Abstract
In this research, we developed a direct-flow surface plasmon resonance (SPR) immunosensor for ampicillin to perform direct, simple, and fast measurements of this important antibiotic. In order to better evaluate the performance, it was compared with a conventional amperometric immunosensor, working with a competitive format with the aim of finding out experimental real advantages and disadvantages of two respective methods. Results showed that certain analytical features of the new SPR immunodevice, such as the lower limit of detection (LOD) value and the width of the linear range, are poorer than those of a conventional amperometric immunosensor, which adversely affects the application to samples such as natural waters. On the other hand, the SPR immunosensor was more selective to ampicillin, and measurements were more easily and quickly attained compared to those performed with the conventional competitive immunosensor.
1. Introduction
Ampicillin is a broad-spectrum, semi-synthetic, beta-lactam penicillin antibiotic with bactericidal activity, used to treat bacterial infections caused by susceptible, usually Gram-positive, organisms. Ampicillin has in vitro activity against Gram-positive and Gram-negative aerobic and anaerobic bacteria. The bactericidal activity of ampicillin results from inhibiting cell wall synthesis and is mediated through ampicillin’s binding to penicillin binding proteins (PBPs). In fact, ampicillin binds to and inactivates penicillin-binding proteins located on the inner membrane of the bacterial cell wall. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity.
This interrupts bacterial cell wall synthesis [1], resulting in a weakening of the bacterial cell wall and causes cell lysis. Ampicillin is stable against hydrolysis by a variety of beta-lactamases including penicillinases, cephalosporinases, and extended spectrum beta-lactamases, and thus can be used in a wide range of Gram-positive and Gram-negative infections [1]. Of course, due to the great importance of antibiotics in the clinical and pharmaceutical fields, several strategies, such as chromatography [2,3,4,5,6], mass spectrometry [7,8,9,10], and microbial screening [11,12], as well as methods based on sensors, biosensors and immunosensors [13,14,15,16,17,18,19,20], have been developed for the analytical determination of ampicillin and other antibiotics. Our research group has devoted several efforts in developing several kind of sensors for different type of antibiotics: for instance, we developed different sensors for β-lactam antibiotic detection, initially ion selective electrodes (ISEs) [21] and most recently amperometric immunosensors [22,23]; these amperometric immunosensors have proven to be very efficient in terms of analytical characteristics (very low LOD, wide linear range, good repeatability, and accuracy) but measurements, of competitive formats, require up to an hour, so analysis time is often too long for the requirements of modern analytical chemistry. Therefore, researchers have been attempting to develop methods for direct instead of competitive measurement to save time. Most of such methods have attempted to achieve this by using more modern transducers, generally of the SPR (surface plasmon resonance) type. This approach generally provides good results when the target molecule has a high molecular weight (proteins and so on). However, when the molecule to be determined has a low molecular weight (as is the case for many drugs), the analytical efficiency of an SPR transducer is significantly reduced. For instance, some researchers in our laboratories have recently attempted to develop an SPR immunosensor [24] to ampicillin based on the sandwich method, but achieved only partial success. Therefore, to better investigate these results, in the present research, we compared a simpler immunosensor for the direct determination of ampicillin using the classical-flow SPR technique and compared its analytical features with a conventional competitive immunosensor device. The two methods were used to analyze ampicillin in different real samples: bovine milk, river water, and spring surface water samples. Ampicillin was chosen for its wide use in the medical and veterinary fields, as it is often found in food samples of biological and environmental interest.
2. Materials and Methods
2.1. Reagents and Samples
Anti-ampicillin, monoclonal antibody was provided by Acris (Acris Antibodies GmbH, Herford, Germany), while magnesium chloride, potassium chloride, and dibasic and monobasic anhydrous potassium phosphate RPE (Reagents for European Pharmacopoeia) were supplied by Carlo Erba Reagents (Carlo Erba, Milan, Italy). Ny+ Immobilion Affinity membrane (porosity 0.65 μm) was provided by Millipore (Millipore Corporation, Billerica, MA, USA). Biotin TagTM Micro Biotinylation Kit, composed of biotinylation Reagent (BAC-SulfoNHS, namely biotinamido hexanoic acid 3-sulfo-n-hydroxysuccinide ester), 5 M sodium chloride solution, micro-spin column (2 mL) (in practice, a small empty cylindrical vessel prepackaged with Sephadex G-50), 0.1 M sodium phosphate buffer pH 7.2, 0.01 M phosphate buffer saline (PBS) pH 7.4 (reconstituted with 1 L of deionized water to give 0.01 M phosphate buffer, 0.138 M NaCl, 2.7 mM KCl, pH 7.4), and ExtrAvidin® peroxidase (containing 0.2 mL of ExtrAvidin Peroxidase conjugate at 2.0 mg·mL−1, with 0.01% thimerosal), Fosfomycin, 6-amino-penicillanic acid, dialysis membrane (art. D-9777), albumin from bovine serum (BSA), TRIS (hydroxymethyl-aminomethane), and TWEEN® 20 were all provided by Sigma (Sigma Aldrich, Milan, Italy). Dicloxacillin, cefotaxime, cefalexin, and potassium penicillin G were provided by IBI (I.B.I. Sud Spa, Milan, Italy). Sodium ampicillin was provided by Farmitalia (Farmitalia, Carlo Erba S.p.A., Milan, Italy). Piperacillin was provided by Lederle (Cynamid Italia, Catania, Italy). Neomycin and bacitracin were produced by Boehringer Ingelheim It Spa (Milan, Italy). Ampicillin was produced by Fluka Analyticals (St. Louis, MO, USA); fosfomycin was produced by Crinos S.P.A. (Villa Guardia, Como, Italy). Sodium dihydrogen phosphate NaH2PO4 (≥99.0%), sodium dibasic phosphate Na2HPO4 (≥99.0%), EtOH (96%), 11-mercaptoundecanoic acid (MUA) (95%), 1-ethyl-3-(3-dimethyl-aminopropyl) carbodiimide (EDC), N-hydroxysuccinimide (NHS) (98%), glycine, ethanolamine (≥99%), ceftriaxone, and erythromycin were supplied by Sigma-Aldrich (St. Louis, MO, USA). The SPR plates, each of which was composed of a layer of Au with a thickness of 50 nm on a glassy support, were supplied by XanTec bioanalytics GmbH (Dusseldorf, Germany). The oil with a refractive index of 1.6100 ± 0.0002 was supplied by Cargille Laboratories (Cedar Grove, NJ, USA).
Real analyzed samples were as follows: cow milk, derived from a farm in Central Italy (Lazio); water samples from Sacco River (Lazio); and spring surface water near Rome. In the case of the amperometric method, 0.5 mL of each sample were added to 5 mL of a buffer, and this solution was then subjected as is for analysis. In the case of the SPR method, only a simple dilution with the buffer was necessary; in particular, 0.5 mL of each sample were added to 0.5 mL of the buffer and analyzed.
2.2. Flow SPR or Amperometric Apparatus
SPR flow measurements were performed by using a BioSuplar 400T apparatus (Analytical μ-Systems-Department of Mivitec GmbH, Sinzing, Germany), with a laser diode with low power (630–670 nm) as a light source. This instrument allows for the quantitative analysis of molecules on the basis of the mass of the antibody complex on the plate, which produces a variation successively processed as a function of time in the form of a sensorgram. For ampicillin analysis, using the conventional immunosensor, a 551 VA-Detector Amel potentiostat was used, connected to a 4006a amperometric hydrogen peroxide electrode and to a d5126-2 Omniscribe analog recorder from Houston Instruments (Houston, NS, USA). The test solution was contained in a thermostated cell at 25 °C under constant magnetic stirring (291/lf, Amel Instruments, Milan, Italy).
2.3. Flow-Immunosensor SPR
As shown in Figure 1, the SPR flow device has a prism as the main component, installed on a rotating plate. The instrument is automatically controlled by a computer, and the samples are introduced into the cell by means of a peristaltic pump. The sensor consists of a gold sheet that is 50 nm thick and is placed on a glassy support using oil with a refractive index equal to that of the prism. To observe the SPR phenomenon, the polarized light is reflected from the wafer on a detector and monitored as a function of the resonance angle. After the formation of the complex between antibody and analyte, the SPR angle changes and this variation is proportional to the concentration of the solutions.
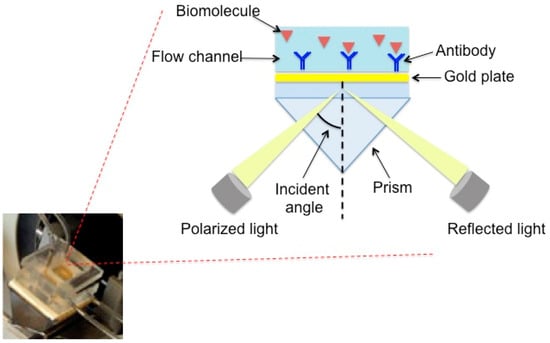
Figure 1.
Measurement device used for surface plasmon resonance (SPR) operating in flow mode. (Of course, only the well “oriented” immobilized antibody, which can contribute to the response, has been reported).
2.3.1. Functionalization of the Gold Plate
The functionalization of the gold surface is carried out by dipping the plate in a solution containing 2 mM 1,1-mercaptoundecanoic acid (MUA) in order to form a self-assembled monolayer (SAM). A SAM structure forms spontaneously with the mercapto groups of the MUA able to form Au–S covalent bonds, while the carboxylic groups, which remain free, are available for subsequent binding with the antibody. After about 12 h, the functionalized plate is washed with ethanol and dried with nitrogen. Then, the SAM-modified gold plate is placed onto the prism surface by means of a drop oil (refractive index <2 × 10−6).
2.3.2. Immobilization of Anti-Ampicillin
The sensorgram reported in Figure 2a is relative to the immobilization of anti-ampicillin. The first operation was the stabilization of the SPR signal with the rehydratation of the MUA by a 0.1 M flowing phosphate buffer at pH 7.4 for about 1 h. After the stabilization of the baseline (Line A, Figure 2a), a mixture of 0.5 mM ethyl (dimethylamminopropyl) carbodiimide and 0.1 mM N-hydroxysuccinimide) was allowed to flow for 15 min in order to activate the carboxylic groups of the SAM, and an increase in the signal was observed (Line B, Figure 2a).
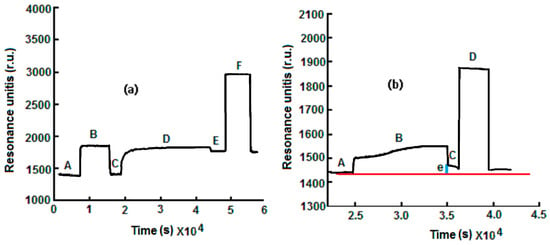
Figure 2.
(a) Immobilization of anti-ampicillin: (A) baseline obtained after rehydratation of the self-assembled monolayer (SAM); (B) activation with 1-ethyl-3-(3-dimethyl-aminopropyl) carbodiimide (EDC)/N-hydroxysuccinimide (NHS) (EDC/NHS); (C) washing with buffer solution; (D) immobilization of 0.5 g·L−1 anti-ampicillin; (E) washing with buffer solution; (F) inactivation of non reacted –COOH groups with ethanolamine; (e = Δ in r.u.). (b) Example of response of anti-ampicillin SPR immunosensor towards ampicillin.
Then, the phosphate buffer was allowed to flow to remove the excess of the EDC/NHS mixture with a lowering of the signal (Line C, Figure 2a). After the activation step, a phosphate buffer containing a concentration of 0.5 g·L−1 of anti-ampicillin antibodies was allowed to flow over the sensor surface for 20–30 min to achieve a covalent cross-linking of the amino reactive groups of antibodies with the aldehyde terminals (Line D, Figure 2a). Finally, after washing with the buffer solution (Line E, Figure 2a), the non-reacted, activated groups were deactivated by treatment with ethanolamine 10−3 M for 10 min in order to avoid non-specific adsorption (Line F, Figure 2a). As shown in Figure 2a, anti-ampicillin was effectively immobilized on the sensor surface. The baseline, after the ampicillin immobilization step, is located at an r.u. (Units of Resonance) value greater than the baseline before the antibody immobilization.
2.3.3. Association of Ampicillin
For the measurement, the following sequence was used: first, the baseline (Line A, Figure 2b), in the presence of the flowing buffer (0.1 M sodium phosphate buffer, pH 7.4), was recorded, and the ampicillin solution was then allowed to flow into the cell, thus generating a signal increase (Line B, Figure 2b). When a plateau was reached, the phosphate buffer was allowed to flow to eliminate the ampicillin that was not bound to the anti-ampicillin (Line C, Figure 2b), and the Δ in r.u. values related to the ampicillin solution were determined (Dotted Line E, Figure 2b). Finally, the surface was regenerated by allowing a 0.1 mM glycine–HCl solution, pH 2.5, to flow in the SPR cell (Line D, Figure 2b), producing the dissociation of the anti-ampicillin-ampicillin complex. All the obtained Δ as units of resonance (r.u. values) were plotted as a function of the relative ampicillin concentration to obtain a calibration curve.
2.4. The Conventional Amperometric Immunosensor
The conventional amperometric immunosensor (Figure 3) was assembled using an Immobilon membrane in which the antibody or the antigen was immobilized and which overlapped a cellulose acetate membrane (0.1 mm thick) placed on the lower end of the plastic cap of an amperometric electrode for H2O2. A nylon net and a rubber O-ring were used to fix the Immobilon membrane to the head of the cap itself. The transducer used consisted of an amperometric electrode for hydrogen peroxide, with a Pt anode polarized at +0.7 V versus an Ag/AgCl/Cl− cathode provided with a plastic cap filled with a 0.1 M KCl solution and screwed onto the body of the electrode. Horseradish peroxidase enzyme was used as a marker for immunocomplex detection.
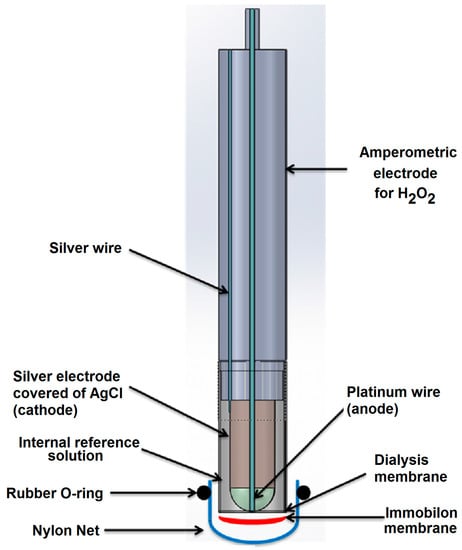
Figure 3.
View of amperometric immunosensor for ampicillin determination using an H2O2 electrode as transducer and peroxidase as enzyme label.
2.4.1. Ampicillin Biotinylation
Exactly 0.2 mL of a 1.0 mg·mL−1 ampicillin solution in a sodium phosphate buffer (pH 7.4; 0.1 M) was prepared. A BAC-SulfoNHS solution (5 mg·mL−1) was also prepared separately by dissolving 5 mg of biotinamido hexanoic acid 3-sulfo-N-hydroxysuccinimide ester in 30 µL of DMSO (dimethylsulfoxide) and adding another sodium phosphate buffer (pH 7.2; 0.1 M) for a final volume of 1 mL. Then, 10 µL of the BAC-SulfoNHS solution were immediately added to the ampicillin solution via gentle stirring, and the mixture incubated under stirring for 30 min at room temperature. The dry Sefadex G-50 resin, contained in a microspin column, was re-suspended in the column by vortexing and equilibrated with 0.2 mL of PBS (pH 7.40; 0.01 M); this buffer is needed as an equilibration buffer of the microspin G-50 column and for the elution of the labeled protein from the column. The biotinylation reaction mixture was applied to the top center of the resin, and the column was centrifuged for 5 min at 700× g. The purified sample was then eluted and collected at the bottom of an Eppendorf test tube. This step was repeated twice more, and a total of three fractions were collected. An ExtrAvidin peroxidase solution (20 µL, 2.0 mg·mL−1) was added to the collected fractions, incubated for 1 h at room temperature, and stored in a freezer at −20 °C.
2.4.2. Anti-Ampicillin Immobilization on the Immobilon Membrane
The positively charged nylon Immobilon Ny+ membranes [22] were cut into 1 cm2 disks; then, 50.0 µL (3.4 × 10−5 M) of an anti-ampicillin solution was prepared in an Eppendorf test tube by dissolving 25 µL (10.3 mg·mL−1) of a standard antibody solution in 500 μL of a 0.1 M phosphate buffer, pH 7.4. This mixture was coated directly onto the surface disk membrane. The Immobilon membrane obtained was then dried at room temperature for about 24 h and stored at 4 °C.
2.4.3. Ampicillin Determination with the “Competitive Format”
The Immobilon membrane, on which the anti-ampicillin was immobilized, as described in Section 2.4.2, was fixed to the head of the amperometric electrode for hydrogen peroxide. Before measurement, the immunosensor was dipped into a Tris-HCl buffer solution, 0.1 M (pH 8.0), containing 0.05% Tween-20 by weight and 2.5% BSA by weight (bovine albumin was used to minimize non-specific adsorption on the membrane).
The same competitive format for penicillin, described in detail in previous papers [22,23], was used, i.e., the ampicillin, free in solution, to be determined was allowed to compete with a fixed supply of the ampicillin labeled with biotin–avidin–peroxidase for the ampicillin antibody immobilized on the membrane (Immobilon) in order to produce the antigen-antibody immunocomplex.
In detail, the ampicillin sample to be determined was added to 5 mL of a new phosphate buffer solution 0.1 M (pH 7.4) restored in the measurement cell, together with a fixed concentration of 50 μL (10 mg·mL−1) of ampicillin–biotin–avidin–peroxidase conjugated (final concentration 0.93 × 10−6 M). For 1 h, the enzyme-conjugated ampicillin was allowed to compete with the non-conjugated ampicillin that was free in solution that try to bind the anti-ampicillin immobilized on the Immobilon membrane. After washing with the same buffer solution to remove all unbound labeled ampicillin, the specific substrate of the enzyme, i.e., 25 μL of a 1% (v/v) H2O2 solution, was added to the renewed buffer solution in which the immunosensor was dipped, and the mixture was then stirred. The ensuing enzyme reaction was catalyzed by the enzymatic marker. The measured signal (nA) correlated directly with the ampicillin concentration to be measured. In this case, hydrogen peroxide produced a signal that increased with increasing concentrations of ampicillin free in solution. The higher the amount of ampicillin bound to the antibody immobilized on the Immobilon membrane, the lower the H2O2 consumed in the enzymatic reaction and the stronger the signal of the H2O2 amperometric electrode. The final signal was obtained by measuring the difference between this signal and that of a blank measured under the same conditions, but the latter was measured using a probe without the antibody complex immobilized on the membrane (see Figure 4).
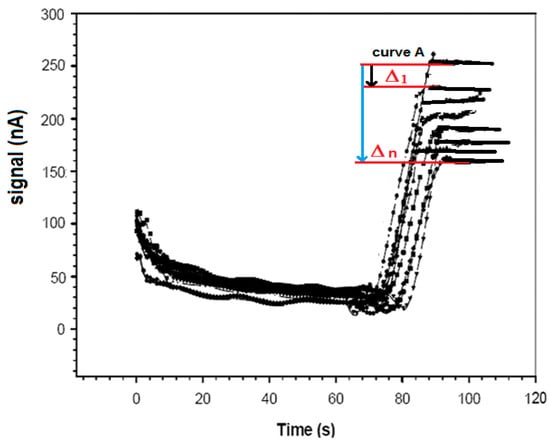
Figure 4.
Trend in amperometric responses in the absence of peroxidase conjugated (curve A) and another curve in the presence of enzyme for H2O2, obtained by the procedure described in Section 2.4.3.
A calibration curve was constructed by plotting the response (in nA) on a semilogarithmic scale as a function of increasing ampicillin concentration in solution and was used to determine the unknown concentration of ampicillin contained in any sample. The schematic “competitive format” for measuring ampicillin is shown in Figure 5. The enzymatic reaction response took about 10 min. Individual measurements were performed, each time using a new membrane.
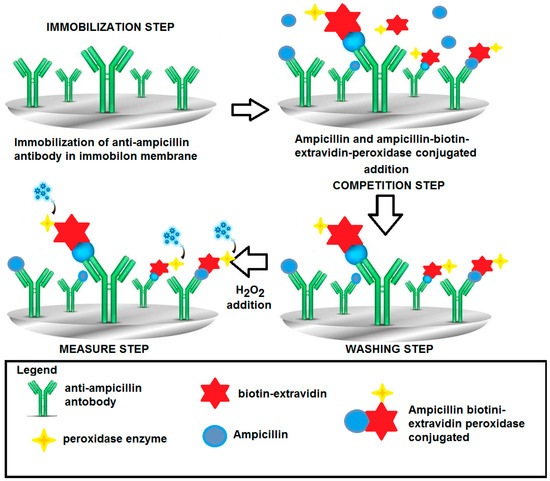
Figure 5.
Scheme for determination of ampicillin by immunosensor using the “competitive format”; peroxidase enzyme as marker and H2O2 electrode as transducer. Test geometry: Competition between ampicillin–biotin–avidin–peroxidase conjugated and ampicillin to be measured, both free in solution, for anti-ampicillin immobilized in membrane. (Of course, only the well “oriented” immobilized antibody, which can contribute to the response, has been reported).
3. Results
In Figure 6a,b, the response behavior and the calibration curve, obtained using the flow SPR immunodevice, are respectively reported. In Figure 7a,b, the response behavior of the conventional amperometric immunosensor and the relative calibration curve are displayed.
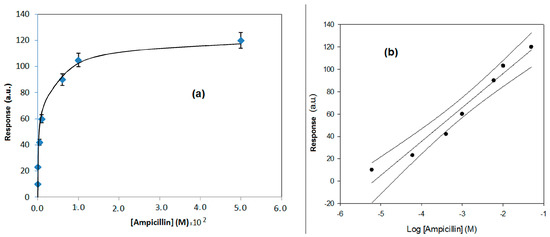
Figure 6.
(a) Behavior of the response of the direct method based on flow SPR vs. concentration, for ampicillin determination. (b) Calibration curve using a semi-logarithmic scale and confidence interval, obtained by the direct method, based on flow SPR, for ampicillin determination.
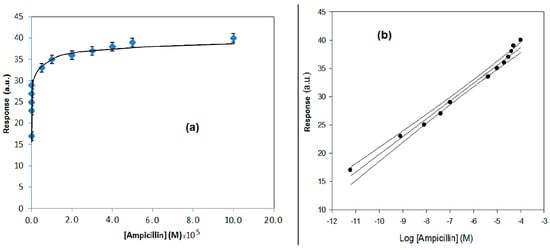
Figure 7.
(a) Behavior of the conventional amperometric immunosensor response using the competitive format as a function of increasing ampicillin concentration; H2O2 electrode as transducer and peroxidase enzyme as marker. (b) Calibration curve for the conventional amperometric immunosensor and confidence interval using the competitive format for ampicillin determination obtained using a semilogarithmic scale.
In Table 1, the main analytical data found using the two immunosensors are summarized.

Table 1.
The main analytical data found using two immunosensors for the determination of ampicillin.
Kaff and IC50 values, obtained using both the SPR device, operating in flow mode, and the conventional amperometric immunosensor, were collected and are shown in Table 2.

Table 2.
IC50 and kaff values for the ampicillin obtained by the SPR immunosensor, and the values found using the conventional amperometric immunosensor.
Selectivity data are shown in Table 3.

Table 3.
Percent cross selectivity values for several common antibiotics.
After both the SPR immunosensor and the ampicillin conventional amperometric immunosensor were analytically standardized, and after Kaff values were evaluated and selectivity checked, both devices were used to determine the concentration of ampicillin in surface spring and river water samples and in cow milk samples (see results reported in Table 4).

Table 4.
“Pool” of β-lactam antibiotic concentration, expressed as M (of ampicillin), found in bovine milk and river and spring surface water samples using the direct SPR immunosensor and the amperometric immunosensor using a competitive format.
The values found were also compared with similar data found in the literature [25,26,27,28], which are reported in Table 4. Furthermore, several recovery tests were carried out in the analyzed samples using both methods (see Table 5 and Table 6 respectively). All values are expressed as M of ampicillin.

Table 5.
Recovery tests for ampicillin using the SPR immunosensor in real samples of bovine milk and of river and spring water samples; values expressed as M (of ampicillin).

Table 6.
Recovery tests for ampicillin using the conventional amperometric immunosensor in real samples of bovine milk and river and spring surface water samples; values expressed as M (of ampicillin).
We carried out a comparison between other β-lactam antibiotic sensors of every type reported in the literature in a previous work [22], wherein the amperometric competitive method was compared with 27 different methods. In that study, it was found that only two of these methods (which are more sophisticated) can reach LOD values lower than that of the conventional competitive format that we developed. Data found using other immunoassay methods are shown in Table 7, where it can be seen that our competitive format method has an LOD of about 0.087 μg·L−1, which is of about the same order, or better, than most immunoassay methods reported in the literature. By comparison, the direct SPR method yielded an LOD value of at least two or three decades higher than LOD values of other immunoassays. The previous developed SPR sandwich method [24] had shown an LOD five or six decades higher.

Table 7.
Data found using other immunoassay methods and immunosensor methods that we have developed.
4. Discussion
Looking to the behavior curves of Figure 6a and Figure 7a showing the response of the immunodevices, an almost logarithmic trend can be observed in both cases. In the case of the conventional immunosensor, this trend was expected and can be easy attributed to the competitive format used, as several previous examples have tested [22,23]. On the contrary, with the SPR direct format, a linear trend was expected [24], and the found almost logarithmic trend has been surprised. We believe that the explanation must attempt to explain the antibody complex dissociation that is, as was demonstrated previously, particularly difficult in the case of ampicillin and its antibody [24]. The hydrolytic dissociation is likely also influenced from the different antigen concentration added in complex formation, such that the result of each measurement, performed at different antigen concentration in solution, depends on not only the step of formation of the antibody complex, but also the hydrolytic dissociation step of the complex, which can influence the result of the measurement, as the original baseline was probably not restored after the regeneration step.
A comparison between the main analytical data of the SPR immunosensor and those of the classical amperometric immunosensor shown in Table 1 and Table 2 indicates that better analytical results were found using the conventional immunosensor regarding sensitivity, linearity range, and LOD and Kaff values, while precision is of the same order; on the contrary, the measurement time is shorter with the SPR method (Table 1) and selectivity is usually improved (see results in Table 3). On the other hand, comparing the analytical data obtained using the direct SPR method, with those that we recently obtained still employing the flow SPR method using a sandwich format [24], it is evident that the present direct method has a wider linearity range and a lower LOD value by about three decades. However, Table 1 shows that the linearity range of the present SPR method is only within about four decades and so is less wide compared with that of the conventional immune-amperometric method (which has a linearity range of about six decades). The latter, above all, is displaced towards lower concentration values. These results are in agreement with the Kaff values of the two methods. In fact, the one relative to the conventional method is about four decades higher than that obtained with the SPR method; therefore, the sensitivity and LOD value of the conventional method is able to analyze all kinds of real samples, whereas the sensitivity of the SPR method is still at least useful for milk analysis, but it cannot be employed for environmental or natural water samples measurements (Table 4). Lastly, recovery data shown in Table 5 and Table 6 are not so different in the case of milk samples for both immunosensors.
Concerning selectivity, it is interesting to observe that the SPR immunosensor responds primarily to ampicillin, while the conventional one responds better to penicillin G. The use of the SPR immunosensor is therefore preferable when determining samples containing primarily ampicillin. Furthermore, Table 3 shows that the response of the conventional amperometric immunosensor to dicloxacillin, piperacillin, amoxicillin, and cefotaxime is very similar to the response to ampicillin, so the conventional device is more useful for determining the β-lactam pool of antibiotics. Additionally, in the case of the SPR immunosensor, the response to ampicillin is higher than that to the aforementioned β-lactam antibiotics.
5. Conclusions
The purpose of this research was to compare the direct determination of ampicillin, using the SPR technique operating in flow conditions, with results found with a conventional competitive immunosensor. The results were perhaps predictable, but we clearly establish here that, in an experimental way, at least for the determination of comparatively low molecular weight compounds (as in the case of β-lactam antibiotics), the amperometric conventional method, compared to direct methods that easily attain data with the SPR transducer, provides the best results from an analytical point of view. Nevertheless, experimental results highlight the great selectivity of the SPR immunosensor in a concentration range from 10−6 M to 10−2 M. Another important positive aspect of the SPR method is the analysis time. In fact, a direct determination of ampicillin was performed in a shorter time. On the other hand, the conventional immunosensor method has a lower LOD and a wide linear range, but its analysis time is very long (about 1 h) due to the competitive format. Further, this competitive immunosensor exhibits low selectivity towards other β-lactam antibiotics, but good selectivity towards antibiotics that do not belong to the same class. This sensor therefore can be useful for the determination of the “pool” of antibiotics of the β-lactam class in common real matrices, such as river or surface natural water, which can be more or less polluted by these types of antibiotics. It is clear that, on the basis of these results, the conventional competitive device, compared to the SPR device that has a very high LOD for this kind of application, is preferred for its much lower LOD values when perform β-lactam antibiotics analysis on real natural water samples, even though recovery data on milk samples obtained with the standard addition method yielded reasonable results with the SPR immunosensor. The real advantages of the SPR method (which is a remarkable advantage) consists in a drastic reduction in analysis time, as well as in the exemplification of the measurement format. In addition, the SPR immunosensor, compared with the conventional immunosensor (that is, for instance, more selective towards penicillin than to ampicillin), is more selective towards ampicillin compared to all other beta-lactam antibiotics.
Acknowledgments
This work was funded by the University of Rome “ Sapienza”, Center “Protezione dell’Ambiente e dei Beni Culturali (CIABC)” and “Istituto per lo Studio dei Materiali Nanostrutturati (ISMN)” of CNR.
Author Contributions
Mauro Tomassetti, Elisabetta Martini, Giovanni Merola, Gabriella Sanzò conceived and designed the experiments; Giovanni Merola, Elisabetta Martini, Gabriella Sanzò performed the experiments; Mauro Tomassetti, Elisabetta Martini, and Gabriella Sanzò analyzed the data; Mauro Tomassetti, Elisabetta Martini, Luigi Campanella, Gabriella Sanzò wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Murray, P.R.; Rosenthal, K.S.; Pfaller, M.A. Microbiologia Medica, 6th ed.; Edra Masson: Milan, Italy, 2015; pp. 167–169, 187–190. [Google Scholar]
- Benito-Peña, E.; Partal-Rodera, A.I.; Léon-González, M.E.; Moreno-Bondi, M.C. Evaluation of mixed mode solid phase extraction cartridges for the preconcentration of beta-lactam antibiotics in wastewater using liquid chromatography with UV-DAD detection. Anal. Chim. Acta 2006, 556, 415–422. [Google Scholar] [CrossRef]
- Marazuela, M.D.; Bogialli, S.A. A review of novel strategies of sample preparation for the determination of antibacterial residues in foodstuffs using liquid chromatography-based analytical methods. Anal. Chim. Acta 2009, 645, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, S.E.; McWhinney, B.C.; Lipman, J.; Roberts, J.A.; Ungerer, J.P.J. A method for determining the free (unbound) concentration of ten beta-lactam antibiotics in human plasma using high performance liquid chromatography with ultraviolet detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 15, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.F.; Evaggelopoulou, E.N.; Papadoyannis, I.N. Development of a validated HPLC method for the determination of four penicillin antibiotics in pharmaceuticals and human biological fluids. J. Sep. Sci. 2006, 2, 1550–1560. [Google Scholar] [CrossRef]
- Jin, H.; Kumar, A.P.; Paik, D.H.; Ha, K.C.; Yoo, K.C.; Lee, Y.I. Trace analysis of tetracycline antibiotics in human urine using UPLC-QTOF mass spectrometry. Microchem. J. 2010, 94, 139–147. [Google Scholar] [CrossRef]
- Mottier, P.; Parisod, V.; Gremaud, E.; Guy, P.A.; Stadler, R.H. Determination of the antibiotic chloramphenicol in meat and seafood products by liquid chromatography–electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2003, 994, 75–84. [Google Scholar] [CrossRef]
- Becker, M.; Zittlau, E.; Petz, M. Residue analysis of 15 penicillins and cephalosporins in bovine muscle, kidney and milk by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2004, 520, 19–32. [Google Scholar] [CrossRef]
- Ohmori, T.; Suzuki, A.; Niwa, T.; Ushikoshi, H.; Shirai, K.; Yoshida, S.; Ogura, S.; Itoh, Y. Simultaneous determination of eight b-lactam antibiotics in human serum by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2011, 879, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Rasic Misic, I.; Miletic, G.; Mitic, S.; Mitic, M.; Pecev-Marinkovic, E. A Simple Method for the Ampicillin Determination in Pharmaceuticals and Human Urine. Chem. Pharm. Bull. 2013, 61, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Pikkemaat, M.G.; Rapallini, M.L.; Dijk, S.O.; Elferink, J.W. Comparison of three microbial screening methods for antibiotics using routine monitoring samples. Anal. Chim. Acta 2009, 637, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Musser, M.B.; Anderson, K.L.; Rushing, J.E.; Moats, W.A. Potential for milk containing penicillin G or amoxicillin to cause residues in calves. J. Dairy Sci. 2001, 84, 126–133. [Google Scholar] [CrossRef]
- Brand, U.; Reinhardt, B.; Rüther, F.; Scheper, T.; Schügerl, K. Bio-field-effect transistors as detectors in flow-injectin analysis. Anal. Chim. Acta 1990, 238, 201–210. [Google Scholar] [CrossRef]
- Goldfinch, M.J.; Lowe, C.R. Solid-phase optoelectronic sensors for biochemical analysis. Anal. Biochem. 1984, 138, 430–436. [Google Scholar] [CrossRef]
- Adrian, J.; Pasche, S.; Voirin, G.; Adrian, J.; Pinacho, D.G.; Font, H.; Sanchez-Baeza, F.; Marco, J.M.P.; Diserens, J.M.; Granier, B. Wavelength-interrogated optical biosensor for multi-analyte screening of sulphonamide, fluoroquinolone, beta-lactam and tetracycline antibiotics in milk. Trends Anal. Chem. 2009, 28, 769–777. [Google Scholar] [CrossRef]
- Thavarungkul, P.; Dawan, S.; Kanatharana, P.; Asawatreratanakul, P. Detecting penicillin G in milk with Impedimetric label-free immunosensor. Biosens. Bioelectron. 2007, 23, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Benito-Peña, E.; Moreno-Bondi, M.C.; Orellana, G.; Maquieira, A.; Van Amerongen, A. Development of a novel and automated fluorescent immunoassay for the analysis of beta-lactam antibiotics. J. Agric. Food Chem. 2005, 53, 6635–6642. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Jung, W.C.; Lee, H.J. Application of a solid-phase fluorescence immunoassay to determine amoxicillin residues in fish tissue. Acta Vet. Hung. 2010, 58, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Gamella, M.; Campuzano, S.; Conzuelo, F.; Esteban-Torres, M.; De Las Rivas, A.B.; Reviejo, A.J.; Muñoz, R.; Pingarrón, J.M. An amperometric affinity penicillin-binding protein magnetosensor for the detection of beta-lactam antibiotics in milk. Analyst 2013, 138, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Huth, S.P.; Warholic, P.S.; Devou, J.M.; Chaney, L.K.; Clark, G.H. Parallux™ beta-lactam: A capillary-based fluorescent immunoassay for the determination of penicillin-G, ampicillin, amoxicillin, cloxacillin, cephapirin, and ceftiofur in bovine milk. J. AOAC Int. 2002, 85, 355–364. [Google Scholar] [PubMed]
- Campanella, L.; Tomassetti, M.; Sbrilli, R. Benzylpenicillinate liquid membrane ion-selective electrode: Preparation and application to a real matrix (Drug). Ann. Chim. 1986, 26, 483–497. [Google Scholar]
- Merola, G.; Martini, E.; Tomassetti, M.; Campanella, L. New immunosensor for β-lactam antibiotics determination in river waste waters. Sens. Actuators B Chem. 2014, 199, 301–313. [Google Scholar] [CrossRef]
- Merola, G.; Martini, E.; Tomassetti, M.; Campanella, L. Simple and suitable immunosensor for β-Lactam antibiotics analysis in real matrixes: Milk, serum, urine. J. Pharm. Biomed. Anal. 2015, 106, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, M.; Conta, G.; Campanella, L.; Favero, G.; Sanzò, G.; Mazzei, F.; Antiochia, R. A Flow SPR Immunosensor Based on a Sandwich Direct Method. Biosensors 2016, 6, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.N.; Smith, M.L.; Chiesa, O.A. LC/MS/MS measurement of penicillin G in bovine plasma, urine, and biopsy samples taken from kidneys of standing animals. J. Chromatogr. B 2006, 830, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, M.; Hu, J.; Zhang, Y.; Chang, H.; Jin, F. Determination of ampicillin and its degradation products in a penicillin production wastewater treatment plant and the receiving river. Water Res. 2008, 42, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed]
- Christian, T.; Schneider, R.J.; Färber, H.A.; Skutlarek, D.; Meyer, M.T.; Goldbach, H.E. Determination of antibiotic residues in manure, soil, and surface waters. Acta Hydrochim. Hydrobiol. 2003, 31, 36–44. [Google Scholar] [CrossRef]
- Grubelnik, A.; Padeste, C.; Tiefenauer, L. Highly Sensitive Enzyme Immunoassays for the Detection of β-Lactam Antibiotics. Food Agric. Immunol. 2001, 13, 161–169. [Google Scholar] [CrossRef]
- Bogdanov, S. Current status of analytical methods for the detection of residues in bee products. Apiacta 2003, 38, 190–197. [Google Scholar]
- Adrian, J.; Pinacho, D.G.; Granier, B.; Diserens, J.M.; Sanchez-Baeza, F.; Marco, M.P. A multianalyte ELISA for immunochemical screening of sulfonamide, fluoroquinolone and β-lactam antibiotics in milk samples using class-selective bioreceptors. Anal. Bioanal. Chem. 2008, 391, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, S.P.; O’Loan, N.; McConnell, R.I.; Benchikh, E.O.; Kane, N.E. Stable competitive enzyme-linked immunosorbent assay kit for rapid measurement of 11 active beta-lactams in milk, tissue, urine, and serum. J. AOAC Int. 2007, 90, 334–342. [Google Scholar] [PubMed]
- Okerman, L.; De Wasch, K.; Van Hoof, J.; Smedts, W. Simultaneous determination of different antibiotic residues in bovine and in porcine kidneys by solid-phase fluorescence immunoassay. J. AOAC Int. 2003, 86, 236–240. [Google Scholar] [PubMed]
- European Communities. Commission Regulation (EEC) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union L 2010, 15, 1–72. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).