Abstract
The present study explored the seasonal dynamics of spat settlement and growth of the Mediterranean mussel (Mytilus galloprovincialis) in the semi-enclosed and eutrophic Maliakos Gulf (Central Aegean, Greece), a coastal system within the Natura 2000 network (GR 2440002, Natura 2000). Spat collectors were deployed at three mussel farms representing different locations in the gulf (north, south, and inner west) and at multiple depths over a year. The results revealed a clear reproductive cycle, with spawning initiated in early January and spat settlement occurring from March to June. Settlement intensity was highest in shallower waters during the beginning of the season (March) and in the end (June), while depth had no significant effect mid-season. Mussel size and weight varied significantly with season and location, with the largest individuals observed during spring and early summer at the north and south sites. Environmental monitoring depicted strong nitrogen enrichment and phosphorus limitation, driven by inputs from the Spercheios River and surrounding agricultural activities. During winter, elevated chlorophyll-a concentrations likely supported early larval development, while nutrient imbalances threaten long-term ecosystem stability. These findings underscore the importance of area- and season-specific management of spat collectors and call for integrated monitoring and regulation of nutrient inputs to safeguard the ecological integrity of the gulf and ensure sustainable mussel farming.
1. Introduction
The Mediterranean mussel Mytilus galloprovincialis (Lamarck, 1819) is the predominant bivalve species cultivated in Greece, with an annual production ranging between 18,000 and 26,000 tons and an estimated potential capacity exceeding 40,000 tons per year [1]. In 2023, bivalve production amounted to 20,100 tons, worth EUR 11.6 million, representing 13% of the volume and just 1% of the value of the total Greek aquaculture production [2]. Among the areas contributing to this production, Maliakos Gulf—an environmentally sensitive, semi-enclosed ecosystem within the Natura 2000 network (GR 2440002)—has emerged as a key aquaculture site since the 1990s, especially for mussel farming as it is considered as an environmentally compatible activity [3,4]. In fact, mussel farming zones are integral parts of the nationwide spatial planning for the aquaculture sector (i.e., the Common Spatial Planning Framework for Aquaculture ratified on 4 November 2011 by the Common Ministerial Decision No 31722/2011, FEK 2505). According to this framework, Maliakos Gulf belongs to the particularly developed areas from an aquaculture viewpoint, and it is currently in the final stage of zoning declarations via Presidential Decrees [5].
The major anthropogenic activity is related with agriculture and the relevant small scale processing plants (i.e., rice, grains, olives, oil refineries) [6]. The gulf hosts approximately 12 floating mussel farms with a combined capacity of about 2000 tons per year [1]. Sustainable mussel farming in Maliakos Gulf is critically dependent on the availability of high-quality mussel seed (with strong byssus attachment but easy harvested in bunches), which exhibits strong seasonal and spatial variability [7]. Predicting spat supply is essential for optimizing farm operations and mitigating economic risks, yet the timing and magnitude of recruitment events remain poorly understood due to complex interactions between environmental factors and reproductive biology [8,9,10]. Previous studies have shown that the reproductive cycle of M. galloprovincialis is highly influenced by local environmental conditions such as temperature, salinity, and food availability, leading to inter-annual and regional variability in spawning and settlement patterns [11,12,13]. M. galloprovincialis’ larval settlement and metamorphosis are dependent also on the biofilm of the attached substrates. The spat collector immersion month (season) or seawater temperature can affect the community structure, including extracellular products of constituent organisms of the biofilm [14]. Furthermore, a wide range of substrates of spat collectors are examined to enhance the recruitment pattern at a level suitable for commercial exploitation [15].
Maliakos Gulf presents unique ecological characteristics, including high nutrient loads from the Spercheios River, which contribute to its eutrophic status [16]. Elevated chlorophyll-a concentrations, particularly during winter, coincide with gametogenesis and may favor larval development and settlement [17]. However, nutrient imbalances and anthropogenic pressures, coupled with climate-induced temperature fluctuations, may alter spawning phenology and settlement success [18]. Despite these challenges, no comprehensive study has yet documented the spatial and temporal patterns of mussel reproduction and spat settlement in Maliakos Gulf, nor assessed the influence of depth—a key factor for larval distribution and food availability—on settlement intensity.
The present study aims to characterize the spat settlement patterns of cultured M. galloprovincialis in Maliakos Gulf, with a focus on the influence of site location and depth. Specifically, the study aimed to achieve the following: (i) identify the timing of spawning and spat settlement in Maliakos Gulf, (ii) evaluate the effect of depth and farm location on settlement intensity, and mussel spat growth, and (iii) provide recommendations for optimizing seed collection and growth under prevailing environmental conditions.
2. Materials and Methods
2.1. Study Area
Maliakos Gulf, located on the eastern side of mainland central Greece (38°51′39.82″ N, 22°41′45.54″ E), is a semi enclosed embayment with a surface area about 110 km2 and connected with north Evvoikos Gulf through a channel of 2 km in width (Figure 1). The depth of the inner-western part of the gulf varies from less than 2 m at its shallowest, down to 20–27 m, covering about 25% and 27% of the gulf surface (91.5 km2 approx.), respectively. There are subaqueous seaward-spreading sedimentation-laden fresh river plumes, favored by limited wave, wave-induced current, and tidal activity [19]. The salinity ranges from 20 to 36 ppt and, according to primary production and nutrients recorded, it is classified as a eutrophic marine ecosystem [18]. In this area with limited wind-generated waves (Mentzafou et al., 2017), there is established intensive farming of the Mediterranean mussel M. galloprovincialis [1,20].
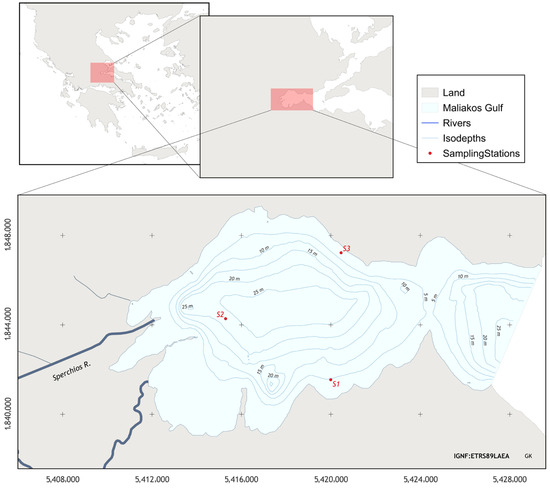
Figure 1.
Sampling stations/mussel farm sites in Maliakos Gulf. S1: South (Molos), S2: West (Ag. Trias), S3: North (Ag. Ioannis).
This study took place within three single long line mussel farms located along the coastal area of the Maliakos Gulf (Figure 1) that are mainly dependent on riverine dynamics [21]. The gulf has significant nutrient loads from the outflows of River Spercheios, with anti-clockwise movement that supports the primary productivity and consequently the existing bivalve populations in the area [3,22].
Maliakos is a shellfishing ground of commercial exploited bivalves, such as mussels (M. galloprovincialis Lamarck, 1819), flat oysters (Ostrea edulis Linnaeus, 1758), bearded horse mussels (Modiolus modiolus Linnaeus, 1758), clams (natives Ruditapes decussatus Linnaeus, 1758 and warty venus Venus verrucosa Linnaeus, 1758), and non-endemic pearl oysters (Pinctada radiata Leach,1814), providing economic incomes to the local fishermen [23,24,25].
2.2. Environmental Parameters
Physicochemical sampling was conducted monthly from August 2004 to August 2005 at three sampling stations (Figure 1). All samples were taken with a Ruttner type sampler (2 L). The water samples were filtered up to 60 μ and were stored in 1.5 LT plastic bottles. Temperature, pH, salinity and dissolved oxygen (DO) were performed in situ at depth intervals (0–10 m) using a profiler equipped with the appropriate sensors (YSI 556) and an Oxy-Guard Handy Gamma portable dissolved oxygen meter.
Chemical analysis of the water samples filtered through Whatman GF/C filters (0.45 μm) were performed for N-NO3, N-NO2, P-PO4, N-NH4 according to [26]. Chlorophyll-a determination was conducted following the spectrophotometric determination after the pigment’s extraction in 90% acetone. Nutrients and chlorophyll-a were measured at three depths (0.5, 3.0 and 6.0 m). The sum of N-NO3, N-NO2, and N-NH4 corresponds to the dissolved inorganic nitrogen and the P-PO4 concentration to the soluble reactive phosphorus (SRP).
2.3. Biotic Sampling
Biotic sampling was conducted from August 2004 to June 2005 and the regional spat settlement pattern is estimated by putting spat collectors in the examined area at three different commercial mussel long line farm sites located in the south sampling station (S1), west sampling station (S2), and northern sampling station (S3) area of Maliakos (Figure 1) at different depths (2, 4, and 6 m) according to [27,28]. Duplicate seed collectors, 5 m lengths of 22 mm diameter using polypropylene rope (polysteel type, manufactured by Koronakis SA, Piraeus-Greece), were suspended from 1 m submerged single long lines at each farm site and used as sampling stations S1, S2, S3 (Figure 1). Ropes were weighed at the bottom ends so that they hung vertically in the water. Every 2 months the seed collectors were exploited and replaced with new ones. The harvested seed collectors were transferred into the laboratory for further analysis.
To count spat, each collector was immersed overnight in sea water [29] after several rinses 10 cm from three different depths (1.5, 3.5, 5.5 m) of each collector was removed. The number of counts per area (counts/m2) was estimated by counting the number of spats on 5 randomly selected fields of view. The size of mussels settled on the collectors was measured with a stereoscope using a digital image analysis system (Seescan, Cambridge, UK).
2.4. Growth Estimation
To estimate the mussel spat growth in Maliakos, a set (X 3 replicates) of tubular net socks (pergolaris) originated from the same stock were placed in each farm site (S1, S2, S3). In September 2004, following the typical production plan of Greek mussel farming (Figure 2), mussel spat bunches originated from the 2nd tubing of seed stock in S1 farm (Molos) were tubed (Φ80 plastic tube) in 9X 3.5 m long pergolaris (Figure 3). The individual mussel average length (n = 125) was about 3.68 cm (min = 2.3 g–max = 4.98 g), (n = 125), and weight (n = 150) was 2.16 g (ranging from 0.57 g to 5.07 g).
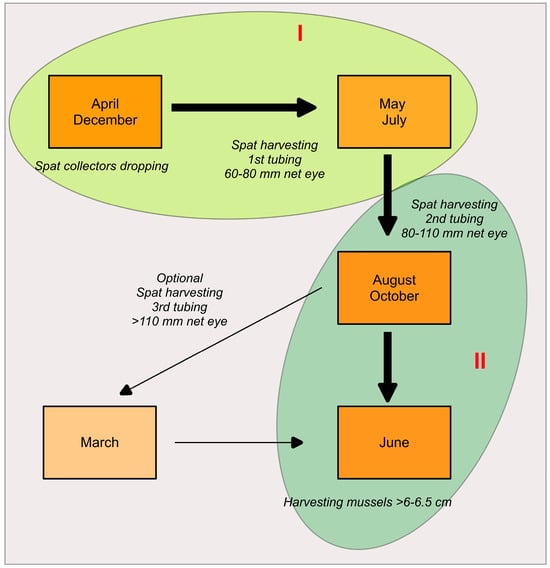
Figure 2.
Experimental design of (I) spat settlement (up) and (II) growth (down) based on the typical production plan of mussel farming. (Adapted from [11]).
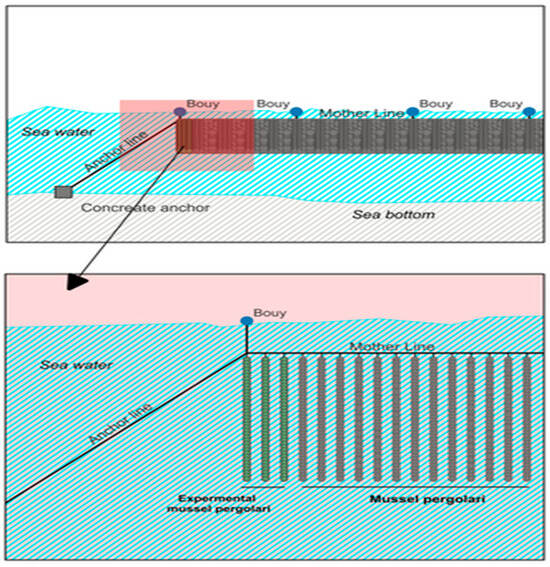
Figure 3.
Experimental mussel pergolari (X3) hanged up to a single mother line of a single longe line farm.
On a bi-monthly basis, 30 individuals were selected randomly along the length of one pergolari per site. Morphometric parameters such as shell length (maximum anterior–posterior axis), width (maximum lateral axis), and height (maximum dorsiventral axis), were measured to the nearest 0.1 mm using a vernier caliper. Individual (total live), Meat and Shell wet weight were measured by blotting them with tissue paper. All were estimated closest to the nearest 0.01 g by using a 2-digit balance [30].
The comparison of the morphometric parameters (i.e., length, width, height) as well as of the weight with season and area was assessed using the two-way Analysis of Variance (two-way ANOVA). For comparative analyses of the abiotic and biotic parameters, a normal distribution of the samples was tested for using the Shapiro–Wilk test (S-W, p < 0.05), and Levene’s test was implemented for testing the homogeneity of variances.
3. Results
3.1. Nutrients
Spatial and temporal variations in nutrients in Maliakos Gulf are presented in Figure 4. This may constitute an impact on water quality due to the effluents or/and sub-products enriching the water column with decomposition products. The most important form of DIN consisted of nitrate–nitrogen (66.4%), followed by ammonium (33.1%). Nitrite–nitrogen had an insignificant role in contributing to the dissolved inorganic nitrogen pool, with an average percentage of 0.5%.
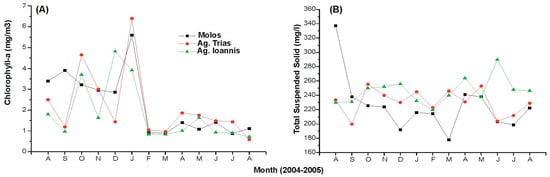
Figure 4.
Indicative productivity profile: (A) chlorophyll-a and (B) total suspended solids (TSSs) of the three sampling stations/mussel farm sites in Maliakos Gulf during September 2004–June 2005. S1: South (Molos), S2: West (Ag. Trias), S3: North (Ag. Ioannis).
Phosphate showed no clear temporal or spatial pattern. Extremely high concentrations were observed at sampling stations S1 and S2 (south and west, respectively) during the high precipitation period and river flow. The DIN/SRP ratio was greater than the Redfield ratio in all sampling stations. Mean values were considered to be quite high (57.8 mg/L), suggesting a phosphorous limitation almost over the whole period. On two occasions, at stations S1 and S2 during the same months (December and January), a nitrogen-limitation system was switched on.
Concerning the vertical profile of nutrients, no clear differences were found in nitrite–nitrogen and phosphate concentrations at the three depths. Nitrates showed a weak stratification, appearing in higher values at 3 m depth, while a strong stratification was noticed in ammonia concentrations during the end of summer.
3.2. Chlorophyll-a, Temperature, Salinity, and Dissolved Oxygen
Chlorophyll-a values ranged between 1.0 and 6.4 μg/L, while the chlorophyll-a pattern did not show any clear spatial or temporal differences. The highest concentrations for each station were measured during the December–January period (5.6, 6.4 and 4.8 μg/L, respectively). Temperature appeared to be the highest in August (25.0–27.5 °C) and the lowest during January (12.4–14 °C) (Figure 5). There were no significant differences between sampling stations during the monitoring period. Furthermore, no thermal stratification was observed when studying the depth temperature profile at 1 m intervals (0–6 m). Salinity in the surface water layer ranged from 36 to 37 ppt at station S1 and from 20 to 36 ppt at station S2. Generally, station S2 exhibited lower salinity values, confirming the influence of the Spercheios river’s inflows. Salinity values at station S3 ranged between 35 and 38 ppt. The same station depicted the highest variability between salinity recorded at the surface and the deeper part (2.0 ppt in October). The DO exhibited a seasonal fluctuation, with the highest surface values shown in January (14.3 mg/L at S3–16.6 mg/L at S2), when the water temperature fluctuations marked low levels. Low values were observed in August, ranging between 1.9 mg/L and 2.9 mg/L. No differences in the DO vertical profile were depicted during almost the whole period, since the variability between surface and bottom DO values ranged from 0.1 to 0.3 mg/L. Higher differences were measured in October at the S2 station ([DO] difference up to 1.5 mg/L) and in December at S3 ([DO] difference up to 1.7 mg/L).
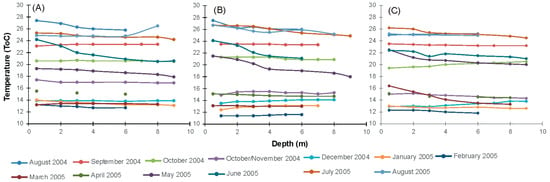
Figure 5.
Temperature profile of the experimental sampling stations/mussel farm sites in Maliakos Gulf during August 2004–August 2005: (A) S1, South (Molos), (B) S2, West (Ag. Trias), (C) North (Ag. Ioannis).
3.3. Spat Collection
The M. galloprovincialis spat started settling on the collectors in early March and lasted for another 3 months until late June. The results showed that there is a stable monthly spat settlement in the inner part of the Gulf (S2) for the whole period tested (Figure 6). In contrast, the south (S1) and north (S3) stations seemed to recruit more spat in the beginning of the season, with a decrease in the following months. The effect of depth on the spat settlement was independent of location and was significant in both the beginning of the season in March and in the ending during June, showing a decreasing trend in the settled spat with the increase in depth. During the mid period, there was no effect of depth on the spat settlement.
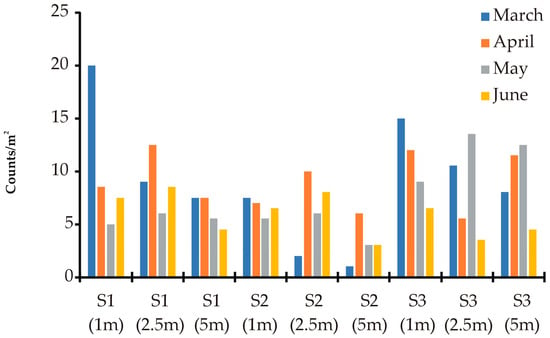
Figure 6.
Seasonal, spatial, and depth variation in the M. galloprovincialis spat settlement in Maliakos Gulf during August 2004–August 2005. Only the months with active spat settlement were shown (all other nil). South (S1), West (S2), North (S3). Depths are given in the parenthesis. Columns represent means of at least 10 individuals on 5 random measurements on 2 spat collectors per location.
3.4. Spat Growth
Length and weight data samples were normally distributed (S-W, p > 0.05) and depicted the homogeneity of their variances (Levene’s test). The mean length and weight of the growing spat was significantly (two-way ANOVA: p < 0.05) influenced by time and the first-order interaction of time–area, with spring and summer significantly (Tukey’s post hoc test: p < 0.05) exhibiting the largest and more weightier individuals compared with autumn and winter (Figure 7A,B). The mean length and weight of the mussels were significantly (two-way ANOVA: p < 0.05) influenced by time and the first-order interaction of time–area, with spring and summer significantly (Tukey’s post hoc test: p < 0.05) exhibiting the largest and more weightier individuals compared with autumn and winter (Figure 7A,B). The season was also the only significant factor (two-way ANOVA: p < 0.05) that also differentiated the mean width and height of the growing spat, with spring and summer exhibition significantly (Tukey’s post hoc test: p < 0.05) higher estimates for both of the aforementioned parameters (Figure 7C,D). In contrast, the meat weight and shell length of the individuals were significantly (two-way ANOVA: p < 0.05) influenced by time, season, and the first–order interaction of time–area, with spring and summer in the north (Ag. Ioannis) and south (Molos) stations exhibiting significantly (Tukey’s post hoc test: p < 0.05) higher estimates for both parameters (Figure 7E,F).
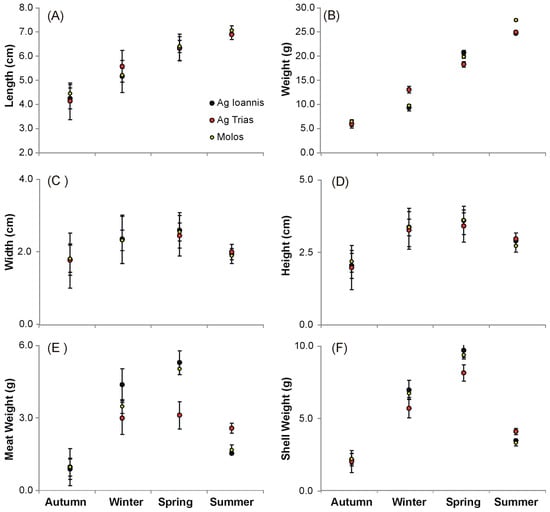
Figure 7.
Season–area comparison of the mean (and standard error) estimates of (A) length, (B) weight, (C) width, (D) height, (E) meat weight, and (F) shell weight of the M. galloprovincialis growth in three different areas (S1, S2, S3) in Maliakos Gulf during August 2004–August 2005.
4. Discussion
The present study provided a detailed insight into the spat settlement dynamics of M. galloprovincialis in the semi-enclosed and eutrophic Maliakos Gulf (CE Greece). The results clearly showed a defined reproductive timeline beginning with spawning in early January, followed by spat settlement from March through June. Notably, settlement patterns and spat growth were significantly affected by depth and area, especially during the beginning of the season (March) and in the end (June), while depth had no significant effect mid-season.
Nutrient analysis revealed consistently increased DIN levels throughout the study period, with nitrate as the dominant form. These high nitrogen concentrations, particularly near the southern (S1) and inner (S2) sites, align with the increased agricultural runoff and riverine inputs, notably from the Spercheios River [26]. The observed phosphorus limitation, as indicated by a consistently high DIN/SRP ratio, reflects an imbalance likely driven by disproportionate nitrogen fertilization. This support previous assessments [31,32] suggesting that phosphorus, rather than nitrogen, is the primary limiting nutrient in the study area and across of the Aegean Sea [33]. Regarding the trophic state of Maliakos Gulf, is evident that the gulf is classified as a eutrophic ecosystem [34], while there are also studies reporting a mesotrophic state of Maliakos Gulf [22]. This difference probably occurred due to the different methodological approaches of characterization and the examined period of time. During the 2010s, the ecological status of the study area in terms of the eutrophic index EI (classifies the seawater body into five ecological status levels: (a) less than 0.04 (High), (b) 0.04–0.38 (Good), (c) 0.38–0.85 (Moderate), (d) 0.85–1.51 (Poor), and (e) more than 1.51 (Bad) [Dimitriou et al., 2015]) was characterized as “Bad” or “Poor” [3] because of the river discharge [4]. Yet, ref. [35] suggested that there are high nutrient flows and a strong impact from the Spercheios river towards Maliakos Gulf, detecting high nutrients concentrations close to the river mouth. As a result, the mean values of DIN in Maliakos Gulf were estimated to be higher than those reported in other Greek eutrophic enclosed systems, such as Thermaikos, Saronikos, and Strymonikos Gulfs [36,37,38,39,40]. Moreover, the high percentage of NO3-N suggested agricultural activity as a serious nutrient source. Coupled with recent urbanization of the coastal zone, these factors may push the ecosystem beyond its carrying capacity unless controlled.
Chlorophyll-a levels peaked in winter in Maliakos, likely fueled by riverine nutrient input, coinciding with the onset of gametogenesis and early larval stages. Similar trophic support for reproduction has been reported in the Venice Lagoon, where phytoplankton availability underpins seasonal reproductive success [17]. However, while nutrient enrichment promoted phytoplankton growth, the imbalance between nitrogen and phosphorus may lead to shifts in phytoplankton community composition, potentially affecting mussel larval development and survival. Chlorophyll-a concentrations were in agreement with recent observations and pointed out a rather homogenous Chl a pattern in integrated water samples throughout Maliakos Gulf, except for the maximum values that were higher to close to the river mouth [36]. High chlorophyll-a values have also been reported during the same period in past research in Maliakos Gulf [31]. Vertical homogeneity in chlorophyll-a and dissolved oxygen indicated limited stratification, supporting the classification of Maliakos Gulf as polymictic system. The chlorophyll-a values of Maliakos Gulf are comparable to those reported in other Aegean and Mediterranean systems affected by anthropogenic influences [20,25,36,37,38,39,40]. Concerning the Maliakos’ ecological status, and according to the WFD requirements, the assessment of the coastal marine waters failed to reach Good environmental status (GES) [35,40].
This study demonstrates that sustainable mussel farming in the Maliakos Gulf depends on both optimizing spat collection practices and addressing nutrient imbalances at the ecosystem scale. A holistic environmental management approach of the activity is recommended by interconnecting the Spercheios estuaries and coastal zone interactions [41]. The observed seasonality of recruitment provides actionable guidance for producers, supporting the efficiency goals of the EU Blue Growth agenda. At the same time, persistent eutrophication highlights the need for integrated watershed–coastal management to meet Water Frame Directive [42] and Marine Strategy Framework Directive requirements [43]. Aligning aquaculture operations with ecological thresholds will be essential to secure long-term productivity while contributing to the European Green Deal and Farm to Fork strategy.
The observed timing of recruitment in Maliakos Gulf confirms that M. galloprovincialis exhibits seasonal reproduction strongly influenced by temperature and food availability. Gametogenesis typically commences in autumn, continuing through winter, with spawning occurring in late winter and spring [12]. Similar phenology has been documented in Tokyo and Boka Kotorska bays [11] and [12], respectively, where reproductive activity is concentrated in cooler months and ceases when water temperature exceeds 25 °C. These observations highlight temperature as a key determinant of reproductive seasonality, with implications for aquaculture scheduling. Climate change further adds complexity to the above-mentioned interactions. Prolonged thermal stress at 28 °C severely reduces sperm motility, disrupts mitochondrial function, and increases DNA fragmentation [18]. Projected warming of the Eastern Mediterranean could shorten spawning windows and increase the frequency of partial or asynchronous spawning events, thereby reducing settlement predictability and farm productivity.
This study also identified that depth significantly influenced spat density, with shallower depths (2 m) exhibiting greater settlement in March and June. This is likely due to higher food concentrations and larval densities in surface waters during those months. During the mid-season (April–May), depth had no significant effect, implying uniform larval distribution potentially driven by hydrodynamic mixing. These findings highlight the importance of collector depth optimization to maximize spat yield, particularly at the beginning and end of the recruitment period. Mussel morphometrics, including shell length, width and height, varied significantly with season and area. Larger and heavier individuals were consistently observed during spring and early summer, especially at the southern (S1) and northern (S3) stations. These results suggest that environmental conditions during these periods, including temperature and food availability, were more favorable for spat growth. Moreover, the interaction of depth and area with time underscored the complexity of environmental influences on spat development, reinforcing the need for area-specific management strategies [44].
While Maliakos Gulf offers a high-potential environment for mussel farming, its ecological sensitivity and exposure to nutrient over-enrichment necessitate a prudent approach. The current results reaffirmed past findings that the gulf experiences high nutrient inputs [3], especially nitrogen, from both natural (riverine) and anthropogenic (agriculture) sources. These inputs not only influence productivity but also increase the risks of harmful algal blooms and hypoxic conditions, especially during the warmer months [44].
Given the clear seasonal and spatial trends in spat settlement and growth, mussel farmers in Maliakos Gulf could benefit from adaptive seeding and harvesting planning that aligns with peak spat abundance and growth periods. In addition, long-term monitoring and integrated abiotic–biotic modeling are essential for identifying the implications of climate change on mussel recruitment dynamics. Further research should also be focused on the estimation of the carrying capacity of Maliakos Gulf, concerning farming activity becoming an eco-sustainable activity.
Our findings highlight that early life stage conditions are critical for mussel aquaculture, as settlement intensity and spat growth depend on environmental variability. Recent studies confirm this sensitivity: ref. [13] showed that reproductive patterns in the Adriatic are tightly controlled by temperature, while Bordignon et al. [17] demonstrated that growth and gene expression in the Venice Lagoon respond strongly to environmental fluctuations. Complementing these field observations, ref. [45] reported that in hatchery trials, larval stocking density determines settlement efficiency and seed quality. The results of our study, together with the above-mentioned research, indicate that early life stages constitute a critical determinant of M. galloprovincialis productivity, influencing both natural ecosystem dynamics and hatchery seed production.
5. Conclusions
This study provided a comprehensive evaluation of the reproductive cycle and spat settlement patterns of M. galloprovincialis in Maliakos Gulf, a productive and ecologically vulnerable coastal ecosystem. The findings confirmed a consistent annual spawning initiation in early January, with spat settlement occurring primarily between March and June. Spat settlement intensity and growth were significantly influenced by depth, season, and farm location, with shallower depths yielding higher settlement at the beginning and end of the spring–summer season, and larger spat individuals observed during spring and early summer. The nutrient and chlorophyll-a profiles indicated a eutrophic environment shaped by strong nitrogen enrichment and phosphorus limitation, likely driven by agricultural runoff and riverine inflows from the Spercheios River. These conditions supported high phytoplankton biomass, which may enhance larval food availability but also pose risks of ecological imbalance, including harmful algal blooms and hypoxia during summer.
To ensure sustainable mussel farming in Maliakos Gulf, future efforts should focus on adaptive management strategies that consider seasonal and spatial variability in spat recruitment and growth. This includes optimizing collector deployment depth and timing, reducing nutrient inputs through improved land-use practices, and enhancing environmental monitoring. Further research is needed to quantify the carrying capacity of the gulf, integrate hydrodynamic and larval dispersal models, and assess the resilience of mussel populations under projected climate change scenarios. Aligning farming operations with ecosystem thresholds will be essential to maintain both productivity and ecological integrity in this sensitive coastal zone.
Author Contributions
Conceptualization, J.A.T.; methodology, J.A.T., I.T. and D.K.M.; resources, J.A.T., I.T., F.K., C.N. and I.K.; data curation, J.A.T., C.N., D.K.M. and G.K.; writing—review and editing, J.A.T., I.T., C.N., I.K., D.K.M. and G.K.; supervision, J.A.T.; project administration, J.A.T.; funding acquisition, J.A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by EU (EPEAEK II—Archimedes contract no 10012-00004, “Environmental Interactions of the Mussel farming”).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are available upon request to the first author.
Acknowledgments
Thanks to the shellfish farms of Maliakos Gulf for the kind field support during the present study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Theodorou, J.A.; Viaene, J.; Sorgeloos, P.; Tzovenis, I. Production and marketing trends of the cultured mediterranean mussel Mytilus galloprovincialis lamarck 1819, in Greece. J. Shellfish. Res. 2011, 30, 859–874. [Google Scholar] [CrossRef]
- HAPO Aquaculture Annual Report 2024. Available online: https://fishfromgreece.com/wp-content/uploads/2024/10/HAPO_AR24_Spreads.pdf (accessed on 9 September 2025).
- Dimitriou, P.D.; Karakassis, I.; Pitta, P.; Tsagaraki, T.M.; Apostolaki, E.T.; Magiopoulos, I.; Nikolioudakis, N.; Diliberto, S.; Theodorou, J.A.; Tzovenis, I.; et al. Mussel farming in Maliakos Gulf and quality indicators of the marine environment: Good benthic below poor pelagic ecological status. Mar. Pollut. Bull. 2015, 101, 784–793. [Google Scholar] [CrossRef]
- Neofitou, N.; Charizopoulos, N.; Vafidis, D.; Skordas, K.; Tziantziou, L.; Neofitou, C. Mussel farming impacts on trophic status and benthic community structure in Maliakos Gulf (Eastern Mediterranean). Aquac. Int. 2014, 22, 843–857. [Google Scholar] [CrossRef]
- Theodorou, J.A.; Leech, B.S.; Perdikaris, C.; Hellio, C.; Katselis, G. Performance of the cultured Mediterranean mussel Mytilus galloprovincialis Lamark, 1819 after summer post-harvest re-immersion. Turk. J. Fish. Aquat. Sci. 2019, 19, 221–229. [Google Scholar] [CrossRef]
- Skoulikidis, N.T. The environmental state of rivers in the Balkans—A review within the DPSIR framework. Sci. Total Environ. 2009, 407, 2501–2516. [Google Scholar] [CrossRef]
- Kamermans, P.E.; Brummelhuis, E. Enhancing mussel seed supply. In Aquaculture Europe 2003—Beyond Monoculture, Compilers; Chopin, T., Reinertsen, H., Eds.; No 33; EAS Special Publication: Gent, Belgium, 2003. [Google Scholar]
- Seed, R. The ecology of the Mytilus edulis L. (Lamelibranchiata) on exposed rock shores. I growth and mortality. Ecologia 1976, 3, 317–350. [Google Scholar]
- Seed, R.; Suchanek, T.H. Population and community ecology of Mytilus. In Developments in Aquaculture and Fisheries Science: The Mussel Mytilus: Ecology, Physiology, Genetics and Culture; Gosling, E., Ed.; Elsevier: London, UK, 1992; Volume 25, pp. 87–170. [Google Scholar]
- Rodhouse, P.G.; Roden, C.M.; Burnell, G.M.; Hensey, M.P.; McMohan, O.B.; Ryan, T.H. Food source, gametogenesis and growth of Mytilus edulis on the shore and suspended culture: Killary Harbour, Ireland. J. Mar. Biol. Assoc. UK 1984, 48, 513–529. [Google Scholar] [CrossRef]
- Lowe, D.M.; Moore, M.N.; Bayne, B.L. Aspects of gametogenesis in the marine mussel Mytilus edulis. J. Mar. Biol. Assoc. UK 1982, 62, 133–145. [Google Scholar] [CrossRef]
- Okaniwa, N.; Miyaji, T.; Sasaki, T.; Tanabe, K. Shell growth and reproductive cycle of the Mediterranean mussel Mytilus galloprovincialis in Tokyo Bay, Japan: Relationship with environmental conditions. Plankton Benthos Res. 2010, 5, 214–220. [Google Scholar] [CrossRef]
- Nikolić, S.; Peraš, I.; Mandić, M. Some Reproductive Patterns of Cultured Mediterranean Mussel (Mytilus galloprovincialis Lamarck, 1819) in Boka Kotorska Bay, Adriatic Sea. J. Agric. For. 2023, 69, 53–65. [Google Scholar] [CrossRef]
- Bao, W.-Y.; Satuito, C.G.; Yang, J.-L.; Kitamura, H. Larval settlement and metamorphosis of the mussel Mytilus galloprovincialis in response to biofilms. Mar. Biol. 2007, 150, 565–574. [Google Scholar] [CrossRef]
- Wu, W.; Jeffs, A.G. Influence of Microstructure of Substrate Surface on the Attachment of Juvenile Mussels. Fishes 2025, 10, 135. [Google Scholar] [CrossRef]
- Mentzafou, A.; Vamvakaki, C.; Zacharias, I.; Gianni, A.; Dimitriou, E. Climate change impacts on a Mediterranean river and the associated interactions with the adjacent coastal area. Environ. Earth Sci. 2017, 76, 259. [Google Scholar] [CrossRef]
- Bordignon, F.; Bertolini, C.; Bernardini, I.; Rovere, G.; Iori, S.; Breggion, C.; Pastres, R.; Boffo, L.; Xiccato, G.; Matozzo, V.; et al. Spatio-temporal variations of growth, chemical composition, and gene expression in Mediterranean mussels (Mytilus galloprovincialis): A two-year study in the Venice lagoon under anthropogenic and climate changing scenarios. Aquaculture 2023, 578, 74011. [Google Scholar] [CrossRef]
- Boni, R.; Gallo, A.; Montanino, M.; Macina, A.; Tosti, E. Dynamic changes in the sperm quality of Mytilus galloprovincialis under continuous thermal stress. Mol. Reprod. Dev. 2016, 83, 162–173. [Google Scholar] [CrossRef]
- Poulos, S.E.; Collins, M.B.; Shaw, H.F. Deltaic sedimentation, including clay mineral deposition patterns, associated with small mountainous rivers and shallow marine embayments of Greece (SE Alpine Europe). J. Coast. Res. 1996, 12, 940–952. [Google Scholar]
- Hatzonikolakis, G.; Tsiaras, K.; Theodorou, J.A.; Petihakis, G.; Sofianos, S.; Triantafyllou, G. Simulation of cultured Mediterranean mussel Mytilus galloprovincialis by a dynamic energy budget (DEB) model; Implementation in the gulfs of Maliakos and Thermaikos, (Aegean Sea, Greece). Aquac. Environ. Interact. 2017, 9, 371–383. [Google Scholar] [CrossRef]
- Akoumianaki, I.; Nicolaidou, A. Spatial variability and dynamics of macrobenthos in a Mediterranean delta front area: The role of physical processes. J. Sea Res. 2007, 57, 47–64. [Google Scholar] [CrossRef]
- Kormas, K.; Kapiris, K.; Thessalou-Legaki, M.; Nikolaidou, A. Quantitative relationships between phytoplankton, bacteria & protists in an Aegean semi-enclosed empayment (Maliakos Gulf, Greece). Aquat. Microb. Ecol. 1998, 15, 255–264. [Google Scholar] [CrossRef]
- Theodorou, J.A.; Tzovenis, I.; Adams, C.M.; Sorgeloos, P.; Vlaene, J. Risk factors affecting the profitability of the mediterranean Mussel (Mytilus galloprovincialis Lamarck 1819) farming in Greece. J. Shellfish. Res. 2014, 33, 695–708. [Google Scholar] [CrossRef]
- Galanidi, M.; Zenetos, A.; Bacher, S. Assessing the socio-economic impacts of priority marine invasive fishes in the Mediterranean with the newly proposed SEICAT methodology. Mediterr. Mar. Sci. 2018, 19, 107–123. [Google Scholar] [CrossRef]
- Zgouridou, A.; Tripidaki, E.; Giantsis, I.; Lattos, A.; Raitsos, D.; Anestis, A.; Theodorou, J.A.; Kalaitzaki, M.; Feidantsis, K.; Staikou, A.; et al. A survey on the microbial load on edible and economical important marine bivalve species in the Greek Seas. Environ. Microbiol. 2021, 24, 1012–1034. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1989. [Google Scholar]
- Margus, D.; Teskeredzic, E. Settlement of mussels (Mytilus galloprovinciallis Lamarck) on rope collectors in the estuary of the river Krka, Yugoslavia. Aquaculture 1986, 55, 285–296. [Google Scholar] [CrossRef]
- Hrs-Brenko, M. The Study of Mussel Larvae and their Settlement in Vela Draga Bay (Pula, The Northern Adriatic Sea). Aquaculture 1973, 2, 173–182. [Google Scholar] [CrossRef]
- Karayücel, S.; Erdem, M.; Uyan, O.; Saygun, S.; Karayücel, I. Spat settlement and growth on a long-line culture system of the mussel, Mytilus galloprovincialis, in the Southern Black Sea. Isr. J. Aquac. 2002, 54, 163–172. [Google Scholar] [CrossRef]
- Karayücel, S.; Karayücel, I.; Erdem, M.; Saygun, S.; Uyan, O. Growth And Production in Long-Line Cultivated Mediterranean Mussel (Mytilus galloprovincialis) In Sinop, Black Sea. Isr. J. Aquac. 2003, 55, 169–178. [Google Scholar] [CrossRef]
- Kormas, K.; Garametsi, V.; Nicolaidou, A. Size-fractionated phytoplankton chlorophyll in an Eastern Mediterranean coastal system (Maliakos Gulf, Greece). Helgol. Mar. Res. 2002, 56, 125–133. [Google Scholar] [CrossRef]
- Turner, R.; Rabalais, N.N.; Justic, D.; Dortch, Q. Global Patterns of Dissolved N, P and Si in Large Rivers. Biogeochemistry 2003, 64, 297–317. [Google Scholar] [CrossRef]
- Stergiou, K.I.; Christou, E.D.; Georgopoulos, D.; Zenetos, A.; Souvermezoglou, C. The Hellenic Seas: Physics, chemistry, biology and fisheries. Oceanogr. Mar. Biol. Annu. Rev. 1997, 35, 415–538. [Google Scholar]
- Ignatiades, L.; Karydis, M.; Vounatsou, P. A possible method for evaluating oligotrophy and eutrophication based on nutrient concentration scales. Mar. Pollut. Bull. 1992, 24, 238–243. [Google Scholar] [CrossRef]
- Markogianni, V.; Varkitzi, I.; Pagou, K.; Pavlidou, A.; Dimitriou, E. Nutrient flows and related impacts between a Mediterranean river and the associated coastal area. Cont. Shelf Res. 2017, 134, 1–14. [Google Scholar] [CrossRef]
- Friligos, N. Contribution to the Study of the Influence of Sewage and Industrial Effluents on the Distribution of Nutrient Salts During the Years 1973–1977. Ph.D. Thesis, University of Athens, Athens, Greece, 1978; p. 208. [Google Scholar]
- Nikolaidis, N.P.; Karageorgis, A.P.; Kapsimalis, V.; Marconis, G.; Drakopoulou, P.; Kontoyiannis, H.; Krasakopoulou, E.; Pavlidou, A.; Pagou, K. Circulation and nutrient modeling of Thermaikos Gulf, Greece. J. Mar. Syst. 2006, 60, 51–62. [Google Scholar] [CrossRef]
- Nikolaidis, N.P.; Karageorgis, A.P.; Kapsimalis, V.; Drakopoulou, P.; Skoulikidis, N.; Behrendt, H.; Levkov, Z. Management of nutrient emissions of Axios River catchment: Their effect in the coastal zone of Thermaikos Gulf, Greece. Ecol. Model. 2009, 220, 383–396. [Google Scholar] [CrossRef]
- Rodrigues Luís, C.; van den Bergh, J.C.J.M.; Massa, F.; Theodorou, J.A.; Ziveri, P.; Gazeau, F. Sensitivity of Mediterranean Bivalve Mollusc Aquaculture to Climate Change and Ocean Acidification: Results from a producers’ survey. J. Shellfish. Res. 2015, 34, 1161–1176. [Google Scholar] [CrossRef]
- Simboura, N.; Tsapakis, M.; Pavlidou, A.; Assimakopoulou, G.; Pagou, K.; Kontoyiannis, H.; Zeri, C.; Krasakopoulou, E.; Rousselaki, E.; Katsiaras, N.; et al. Assessment of the environmental status in the Hellenic coastal waters (Eastern Mediterranean): From the Water Framework Directive to the Marine Strategy Framework Directive. Mediterr. Mar. Sci. 2015, 16, 46–64. [Google Scholar] [CrossRef]
- Wolanski, E.; Boorman, L.A.; Chicharo, L.; Langlois-Saliou, E.; Lara, R.; Plater, A.J.; Uncles, R.J.; Zalewski, M. Ecohydrology as a new tool for sustainable management of estuaries and coastal waters. Wetl. Ecol. Manag. 2004, 12, 235–276. [Google Scholar] [CrossRef]
- WFD 2000/60/EC: Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:5c835afb-2ec6-4577-bdf8-756d3d694eeb.0004.02/doc_1&format=pdf (accessed on 9 September 2025).
- MSFD 2008/56/EC: European Commission 2008. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008L0056 (accessed on 9 September 2025).
- Theodorou, J.A.; Moutopoulos, D.K.; Tzovenis, I. Semi-quantitative risk assessment of Mediterranean mussel (Mytilus galloprovincialis L.) harvesting bans due to harmful algal bloom (HAB) incidents in Greece. Aquac. Econ. Manag. 2020, 24, 273–293. [Google Scholar] [CrossRef]
- Janah, H.; Aghzar, A.; Presa, P.; Ouagajjou, Y. Influence of Pediveliger Larvae Stocking Density on Settlement Efficiency and Seed Production in Captivity of Mytilus galloprovincialis in Amsa Bay, Tetouan. Animals 2024, 14, 239. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).