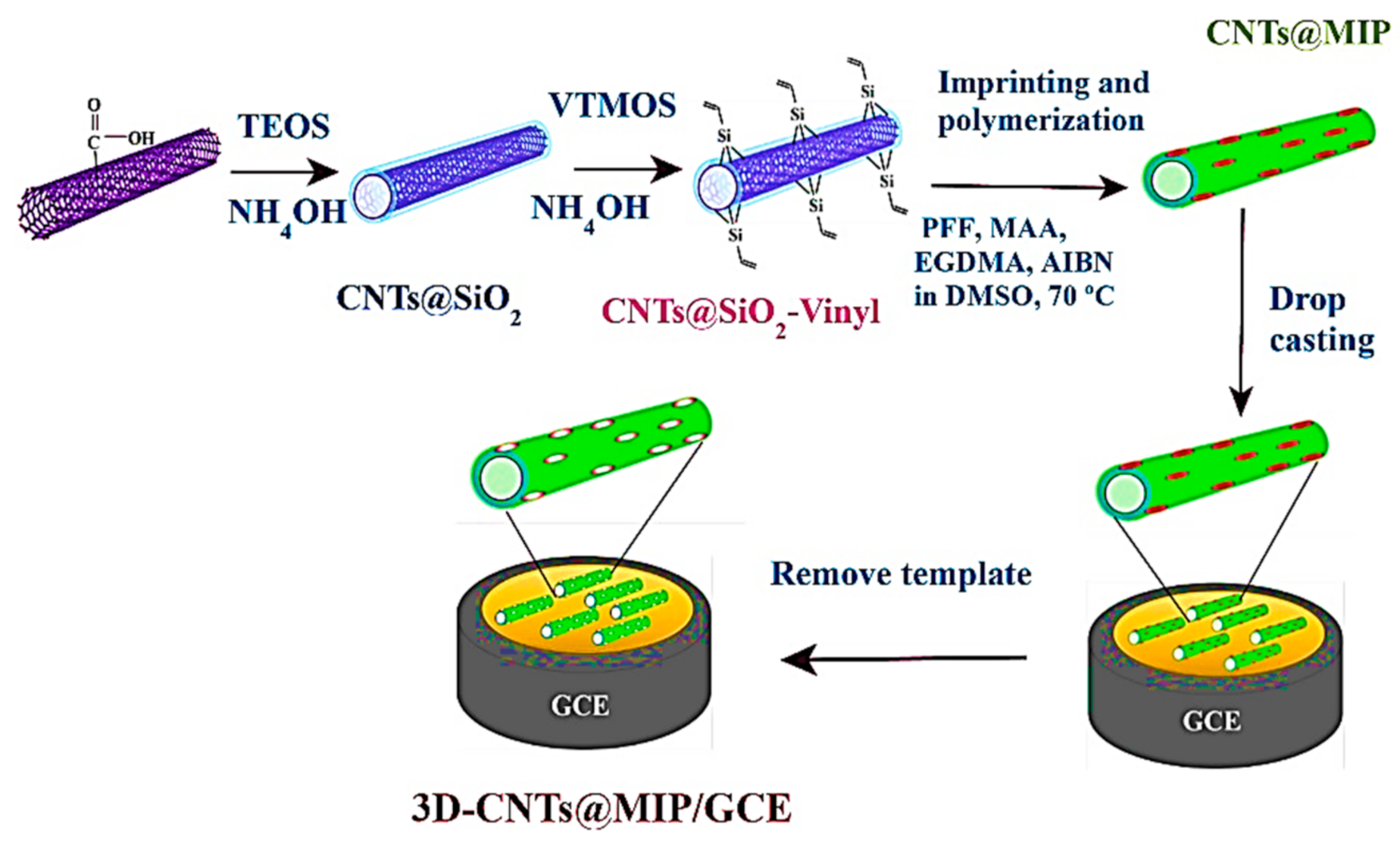
- Amatatongchai, M.; Sroysee, W.; Sodkrathok, P.; Kesangam, N.; Chairam, S.; Jarujamrus, P. Novel three-Dimensional molecularly imprinted polymer-coated carbon nanotubes (3D-CNTs@MIP) for selective detection of profenofos in food. Anal. Chim. Acta 2019, 1076, 64–72. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, H.T.; Xie, T.J.; Yang, X.; Dong, A.J.; Zhang, H.; Wang, J.; Wang, Z.Y. Molecularly imprinted polymer on graphene surface for selective and sensitive electrochemical sensing imidacloprid. Sens. Actuators B Chem. 2017, 252, 991–1002. [Google Scholar] [CrossRef]
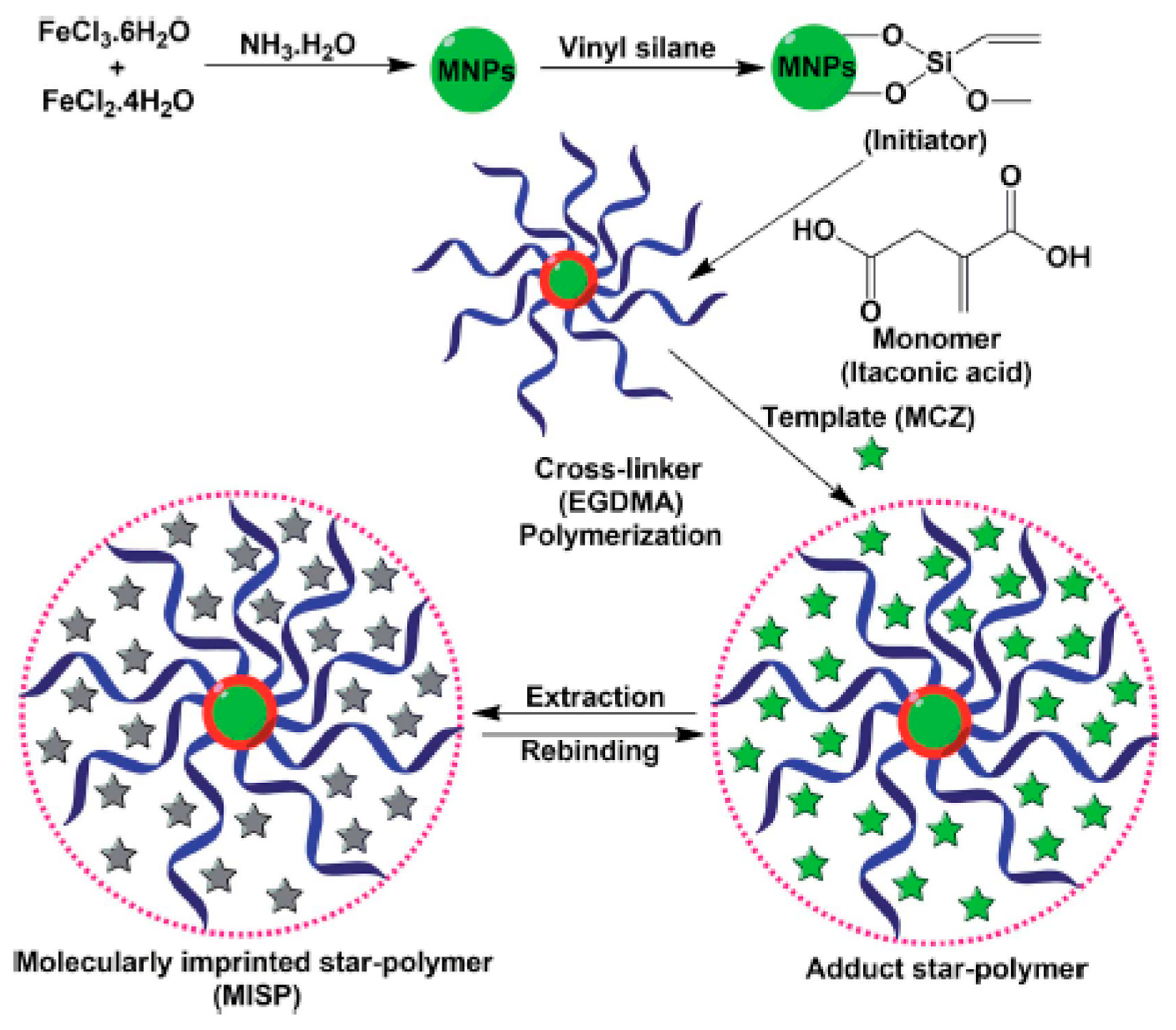
- Kumar, S.; Karfa, P.; Patra, S.; Madhuri, R.; Sharma, P.K. Molecularly imprinted star polymer-modified superparamagnetic iron oxide nanoparticle for trace level sensing and separation of mancozeb. RSC Adv. 2016, 6, 36751–36760. [Google Scholar] [CrossRef]
- El-Moghazy, A.Y.; Soliman, E.A.; Ibrahim, H.Z.; Marty, J.L.; Istamboulie, G.; Noguer, T. Biosensor based on electrospun blended chitosan-poly (vinyl alcohol) nanofibrous enzymatically sensitized membranes for pirimiphos-methyl detection in olive oil. Talanta 2016, 155, 258–264. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, J.; Huang, G.; Wei, W.; Huang, T. Conducting microporous organic polymer with –OH functional groups: Special structure and multi-functional integrated property for organophosphorus biosensor. Chem. Eng. J. 2021, 405, 126682. [Google Scholar] [CrossRef]
- Yasa, M.; Deniz, A.; Forough, M.; Yildirim, E.; Persil Cetinkol, O.; Udum, Y.A.; Toppare, L. Construction of amperometric biosensor modified with conducting polymer/carbon dots for the analysis of catechol. J. Polym. Sci. 2020, 58, 3336–3348. [Google Scholar] [CrossRef]
- Akdag, A.; Işık, M.; Göktaş, H. Conducting polymer-based electrochemical biosensor for the detection of acetylthiocholine and pesticide via acetylcholinesterase. Biotechnol. Appl. Biochem. 2020. [Google Scholar] [CrossRef]
- Kondawar, S.B.; Virutkar, P.D.; Mahajan, A.P.; Meshram, B.H. Conductive polymer nanocomposite enzyme immobilized biosensor for pesticide detection. J. Mater. Nanosci. 2019, 6, 7–12. [Google Scholar]
- Turan, J.; Kesik, M.; Soylemez, S.; Goker, S.; Coskun, S.; Unalan, H.E.; Toppare, L. An effective surface design based on a conjugated polymer and silver nanowires for the detection of paraoxon in tap water and milk. Sens. Actuators B Chem. 2016, 228, 278–286. [Google Scholar] [CrossRef]
- Guler, M.; Turkoglu, V.; Kivrak, A. Electrochemical detection of malathion pesticide using acetylcholinesterase biosensor based on glassy carbon electrode modified with conducting polymer film. Environ. Sci. Pollut. Res. 2016, 23, 12343–12351. [Google Scholar] [CrossRef]
- Bhardwaj, S.K.; Bhardwaj, N.; Mohanta, G.C.; Kumar, P.; Sharma, A.L.; Kim, K.-H.; Deep, A. Immunosensing of Atrazine with Antibody-Functionalized Cu-MOF Conducting Thin Films. Acs Appl. Mater. Interfaces 2015, 7, 26124–26130. [Google Scholar] [CrossRef]
- Salih, F.E.; Oularbi, L.; Halim, E.; Elbasri, M.; Ouarzane, A.; El Rhazi, M. Conducting Polymer/Ionic Liquid Composite Modified Carbon Paste Electrode for the Determination of Carbaryl in Real Samples. Electroanalysis 2018, 30, 1855–1864. [Google Scholar] [CrossRef]
- Rao, T.P.; Kala, R.; Daniel, S. Metal ion-imprinted polymers—novel materials for selective recognition of inorganics. Anal. Chim. Acta 2006, 578, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Yolcu, M.; Dere, N. A novel copper selective sensor based on ion imprinted 2-vinylpyridine polymer. Can. J. Chem. 2018, 96, 1027–1036. [Google Scholar] [CrossRef]
- Shamsipur, M.; Samandari, L.; Besharati-Seidani, A.; Pashabadi, A. Synthesis, characterization and using a new terpyridine moiety-based ion-imprinted polymer nanoparticle: Sub-nanomolar detection of Pb (II) in biological and water samples. Chem. Pap. 2018, 72, 2707–2717. [Google Scholar] [CrossRef]
- Shamsipur, M.; Samandari, L.; Farzin, L.; Besharati-Seidani, A. Development of an ultrasensitive electrochemical genosensor for detection of HIV-1 pol gene using a gold nanoparticles coated carbon paste electrode impregnated with lead ion-imprinted polymer nanomaterials as a novel electrochemical probe. Microchem. J. 2021, 160, 105714. [Google Scholar] [CrossRef]
- Alizadeh, T.; Hamidi, N.; Ganjali, M.R.; Rafiei, F. An extraordinarily sensitive voltammetric sensor with picomolar detection limit for Pb2+ determination based on carbon paste electrode impregnated with nano-sized imprinted polymer and multi-walled carbon nanotubes. J. Environ. Chem. Eng. 2017, 5, 4327–4336. [Google Scholar] [CrossRef]
- Topcu, C.; Lacin, G.; Yilmaz, V.; Coldur, F.; Caglar, B.; Cubuk, O.; Isildak, I. Electrochemical determination of copper (II) in water samples using a novel ion-selective electrode based on a graphite oxide–imprinted polymer composite. Anal. Lett. 2018, 51, 1890–1910. [Google Scholar] [CrossRef]
- Alizadeh, T.; Hamidi, N.; Ganjali, M.R.; Nourozi, P. Development of a highly selective and sensitive electrochemical sensor for Bi3+ determination based on nano-structured bismuth-imprinted polymer modified carbon/carbon nanotube paste electrode. Sens. Actuators B Chem. 2017, 245, 605–614. [Google Scholar] [CrossRef]
- Chen, J.; Bai, H.; Li, Y.; Zhang, J.; Liu, P.; Cao, Q. Stripping voltammetric determination of europium via ultraviolet-trigger synthesis of ion imprinted membrane. Sens. Actuators B Chem. 2018, 271, 329–335. [Google Scholar] [CrossRef]
- Ying-Lu, H.; Wen-Jun, L.; Ming, G.; Jue, W. Preparation and Cadmium Ion Sensing Properties of Ionic Imprinted Materials Based on HNTs. Chin. J. Inorg. Chem. 2019, 35, 1755–1766. [Google Scholar]
- Pereira, E.; Rivas, B.L.; Heitzman, M.; Moutet, J.C.; Bucher, C.; Royal, G.; Aman, E.S. Complexing polymer films in the preparation of modified electrodes for detection of metal ions. Macromol. Symp. 2011, 304, 115–125. [Google Scholar] [CrossRef]
- Sakhraoui, H.E.E.Y.; Mazouz, Z.; Attia, G.; Fourati, N.; Zerrouki, C.; Maouche, N.; Othmane, A.; Yaakoubi, N.; Kalfat, R.; Madani, A. Design of L-cysteine and acrylic acid imprinted polypyrrole sensors for picomolar detection of lead ions in simple and real media. IEEE Sens. J. 2019, 20, 4147–4155. [Google Scholar] [CrossRef]
- Villis, P.C.M.; Sampaio Filho, J.C.; Gomes, W.C.; de Miranda, R.d.C.M.; Nunes, G.S.; Pissetti, F.L.; Gushikem, Y.; Lucho, A.M.S. Diethylenetriamine ion-imprinted silica gel for copper determination in tap water. J. Appl. Electrochem. 2018, 48, 867–883. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, M.; Taher, M. Novel imprinted polymeric nanoparticles prepared by sol–gel technique for electrochemical detection of toxic cadmium (II) ions. Chem. Eng. J. 2017, 327, 135–141. [Google Scholar] [CrossRef]
- Güney, S.; Güney, O. A novel electrochemical sensor for selective determination of uranyl ion based on imprinted polymer sol–gel modified carbon paste electrode. Sens. Actuators B Chem. 2016, 231, 45–53. [Google Scholar] [CrossRef]
- Chen, J.; Bai, H.; Xia, J.; Li, Z.; Liu, P.; Cao, Q. Electrochemical sensor for detection of europium based on poly-catechol and ion-imprinted sol-gel film modified screen-printed electrode. J. Electroanal. Chem. 2018, 824, 32–38. [Google Scholar] [CrossRef]
- Adauto, A.; Wong, A.; Khan, S.; Picasso, G.; Sotomayor, M. A selective electrochemical sensor for the detection of Cd (II) based on a carbon paste electrode impregnated with a novel ion-imprinted hybrid polymer. Electroanalysis 2021, 33, 1557–1566. [Google Scholar] [CrossRef]
- Roushani, M.; Saedi, Z.; Hamdi, F.; Dizajdizi, B.Z. Preparation an electrochemical sensor for detection of manganese (II) ions using glassy carbon electrode modified with multi walled carbon nanotube-chitosan-ionic liquid nanocomposite decorated with ion imprinted polymer. J. Electroanal. Chem. 2017, 804, 1–6. [Google Scholar] [CrossRef]
- Velempini, T.; Pillay, K.; Mbianda, X.Y.; Arotiba, O.A. Application of a polypyrrole/carboxy methyl cellulose ion imprinted polymer in the electrochemical detection of mercury in water. Electroanalysis 2018, 30, 2612–2619. [Google Scholar] [CrossRef]
- Ait-Touchente, Z.; Sakhraoui, H.E.E.Y.; Fourati, N.; Zerrouki, C.; Maouche, N.; Touzani, R.; Yaakoubi, N.; Chehimi, M.M. Zinc oxide nanorods wrapped with ion-imprinted polypyrrole polymer for picomolar selective and electrochemical detection of mercury II ions. Proceedings 2018, 2, 1004. [Google Scholar] [CrossRef] [Green Version]
- Di Masi, S.; Pennetta, A.; Guerreiro, A.; Canfarotta, F.; De Benedetto, G.E.; Malitesta, C. Sensor based on electrosynthesised imprinted polymeric film for rapid and trace detection of copper (II) ions. Sens. Actuators B Chem. 2020, 307, 127648. [Google Scholar] [CrossRef]
- Jafari, H.; Amiri, M.; Abdi, E.; Navid, S.L.; Bouckaert, J.; Jijie, R.; Boukherroub, R.; Szunerits, S. Entrapment of uropathogenic E. coli cells into ultra-thin sol-gel matrices on gold thin films: A low cost alternative for impedimetric bacteria sensing. Biosens. Bioelectron. 2019, 124, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.R.; Moreira, F.T.; Riu, J.; Sales, M.G.F. Plastic antibody for the electrochemical detection of bacterial surface proteins. Sens. Actuators B Chem. 2016, 233, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.R.; Cardoso, A.R.A.; Sales, M.G.F.; Merino, S.; Tomás, J.M.; Rius, F.X.; Riu, J. Artificial receptors for the electrochemical detection of bacterial flagellar filaments from Proteus mirabilis. Sens. Actuators B Chem. 2017, 244, 732–741. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Arduini, F.; Lista, F.; Amine, A. Label-free electrochemical sensor based on spore-imprinted polymer for Bacillus cereus spore detection. Sens. Actuators B Chem. 2018, 276, 114–120. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, D.; Shao, J.; Sun, X. Magnetic molecularly imprinted polymer nanoparticles based electrochemical sensor for the measurement of Gram-negative bacterial quorum signaling molecules (N-acyl-homoserine-lactones). Biosens. Bioelectron. 2016, 75, 411–419. [Google Scholar] [CrossRef]
- Cho, J.H.; Gao, Y.; Choi, S. A portable, single-use, paper-based microbial fuel cell sensor for rapid, on-site water quality monitoring. Sensors 2019, 19, 5452. [Google Scholar] [CrossRef] [Green Version]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for commercial application in low-cost sensors and assays–An overview of the current status quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef]
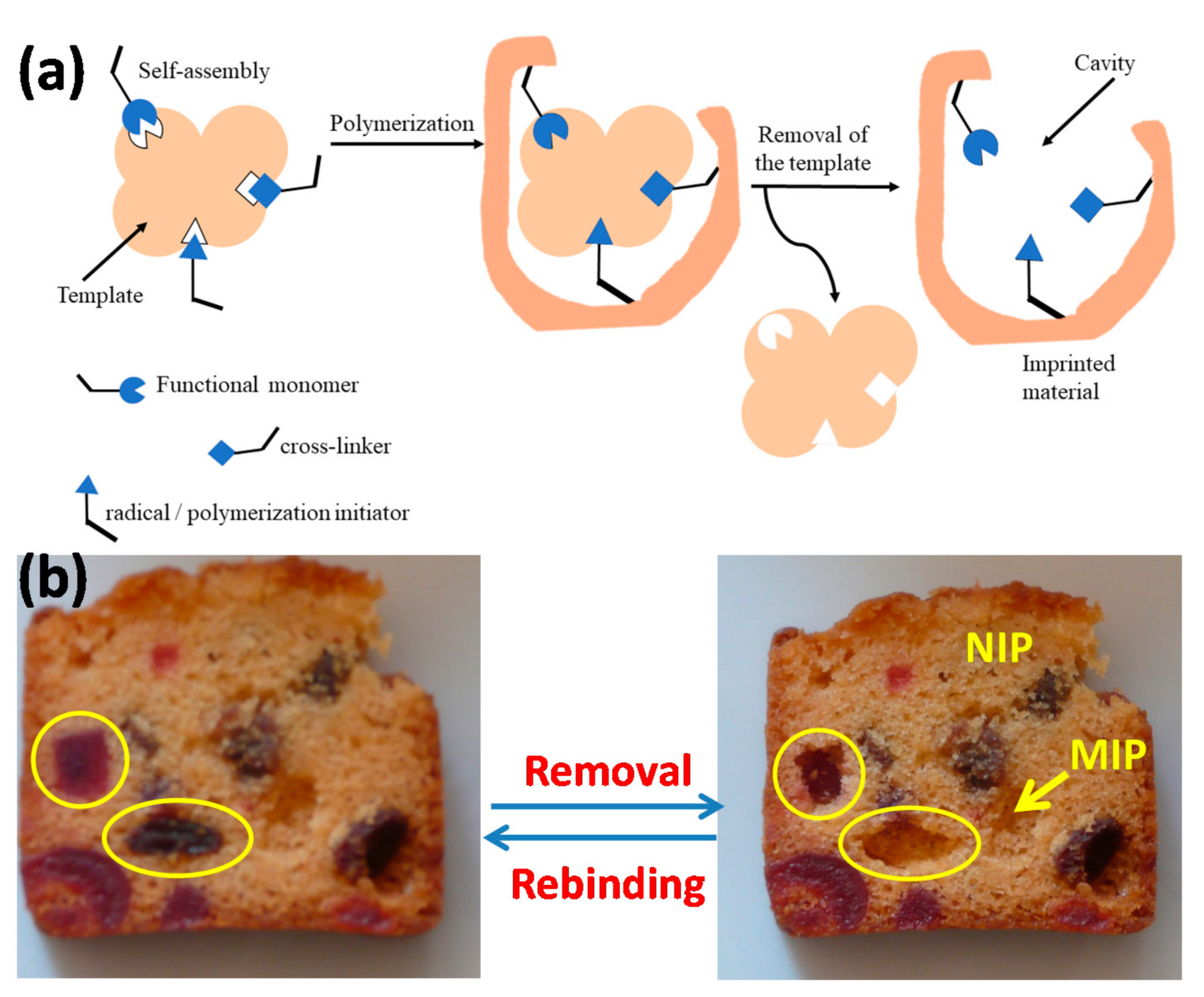
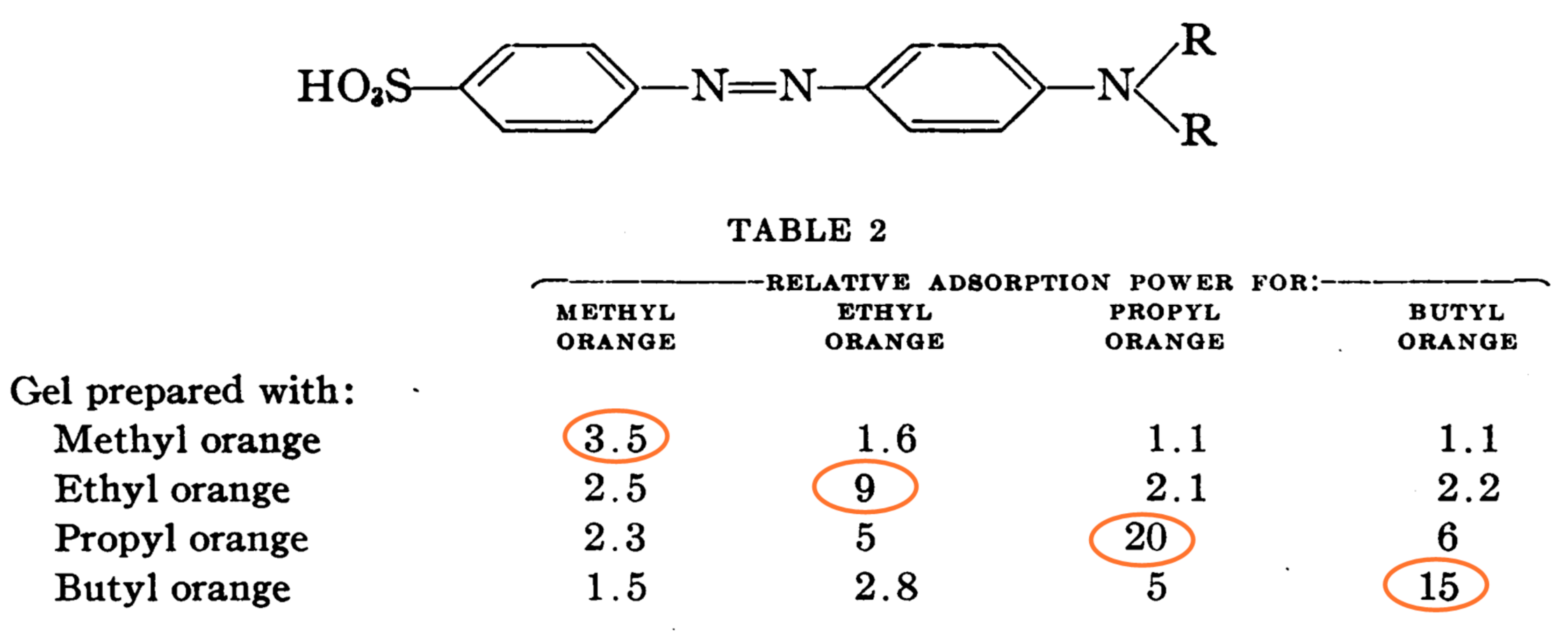

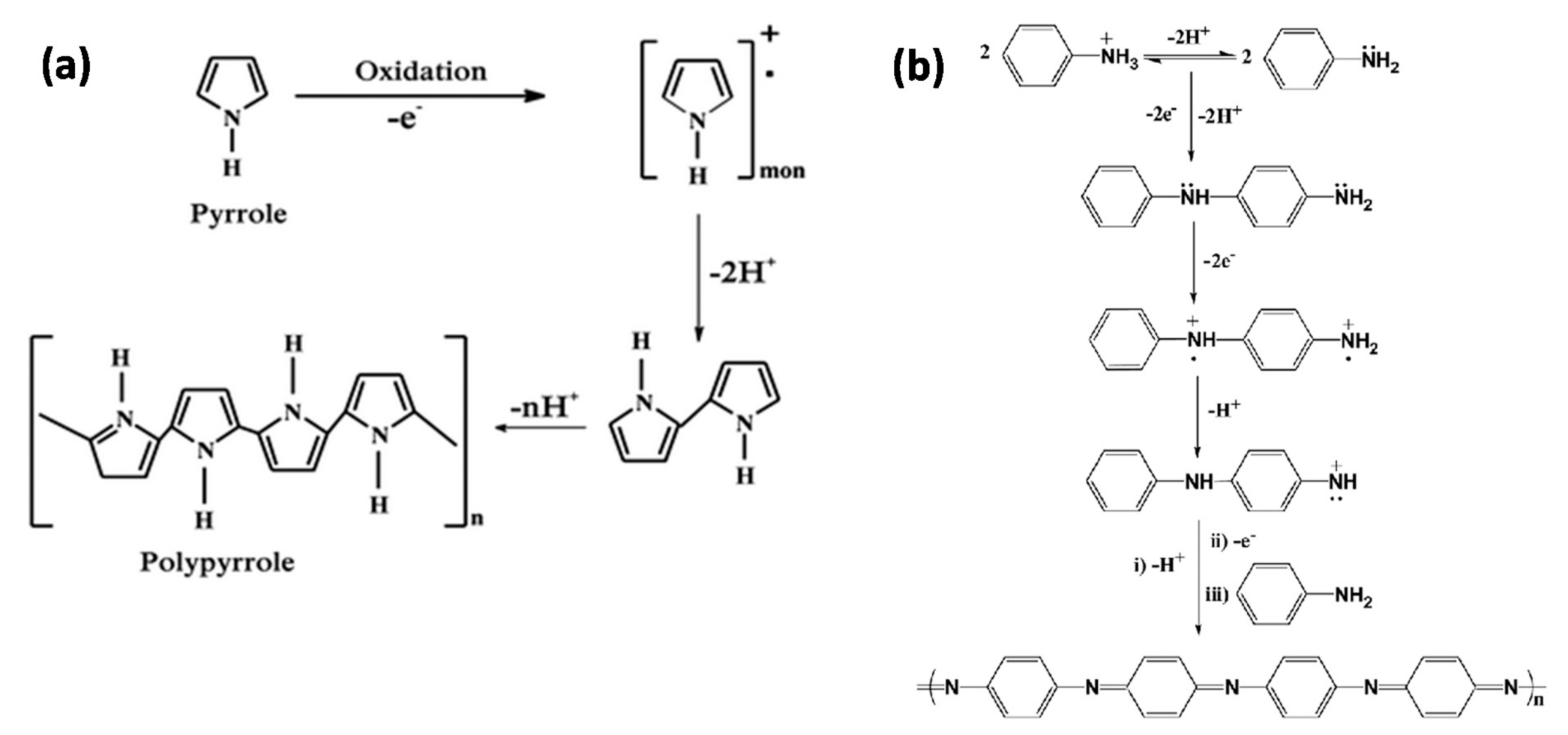
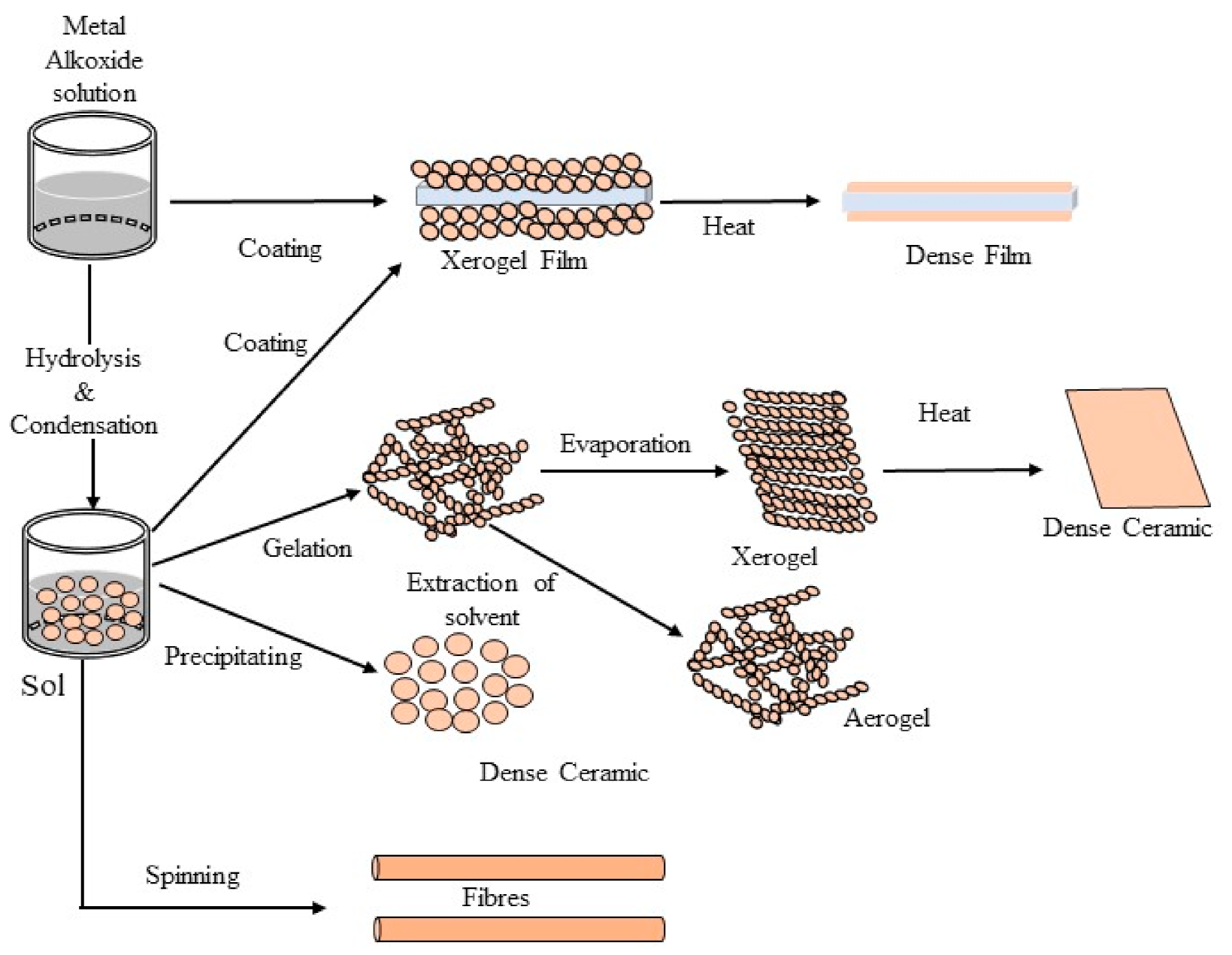









| Running Title | Scope of Review | Year of Publication | Refs. |
|---|---|---|---|
| Monitoring of metals using IIP as | Overview of IIP fabrication and applications in different domains. | 2015 | [18] |
| MIP for electrochemical detection of drugs | This paper critically reviews applications of MIP-based electrochemical sensors for the detection of drugs. | 2018 | [19] |
| MIP-based sensor for detection of food hazard | General overview of MIP-based optical, electrochemical and gravimetric sensors of hazardous compounds in food. | 2019 | [9] |
| Electrochemical sensors based on MIP and nanomaterials | Recent advances on MIP- and nanomaterial-based electrochemical sensors, without specific targets. | 2019 | [20] |
| Overview of recent nanostructured MIP based sensors for pesticide detection | A study on existing NP b-MIP b based sensors for pesticide, showing their fabrication method and experimental result. | 2020 | [21] |
| Applications of chitosan in molecularly and ion imprinted polymers | A brief overview of recent applications of chitosan-based MIPs and MIP composites. | 2020 | [22] |
| MIPs—towards electrochemical sensors and electronic tongues | The paper discusses the combination of chemometrics and MIP technology in view of developing electronic tongues | 2021 | [23] |
| Functional Monomers | Crosslinkers | ||
|---|---|---|---|
| Vinylic Monomers | |||
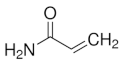 | 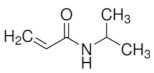 | 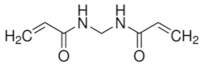 | |
| Acrylamide | N -Isopropylacrylamide | N -Isopropylacrylamide | |
 | 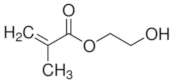 |  | |
| Methacrylic acid | 2-Hydroxyethyl methacrylate | Ethylene glycol dimethacrylate | |
 | 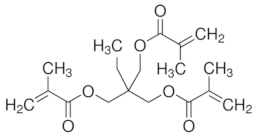 | ||
| Itaconic acid | Trimethylolpropane trimethacrylate | ||
 | 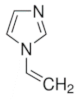 |  | |
| 4-Vinylpyridine | 1-Vinylimidazole | p-Divinylbenzene | |
| Conjugated Monomers | |||
 |  | 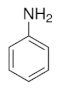 | 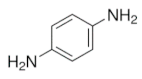 |
| Pyrrole | 2D PPy microstructure | Aniline | p-Phenylenediamine |
| Silanes | |||
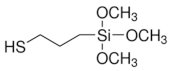 | 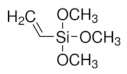 | 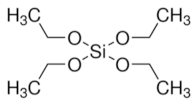 Tetraethyl orthosilicate | |
| (3-Mercaptopropyl) trimethoxysilane | Vinyltrimethoxysilane | ||
 | |||
| 3-(Trimethoxysilyl)propyl methacrylate | |||
| Electrochemical Technique | Principals | General Features and Applications |
|---|---|---|
| Cyclic voltammetry | Current measurement as a function of the linear applied potential |
|
| Differential Pulse Voltammetry | Current measurement between increased pulses of potential with equal increments. |
|
| Square Wave Voltammetry | Current is determined when an increasing square wave potential is applied. |
|
| Amperometric techniques/Chronoamperometry | The application of a constant potential induces the appearance of a corresponding current |
|
| Stripping voltammetry | A step of analyte pre-concentration precedes its stripping by scan potential application |
|
| Electrochemical impedance spectroscopy | Small sinusoidal voltage is applied and complex impedance is measured at the electrode/electrolyte interface |
|
| Pesticide | Sensing Material | Method of Detection | Detection Media | LOD, Sensitivity, Detection Range (DR) | Refs |
|---|---|---|---|---|---|
| Hydrazine | ZnO, NF | CV | Tap, sea, and mineral water | LOD = 5 μM S = 0.14 μA·μM−1·cm−2 | [85] |
| Dichlorvos | TiO2/CS | CV, DPV | Cabbage juice | LOD = 0.23 nM DR = 1.13 nM to 22.6 μM | [89] |
| Dichlorvos | TiO2/CS | DPV | Cabbage juice | LOD = 29 nM DR = 0.036 μM to 22.6 μM | [90] |
| Dichlorvos | TiO2/CS | DPV | Juice samples | LOD = 7.4 nM | [91] |
| Carbamate | MPS | CV | Fruit samples | LOD = 1 nM S = 32.0 μA·cm−2.M−1 | [92] |
| Fipronil | - | CV | Spiked water samples | LOD = 34 × 10−5 μM | [93] |
| Fenitrothion | GdM | DPV | Soil and water samples | LOD = 5 nM S = 1.36 μA·μM−1 cm−2 | [94] |
| Trichlorfon | TiO2/CMCS | CV, DPV | Food | LOD = 4 × 10−7 M S = 0.5077 µA·M−1 | [95] |
| Cypermethrin | MMA (FM), EDGMA (CL), AIBN (In) | CV | Vegetable juice | LOD = 15 ppb S = 0.094 μA·ppm−1 | [97] |
| Cypermethrin | CHAC, resorcinol, dopamine | CV | Crayfish, squid, soil and water | LOD = 6.7 × 10−14 M | [98] |
| Glyphosate | CS | EIS, CV | River water | LOD = 0.001 pg/mL | [99] |
| Glyphosate | PPy | SWV | Spiked water samples | LOD = 1 pM | [13] |
| Malathion | Bisacrylamide, TMEDA, APS | EIS, CV, DPV | Olive oil and fruit samples | LOD = 0.06 pg·mL−1 | [100] |
| Methyl parathion | MAA (FM), EGDMA (CL), AIBN (In) | - | Fish samples | LOD = 1.22 × 10−6 mg·L−1 | [101] |
| Methyl parathion | quercetin, resorcinol | CV | Water, fruit and vegetable juice | LOD = 0.01 μM | [102] |
| Methyl parathion | Zinc porphyrin, EGDMA (CL), AIBN (In) | DPV | Apple samples | LOD = 31.6 nM | [103] |
| Phosalone | - | SWV | Fruit, lake water, and soil | LOD = 0.078 nM | [104] |
| Profenofos | SiO2-vinylcarboxylat | - | Vegetable samples | LOD = 2 nM S = 0.573 A·M−1 | [105] |
| Imidacloprid | VBA, EGDMA (CL) | LSV | Brown rice samples | LOD = 0.10 μM | [106] |
| Mancozeb | IA (FN), EGDMA (CL) | SWV | Soil and vegetable samples | LOD = 0.96 mg·L−1 DR = 5.96–257 mg·L−1 | [107] |
| Pirimiphos-methyl | CS-PVA, Gl, PMO | - | Olive oil | LOD = 0.2 nm | [108] |
| Methyl parathion | Phloroglucinol, NF | DPV | Lettuce | LOD = 1.5 × 10−13 g·mL−1 DR = 5 × 10−13 to 1.0 × 10−8 g·mL−1 | [109] |
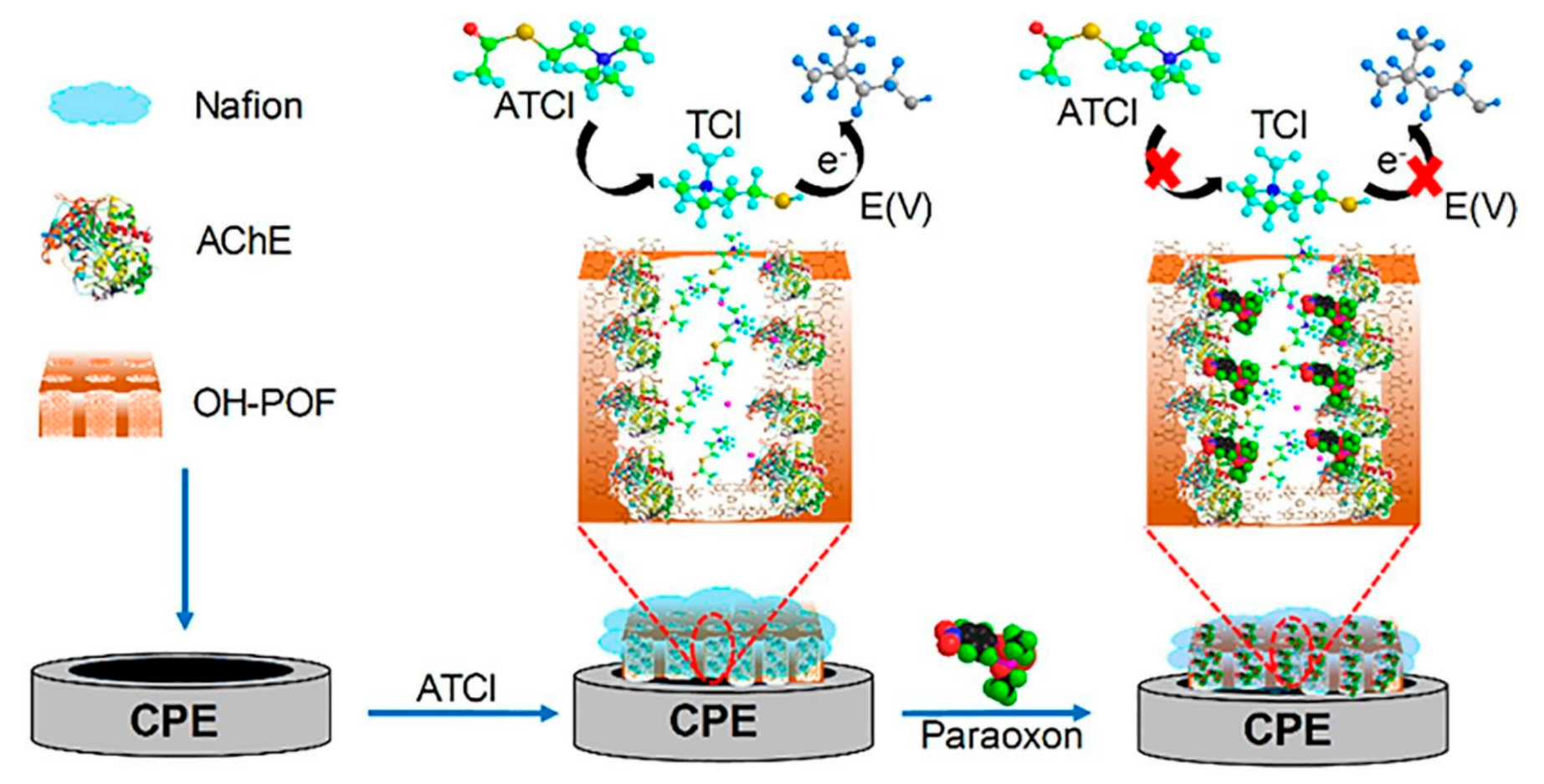
| Paraoxon | Phloroglucinol, NF | DPV | Lettuce | LOD = 3.4 × 10−14 g·mL−1 DR = 1.0 × 10−13 to 1.0 × 10−9 g·mL−1 | [109] |
| Catechol | Thienopyrrole, PFTBDT, Gl | - | Tap water | LOD = 1.23 μM S = 737.4 μA·mM−1·cm−2 DR = 1.25 to 175 μM | [110] |
| Paraoxon | PPy, CS | DPV | Spiked water samples | LOD = 0.17 nM | [111] |
| Acephate | PPy, aniline | CA | Spiked water samples | LOD = 0.007 ppm | [112] |
| Paraoxon | TTBO, Gl | - | Milk and tap water | LOD = 0.212 μM S = 8.076 μA μM−1 cm−2 | [113] |
| Malathion | PTT | CV | Parsley leaves samples | LOD = 4.08 nM S = 183.19 μA/mM | [114] |
| Atrazine | NH2-BDC, PANI | - | Spiked water samples | LOD = 0.01 nM | [115] |
| Carbaryl | p-PD, IL | DPV | Spring water and fruit | LOD = 0.09 mmol·L−1 | [116] |
| Template/Ligand/Monomers/Initiator | Synthesis Conditions | Final Ion Imprinted Material | Detection Technique | Performances (Water Source) | Year, Ref. |
|---|---|---|---|---|---|
| Vinylic polymers | |||||
| Mn(II)/1-(2-Pyridylazo)-2-naphthol/MAA & EGDMA/AIBN | Thermal radical polymerization at 60 °C, 24 h; acid wash for 24 h then coating on MWCNT-Chit-IL-modified GCE | Mn(II)-IIP/MWCNT-Chit-IL coated on GCE | SWASV in acetate buffer, pH 6. 1.0 mg IIP, 2 min preconcentration at −1.4 V | LOD: 0.15 µM; sensitivity 130.5 nA μM−1 cm−2). (Wastewater) | [133] |
| Pb(II)/2,2′:6′,6″-terpyridine/EGDMA/AIBN | Thermal polymerization at 60 °C, 24 h in DMF. 0.1 M HCl to remove Pb(II) | IIP-CPE-oil = 15/55/30% | DPASV in acetate buffer, pH 5. 6 min preconcentration at −1 V. | LOD: 0.11 nM; sensitivity 694 nA nM−1 cm−2) for Pb(II) in the 0.4–10 nM range. (Tap or well water) | [119] |
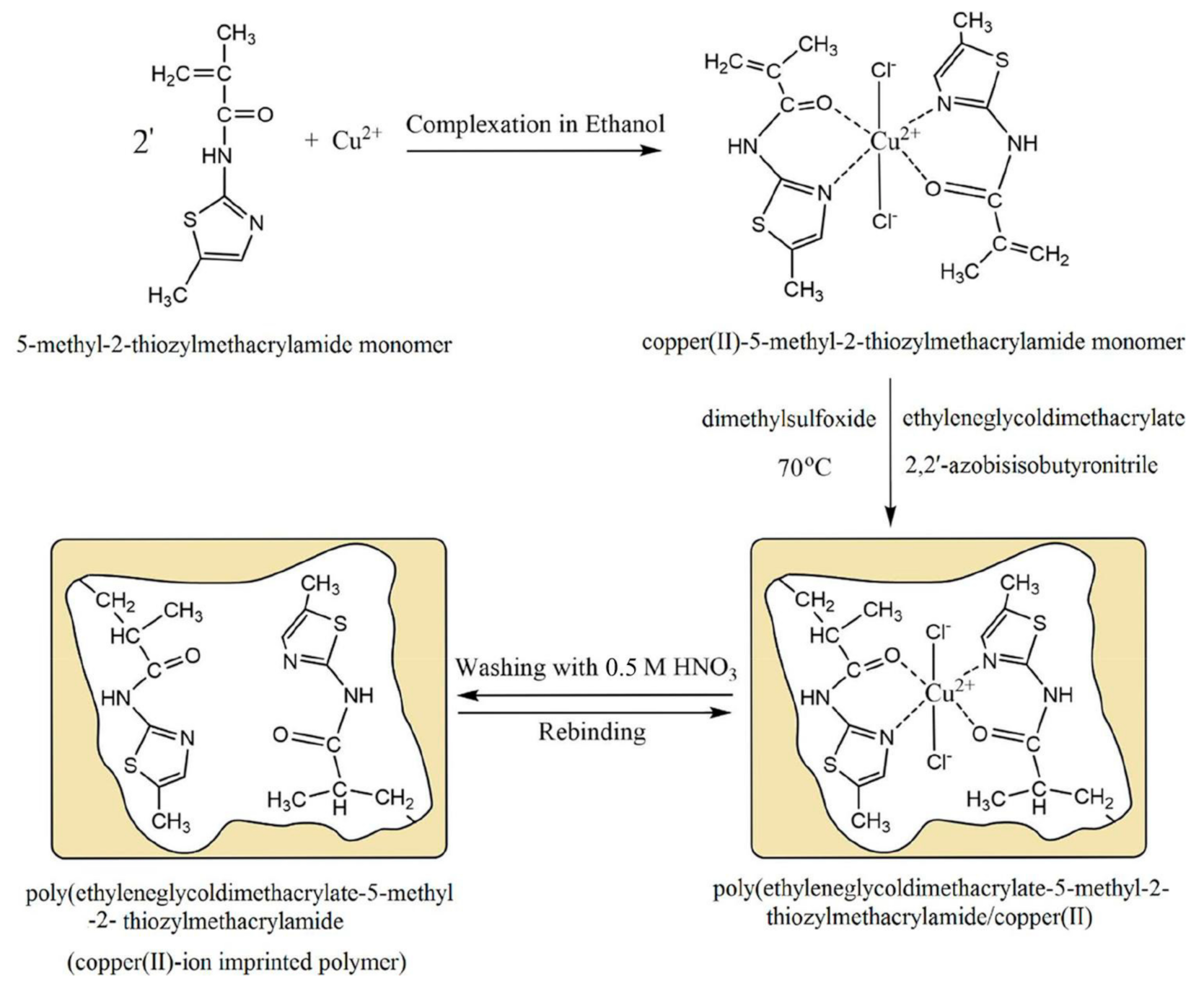
| Cu(II)/5-methyl-2-thiozylmethacrylamide/ EGDMA/AIBN | Thermal polymerization at 70 °C/12 h then 80 °C/3 h in DMSO. Cu(II) was removed in 0.5 M HNO3. | Carbon paste: Cu(II) IIP 20%/65% C/5% MWCNTs/Parrafin oil 10% | Potentiometric titration of Cu(II) in EDTA at pH 6 | Cu selective electrode. LOD 4.0 × 10−7 M; stable at 4.0–8.0 pH range. Linear range: 1.0 × 10−6–1.0 × 10−1 M Cu(II); Sensitivity: 26.1 ± 0.9 mV/decade. Stable 1 year. (Tap or dam or river water) | [122] |
| Pb(II)/IA/EGDMA/AIBN | 1 mmol Pb(ClO4)2 + 2 mmol IA in 35 mL CAN mixed for 30 min then 8 mmol EGDMA and 0.08 g AIBN added. Polymerization at 70 °C for 24 h. Pb(II) leached using EDTA. | CPE: IIP/MWCNT/graphite/oil = 7/6/74.8/12.2% w/w. | SWV in −0.7 to −0.2 V vs. calomel; and scan rate = 500 mVs−1, pH 5, preconcentration at −1 V for 60 s. | LOD = 3.8 pmol L−1; Linear range = 1.0 × 10−11–8.0 × 10−8 mol L−1. (Sea or river water). | [121] |
| Eu(III)/AM/EGDMA/AIBN | 0.0125 mmol of EuCl3 in 30 μL methanol+ 0.05 mmol AM in 0.47 mL + sonication + 30 dwell time + addition of 0.5 mmol EGDMA and 0.04 mmol AIBN. 1.5 µL of solution dropped on MWCNT-coated SPE. UV-triggered photopolyerization for 3 h. | 1.5 µL of template in monomer and AIBN solution was dropped on MWCNT-coated SPE. UV-triggered photopolyerization for 3 h. Eu(III) removed in 0.6 M HCl at −1 V vs. Ag/AgCl. | DPV: −1.2 V to −0.6 V vs. Ag/AgCl at pH 4.7; scan rate = 100 mV s−1; see reference for details. Response of the sensor using 3.0 × 10−5 mol L−1 Eu(III) is ~4 times higher for SPE/MWCNT-IIVP compared to SPE-IIVP. | LOD = 4.0 × 10−8 mol L−1; linear range = 1.0 × 10−7–1.0 × 10−3 mol L−1. 95% of original response 30 uses or 2 month storage in water. Change in response less than 5% in the presence of 30–200 fold excess metal ions. (River or lake water) | [124] |
| Conjugated polymers | |||||
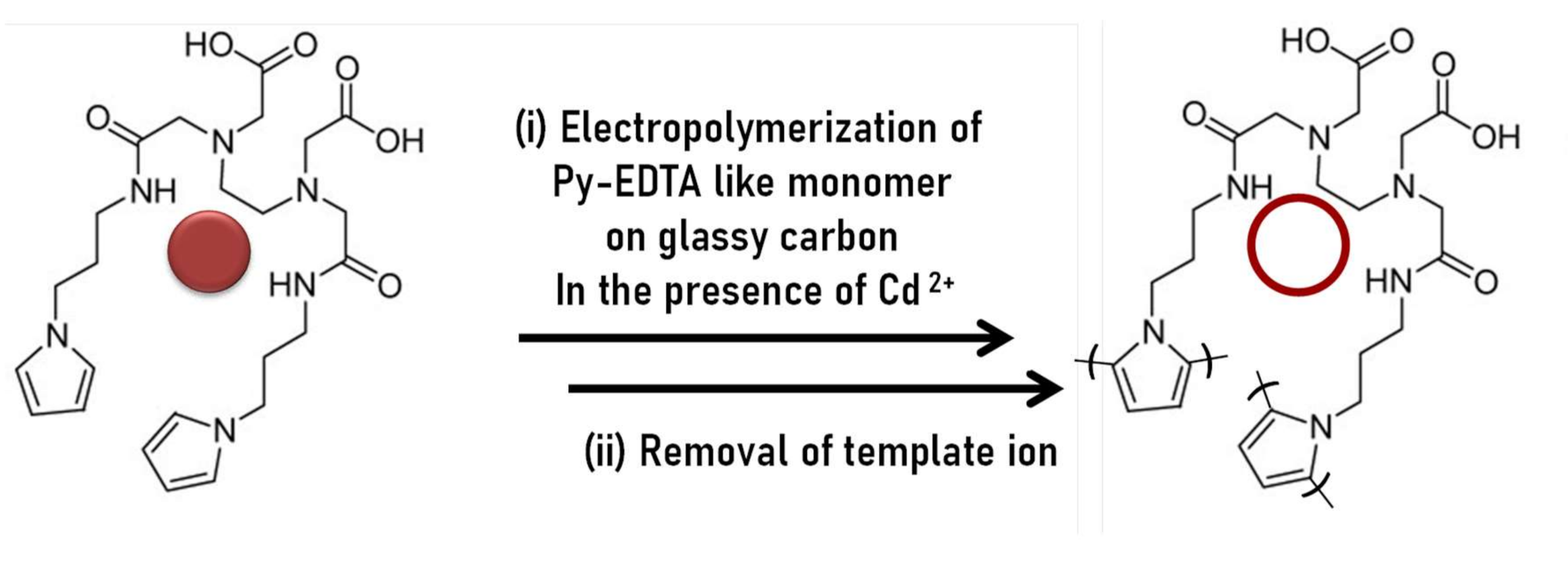
| Hg(II), Pb2+ Cd2+ Cu2+/pyrrole-EDTA like | Oxidative electropolymerization in CH3CN + TBAP | Film/CD | SWV at pH 4.4 pre-concentration anodic time = 40 s at 0.4 V vs. SCE,; scan rate = 50 mV s−1. | Hg2+: LR = 510−8 to 5.10−6, LOD = 10−7; Pb2+: −10−8 to 10−6, LOD = 5.10−10 Cd2+: 10−7 to 10−5 LOD = 510−7; Cu2+: 510−8 to 2.510−7 LOD = 5.10−9 (Tap water) | [126] |
| Hg(II)/CMC/pyrrole | Electropolymerization aqueous solution in KCL | Film/GCE | SWASV at pH of 3, in the −1 to 1.25 V potential rang, pre-concentration time = 60 s Ered = −1 V/SCE Scan rate 50 mV·s−1 | 20–800 µg·L−1. LOD = 0.1 µg·L−1 (Ground or tap water) | [134] |
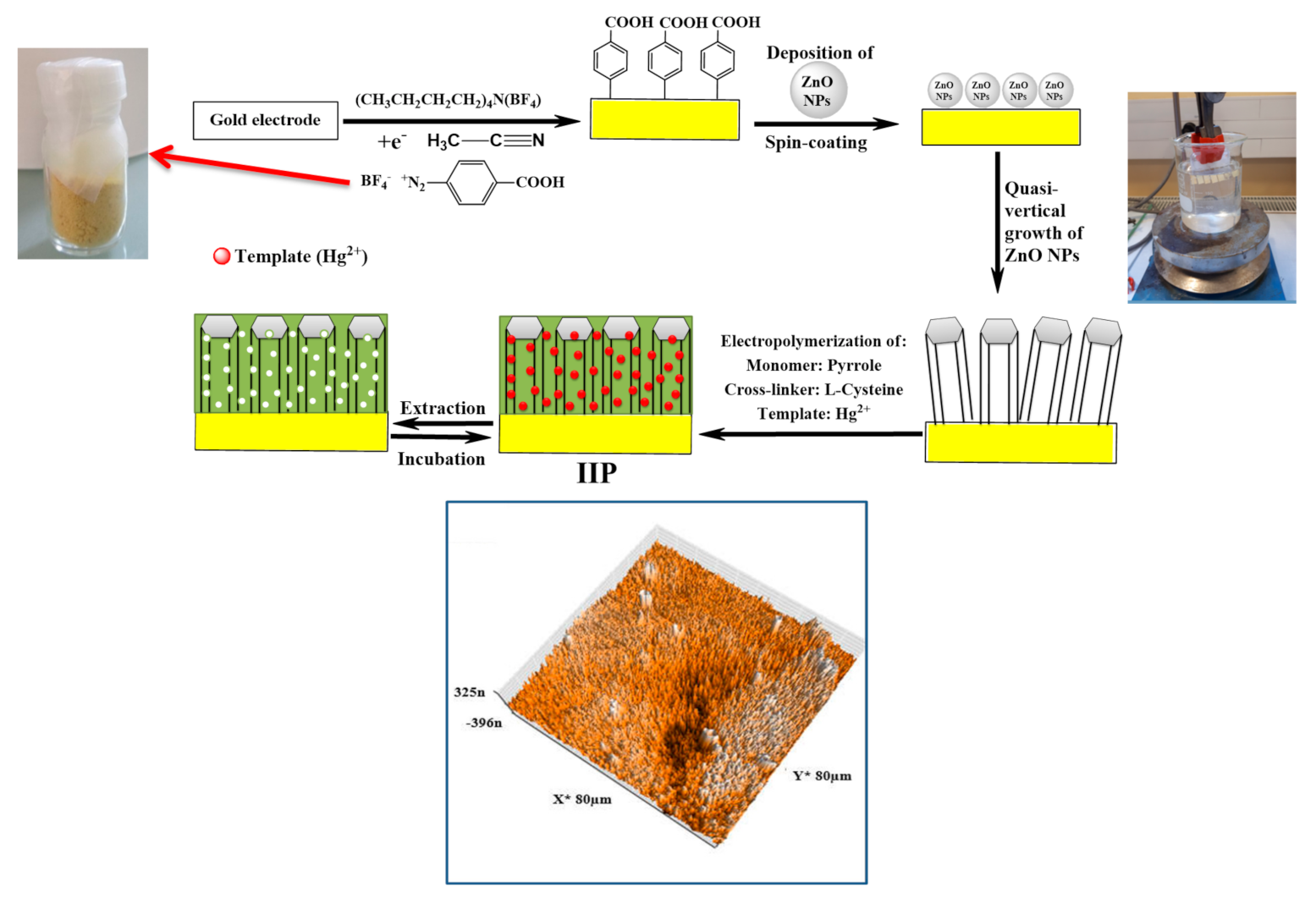
| Hg(II)/pyrrole | Aqueous medium + NaCl Chronoamperometry performed on diazonium-modified gold electrode decorated with ZnO nanorods | IIPPy@ZnO NRs film coated on Au | SWV method, in the −0.6 to 0.9 potential range; ZnO/Hg(II)-IIP electrodes incubated solutions of either mercury, cadmium, lead or copper ions for 20 min. | Sensitivity: 7.17 ± 0.15 μA/M; LOD: 10−12 M (Drinking water) | [135] |
| Pb(II)/L-Cys/AA/pyrrole | Electropolymerization by CA on SAW sensor gold electrode. Conditions: 0.9 V/SCE, in water, pyrrole:10−2 M, L-Cys or AA: 10−4 M, Pb2+: 10−3 M, LiClO4: 0.1 M. | Sensing imprinted L-Cys/PPy or AA/PPy | SWASV in a 0.1 M buffer solution with duration: 0.02 s, Amplitude: 2 mV, Pulse: 50 mV, −0.8 to 0 V vs. SCE potential range, Pb(II)/L-Cys/AA/pyrrole electrodes incubated solution for 20 min in solution of lead. | LOD in the picomolar regime. Pb(II) detected in Bousselem river = 14 μg/L. (River water) | [127] |
| Cu(II)/para-phenylene diamine | CV in H2SO4 0.5 M, 10 mM of Cu2+ and 5 mM pPD on SPPtEs; 50 mV/s for 40 cycles. | Thin copper ion imprinted poly(para-phenylene diamine) films on SPPtED | DPV in the −0.2 V to + 0.6 V range, in acetate buffer pH 5.2, | LOD: 2.7 × 10−9; LR = 9.0 × 10−10–1.5 × 10−8, sensitivity = 1.30 μA nM−1 (Commercial drinking water) | [136] |
| Sol-gel polymers | |||||
| Cu(II)/TPDT | Complexation of Cu(II) by ligand-functionalized silane in ethanol followed by condensation of the silanols at reflux for 24 h in water/ethanol. | Carbon paste of diethylenetriamine-functionalized copper ion-imprinted silica gel. | DPSAV at pH 5.2, in the −0.8 to +0.8 V potential range, pre-concentration time = 1800 s at Ered = − 0.51 V vs. SCE; scan rate = 20 mV s−1. | LOD = 1.82 × 10−7 mmol L−1. No significant change in sensor response in the presence of Fe(II), Ni(II), Zn(II) or Pb(II). (Tap water) | [128] |
| Cd(II)/AAAPTS/ECH/TEOS | 1 mmol of AAAPTS and 0.5 mmol CdCl2 mixed in 100 mL anhydrous ethanol, 1 h stirring and heating. Then 1 mmol of ECH added and stirring at 60 °C was conducted for 2 h. Finally 5 mmol TEOS and 2.5 mL NH4OH (14%) were added to the mixture under stirring and reactionleft to proceed for 12 h.Sol-gel material was washed with ethanol than in 30 mL HCl (1 mol/L) to remove Cd(II). | CPE: graphite powder (57–75% (w/w)), IISG (0–13% (w/w)) and paraffin oil (25% (w/w)) | DPASV in the −1 to −0.4 V at pH 5, after 300 s accumulation in Cd(II) solution at −1.1 V vs. Ag/AgCl, | 10% IISG in CPE, LOD = LOD is 0.15 μg·L−1, selective to Cd(II) in the presence of 30–100 fold excess competitive metal ions. (Dam or aqueduct or tap or river or wastewater). | [129] |
| Eu(III)/PTMOS/MTMOS/TEOS/HCl in ethanol | Mixture of 50 μL TEOS, 50 μL ethanol, 30 μL PTMOS, 28 μL of MTMOS, 10 μL of 1 × 10−4 mol L−1 HCl and 50 μL of water left for 2 h. deionized. 10 μL of 10 mmol L−1 Eu3+ added to 90 μL of this mixture to obtain PPC. 1.5 µL of PCC solution dropped on SPE-polycatechol and left to gelify. IISG washing with HCl to remove Eu(III) template. | SPE-polycatechol-IISG membrane. | DPV in buffer (pH 4.7) Eu(III): 3 × 10−7 to 10−3 M; accumulation at −0.2 V for 300 s; scan range: −1.2 to −0.6 V vs. Ag/AgCl; scan rate = 100 mV s−1; amplitude = 0.05 V. | LOD = 1.0 × 10−7 mol·L−1; linear range = 0.3–1000 μmol·L−1; selectivity over Ni2+, Co2+, Cu2+, Fe3+ or Gd3+ with 50–100 fold excess concentration. (Application to tap water, Greenlake water and Panlong river water). | [131] |
| Cd(II)/{MPS/TMSPMA/TEOS}/{VIN/TRIM/AIBN} | 0.18 g of Cd(NO3)24H2O in 10 mL of ethanol + 0.90 mL VIN + 1 mL MPS, 1.2 mL TMSPMA + 1.1 mL TRIM + 0.075 g AIBN. 10 min purge in N2, then addition of 2 mL TEOS dissolved in ethanol and 0.95 mL of NaOH pH(1 mol·L−1). Polymerization: 60 °C for 24 h in absence of oxygen. Template removed with HNO3 (1 mol·L−1). | CPE-ion-imprinted hybrid polymer (IIHP). 80 mg of graphite + 20 mg IIHP+ 1 mL of 0.1 M KCl. After 12 h drying, 85 μL mineral oil was added to obtain a compact paste. | Accumulation: 2000 μg/L−1 of Cd(II) at pH 1, −1.2 V vs. Ag/AgCl, for 300 s. DPASV in the −1 to −0.6 V in HCl 0.1 mol·L−1. | Linear ranges: Cd(II) in the 1 to 100 μg·L−1 and 2.75–5.0 mg·L−1. LOD = 0.10 µg·L−1. Recovery > 93.6% in rivers and drinking water (Peru and Brazil). No interference with other metal ions, except for Hg(II) at 50 fold excess. (Drinking or river water) | [132] |
| UO2(II)/QFS/TMOS | Pre-gel: 40 mmol TMOS + 12 mL of propanol + 0.4 mL of 0.02 M HCl refluxed at 70 °C for 3 h. Sol: TMOS/QFS mixture. 0.1 mL of 0.1 M TEA added to catalyse sol-gel synthesis for 48 h at RT and 24 h at 100 °C. Final imprinted powder was crushed. | CPE preparation: carbon powder (CP) + IISG + paraffin oil (55:15:30) (% w/w). | DPCSV in the −0.4–+0.4 V vs. Ag/AgCl; accumulation time = 5 min. | LOD = 3.07 × 10−10 mol·L−1; linear range = 2.0 × 10−9–3.0 × 10−7 mol·L−1. No competitive effect of other metal ions. (Application in tap, pond and waste waters). | [130] |
| Target | Functional Monomer | Electrode Material | Polymerization Technique | Detection Method | Detection Medium/LOD | Ref. |
|---|---|---|---|---|---|---|
| E. Coli | TEOS | Gold | Sol-gel imprinting | EIS | Urine; 1 to 106 cfu/mL LOD = 1 cfu/mL | [137] |
| S. Aureus | AP | Carbon | Electropolymerization | CV, EIS | Tap water LOD = 0.60 nM | [138] |
| Aeromas hydrophila (AHLs) | MAA, DMHF | Magnetic Glassy carbon | Controlled Radical polymerization | DPV | Solutions prepared in lab and spiked; 2.5.10−9 to 1.O.10−7 mol/L LOD = 8.10−10 mol/L | [141] |
| Bacillus cereus (spore) | Pyrrole | Carbon paste | Electropolymerization | CV | Solutions prepared in lab and spiked; 102 to 105 cfu/mL LOD = 102 cfu/mL | [140] |
| Proteus mirabilis (flagella) | Phenol | Carbon | Electropolymerization | CV, EIS, SWV | Tap water LOD = 0.9 ng/mL | [139] |
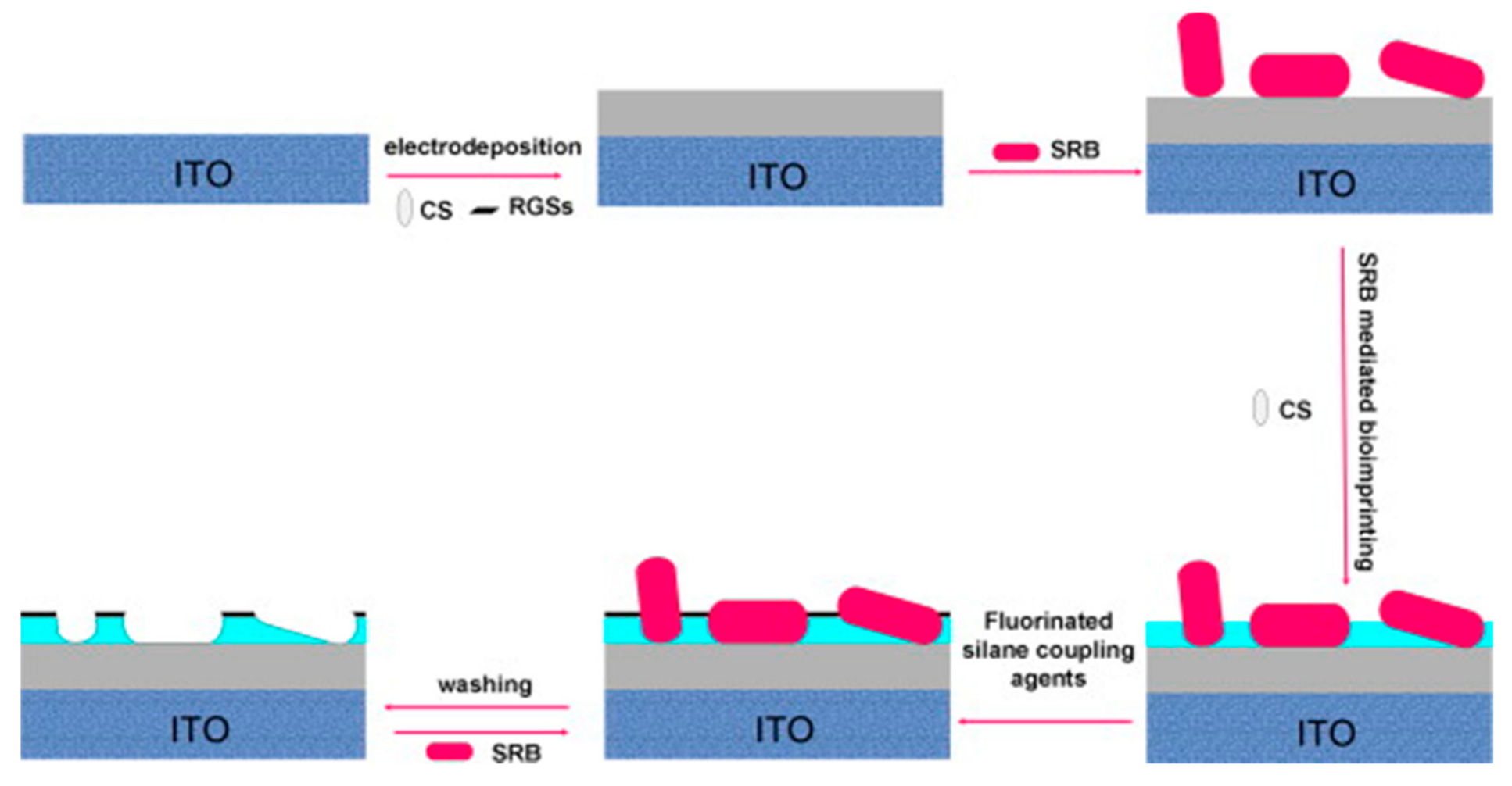
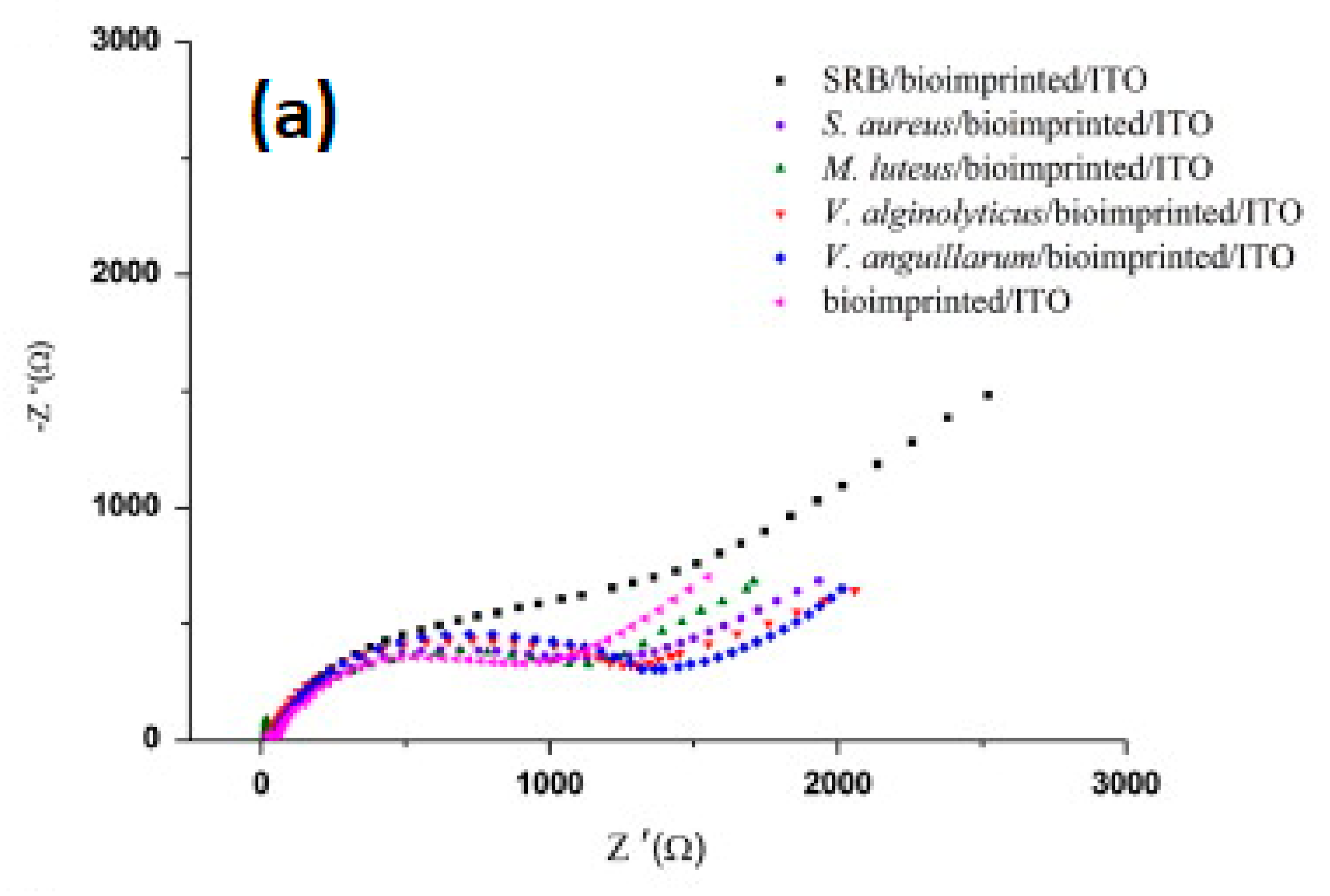
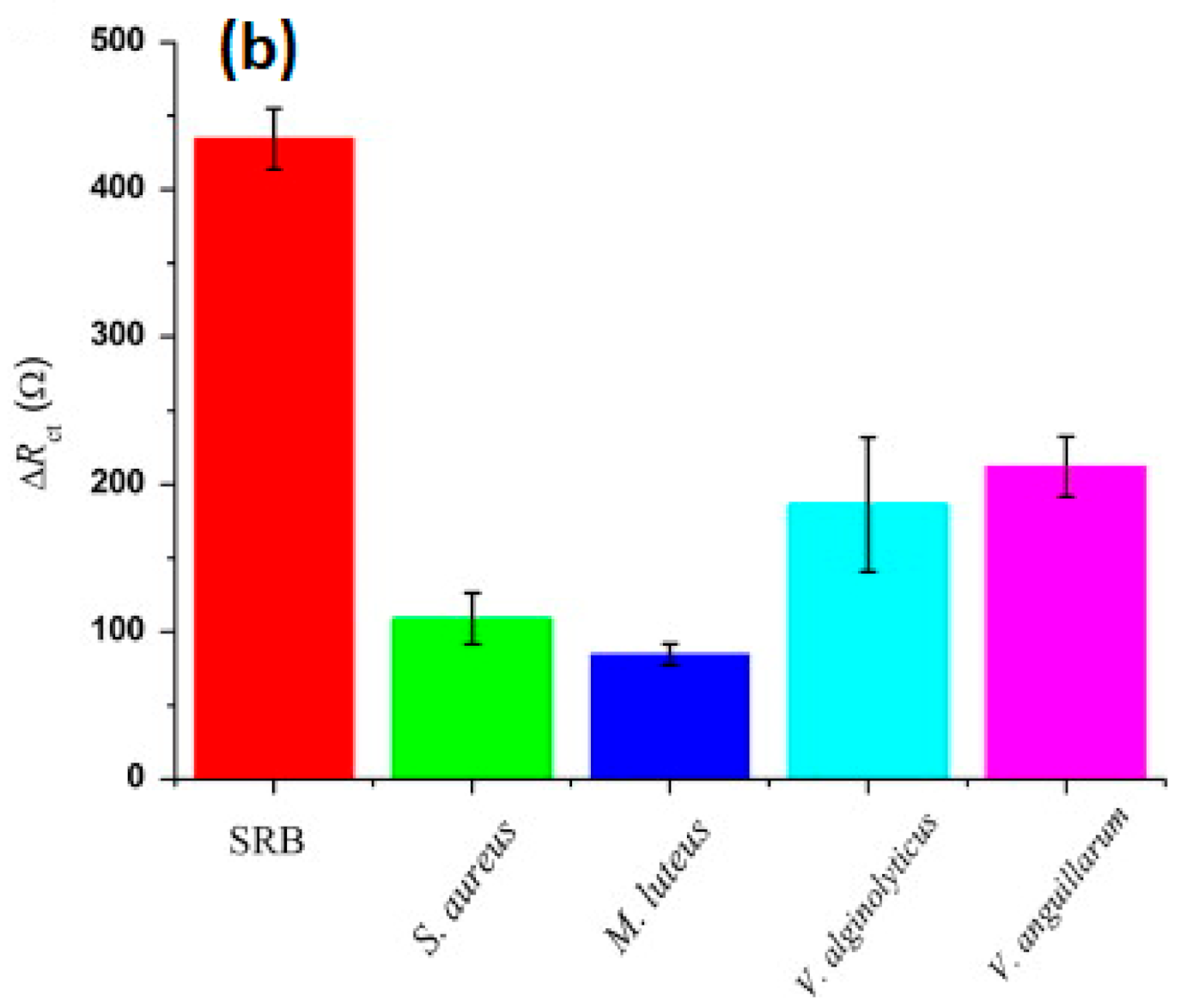
| Sulfate-reducing bacteria | CS | ITO/graphene | Electrodeposition | EIS | Solution prepared in lab and spiked; 1 to 108 cfu/mL LOD = 0.7.104 cfu/mL | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Khaoulani, S.; Ktari, N.; Lo, M.; Khalil, A.M.; Zerrouki, C.; Fourati, N.; Chehimi, M.M. Towards Clean and Safe Water: A Review on the Emerging Role of Imprinted Polymer-Based Electrochemical Sensors. Sensors 2021, 21, 4300. https://doi.org/10.3390/s21134300
Zheng X, Khaoulani S, Ktari N, Lo M, Khalil AM, Zerrouki C, Fourati N, Chehimi MM. Towards Clean and Safe Water: A Review on the Emerging Role of Imprinted Polymer-Based Electrochemical Sensors. Sensors. 2021; 21(13):4300. https://doi.org/10.3390/s21134300
Chicago/Turabian StyleZheng, Xiaofeng, Sohayb Khaoulani, Nadia Ktari, Momath Lo, Ahmed M. Khalil, Chouki Zerrouki, Najla Fourati, and Mohamed M. Chehimi. 2021. "Towards Clean and Safe Water: A Review on the Emerging Role of Imprinted Polymer-Based Electrochemical Sensors" Sensors 21, no. 13: 4300. https://doi.org/10.3390/s21134300
APA StyleZheng, X., Khaoulani, S., Ktari, N., Lo, M., Khalil, A. M., Zerrouki, C., Fourati, N., & Chehimi, M. M. (2021). Towards Clean and Safe Water: A Review on the Emerging Role of Imprinted Polymer-Based Electrochemical Sensors. Sensors, 21(13), 4300. https://doi.org/10.3390/s21134300









