Internet of Food (IoF), Tailor-Made Metal Oxide Gas Sensors to Support Tea Supply Chain
Abstract
:1. Introduction
- Fermented tea: black and Pu-Erh tea
- Unfermented tea: green and white tea,
- Partially or semi-fermented tea: Oolong tea.
2. Materials and Methods
2.1. GC-MS Analysis Conditions
2.2. S3 Analysis Conditions
- (A)
- The sensors are housed inside a steel chamber isolated from the external environment, except for an inlet and an outlet path for the passage of volatile compounds. In addition to the MOX sensors, a temperature, humidity sensor, and a flow sensor are also allocated as necessary to take into account the number of variables during the analysis. The dimensions of the chamber are 11 × 6.5 × 1.3 cm.
- (B)
- The fluid dynamic circuit consists of a pump (Knf, model: NMP05B), polyurethane pipes, a solenoid valve, and a metal cylinder containing activated carbon for filtering possible interfering odors present in the environment. The pump flow is regulated by a needle valve placed at the chamber inlet; the flow range for tea analysis was set to 100 sccm.
- (C)
- The electronic boards make it possible to acquire the resistances of the sensors, the correct heating of the sensors themselves to their operating temperature, and the sending of data to the Web App dedicated to the S3 device through an internet connection. In addition, it allows communication and synchronization with an autosampler. This is an autosampler of the company HTA S.r.l. (model HT2010H) which allows one to prepare batches of 42 samples per measurement session. Tea samples were conditioned for 5 min at 40 °C with 1 min in a shaking mode in order to equilibrate the headspace (HS).
3. Results
3.1. GC-MS Results
- Cyanoacetic acid: (C3H3NO2) is an organic compound that has two functional groups: COOH typical of carboxylic acids and NC with triple bond typical of nitriles. It is obtained from the treatment of chloroacetate with sodium cyanide followed by acidification or electrolysis by cathodic reduction of carbon dioxide or the anodic oxidation of acetonitrile. It is a precursor of synthetic caffeine by theophylline [33].
- Hexanal: (C6H10O) is an aldehyde. In the cell, it is contained in the cytoplasm. It has a sweet almond and honey flavor and is found in several foods including soy, cucumber, black elderberry, and black currant [33].
- Limonene: (C10H16) is the most widespread and most important monoterpene. It has a lemon smell and turpentine-like notes. It is obtained by steam distillation of citrus peel and pulp obtained from the production of juice [33]. It should be specified that it is present as two isomers: R-LIMONENE and D-LIMONENE. In particular, it is R-LIMONENE in the SGP, while in the others, it is D-LIMONENE, and both isomers are present in the SGM.
- 6-methyl, 5-hepten-2-one: (C8H14O) is an unsaturated ketone called sulcatone. It has a strong, greasy, green, citrus smell and tastes reminiscent of pear. It is obtained from citronella or citral oil by mixing for 12 h in aqueous solution with K2CO3 and subsequent distillation and fractionation under vacuum. It was originally identified in lemongrass; later, it was also discovered in the essential oils of lemons and geraniums. We also find this ketone in grapes, melon, peaches, avocados, cognac, mangoes, rice, olives, blueberries, and more [33].
- Nonanal: (C9H18O) is an aldehyde. It has a strong and greasy odor which develops notes of orange and rose when diluted. The fat recalls the flavor of citrus fruits. It is synthesized by the catalytic oxidation of the corresponding alcohol or by the reduction of the respective acid. In nature, we find it in orange, mandarin, lemon, and lime oils. It is also found in more than 200 foods and beverages including apples, tomatoes, rum, wine, plum, coconut, cardamom, avocado, corn oil, broccoli, milk, eggs, tea, and others [34].
- α-Terpineol: (C10H18O) is a monoterpenic alcohol. It has a characteristic smell of lilac with a sweet flavor reminiscent of peach. It is obtained from the hydration of the terpene or from the pentane tricarboxylic acid by cyclization or from the isoprene and methyl-vinyl-ketone. It is present in more than 150 derivatives of herbs, leaves, and flowers. Form D is found in cardamom, star anise, sage, and marjoram oil. The L form is present in lavender, lime, and cinnamon leaves. The racemic form is the eucalyptus [33,34].
- 5,6,7,7a-Tetrahydro, 4,4,7a-Trimethyl-2(4H)–benzofuranone: (C11H16O2) is a heterocyclic compound. It has a coumarin and musky smell. This compound is formed from the photo-oxidation of carotene. The flavor is linked to the fruit and in particular to their point of ripeness. It is obtained from the degradation process of β-carotene in the presence of nitrogen and air. It occurs naturally in lemongrass and sweet grass oil [33].
- Nonanal: (C9H18O) is an aldehyde. It has a strong and greasy odor which develops notes of orange and rose when diluted. The fat recalls the flavor of citrus fruits. It is synthesized by the catalytic oxidation of the corresponding alcohol or by the reduction of the respective acid. In nature, we find it in orange, mandarin, lemon, and lime oils. It is also found in more than 200 foods and drinks including apples, tomatoes, rum, wine, plum, coconut, cardamom, avocado, corn oil, broccoli, milk, egg, tea, and others [34].
- Ammonium acetate: (C2H7NO2) is an ammonium salt obtained from the reaction between ammonia and acetic acid. It is used to regulate acidity in food, even though the EU decided to ban its use as a food additive [33].
- Phenylethyl alcohol: (C8H10O) is an alcohol. It has a characteristic rose odor and initially a slight bitter taste. The dessert is reminiscent of peaches. It is synthesized from toluene, benzene, or styrene. It is found in esterified form in rose concentrate or distilled rose water. It is present in the essential oil of lily, narcissus, and tea leaves but not only because it has been found in more than 200 foods and drinks including peaches, grapes, coffee, tea, mushrooms, mango, kiwi, rum, whiskey, milk, butter, cheese, and more [33,34].
- 5,6,7,7a-Tetrahydro, 4,4,7a- trimethyl 2 (4H)—benzofuranone: (C11H16O2) is a heterocyclic compound. It has a coumarin and musky smell. This compound is formed from the photo-oxidation of carotene. The flavor is linked to the fruit and in particular to their point of ripeness. It is obtained from the degradation process of β-carotene in the presence of nitrogen and air. It occurs naturally in lemongrass and sweet grass oil [33].
3.2. S3 Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| RT | Compound Name | Abundance Mean |
|---|---|---|
| 0.745 | Acetic acid, cyano- | 168,502 |
| 0.830 | 2-Amino-4-dimethylaminomethylenepentanedinitrile | 108,883 |
| 1.054 | Ethane-1,2-diimine, N,N′-diamino- | 109,436 |
| 1.065 | Acetone | 114,706 |
| 2.575 | Butanoic acid, ethyl ester | 3,839,625.5 |
| 3.264 | Hexanal | 722,081 |
| 4.235 | 9-Tetradecen-1-ol, acetate, (E)- | 256,238 |
| 5.942 | D-Limonene | 13,781,429.5 |
| 6.678 | 2-Hexenal | 213,594 |
| 6.993 | N-(Trifluoroacetyl)-N,O,O′,O″-tetrakis(trimethylsilyl)norepinephrine | 377,968 |
| 7.628 | (+)-3-Carene, 10-(acetylmethyl)- | 259,938 |
| 7.630 | 3(10)-Caren-4-ol, acetoacetic acid ester | 498,827 |
| 8.286 | 1-Pentanol | 208,402 |
| 8.518 | Nonane, 5-(2-methylpropyl)- | 239,091 |
| 9.647 | Octanal | 165,442.5 |
| 10.755 | 3-Ethyl-3-methylheptane | 114,704 |
| 10.976 | 2-Heptenal, (Z)- | 207,932.5 |
| 11.957 | 5-Hepten-2-one, 6-methyl- | 623,348 |
| 14.761 | Nonanal | 581,017.5 |
| 15.175 | Oxirane, [(tetradecyloxy)methyl]- | 193,068 |
| 15.666 | Oxalic acid, propyl undecyl ester | 165,284 |
| 16.277 | 2-Undecenal, E- | 121,169 |
| 16.306 | Oxirane, 2,2′-(1,4-butanediyl)bis- | 119,155 |
| 17.193 | 2-Furanmethanol, 5-ethenyltetrahydro-.alpha.,.alpha.,5-trimethyl-, cis- | 1,799,498 |
| 17.647 | Undecanol-4 | 291,287 |
| 17.678 | 3-Octanol, 3,6-dimethyl- | 239,713 |
| 18.343 | Ammonium acetate | 1,079,115.5 |
| 18.768 | 2-Furanmethanol, 5-ethenyltetrahydro-.alpha.,.alpha.,5-trimethyl-, cis- | 2,039,755 |
| 19.085 | Octadecane, 1-chloro- | 603,170 |
| 19.587 | Acetic acid, hexyl ester | 601,861 |
| 19.760 | 1,6-Heptadiene, 3,5-dimethyl- | 80,227 |
| 20.519 | Decanal | 622,713 |
| 21.134 | Benzaldehyde | 94,325 |
| 22.424 | 3-Cyclohexene-1-ethanol, .beta.,4-dimethyl- | 131,133 |
| 22.431 | 1-Cyclohexene-1-methanol, 4-(1-methylethenyl)- | 140,779 |
| 22.670 | 3-Heptyne-2,6-dione, 5-methyl-5-(1-methylethyl)- | 134,078 |
| 22.801 | Cyclobutane, 1,2-bis(1-methylethenyl)-, trans- | 214,018 |
| 22.816 | 1-Isopropenyl-3-propenylcyclopentane | 189,887 |
| 23.354 | Cyclohexanol, 2,2,6,6-tetramethyl- | 1,107,971 |
| 23.360 | Citronellyl butyrate | 1,018,024 |
| 24.182 | 1,6-Octadien-3-ol, 3,7-dimethyl-, 2-aminobenzoate | 6,824,657.5 |
| 24.495 | 1-Octanol | 905,951.5 |
| 24.911 | Cyclohexane, 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl)-, [1S-(1.alpha.,2.beta.,4.beta.)]- | 122,847.5 |
| 25.385 | 1,1-Dimethyl-4-methylenecyclohexane | 277,392 |
| 25.417 | Cyclohexanone, 2-methyl-5-(1-methylethenyl)-, trans- | 243,765 |
| 27.500 | Hotrienol | 80,758 |
| 27.795 | Carveol | 545,775 |
| 27.797 | Dispiro[2 .1.2.4]undecane, 8-methylene- | 617,343 |
| 28.060 | Acetophenone | 82,331 |
| 28.301 | Hexanoic acid, 6-bromo- | 217,313 |
| 29.001 | 2-Cyclohexen-1-one, 2-methyl-5-(1-methylethyl)-, (S)- | 532,374.5 |
| 30.125 | Carveol | 474,827 |
| 30.130 | 6-Isopropenyl-3-methoxymethoxy-3-methyl-cyclohexene | 465,247 |
| 30.242 | 1-Nonanol | 768,727.5 |
| 30.972 | cis-p-Mentha-2,8-dien-1-ol | 560,885 |
| 30.974 | trans-p-mentha-1(7),8-dien-2-ol | 662,768 |
| 31.523 | .alpha.-Terpineol | 1,098,969 |
| 32.232 | (-)-Carvone | 3,383,059 |
| 32.611 | Undecane, 2-methyl- | 74,437 |
| 32.624 | Pentadecane | 155,418 |
| 33.721 | 2H-Pyran-3-ol, 6-ethenyltetrahydro-2,2,6-trimethyl- | 203,784 |
| 33.982 | Carveol | 450,597 |
| 34.200 | Ethanone, 1-(3-methylphenyl)- | 150,098 |
| 34.340 | 1-Cyclohexene-1-carboxaldehyde, 4-(1-methylethenyl)- | 262,883 |
| 34.627 | Decane, 1,1′-oxybis- | 92,683 |
| 35.010 | 2H-Pyran-3-ol, 6-ethenyltetrahydro-2,2,6-trimethyl- | 109,855 |
| 35.173 | Cyclopropane, octyl- | 181,727 |
| 35.176 | Decane, 3-chloro- | 131,903 |
| 35.319 | 6-Octen-1-ol, 3,7-dimethyl-, (R)- | 158,561 |
| 35.332 | Citronellal | 169,686 |
| 35.896 | p-Mentha-1(7),8-dien-2-ol | 152,465 |
| 36.474 | 2-Cyclohexen-1-one, 3-methyl-6-(1-methylethenyl)-, (S)- | 98,294 |
| 36.694 | 3-hydroxy-2-methyl-5-(prop-1-en-2-yl)cyclohexanone | 250,595 |
| 37.280 | 2-Cyclohexen-1-ol, 2-methyl-5-(1-methylethenyl)-, acetate, (1R-cis)- | 1,127,661 |
| 37.837 | Heptanoic acid | 1,468,435.5 |
| 38.200 | trans-p-mentha-1(7),8-dien-2-ol | 380,766 |
| 38.414 | Benzyl-diseryl phosphate | 162,123.5 |
| 38.840 | (2R,4R)-p-Mentha-[1(7),8]-diene, 2-hydroperoxide | 97,386 |
| 40.616 | (-)-trans-Myrtanyl acatate | 105,666 |
| 40.623 | Myrcenylacetat | 146,060 |
| 40.857 | Cyclododeca-5,9-dien-1-ol, 2-methyl-, (Z,Z)- | 317,443 |
| 40.859 | (2R)-Bornane-10,2-sultam | 219,341 |
| 41.850 | Benzaldehyde, 4-methoxy- | 233,353 |
| 43.263 | Octanoic acid | 191,580 |
| 43.436 | Cyclohexanemethanol, 4-ethenyl-.alpha.,.alpha.,4-trimethyl-3-(1-methylethenyl)-, [1R-(1.alpha.,3.alpha.,4.beta.)]- | 103,468 |
| 44.618 | 2-Pentadecanone, 6,10,14-trimethyl- | 69,726 |
| 45.482 | Dodecanoic acid, 3-hydroxy- | 256,424 |
| 45.502 | n-Hexadecanoic acid | 335,278 |
| 47.527 | 2,6-Octadiene-1,8-diol, 2,6-dimethyl- | 896,585.5 |
| 47.940 | 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | 344,852.5 |
| 50.351 | Benzophenone | 173,741.5 |
| 51.518 | Vanillin | 206,667.5 |
| RT | Compound Name | Abundance Mean |
|---|---|---|
| 1.073 | Cyclopropyl methyl carbinol | 120,514 |
| 3.273 | Hexanal | 575,125.5 |
| 3.405 | Methoxyacetic acid, tridecyl ester | 68,993 |
| 3.430 | 3-Tridecene, (Z)- | 68,920 |
| 4.183 | 4-Pentenal, 2-ethyl- | 115,550 |
| 4.460 | Sydnone, 3,3′-trimethylenedi- | 22,564 |
| 5.214 | .beta.-Myrcene | 1,018,173.5 |
| 5.260 | .beta.-Myrcene | 619,254 |
| 5.490 | Ethanol, 2-[2-(4-pyridyl)ethylamino]- | 42,808 |
| 5.921 | Cyclohexene, 4-ethenyl-1,4-dimethyl- | 639,379 |
| 6.702 | 5-Aminoisoxazole | 42,411 |
| 6.706 | 3-Hexenal, (Z)- | 364,986 |
| 7.009 | N-(Trifluoroacetyl)-N,O,O′,O″-tetrakis(trimethylsilyl)norepinephrine | 118,904 |
| 7.601 | .beta.-Ocimene | 110,410 |
| 7.615 | .alpha.-Pinene | 433,550 |
| 8.170 | 3-Carene | 119,228 |
| 8.215 | Tricyclo[2.2.1.0(2,6)]heptane, 1,7,7-trimethyl- | 156,509 |
| 8.229 | .beta.-Ocimene | 630,021 |
| 8.464 | Dodecane, 4,6-dimethyl- | 129,460 |
| 8.475 | Undecane, 3,8-dimethyl- | 507,030 |
| 10.385 | trans,cis-2,6-Nonadienyl acetate | 34,989 |
| 10.467 | Tridecane, 6-methyl- | 210,574 |
| 10.497 | Nonane, 4,5-dimethyl- | 37,215 |
| 10.734 | Dodecane, 4,6-dimethyl- | 338,715.5 |
| 11.000 | 3-Hepten-1-ol, (Z)- | 226,106 |
| 11.151 | 3-Ethyl-3-methylheptane | 239,089 |
| 11.425 | cis-Aconitic anhydride | 27,787 |
| 11.556 | 4-Heptafluorobutyroxytridecane | 524,738 |
| 11.586 | Nonane, 4,5-dimethyl- | 150,122 |
| 11.930 | 5-Hepten-2-one, 6-methyl- | 378,933 |
| 12.318 | 2-Isopropyl-5-methyl-1-heptanol | 195,059 |
| 12.323 | Oxalic acid, allyl octadecyl ester | 89,397 |
| 12.380 | l-Alanine, N-(cyclohexylcarbonyl)-, heptadecyl ester | 37,359 |
| 14.688 | Nonanal | 113,348 |
| 15.129 | 1-Methoxy-3-hydroxymethylheptane | 119,355 |
| 15.620 | Heptadecane, 2,6,10,14-tetramethyl- | 65,889 |
| 15.635 | Tridecane | 183,448 |
| 16.195 | 3-Undecene, (Z)- | 63,096 |
| 16.220 | 2-Nonenal, (Z)- | 147,528 |
| 16.460 | 2-(3-Bromopropyl)-[1,3]dioxolane | 28,267 |
| 18.280 | Ammonium acetate | 187,763 |
| 18.330 | Methoxyacetic acid, hexyl ester | 48,206 |
| 18.390 | 1,4-Hexadiene, 5-methyl- | 261,153 |
| 18.429 | Cyclohexan-1,4,5-triol-3-one-1-carboxylic acid | 619,199 |
| 18.661 | Furfural | 345,710 |
| 19.005 | Methoxyacetic acid, 2-tridecyl ester | 57,046 |
| 19.022 | Nonane, 3-methyl-5-propyl- | 417,744 |
| 19.055 | Nonane, 4,5-dimethyl- | 72,220 |
| 19.600 | Butane, 1-(ethenyloxy)- | 23,034 |
| 19.715 | 2,3-Hexadiene, 2-methyl- | 388,031 |
| 20.635 | 2-Propyl-1-pentanol | 135,168 |
| 21.037 | Benzaldehyde | 246,318 |
| 21.250 | Undecane, 3,6-dimethyl- | 147,124 |
| 21.466 | R-Limonene | 197,652 |
| 21.730 | Pyrrolidine-2,4-dione | 21,913 |
| 21.764 | Dodecane, 2,6,11-trimethyl- | 130,862 |
| 21.770 | Decane, 2-methyl- | 37,031 |
| 22.158 | 1,5-Heptadiene, 2,3,6-trimethyl- | 635,307.5 |
| 23.267 | 6-Octen-1-ol, 3,7-dimethyl-, acetate | 589,174 |
| 23.301 | Cyclopropanemethanol, .alpha.,2-dimethyl-2-(4-methyl-3-pentenyl)-, [1.alpha.(R*),2.alpha.]- | 1,041,462 |
| 23.660 | N-[2,2,2-Trifluoro-1-(isopropylamino)-1-(trifluoromethyl)ethyl]isovaleramide | 197,837 |
| 24.198 | 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl- | 18,728,058 |
| 24.205 | 1-Bromo-3,7-dimethyl-2,6-octadiene | 31,337,534 |
| 24.306 | Linalyl acetate | 10,646,193 |
| 25.300 | 3-hydroxy-2-methyl-5-(prop-1-en-2-yl)cyclohexanone | 105,570 |
| 26.919 | Oxalic acid, ethyl neopentyl ester | 276,546 |
| 26.940 | Undecane, 2-methyl- | 248,945 |
| 27.153 | Ethanol, 2-(2-ethoxyethoxy)- | 337,777 |
| 27.405 | 1,5-Heptadiene, 2,5-dimethyl-3-methylene- | 127,621 |
| 27.590 | Cyclohexane, 1R-acetamido-2,3-cis-epoxy-4-cis-formyloxy- | 41,150 |
| 27.948 | Acetophenone | 217,637 |
| 28.322 | d-Menthol | 135,041 |
| 30.080 | 1-Pentene, 5-(2,2-dimethylcyclopropyl)-2-methyl-4-methylene- | 495,428 |
| 30.086 | (-)-cis-Myrtanol | 169,778 |
| 30.505 | Acetic acid, cyano- | 116,530 |
| 30.842 | Cyclobutane, 1,2-bis(1-methylethenyl)-, trans- | 607,249.5 |
| 31.440 | .alpha.-Terpineol | 3,145,562.5 |
| 32.278 | Heptafluorobutanoic acid, 2-(1-adamantyl)ethyl ester | 611,947 |
| 32.279 | 2,4-Methano-1H-indene, 4-chlorooctahydro- | 186,219 |
| 32.657 | .beta.-Bisabolene | 192,658.5 |
| 32.958 | 2,6-Octadienal, 3,7-dimethyl-, (E)- | 342,345.5 |
| 33.300 | 1-Bromo-3,7-dimethyl-2,6-octadiene | 1,139,696 |
| 33.680 | 2H-Pyran-3-ol, 6-ethenyltetrahydro-2,2,6-trimethyl- | 138,117 |
| 34.317 | Dispiro[4.2.4.2]tetradecane | 118,050 |
| 34.327 | Cis-8-ethyl-exo-tricyclo[5.2.1.0(2.6)]decane | 66,678 |
| 34.660 | 1-Bromo-3,7-dimethyl-2,6-octadiene | 1,387,273 |
| 36.273 | 5-Hepten-1-ol, 2-ethenyl-6-methyl- | 386,406 |
| 36.587 | Anethole | 232,191 |
| 37.173 | Lanceol, cis | 198,363 |
| 37.177 | Ionone | 218,275 |
| 37.344 | 1,4-Methanobenzocyclodecene, 1,2,3,4,4a,5,8,9,12,12a-decahydro- | 517,247.3 |
| 37.793 | 1,2,4-Trioxolane, 3,5-dipropyl- | 599,431 |
| 37.811 | Octane, 1-azido- | 718,189 |
| 37.900 | 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-, (Z,E)- | 332,897 |
| 38.200 | 2H-Azepin-2-one, hexahydro-4-methyl- | 111,168 |
| 38.205 | 1-Undecene, 5-methyl- | 150,120 |
| 38.453 | 2-Methylvaleroyl chloride | 184,447 |
| 38.730 | Geranyl acetate, 2,3-epoxy- | 139,279 |
| 39.503 | 2-Dimethyl(trimethylsilyl)silyloxytridecane | 37,938 |
| 39.686 | 2-Butanone, 4-(2,6,6-trimethyl-2-cyclohexen-1-ylidene)- | 212,113.5 |
| 40.236 | 3-Decyn-2-ol | 108,829 |
| 40.269 | 3-Tetradecyn-1-ol | 127,313 |
| 40.807 | Cyclododeca-5,9-dien-1-ol, 2-methyl-, (Z,Z)- | 151,636 |
| 41.095 | 1,5,5-Trimethyl-6-methylene-cyclohexene | 370,901 |
| 41.107 | 1,3,6-Heptatriene, 2,5,6-trimethyl- | 312,389 |
| 44.479 | 1-Heptyn-6-one | 88,109 |
| 44.600 | 9-Decen-2-one, 5-methylene- | 126,372 |
| 44.613 | 1-Heptyn-6-one | 278,250 |
| 44.807 | 10-Undecyn-1-ol | 104,806 |
| 44.819 | Cyclopropane, hexylidene- | 158,800 |
| 46.771 | Acetic acid, cyano- | 106,057 |
| 47.899 | 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | 484,359.5 |
| 57.725 | Imidodicarbonic acid, diethyl ester | 132,177 |
| RT | Compound Name | Abundance Mean |
|---|---|---|
| 0.879 | Acetic acid, cyano- | 56,772 |
| 1.040 | Pentanal, 2,4-dimethyl- | 57,277 |
| 1.071 | 1-Propen-2-ol, formate | 167,675 |
| 2.285 | .alpha.-Pinene | 170,241.5 |
| 2.328 | 1,3,6-Octatriene, 3,7-dimethyl-, (Z)- | 363,519 |
| 2.385 | (R)-(+)-Citronellic acid | 85,004 |
| 2.460 | 3-Undecen-1-yne, (E)- | 7687 |
| 3.225 | Hexanal | 28,897 |
| 3.965 | Methanone, 1,3-dithian-2-ylphenyl- | 64,181 |
| 6.013 | D-Limonene | 44,500,934 |
| 6.715 | 4-Pentenal, 2-methyl- | 50,578 |
| 7.642 | .gamma.-Terpinene | 6,561,102 |
| 8.201 | .beta.-Ocimene | 328,361 |
| 8.607 | Benzene, 1-methyl-3-(1-methylethyl)- | 2,692,739.5 |
| 9.068 | 2-Carene | 3,351,302 |
| 9.078 | (+)-4-Carene | 3,540,274 |
| 10.465 | Octane, 2,3,3-trimethyl- | 44,003 |
| 10.752 | Undecane, 3,8-dimethyl- | 74,779 |
| 11.947 | 5-Hepten-2-one, 6-methyl- | 273,176.5 |
| 13.846 | 1-Octanol, 3,7-dimethyl-, (S)- | 180,675 |
| 14.722 | Nonanal | 92,985 |
| 15.668 | Tridecane | 139,294 |
| 16.709 | Benzene, 2-ethenyl-1,3-dimethyl- | 191,674 |
| 16.740 | o-Isopropenyltoluene | 194,054 |
| 18.399 | Cyclohexan-1,4,5-triol-3-one-1-carboxylic acid | 361,809 |
| 18.408 | Glycidol | 717,548 |
| 18.670 | Furfural | 621,339 |
| 18.970 | Copaene | 551,737 |
| 18.986 | .alfa.-Copaene | 507,260 |
| 19.180 | Decane, 5-propyl- | 88,193 |
| 19.434 | 6-Octenal, 3,7-dimethyl-, (R)- | 837,850 |
| 19.699 | 3-Octen-2-ol, (Z)- | 451,255 |
| 19.722 | Cyclopropane, trimethylmethylene- | 866,370 |
| 20.190 | E-1,5,9-Decatriene | 64,911 |
| 20.665 | 1-Methoxy-3-hydroxymethylheptane | 126,181 |
| 21.051 | 1,2,4-Trioxolane, 3-methyl-5-phenyl- | 59,002 |
| 21.430 | Octane, 5-ethyl-2-methyl- | 168,365 |
| 21.683 | (Z,Z)-.alpha.-Farnesene | 330,622 |
| 21.813 | Nonane, 5-(2-methylpropyl)- | 161,847 |
| 22.189 | 3-Ethyl-1,5-octadiene | 224,260 |
| 23.256 | trans-p-mentha-1(7),8-dien-2-ol | 842,313 |
| 23.272 | cis-p-mentha-1(7),8-dien-2-ol | 736,608 |
| 24.139 | 1-Bromo-3,7-dimethyl-2,6-octadiene | 8,422,457 |
| 24.146 | .beta.-Myrcene | 7,426,440 |
| 24.450 | Caryophyllene | 2,412,339 |
| 24.803 | Cyclohexane, 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl)- | 1,661,763 |
| 24.828 | cis-.alpha.-Bisabolene | 1,997,395 |
| 25.130 | Dodecanal | 957,818 |
| 25.148 | trans-2-Dodecen-1-ol, trifluoroacetate | 522,026 |
| 26.458 | Cyclohexane, 4-methyl-2-methylene-1-(1-methylethylidene)- | 236,924 |
| 27.055 | Pentadecane | 615,120 |
| 27.122 | Heptadecane, 2,6,10,14-tetramethyl- | 369,320 |
| 27.928 | 3-Tetradecyn-1-ol | 964,333 |
| 27.958 | trans-2-Dodecen-1-ol, trifluoroacetate | 840,881 |
| 28.359 | 1,4,7,-Cycloundecatriene, 1,5,9,9-tetramethyl-, Z,Z,Z- | 245,910 |
| 28.761 | 2,2-Dimethylpropanoic acid, 2-adamantyl ester | 255,092 |
| 29.215 | Cycloisolongifolene | 97,234 |
| 30.355 | 2,6-Octadienal, 3,7-dimethyl-, (E)- | 51,100,548.5 |
| 31.498 | .alpha.-Terpineol | 8,024,237 |
| 32.200 | (-)-Carvone | 653,884 |
| 32.388 | Dispiro[4.2.4.2]tetradecane | 107,256 |
| 32.450 | 3-Adamantanecarboxylic acid, phenyl ester | 701,488 |
| 33.255 | 2,6-Octadienal, 3,7-dimethyl-, (E)- | 71,369,488.5 |
| 33.958 | Carveol | 117,489 |
| 33.989 | Cycloheptane, 1,3,5-tris(methylene)- | 157,810 |
| 34.465 | Naphthalene, 1,2,3,4,4a,5,6,7-octahydro-4a-methyl- | 102,571 |
| 34.475 | 1H-3a,7-Methanoazulene, 2,3,6,7,8,8a-hexahydro-1,4,9,9-tetramethyl-, (1.alpha.,3a.alpha.,7.alpha.,8a.beta.)- | 90,857 |
| 34.689 | 1-Bromo-3,7-dimethyl-2,6-octadiene | 594,352 |
| 35.305 | 7-Octen-1-ol, 3,7-dimethyl-, (S)- | 473,977 |
| 36.040 | Z,Z,Z-4,6,9-Nonadecatriene | 914,01 |
| 36.294 | 5-Hepten-1-ol, 2-ethenyl-6-methyl- | 1,012,776 |
| 36.612 | Anethole | 319,573 |
| 37.369 | 1,4-Methanobenzocyclodecene, 1,2,3,4,4a,5,8,9,12,12a-decahydro- | 867,511.5 |
| 37.929 | 5-Hepten-1-ol, 2-ethenyl-6-methyl- | 1,881,320 |
| 37.939 | 1-Bromo-3,7-dimethyl-2,6-octadiene | 2,378,768 |
| 38.473 | 1-Hexene, 3,4,5-trimethyl- | 2,227,084.5 |
| 39.175 | 2-Bromosebacic acid, bis(trimethylsilyl) ester | 120,406 |
| 39.715 | 2-Butanone, 4-(2,6,6-trimethyl-2-cyclohexen-1-ylidene)- | 172,929 |
| 40.354 | Caryophyllene | 167,562 |
| 41.044 | 2-Butanone, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)- | 182,625 |
| 41.120 | 11,11-Dimethyl-spiro[2,9]dodeca-3,7-dien | 156,106 |
| 41.352 | 5-Hepten-2-one, 6-methyl- | 57,635 |
| 41.943 | 2-Cyclohexen-1-one, 6-(1-hydroxy-1-methylethyl)-3-methyl- | 71,111 |
| 43.753 | 1-Nitro-2-propanone | 369,292 |
| 44.620 | 1-Heptyn-6-one | 806,489 |
| 44.628 | Methyl Isobutyl Ketone | 840,137 |
| 44.750 | trans,trans-2,6-Dimethyl-2,6-octadiene-1,8-diol | 174,272 |
| 47.500 | Cyclopropaneoctanoic acid, 2-[[2-[(2-ethylcyclopropyl)methyl]cyclopropyl]methyl]-, methyl ester | 105,937 |
| 47.515 | Tetraacetyl-d-xylonic nitrile | 34,187 |
| 47.917 | 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | 481,958 |
| 48.378 | Cycloheptane, 4-methylene-1-methyl-2-(2-methyl-1-propen-1-yl)-1-vinyl- | 99,068 |
| 52.790 | 1-Propanamine, N-nitro- | 37,512 |
| 52.992 | 1-Butanol | 59,088 |
| RT | Compound Name | Abundance Mean |
|---|---|---|
| 0.018 | Acetic acid, cyano- | 63,013 |
| 0.825 | Ethanol, 2-(vinyloxy)- | 60,490 |
| 1.055 | Hydroperoxide, 1-methylethyl | 85,713 |
| 1.120 | Ether, 2-chloro-1-propyl isopropyl | 63,783 |
| 3.259 | Hexanal | 352,944 |
| 3.350 | Glutaraldehyde | 150,508 |
| 3.355 | 3-Penten-2-ol | 315,320 |
| 3.440 | 1,2,15-Pentadecanetriol | 149,001 |
| 3.490 | Trifluoromethanesulfonyl imidazole | 146,098 |
| 3.540 | Acetic acid, cyano- | 63,215 |
| 4.239 | 9-Tetradecen-1-ol, acetate, (E)- | 81,718 |
| 5.184 | 2-Octyn-1-ol | 825,999 |
| 5.211 | .beta.-Myrcene | 501,590 |
| 5.665 | 2-Heptanone | 127,653 |
| 5.912 | D-Limonene | 201,138 |
| 5.918 | Cyclohexene, 4-ethenyl-1,4-dimethyl- | 109,577 |
| 6.190 | 3-Pyridinecarbonitrile, 4-(methoxymethyl)-6-methyl-2-(2-propenyloxy)- | 64,607 |
| 6.706 | 2-Hexenal | 151,942 |
| 6.714 | (1-Allylcyclopropyl)methanol | 133,747 |
| 6.957 | N-(Trifluoroacetyl)-N,O,O′,O″-tetrakis(trimethylsilyl)norepinephrine | 55,876 |
| 7.511 | Furfuryl heptanoate | 99,402 |
| 7.556 | .alpha.-Pinene | 97,319 |
| 7.630 | 4-Terpinenyl acetate | 61,282 |
| 8.220 | .beta.-Ocimene | 235,507 |
| 8.486 | Undecane, 3,8-dimethyl- | 261,838 |
| 8.499 | Dodecane, 4,6-dimethyl- | 334,363 |
| 8.784 | Octane, 2,3,3-trimethyl- | 140,125 |
| 9.045 | (+)-4-Carene | 55,258 |
| 10.420 | trans-.beta.-Terpinyl pentanoate | 98,048 |
| 10.485 | Methoxyacetic acid, 2-methylpropyl ester | 72,844 |
| 10.729 | Undecane, 2-methyl- | 153,807 |
| 10.739 | Nonane, 5-(2-methylpropyl)- | 210,592 |
| 10.963 | Butanamide, 3-cyclohexylamino-4-hydroxy-N-cyclohexyl- | 233,226 |
| 10.972 | Z-1,8-Dodecadiene | 197,182 |
| 11.190 | Nonane, 1-iodo- | 843,66 |
| 11.265 | 2,4-Pentanedione, 3-ethyl- | 65,334 |
| 11.453 | 1-Pentene, 5-chloro- | 326,317.5 |
| 11.595 | Undecane, 2,8-dimethyl- | 101,042 |
| 11.910 | 5-Hepten-2-one, 6-methyl- | 361,742.5 |
| 12.318 | Trichloroacetic acid, tridecyl ester | 59,185 |
| 14.315 | Diazene, dicyclohexyl-, 1,2-dioxide | 61,943 |
| 14.698 | Nonanal | 167,658 |
| 15.146 | 2-Decen-1-ol | 146,286 |
| 15.591 | Heptadecane, 2,6,10,14-tetramethyl- | 59,042 |
| 15.627 | Dodecane, 2-methyl- | 75,909 |
| 16.206 | 5-Tridecene, (Z)- | 68,987 |
| 16.267 | 2-Octyn-1-ol | 71,981 |
| 18.250 | 2,3-Epoxybutane | 411,295 |
| 18.445 | 6-Nonenal, (Z)- | 530,076 |
| 18.452 | Imidazole, 2-amino-5-[(2-carboxy)vinyl]- | 245,253 |
| 18.654 | Furfural | 266,284.5 |
| 19.036 | Nonane, 5-(1-methylpropyl)- | 138,397 |
| 19.039 | Nonane, 3-methyl-5-propyl- | 243,464 |
| 19.698 | 2,3-Hexadiene, 2-methyl- | 277,170 |
| 20.606 | 1-Pentanol, 2-ethyl-4-methyl- | 129,402 |
| 20.995 | Benzaldehyde | 165,013 |
| 21.054 | 1-Benzamido-N-benzyl-1-[.alpha.-(2-pyridylthio)benzylidene]acetamide | 119,058 |
| 21.205 | Nonane, 4,5-dimethyl- | 83,886 |
| 21.426 | R-Limonene | 119,271 |
| 21.758 | Dodecane, 4-methyl- | 78,992 |
| 22.139 | 1,5-Heptadiene, 2,3,6-trimethyl- | 209,903 |
| 23.194 | Heptanal, 2-methyl- | 278,302 |
| 23.262 | 2-Octen-1-ol, 3,7-dimethyl- | 450,603 |
| 23.615 | 2-Heptafluorobutyroxydodecane | 70,417 |
| 24.087 | 1-Bromo-3,7-dimethyl-2,6-octadiene | 9,842,404 |
| 25.104 | Oxirane, decyl- | 134,290 |
| 25.211 | Dihydrocarvyl acetate | 90,710 |
| 26.920 | Tridecane | 151,803 |
| 26.937 | Pentadecane | 175,135 |
| 27.128 | Ethanol, 2-(2-ethoxyethoxy)- | 248,708.5 |
| 27.364 | Dispiro[2.0.2.5]undecane, 8-methylene- | 120,439 |
| 27.894 | S-Benzoyl-N-(O-hydroxybenzylidene)thiohydroxylamine | 87,628 |
| 28.021 | 2-Decene, (Z)- | 71,624 |
| 28.284 | Cyclohexanol, 5-methyl-2-(1-methylethyl)-, [1S-(1.alpha.,2.alpha.,5.beta.)]- | 60,158 |
| 30.084 | Longipinene epoxide | 200,527 |
| 30.135 | cis-p-mentha-1(7),8-dien-2-ol | 89,537 |
| 30.355 | Valeric acid, 3-tridecyl ester | 80,075 |
| 30.565 | Acetaldoxime | 68,044 |
| 30.803 | Cyclobutane, 1,2-bis(1-methylethenyl)-, trans- | 211,358.5 |
| 31.404 | .alpha.-Terpineol | 762,821.5 |
| 32.214 | 2,2-Dimethylpropanoic acid, 2-adamantyl ester | 60,028 |
| 32.296 | 2,4-Methano-1H-indene, 4-chlorooctahydro- | 118,582 |
| 32.590 | cis-sesquisabinene hydrate | 117,388 |
| 32.929 | 2,6-Octadien-1-ol, 2,7-dimethyl- | 89,883.5 |
| 33.278 | 1-Bromo-3,7-dimethyl-2,6-octadiene | 288,750 |
| 33.284 | 4-Hexen-1-ol, 5-methyl-2-(1-methylethenyl)-, acetate | 343,381 |
| 33.572 | Cyclopentane, 1-ethyl-3-methyl-, trans- | 52,106 |
| 33.967 | Pentanal | 185,683 |
| 34.280 | Cyclododeca-5,9-dien-1-ol, 2-methyl-, (Z,Z)- | 84,052 |
| 34.310 | Cis-8-ethyl-exo-tricyclo[5.2.1.0(2.6)]decane | 65,264 |
| 34.643 | 2,6-Octadien-1-ol, 2,7-dimethyl- | 492,969 |
| 34.648 | .beta.-Myrcene | 508,110 |
| 36.243 | 5-Hepten-1-ol, 2-ethenyl-6-methyl- | 133,316 |
| 36.577 | Anethole | 199,512 |
| 37.160 | Andrographolide | 77,139 |
| 37.584 | 1,4-Methanobenzocyclodecene, 1,2,3,4,4a,5,8,9,12,12a-decahydro- | 696,692.5 |
| 37.779 | Hexanal | 1,321,771 |
| 37.793 | 1,2,4-Trioxolane, 3,5-dipropyl- | 219,894 |
| 37.875 | .beta.-Myrcene | 172,198 |
| 38.180 | 2,5-Pyrrolidinedione, 1-ethyl- | 131,028 |
| 38.429 | 2-Butanone, 3-methyl-1-phenyl- | 153,622 |
| 38.435 | 4-Ethyl-1-hexyn-3-ol | 192,370 |
| 38.771 | Cyclopropane, 1-(1′-propenyl)-2-hydroxymethyl- | 75,503 |
| 39.686 | Acetic acid, 6,6-dimethyl-2-methylene-7-(3-oxobutylidene)oxepan-3-ylmethyl ester | 61,416 |
| 40.818 | 3-Tridecen-1-yne, (Z)- | 69,714 |
| 40.819 | 9,12,15-Octadecatrienal | 97,882 |
| 41.011 | 2-Butanone, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)- | 102,986 |
| 41.093 | cis-sesquisabinene hydrate | 224,962 |
| 44.166 | Adenine-9-propanoic acid, alpha.-t-butoxycarbonylamino- | 102,494 |
| 44.463 | 1-Heptyn-6-one | 98,989 |
| 44.469 | Methyl Isobutyl Ketone | 63,708 |
| 44.595 | 9-Decen-2-one, 5-methylene- | 143,812 |
| 45.240 | Propanal, 2-methyl-, 2-propenylhydrazone | 52,896 |
| 45.470 | Cyclopentaneundecanoic acid | 64,619 |
| 47.888 | 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | 283,652 |
| RT | Compound Name | Abundance Mean |
|---|---|---|
| 0.730 | Acetic acid, cyano- | 94,784 |
| 0.815 | Propane | 115,993 |
| 0.881 | N-(2-Methylacryloyl)imidazola | 114,248 |
| 1.040 | Allyl acetate | 49,897 |
| 1.081 | 2-Hexanone, 4-hydroxy-5-methyl-3-propyl- | 221,553 |
| 1.425 | Pentan-2-ol, 1-tert-buthylamino-4-methyl- | 37,600 |
| 1.438 | Butanal, 2-methyl- | 100,607 |
| 1.490 | Butanal, 3-methyl- | 84,716 |
| 1.947 | 3-Aminopyrrolidine | 207,498 |
| 1.951 | 1,3-Dioxane-4,6-dione, 2,2-dimethyl- | 225,463 |
| 2.030 | Acetamide, N-[2-(4-methylphenoxy)ethyl]- | 65,728 |
| 2.045 | Azacyclodecan-5-ol | 45,434 |
| 3.284 | Hexanal | 1,398,458.5 |
| 3.655 | Triallyl phosphate | 23,541 |
| 5.575 | Acetonitrile, bromo- | 26,297 |
| 5.675 | Propane, 2-(ethenyloxy)- | 24,723 |
| 5.730 | 2,3-Anhydro-d-galactosan | 30,652 |
| 5.740 | Heptanal | 147,257 |
| 5.765 | Butanal, 3-methyl- | 149,962 |
| 5.953 | Tetrahydropyrrolo[1,2-a]azetidin-2-one | 193,616 |
| 5.957 | Murexide | 90,456 |
| 6.030 | 5-[2-Thienyl]hydantoin | 77,334 |
| 6.702 | 2-Hexenal | 676,577 |
| 6.985 | N-(Trifluoroacetyl)-N,O,O′,O″-tetrakis(trimethylsilyl)norepinephrine | 706,731 |
| 8.505 | Dodecane, 4-methyl- | 90,410 |
| 9.655 | Octanal | 66,979 |
| 9.658 | 1,6-Anhydro-3,4-dideoxy-.beta.-D-manno-hexapyranose | 54,785 |
| 11.959 | 5-Hepten-2-one, 6-methyl- | 262,786 |
| 14.740 | Nonanal | 373,934.5 |
| 15.147 | Ethanol, 2-butoxy- | 138,163 |
| 15.165 | 2,5-Dimethyl-1-hepten-4-ol | 121,419 |
| 18.282 | Ammonium acetate | 2,141,214 |
| 18.701 | Furfural | 1,136,801 |
| 19.103 | Nonane, 5-(2-methylpropyl)- | 246,297 |
| 20.714 | 1-Hexanol, 2-ethyl- | 75,528 |
| 21.122 | Benzaldehyde | 4,085,838.5 |
| 21.846 | Undecane, 3,7-dimethyl- | 316,312 |
| 22.135 | Cycloheptano[d]imidazolidine, 1,3-dihydroxy-2-methyl- | 103,470 |
| 23.290 | Propanoic acid | 200,796.5 |
| 24.141 | 5-Hepten-1-ol, 2-ethenyl-6-methyl- | 197,773 |
| 24.456 | 1H-Pyrazole, 1,3,5-trimethyl- | 783,099 |
| 24.467 | 2-Furancarboxaldehyde, 5-methyl- | 686,396 |
| 26.464 | Benzenemethanol, .alpha.-(1-ethenylpentyl)-.alpha.-methyl- | 96,576 |
| 27.076 | Undecane, 2-methyl- | 255,153 |
| 27.700 | Benzaldehyde, 2-methyl- | 129,230.5 |
| 28.036 | Acetophenone | 69,030 |
| 30.240 | Pent-3-en-2-one, 4-methyl-, oxime | 127,754 |
| 30.255 | 4-Methoxy-2,3-dimethyl-2,3-dihydroazete | 103,964 |
| 30.487 | 2(3H)-Furanone, 5-ethyldihydro- | 554,528.5 |
| 32.606 | 2,3-Dimethyldodecane | 55,910 |
| 32.606 | Tetradecane, 2-methyl- | 71,677 |
| 33.011 | Propanoic acid, 3-hydroxy-2-[2-{[benzyloxy)carbonyl]amino}acetyl)amino] | 43,642 |
| 34.024 | Pentanoic acid | 72,953.5 |
| 34.312 | Methyl salicylate | 34,235 |
| 35.313 | 2(3H)-Furanone, dihydro-5-propyl- | 3,103,701.5 |
| 36.641 | 1-Octadecanesulphonyl chloride | 85,226.5 |
| 37.648 | 1,4-Methanobenzocyclodecene, 1,2,3,4,4a,5,8,9,12,12a-decahydro- | 91,628.5 |
| 37.840 | Hexanoic acid | 887,087.5 |
| 37.928 | Phenol, 2-methoxy- | 624,495 |
| 38.239 | 2,5-Pyrrolidinedione, 1-ethyl- | 345,675.5 |
| 38.411 | Benzyl-diseryl phosphate | 193,527 |
| 38.413 | Benzene, [(2-propenyloxy)methyl]- | 326,206 |
| 38.993 | 2(3H)-Furanone, 5-butyldihydro- | 567,940.5 |
| 39.296 | Phenylethyl Alcohol | 81,672 |
| 40.533 | Maltol | 3,012,548.5 |
| 40.765 | Heptanoic acid | 213,937 |
| 40.869 | 2-Pentenenitrile, 4,4-dimethyl- | 282,191 |
| 40.879 | 7-Nonynoic acid | 362,88 |
| 41.025 | 2-Hexenoic acid | 206,927 |
| 41.060 | 1H-Azepine, hexahydro-1-nitroso- | 33,836 |
| 41.175 | 1-Dodecene | 170,528 |
| 41.180 | E-11,13-Tetradecadien-1-ol | 25,182 |
| 41.435 | Furan, 2,2′-[oxybis(methylene)]bis- | 124,175 |
| 41.845 | Benzaldehyde, 4-methoxy- | 2,417,723 |
| 42.050 | 1H-Pyrrole-2-carboxaldehyde | 73,396 |
| 42.195 | Benzene, (2-methyl-1-methylenebutyl)- | 90,494 |
| 42.201 | Cinnamaldehyde, (E)- | 126,790 |
| 42.348 | Benzene, 1,4-dimethoxy-2-methyl- | 85,105 |
| 43.259 | Octanoic acid | 731,575 |
| 44.420 | 4-Acetylanisole | 102,679 |
| 44.425 | 3-Methoxyacetophenone | 138,444 |
| 44.526 | 2(3H)-Furanone, 5-hexyldihydro- | 597,573 |
| 44.745 | Ethanol, 2-phenoxy- | 39,625 |
| 44.909 | 1,3,5-Cycloheptatriene, 1-methoxy- | 58,650.5 |
| 45.506 | Nonanoic acid | 69,740.5 |
| 46.205 | Piperonal | 87,421.5 |
| 46.704 | 2-n-Butyl furan | 23,192 |
| 47.458 | Benzenemethanol, 4-methoxy- | 421,101.5 |
| 47.590 | n-Decanoic acid | 300,368 |
| 47.938 | 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | 278,448.5 |
| 48.989 | .gamma.-Dodecalactone | 258,188 |
| 49.980 | Benzoic acid | 62,533 |
| 51.376 | 1,2-Benzenedicarboxylic acid, dihexyl ester | 26,249 |
| 51.531 | Vanillin | 8,287,611.5 |
| 54.111 | 3-Hydroxy-4-methoxybenzyl alcohol | 51,514 |
| RT | Compound Name | Abundance Mean |
|---|---|---|
| 0.189 | Carbamic acid, (cyanoacetyl)-, ethyl ester | 40,219 |
| 0.690 | Acetic acid, cyano- | 71,328 |
| 0.859 | 4-Heptanone, dimethylhydrazone | 68,187 |
| 0.931 | 1-(4-Acetamidoanilino)-3,7-dimethylbenzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile | 42,303 |
| 0.980 | Sulfide, methyl 1-methyl-2-butenyl | 44,455 |
| 1.390 | 4H,8H-[1,2,4]Triazino[3,4-b][1,3,4]thiadiazin-4-one, 7-amino-3-methyl- | 48,156 |
| 1.430 | Butanal, 3-methyl- | 54,247 |
| 1.504 | Acetic acid, cyano- | 24,296 |
| 1.547 | 4-Penten-2-ol, 4-methyl- | 36,478 |
| 1.719 | 2-Butyne-1,4-diol bis(.beta.-hydroxyethyl ether) | 29,900 |
| 2.525 | 3-Hexenoic acid, ethyl ester, (Z)- | 21,487 |
| 2.599 | dl-Ornithine | 27,137 |
| 2.618 | Thiocyanic acid, 5-amino-3-methyl-4-isoxazolyl ester | 22,308 |
| 2.740 | 5-Ethyl-2-methyl-pyridin-4-amine | 27,062 |
| 3.220 | Hexanal | 95,779 |
| 3.273 | Hexanal | 32,777 |
| 3.295 | 1,3-Dioxane-4,6-dione, 5,5-dimethyl-2-(1-methylethylidene)- | 36,361 |
| 3.308 | Butane, 1-(ethenyloxy)- | 82,998 |
| 3.335 | 1-Propene, 3-methoxy- | 92,869 |
| 3.400 | 5-Aminoisoxazole | 93,919 |
| 3.420 | cis-Aconitic anhydride | 33,724 |
| 3.435 | 2-Propanamine, N-ethyl-N-nitroso- | 24,872 |
| 3.460 | Tris(aziridinomethyl)hydrazine | 24,606 |
| 3.504 | Butane, 1-(ethenyloxy)- | 22,708 |
| 3.505 | 4,5-Dicarboxy-1,2,3-triazole | 23,452 |
| 5.125 | Formic acid, 1,1-dimethylethyl ester | 23,031 |
| 5.150 | 1,2,4-Triazol-5-acetic acid, 3-amino- | 24,912 |
| 5.212 | Ethanediamide, N-(1-methylpropyl)-N′-(3-pyridinylmethyl)- | 21,740 |
| 5.225 | cis-Aconitic anhydride | 26,678 |
| 5.330 | (+)-2-Carene, 4-.alpha.-isopropenyl- | 21,260 |
| 5.740 | 3-(4-Methyl-piperazin-1-yl)-N-(4-trifluoromethoxy-phenyl)-propionamide | 25,237 |
| 6.677 | Allyl methallyl ether | 54,578 |
| 6.974 | N-(Trifluoroacetyl)-N,O,O′,O″-tetrakis(trimethylsilyl)norepinephrine | 69,040 |
| 7.040 | N-Methyladrenaline, tri-TMS | 28,857 |
| 7.503 | Cyclopropanecarboxylic acid | 21,350 |
| 8.163 | Chlorocarbonyl t-butoxy sulfide | 30,269 |
| 8.186 | 3-Buten-2-ol, 3-methyl- | 26,893 |
| 8.455 | 3-Ethyl-3-methylheptane | 66,659 |
| 9.740 | 1,4-Dioxa-2-decalone | 28,660 |
| 10.738 | Decane, 3,7-dimethyl- | 46,542 |
| 11.560 | 2-Bromononane | 23,532 |
| 11.905 | 2-Ethyl-3-vinyloxirane | 23,682 |
| 11.925 | 2-Pentenal, 2,4,4-trimethyl- | 28,175 |
| 13.315 | Cyclohexanemethanol, .alpha.-ethyl- | 31,869 |
| 14.310 | 6-Chloro-2,4-dihydroxy-1,3-dimethylpyrimidine | 21,426 |
| 14.469 | Methyl piperidin-4-carboxylate | 21,730 |
| 14.610 | Pyrrolizidine-3-one-5-ol, ethyl ether | 27,717 |
| 14.672 | Nonanal | 61,648 |
| 14.690 | p-Nitro carbanilic acid, n-heptyl ester | 23,421 |
| 14.785 | 3,4,4,-Trimethyl-1-pentyn-3-ol | 24,211 |
| 14.834 | 3,3-Dimethyl-2-hydroxy-2-phenylthiomorpholine | 28,086 |
| 15.047 | Cyclohexane, 1,2-bis(t-butoxycarbonylmethoxy)- | 27,138 |
| 17.085 | Cyanamide, N-allyl-N-[2-(2-hydroxy-2-methylpropyl)-3,3-dimethylcyclopropyl]methyl- | 34,017 |
| 17.175 | 8.beta.-17.alpha.-Dihydroxy-desoxycorticosterone | 27,911 |
| 17.505 | 1,2,4-Triazole, 4-[N-(2-hydroxyethyl)-N-nitro]amino- | 22,875 |
| 17.557 | 4-Heptanol, 2-methyl- | 43,238 |
| 18.316 | Ammonium acetate | 46,413 |
| 18.370 | 1,2,4-Trioxolane, 3,5-dipropyl- | 46,649 |
| 18.566 | 1,2-Cyclobutanedicarboxylic acid, 3-methyl-, dimethyl ester | 26,028 |
| 18.639 | 2H-Pyran, 2-(3-butynyloxy)tetrahydro- | 33,812 |
| 18.666 | trans-2,7-Dimethyl-3,6-octadien-2-ol | 41,759 |
| 19.001 | 5-Ethyl-4-tridecanone | 32,467 |
| 19.037 | Tetradecane, 5-methyl- | 27,346 |
| 19.055 | 1-Butanol, 3-methyl-, nitrate | 30,550 |
| 20.405 | Propenone, 3-dimethylamino-1-[3-(3-dimethylaminoacryloyl)-2,6-dihydroxyphenyl]- | 22,794 |
| 20.461 | Sulfide, di(1,3-butadienyl)- | 28,315 |
| 20.545 | 5,6,6-Trimethyl-hept-3-yne-2,5-diol | 26,021 |
| 20.579 | 4-Hexen-2-one, 3-methyl- | 29,025 |
| 20.582 | 1-Decene, 2,4-dimethyl- | 45,611 |
| 20.970 | 3-tert-Butyl-5-chloro-2-hydroxybenzophenone | 26,089 |
| 21.017 | 1-Benzamido-N-benzyl-1-[.alpha.-(2-pyridylthio)benzylidene]acetamide | 43,002 |
| 21.020 | Bis[4-acetamidophenylsulfonyl]phenyl methane | 43,423 |
| 21.105 | 1,2,5-Oxadiazol-3-amine, N-cyclopropyl-4-[5-(trichloromethyl)-1,2,4-oxadiazol-3-yl]- | 31,391 |
| 21.172 | Oxalic acid, allyl decyl ester | 48,664 |
| 21.714 | 5-Hydroxy-2,4,4-trimethyl-cyclopentane-1,3-dione | 28,464 |
| 21.726 | Tetradecane, 4-ethyl- | 49,899 |
| 22.143 | 3-Ethyl-1,5-octadiene | 65,512 |
| 23.217 | 2-Norbornanone, 6-chloro-3,3-dimethyl-, exo- | 32,271 |
| 23.230 | 1,2-Dihydrolinalool | 82,631 |
| 23.265 | 2-Propenyl-3-vinyloxirane | 35,236 |
| 24.021 | 2,6-Octadien-1-ol, 2,7-dimethyl- | 827,705 |
| 24.036 | Linalyl acetate | 886,873 |
| 24.803 | 2H-Pyran, 2-(3-butynyloxy)tetrahydro- | 25,758 |
| 26.907 | Malonic acid, bis(2-trimethylsilylethyl ester | 52,354 |
| 27.985 | 1,8-Dichlorooctane | 25,086 |
| 28.235 | Methylene asparagine | 26,750 |
| 28.269 | 2-Pyrrolidinone, 1-methyl- | 85,192 |
| 28.271 | 3,3,3-Trifluoro-N-(2-fluorophenyl)-2-(trifluoromethyl)propionamide | 31,186 |
| 30.675 | Oxalic acid, allyl nonyl ester | 26,882 |
| 31.325 | .alpha.-Terpineol | 63,064.5 |
| 31.374 | 3-Methyl-2-methylene-5-oxopyrrolidine-3-carbonitrile | 31,392 |
| 31.400 | trans-2,7-Dimethyl-3,6-octadien-2-ol | 26,447 |
| 33.244 | trans,cis-2,6-Nonadien-1-ol | 25,713 |
| 33.895 | Propanedioic acid, propyl- | 35,831 |
| 34.580 | 5-(3,7-Dimethylocta-2,6-dienyl)-4-methyl-2,3-dihydrothiophene 1,1-dioxide | 22,011 |
| 34.904 | 2H-Pyran-3-ol, 6-ethenyltetrahydro-2,2,6-trimethyl- | 25,268 |
| 36.573 | Allyl heptanoate | 22,998 |
| 37.752 | Heptanoic acid | 56,211.5 |
| 37.880 | .beta.-Myrcene | 29,088 |
| 38.160 | 2,5-Pyrrolidinedione, 1-ethyl- | 62,446 |
| 38.341 | Benzene, [(2-propenyloxy)methyl]- | 72,902 |
| 38.344 | Benzyl alcohol | 84,220 |
| 39.227 | Phenylethyl Alcohol | 46,827 |
| 40.794 | Ethanone, 1-(1H-pyrrol-2-yl)- | 29,239.5 |
| 42.939 | 2-Butenediamide, 2-methyl-, (E)- | 24,382 |
| 45.070 | Benzylamine, N-(3-chloro-2,2-dimethyl-1-phenylpropylidene)- | 25,983 |
| 45.373 | Cyclopropanecarboxylic acid, cyclohexylmethyl ester | 23,678 |
| 46.290 | 2-Amino-4-dimethylaminomethylenepentanedinitrile | 30,668 |
| 47.859 | 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | 35,368 |
| 48.860 | 2H-Pyran-2-one, 5,6-dihydro-4-(2-methyl-3-methylene-1-buten-4-yl)- | 76,496 |
| 48.921 | 3H-1,2,4-Triazole-3-thione, 2,4-dihydro-4-phenyl- | 36,542 |
| 49.912 | 4-Piperidinepropanoic acid, 1-benzoyl-3-(2-chloroethyl)-, ethyl ester | 44,931 |
| 52.555 | 1,2,3-Butanetriol | 25,538 |
| 53.001 | Oxetane, 2-methyl-4-propyl- | 30,545 |
| 56.669 | 2,5-Furandione, dihydro-3-methylene- | 29,961 |
| RT | Compound Name | Abundance Mean |
|---|---|---|
| 0.886 | p-Dioxane, methylene- | 60,857 |
| 2.270 | Hexanamide, 6-(2-oxocyclopentyl)-N-phenyl- | 97,276 |
| 2.296 | 2-Hydroxy-3-pyrazin-2-ylacrylic acid | 53,348 |
| 2.410 | 1,1′-(4-Methyl-1,3-phenylene)bis[3-(5-benzyl-1,3,4-thiadiazol-2-yl)urea] | 66,587 |
| 3.230 | Hexanal | 125,407 |
| 4.240 | Cyclohexene, 1-methyl-4-(1-methylethyl)- | 415,553 |
| 5.165 | .beta.-Myrcene | 932,205.5 |
| 6.028 | D-Limonene | 20,772,492 |
| 6.083 | Cyclobutane, 1,2-bis(1-methylethenyl)-, trans- | 28,705,656 |
| 6.680 | 2-Hexenal | 350,397 |
| 6.970 | 4-Methylcatechol, bis(trimethylsilyl) ether | 99,509 |
| 7.608 | .gamma.-Terpinene | 2,668,285.5 |
| 8.218 | 3-Nonen-1-yne, (Z)- | 185,302 |
| 8.577 | Benzene, 1-methyl-3-(1-methylethyl)- | 1,380,619 |
| 9.032 | (+)-4-Carene | 369,547.5 |
| 10.477 | Decane, 2,4-dimethyl- | 59,486 |
| 10.489 | Undecane, 2,7-dimethyl- | 147,641 |
| 11.408 | Cyclopropaneethanol | 87,457.5 |
| 11.920 | 1-Hepten-6-one, 2-methyl- | 216,541 |
| 13.323 | 1-Butanol, 3-methoxy- | 82,569 |
| 14.474 | 3-Hexen-1-ol | 72,691 |
| 14.653 | Nonanal | 227,838 |
| 15.801 | 2-Penten-1-ol, 4-methyl- | 123,248 |
| 17.298 | 2,6-Octadiene-1,8-diol, 2,6-dimethyl- | 143,284 |
| 18.203 | Ammonium acetate | 594,590.5 |
| 18.619 | Pyrazole, 1,4-dimethyl- | 146,710 |
| 18.640 | Furfural | 251,584 |
| 18.926 | Copaene | 187,151 |
| 19.482 | Acetic acid, hexyl ester | 223,391 |
| 20.390 | Octane, 1-azido- | 48,281 |
| 20.413 | Decanal | 82,253 |
| 21.002 | Benzaldehyde | 147,588 |
| 22.121 | 1,6-Heptadiene, 2,5,5-trimethyl- | 81,076 |
| 22.161 | 1,5-Heptadiene, 2,3,6-trimethyl- | 245,420 |
| 23.162 | 5-Nonenoic acid, methyl ester | 231,994 |
| 23.204 | 1,2-Dihydrolinalool | 226,441 |
| 23.628 | Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1.alpha.,2.beta.,5.beta.)- | 54,549 |
| 23.656 | Menthyl acetate | 82,100 |
| 24.079 | Linalyl acetate | 3,330,821 |
| 24.326 | 1,1-Cyclopropanedicarbonitrile, 2-butyl-2-methyl- | 176,787 |
| 24.694 | trans-.alpha.-Bergamotene | 209,911 |
| 26.318 | Benzenemethanol, .alpha.-(1-ethenylpentyl)-.alpha.-methyl- | 92,881 |
| 26.945 | Arabino-Hex-1-enitol, 1,5-anhydro-2-deoxy- | 102,198 |
| 27.845 | Oxiranemethanol, 2-phenyl- | 190,581 |
| 27.871 | Cyclopropane, 1-bromo-2,2,3,3-tetramethyl-1-prop-1-ynyl- | 49,869 |
| 28.261 | d-Menthol | 142,424.5 |
| 28.595 | 2-Bromopropionic acid, 2-pentyl ester | 79,505 |
| 29.070 | Cycloisolongifolene | 120,477 |
| 30.001 | 2,6-Octadienal, 3,7-dimethyl-, (Z)- | 535,579.5 |
| 30.495 | 2-Methyl-l-methylmannopyranoside | 167,380 |
| 30.806 | 3-Cyclohexene-1-methanol, .alpha.,.alpha.,4-trimethyl-, acetate | 635,343 |
| 30.824 | .alpha.-Terpineol | 702,887 |
| 31.110 | 1-Cyclohexyl-2,2-dimethyl-1-propanol acetate | 79,379 |
| 31.364 | L-.alpha.-Terpineol | 953,500.5 |
| 31.644 | Acetic acid, 1-(R)-phenylethyl ester | 152,399.5 |
| 32.078 | (-)-Carvone | 238,609 |
| 32.618 | .beta.-Bisabolene | 292,323 |
| 32.907 | 2,6-Octadienal, 3,7-dimethyl-, (Z)- | 517,386.5 |
| 33.259 | Linalyl acetate | 180,568.5 |
| 33.570 | 7,8-Dibromo-4,4,7-trimethyl-hexahydro-benzo[1,3]dioxin-2-one | 31,710 |
| 33.899 | Butanoic acid, 3-methyl- | 116,192 |
| 34.202 | Methyl salicylate | 140,347 |
| 34.653 | 2,6-Octadien-1-ol, 2,7-dimethyl- | 735,215 |
| 34.653 | Benzene, 1-(1,5-dimethyl-4-hexenyl)-4-methyl- | 439,300 |
| 35.221 | 6-Octen-1-ol, 3,7-dimethyl-, (R)- | 41,711 |
| 35.225 | Citronellol | 91,789 |
| 35.688 | Ethanol, 2-(2-butoxyethoxy)- | 238,659.5 |
| 36.222 | 2,6-Octadien-1-ol, 2,7-dimethyl- | 98,600 |
| 36.242 | 2,6-Octadiene, 3,7-dimethyl-1-(2-propenyloxy)- | 208,993 |
| 36.563 | Anethole | 84,862 |
| 36.595 | Estragole | 89,942 |
| 37.181 | p-Mentha-1(7),8-dien-2-ol | 133,232 |
| 37.554 | 1,4-Methanobenzocyclodecene, 1,2,3,4,4a,5,8,9,12,12a-decahydro- | 127,758 |
| 37.742 | Butanoic acid, 3-methyl- | 566,726 |
| 37.757 | Heptanoic acid | 847,338 |
| 37.870 | 2,6-Nonadienal, (E,Z)- | 263,050 |
| 38.143 | 2,5-Pyrrolidinedione, 1-ethyl- | 108,795 |
| 38.328 | Benzyl alcohol | 130,915.5 |
| 39.218 | Phenylethyl Alcohol | 42,435 |
| 39.663 | Acetic acid, 6,6-dimethyl-2-methylene-7-(3-oxobutylidene)oxepan-3-ylmethyl ester | 76,029 |
| 39.671 | trans-.beta.-Ionone | 110,928 |
| 40.302 | Isoaromadendrene epoxide | 90,637 |
| 40.799 | 11-(2-Cyclopenten-1-yl)undecanoic acid, (+)- | 66,300.5 |
| 40.980 | Acetic acid, 6,6-dimethyl-2-methylene-7-(3-oxobutylidene)oxepan-3-ylmethyl ester | 79,824 |
| 43.179 | Octanoic acid | 184,543.5 |
| 46.331 | Phenol, 2-ethyl-4,5-dimethyl- | 50,752 |
| 46.839 | 2,4,6-Octatrien-1-ol, 3,7-dimethyl-(E,E)- | 36,117 |
| 47.457 | 1,2-Cyclohexanediol, 1-methyl-4-(1-methylethenyl)- | 118,730 |
| 47.870 | 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | 147,328.5 |
| 48.345 | Cyclobutene, 4,4-dimethyl-1-(2,7-octadienyl)- | 164,733 |
| 49.926 | Benzoic acid | 163,139 |
| RT | Compound Name | Abundance Mean |
|---|---|---|
| 0.840 | Glycidol | 75,856.5 |
| 0.950 | .alpha.-Chloroacrylic acid | 59,320 |
| 1.530 | 2-Pentanol, 3-chloro-4-methyl-, (R*,R*)-(.+/−.)- | 299,363 |
| 1.560 | 1-Methoxy-3-methyl-3-butene | 289,385 |
| 3.484 | .beta.-Myrcene | 64,340 |
| 5.194 | .beta.-Myrcene | 1,191,105.5 |
| 5.938 | D-Limonene | 4,419,800.5 |
| 6.681 | 3-Hexenal, (Z)- | 472,247.5 |
| 6.964 | N-(Trifluoroacetyl)-N,O,O′,O″-tetrakis(trimethylsilyl)norepinephrine | 122,798 |
| 7.596 | Tricyclo[2.2.1.0(2,6)]heptane, 1,7,7-trimethyl- | 510,285 |
| 7.607 | 4-Carene, (1S,3R,6R)-(-)- | 531,680 |
| 8.187 | 1,3,7-Octatriene, 3,7-dimethyl- | 520,381.5 |
| 8.550 | p-Cymene | 182,369 |
| 9.023 | (+)-4-Carene | 47,706 |
| 10.463 | Dodecane, 2-methyl- | 128,492 |
| 10.481 | Nonane | 91,987 |
| 10.740 | Undecane, 3,7-dimethyl- | 45,039 |
| 11.600 | Sulfurous acid, hexyl pentadecyl ester | 39,988 |
| 11.911 | 5-Hepten-2-one, 6-methyl- | 99,068 |
| 11.943 | 1-Hepten-6-one, 2-methyl- | 95,405 |
| 13.370 | 1-Butanol, 3-methoxy- | 97,327.5 |
| 14.450 | 3-Hexen-1-ol | 49,402 |
| 14.475 | 3-Hexen-1-ol, (E)- | 58,769 |
| 14.669 | Nonanal | 112,987 |
| 15.135 | Oxalic acid, allyl tetradecyl ester | 53,901 |
| 15.617 | Decane, 2-methyl- | 41,084 |
| 15.875 | 2-Allyloxy-4,6-bis-phenylsulfanyl-[1,3,5]triazine | 37,390 |
| 15.952 | 3-Decene | 61,937 |
| 15.952 | 1-Hexanol, 3-methyl- | 87,038 |
| 17.110 | 2-Furanmethanol, 5-ethenyltetrahydro-.alpha.,.alpha.,5-trimethyl-, cis- | 46,232 |
| 17.569 | Undecanol-4 | 52,905.5 |
| 18.168 | Ammonium acetate | 686,834 |
| 18.610 | Furfural | 171,566 |
| 18.646 | Furan-2-carbohydrazide, N2-(1-methylhexylideno)- | 191,841 |
| 19.639 | 3-Oxatricyclo[4.2.0.0(2,4)]octan-7-one | 65,832 |
| 21.010 | Benzaldehyde | 113,463.5 |
| 21.766 | Tetradecane, 4-ethyl- | 42,793 |
| 22.163 | 1,7-Nonadiene, 4,8-dimethyl- | 635,615 |
| 23.237 | Citronellyl butyrate | 720,225 |
| 23.251 | 1,2-Dihydrolinalool | 778,269 |
| 23.565 | Cyclohexane, 1-methyl-4-(2-hydroxyethyl)- | 104,217 |
| 24.198 | Linalyl acetate | 20,228,501.5 |
| 24.811 | trans-.alpha.-Bergamotene | 91,781 |
| 25.091 | Fenchol, exo- | 39,940 |
| 25.813 | (+)-(E)-Limonene oxide | 64,200 |
| 27.380 | 3,4-Dimethyl-2-prop-2-enyl-2,5-dihydrothiophene 1,1-dioxide | 66,881 |
| 27.385 | Hotrienol | 67,814 |
| 27.856 | 1-(Phenylmethyl)-1,2,3,6-tetrahydropyridin-3-ol | 122,925 |
| 27.880 | Oxiranemethanol, 2-phenyl- | 180,446 |
| 28.535 | 10-Heptadecen-8-ynoic acid, methyl ester, (E)- | 52,188 |
| 28.960 | 4-Pentenoic acid, 2-acetyl-, ethyl ester | 785,25 |
| 30.035 | 2,6-Octadienal, 3,7-dimethyl-, (Z)- | 367,457 |
| 30.069 | Longipinene epoxide | 249,326 |
| 30.305 | Sulfurous acid, hexyl pentadecyl ester | 200,957 |
| 30.358 | Valeric acid, 3-pentadecyl ester | 89,012 |
| 30.832 | .alpha.-Terpineol | 566,609 |
| 31.429 | .alpha.-Terpineol | 2,871,361 |
| 31.445 | L-.alpha.-Terpineol | 3,859,251 |
| 32.063 | (-)-Carvone | 168,274.5 |
| 32.623 | .beta.-Bisabolene | 183,784 |
| 32.918 | 2,6-Octadienal, 3,7-dimethyl-, (Z)- | 153,040.5 |
| 33.281 | 1-Bromo-3,7-dimethyl-2,6-octadiene | 539,860 |
| 33.285 | Linalyl acetate | 735,657 |
| 33.613 | 2H-Pyran-3-ol, 6-ethenyltetrahydro-2,2,6-trimethyl- | 78,347 |
| 33.935 | Propanedioic acid, propyl- | 60,942 |
| 34.239 | Methyl salicylate | 44,184 |
| 34.260 | Tricyclo[7.1.0.0[1,3]]decane-2-carbaldehyde | 53,084 |
| 34.652 | 2,6-Octadien-1-ol, 2,7-dimethyl- | 764,472 |
| 34.935 | 2H-Pyran-3-ol, 6-ethenyltetrahydro-2,2,6-trimethyl- | 93,514.5 |
| 35.255 | 2-Pentadecyn-1-ol | 70,853 |
| 35.255 | 9,15-Octadecadienoic acid, methyl ester | 47,285 |
| 35.697 | Ethanol, 2-(2-butoxyethoxy)- | 110,812.5 |
| 36.251 | 2,6-Octadien-1-ol, 2,7-dimethyl- | 618,537.5 |
| 37.164 | Dihydrocarvyl acetate | 78,561 |
| 37.200 | 2-Cyclohexen-1-ol, 2-methyl-5-(1-methylethenyl)-, acetate, (1R-cis)- | 79,721 |
| 37.889 | trans,cis-2,6-Nonadien-1-ol | 1,039,600 |
| 37.891 | 2,6-Nonadienal, (E,Z)- | 1,143,942 |
| 38.153 | 2,5-Pyrrolidinedione, 1-ethyl- | 129,426.5 |
| 38.335 | Benzyl alcohol | 119,005 |
| 38.380 | Benzene, (2,2-dimethylbutyl)- | 99,835 |
| 38.721 | Geranyl acetate, 2,3-epoxy- | 163,962 |
| 39.235 | Phenylethyl Alcohol | 75,575 |
| 39.670 | Acetic acid, 6,6-dimethyl-2-methylene-7-(3-oxobutylidene)oxepan-3-ylmethyl ester | 134,033 |
| 40.314 | Isoaromadendrene epoxide | 143,108 |
| 40.585 | Pyruvic acid, 3-hexenyl ester | 55,982 |
| 40.793 | Myrcenylacetat | 109,085 |
| 40.799 | 8,11,14-Eicosatrienoic acid, methyl ester, (Z,Z,Z)- | 96,145 |
| 41.084 | 3(10)-Caren-4-ol, acetoacetic acid ester | 176,125 |
| 41.087 | 1,6-Octadien-3-ol, 3,7-dimethyl-, 2-aminobenzoate | 179,443 |
| 43.193 | Octanoic acid | 54,969 |
| 43.780 | Cholestane-3,6,7-triol, (3.beta.,5.alpha.,6.beta.,7.beta.)- | 100,688 |
| 44.784 | Cyclododeca-5,9-dien-1-ol, 2-methyl-, (Z,Z)- | 82,400 |
| 45.205 | 4-Piperidin-1-yl-6-(4-tetrazol-1-yl-phenoxymethyl)-[1,3,5]triazin-2-ylamine | 59,889 |
| 45.626 | 2-Cyclohexen-1-ol, 2-methyl-5-(1-methylethenyl)-, acetate, (1R-cis)- | 111,323 |
| 46.351 | Phenol, 2,3,4,6-tetramethyl- | 98,885 |
| 47.882 | 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | 75,435.5 |
| RT | Compound Name | Abundance Mean |
|---|---|---|
| 1.084 | Acetone | 331,948 |
| 1.185 | 2-Methyl-2-(4-nitrobenzenesulfonamido)propyl N-methylcarbamate | 74,669 |
| 1.210 | Cyclobutane, methylene- | 110,395 |
| 1.270 | Ecgonine, o-pentafluoropropionyl-, pentafluoropropyl ester | 84,216 |
| 1.380 | Tetrahydropyran | 62,535 |
| 1.430 | Diallyl carbonate | 72,075 |
| 1.435 | 2-Propen-1-amine, N-ethyl- | 93,704 |
| 1.605 | Cyclobutaneoctol | 50,049 |
| 3.250 | 2-Propenamide, N-(1-cyclohexylethyl)- | 72,158 |
| 3.285 | Hexanal | 139,699 |
| 3.361 | 1,2,4-Triazole, 4-[N-(2-hydroxyethyl)-N-nitro]amino- | 139,316 |
| 3.555 | .beta.-Pinene | 432,956 |
| 3.593 | Cyclohexane, 1-methylene-4-(1-methylethenyl)- | 495,699 |
| 3.855 | 2,6-Octadiene, 3,7-dimethyl-1-(2-propenyloxy)- | 52,281 |
| 3.870 | Pyridine, 2-(4-pyridylmethylenamino)- | 66,726 |
| 4.450 | 2-Keto-3-methylene-5-methyltetrahydrothiophene | 54,550 |
| 5.180 | 1-Pentene, 5-(2,2-dimethylcyclopropyl)-2-methyl-4-methylene- | 61,122 |
| 5.180 | 1,6,10-Dodecatriene, 7,11-dimethyl-3-methylene- | 66,379 |
| 6.024 | D-Limonene | 12,325,691.5 |
| 6.727 | 2-Hexenal | 147,443 |
| 7.639 | .gamma.-Terpinene | 163,504.5 |
| 7.720 | 2-(3-Methyl-but-1-ynyl)-cyclohexene-1-carboxaldehyde | 77,433 |
| 8.645 | Benzene, 1-methyl-3-(1-methylethyl)- | 824,049.5 |
| 9.130 | trans-.beta.-Terpinyl butanoate | 54,444 |
| 9.689 | Octanal | 70,984.5 |
| 10.410 | 6-Chloro-2,2,9,9-tetramethyl-3,7-decadiyn-5-ol | 53,045 |
| 10.838 | Dodecane, 5-methyl- | 66,791 |
| 11.983 | 1-Hepten-6-one, 2-methyl- | 171,591 |
| 12.000 | 5-Hepten-2-one, 6-methyl- | 214,793 |
| 14.540 | 1,3,3-Trimethylcyclopropene | 71,027 |
| 14.763 | Nonanal | 104,120 |
| 16.748 | Cyclobutane, 1,2-bis(1-methylethenyl)-, trans- | 265,149.5 |
| 17.190 | 2-Furanmethanol, 5-ethenyltetrahydro-.alpha.,.alpha.,5-trimethyl-, cis- | 76,018 |
| 17.211 | cis-5-Methyl-2-isopropyl-2-hexen-1-al | 57,488 |
| 17.412 | Cyclobutane, 1,2-bis(1-methylethenyl)-, trans- | 303,970 |
| 18.334 | Ammonium acetate | 216,233 |
| 18.712 | Furfural | 415,801 |
| 19.127 | Octane, 5-ethyl-2-methyl- | 100,177 |
| 19.789 | 2,5-Heptadiene, (E,E)- | 78,358 |
| 20.498 | 1,1-Dodecanediol, diacetate | 57,452 |
| 20.663 | 1-Decene, 4-methyl- | 186,582 |
| 20.683 | 1-Hexanol, 2-ethyl- | 133,849 |
| 21.128 | Benzaldehyde | 193,767 |
| 21.270 | Decane, 3,7-dimethyl- | 126,527 |
| 21.350 | Heptane, 2,3,4-trimethyl- | 67,035 |
| 21.509 | 3,5-Octadien-2-one, (E,E)- | 102,421.5 |
| 21.858 | Undecane, 3,7-dimethyl- | 115,470 |
| 22.150 | 1-Decanol, 5,9-dimethyl- | 92,827 |
| 22.402 | Cyclobutane, 1,2-bis(1-methylethenyl)-, trans- | 81,623 |
| 22.419 | Cyclohexene, 1-methyl-5-(1-methylethenyl)- | 98,513 |
| 22.829 | 1-Isopropenyl-3-propenylcyclopentane | 124,022 |
| 23.281 | 1,2-Dihydrolinalool | 101,149 |
| 23.317 | Cyclopropanemethanol, .alpha.,2-dimethyl-2-(4-methyl-3-pentenyl)-, [1.alpha.(R*),2.alpha.]- | 113,237 |
| 24.144 | Linalyl acetate | 2,220,364 |
| 24.430 | 2-Decenal, (Z)- | 162,906 |
| 24.448 | Pentane, 1-(2,2-dibromocyclopropyl)- | 114,649 |
| 25.170 | Oxirane, octyl- | 783,66 |
| 25.384 | 5-Decen-1-ol, acetate, (E)- | 100,866 |
| 25.437 | 1-(1H-Imidazol-2-yl)-ethanone | 92,763 |
| 25.862 | 1H-Pyrrole-2-carboxaldehyde, 1-ethyl- | 56,395.5 |
| 26.174 | 2-Pentanol, 3-chloro-4-methyl-, (R*,S*)-(.+/−.)- | 245,217.5 |
| 26.420 | Butyrolactone | 60,795 |
| 27.010 | Sulfurous acid, octyl 2-propyl ester | 85,408 |
| 27.028 | Sulfurous acid, dodecyl 2-propyl ester | 73,132 |
| 30.159 | 2,6-Octadienal, 3,7-dimethyl-, (Z)- | 928,389 |
| 30.420 | 2,5-Dihydroxyheptane | 73,287 |
| 30.480 | Oxetane, 2-ethyl-3-methyl- | 81,149 |
| 30.640 | Thiophene, 3-methylsulfonyl- | 69,756 |
| 30.909 | p-Mentha-1(7),8(10)-dien-9-ol | 143,103 |
| 31.487 | .alpha.-Terpineol | 408,707 |
| 32.192 | (-)-Carvone | 402,266.5 |
| 33.032 | 2,6-Octadienal, 3,7-dimethyl-, (Z)- | 1,115,315.5 |
| 33.362 | Linalyl acetate | 167,765.5 |
| 33.670 | Cyclopropanemethanol, 2-isopropylidene-.alpha.-methyl- | 55,873 |
| 33.949 | cis-p-Mentha-2,8-dien-1-ol | 843,89,5 |
| 34.711 | 2,6-Octadien-1-ol, 2,7-dimethyl- | 253,900 |
| 34.970 | Cyclohexene, 3-acetoxy-4-(1-hydroxy-1-methylethyl)-1-methyl- | 68,155 |
| 35.301 | Citronellal | 77,794.5 |
| 35.581 | p-Mentha-1(7),8(10)-dien-9-ol | 51,607 |
| 36.309 | .beta.-Myrcene | 76,695.5 |
| 36.649 | 1-Heptadec-1-ynyl-cyclohexanol | 86,850 |
| 36.697 | 5-Isopropenyl-1,2-dimethylcyclohex-2-enol | 50,040 |
| 37.261 | trans-p-mentha-1(7),8-dien-2-ol | 262,206 |
| 37.270 | Carveol | 221,405 |
| 37.820 | Butanoic acid, 3-methyl- | 378,023.5 |
| 37.943 | .beta.-Myrcene | 279,721 |
| 38.222 | 2,5-Pyrrolidinedione, 1-ethyl- | 221,432.5 |
| 38.401 | Benzyl alcohol | 196,719 |
| 38.417 | Benzene, [(2-propenyloxy)methyl]- | 158,666 |
| 38.773 | 3-hydroxy-2-methyl-5-(prop-1-en-2-yl)cyclohexanone | 108,854 |
| 39.288 | Phenylethyl Alcohol | 64,612 |
| 39.733 | Acetic acid, 6,6-dimethyl-2-methylene-7-(3-oxobutylidene)oxepan-3-ylmethyl ester | 71,831 |
| 39.739 | trans-.beta.-Ionone | 98,394 |
| 40.358 | Cyclododeca-5,9-dien-1-ol, 2-methyl-, (Z,Z)- | 58,035 |
| 40.850 | 1-Ethynyl-1-cyclooctanol | 65,930 |
| 41.063 | Acetic acid, 2,6,6-trimethyl-3-methylene-7-(3-oxobutylidene)oxepan-2-yl ester | 92,499 |
| 43.248 | Octanoic acid | 53,587 |
| 43.765 | Triacetin | 72,394 |
| 47.941 | 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | 107,351 |
| RT | Compound Name | Abundance Mean |
|---|---|---|
| 0.715 | Acetic acid, cyano- | 58,385 |
| 0.814 | Acetic acid, cyano- | 41,951 |
| 0.890 | Disulfide, isopentyl methyl | 32,293 |
| 0.891 | 5-Aminoisoxazole | 77,786 |
| 0.967 | Thiocyanic acid, 5-amino-3-methyl-4-isoxazolyl ester | 59,100 |
| 0.970 | 3-Butynoic acid | 63,100 |
| 1.083 | Isobutyl nitrite | 180,084 |
| 1.086 | Propanal, 2-methyl- | 118,133 |
| 1.305 | 1,4-Dioxane, 2,5-dimethyl- | 52,275 |
| 1.319 | 4-Oxa-6-hepten-2-one, 6-bromo-3-methyl- | 76,789 |
| 1.452 | Oxalic acid, butyl propyl ester | 205,128 |
| 1.486 | Butanal, 2-methyl- | 454,443 |
| 1.495 | Oxirane, trimethyl- | 64,342 |
| 1.520 | Neburon | 152,396 |
| 1.590 | Carbonic acid, allyl 2-ethoxyethyl ester | 85,342 |
| 1.690 | Carbonic acid, allyl isohexyl ester | 65,883 |
| 1.695 | 1,2-Pentadiene, 4,4-dimethyl- | 47,310 |
| 1.750 | 2-Oxazolamine, 4,5-dihydro-5-(phenoxymethyl)- | 50,644 |
| 1.998 | Butanal, 3-methyl- | 97,682 |
| 2.602 | Octane, 2,3,6,7-tetramethyl- | 46,275 |
| 3.302 | Hexanal | 153,104.5 |
| 3.375 | (1RS)-propanol, 1-cyano-(2S)-(tert.butyloxycarbonyl)amino- | 62,614 |
| 3.450 | 4-Chloro-3-methylbut-2-en-1-ol | 101,437 |
| 3.485 | 3,4-Dimethylcyclohexanol | 42,683 |
| 5.165 | Cyclopropanemethanol, 2-isopropylidene-.alpha.-methyl- | 42,196 |
| 5.219 | Sulfide, cyclopentyl isopropyl | 70,260 |
| 6.045 | 3-Cyclohexene-1-methanol, .alpha.,.alpha.,4-trimethyl-, acetate | 33,625 |
| 6.732 | 2-Hexenal | 213,047 |
| 6.744 | 3-Hexenal, (Z)- | 523,559 |
| 6.760 | Heptanonitrile | 257,029 |
| 6.998 | 2,6-Nonadienal, (E,Z)- | 40,726 |
| 7.370 | Homopiperazine | 32,993 |
| 8.270 | 4-Methyl-2-oxopentanenitrile | 45,025 |
| 8.297 | 1-Pentanol | 91,705 |
| 8.478 | Sulfurous acid, hexyl octyl ester | 64,375 |
| 8.941 | Cyclohexane, 1,2,4-tris(methylene)- | 30,691 |
| 10.065 | 1,3-Pentanedione, 2,4-dimethyl-1-phenyl- | 50,566 |
| 11.997 | 1-Hepten-6-one, 2-methyl- | 43,920 |
| 12.959 | Spiro[3.5]nona-5,7-dien-1-one, 5,9,9-trimethyl- | 40,729 |
| 13.104 | 2-Trifluoroacetoxydodecane | 38,203 |
| 14.530 | Cycloheptano[d]imidazolidine, 1,3-dihydroxy-2-methyl- | 73,913 |
| 14.567 | 3-Hexen-1-ol, (E)- | 106,729.5 |
| 14.605 | 1,3,2-Dioxaphospholane, 2-cyclohexyl-4,5-dimethyl- | 56,722 |
| 14.720 | 1-Cyclohexylethanol | 38,396 |
| 14.763 | Nonanal | 119,865 |
| 14.770 | 1,2,4-Triazol-5-acetic acid, 3-amino- | 50,306 |
| 14.805 | 5-t-Butyl-cycloheptene | 36,888 |
| 15.219 | Oxirane, 2-butyl-3-methyl-, cis- | 47,050 |
| 15.665 | 3,6-Heptanedione | 166,183 |
| 15.694 | Dodecane, 2-methyl- | 406,601 |
| 15.706 | Decane, 2,4-dimethyl- | 203,345 |
| 15.790 | 2-Furannonanoic acid, 5-(21,23-dimethylpentacosyl)tetrahydro-, methyl ester | 45,108 |
| 15.825 | Piperidine-4-carboxamide, 1-(3,4,5-trimethoxybenzoyl)- | 47,258 |
| 15.845 | 5-Bromo-1-hexene | 75,733 |
| 15.885 | .alpha.-Chlorocyclooctanone oxime | 66,635 |
| 15.910 | 7-Octenoic acid, methyl ester | 39,237 |
| 16.203 | Benzene, 1-ethyl-3,5-dimethyl- | 41,469 |
| 18.342 | Ammonium acetate | 102,343 |
| 18.415 | Acetic acid | 64,804 |
| 18.729 | 2-Nonenal, 8-oxo- | 173,371 |
| 18.749 | 4-Pentenoic acid, 2-methylene-, methyl ester | 182,615 |
| 18.785 | 6-Tetradecanol | 49,218 |
| 20.671 | 1-Hexanol, 2-ethyl- | 721,868 |
| 21.081 | Benzaldehyde | 163,232.5 |
| 21.305 | 3(2H)-Furanone, dihydro-5-isopropyl- | 65,684 |
| 21.495 | 1,3-Butanedione, 1-(2-furanyl)- | 55,641 |
| 23.285 | Citronellyl butyrate | 39,321 |
| 24.110 | Linalyl acetate | 840,872 |
| 24.413 | Octanal | 45,631 |
| 24.423 | Cyclopropane, 1-heptyl-2-methyl- | 64,716 |
| 25.172 | Oxirane, dodecyl- | 94,251.5 |
| 26.065 | 3-Isopropylidene-5-methyl-hex-4-en-2-one | 33,496 |
| 26.418 | Butyrolactone | 47,210 |
| 27.021 | Decane, 2,4,6-trimethyl- | 80,426 |
| 27.023 | Sulfurous acid, dodecyl 2-propyl ester | 91,716 |
| 27.940 | 2,4,6-Cycloheptatrien-1-one, 4-methyl- | 88,296 |
| 28.746 | Tricyclo[3.3.1.1(3,7)]decane, 2-nitro- | 276,194.5 |
| 29.028 | Dispiro[4.2.4.2]tetradecane | 72,071 |
| 29.038 | Tricyclo[3.3.1.1(3,7)]decane, 2-nitro- | 117,874 |
| 30.175 | 1,3-Pentadiene, 5-(2,2-dimethylcyclopropyl)-2,4-dimethyl-, (Z or E)- | 60,534 |
| 30.229 | .alpha.-Chlorocyclooctanone oxime | 35,753 |
| 30.524 | Aziridinone, 1-(1,1-dimethylethyl)-3-tricyclo[3.3.1.1(3,7)]dec-1-yl- | 73,998 |
| 30.533 | 8,11,14-Eicosatrienoic acid, methyl ester, (Z,Z,Z)- | 93,089 |
| 31.424 | .alpha.-Terpineol | 47,609 |
| 32.351 | Dispiro[4.2.4.2]tetradecane | 367,543.5 |
| 33.945 | Pentanoic acid | 44,424 |
| 34.295 | Methyl salicylate | 132,619.5 |
| 34.425 | Benzenamine, N-[4-(1-methylethyl)benzylidene]-4-(1-pyrrolidylsulfonyl)- | 40,434 |
| 34.685 | 2,6-Octadien-1-ol, 2,7-dimethyl- | 93,110 |
| 34.986 | Cyclopropanemethanol, 2-isopropylidene-.alpha.-methyl- | 81,101 |
| 35.015 | 2H-Pyran-3-ol, 6-ethenyltetrahydro-2,2,6-trimethyl- | 107,522 |
| 35.648 | 1-Phenyl-2-butanone | 77,853.5 |
| 37.437 | 1,4-Methanobenzocyclodecene, 1,2,3,4,4a,5,8,9,12,12a-decahydro- | 182,739.5 |
| 37.818 | Heptanoic acid | 327,611 |
| 37.826 | Formic acid, 2-methylpropyl ester | 270,250 |
| 38.221 | 2,5-Pyrrolidinedione, 1-ethyl- | 111,221.5 |
| 38.402 | Benzyl-diseryl phosphate | 132,761 |
| 38.403 | Benzyl alcohol | 126,413 |
| 39.052 | Pregnane-3,8,12,14,17,20-hexol, (3.beta.,5.alpha.,12.beta.,14.beta.,17.alpha.,20S)- | 34,151 |
| 39.285 | Phenylethyl Alcohol | 55,509 |
| 39.726 | trans-.beta.-Ionone | 77,701 |
| 39.727 | 3-Buten-2-one, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)- | 97,960 |
| 40.852 | 11-(2-Cyclopenten-1-yl)undecanoic acid, (+)- | 52,398 |
| 41.019 | Pentane, 2-bromo- | 31,014 |
| 41.044 | Acetic acid, 6,6-dimethyl-2-methylene-7-(3-oxobutylidene)oxepan-3-ylmethyl ester | 78,871 |
| 43.085 | 3-Hepten-2-one, O-methyloxime | 33,472 |
| 44.698 | 1,5-Hexadiene, 2,5-dipropyl- | 38,397 |
| 47.938 | 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | 105,392 |
| 52.435 | Methyl 4,6-benzylidene-3-deoxy-4-hexopyranoside | 41,183 |
| 52.725 | 2-(Imidazole-1-sulfonyl)-benzoic acid methyl ester | 34,590 |
| RT | Compound Name | Abundance Mean |
|---|---|---|
| 0.103 | Borane carbonyl | 72,523 |
| 0.770 | Acetic acid, cyano- | 119,964 |
| 0.840 | aS-Triazine-3,5(2H,4H)-dione, 6-(dimethylamino)- | 109,600 |
| 1.096 | Acetone | 194,968 |
| 2.590 | Benzeneacetic acid, 2-tetradecyl ester | 142,487 |
| 3.265 | Hexanal | 1,020,449 |
| 3.455 | Allyl trifluoroacetate | 100,639 |
| 6.689 | 2-Hexenal | 165,126.5 |
| 6.982 | N-(Trifluoroacetyl)-N,O,O′,O″-tetrakis(trimethylsilyl)norepinephrine | 474,946.5 |
| 8.491 | Dodecane, 4-methyl- | 154,490 |
| 8.511 | Octane, 5-ethyl-2-methyl- | 209,142 |
| 9.656 | Octanal | 133,742 |
| 10.964 | 2-Heptenal, (Z)- | 132,743.5 |
| 11.957 | 5-Hepten-2-one, 6-methyl- | 3,391,388 |
| 12.325 | trans-Rose oxide | 65,810 |
| 14.753 | Nonanal | 36,0131 |
| 15.167 | trans,cis-2,6-Nonadien-1-ol | 166,441.5 |
| 18.350 | Ammonium acetate | 1,197,691.5 |
| 18.705 | Furfural | 245,261 |
| 19.132 | Nonane, 5-(1-methylpropyl)- | 192,955 |
| 19.135 | Tetradecane, 4-methyl- | 137,144 |
| 20.725 | Tridecane, 6-methyl- | 108,712 |
| 21.129 | Benzaldehyde | 309,445.5 |
| 21.375 | Nonane, 5-(2-methylpropyl)- | 90,406 |
| 24.145 | 5-Hepten-1-ol, 2-ethenyl-6-methyl- | 355,464,5 |
| 24.473 | 1-Octanol | 104,288 |
| 24.493 | 1-Nonene | 128,456 |
| 25.494 | (S)-(-)-1,2,4-Butanetriol, 2-acetate | 89,072 |
| 25.844 | 2(5H)-Furanone, 5,5-dimethyl- | 107,360 |
| 26.274 | (S)-(+)-1,2-Propanediol | 3,130,504 |
| 26.282 | R-(-)-1,2-propanediol | 3,474,497 |
| 27.054 | Sulfurous acid, 2-propyl undecyl ester | 407,374 |
| 28.037 | Acetophenone | 134,811 |
| 28.823 | Dodecane, 2,5-dimethyl- | 90,189 |
| 30.205 | 2,6-Octadien-1-ol, 2,7-dimethyl- | 224,600 |
| 30.445 | Nonane, 4,5-dimethyl- | 125,045 |
| 30.657 | Verbenol | 121,652 |
| 31.525 | .alpha.-Terpineol | 345,922 |
| 31.755 | 2′-Ethyl-3-[(3-phenylpropionyl)hydrazono]butyranilide | 86,101 |
| 32.640 | Tetradecane | 365,951 |
| 32.666 | Eicosane, 10-methyl- | 386,657 |
| 33.025 | 2,6-Octadienal, 3,7-dimethyl-, (Z)- | 132,753 |
| 34.015 | Pentanoic acid | 82,703 |
| 34.325 | Oxalic acid, octadecyl propyl ester | 88,447 |
| 34.377 | 2-methyltetracosane | 115,659 |
| 34.697 | 2-Dodecenal, (E)- | 163,451 |
| 34.697 | Oxalic acid, propyl tridecyl ester | 104,743 |
| 34.945 | 4-Methyl-2-oxopentanenitrile | 101,744 |
| 35.141 | Heptadecane, 2,6,10,14-tetramethyl- | 101,875 |
| 35.145 | Nonadecane, 2-methyl- | 124,979 |
| 35.319 | 6-Octen-1-ol, 3,7-dimethyl-, (R)- | 507,414.5 |
| 35.505 | Hexadecane, 2-methyl- | 83,391 |
| 35.506 | Heptadecane, 3-methyl- | 103,232 |
| 35.765 | Ethanol, 2-(2-butoxyethoxy)- | 106,602.5 |
| 36.134 | Eicosane | 155,798 |
| 36.343 | 2,6-Octadien-1-ol, 2,7-dimethyl- | 242,610 |
| 36.468 | Cyclohexane, eicosyl- | 249,746 |
| 36.651 | Eicosane, 10-methyl- | 298,746.5 |
| 37.665 | 1,4-Methanobenzocyclodecene, 1,2,3,4,4a,5,8,9,12,12a-decahydro- | 72,802.5 |
| 37.838 | Hexanoic acid | 886,987 |
| 37.953 | 1-Bromo-3,7-dimethyl-2,6-octadiene | 1,140,624.5 |
| 38.241 | 2,5-Pyrrolidinedione, 1-ethyl- | 368,554.5 |
| 38.419 | Benzyl-diseryl phosphate | 533,200 |
| 38.420 | Benzyl alcohol | 754,339 |
| 38.705 | Thiazolo[3,2-a]pyridinium, 3-hydroxy-2-methyl-, acetate | 92,220 |
| 38.915 | Methanesulfonylacetic acid | 98,645 |
| 39.303 | Phenylethyl Alcohol | 91,829 |
| 39.585 | Octane, 2,3,3-trimethyl- | 109,959 |
| 39.596 | Nonadecane, 2-methyl- | 159,690 |
| 40.285 | Benzene, (1-butyloctyl)- | 115,894 |
| 40.540 | Creosol | 139,225.5 |
| 40.715 | Hexanoic acid, 2-ethyl- | 155,344 |
| 40.754 | Heptanoic acid | 284,358 |
| 40.871 | Ethanone, 1-(1H-pyrrol-2-yl)- | 163,370.5 |
| 41.055 | Pregan-20-one, 2-hydroxy-5,6-epoxy-15-methyl- | 107,105 |
| 41.205 | Pentafluoropropionic acid, decyl ester | 85,453 |
| 41.425 | m-Toluic acid, 2-ethylcyclohexyl ester | 84,728 |
| 41.847 | Benzaldehyde, 4-methoxy- | 534,873.5 |
| 42.365 | 3-Isopropylidene-5-methyl-hex-4-en-2-one | 103,040 |
| 42.505 | (+)-3-Carene, 10-(acetylmethyl)- | 66,804 |
| 43.255 | Octanoic acid | 3,876,198 |
| 44.623 | 2-Pentadecanone, 6,10,14-trimethyl- | 225,638 |
| 44.755 | 2,6-Octadiene-1,8-diol, 2,6-dimethyl- | 63,621 |
| 44.755 | p-Mentha-1,8-dien-7-yl acetate | 96,497 |
| 45.306 | 3-Ethyl-1-heptyne-3-ol | 106,999.5 |
| 45.515 | n-Hexadecanoic acid | 282,887 |
| 45.899 | .alpha.-Bulnesene | 79,896 |
| 46.293 | Cycloheptane, 4-methylene-1-methyl-2-(2-methyl-1-propen-1-yl)-1-vinyl- | 87,168 |
| 46.294 | (-)-Isolongifolol, acetate | 122,326 |
| 46.424 | 2-(p-Tolylmethyl)-p-xylene | 171,310 |
| 46.430 | Ethane, 1-(o-ethylphenyl)-1-phenyl- | 144,701 |
| 47.579 | Pyrrole-2-carboxylic acid, 4-(1-chlorodec-1-enyl)-3,5-dimethyl-, ethyl ester | 98,928 |
| 47.605 | n-Decanoic acid | 2,589,191 |
| 47.940 | 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | 416,391.5 |
| 50.353 | Benzophenone | 885,368 |
| 51.520 | Vanillin | 684,992 |
| 52.990 | 1,2-Benzenedicarboxylic acid, butyl 2-ethylhexyl ester | 304,646 |
| 52.991 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 390,059 |
References
- Uhl, W.J. L’Arte del Tè, Guida Alla Selezione, Infusione e Presentazione Di Tè Squisiti, 1st ed.; Il Castello: Milan, Italy, 2017. [Google Scholar]
- Deka, H.; Barman, T.; Dutta, J.; Devi, A.; Tamuly, P.; Paul, R.K.; Karak, T. Catechin and caffeine content of tea (Camellia sinensis L.) leaf significantly differ with seasonal variation: A study on popular cultivars in North East India. J. Food Compos. Anal. 2020, 103684. [Google Scholar] [CrossRef]
- Yu, L.; Wang, S.; Li, T.; Han, L. Response of soil faunal communities to tea tree cultivars in the hilly region of western Sichuan, China. Sci. Hortic. 2021, 275, 109701. [Google Scholar] [CrossRef]
- Wang, M.; Bai, Y.; Wang, Z.; Zhang, Z.; Liu, D.; Lian, X. Higher tea consumption is associated with decreased risk of small vessel stroke. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ody, P. Complete Guide to Medicinal Herbs, 2nd ed.; Dorling Kindersley Publishing: London, UK, 2008; pp. 48–53. [Google Scholar]
- Zhang, P.; Wang, W.; Liu, X.-H.; Yang, Z.; Gaur, R.; Wang, J.-J.; Ke, J.-P.; Bao, G.-H. Detection and quantification of flavoalkaloids in different tea cultivars and during tea processing using UPLC-TOF-MS/MS. Food Chem. 2021, 339, 127864. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.J.; Yao, Y.; Hua, J.; Zhou, Q.; Jiang, Y.; Deng, Y.; Yang, Y.; Wang, J.; Yuan, H.; Dong, C. Phytochemical comparison of different tea (Camellia sinensis) cultivars and its association with sensory quality of finished tea. LWT 2020, 117, 108595. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Wei, K.; Ruan, L.; Wu, L.; He, M.; Tong, H.; Cheng, H. Differential regulatory mechanisms of secondary metabolites revealed at different leaf positions in two related tea cultivars. Sci. Hortic. 2020, 270, 109579. [Google Scholar] [CrossRef]
- Okakura, K. Il Libro del Tè, 1st ed.; Garzanti: Milan, Italy, 2016. [Google Scholar]
- Mu, B.; Zhu, B.; Lv, H.-P.; Yan, H.; Peng, Q.-H.; Lin, Z. The enantiomeric distributions of volatile constituents in different tea cultivars. Food Chem. 2018, 265, 329–336. [Google Scholar] [CrossRef]
- Zhao, Y.; Lai, W.; Xu, A.; Jin, J.; Wang, Y.; Xu, P. Characterizing relationships among chemicals, sensory attributes and in vitro bioactivities of black tea made from an anthocyanins-enriched tea cultivar. LWT 2020, 132, 109814. [Google Scholar] [CrossRef]
- Liao, X.; Yan, J.; Wang, B.; Meng, Q.; Zhang, L.; Tong, H. Identification of key odorants responsible for cooked corn-like aroma of green teas made by tea cultivar ‘Zhonghuang 1’. Food Res. Int. 2020, 136, 109355. [Google Scholar] [CrossRef]
- Ji, H.-G.; Lee, Y.-R.; Lee, M.-S.; Hwang, K.-H.; Kim, E.-H.; Park, J.-S.; Hong, Y.-S. Metabolic phenotyping of various tea (Camellia sinensis L.) cultivars and understanding of their intrinsic metabolism. Food Chem. 2017, 233, 321–330. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Kong, Y.; Peng, X.; Li, C.; Liu, S.; Du, L.; Xiao, D.; Xu, Y. A comparative study of volatile components in Dianhong teas from fresh leaves of four tea cultivars by using chromatography-mass spectrometry, multivariate data analysis, and descriptive sensory analysis. Food Res. Int. 2017, 100, 267–275. [Google Scholar] [CrossRef]
- Ye, N.; Zhang, L.; Gu, X. Discrimination of Green Teas from Different Geographical Origins by Using HS-SPME/GC–MS and Pattern Recognition Methods. Food Anal. Methods 2012, 5, 856–860. [Google Scholar] [CrossRef]
- Il Mondo del Tè. Available online: https://mondodelte.wordpress.com/2014/09/10/icomposti-chimici-nel-te/ (accessed on 30 June 2020).
- Zheng, X.-Q.; Li, Q.-S.; Xiang, L.-P.; Liang, Y.-R. Recent Advances in Volatiles of Teas. Molecules 2016, 21, 338. [Google Scholar] [CrossRef]
- Collings, E.R.; Alamar, M.C.; Redfern, S.; Cools, K.; Terry, L.A. Spatial changes in leaf biochemical profile of two tea cultivars following cold storage under two different vapour pressure deficit (VPD) conditions. Food Chem. 2019, 277, 179–185. [Google Scholar] [CrossRef]
- Han, Z.-X.; Rana, M.-M.; Liu, G.-F.; Gao, M.-J.; Li, D.-X.; Wu, F.-G.; Li, X.-B.; Wan, X.-C.; Wei, S. Data on green tea flavor determinantes as affected by cultivars and manufacturing processes. Data Brief 2017, 10, 492–498. [Google Scholar] [CrossRef]
- Abbatangelo, M.; Núñez-Carmona, E.; Sberveglieri, V.; Zappa, D.; Comini, E.; Sberveglieri, G. Application of a Novel S3 Nanowire Gas Sensor Device in Parallel with GC-MS for the Identification of Rind Percentage of Grated Parmigiano Reggiano. Sensors 2018, 18, 1617. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Carmona, E.; Abbatangelo, M.; Sberveglieri, V. Innovative Sensor Approach to Follow Campylobacter jejuni Development. Biosensors 2019, 9, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbatangelo, M.; Núñez-Carmona, E.; Duina, G.; Sberveglieri, V.; Núñez-Carmona, E. Multidisciplinary Approach to Characterizing the Fingerprint of Italian EVOO. Molecules 2019, 24, 1457. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Carmona, E.; Abbatangelo, M.; Zottele, I.; Piccoli, P.; Tamanini, A.; Comini, E.; Sberveglieri, G.; Sberveglieri, V. Nanomaterial Gas Sensors for Online Monitoring System of Fruit Jams. Foods 2019, 8, 632. [Google Scholar] [CrossRef] [Green Version]
- Sberveglieri, V.; Bhandari, M.P.; Carmona, E.N.; Betto, G.; Sberveglieri, G. A novel MOS nanowire gas sensor device (S3) and GC-MS-based approach for the characterization of grated Parmigiano Reggiano cheese. Biosensors 2016, 6, 60. [Google Scholar] [CrossRef] [Green Version]
- Sberveglieri, G. Recent developments in semiconducting thin-film gas sensors. Sens. Actuators B Chem. 1995, 23, 103–109. [Google Scholar] [CrossRef]
- Comini, E.; Ottini, L.; Faglia, G.; Sberveglieri, G. SnO2 RGTO UV Activation for CO Monitoring. IEEE Sens. J. 2004, 4, 17–20. [Google Scholar] [CrossRef]
- Dieguez, A.; Romano-Rodrıguez, A.; Morante, J.; Sangaletti, L.; Depero, L.E.; Comini, E.; Faglia, G.; Sberveglieri, G. Influence of the completion of oxidation on the long-term response of RGTO SnO2 gas sensors. Sens. Actuators B Chem. 2000, 66, 40–42. [Google Scholar] [CrossRef]
- Comini, E.; Faglia, G.; Sberveglieri, G.; Pan, Z.; Wang, Z.L. Stable and highly sensitive gas sensors based on semiconducting oxide nanobelts. Appl. Phys. Lett. 2002, 81, 1869–1871. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Concina, I.; Comini, E.; Falasconi, M.; Ferroni, M.; Sberveglieri, V. Synthesis and integration of tin oxide nanowires into an electronic nose. Vacuum 2012, 86, 532–535. [Google Scholar] [CrossRef]
- Zappa, D.; Comini, E.; Zamani, R.; Arbiol, J.; Morante, J.; Sberveglieri, G. Preparation of copper oxide nanowire-based conductometric chemical sensors. Sens. Actuators B Chem. 2013, 182, 7–15. [Google Scholar] [CrossRef]
- Abbatangelo, M.; Núñez-Carmona, E.; Sberveglieri, V. Application of a novel S3 nanowire gas sensor device in parallel with GC-MS for the identification of Parmigiano Reggiano from US and European competitors. J. Food Eng. 2018, 236, 36–43. [Google Scholar] [CrossRef]
- Abbatangelo, M.; Núñez-Carmona, E.; Sberveglieri, V. Novel equipment for food quality control: An IoT nanowire gas sensors array. Chem. Eng. Trans. 2019, 75, 25–30. [Google Scholar]
- Yannai, S. Dictionary of Food Compounds with CD-ROM, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 5th ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
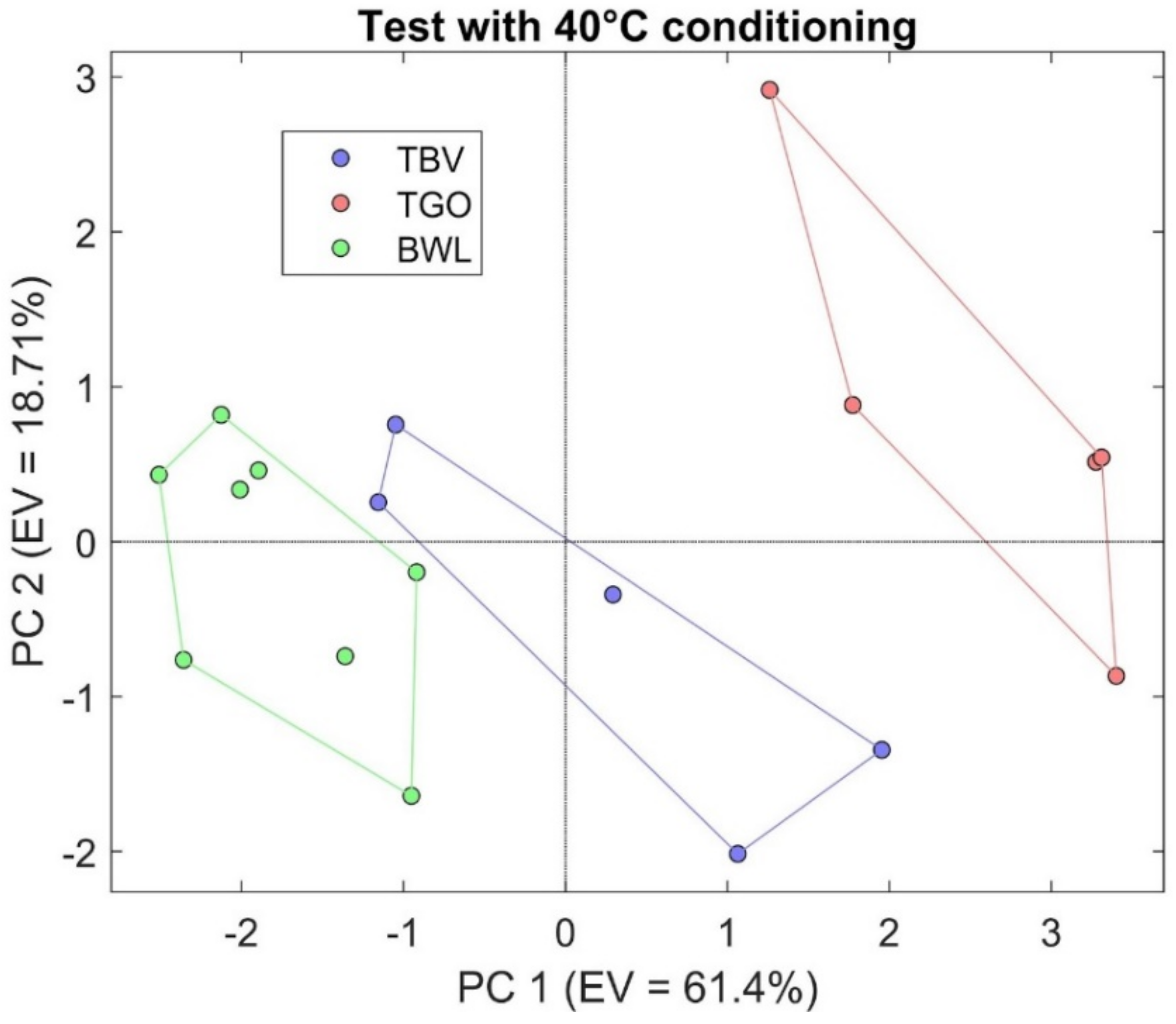
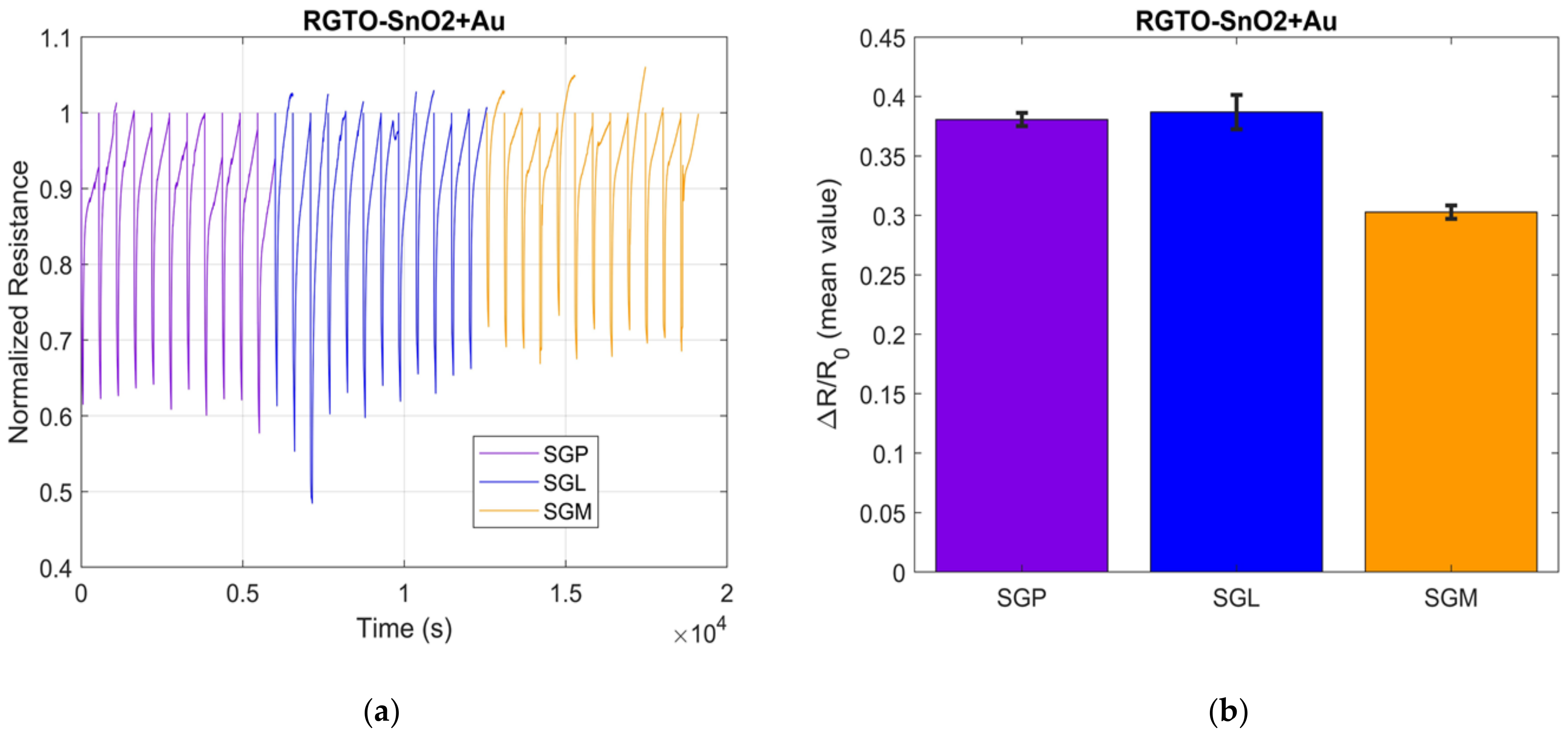
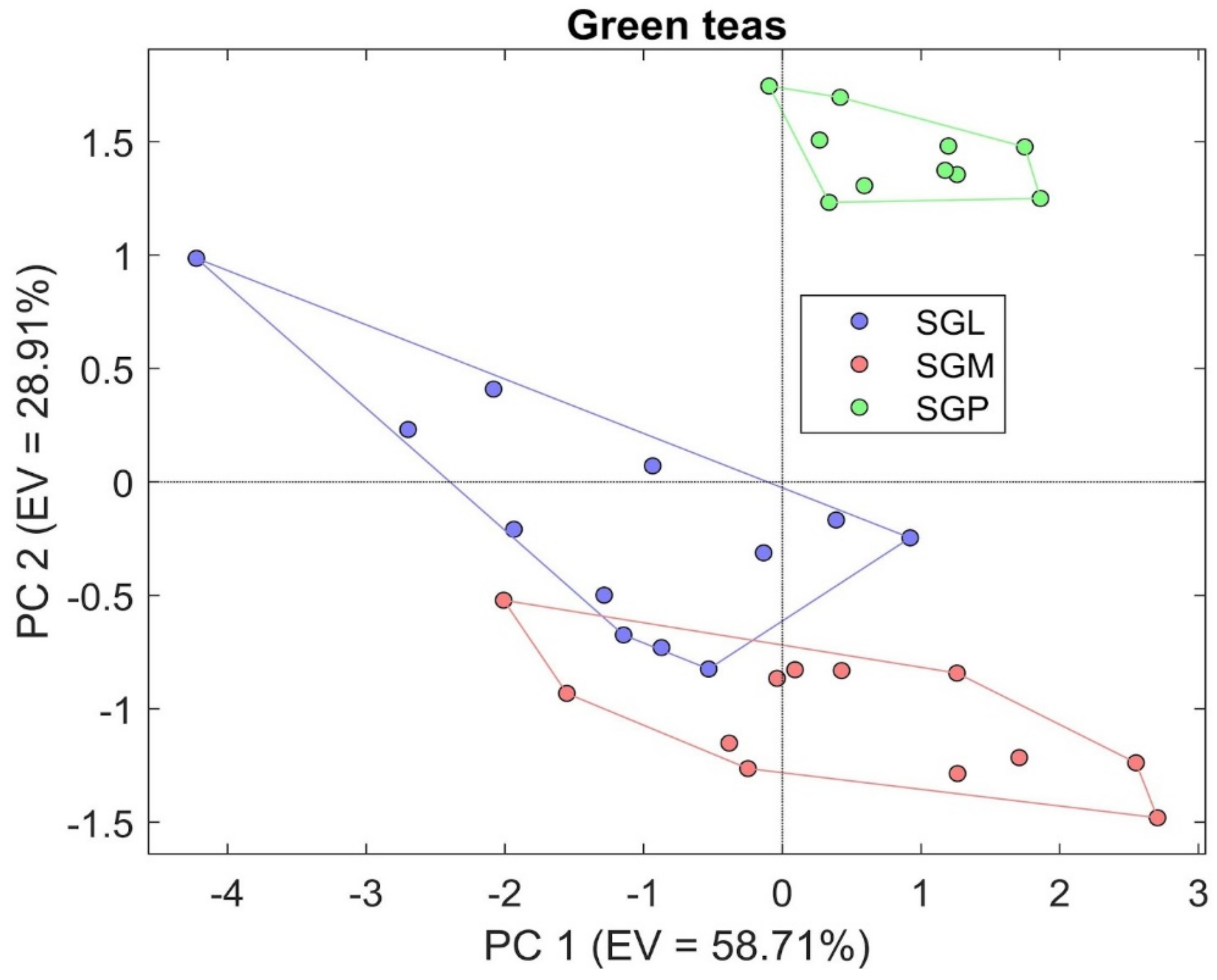
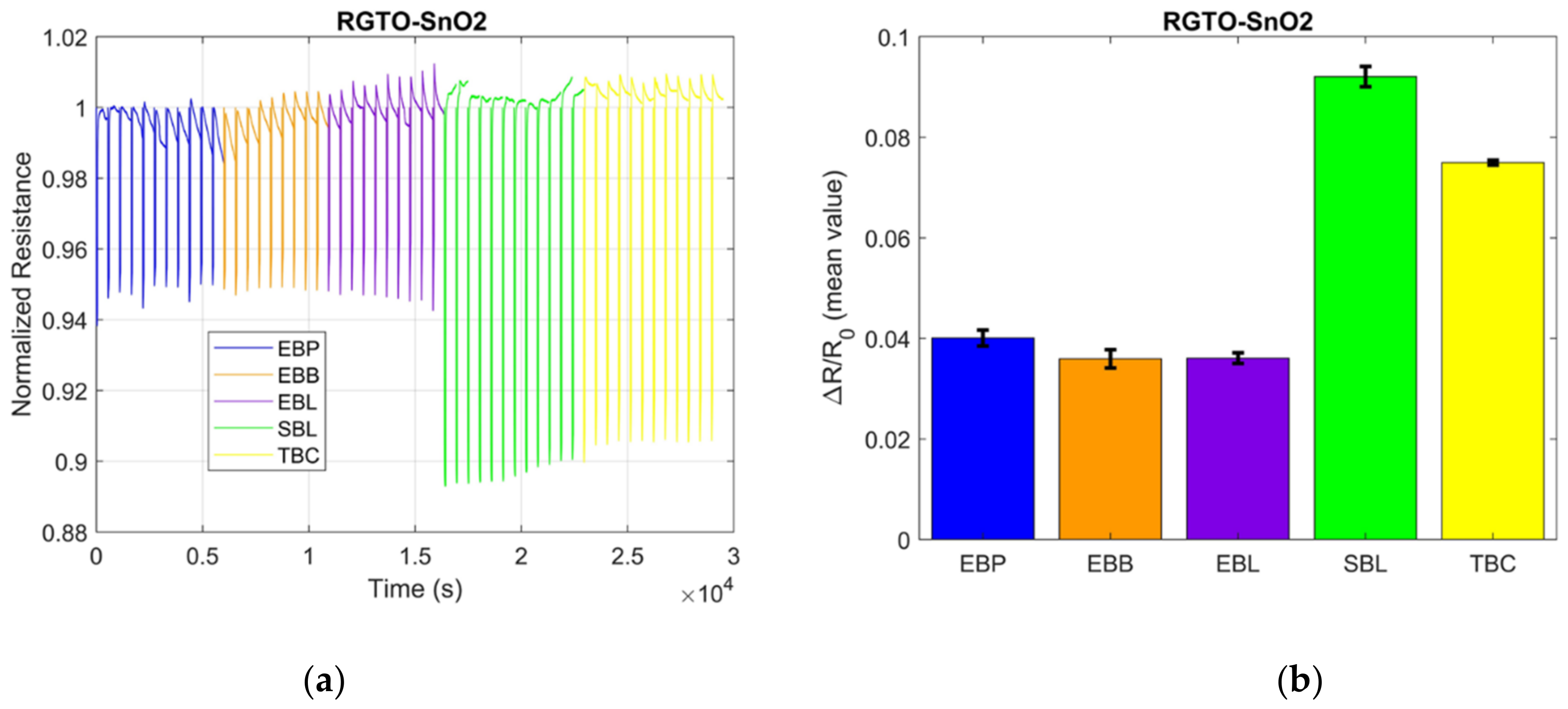
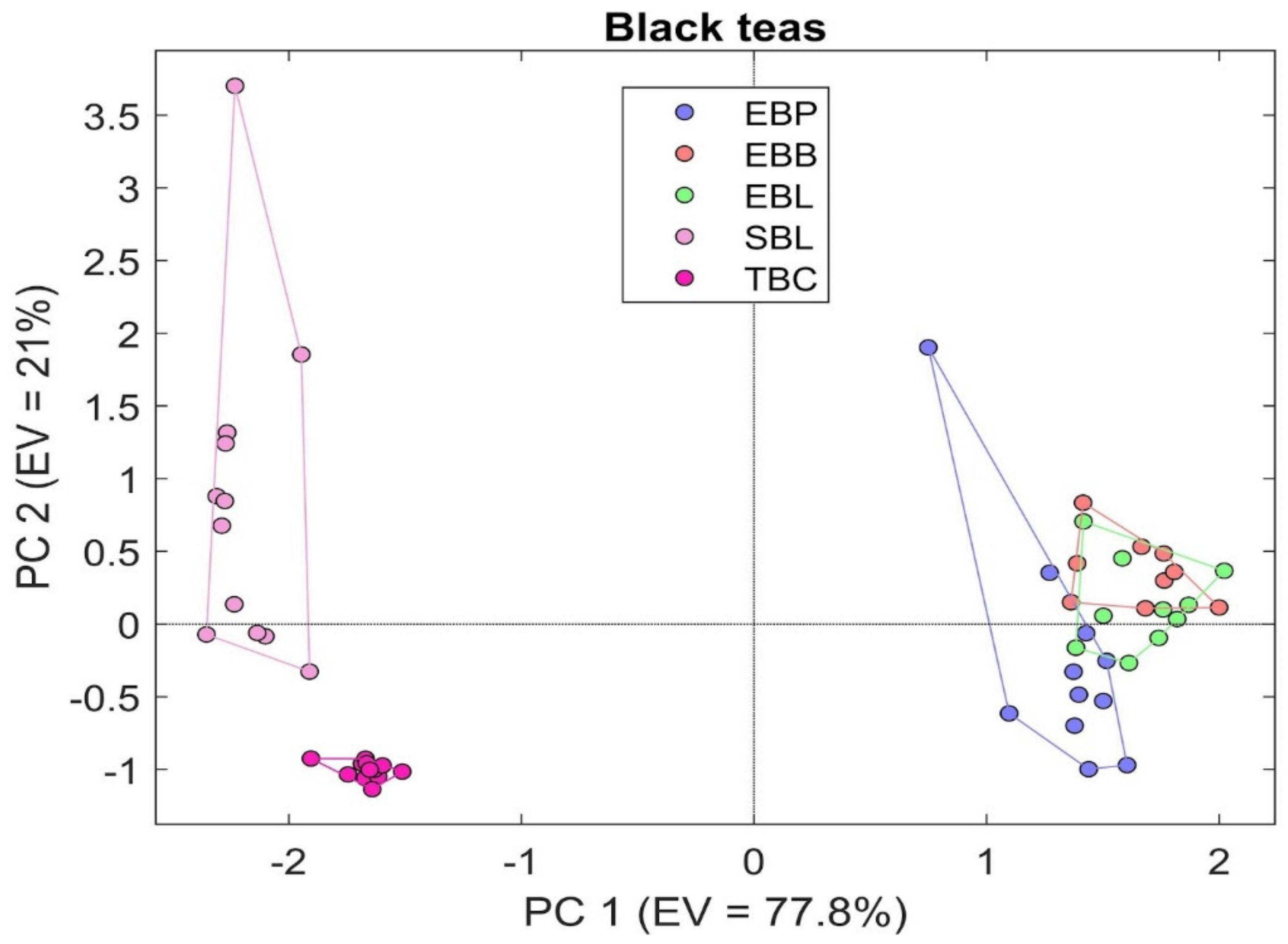
| Kind | Code | Description | Components | Number of Replicates | |
|---|---|---|---|---|---|
| GC-MS | S3 | ||||
| Green tea | TGO | Green tea with orange aroma | 88% Green tea + 10% natural orange aroma + 1% loto flower + 1% orange skin | 3 | 5 |
| SGP | Pure green tea | 100% Green tea | 3 | 12 | |
| SGL | Green tea with lemon aroma | Green tea 83% + lemon aroma + lemon juice concentrate 4% | 3 | 12 | |
| SGM | Green tea with Matcha tea | Green tea + green tea Matcha | 3 | 12 | |
| Black tea | TBV | Black tea with vanilla aroma | 91,5% tea + 8% aroma + 0,5% vanilla | 3 | 5 |
| TBC | Pure black tea from Ceylon | 100% Black tea Ceylon Sri Lanka | 3 | 12 | |
| EBL | Black tea with lemon aroma | Black tea from India and lemon aroma | 3 | 12 | |
| EBB | Black tea Earl Grey | Black Tea from India + bergamot juice | 3 | 12 | |
| EBP | Pure black tea | Biological black Tea | 3 | 12 | |
| SBL | Black tea with lemon aroma | Black tea + aroma + 1.04% powder of lemon juice | 3 | 12 | |
| White tea | BWL | White tea with lemongrass | White tea leaf 90%, dried lemongrass 10% | 3 | 8 |
| Total number of samples for each technique | 33 | 114 | |||
| Material | Kind | Working Temperature |
|---|---|---|
| SnO2 + Au | RGTO | 400 °C |
| SnO2 | RGTO | 400 °C |
| CuO | Nanowire | 350 °C |
| SnO2 + Au | Nanowire | 350 °C |
| SnO2 | Nanowire | 350 °C |
| Compound | TGO | SPG | SGL | SGM |
|---|---|---|---|---|
| Cyanoacetic acid | 1.685 × 105 | 1.165 × 105 | 5.677 × 104 | 6.301 × 104 |
| Hexanal | 7.22 × 105 | 5.751 × 105 | 2.889 × 104 | 3.529 × 105 |
| Limonene | 1.378 × 107 | 1.976 × 105 | 2.602 × 108 | 2.011 × 105 |
| 6-metile 5-epten-2-one | 6.233 × 105 | 3.789 × 105 | 2.731 × 105 | 3.617 × 105 |
| Nonanal | 5.81 × 105 | 1.133 × 105 | 9.298 × 104 | 1.462 × 105 |
| α-terpineol | 1.098 × 106 | 3.145 × 106 | 8.024 × 106 | 7.628 × 105 |
| 5,6,7,7a-tetrahydro, 4,4,7a- Trimethyl 2 (4H)—benzofuranone | 3.448 × 105 | 4.843 × 105 | 4.819 × 105 | 2.836 × 105 |
| Compound | TBV | EBP | EBL | EBB | SBL | TBC |
|---|---|---|---|---|---|---|
| Nonanal | 3.739 × 105 | 6.164 × 104 | 2.278 × 105 | 1.129 × 105 | 1.041 × 105 | 1.198 × 105 |
| Ammonium acetate | 2.141× 106 | 4.641 × 104 | 5.945 × 105 | 6.868 × 105 | 2.162 × 105 | 1.023 × 105 |
| Phenylethyl alcohol | 8.167× 104 | 4.682 × 104 | 4.243 × 104 | 7.557 × 104 | 6.461 × 104 | 5.550 × 104 |
| 5,6,7,7a-tetrahydro, 4,4,7a- Trimethyl 2 (4H)—benzofuranone | 2.784 × 105 | 3.536 × 104 | 1.473 × 105 | 7.543 × 104 | 1.073 × 105 | 1.053 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Carmona, E.; Abbatangelo, M.; Sberveglieri, V. Internet of Food (IoF), Tailor-Made Metal Oxide Gas Sensors to Support Tea Supply Chain. Sensors 2021, 21, 4266. https://doi.org/10.3390/s21134266
Núñez-Carmona E, Abbatangelo M, Sberveglieri V. Internet of Food (IoF), Tailor-Made Metal Oxide Gas Sensors to Support Tea Supply Chain. Sensors. 2021; 21(13):4266. https://doi.org/10.3390/s21134266
Chicago/Turabian StyleNúñez-Carmona, Estefanía, Marco Abbatangelo, and Veronica Sberveglieri. 2021. "Internet of Food (IoF), Tailor-Made Metal Oxide Gas Sensors to Support Tea Supply Chain" Sensors 21, no. 13: 4266. https://doi.org/10.3390/s21134266
APA StyleNúñez-Carmona, E., Abbatangelo, M., & Sberveglieri, V. (2021). Internet of Food (IoF), Tailor-Made Metal Oxide Gas Sensors to Support Tea Supply Chain. Sensors, 21(13), 4266. https://doi.org/10.3390/s21134266








