Abstract
Epiphytic corticolous lichens are vital components of forest ecosystems, yet their species composition and distribution patterns along altitudinal gradients in the Populus tremula forests of Barluk Mountain National Nature Reserve, Xinjiang, China, remain understudied. This study analyzes the diversity and distribution of epiphytic corticolous lichens in these forests along an altitudinal gradient. Field research was conducted at six sites (940–1450 m) from June to July 2023–2024, with samples collected from 576 quadrats on 48 Populus tremula trees. Lichen identification involved morphological, anatomical, and chemical analyses. Data on cover and frequency were analyzed to calculate importance values (IV), diversity indices, and floristic similarity (Sørensen’s index). NMDS and TWINSPAN were used to explore distribution patterns along the altitude gradient. In total, 28 epiphytic lichen species were identified, with diversity indices peaking at 1040 m. Dominant species exhibited significant variations in IV across altitudes (P < 0.05), and NMDS/TWINSPAN revealed distinct community clustering associated with elevation. Sørensen’s index indicated a low similarity (<30%) between the highest and lowest altitude sites. This study provides a baseline for biodiversity conservation and forest management in arid and semi-arid land mountain ecosystems.
1. Introduction
Lichens, a symbiotic association of fungi and algae or cyanobacteria, are important components of ecosystems [1]. In many forest environments, lichens are common and could contribute significantly to the overall biodiversity [2,3]. There is abundant evidence that epiphytic lichens play a crucial role in ecology as food for a variety of invertebrates, including oribatid mites, springtails, and slugs, providing microhabitats and resources for successional species [4]. They also alter canopy water regimes, impact nitrogen cycling, and boost structural complexity [5]. Epiphytic corticolous lichens are components of epiphyte communities, contributing to nutrient cycling (e.g., fixing atmospheric nitrogen), regulating microclimates on bark surfaces, and serving as sensitive bio-indicators of air quality and forest integrity [6,7,8]. Because of the influence of microhabitat characteristics like diameter, height, and architecture, epiphyte lichen composition varies within forests [9]. Rapid changes in forest structure lead to changing microclimates, which have a significant impact on the epiphytic lichen flora [10,11].
Geographical and topographical conditions, forest protection measures, host species, and the spatial distribution patterns of the trees can all significantly affect the species diversity, composition, biomass, and distribution of epiphytic lichen communities [12,13]. Their survival is tightly linked to environmental conditions, as their poikilohydric, epiphytic lifestyle makes them dependent on external resources such as light, moisture, and substrate chemistry. These factors are further affected by topographic gradients, particularly elevation [14,15,16].
Among environmental gradients, altitudinal gradients are among the most suitable model templates for exploring the relationship between biodiversity and climate [17,18]. With increasing elevation, predictable changes occur in temperature, precipitation patterns, solar radiation, and vegetation structure [19,20]. These changes, in turn, modify the availability of suitable microhabitats for epiphytic corticolous lichens. For instance, lower temperatures at high altitudes may slow metabolic rates, while increased moisture in cloud forests can favor hygrophilic species [21,22]. Additionally, altitude induces shifts in tree species composition that alter substrate properties (e.g., bark pH, texture, and nutrient content), which further affect lichen communities [23,24].
Barluk Mountain in Xinjiang, China, stands out for its high biodiversity, including lichens [25,26]. It is also an increasingly important destination for tourism with significant conservation challenges. Therefore, the People’s Government of Xinjiang Uygur Autonomous Region, China, approved the establishment of the Xinjiang Wild Almond Nature Reserve in April 1980; this was expanded and renamed the Xinjiang Barluk Mountain National Nature Reserve on 18 January 2005 [25]. Subsequently studies have explored the lichen biodiversity and floristic characteristics of the Barluk Mountain National Nature Reserve (BMNNR) [27,28], including crustose and ground-dwelling macro-lichens, their ecological indicator values, and the distribution characteristics of epiphytic lichens on Populus tremula trunks [29,30,31]. However, the species composition and altitudinal distribution patterns of epiphytic corticolous lichen in the Barluk Mountain National Nature Reserve have not been studied.
The present study investigates the distribution of epiphytic corticolous lichen communities across a continuous elevation gradient in the montane forests of the Barluk Mountain National Nature Reserve (BMNNR). Specifically, the study aims to (1) quantify changes in epiphytic corticolous lichen species composition, richness, and abundance in European aspen (Populus tremula L.) forest along the altitudinal gradient; and (2) test whether lichen functional traits (e.g., growth form, photobiont type) exhibit predictable responses to altitude, reflecting adaptation to changing microconditions.
By addressing these objectives, this research will advance our understanding of how altitudinal gradients structure epiphytic lichen communities, and may provide early warning signs regarding the loss of forest biodiversity and ecosystem functions as a result of climate change. The findings will also fill a regional knowledge gap in the Barluk Mountain and complement regional efforts to map and protect epiphyte diversity.
2. Materials and Methods
2.1. Study Area
The BMNNR is located in the Barluk Mountains (Figure 1). The average annual temperature is 6.2 °C, with extreme maximum and minimum temperatures of 38.6 °C and −35.9 °C, respectively. The annual accumulated temperature ≥ 10.0 °C is 2869 °C, the annual precipitation is 289.2 mm, and the annual evaporation is 1882.7 mm [25]. Vegetation varies with altitude and climate types; the subalpine zone is above 2300 m, the mid-mountain zone is at 1400–2300 m, the low-mountain zone is at 900–1400 m, and the piedmont plain area at 500–900 m has a temperate continental arid climate [32,33].
Figure 1.
Sampling sites at Barluk Mountain National Nature Reserve in Xinjiang, China. (a) People’s Republic of China; (b)Xinjiang Uygur Autonomous region;(c) Sampling sites in Barluk Mountain National Nature Reserve.
2.2. Sampling Design
Six sites were selected, matching the requirement of containing Populus tremula-dominated forests within the whole elevation range of this forest type (940–1450 m a.s.l). The site selection spanned the entire regional rainfall gradient (c. 280–350 mm/year) (Table 1). At each site, we attempted to minimize potentially confounding effects of within-site variation by sampling a single slope and habitat type per site. Canopy openness was visually estimated in each sampling site. During sampling, according to the species accumulation curve calculated to determine sampling efforts, the lichen samples were taken from eight Populus tremula trees (DBH > 40 cm) that were 1.5 to 2 m close to each other (basically straight) [34], and each trunk was sampled at 100 cm–130 cm intervals using a 20 × 30 cm quadrat, divided into six subunits of 10 × 10 cm, which was placed on the north and south side for each trunk [35]. In total, lichen samples were collected from 576 sample units.

Table 1.
Information of sampling sites.
2.3. Species Identification and Lichen Functional Traits
Common lichen species were identified in the field using handheld magnifying glasses and color test methods. For those species that could not be identified in the field, samples were collected and taken to the laboratory for identification based on a study of their morphology, anatomy, and chemistry [26,36] and on regional keys [26], and also the keys of North America lichen [37], and the nomenclature generally follows the Index Fungorum (https://speciesfungorum.org/ accessed on 23 October 2025). We collected lichen information regarding three biological traits, thallus growth form, photobiont type, and reproductive strategy [26].
2.4. Data Analysis
Lichen samples were quantitatively analyzed for cover (Table A1), frequency, and importance, which was calculated as IV = (Relative frequency (RF) and relative cover (RC)/2) [35] (p. 2663), where RF = 100 × (frequency of species i/sum of frequency values of all species) and RC = 100 × (cover of species i/sum of cover values of all species) [35]. Epiphytic corticolous lichen diversity and evenness indices at different altitudes were also calculated using PAST version 3.17. Floristic similarities were calculated using Sørensen’s similarity index [38]. The relationship between the distribution of the epiphytic corticolous lichen and altitude was also explored. A total of 28 species occurring at more than one site were included in the statistical analysis. A data matrix of 28 species × 48 samples was used to examine the distribution and community structure of epiphytic corticolous lichens along the altitudinal gradient via Non-metric Multidimensional Scaling (NMDS) using PCORD 5 and multivariate classification techniques (Two-Way Indicator Species Analysis = TWINSPAN) [39]. Significant differences in importance values for species across altitudes were determined using one-way ANOVA with Bonferroni correction. All statistical analyses were performed using SPSS for Windows (Version 11.5) and assessed at a 95% confidence level.
3. Results
3.1. Lichen Species Composition
The 653 epiphytic corticolous lichen specimens collected from 48 trunks at 6 different altitudes in BMNNR included 28 species belonging to 14 genera and 7 families (Table A2). Among these lichens, 17 species (60.7%) were foliose, 8 species (28.5%) were crustose, and 3 species (10.7%) were fruticose. There are 2 dominant families, namely Physciaceae and Parmeliaceae, which are composed of 7 genera and 17 species, and account for 60.7% of the species. There were also 3 oligotypic families (2 ≤ species ≤ 5) consisting of 5 genera and 9 species (35.7% and 32.1% of the total number of genera and species, respectively). We detected a single species from eight genera, Bryoria Brodo & D. Hawksw., Flavoplaca Arup, Flavopunctelia (Krog) Hale, Lecidella Körb., Myriolecis Clem., Physconia Poelt, Ramalina Ach., and Usnea Dill. ex Adans., which account for 28.5% of the total number of species.
3.2. The Relationship Between Species Richness and Elevation
The richness of epiphytic corticolous lichens was the highest at an altitude of 1040 m, with 23 species, followed by 940 m, with 16 species, then 1350 m and 1150 m, with 14 and 12 species, respectively, and 1250 m, with 11 species. The richness at 1450 m was the lowest, with only five species. Only two species (7.1%) occurred across all altitudes, and five species (17.8%) were rare and occurred at only two different altitudes (Table A3).
Species richness and evenness were highest at lower altitudes, regardless of the metric examined (Table 2).

Table 2.
Diversity index for epiphytic corticolous lichens on the trunks of Populus tremula at six altitudes.
Sørensen’s similarities between altitudes ranged from 0.222 to 0.413, with an average of 0.320. The highest similarity (0.424) was observed between 1040 m and 1150 m; since environmental conditions are relatively similar between adjacent altitudes, the species turnover rate is relatively low, and there are more similar species, whereas the lowest level (0.222) was between 940 m and 1450 m (Table 3). As the altitude rises from 940 to 1450 m, the environment undergoes changes, resulting in a significant turnover of lichen species and a subsequent decrease in overall similarity.

Table 3.
The Sørensen’s similarities between six altitudes.
3.3. The Relationship Between Lichen Functional Traits and Elevation
The foliose and crustose growth forms showed a bimodal pattern with elevation. Foliose and crustose species had the highest values of 14 and 7 species, respectively, at 1040 m (Figure 2). Due to the extremely small number of fruticose lichens, it is impossible to analyze their altitudinal distribution characteristics.
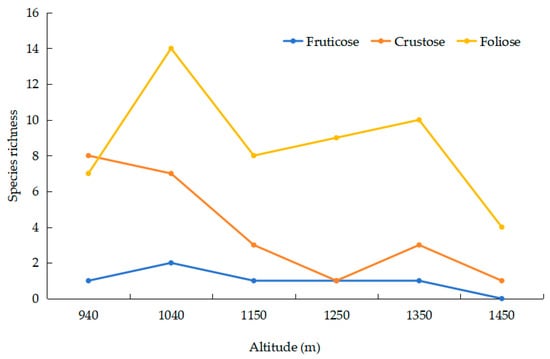
Figure 2.
Relationship between elevation and lichens growth form.
The analysis of biological traits showed that the main reproductive strategy was by vegetative propagules (9 sorediates and 6 isidiates), followed by sexual reproduction by ascospores (12 species) and thallus fragmentation (1 species). The species richness of sexually reproducing species shows a unimodal model along the elevation gradient. The richness reaches its maximum of 11 at an elevation of 1040 m and then decreases with increasing elevation. In contrast, the species richness of asexually reproducing species exhibits a bimodal model along the elevation gradient (Figure 3).
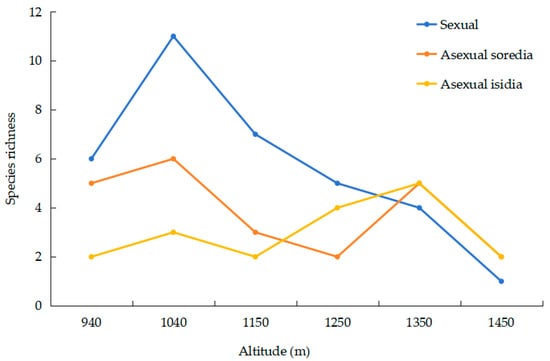
Figure 3.
Relationship between elevation and reproductive types.
Regarding photobionts, the species of chlorolichens shows a trend of first increasing, reaching a maximum at 1040 m with 22 species, then decreasing as altitude rises. Because there are only two cyanolichen species, the cyanolichen did not show a meaningful correlation with an altitudinal gradient (Figure 4).
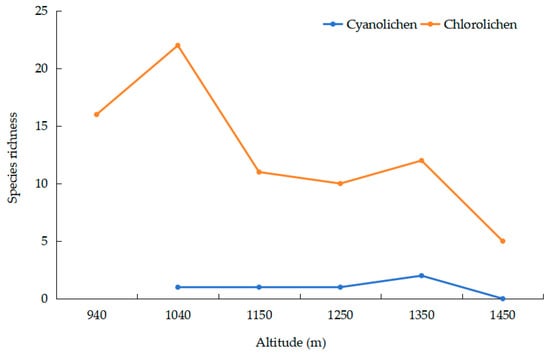
Figure 4.
Relationship between elevation and photobiont.
3.4. Distribution of Epiphytic Corticolous Lichens
The most common species were Physcia stellaris and P. dimidiata, which occurred at all sampling sites. Lecanora chlarotera, Melanohalea exasperatula, and Physcia tribacia were shown to occur up to 1250 m. Candelariella xanthostigma, Collema oleifera, C. subconveniens, Flavopunctelia flaventior, Lecanora xylophila, Phaeophyscia sp., and P. limbata were shown to occur up to 1350 m. Collema subflaccidum, Physcia dubia, Physconia grisea, and Ramalina sinensis occurred between altitudes 1250 m to 1350 m. The distributions of some species, such as Melanohalea sp., Phaeophyscia sp., and P. limbate, were not significantly different between altitudes. Physcia tribacia had a high importance value at medium altitude of 1040 m to 1250 m (Table A4).
The NMDS ordination of lichen samples and species is shown in Figure 5.
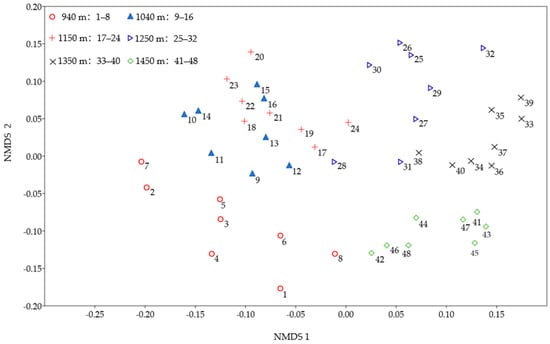
Figure 5.
NMDS ordination of 48 sample trees at 6 different altitudes related to importance value of lichens.
The NMDS ordination reveals differences in the epiphytic corticolous lichen species distributed at low versus high altitudes; species from low altitudes (e.g., 940 m) are located in the lower left of the ordination diagram, while those from 1040 m and 1150 m are in the upper left; species from 1250 m and 1350 m are in the upper right, and those from 1450 m are in the lower right.
TWINSPAN was used to group the samples (Figure 6).
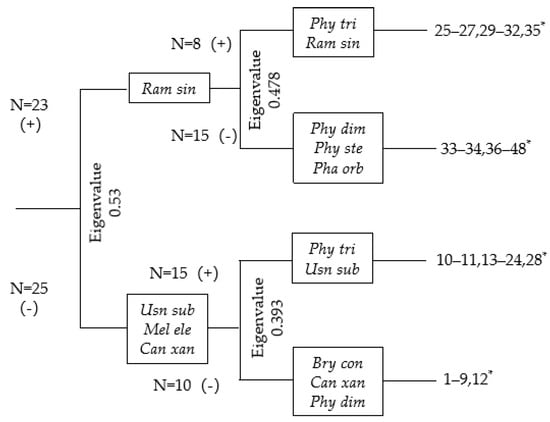
Figure 6.
TWINSPAN dendrogram of 48 samples at 6 different altitudes. *: Number of samples at each altitude: 940 m = 1–8, 1040 m = 9–16, 1150 m = 17–24, 1250 m = 25–32, 1350 m = 33–40, and 1450 m = 41–48. For abbreviations of species names, see Table A2.
The cut levels in the TWINSPAN analysis were set at the importance value percentages of 0, 3, 7, 13, and 20 [35]. At the first level, the samples were divided into two clusters. The first cluster comprises 23 samples from 1250 m to 1450 m, with Ramalina sinensis as the indicator species; the second cluster includes samples from 940 m to 1150 m, with indicator species Usnea subfloridana, Melanohalea elengantula, and Candelariella xanthostigma. At the second level, the first cluster was divided into two groups, with an Eigenvalue of 0.478. Group one consists of the samples from 1250 m, with indicator species Physcia tribacia and Ramalina sinensis. Group two includes samples from 1350 m, with indicator species Physcia dimidiata, P. stellaris, and Phaeophyscia orbicularis. The second cluster from the first level was divided into two groups, with an Eigenvalue of 0.393. The first group comprised samples from 1040 m and 1150 m, with indicator species Physcia tribacia and Usnea subfloridana. The second group consists of 10 samples from 940 m, with indicator species Bryoria confusa, Candelariella xanthostigma, and Physcia dimidiata.
4. Discussion
Studies on the altitudinal gradient distribution patterns of epiphytic lichen diversity in managed spruce forests have shown that lichen species richness showed a linear positive relationship with elevation, and, along the whole elevational gradient, species richness was significantly explained by temperature, with a negative nonlinear effect resulting in a peak close to the lower end of the temperature gradient [17]. However, the nonlinear relationship between lichen diversity, trait composition, and temperature indicated that the response of lichen species richness to temperature was relatively non-intense up to a certain level and then became very strong, i.e., even a slight temperature increase resulted in a reduction in species richness. This relationship may imply that, under climate warming scenarios, major changes could be expected in the intermediate part of the elevational gradient [17].
The canopy openness, a proxy for the combination of light, humidity and temperature, proved to be the most important factor affecting epiphytic lichen distribution [7]. The species composition of epiphytic lichens growing on Fagus orientalis changed with altitude at Uludağ Mountain, Turkey, with nine species occurring from 900 m to 1200 m, while only four species occurred from 1300 m to 1400 m [35]. Similarly, our study found that the species diversity of epiphytic corticolous lichens on Populus tremula was higher at intermediate altitudes than at higher altitudes in the BMNNR. Temperature was the main drive of lichen diversity [17]. The negative relationship with species richness reflects the negative effects of increasing temperature on key eco-physiological processes, such as an increase in respiratory carbon loss [40], that may limit the distribution of many lichen species. It further indicates that temperatures in the intermediate elevation zone are relatively moderate, which is suitable for the survival of various lichen species.
Biological traits strongly influenced lichen species in the community. In particular, thallus growth form and reproduction type were the most important traits influencing both the lichens’ occupancy capability and role in the network [18]. Concerning the corticolous lichens, the growth forms are closely related to forest types and host tree characteristics. For example, some of the foliose and fruticose growth forms are more distributed in open habitats than in dense overgrowing stands, with foliose lichens requiring high light levels [41], and the crustose lichens have a lower surface-to-volume ratio, resulting in a higher tolerance to desiccation, water loss being restricted to the upper exposed surface [42]. Diversity and distribution of lichen in relation to altitude within a protected biodiversity hot spot and a high number of foliose species at intermediate altitudes and the absence of fruticose lichens at lower altitudes in north-east India [43]. Our result indicates that, among the corticolous lichens, 17 species (60.7% of total species) were foliose and 8 were crustose species (28.5%), but only 3 were fruticose species (10.7%). The European aspen zone of the BMNNR forms an open forest form at lower altitudes, leaving us to suggest that arid conditions and intense solar radiation are conducive to the growth of corticolous lichens and most of the foliose lichen distribute at intermediate altitudes.
Lichens may reproduce both sexually and asexually, and this influences the capability of the species to disperse and establish [44]. Asexual reproduction allows for effective local recruitment since the two symbionts (the mycobiont and the photobiont) are simultaneously dispersed, thus avoiding the uncertainty of reestablishing the lichen symbiosis, as in the case of sexually dispersed species whose spores have to meet a new photobiont [18]. This situation is particularly favorable in forest ecosystems, where the vitality of vegetative propagules is not hampered by harsh conditions [45]. Our results indicate that the main reproductive strategy of corticolous lichens in the BRMNNR Nature Reserve is also via vegetative propagules, which represents a form of asexual reproduction, which is consistent with the findings of Saiz et al. [18].
Concerning the photobiont, cyanolichens (those lichens with a cyanobacterial photobiont) have greater hydration requirements than chlorolichens (with chlorophyte algae) and cephalolichens (with both algae and cyanobacteria) [1] (pp. 1). Consequently, cyanolichens are particularly sensitive to changes in the microclimate, such as those that result from forest harvesting [46]. Our result indicates that, in the BMNNR, chlorolichens are dominant and widely distributed across different elevations, which further reflects lichens’ adaptability to the changes in environment factors at different elevations. Climate parameters such as temperature, rainfall, and humidity are known to be closely related to altitude [47]. There is a positive relationship between lichen biomass and location humidity [48]. So, the change in species composition of the epiphytic lichen communities along the elevation gradient might reflect the different ecological condition of the region besides the forest nature and age of the tree [35].
In forest ecosystems, the richness of epiphytic lichens on south-facing vs. north-facing trunk surfaces of trees is primarily determined by differences in micro-environmental conditions—especially light, temperature, and moisture. In most forests, the epiphytic lichen richness of north-facing tree trunks is affected by the lower light intensity, cooler temperatures, and greater moisture availability [49]. A recent study reported that, in the BMNNR, the distribution of epiphytic lichens is affected by factors such as altitude and aspect [31]. In this study, the result shows that, in the BMNNR, both the number of epiphytic lichen species and the total relative coverage on the north-facing trunks of European aspens were significantly higher than those on the south-facing trunks.
Therefore, we speculate that, due to the differences in altitude and slope aspect in this reserve, the light intensity, temperature, and evaporation rate on the south-facing trunks of European aspens are all higher than those on the north-facing ones, which are unfavorable for the growth of epiphytic lichens.
5. Conclusions
In conclusion, the epiphytic corticolous lichen community in the BMNNR is structured by altitude, with mid-altitudes (1040 m) supporting maximum diversity due to moderate environmental conditions. This is closely related to the canopy openness because altitude light, humidity, and temperature changed with canopy openness, which is one of most important factors affecting epiphytic lichen distribution. A taxonomic dominance of Physciaceae and Parmeliaceae and dominance of foliose growth forms and chlorophotobionts are found at mid-altitude peaks. Ordination/clustering confirm a clear altitudinal partitioning. These findings highlight the importance of preserving mid-altitude forest habitats in the BMNNR to maintain lichen diversity.
The BMNNR is located in an area with significant ecosystem diversity and heterogeneity, comprising deserts, mountains, and wetlands. Populus tremula is widely distributed in the reserve’s forest ecosystem, providing important habitats for epiphytic corticolous lichens. Therefore, scientifically managing the European aspen in the reserve and appropriately expanding the distribution of Populus tremula in the mid-altitude would be of great value for enhancing the diversity of epiphytic corticolous lichens.
Author Contributions
N.A.: Field survey, methodology, writing—original draft; R.M.: Field survey, species identification of macro-lichens, data analysis; D.T.: Field survey, species identification of micro-lichens; M.S.I.: Formal analysis, review and editing. A.T.: Conceptualization, funding acquisition, project administration, formal analysis, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the National Nature Science Foundation of China (grant number 32160046).
Institutional Review Board Statement
This study did not require ethical approval.
Data Availability Statement
Data are contained within article.
Acknowledgments
We are grateful to David H. S. Richardson (Environmental Science, Saint Mary’s University, Canada) for many additional helpful suggestion and comments, and thanks to Jinsiguli Bahenuer and Yong Hai Ying (graduate students of Xinjiang University, P.R., China) for their help with the fieldwork and to the Administration Office of Barluk Mountain National Nature Reserve for permission to undertake the field survey.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BMNNR | Barluk Mountain National Nature Reserve |
Appendix A

Table A1.
Coverage of 28 epiphytic corticolous lichens in 48 sampling plots.
Table A1.
Coverage of 28 epiphytic corticolous lichens in 48 sampling plots.
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | P14 | P15 | P16 | P17 | P18 | P19 | P20 | P21 | P22 | P23 | P24 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bry con | 2.12 | 0.00 | 0.36 | 1.28 | 0.00 | 0.00 | 0.86 | 0.00 | 0.00 | 3.21 | 1.24 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Can ole | 0.00 | 2.35 | 0.89 | 0.00 | 0.00 | 0.00 | 0.40 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Can xan | 0.00 | 0.23 | 0.21 | 0.52 | 3.54 | 0.00 | 2.89 | 0.00 | 0.00 | 0.54 | 1.25 | 0.00 | 0.65 | 5.23 | 0.00 | 0.00 | 2.03 | 1.28 | 0.58 | 0.00 | 0.62 | 1.11 | 2.65 | 0.00 |
| Col sub | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.20 | 0.00 | 0.26 | 0.36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.87 | 0.00 | 0.00 | 0.25 | 0.41 | 0.21 | 0.00 | 0.35 |
| Col subf | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fla cor | 1.21 | 0.00 | 0.98 | 0.00 | 1.38 | 1.26 | 0.00 | 0.66 | 0.20 | 0.00 | 0.33 | 0.70 | 0.00 | 0.00 | 0.00 | 0.25 | 0.00 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fla fla | 0.00 | 2.00 | 0.00 | 1.02 | 1.30 | 0.36 | 0.00 | 0.03 | 0.35 | 0.00 | 0.48 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Lec chl | 0.00 | 0.22 | 0.69 | 0.00 | 0.00 | 0.00 | 0.28 | 0.00 | 0.37 | 0.36 | 1.12 | 0.00 | 0.17 | 0.00 | 0.29 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Lec sal | 0.00 | 0.00 | 0.00 | 0.65 | 0.00 | 0.72 | 0.25 | 0.97 | 5.52 | 0.11 | 1.04 | 0.28 | 0.00 | 2.42 | 0.75 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Lec xyl | 0.00 | 0.00 | 0.22 | 0.00 | 2.00 | 0.00 | 0.23 | 0.00 | 0.92 | 0.47 | 0.40 | 0.00 | 0.00 | 0.26 | 0.00 | 0.00 | 1.12 | 0.65 | 2.05 | 1.87 | 0.65 | 1.36 | 0.87 | 0.00 |
| Lec ela | 0.00 | 1.25 | 0.87 | 0.00 | 0.00 | 0.12 | 0.41 | 0.00 | 0.41 | 0.44 | 0.29 | 0.00 | 0.00 | 0.16 | 0.71 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Myr hag | 0.00 | 0.00 | 0.00 | 1.87 | 0.00 | 0.00 | 0.35 | 0.00 | 0.70 | 0.24 | 2.46 | 0.00 | 1.18 | 0.53 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mel exa | 0.00 | 0.28 | 0.00 | 0.00 | 0.14 | 0.44 | 0.35 | 0.00 | 2.26 | 0.00 | 0.00 | 0.22 | 0.18 | 0.13 | 0.00 | 0.00 | 0.00 | 0.36 | 2.39 | 1.12 | 1.47 | 0.00 | 0.45 | 1.39 |
| Mel ele | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.45 | 0.52 | 1.34 | 0.09 | 0.35 | 0.05 | 1.23 | 0.57 | 0.21 | 1.36 | 0.25 | 0.00 | 0.66 | 0.35 | 0.23 | 0.00 |
| Mel sub | 0.00 | 0.00 | 0.00 | 1.54 | 0.00 | 0.98 | 0.00 | 0.00 | 0.38 | 0.00 | 0.00 | 0.28 | 0.63 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mel sp | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.56 | 0.00 | 0.27 | 0.12 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 6.25 |
| Pha sp | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 | 0.00 | 0.00 | 0.44 | 1.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pha his | 0.00 | 0.00 | 0.00 | 0.58 | 0.00 | 1.24 | 0.00 | 0.00 | 0.37 | 0.00 | 0.32 | 2.51 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pha lim | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.80 | 0.00 | 0.00 | 0.00 | 0.41 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pha orb | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.49 | 0.00 | 0.00 | 2.16 | 0.87 | 0.00 | 2.98 | 0.00 | 0.25 | 0.00 | 0.52 | 0.00 | 0.36 | 0.00 | 0.00 | 0.47 |
| Phy aip | 0.00 | 1.24 | 0.00 | 0.00 | 6.31 | 0.00 | 0.00 | 0.00 | 0.65 | 0.00 | 0.37 | 1.23 | 0.25 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Phy dim | 0.65 | 0.00 | 0.00 | 1.35 | 1.36 | 0.68 | 0.00 | 1.14 | 0.88 | 0.00 | 0.38 | 0.78 | 0.00 | 0.00 | 0.00 | 0.15 | 1.75 | 1.37 | 0.00 | 0.00 | 0.64 | 2.3 | 0.00 | 0.00 |
| Phy dub | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Phy ste | 0.00 | 0.00 | 1.36 | 0.00 | 0.00 | 0.49 | 0.00 | 0.71 | 0.28 | 0.00 | 0.00 | 2.38 | 0.01 | 0.00 | 0.00 | 0.00 | 0.54 | 0.00 | 5.64 | 0.00 | 0.00 | 0.00 | 0.00 | 12.63 |
| Phy tri | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.10 | 2.35 | 0.25 | 0.98 | 1.36 | 2.06 | 2.25 | 0.25 | 2.58 | 0.69 | 5.24 | 2.36 | 1.58 | 0.65 | 3.36 |
| Phy gri | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ram sin | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Usn sub | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 | 2.35 | 1.32 | 0.25 | 0.78 | 1.26 | 0.98 | 2.25 | 3.25 | 0.54 | 1.65 | 0.68 | 2.35 | 4.25 | 3.28 | 1.14 |
| Bry con | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Can ole | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.2 | 1.49 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Can xan | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.64 | 0.00 | 1.53 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Col sub | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.77 | 11.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Col subf | 0.54 | 0.00 | 0.25 | 0.00 | 2.44 | 0.00 | 0.00 | 0.44 | 0.32 | 0.00 | 0.97 | 0.00 | 0.00 | 0.00 | 0.56 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fla cor | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fla fla | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.58 | 0.00 | 0.00 | 0.00 | 0.00 | 1.46 | 2.81 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Lec chl | 2.22 | 0.00 | 1.19 | 0.25 | 0.00 | 3.75 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Lec sal | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Lec xyl | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.35 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Lec ela | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Myr hag | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mel exa | 0.14 | 0.38 | 0.00 | 0.78 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mel ele | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mel sub | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mel sp | 8.2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pha sp | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pha his | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pha lim | 0.00 | 0.00 | 0.00 | 0.00 | 1.25 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.88 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pha orb | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.31 | 0.52 | 1.86 | 0.69 | 3.28 | 1.65 | 0.32 | 0.35 | 0.00 | 0.88 | 2.15 | 0.24 | 0.00 | 0.68 | 0.00 |
| Phy aip | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Phy dim | 0.00 | 0.00 | 1.75 | 1.23 | 0.00 | 0.56 | 0.8 | 0.00 | 0.00 | 0.00 | 1.18 | 0.00 | 0.00 | 0.12 | 0.00 | 0.00 | 0.00 | 0.16 | 0.00 | 7.92 | 0.00 | 1.22 | 0.00 | 0.05 |
| Phy dub | 2.03 | 0.00 | 0.69 | 0.00 | 2.52 | 0.00 | 0.00 | 2.49 | 0.79 | 0.00 | 1.45 | 3.01 | 0.47 | 0.87 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Phy ste | 0.00 | 0.00 | 3.25 | 0.87 | 1.25 | 0.00 | 1.47 | 0.00 | 0.05 | 0.56 | 0.00 | 0.87 | 1.25 | 0.65 | 0.00 | 3.27 | 6.32 | 8.54 | 10.2 | 0.68 | 3.25 | 5.32 | 0.25 | 6.35 |
| Phy tri | 3.25 | 2.02 | 1.65 | 6.38 | 2.38 | 0.58 | 4.25 | 6.91 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Phy gri | 0.02 | 0.25 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.18 | 0.70 | 0.24 | 2.46 | 0.00 | 1.18 | 0.53 | 0.02 | 3.21 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ram sin | 2.14 | 0.95 | 1.05 | 0.00 | 0.54 | 0.89 | 1.12 | 0.65 | 0 | 0.05 | 1.02 | 0.32 | 0.01 | 0.02 | 0.57 | 0.35 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Usn sub | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |

Table A2.
Species composition of epiphytic corticolous lichens on the trunks of Populus tremula in BMNNR.
Table A2.
Species composition of epiphytic corticolous lichens on the trunks of Populus tremula in BMNNR.
| Family | Genus | Species | Abbr. | Photobiont | Growth Form | Reproduction Strategy |
|---|---|---|---|---|---|---|
| Candelariaceae | Candelariella | C. oleifera H. Magn. | Can ole | Ch | Crustose | Sex |
| C. xanthostigma (Ach.) Lettau | Can xan | Ch | Crustose | Sex | ||
| Physciaceae | Physcia | P.aipolia (Ehrh. ex Humb.) Fürnr. | Phy aip | Ch | Foliose | Sex |
| P.dimidiata (Arnold) Nyl. | Phy dim | Ch | Foliose | A.s | ||
| P.dubia (Hoffm.) Lettau | Phy dub | Ch | Foliose | Sex | ||
| P.stellaris (Linn.) Nyl. | Phy ste | Ch | Foliose | Sex | ||
| P.tribacia (Ach.) Nyl. | Phy tri | Ch | Foliose | A.s | ||
| Phaeophyscia | Phaeophyscia sp. | Pha sp | Ch | Foliose | Sex | |
| P. hispidula (Ach.) Moberg | Pha his | Ch | Foliose | A.s | ||
| P. limbata (Poelt) Kashiw. | Pha lim | Ch | Foliose | A.s | ||
| P.orbicularis (Neck.) Moberg | Pha orb | Ch | Foliose | A.s | ||
| Physconia | P.grisea (Lam.) Poelt | Phys gri | Ch | Foliose | A.s | |
| Lecanoraceae | Lecanora | L.chlarotera Nyl. | Lec chl | Ch | Crustose | Sex |
| L. saligna (Schrad.) Zahlbr. | Lec sal | Ch | Crustose | Sex | ||
| L.xylophila Hue | Lec xyl | Ch | Crustose | Sex | ||
| Lecidella | L.elaeochroma (Ach.) M. Choisy | Lec ela | Ch | Crustose | Sex | |
| Myriolecis | M. hagenii (Ach.) Śliwa, Zhao Xin et Lumbsch | Mys hag | Ch | Crustose | Sex | |
| Parmeliaceae | Bryoria | B.confusa (D. D. Awasthi) Brodo & D. Hawksw. | Bry con | Ch | Fruticose | A.f |
| Flavopunctelia | F. flaventior (Stirt.) Hale | Fla fla | Ch | Foliose | A.s | |
| Melanohalea | M.exasperatula (Nyl.) Essl. | Mel exa | Ch | Foliose | A.i | |
| M.elegantula (Zahlbr.) O. Blanco et al. | Mel ele | Ch | Foliose | A.i | ||
| M. subelegantula (Essl.) O. Blanco et al. | Mel sub | Ch | Foliose | A.i | ||
| Melanohalae sp. | Mel sp | Ch | Foliose | A.i | ||
| Usnea | U. subfloridana Stirt. | Usn sub | Ch | Fruticose | A.s | |
| Ramalinaceae | Ramalina | R.sinensis Jatta | Ram sin | Ch | Fruticose | Sex |
| Collemataceae | Collema | C.subconveniens Nyl. | Col subc | Nos | Foliose | A.i |
| C.subflaccidum Degel. | Col sub | Nos | Foliose | A.i | ||
| Teloschistaceae | Flavoplaca | F. coronata (Kremp. ex Körb.) Arup | Fla cor | Ch | Crustose | Sex |
Notes: Photobiont: Ch, chlorococcoid green algae; Nos, Nostoc. Reproduction strategy: S, sexual by ascospores; A.s., asexual by soredia; A.i., asexual by isidia; A.f., asexual by thallus fragmentation.

Table A3.
Species occurrence and richness of corticolous lichen on the trunk of Populus tremula at different altitudes.
Table A3.
Species occurrence and richness of corticolous lichen on the trunk of Populus tremula at different altitudes.
| Name of Species | Altitude of Sampling Sites (m) | |||||
|---|---|---|---|---|---|---|
| 940 | 1040 | 1150 | 1250 | 1350 | 1450 | |
| Bryoria confusa | 6 | 2 | 0 | 0 | 0 | 0 |
| Candelariella oleifera | 8 | 0 | 0 | 0 | 4 | 0 |
| Candelariella xanthostigma | 12 | 10 | 14 | 0 | 6 | 0 |
| Collema subconveniens | 0 | 4 | 5 | 0 | 14 | 0 |
| Collema subflaccidum | 0 | 0 | 0 | 8 | 4 | 0 |
| Flavoplaca coronata | 8 | 4 | 1 | 0 | 0 | 0 |
| Flavopunctelia flaventior | 6 | 6 | 0 | 0 | 6 | 0 |
| Lecanora chlarotera | 5 | 8 | 0 | 14 | 0 | 0 |
| Lecanora saligna | 4 | 13 | 0 | 0 | 0 | 2 |
| Lecanora xylophila | 4 | 4 | 3 | 0 | 4 | 0 |
| Lecidella elaeochroma | 5 | 6 | 0 | 0 | 0 | 0 |
| Myriolecis hagenii | 2 | 8 | 0 | 0 | 0 | 0 |
| Melanohalea exasperatula | 8 | 6 | 12 | 7 | 0 | 0 |
| Melanohalea elegantula | 0 | 13 | 10 | 0 | 0 | 0 |
| Melanohalea subelegantula | 2 | 6 | 0 | 0 | 0 | 0 |
| Melanohalea sp. | 0 | 5 | 7 | 9 | 0 | 3 |
| Phaeophyscia sp. | 0 | 4 | 0 | 0 | 5 | 0 |
| Phaeophyscia hispidula | 2 | 7 | 0 | 0 | 0 | 0 |
| Phaeophyscia limbata | 0 | 2 | 0 | 2 | 2 | 0 |
| Phaeophyscia orbicularis | 0 | 16 | 4 | 0 | 19 | 14 |
| Physcia aipolia | 3 | 6 | 0 | 0 | 0 | 0 |
| Physcia dimidiata | 9 | 5 | 11 | 9 | 5 | 15 |
| Physcia dubia | 0 | 0 | 0 | 10 | 11 | 0 |
| Physcia stellaris | 6 | 5 | 5 | 5 | 5 | 5 |
| Physcia tribacia | 0 | 21 | 24 | 27 | 0 | 0 |
| Physconia grisea | 0 | 0 | 0 | 3 | 16 | 0 |
| Ramalina sinensis | 0 | 0 | 0 | 14 | 17 | 0 |
| Usnea subfloridana | 0 | 16 | 25 | 0 | 0 | 0 |
| Species richness | 16 | 23 | 12 | 11 | 14 | 5 |
| Number of collected specimens | 90 | 177 | 121 | 108 | 118 | 39 |

Table A4.
Important values of the corticolous lichen species along the altitudinal gradient on the trunk of Populus tremula.
Table A4.
Important values of the corticolous lichen species along the altitudinal gradient on the trunk of Populus tremula.
| Species | Altitude (m) | One-Way ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|
| 940 | 1040 | 1150 | 1250 | 1350 | 1450 | F | Sig. | |
| Bryoria confusa | 12.15 ± 6.73 | 4.87 ± 3.02 | 0.00 | 0.00 | 0.00 | 0.00 | 3.26 | 0.01 |
| Candelariella oleifera | 7.77 ± 4.91 | 0.00 | 0.00 | 0.00 | 1.63 ± 0.87 | 0.00 | 2.26 | 0.06 |
| Candelariella xanthostigma | 12.86 ± 5.14 | 7.75 ± 4.53 | 10.96 ± 3.99 | 0.00 | 3.95 ± 2.63 | 0.00 | 2.54 | 0.04 |
| Collema subconveniens | 0.00 | 1.56 ± 0.82 | 2.82 ± 0.94 | 0.00 | 10.76 ± 7.12 | 0.00 | 2.02 | 0.09 |
| Collema subflaccidum | 0.00 | 0.00 | 0.00 | 5.05 ± 2.61 | 4.81 ± 2.48 | 0.00 | 2.94 | 0.02 |
| Flavoplaca coronata | 12.67 ± 4.17 | 2.01 ± 0.87 | 0.48 ± 0.21 | 0.00 | 0.00 | 0.00 | 8.15 | 0.00 |
| Flavopunctelia flaventior | 9.27 ± 3.23 | 0.93 ± 0.45 | 0.00 | 0.00 | 7.93 ± 3.91 | 0.00 | 4.14 | 0.00 |
| Lecanora chlarotera | 3.54 ± 1.85 | 3.81 ± 1.33 | 0.00 | 11.43 ± 6.54 | 0.00 | 0.00 | 2.38 | 0.05 |
| Lecanora saligna | 7.17 ± 3.22 | 7.68 ± 3.06 | 0.00 | 0.00 | 0.00 | 1.48 ± 0.98 | 3.59 | 0.01 |
| Lecanora xylophila | 3.93 ± 2.47 | 2.25 ± 0.93 | 11.16 ± 2.62 | 0.00 | 0.46 ± 0.21 | 0.00 | 8.30 | 0.00 |
| Lecidella elaeochroma | 5.28 ± 2.51 | 3.12 ± 1.2 | 0.00 | 0.00 | 0.00 | 0.00 | 3.66 | 0.01 |
| Myriolecis hagenii | 5.01 ± 3.17 | 5.18 ± 2.07 | 0.00 | 0.00 | 0.00 | 0.00 | 5.54 | 0.00 |
| Melanohalea exasperatula | 5.25 ± 2.24 | 2.56 ± 1.27 | 7.71 ± 2.16 | 5.48 ± 3.24 | 0.00 | 0.00 | 2.72 | 0.03 |
| Melanohalea elegantula | 0.00 | 7.57 ± 2.22 | 5.9 ± 2.01 | 0.00 | 0.00 | 0.00 | 9.85 | 0.00 |
| Melanohalea subelegantula | 5.01 ± 2.11 | 2.31 ± 1.25 | 0.00 | 0.00 | 0.00 | 0.00 | 2.35 | 0.06 |
| Melanohalea sp. | 0.00 | 1.68 ± 0.68 | 2.93 ± 1.94 | 5.01 ± 1.24 | 0.00 | 3.09 ± 1.87 | 0.58 | 0.71 |
| Phaeophyscia sp. | 0.00 | 2.31 ± 1.61 | 0.00 | 0.00 | 2.66 ± 0.61 | 0.00 | 0.84 | 0.52 |
| Phaeophyscia hispidula | 4.77 ± 2.32 | 2.87 ± 2.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.58 | 0.18 |
| Phaeophyscia limbata | 0.00 | 1.12 ± 0.76 | 0.00 | 1.53 ± 0.58 | 1.50 ± 0.58 | 0.00 | 0.46 | 0.80 |
| Phaeophyscia orbicularis | 0.00 | 8.93 ± 4.01 | 1.96 ± 0.78 | 0.00 | 16.81 ± 4.25 | 16.99 ± 7.36 | 4.14 | 0.00 |
| Physcia aipolia | 8.87 ± 3.87 | 2.81 ± 1.25 | 0.00 | 0.00 | 0.00 | 0.00 | 3.16 | 0.02 |
| Physcia dimidiata | 13.26 ± 4.17 | 2.69 ± 1.17 | 7.80 ± 2.91 | 7.58 ± 2.09 | 2.73 ± 1.21 | 13.50 ± 7.82 | 1.35 | 0.26 |
| Physcia dubia | 0.00 | 0.00 | 0.00 | 7.82 ± 3.49 | 12.75 ± 4.86 | 0.00 | 4.65 | 0.00 |
| Physcia stellaris | 7.58 ± 3.79 | 2.37 ± 1.96 | 11.02 ± 6.47 | 8.40 ± 3.55 | 10.26 ± 3.61 | 64.91 ± 9.74 | 18.52 | 0.00 |
| Physcia tribacia | 0.00 | 16.01 ± 3.82 | 17.86 ± 4.82 | 33.51 ± 7.19 | 0.00 | 0.00 | 13.47 | 0.00 |
| Physconia grisea | 0.00 | 0.00 | 0.00 | 2.17 ± 1.32 | 14.24 ± 3.76 | 0.00 | 10.29 | 0.00 |
| Ramalina sinensis | 0.00 | 0.00 | 0.00 | 12.30 ± 2.54 | 10.48 ± 3.71 | 0.00 | 8.65 | 0.00 |
| Usnea subfloridana | 0.00 | 13.01 ± 3.81 | 19.33 ± 4.34 | 0.00 | 0.00 | 0.00 | 14.76 | 0.00 |
References
- Rinas, C.R.; Haughian, S.R.; Harper, K.A. Diversity, composition, and gastropod grazing of epiphytic lichen communities of forested wetlands at clearcut and intact edges in Nova Scotia, Canada. For. Ecol. Manag. 2025, 595, 123045. [Google Scholar] [CrossRef]
- Faisal, S.; Iqbal, Z.; Shah, G.M.; Haq, F. Diversity, distribution, and environmental influences on epiphytic lichens in the Hazara Division, Khyber Pakhtunkhwa. Ecol. Fron. 2025, 46, 122–134. [Google Scholar] [CrossRef]
- Moller, T.; Kaufmann, S.; Hauck, M. Tree, stand, and landscape scale effect on epiphytic lichen and bryophyte diversity in temperate mountain forests. For. Ecol. Manag. 2025, 594, 122967. [Google Scholar] [CrossRef]
- James, W.H.; Patricia, L.H. The Macrolichens of New England; The New York Botanical Garden Press: New York, NY, USA, 2007; pp. 17–21. [Google Scholar]
- Galloway, D.J. Biodiversity: A lichenological perspective. Biodivers. Conserv. 1992, 1, 312–323. [Google Scholar] [CrossRef]
- Ellis, C.J. Lichen epiphyte diversity: A species, community and trait-based review. Perspect. Plant Ecol. 2012, 14, 131–152. [Google Scholar] [CrossRef]
- Li, S.; Liu, W.Y.; Li, D.W. Bole epiphytic lichens as potential indicators of environmental change in subtropical forest ecosystems in southwest China. Ecol. Indic. 2013, 29, 93–104. [Google Scholar] [CrossRef]
- Brunialti, G.; Frati, L.; Aleffi, M.; Marignani, M.; Rosati, L.; Burrascano, S. Lichens and bryophytes as indicators of old-growth features in Mediterranean forests. Plant Biosyst. 2010, 144, 221–233. [Google Scholar] [CrossRef]
- Liu, C.; Ilvesniemi, H.; Westman, C.J. Biomass of arboreal lichens and its vertical distribution in a boreal coniferous forest in central Finland. Lichenologist 2000, 32, 495–504. [Google Scholar] [CrossRef]
- Aragón, G.; Martínez, I.; Izquierdo, P.; Belinchón, R.; Escudero, A. Effects of forest management on epiphytic lichen diversity in Mediterranean forests. Appl. Veg. Sci. 2010, 13, 183–194. [Google Scholar] [CrossRef]
- Johansson, P. Consequences of disturbance on epiphytic lichens in boreal and near boreal forests. Biol. Conser. 2008, 141, 1933–1944. [Google Scholar] [CrossRef]
- Trobajo, S.; Martinez, I.; Prieto, M.; Fernandez-Salegui, A.B.; Terron, A.; Hurtado, P. Multi-scale environmental drives of lichen diversity: Insights for forest management. For. Ecol. Manag. 2025, 585, 122671. [Google Scholar] [CrossRef]
- Lubek, A.; Adamowski, W.; Dyderski, M.K.; Wierzcholska, S.; Czortek, P. Invasive Prunus cerasifera Ehrh. hosts more lichens than native tree species does quantity reflect quality? For. Ecol. Manag. 2025, 590, 122812. [Google Scholar] [CrossRef]
- Łubek, A.; Kukwa, M.; Czortek, P.; Jaroszewicz, B. Impact of Fraxinus excelsior dieback on biota of ash-associated lichen epiphytes at the landscape and community level. Biodivers. Conserv. 2019, 29, 431–450. [Google Scholar] [CrossRef]
- Marini, L.; Nascimbene, J.; Nimis, P.L. Large-scale patterns of epiphytic lichen species richness: Photobiont-dependent response to climate and forest structure. Sci. Total Environ. 2011, 409, 4381–4386. [Google Scholar] [CrossRef]
- Lomolino, M.V. Elevational gradients of species-density: Historical and prospective views. Glob. Ecol. Biogeogr. 2001, 10, 3–13. [Google Scholar] [CrossRef]
- Nascimbene, J.; Marini, L. Epiphytic lichen diversity along elevational gradients: Biological traits reveal a complex response to water and energy. J. Biogeogr. 2015, 42, 1222–1232. [Google Scholar] [CrossRef]
- Saiz, H.; Dainese, M.; Chiarucci, A.; Nascimbene, J. Networks of epiphytic lichens and host trees along elevation gradients: Climate change implications in mountain ranges. J. Ecol. 2021, 109, 1122–1132. [Google Scholar] [CrossRef]
- Asplundm, J.; Roos, R.E.; Klanderud, K.; Zuijlen, K.; Lang, S.I.; Birkemoe, T. Divergent responses of functional diversity to an elevational gradient for vascular plants, bryophytes and lichens. J. Vegetat. Sci. 2022, 33, e13105. [Google Scholar] [CrossRef]
- Worthy, F.R.; Douglas, A.S.; Stefanie, D.G.; Dhanushka, W.; Hui, L.L.; Vinodhini, T. Simulated climate change impacts health, growth, photosynthesis, and reproduction of high-elevation epiphytic lichens. Ecosphere 2025, 16, e70224. [Google Scholar] [CrossRef]
- Hauck, M.; Hofmann, E.; Schmull, M. Site factors determining epiphytic lichen distribution in a dieback-affected spruce-fir forest on Whiteface Mountain, New York: Microclimate. Ann. Bot. Fen. 2006, 43, 1–12. [Google Scholar]
- Huang, M.R. Altitudinal patterns of Stereocaulon (Lichenized Ascomycota) in China. Acta. Oecol. 2010, 36, 173–178. (In Chinese) [Google Scholar] [CrossRef]
- Baniya, C.B.; Solhoy, T.; Gauslaa, Y.; Palmer, M.W. The elevation gradient of lichen species richness in Nepal. Lichenologist 2010, 42, 83–96. [Google Scholar] [CrossRef]
- Rinas, C.L.; McMullin, R.T.; Rousseu, F.; Vellend, M. Diversity and assembly of lichens and bryophytes on tree trunks along a temperate to boreal elevation gradient. Oecologia 2023, 202, 55–67. [Google Scholar] [CrossRef]
- Abdushalik, N. Universal Scientific Expedition Report of Barluk Mountain Nature Reserve in Xinjiang, China; Xinjiang University Press: Urumqi, China, 2013; pp. 1–6. (In Chinese) [Google Scholar]
- Abbas, A.; Wu, J.R. Lichens of Xinjiang; Science Technology & Hygiene Publishing House of Xinjiang: Urumqi, China, 1998; pp. 1–16. (In Chinese) [Google Scholar]
- Mamatali, R.; Yong, H.Y.; Tosun, D.; Tumur, A. Diversity of macrolichen in Barluk Mountain National Nature Reserve, in Xinjiang, China. BIO Web Conf. 2024, 100, 02024. [Google Scholar] [CrossRef]
- Toksun, D.; Mamatali, R.; Yong, H.Y.; Tumur, A. The macrolichens of Barluk Mountain National Nature Reserve, Xinjiang Province, China. Open. J. For. 2024, 14, 413–432. [Google Scholar] [CrossRef]
- Mamatali, R.; Imin, B.; Kahriman; Tumur, A. The diversity of crustose lichens in Barluk Mountain National Nature Reserve, Xinjiang, China. In Proceedings of the 2023 Science and Technology Annual Conference of the Chinese Society of Environmental Sciences, Nanchang, China, 22–23 April 2023. (In Chinese). [Google Scholar]
- Toksun, D.; Mamatali, R.; Yong, H.Y.; Tumur, A. Species diversity of ground-dwelling macrolichens and their ecological indicator values in the Barluk Mountain National Nature Reserve. J. Northeast. For. Univ. 2025, 53, 1998–2008. (In Chinese) [Google Scholar]
- Toksun, D.; Bahenuer, J.; Yong, H.Y.; Tumur, A. Distribution characteristics of epiphytic lichens on Populus tremula trunks in Barluk Mountain National Nature Reserve of Xinjiang, China. J. Plant Resour. Environ. 2025, 34, 62–71. (In Chinese) [Google Scholar]
- Tajiguli, A.; Nurbay, A.; Wang, Y.Y. Biodiversity and protection countermeasures research on Natural Reserve of Xinjiang Barluk Mountain. North. Hortic. 2011, 21, 73–77. (In Chinese) [Google Scholar]
- Duan, X.B.; Nurbay, A. Research on wild plant resources in Barluk Mountain Natural Reserve of Xinjiang. J. Anhui. Agric. Sci. 2011, 39, 5996–5999. (In Chinese) [Google Scholar]
- Kubiak, D.; Osyczka, P. Non-forest vs forest environments: The effect of habitat conditions on host tree parameters and the occurrence of associated epiphytic lichens. Fungal Ecol. 2020, 47, 100957. [Google Scholar] [CrossRef]
- Ozturk, S.; Oran, S.; Guvenc, S.; Dalkiran, N. Analysis of the distribution of epiphytic lichens in the oriental beech (Fagus orientalis Lipsky) forests along an altitudinal gradient in Uludag mountain, bursa—Turkey. Pak. J. Bot. 2010, 42, 2661–2670. [Google Scholar]
- Orange, A.; James, P.W.; White, F.J. Microchemical Methods for the Identification of Lichens, 2nd ed.; British Lichen Society: London, England, 2010. [Google Scholar]
- Irwin, M.B. Keys to Lichens of North America: Revised and Expanded; Yale University Press: New Haven, CT, USA, 2016; pp. 3–423. [Google Scholar]
- Cobanoglu, G.; Sevgi, O. Analysis of the distribution of epiphytic lichens on Cedrus libani in Elmali Research Forest (Antalya, Turkey). J. Environ. Biol. 2009, 2, 205–212. [Google Scholar]
- Guo, S.L.; Yu, J.; Chen, G.Q. Ecological Data Analyses-Methods, Programs and Software; Science Publishing House: Beijing, China, 2014; pp. 160–170. (In Chinese) [Google Scholar]
- Schroeter, B.; Kappen, L.; Sancho, L.G. Seasonal variation in the carbon balance of lichens in the maritime Antarctic: Long-term measurements of photosynthetic activity in Usnea aurantiaco-atra. In Antarctic Ecosystems: Models for Wider Ecological Understanding; Davison, W., Howard-Williams, C., Broady, P., Eds.; The Caxton Press: London, UK, 2000; pp. 258–262. [Google Scholar]
- Leppik, E.; Jüriado, I. Factors important for epiphytic lichen communities in wooded meadows of Estonia. Folia. Cryptog. 2008, 44, 75–87. [Google Scholar]
- Büdel, B.; Scheidegger, C. Thallus morphology and anatomy. In Lichen Biology, 2nd ed.; Nash, I.T.H., Ed.; 2008; pp. 40–68. [Google Scholar]
- Pinokiyo, A.; Singh, K.P.; Singh, J.S. Diversity and distribution of lichen in relation to altitude within a protected biodiversity hot spot, north-east India. Lichenologist 2008, 40, 47–62. [Google Scholar] [CrossRef]
- Morando, M.; Matteucci, E.; Nascimbene, J.; Borghi, A.; Piervittori, R.; Favero-Longo, S.E. Effectiveness of aerobiological dispersal and microenvironmental requirements together influence spatial colonization patterns of lichen species on the stone cultural heritage. Sci. Total Environ. 2019, 685, 1066–1074. [Google Scholar] [CrossRef]
- Nimis, P.; Martellos, S. On the ecology of sorediate lichens in Italy. Bibl. Lichenol. 2003, 86, 393–406. [Google Scholar]
- Rhoades, F.M. Nonvascular epiphytes in forest canopies: Worldwide distribution, abundance, and ecological roles. In Forest Canopies; Lowman, M.D., Nadkarni, N.M., Eds.; Academic Press: Cambridge, MA, USA, 1995; pp. 353–408. [Google Scholar]
- Loppi, S.; Printsos, S.A.; Dominics, V.D. Analysis of the distribution of epiphytic lichens on Quercus pubescens along an altitudinal gradient in a mediterranean area (Tuscany, Central Italy). Isr. J. Plant Sci. 1997, 45, 53–58. [Google Scholar] [CrossRef]
- Caldiz, M.S.; Brunet, J. Litter fall of epiphytic macrolichens in Nothofagus forest of northern Patagonia Argentina: Relation to stand age and precipitation. Austral Ecol. 2006, 31, 301–309. [Google Scholar] [CrossRef]
- Marmor, L.; Tõrra, T.; Saag, L.; Randlane, T. Species Richness of Epiphytic Lichens in Coniferous Forests: The Effect of Canopy Openness. Ann. Bot. Fenn. 2012, 49, 352–358. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.