Abstract
Enhanced hydrogen sensing performance of Pt Schottky diodes on ZnO single crystal wafers in humid ambient conditions is reported using a polymethylmethacrylate (PMMA) membrane layer. ZnO diode sensors showed little change in forward current when switching to wet ambient H2 conditions with 100% relative humidity. This sensitivity drop in the presence of water vapor can be attributed to surface coverage of hydroxyl groups on the Pt surface in humid ambient conditions. The hydrogen sensitivity of PMMA-coated diode sensors recovered up to 805% in wet H2 ambient conditions at room temperature. The PMMA layer can selectively filter water vapor and allow H2 molecules to pass through the membrane layer. It is clear that the PMMA layer can effectively serve as a moisture barrier because of low water vapor permeability and its hydrophobicity. In both dry and wet conditions, ZnO diodes exhibited relatively fast and stable on/off switching in each cycle with good repeatability.
1. Introduction
Gas sensors based on metal oxide (MOX) semiconductors have been widely used for toxic gas detection and quality air control. Extensive efforts have been devoted to developing highly sensitive, selective, reliable, stable, and low-cost sensors [1,2,3,4,5,6,7]. Various MOXs such as SnO2, TiO2, ZnO, WO3, NiO, and Ga2O3 have shown significant change in the conductance through surface interaction and charge transfer between gas molecules and oxide surface atoms. Among those MOXs, ZnO is highly promising for the potential use for gas sensors due to high conductance change, thermal and chemical stability, and facile synthesis into nanostructures having high surface-to-volume ratios [8,9,10,11,12]. Surface modification with ZnO nanorods and doping with specific dopants have yielded dramatic increases of its sensitivity and selectivity in gas sensor applications [13,14,15].
One of the most challenging issues for highly precise gas sensors is the selectivity, which detects the specific gas molecules under other reducing gas mixtures as well as in humid ambient conditions. In the presence of water vapor, SnO2-based gas sensors have shown the increased sensitivity as well as degraded sensor signals over a certain period of time [16,17,18]. The increase in operation temperature and the addition of select dopants were proposed to mitigate the effects of water on the sensitivity [19,20]. In the case of diode-based gas sensors, the sensitivity and selectivity were reported to be severely degraded in humid air conditions [21,22,23]. Under the exposure of water vapor, the surface-active sites for gas adsorption can be blocked by water molecules, thus resulting in the sensitivity drop in the presence of water vapor. There are rapidly growing demands for stable gas sensors to detect a small amount of hydrogen in extreme conditions, especially in hydrogen-fueled vehicles that require conditions above 50% relative humidity (RH) at low temperatures to operate. Several methods have been proposed to solve the humidity problem in gas sensing, including polymer-based membrane layers such as polyvinyl fluoride, polytetrafluoroethylene, polymethylmethacrylate (PMMA), and polyimide, as well as inorganic membranes [24,25,26,27]. The permeability and its selectivity of polymer membrane are significantly influenced by various factors, such as the polarity of membrane materials, the degree of crystallization, and glass transition temperatures. Non-polar polymers exhibit a lower permeability to water vapor and polar gas molecules. Thus, a hydrophobic PMMA layer can serve as a selective filter due to its low permeability coefficient for moisture to water vapor by reducing water adsorption on the oxide surface. In this paper, we investigated the hydrogen sensing performance in high humidity conditions using PMMA protective layers directly coated on Pt/ZnO Schottky diode sensors.
2. Materials and Methods
The 5 mm × 5 mm ZnO single crystal substrates with the c-axis (0001) direction were purchased from a commercial supplier. Both substrates were double sided polished to a thickness of 0.5 mm. Undoped ZnO substrates were characterized by scanning electron microscopy, atomic force microscope, and high-resolution X-ray diffraction analysis. The root-mean-square roughness of the ZnO substrates was measured to be in the range of 1–5 nm with a scan size of 5 × 5 μm2. As can be seen in Figure 1, the full width at half maximum of the X-ray rocking curve of the (0001) ZnO single crystal was measured to be 112 arcsec, indicating high crystalline quality. The electron carrier concentration of ZnO single crystal wafers was measured to be ~5 × 1017 cm−3. Ti/Al (20/100 nm) metal layers were evaporated on ZnO substrates as Ohmic contact, formed by a photolithography and lift-off process. The Ohmic metallization was achieved by annealing at 300 °C for 1 min in ambient nitrogen in a rapid thermal annealer. Then, 200 nm-thick Si3N4 was deposited by plasma-enhanced chemical vapor deposition for the passivation layer. Wet etching with buffered HF solution was used for window opening for the active gas sensing surface. Schottky contact was formed by the evaporation of the catalytic Pt layer with 10 nm thickness. Ti/Au (20/100 nm) were evaporated on both Schottky and Ohmic contacts for metal probe pads. The current–voltage (I–V) characteristics were recorded using an Agilent 4155C semiconductor parameter analyzer (Agilent technologies, Santa Clara, CA, USA) under various H2 concentrations balanced with nitrogen. In order to prepare the PMMA protective layer on the device, PMMA with a molecular weight of 996,000 was dissolved in solvent (anisole) with a concentration of 40 mg/mL, followed by mixing of the solution for 12 h. PMMA was spin coated at 4000 rpm for 30 s, resulting in a thickness of 145 nm. The morphologies of the PMMA surface and cross-section were observed using a scanning electron microscope, as shown in the author’s previous literature [22]. The contact angle measurement for the PMMA dissolved in anisole on the Pt surface was 15.91°, whereas that on Si was 68.34°, clearly indicating that PMMA was more hydrophilic on the Pt layer. The hydrogen gas responses of Schottky diodes with and without PMMA membrane layers were measured when loaded in a gas test chamber with 4% dry H2 in ambient nitrogen. A water bubbler was utilized to produce wet ambient H2 for humidity conditions with 100% RH.
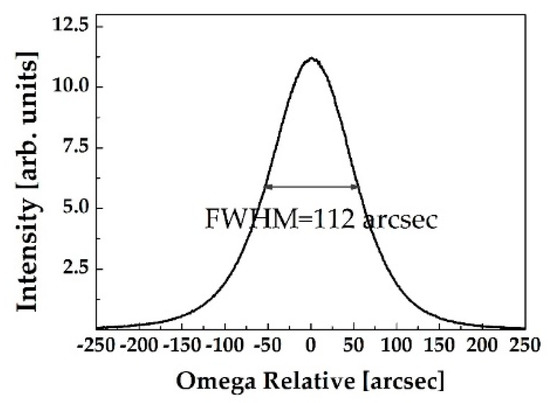
Figure 1.
The relative w-rocking curve of c-plane (0001) ZnO single crystal wafer showing the full-width at half maximum (FWHM) value of 112 arcsec.
3. Results and Discussion
Figure 2a shows the top-view optical microscope image of the fabricated Schottky barrier diode (SBD) on a ZnO single crystal wafer. Figure 2b presents the schematic cross-section of the PMMA-coated diode on the catalytic Pt surface with Ohmic contact and a Si3N4 passivation layer.
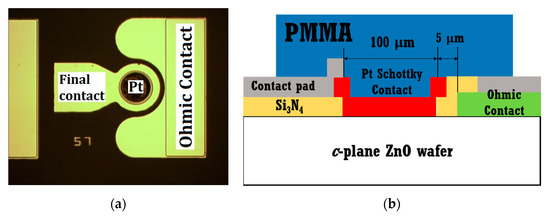
Figure 2.
(a) Top-view optical microscope image of fabricated Schottky diode sensor; (b) cross-sectional schematic of polymethylmethacrylate (PMMA)-coated Pt/ZnO Schottky diode.
Figure 3a shows current–voltage (I–V) characteristics in-log scale of Pt/ZnO Schottky barrier diodes (SBD) before and after 4% H2 exposure in dry ambient as well as in wet ambient conditions. As clearly seen in Figure 3b of I–V curves in linear scale, Pt/ZnO SBDs exhibited excellent response to hydrogen at room temperature. Under H2 exposure, hydrogen gas molecules adsorbed on the Pt catalytic layer, thus leading to the increase in forward current by reducing the effective Schottky barrier height (SBH). When switching to wet ambient conditions (100% RH), little change in forward current was observed upon H2 gas exposure. This sensitivity drop in the presence of water vapor can be attributed to surface coverage of hydroxyl groups on the Pt surface in humid ambient conditions. As water vapor chemisorbed on the Pt catalytic surface, it resulted in the decrease of surface adsorption sites available for hydrogen. The cross-sensitivity toward water vapor has been well reported in SnO2-based MOX gas sensors [18,19,20]. Water vapor has been shown not only to decrease the resistance of SnO2-based materials, but also to significantly interfere with most target gases, resulting in the selectivity issues of gas sensors in humid air.
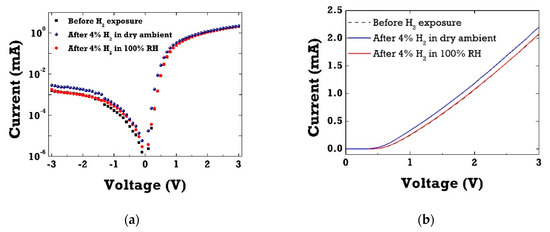
Figure 3.
(a) Current–voltage (I–V) curve of ZnO diodes in log-scale before and after 4% hydrogen exposure in dry and wet ambient conditions; (b) corresponding I–V characteristics of ZnO diodes in linear-scale before and after 4% hydrogen exposure in dry and wet ambient conditions.
Figure 4a shows the I–V characteristics of Pt/ZnO SBD diodes with the PMMA membrane layer before and after 4% H2 exposure in wet ambient conditions (RH 100%). The hydrogen sensitivity of PMMA-coated diode sensors in wet H2 almost recovered to the same level with the one in dry H2 ambient conditions. The relative current change for PMMA-coated diode sensors reached the maximum value of 805% in wet H2 ambient conditions at room temperature. The PMMA layer performed well in ZnO SBDs as a membrane layer, which selectively blocked water molecules, as previously reported by the authors [21,22]. Figure 4b presents the transient change in SBH of Pt/ZnO SBDs in dry H2 and PMMA-coated Pt/ZnO diodes in wet H2 ambient conditions. Note that the SBH values were extracted from the I–V curves using the thermionic emission model. The SBH changes were measured to be 72–75 meV for both SBD diodes upon exposure to hydrogen. After switching back to ambient nitrogen, the SBH of PMMA-coated diodes showed faster recovery.
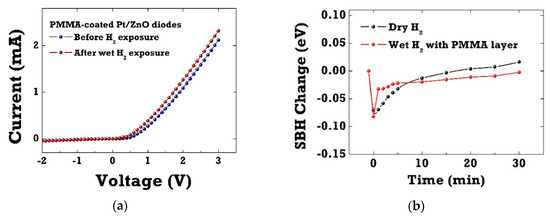
Figure 4.
(a) I–V characteristics of PMMA-coated Pt/ZnO SBDs before and after wet 4% hydrogen exposure; (b) the transient change in Schottky barrier height (SBH) of Pt/ZnO SBDs in dry H2 and PMMA-coated Pt/ZnO diodes in wet H2 ambient conditions.
Figure 5 shows the relative current change (ΔI) in percentage of PMMA-coated Pt/ZnO SBDs with and without the PMMA membrane layer upon exposure to 4% H2 in dry and wet ambient conditions (100% RH). Clearly, the SBD diodes without any protective layer exhibited only a negligible current response to 4% H2 in wet conditions (RH 100%). This H2 sensitivity drop in the presence of water vapor can be attributed to surface coverage of hydroxyl groups on the Pt surface in humid ambient conditions. As water vapor chemisorbed on the Pt catalytic surface, it resulted in the decrease of surface adsorption sites available for hydrogen. It suggests that there is competition and strong interference between water vapor and H2 for the active adsorption sites. The sensitivity, the relative current change as a percentage, is defined as ΔI [–)/] in percentage where and denote diode currents measured in H2 and N2 ambient, respectively. The water-induced effect has been well established in metal oxide-based gas sensors. Water vapor has been shown not only to decrease the resistance of SnO2-based materials, but also to significantly interfere with most target gases, resulting in selectivity issues of gas sensors in humid air. In contrast, PMMA-coated SBD diodes demonstrated little change in sensitivity up to 800% at 0.2 V in high humid condition. The PMMA layer can selectively filter water vapor and allow H2 molecules to pass through the membrane layer. H2 molecules with small kinetic diameter (2.89 Å) can permeate easily thorough the PMMA layer when compared with other gases, such as CO2 (3.3 Å), N2 (3.64 Å), and CH4 (3.8 Å).
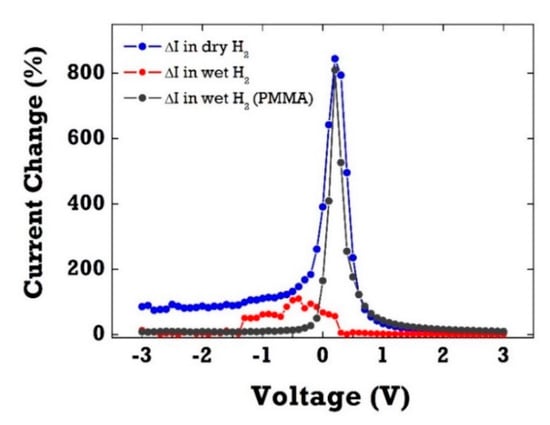
Figure 5.
The current change as a function of bias voltage of ZnO diode sensors with and without the PMMA membrane layer in dry and wet H2 exposure. H2 (4%) was injected into the gas chamber for 30 s.
Figure 6a shows the cyclic response of the Pt/ZnO Schottky diode upon switching 4% H2 on and off in dry and wet ambient conditions. One can see a dramatic decrease in forward currents in wet H2 ambient conditions measured at 0.2 V at room temperature. The inset figures show the negligible current response to hydrogen in the range of a few nA. It suggests that there would be competition and strong interference between water vapor and H2 for the adsorption sites. Water molecules significantly interfere with most target gases, resulting in the selectivity issues of gas sensors in humid air. In the case of PMMA-coated diodes, the hydrogen sensitivity in wet H2 recovered to the same level in dry H2 ambient conditions as can be seen in Figure 6b. Clearly, the PMMA layer could effectively act as effective moisture barrier because of low water vapor permeability. In both dry and wet conditions, SBD diodes exhibited relatively fast and stable on/off switching in each cycle with good repeatability.
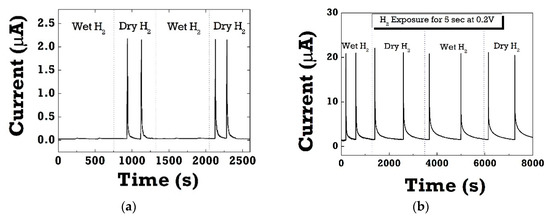
Figure 6.
(a) Cyclic response curves of Pt/ZnO diode sensors without PMMA layer; (b) cyclic response curves of PMMA-coated Pt/ZnO diode sensors, which were measured at 0.2 V forward voltage when switching H2 on and off in dry and wet ambient conditions. The same level of sensitivity was observed in PMMA-coated diode sensors even in repeated cycles of switching dry and wet H2 ambient conditions in 100% RH.
4. Conclusions
Pt/ZnO Schottky diode sensors showed a drastic decrease of hydrogen sensitivity in the presence of water vapor. This sensitivity drop in the presence of water vapor resulted from the surface coverage of hydroxyl groups on the Pt surface, thus leading to the interference of surface adsorption sites available for hydrogen. By employing a PMMA membrane layer on the catalytic Pt surface, enhanced hydrogen sensing performance was achieved in humid ambient at room temperature. The hydrogen sensitivity up to 805% was measured in 100% relative humidity. The PMMA-coated diode sensors also showed stable sensor operation in repeated cycles of H2 on and off without any degradation. PMMA membrane layers can be effectively integrated on Pt surface of diode sensor and work as a selective filter of water molecules in humid ambient conditions.
Author Contributions
S.J. (Soohwan Jang) proposed the research idea and designed the experiments and contributed to revising and proofreading the article. S.J. (Sunwoo Jung) performed the experiments and analyzed the data. K.H.B. guided the experiments and analyzed the data and wrote the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1A09083988, 2017R1D1A3B03035420), and Nano Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2015M3A7B7045185). This present research was also supported by 2019 Hongik University Research Fund.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef]
- Neri, G. First fifty years of chemoresistive gas sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Patil, S.J.; Patil, A.V.; Dighavkar, C.G.; Thakare, K.S.; Borase, R.Y.; Nandre, S.J.; Deshpande, N.G.; Ahire, R.R. Semiconductor metal oxide compounds based gas sensors: A literature review. Front. Mater. Sci. 2015, 9, 14–37. [Google Scholar] [CrossRef]
- Jiang, H.; Huang, M.; Yu, Y.; Tian, X.; Zhao, X.; Zhang, W.; Zhang, J.; Huang, Y.; Yu, K. Integrated Temperature and Hydrogen Sensors with MEMS Technology. Sensors 2018, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yu, Y.; Zhang, L.; Zhu, J.; Zhao, X.; Zhang, W. Flexible and Highly Sensitive Hydrogen Sensor Based on Organic Nanofibers Decorated by Pd Nanoparticles. Sensors 2019, 19, 1290. [Google Scholar] [CrossRef]
- Anderson, T.; Ren, F.; Pearton, S.; Kang, B.S.; Wang, H.-T.; Chang, C.-Y.; Lin, J. Advances in hydrogen, carbon dioxide, and hydrocarbon gas sensor technology using GaN and ZnO-based devices. Sensors 2009, 9, 4669–4694. [Google Scholar] [CrossRef]
- Wan, Q.; Li, Q.H.; Chen, Y.J.; Wang, T.H.; He, X.L.; Li, J.P.; Lin, C.L. Fabrication and ethanol sensing characteristics of ZnO nanowire gas sensors. Appl. Phys. Lett. 2004, 84, 3654–3656. [Google Scholar] [CrossRef]
- Leonardi, S.G. Two-dimensional zinc oxide nanostructures for gas sensor applications. Chemosensors 2017, 5, 17. [Google Scholar] [CrossRef]
- Chava, R.K.; Oh, S.-Y.; Yu, Y.T. Enhanced H2 gas sensing properties of Au@In2O3 core–shell hybrid metal–semiconductor heteronanostructures. CrystEngComm 2016, 18, 3655–3666. [Google Scholar] [CrossRef]
- Majhi, S.M.; Rai, P.; Yu, Y.-T. Facile Approach to Synthesize Au@ZnO Core–Shell Nanoparticles and Their Application for Highly Sensitive and Selective Gas Sensors. ACS Appl. Mater. Interfaces 2015, 7, 9462–9468. [Google Scholar] [CrossRef] [PubMed]
- Gurav, K.V.; Gang, M.G.; Shin, S.W.; Patil, U.M.; Deshmukh, P.R.; Agawane, G.L.; Suryawanshi, M.P.; Pawar, S.M.; Patil, P.S.; Lokhande, C.D.; et al. Gas sensing properties of hydrothermally grown ZnO nanorods with different aspect ratios. Sens. Actuators B Chem. 2014, 190, 439–445. [Google Scholar] [CrossRef]
- Chang, J.; Ahmad, M.Z.; Wlodarski, W.; Waclawik, E.R. Self-assembled 3D ZnO porous structures with exposed reactive {0001} facets and their enhanced gas sensitivity. Sensors 2013, 13, 8445–8460. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Wang, L.; Yang, T.; Guo, X.; Wu, S.; Wang, S. 3D hierarchically porous ZnO structures and their functionalization by au nanoparticles for gas sensors. J. Mater. Chem. 2011, 21, 349–356. [Google Scholar] [CrossRef]
- Ionescu, R.; Vancu, A.; Moise, C.; Tomescu, A. Role of water vapour in the interaction of SnO2 gas sensors with CO and CH4. Sens. Actuators B Chem. 1999, 61, 39–42. [Google Scholar] [CrossRef]
- Ghiotti, G.; Chiorino, A.; Martinelli, G.; Carotta, M.C. Moisture effects on pure and Pd-doped SnO2 thick films analysed by FTIR spectroscopy and conductance measurements. Sens. Actuators B Chem. 1995, 25, 520–524. [Google Scholar] [CrossRef]
- Fukui, K.; Katsuki, A. Improvement of humidity dependence in gas sensor based on SnO2. Sens. Actuators B Chem. 2000, 65, 316–318. [Google Scholar] [CrossRef]
- Itoh, T.; Matsubara, I.; Kadosaki, M.; Sakai, Y.; Shin, W.; Izu, N.; Nishibori, M. Effects of high-humidity aging on platinum, palladium, and gold loaded tin oxide—Volatile organic compound sensors. Sensors 2010, 10, 6513–6521. [Google Scholar] [CrossRef]
- Kim, H.-R.; Haensch, A.; Kim, I.-D.; Barsan, N.; Weimar, U.; Lee, J.-H. The role of NiO doping in reducing the impact of humidity on the performance of SnO2-based gas sensors: Synthesis strategies, and phenomenological and spectroscopic studies. Adv. Funct. Mater. 2011, 21, 4456–4463. [Google Scholar] [CrossRef]
- Baik, K.H.; Jung, S.; Ren, F.; Pearton, S.J.; Jang, S. Moisture insensitive PMMA coated Pt-AlGaN/GaN diode hydrogen sensor and its thermal stability. ECS J. Solid State Sci. Technol. 2018, 7, Q3009–Q3013. [Google Scholar] [CrossRef]
- Jung, S.; Baik, K.H.; Ren, F.; Pearton, S.J.; Jang, S. Pt-AlGaN/GaN hydrogen sensor with water-blocking PMMA layer. IEEE Electron Dev. Lett. 2017, 38, 657–660. [Google Scholar] [CrossRef]
- Bazylak, A. Liquid water visualization in PEM fuel cells: A review. Int. J. Hydrogen Energy 2009, 34, 3845–3857. [Google Scholar] [CrossRef]
- Graunke, T.; Schmitt, K.; Raible, S.; Wollenstein, J. Towards enhanced gas sensor performance with fluoropolymer membranes. Sensors 2016, 16, 1605. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, I.; Nakamura, M.; Kuwano, H. Organic gas sorption by chemical-sensing fluoropolymer films prepared by radio-frequency sputtering. Thin Solid Films 1994, 249, 118–125. [Google Scholar] [CrossRef]
- Samuel, J.J.S.; Ruther, P.; Frerichs, H.-P.; Lehmann, M.; Paul, O.; Rühe, J. A simple route towards the reduction of surface conductivity in gas sensor devices. Sens. Actuators B Chem. 2005, 110, 218–224. [Google Scholar] [CrossRef]
- Jansen, J.C.; Clariziaa, G.; Bernardoa, P.; Bazzarellia, F.; Friessb, K.; Randováa, A.; Schauerc, J.; Kubickad, D.; Kacirkováe, M.; Izake, P. Gas transport properties and pervaporation performance of fluoropolymer gel membranes based on pure and mixed ionic liquids. Sep. Purif. Technol. 2013, 109, 87–97. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).