Abstract
The Great Barrier Reef (GBR) is currently experiencing widespread crown of thorns starfish (CoTS) outbreaks, as part of the fourth wave of outbreaks since 1962. It is believed that these outbreaks have become more frequent on the GBR and elsewhere in the Indo-Pacific and are associated with anthropogenic causes. The two widely accepted potential causes are (1) anthropogenic nutrient enrichment leading to the increased biomass of phytoplankton, the food of the planktonic stage of larval CoTS; and (2) the overfishing of predators in the juvenile to adult stages of CoTS, for example, commercially fished species such as coral trout. In this study, we show that the evidence for the nutrient enrichment causation hypothesis is strongly based on a large number of recent studies in the GBR. We also hypothesise that secondary outbreaks in the region between Cairns and Townsville can also be enhanced by nutrient enriched conditions associated with the annual nutrient discharge from Wet Tropics rivers.
1. Introduction
The crown of thorns starfish (Acanthaster sp—CoTS) is a specialized coral-feeder and is found across the Indo-Pacific. Populations of CoTS display cyclic oscillations between extended periods of low-density with individuals sparsely distributed among large reef areas, and episodes of unsustainable high densities, commonly termed ”outbreaks”. The outbreaks result in mass mortalities of corals, with typically second-order and long-term consequences on various reef communities. CoTS outbreaks usually spread widely across reef ecosystems in the Indo-Pacific and are driven over large distances by larval transport, commonly leading to increases in benthic algae, a loss of coral-feeding assemblages, an overall collapse of reef structural complexity, and a decline in coral biodiversity and productivity [1,2].
CoTS is a naturally occurring animal on the Indo-Pacific coral reefs and seems to “normally” occur in low densities on most reefs. However, our knowledge of the density of CoTS on reefs before 1960 is very limited. CoTS have caused widespread damage to many coral reefs in the Indo-Pacific over the past six decades, as population”explosions” have occurred at regular intervals [1,3,4,5]. There have been several opposing views as to the origin of the outbreaks, including both a “natural occurrence” hypothesis and various anthropogenic causes’ hypotheses [6]. However, given the huge number of animals which were found on reefs across the Indo-Pacific by the 1970s [1], it has been suggested that these “outbreaks” could not have occurred in the past, or alternatively, not at the frequency at which they were occurring by the 1990s. Therefore, a range of anthropogenic possible “causes” were postulated (reviewed in [1,5,6,7]).
The human causes which have been suggested for the CoTS population outbreaks include the removal of predators (especially fish, such as the commercially fished coral trout) [8,9] and/or increased larval survivorship due to increased phytoplankton food stocks associated with nutrient enrichment [6,10,11,12]. However, the cause (or causes) of the outbreaks remains a controversial issue and there are several opposing views in the literature [5]. One view postulates that population outbreaks are a natural phenomenon, due to the inherently unstable population sizes of highly fecund organisms such as CoTS [13]. Outbreaks have also been attributed to anthropogenic changes to the environment of the starfish, with a range of possible causes including: the removal of adult predators, particularly fish [8] and the gastropod Charonia tritonis [1]; changes to population structures of predators in larval and juvenile stages, caused by chemical (possibly pesticide) pollution [14,15]; the destruction of larval predators, particularly corals, by construction activities on reefs; and larval food supply (phytoplankton) enhancement from nutrient enriched terrestrial runoff [6].
Outbreaks of the pacific CoTS species, A. cf. solaris, on the Great Barrier Reef (GBR) have been traditionally divided into primary outbreaks and secondary outbreaks (Figure 1). Primary outbreaks originate in the “initiation area” between Cairns and Lizard Island. The conditions which are believed to be necessary for the initiation of an outbreak wave include a sufficient coral biomass for food [12], nutrient enrichment and a high biomass of phytoplankton to sustain larval survivorship [11], and suitable oceanographic conditions so as to retain high local connectivity and/or within-reef larval retention [16,17]. Following the initiation of a primary outbreak, massive larval production [18] leads to secondary outbreaks to the north and south of the initiation area, with a wave of secondary outbreaks occurring to the south of the initiation area from Cairns towards Townsville, over approximately eight to nine years after the primary outbreak and then offshore from Mackay (12 years after primary outbreak), after which the outbreaks appear to diminish [5]. This contribution examines the latest research on the nutrient enrichment hypothesis for CoTS outbreaks, with a specific focus on the GBR, north-eastern Australia. We highlight the importance of understanding the life history stages of CoTS development and the influence of nutrients on the larval stage, and for the first time, we examine the potential role of river-discharged nutrients on triggering and sustaining secondary CoTS outbreaks on the central GBR. We also reinterpret recent works on the potential causes of CoTS outbreaks in the southern GBR.
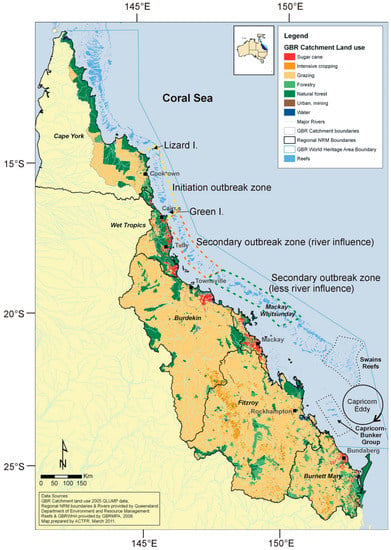
Figure 1.
The Great Barrier Reef. The CoTS initiation zone is shown in yellow (dotted); the CoTS secondary outbreak zone strongly influenced by river discharge is shown in orange (dotted); the CoTS secondary outbreak zone weakly or not at all influenced by river discharge is shown in green (dotted).
2. Mortality of COTS at Different Life History Stages
The nutrient enrichment hypothesis for CoTS outbreaks centers on the increased survivorship during the larval stage of development, although there is little robust knowledge on the rates of mortality at their different life stages. Keesing and Halford [19,20] studied mortality rates at the small settled juvenile stage (0.5–5.5 mm) and found high rates (6.49% d−1) for one month old starfish (mean size = 1.1 mm). The mortality rates decreased considerably with increasing age, revealing a value of 1.24% d−1 for four month old starfish (2.7 mm) and 0.45% d−1 for seven month old starfish (5.5 mm) [19,20]. The results highlighted that mortality rates in these early life stages may be strongly influenced by both predators (epibenthic fauna) and food availability [21]. These experiments, involving the exclusion of predators, indicated that the major source of mortality was predation by epibenthic fauna for juvenile CoTS. Results also show that, in the presence of an adequate food supply, juvenile CoTS move very little, suggesting that juvenile survival is enhanced by settling in areas, whereas predation is minimal.
It has been suggested that the larval stage, especially at the brachiolaria stage where the larvae requires an external food source, is likely to display high mortality rates [6,10], but no field data exist to support this hypothesis [7]. However, laboratory experiments show that very low numbers of larvae are able to reach competency in food limited conditions [10,12,22,23,24]. In addition, predation would also be a factor contributing to the mortality rate, with fish, such as damselfish, preying on CoTS larvae [25] in experimental aquarium conditions; however, the extent of predation in reef waters is not known.
3. Conceptual Understanding of the Nutrient Enrichment-CoTS Link
In the 1970s, Charles Birkeland formulated the hypothesis that CoTS population outbreaks were more common on high islands in the Pacific islands due to the terrestrial runoff of nutrients (nitrogen and phosphorus), than they were on atoll islands, where there is no natural runoff [26]. This hypothesis was founded on the fact that CoTS have a planktonic larval stage (of a few weeks) which requires feeding on a minimum amount of phytoplankton to reach a level of viability, such that they can settle on a reef. In “normal” nutrient conditions, the larvae have insufficient phytoplankton food to reach competence [10] and thus cannot provide the conditions required for the survival of high population numbers. However, in the areas where nutrient runoff in river discharge from the high islands occurs, the conditions required for phytoplankton to bloom can be achieved, thus providing the food source for this larval stage to develop [6,27].
The anthropogenic causation theory of nutrient enrichment is strongly linked to the increase in human activities such as sewage discharge, fertiliser runoff, and increased erosion of nutrient-rich soil. These activities lead to the enrichment of adjacent coastal waters [28], a higher phytoplankton biomass, and a shift to larger species of phytoplankton which are more suitable as CoTS larval food [29], resulting in better rates of survivorship of CoTS larvae, ultimately leading to more frequent outbreaks [6,11].
The analysis of larval growth, the effects of environmental factors, and the experimental testing of the nutrient enrichment hypothesis were first carried out in the 1970s [10,30,31,32], and showed that COTS larvae were food limited and did not develop well in low-nutrient and phytoplankton biomass conditions. In contrast, experimental testing using more sophisticated apparatus [33,34] tested the growth of larvae in a range of nutrient conditions, with results suggesting no nutrient effect. However, the experiments were repeated, this time with more care given to strict protocols regarding nutrient supply, with results supporting the nutrient enrichment hypothesis [12,20,21,35]. CoTS outbreaks associated with anthropogenic nutrient enrichment are now seen as one of the “signals” of partial eutrophication of the GBR [28,36]. Recently, refinements of the experiments have further supported the hypothesis [37,38,39], by establishing that larval survivorship increases with an increased food supply of phytoplankton biomass. Optimal survival is represented by chlorophyll a concentrations of about 1 μg·L−1 [38,39] or at algal food densities above 1000 cells·mL−1 [34]. Both low chlorophyll a concentrations (0.1 μg·L−1) and higher chlorophyll a concentrations (~10 μg·L−1) appear to retard the development of CoTS larvae [38,39,40], indicating that there is an optimum range of phytoplankton biomass or cell counts for larval survivorship.
Concentrations of chlorophyll a in the GBR lagoon are generally less than 0.5 μg·L−1 in non-river discharge conditions [41], but can be in the range of 0.5–3 μg·L−1 where influenced by major river discharge, particularly in the Burdekin—Wet Tropics regions, where flood plumes reach the mid- and outer shelf reef areas [42,43,44,45,46]. The phytoplankton biomass, measured as chlorophyll a during these peak and post flow conditions, is able to support the high larval survivorship of CoTS juveniles if the high nutrient conditions intersect with periods of high larval counts.
4. Crown of Thorns Starfish and the Great Barrier Reef
The cycles (waves) of outbreaks on the GBR have occurred from 1962 to 1976, 1979 to 1991, 1993 to 2005 [11,12], and 2009—current [5,16]. Each wave has severely reduced the coral cover on the GBR, especially in the mid-shelf reefs of the central section [47] (Figure 1). CoTS outbreaks have been less frequent on the inner and outer shelf reefs, for not-well understood reasons. There is evidence that CoTS outbreaks have occurred on the GBR over the past 7000 years [48], although historical records suggest that the waves of outbreaks post 1962 appear to be an unusual phenomenon and that outbreaks have become more frequent in recent times (see below) [11,12].
Oceanographic conditions associated with the ENSO cycle [49] also play a part in initiating CoTS outbreaks in the Cairns area of the GBR, with nutrient enrichment from river runoff and increased local connectivity and/or within-reef larval retention being due to ENSO conditions, both of which provide conditions that result in the initiation of waves of outbreaks [16]. Indeed, this new climate association suggests that the “natural” frequency of CoTS outbreaks may have also been highly variable on the GBR over the past 7000 years, given changes in the strength of ENSO forcing during this period. However, the modern conditions of strong ENSO coupled with increased nutrient sources, likely creates a scenario where CoTS outbreaks become an almost continuous feature on the GBR.
It is also important to note that CoTS outbreaks can occur at sites with no obvious nutrient enrichment [50], but where, in reality, upwelling may be present but not documented and at sites with possible nutrient enrichment associated with natural upwelling systems (e.g., in the Chagos archipelago—[51]) or at oceanic nutrient/productivity fronts [52,53].
There has also been a persistent outbreak area in the Swains reefs (Figure 1), but this is not considered to be associated with the outbreaks to the north and the cycle of anthropogenic drivers and higher larval survivorship. This long-standing situation of CoTS outbreaks in the Swains reefs in the southern GBR is likely associated with the known upwelling systems in that region [11]. This has been identified as an area of upwelling associated with the Capricorn Eddy [54,55,56] and the resultant phytoplankton blooms are associated with other enrichment phenomena, such as manta ray feeding aggregations [57] and plankton enrichment [58]. It is likely that these factors help to explain the CoTS outbreaks in the Swains and Capricorn-Bunker Group reefs of the southern GBR [50]. In addition, the recent outbreaks in the southern Capricorn Bunker Group [50] may be associated with record river discharge from the Burnett and Mary Rivers in early 2011 and 2013 [59].
Miller et al. [50] considered the role of one large southern river, the Fitzroy, but did not show that the discharge from this river had a significant effect on the CoTS outbreaks in the Capricorn Bunker group. However, work on the extent of all southern rivers, i.e., Mary, Burrum, Burnett, Kolan and Baffle in the Burnett Mary NRM Region(Figure 1), clearly showed the discharge plume extent of these rivers impinged on the southern Capricorn Bunkers [59]. While the Fitzroy River discharge only influences the northern section of the Capricorn-Bunker Group reefs during rare large flood events that are coupled with favorable wind conditions [42,60], the five rivers to the south of the Fitzroy are known to influence phytoplankton dynamics in the southern Capricorn-Bunker group [57] and may influence the survivorship of CoTS larvae in this region.
5. Nutrient Enrichment and Secondary Outbreaks
While it often assumed that nutrient enrichment is primarily involved in producing “primary” CoTS outbreaks in the initiation area (Cairns—Lizard Island), there is now evidence that nutrient enrichment can enhance “secondary” outbreaks, especially in the offshore Wet Tropics region. Nutrient loading from these rivers has greatly increased with the proliferation of agriculture and associated fertiliser use in the region [61,62]. The period in which CoTS larvae are in the plankton stages, during November to February, coincides with the point at which the Wet Tropics rivers exhibit high discharge levels each year (Figure 2), with high levels of intra-annual variability, and regularly produce phytoplankton blooms on the GBR shelf in this region [42,43,63,64,65,66,67]. Measurements of high chlorophyll a concentrations during this period represent an extended period of time, in which conditions of high phytoplankton biomass are optimal for CoTS larval development (see also [38,67]).
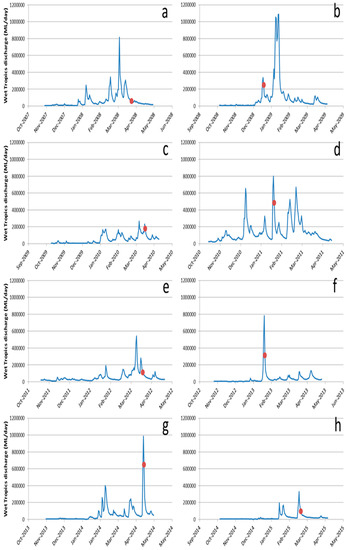
Figure 2.
Total daily wet season discharge (November to April, inclusive, ML/day) for the Wet Tropics rivers for the wet seasons of 2007–2008 (a), 2008–2009 (b), 2009–2010 (c), 2010–2011 (d), 2011–2012 (e), 2012–2013 (f), 2013–2014 (g), 2014–2015 (h). Red dots in parts (d), (f), and (h) stand for the day on which the satellite images shown in Figure 3 were taken and are within the CoTS spawning period.
Images of river discharge are easily identifiable using remote sensed imagery and are available on a daily basis, where the brown turbid, coastal waters are clearly contrasted with the greener offshore waters associated with increased phytoplankton production, as well as the blue oceanic waters (Figure 3 and Figure 4); although it is worth noting that these images are subject to cloud cover. In Figure 4, we show a series of MODIS images in true colour from 2007, demonstrating the formation and dispersal of a phytoplankton bloom driven by high discharge from Wet Tropics rivers over a five day period. By the 13 February, the plume/bloom has extended across the outer-shelf reefs of this area and into the Coral Sea.
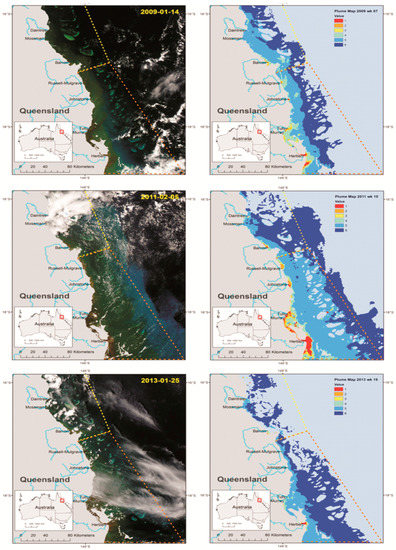
Figure 3.
Selected satellite images showing plume water intrusion into the CoTS initiation zone (south) and the region south to the Palm Island Group over three years (left column) and the corresponding weekly plume colour class map (right column). Dashed yellow lines on maps stand for the south delimitation of the CoTS initiation zone and the secondary outbreak zone is shown by the red dashed lines. Plume colour classes vary from one to six, as described in the text. The stage of river discharge on each occasion is shown in Figure 2.
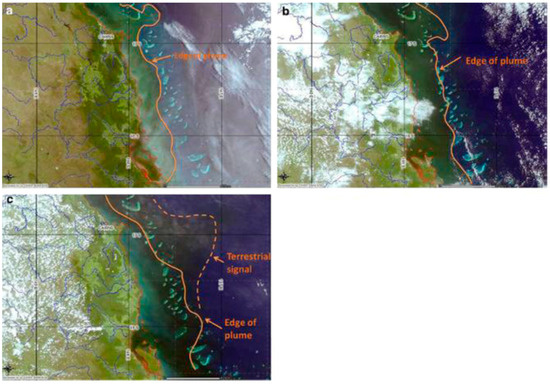
Figure 4.
Progression (a–c) of multiple river plumes in the Wet Tropics (9, 11, 13 February 2007, respectively) extending from the coast to beyond the outer reef. The lines show the outer edge of the plume made visible due to Colored Dissolved Organic Matter and phytoplankton. Images (a–c) show the transformation from a plume dominated by terrestrial particulate matter (brown colour near river mouths) into a plume dominated by a dissolved nutrient, driven phytoplankton bloom (green colour). A proportion of the contained nutrients in the plume may be seen “escaping” to the Coral Sea in part c. The images show a period of enrichment on the mid- and outer- shelf of the Wet Tropics GBR lasting at least five days. Images courtesy of CSIRO.
The mapping and modelling of the extent and composition of flood plumes has been employed as a monitoring tool since 1991. More recently, the classification of plume water types has allowed a more detailed modelling of the extent and composition of plume waters at a greater frequency. The frequency and extent of the plumes, correlated with water quality values, provides mechanisms to predict the mean chlorophyll values within the plume waters across the wet season [44]. Work focused on the Tully region [68] showed that plume waters were mapped on a weekly basis and secondary waters extended out into the mid shelf areas for 14 out of the 22 weeks of the wet season in 2010/2011, highlighting the extended period of time that optimal conditions existed for supporting phytoplankton growth.
A daily image of flood plumes was selected from remote sensed imagery (MODIS, Aqua, 250 m) (Figure 3), at a period of elevated discharge (or in the days following) (Figure 2) and at periods of low clouds, to ensure that the plume was visible. The three selectedimages from plumes associated with the Wet Tropics rivers during the period of CoTS spawning, show an elevated phytoplankton biomass on the mid-shelf reef area of the GBR between Cairns and Townsville, with examples presented for the period between November to March (Figure 3) for each year.
These conditions of elevated phytoplankton biomass are seen both visually, and by water type analysis [44,64,65,67] and ascribed water types (right column panels of Figure 3), following the methodology of Devlin et al. [44] and Petus et al. [69,70].
Each image represents a period of time of optimal conditions for high phytoplankton biomass, and potentially a source of increased CoTS larval survivorship. Chlorophyll a concentrations measured on the Cairns—Townsville mid-shelf area during major river discharge periods (December to March), are 0.5–3 μg·L−1 at salinities in the range 30–35 [42,64,68]. Water type descriptions [44,65,70] are derived from true colour MODIS imagery, calibrated against the direct measurement of salinity, temperature, particulate and dissolved nutrients, phytoplankton, total suspended solids (TSS), the diffuse attenuation coefficient of photosynthetically active radiation (Kd(PAR)), colored dissolved organic matter (CDOM), chlorophyll a, and herbicides [70]. The ranges and mean values of chlorophyll a for the water types are summarized in Table 1, drawn from Devlin et al. [44].

Table 1.
Chlorophyll a concentrations in the different water types and colour classes.
Water types and classes are a necessary step in estimating likely chlorophyll a concentrations in high river discharge conditions, as the algorithms for estimated chlorophyll a from satellites such as MODIS do not work well in the Case 2 waters of the GBR [71], with errors, even in the deeper, clearer waters of the outer GBR lagoon, of typically 100%. While other researchers [38,72] have used estimates of chlorophyll a concentrations from MODIS, it should be noted these estimates can have very large errors. It should also be noted that regular direct measurements of chlorophyll in the GBR lagoon ceased in 2006. The results of the 1991–2006 program can be found in Brodie et al. [41]. Since 2006, only irregular direct measurements of chlorophyll a have occurred during flood plume studies [44] and estimates from remote sensing algorithms, which can be problematic in the Case 2 coastal waters of the GBR [71].
The concentrations of chlorophyll a in colour class six (and to some extent five) waters are in the range at which CoTS larval development is most favorable, estimated to be at 1 μg·L−1 (compared to 0.1 and 10 μg·L−1) in Pratchett et al. [40]; at 1 μg·L−1 compared to much lower and higher concentrations in Wolfe et al. [38]; and at 1 μg·L−1 in Fabricius et al. [12]. While Wolfe et al. [38] claim “it seems that mid-shelf regions on the GBR have a high background level of phytoplankton (0.4–0.6 μg chl a·L−1; Table 1, S1 Table), which may support successful development of A. planci larvae irrespective of flood or storm events”, these results for chlorophyll a are estimated from the MODIS sensor using algorithms which are known to have serious errors and which generally overestimate chlorophyll a concentrations (see above). From the measured results, the only periods when chlorophyll a concentrations are in this range on the mid-shelf of the GBR between Townsville and Lizard Island is during high river discharge conditions.
At higher chlorophyll a concentrations, larvae develop less strongly [38,40]. CoTS larvae generally also grow slowly and do not easily reach a competent stage at lower concentrations of chlorophyll a (<0.2 μg·L−1) or low phytoplankton biomass [12,37,38,40]. An exception to this situation seems to be in the case where female CoTS have been fed on Acropora corals. In these circumstances, the weight of the females increased, as well as the gonads, and also produced larger oocytes compared to Porites-fed and starved females [73]. The CoTS larvae under these conditions may be able to reach competency faster, without significant phytoplankton feeding [73], but this has not actually been demonstrated.
The remote sensed imagery only examines a single day during the high discharge period and a further analysis to extend the period over the primary four months associated with CoTS spawning i.e., November to March inclusive, would be needed to further advance this speculative hypothesis. However, it is worth noting that the river flow conditions for all of the images were variable, and represent periods of flow from 80th to 99th percentile conditions. Optimal growth conditions can still occur at the lower flow rates, indicating that high phytoplankton biomass can be stimulated in first flush, medium to high flow conditions, and may represent a period of time from days to weeks. Extended periods of high biomass conditions can increase the probability of time that larval bloom and enhanced plume conditions can intersect and increase survivorship, supporting the growth associated with secondary outbreaks.
6. Conclusions
The range of phytoplankton biomass, as measured via the chlorophyll a concentration, for the favorable development of CoTS, is 0.6–1 μg·L−1. It appears that well-nourished (from their mother) [73] larvae may be able to reach competency at a lower phytoplankton biomass, although this has not been demonstrated. This may correspond to the low chlorophyll results of Wolfe et al. [72], where larvae were able to reach competency at chlorophyll a concentrations in the range of 0.1 to 0.4 μg·L−1; conversely, in this study, the CoTS larvae were fed the microalga Proteomonas, an algae with hardly any chlorophyll, and so the results are hard to interpret compared to other phytoplankton food. However, most larvae need chlorophyll a concentrations in the range 0.6 to 1.0 μg·L−1 for adequate development [12,38,40]. Larvae develop poorly when chlorophyll a concentrations are above about 1.5 μg·L−1 [38,40].
This chlorophyll a range (0.6–1.0 μg·L−1) has been shown to occur commonly in the wet season on the mid-shelf waters of the GBR between Cairns and Townsville, where secondary CoTS outbreaks occur. As such, it is possible that the enhanced survivorship of CoTS larvae is occurring in this region on a regular basis, as a result of increased nutrient delivery during periods of high discharge from the Wet Tropics rivers [42], and also during the much more irregular high discharge periods from the Burdekin River (e.g., [46,69]). Thus, these secondary outbreaks of CoTS, while mainly driven by massive larval supply, may also be accelerated by suitable phytoplankton conditions provided by increased nutrient discharges from the relevant rivers [61]. This finding also explains the query in Babcock et al. [74], expressed as follows: “Moreover, if the productivity of mid-shelf waters on the GBR are consistently below levels (0.25 μg·L−1) at which there is almost zero survival of COTS larvae, it is hard to explain how the southward propagating waves of outbreaks (that subsequently cause widespread devastation) are maintained.” In fact, chlorophyll a concentrations in the mid-shelf waters of the GBR (at least in the area between Townsville and Lizard Island) are well above 0.25 μg·L−1 for extended periods during the wet season.
Further studies are required to assess the strength of the nutrient enrichment element in secondary outbreaks of CoTS, especially in the mid-shelf area between Townsville and Cairns, where river plumes reach this area on a regular basis. Further south from Townsville, river plumes (from rivers south of the Burdekin) rarely reach the mid-shelf of the GBR and it may be speculated that this effect does not play a part in enhancing CoTS outbreaks. An exception may be associated with the very large discharge events from the Mary and Burnett Rivers and CoTS outbreaks in the southern Capricorn Bunker Group reefs, but further research is required to confirm this connection.
Unfortunately, the direct measurement of chlorophyll a in the GBR lagoon is still limited by sample numbers and locations of sampling. Estimates of chlorophyll a concentrations can be made from water type analysis [44,70], and by using the eReefs model in conjunction with direct measurements [75]. However, a more intensive direct measurement program is still required to be able to answer questions regarding the influence of nutrient enrichment on populations of CoTS.
Acknowledgments
Author Contributions
Jon Brodie conceived the intent and structure of the paper, while all authors contributed to writing the text.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Birkeland, C.; Lucas, J.S. Acanthaster planci: Major Management Problem of Coral Reefs; CRC Press, Inc.: Boca Raton, FL, USA, 1990. [Google Scholar]
- Moran, P.J. The Acanthaster phenomenon. Oceanogr. Mar. Biol. Ann. Rev. 1986, 24, 379–480. [Google Scholar]
- Zann, L.; Brodie, J.; Berryman, C.; Naqasima, M. Recruitment, ecology, growth and behavior of juvenile Acanthaster planci (L.)(Echinodermata: Asteroidea). Bull. Mar. Sci. 1987, 41, 561–575. [Google Scholar]
- Zann, L.; Brodie, J.; Vuki, V. History and dynamics of the crown-of-thorns starfish Acanthaster planci (L.) in the Suva area, Fiji. Coral Reef. 1990, 9, 135–144. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Caballes, C.F.; Rivera-Posada, J.A.; Sweatman, H.P.A. Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). Oceanogr. Mar. Biol. Ann. Rev. 2014, 52, 133–200. [Google Scholar]
- Brodie, J.E. Enhancement of larval and juvenile survival and recruitment in Acanthaster planci from the effects of terrestrial runoff: A review. Aust. J. Mar. Freshw. Res. 1992, 43, 539–554. [Google Scholar] [CrossRef]
- Caballes, C.F.; Pratchett, M.S. Reproductive biology and early life history of the crown-of-thorns starfish. In Echinoderms: Ecology, Habitats and Reproductive Biology; Eric, W., Ed.; Nova Science Publishers: New York, NY, USA, 2014; pp. 101–146. [Google Scholar]
- Sweatman, H.P.A. A field study of fish predation on juvenile crown of- thorns starfish. Coral Reefs 1995, 14, 47–53. [Google Scholar] [CrossRef]
- Mendonça, V.M.; Al Jabri, M.M.; Al Ajmi, I.; Al Muharrami, M.; Al Areimi, M.; Al Aghbari, H.A. Starfish Acanthaster planci in the Northwestern Indian Ocean: Are they really a consequence of unsustainable starfish predator removal through overfishing in coral reefs, or a response to a changing environment? Zool. Stud. 2010, 49, 108–123. [Google Scholar]
- Lucas, J.S. Quantitative studies of feeding and nutrition during larval development of the coral reef asteroid Acanthaster planci (L.). J. Exp. Mar. Biol. Ecol. 1982, 65, 173–193. [Google Scholar] [CrossRef]
- Brodie, J.; Fabricius, K.; De’ath, G.; Okaji, K. Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar. Pollut. Bull. 2005, 51, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, K.E.; Okaji, K.; De’ath, G. Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 2010, 29, 593–605. [Google Scholar] [CrossRef]
- Potts, D.C. Crown-of-thorns starfish—Man-induced pest or natural phenomenon. In the Ecology of Pests; Commonwealth Scientific and Industrial Research Organization: Melbourne, Australia, 1981; pp. 55–86. [Google Scholar]
- Chesher, R.H. Destruction of the Pacific Corals by the Sea Star Acanthaster planci. Science 1969, 165, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.E. Chemical pollution in the sea and the crown-of-thorns starfish (Acanthaster planci). Biotropica 1972, 4, 132–144. [Google Scholar] [CrossRef]
- Wooldridge, S.; Brodie, J. Environmental triggers for primary outbreaks of crown-of-thorns starfish on the Great Barrier Reef, Australia. Mar. Pollut. Bull. 2015, 101, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Hock, K.; Wolff, N.H.; Condie, S.A.; Anthony, K.; Mumby, P.J. Connectivity networks reveal the risks of crown-of-thorns starfish outbreaks on the Great Barrier Reef. J. Appl. Ecol. 2014, 51, 1188–1196. [Google Scholar] [CrossRef]
- Uthicke, S.; Doyle, J.; Duggan, S.; Yasuda, N.; McKinnon, A.D. Outbreak of coral-eating Crown-of-Thorns creates continuous cloud of larvae over 320 km of the Great Barrier Reef. Sci. Rep. 2015, 5, 16885. [Google Scholar] [CrossRef] [PubMed]
- Keesing, J.K.; Halford, A.R. Importance of post settlement processes for the population dynamics of Acanthaster planci (L.). Mar. Freshw. Res. 1992, 43, 635–651. [Google Scholar] [CrossRef]
- Keesing, J.K.; Halford, A.R. Field measurement of survival rates of juvenile Acanthaster planci: Techniques and preliminary results. Mar. Ecol. Prog. Ser. 1992, 85, 107–114. [Google Scholar] [CrossRef]
- Keesing, J.K.; Wiedermeyer, W.L.; Okaji, K.; Halford, A.R.; Hall, K.C.; Cartwright, C.M. Mortality rates of juvenile starfish Acanthaster planci and Nardoa spp. measured on the Great Barrier Reef, Australia and in Okinawa, Japan. Oceanol. Acta 1996, 19, 441–448. [Google Scholar]
- Ayukai, T. Ingestion of ultraplankton by the planktonic larvae of the crown-of-thorns starfish Acanthaster planci. Biol. Bull. 1994, 186, 90–100. [Google Scholar] [CrossRef]
- Okaji, K.; Ayukai, T.; Lucas, J. Selective feeding by larvae of the crown-of-thorns starfish, Acanthaster planci (L.). Coral Reefs 1997, 16, 47–50. [Google Scholar] [CrossRef]
- Okaji, K.; Ayukai, T.; Lucas, J. Are Acanthaster planci larvae food limited in the Great Barrier Reef waters. In Proceedings of the 8th International Coral Reef Symposium, Panama, Panama, 24–29 June 1996.
- Cowan, Z.L.; Dworjanyn, S.A.; Caballes, C.F.; Pratchett, M.S. Predation on crown-of-thorns starfish larvae by damselfishes. Coral Reefs 2016, 35, 1253–1262. [Google Scholar] [CrossRef]
- Birkeland, C. Acanthaster in the cultures of high islands. Atoll Res. Bull. 1981, 255, 55–58. [Google Scholar]
- Birkeland, C. Terrestrial runoff as a cause of outbreaks of Acanthaster planci (Echinodermata: Asteroidea). Mar. Biol. 1982, 69, 175–185. [Google Scholar] [CrossRef]
- Brodie, J.E.; Devlin, M.; Haynes, D.; Waterhouse, J. Assessment of the eutrophication status of the Great Barrier Reef lagoon (Australia). Biogeochemistry 2011, 106, 281–302. [Google Scholar] [CrossRef]
- Mellin, C.; Lugrin, C.; Okaji, K.; Francis, D.S.; Uthicke, S. Selective feeding and microalgal consumption rates by Crown-of-Thorns Seastar (Acanthaster cf. solaris) larvae. Diversity 2017, 9, 8. [Google Scholar] [CrossRef]
- Henderson, J.A.; Lucas, J.S. Larval development and metamorphosis of Acanthaster planci (Asteroidea). Nature 1971, 232, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.S. Reproductive and larval biology of Acanthaster planci (L.) in Great Barrier Reef waters. Micronesica 1973, 9, 197–203. [Google Scholar]
- Lucas, J.S. Growth, maturation and effects of diet in Acanthaster planci (L.)(Asteroidea) and hybrids reared in the laboratory. J. Exp. Mar. Biol. Ecol. 1984, 79, 129–147. [Google Scholar] [CrossRef]
- Olson, R.R. In situ culturing as a test of the larval starvation hypothesis for the crown-of-thorns starfish, Acanthaster planci. Limnol. Oceanogr. 1987, 32, 895–904. [Google Scholar] [CrossRef]
- Olson, R.R.; Olson, M.H. Food limitation of planktotrophic marine invertebrate larvae: Does it control recruitment success? Ann. Rev. Ecol. Syst. 1989, 20, 225–247. [Google Scholar] [CrossRef]
- Ayukai, T.; Okaji, K.; Lucas, J.S. Food limitation in the growth and development of crown-of-thorns starfish in the Great Barrier Reef. In Proceedings of the 8th International Coral Reef Symposium, Panama, Panama, 24–29 June 1996; pp. 621–626.
- Fabricius, K.E. Factors determining the resilience of coral reefs to eutrophication: A review and conceptual model. In Coral Reefs: An Ecosystem in Transition; Dubinski, Z., Stambler, N., Eds.; Springer: New York, NY, USA, 2011; pp. 493–509. [Google Scholar]
- Uthicke, S.; Logan, M.; Liddy, M.; Francis, D.; Hardy, N.; Lamare, M. Climate change as an unexpected co-factor promoting coral eating seastar (Acanthaster planci) outbreaks. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.; Graba-Landry, A.; Dworjanyn, S.A.; Byrne, M. Larval starvation to satiation: Influence of nutrient regime on the success of Acanthaster planci. PLoS ONE 2015, 10, e0122010. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.; Graba-Landry, A.; Dworjanyn, S.A.; Byrne, M. Larval phenotypic plasticity in the boom-and-bust crown-of-thorns seastar, Acanthaster planci. Mar. Ecol. Prog. Ser. 2015, 539, 179–189. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Dworjanyn, S.; Mos, B.; Caballes, C.F.; Thompson, C.A.; Blowes, S. Larval Survivorship and Settlement of Crown-of-Thorns Starfish (Acanthaster cf. solaris) at Varying Algal Cell Densities. Diversity 2017, 9, 2. [Google Scholar] [CrossRef]
- Brodie, J.; De’ath, G.; Devlin, M.; Furnas, M.; Wright, M. Spatial and temporal patterns of near-surface chlorophyll a in the Great Barrier Reef lagoon. Mar. Freshw. Res. 2007, 58, 342–353. [Google Scholar] [CrossRef]
- Devlin, M.J.; Brodie, J. Terrestrial discharge into the Great Barrier Reef Lagoon: Nutrient behaviour in coastal waters. Mar. Pollut. Bull. 2005, 51, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Devlin, M.; Schaffelke, B. Spatial extent of riverine flood plumes and exposure of marine ecosystems in the Tully coastal region, Great Barrier Reef. Mar. Freshw. Res. 2009, 60, 1109–1122. [Google Scholar] [CrossRef]
- Devlin, M.J.; Da Silva, E.T.; Petus, C.; Wenger, A.; Zeh, D.; Tracey, D.; Álvarez-Romero, J.G.; Brodie, J. Combining in-situ water quality and remotely sensed data across spatial and temporal scales to measure variability in wet season chlorophyll-a: Great Barrier Reef lagoon (Queensland, Australia). Ecol. Process. 2013, 2, 1–22. [Google Scholar] [CrossRef]
- Schroeder, T.; Devlin, M.J.; Brando, V.E.; Dekker, A.G.; Brodie, J.E.; Clementson, L.A.; McKinna, L. Inter-annual variability of wet season freshwater plume extent into the Great Barrier Reef lagoon based on satellite coastal ocean colour observations. Mar. Pollut. Bull. 2012, 65, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, Z.T.; Wolanski, E.; Álvarez-Romero, J.G.; Lewis, S.E.; Brodie, J.E. Fine sediment and nutrient dynamics related to particle size and floc formation in a Burdekin River flood plume, Australia. Mar. Pollut. Bull. 2012, 65, 236–248. [Google Scholar] [CrossRef] [PubMed]
- De’ath, G.; Fabricius, K.E.; Sweatman, H.; Puotinen, M. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl. Acad. Sci. USA 2012, 109, 17995–17999. [Google Scholar] [CrossRef] [PubMed]
- Walbran, P.D.; Henderson, R.A.; Faithful, J.W.; Polach, H.A.; Sparks, R.J.; Wallace, G.; Lowe, D.C. Crown of thorns starfish outbreaks on the Great Barrier Reef: A geological perspective based upon the geological record. Coral Reefs 1989, 8, 67–78. [Google Scholar] [CrossRef]
- Hock, K.; Wolff, N.H.; Beeden, R.; Hoey, J.; Condie, S.A.; Anthony, K.; Possingham, H.P.; Mumby, P.I. Controlling range expansion in habitat networks by adaptively targeting source populations. Conserv. Biol. 2016, 30, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Sweatman, H.; Cheal, A.; Emslie, M.; Johns, K.; Jonker, M.; Osborne, K. Origins and implications of a primary crown-of-thorns starfish outbreak in the southern Great Barrier Reef. J. Mar. Biol. 2015, 2015, 809624. [Google Scholar] [CrossRef]
- Roche, R.C.; Pratchett, M.S.; Carr, P.; Turner, J.R.; Wagner, D.; Head, C.; Sheppard, C.R.C. Localized outbreaks of Acanthaster planci at an isolated and unpopulated reef atoll in the Chagos Archipelago. Mar. Biol. 2015, 162, 1695–1704. [Google Scholar] [CrossRef]
- Houk, P.; Raubani, J. Acanthaster planci outbreaks in Vanuatu coincide with ocean productivity, furthering trends throughout the Pacific Ocean. J. Oceanogr. 2010, 66, 435–438. [Google Scholar] [CrossRef]
- Houk, P.; Bograd, S.; van Woesik, R. The transition zone chlorophyll front can trigger Acanthaster planci outbreaks in the Pacific Ocean: Historical confirmation. J. Oceanogr. 2007, 63, 149–154. [Google Scholar] [CrossRef]
- Weeks, S.; Bakun, A.; Steinberg, C.; Brinkman, R.; Hoegh-Guldberg, O. The Capricorn Eddy: A prominent driver of the ecology and future of the southern Great Barrier Reef. Coral Reefs 2010, 29, 975–985. [Google Scholar] [CrossRef]
- Mao, Y.; Luick, J.L. Circulation in the southern Great Barrier Reef studied through an integration of multiple remote sensing and in situ measurements. J. Geophys. Res. 2014, 119, 1621–1643. [Google Scholar] [CrossRef]
- Condie, S.; Condie, R. Retention of plankton within ocean eddies. Glob. Ecol. Biogeogr. 2016, 25, 1264–1277. [Google Scholar] [CrossRef]
- Weeks, S.J.; Magno-Canto, M.M.; Jaine, F.R.A.; Brodie, J.; Richardson, A.J. Unique sequence of events triggers manta ray feeding frenzy in the southern Great Barrier Reef, Australia. Remote Sens. 2015, 7, 3138–3152. [Google Scholar] [CrossRef]
- Alongi, D.M.; Patten, N.L.; McKinnon, D.; Köstner, N.; Bourne, D.G.; Brinkman, R. Phytoplankton, bacterioplankton and virioplankton structure and function across the southern Great Barrier Reef shelf. J. Mar. Syst. 2015, 142, 25–39. [Google Scholar] [CrossRef]
- Da Silva, E.T.; Devlin, M.; Wenger, A.; Petus, C. Burnett-Mary Wet Season 2012–2013: Water Quality Data Sampling, Analysis and Comparison against Wet Season 2010–2011 Data.; Centre for Tropical Water & Aquatic Ecosystem Research (TropWATER) Publication: James Cook University, Townsville, Australia, 2013; p. 31. [Google Scholar]
- Brodie, J.E.; Mitchell, A.W. Nutrient composition of the January (1991) fitzroy river flood plume. In Workshop on the Impacts of Flooding; GBRMPA Workshop Series No. 17; Byron, G.T., Ed.; Great Barrier Reef Marine Park Authority: Townsville, Australia, 1992; pp. 56–74. [Google Scholar]
- Kroon, F.J.; Kuhnert, P.M.; Henderson, B.L.; Wilkinson, S.N.; Kinsey-Henderson, A.; Abbott, B.; Brodie, J.E.; Turner, R.D.R. River loads of suspended solids, nitrogen, phosphorus and herbicides delivered to the Great Barrier Reef lagoon. Mar. Pollut. Bull. 2012, 65, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, J.; Brodie, J.; Lewis, S.; Mitchell, A. Quantifying the sources of pollutants in the Great Barrier Reef catchments and the relative risk to reef ecosystems. Mar. Pollut. Bull. 2012, 65, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Furnas, M.; Mitchell, A.; Skuza, M.; Brodie, J. In the other 90%: Phytoplankton responses to enhanced nutrient availability in the Great Barrier Reef Lagoon. Mar. Pollut. Bull. 2005, 51, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Devlin, M.J.; McKinna, L.W.; Álvarez-Romero, J.G.; Petus, C.; Abott, B.; Harkness, P.; Brodie, J. Mapping the pollutants in surface riverine flood plume waters in the Great Barrier Reef, Australia. Mar. Pollut. Bull. 2012, 65, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Romero, J.G.; Devlin, M.; Teixeira da Silva, E.; Petus, C.; Ban, N.C.; Pressey, R.L.; Kool, J.; Roberts, J.J.; Cerdeira-Estrada, S.; Wenger, A.S.; et al. A novel approach to model exposure of coastal-marine ecosystems to riverine flood plumes based on remote sensing techniques. J. Environ. Manag. 2013, 119, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Blondeau-Patissier, D.; Schroeder, T.; Brando, V.E.; Maier, S.W.; Dekker, A.G.; Phinn, S. ESA-MERIS 10-year mission reveals contrasting phytoplankton bloom dynamics in two tropical regions of Northern Australia. Remote Sens. 2014, 6, 2963–2988. [Google Scholar] [CrossRef]
- Devlin, M.; Petus, C.; da Silva, E.; Tracey, D.; Wolff, N.; Waterhouse, J.; Brodie, J. Water quality and river plume monitoring in the Great Barrier Reef: An overview of methods based on ocean colour satellite data. Remote Sens. 2015, 7, 12909–12941. [Google Scholar] [CrossRef]
- Devlin, M.; Waterhouse, J.; Taylor, J.; Brodie, J. Flood Plumes in the Great Barrier Reef: Spatial and Temporal Patterns in Composition and Distribution; GBRMPA Research Publication No. 68; Great Barrier Reef Marine Park Authority: Townsville, Australia, 2001. [Google Scholar]
- Petus, C.; Collier, C.; Devlin, M.; Rasheed, M.; McKenna, S. Using MODIS data for understanding changes in seagrass meadow health: A case study in the Great Barrier Reef (Australia). Mar. Environ. Res. 2014, 98, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Petus, C.; Devlin, M.; Thompson, A.; McKenzie, L.; Teixeira da Silva, E.; Collier, C.; Tracey, D.; Martin, K. Estimating the Exposure of Coral Reefs and Seagrass Meadows to Land-Sourced Contaminants in River Flood Plumes of the Great Barrier Reef: Validating a Simple Satellite Risk Framework with Environmental Data. Remote Sens. 2016, 8, 210. [Google Scholar] [CrossRef]
- Waterhouse, J.; Brodie, J.; Petus, C.; Devlin, M.; da Silva, E.; Maynard, J.; Heron, S.; Tracey, D. Recent Findings of an Assessment of Remote Sensing Data for Water Quality Measurement in the Great Barrier Reef: Supporting Information for the GBR Water Quality Improvement Plans; Centre for Tropical Water & Aquatic Ecosystem Research (TropWATER) Publication: James Cook University, Townsville, Australia, 2015; p. 71. [Google Scholar]
- Wolfe, K.; Graba-Landry, A.; Dworjanyn, S.A.; Byrne, M. Superstars: Assessing nutrient thresholds for enhanced larval success of Acanthaster planci, a review of the evidence. Mar. Pollut. Bull. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Caballes, C.F.; Pratchett, M.S.; Kerr, A.M.; Rivera-Posada, J.A. The role of maternal nutrition on oocyte size and quality, with respect to early larval development in the coral-eating starfish, Acanthaster planci. PLoS ONE 2016, 11, e0158007. [Google Scholar] [CrossRef] [PubMed]
- Babcock, R.C.; Dambacher, J.M.; Morello, E.B.; Plaganyi, É.E.; Hayes, K.R.; Sweatman, H.P.A.; Pratchett, M.S. Assessing Different Causes of Crown-of-Thorns Starfish Outbreaks and Appropriate Responses for Management on the Great Barrier Reef. PLoS ONE 2016, 11, e0169048. [Google Scholar] [CrossRef] [PubMed]
- Baird, M.E.; Cherukuru, N.; Jones, E.; Margvelashvili, N.; Mongin, M.; Oubelkheir, K.; Ralph, P.J.; Rizwi, F.; Robson, B.J.; Schroeder, T.; et al. Remote-sensing reflectance and true colour produced by a coupled hydrodynamic, optical, sediment, biogeochemical model of the Great Barrier Reef, Australia: Comparison with satellite data. Environ. Model. Softw. 2016, 78, 79–96. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).