Abstract
Environmental changes may affect animal hoarding behavior through changes to plant diversity. Food hoarding behavior in small mammals can affect the seed dispersal process and thus the regeneration of the plant population. However, little is known about how small mammals select seeds of different sizes under different forest types. Here, we tracked the fates of 3360 seeds in the field. We used a generalized linear mixed model to analyze the effects of small mammals on three fates (predation in situ, predation after dispersal, hoarding after dispersal) and two dispersal distances (predation distance after dispersal and hoarding distance after dispersal) of different seeds by size and forest type. The results showed that small mammals consume smaller seeds in situ and cache larger seeds after dispersal. The predation distance after dispersal and hoarding distance after dispersal of the large seeds were significantly higher than those of small seeds. Forest types with dense vegetation conditions exhibited lower hoarding rates after dispersal, while those with poor vegetation conditions had increased predation and hoarding distances after dispersal. Our results suggest that larger seeds are dispersed to further distances, potentially enhancing plant population regeneration. However, seeds are more often scatter-hoarded and dispersed over longer distances in forests with poor vegetation conditions, which may also benefit plant population regeneration. These results provide insights into how seed size and forest type influence seed predation and dispersal by small mammals.
1. Introduction
The environmental changes caused by recent human activities have destroyed large areas of forests, seriously affecting the plant diversity, structure, and dynamics of forests and ultimately affecting the functional stability of the ecosystem [1,2,3]. The type of forest affects the distribution and consumption activity of small mammals and, subsequently, affects the spatial distribution, dispersal distance, and survival of seeds [4,5,6]. For example, the dispersal and caching of Armeniaea sibiriea seeds happens significantly differently to those of Quercus wutaishanica [7,8]. Forests with good protective conditions have a closer distance of dispersed seeds than those with poor protective conditions, and a higher proportion of seeds close to food sources are recovered and utilized [9,10]. In addition, forest types with better protective conditions are beneficial for small mammal activity but may lead to increased intraspecific competition, which increases the consumption and larder-hoarding of seeds by small mammals [11,12,13]. Thus, it is necessary to explore how forest type influences the dispersal of seeds by small mammals.
Food hoarding by small mammals not only contributes to their own reproduction and survival, but also plays a vital role in the natural regeneration of plants [6,9,14]. During the seed maturity season, small mammals hoard large quantities of seeds to secure nutrient supplies for survival and successful reproduction during food shortages [6,14]. On the other hand, hoarded (especially scatter-hoarded) seeds may have a higher chance of escaping predation and successfully germinating [15,16]. According to the Janzen−Connell hypothesis [17,18], the further a seed is dispersed from the parent tree, the higher the chance of it escaping predation and establishing as a seedling. Generally, the hoarding behavior of animals is classified into larder-hoarding and scatter-hoarding [19]. Larder-hoarding means that the animal places a large amount of food in the nest or at one site, while scatter-hoarding means that the animal caches the food in multiple sites, with only a small amount of food at each site [9,14]. Many factors influence the hoarding behavior and therefore the seed dispersal process, ultimately affecting plant population regeneration [20]. However, few studies have examined how small mammals disperse seeds of different seed sizes under different forest types.
Optimal foraging theory suggests that animals usually choose to consume higher-quality food in order to obtain higher energy returns during foraging [21,22]. Seed size serves as an important indicator of seed quality and nutritional value [23,24]. Large seeds generally have more nutrients, and when small mammals are faced with multiple choices, they may preferentially consume large seeds to obtain higher energy gain [25,26]. However, this view remains debated. Scatter-hoarding animals usually prefer to disperse and hoard large, nutrient-rich seeds over longer distances, while consuming smaller, less nutritious seeds in situ [27,28,29,30,31]. Because large seeds have more nutrients, this helps animals to obtain higher nutrient returns while retrieving the same number of seed points during food shortages [32,33]. Therefore, there may be a trade-off mechanism between the benefits and inputs of hoarding seeds of different sizes [34,35]. More empirical evidence is needed to clarify the seed size selection strategies of small mammals.
Liaodong oak (Q. wutaishanica) is a dominant tree species in warm temperate deciduous forests in China, and exerts a major influence on the characteristics, structure, dynamics, and species composition of these ecosystems [24,31,36]. Previous studies have mainly addressed the effects of seed size, seed density, seed burial, seed frequency, and other factors on rodent-mediated seed dispersal. However, there has been relatively little research on the impacts of forest types and plant diversity on rodent-mediated seed dispersal [24,30,31]. Our study aims to investigate the influence of seed size and forest type on seed predation and dispersal by small mammals. Specifically, we aim to understand (1) the impact of seed size on seed dispersal; (2) the influence of forest type on seed dispersal; and (3) whether there is an interaction effect between seed size and forest type in shaping seed dispersal.
2. Materials and Methods
2.1. Study Sites
The study sites focus on Larix principis-rupprechtii forest and Q. wutaishanica shrub located in the Dadaogou forest area (35°23′ N, 106°21′ E, 1900 m) of Longtan Forest Farm, part of the Liupanshan National Nature Reserve in Ningxia (Figure 1A,B) [24]. The tree cover of L. principis-rupprechtii forest exceeds 85%, but the shrub vegetation is sparse, with a small number of Q. wutaishanica saplings, accounting for approximately 5% cover. In contrast, the Q. wutaishanica shrub has high vegetation density, with a shrub layer cover exceeding 90%. Common tree species are Q. wutaishanica, Populus davidiana and Betula platyphylla of young trees. Shrubs include spear Euonymus phellomanus, Cotoneaster multiflorus, Fargesia nitida, Viburnum lobophyllum, Abelia dielsii, and Aralia chinensis [36]. Small mammals were investigated in the study area. The main small mammals were Niviventer confucianus, Apodemus peninsulae, Sciurotamias davidianus, Apodemus agrarius, etc.
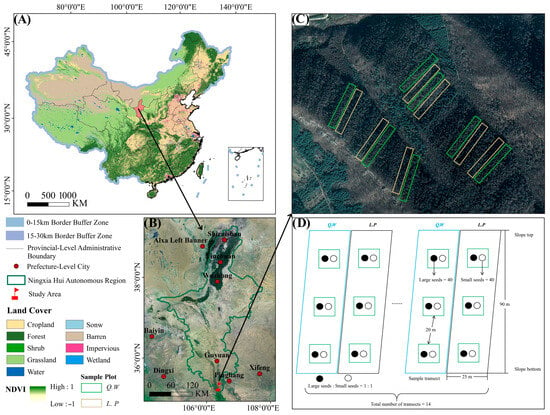
Figure 1.
Overview of the study area and schematic diagram of seed release. Note: Q.W: Quercus wutaishanica shrub; L.P: Larix principis-rupprechtii forest. (A,B): The specific geographical location of the study area. (C): Schematic diagram of plot selection. (D): Schematic diagram of seed release point.
2.2. Plants and Animals Survey
In July 2017, in L. principis-rupprechtii forest and Q. wutaishanica shrub, four transects were randomly selected, and one 10 m × 10 m quadrat was set up in each transect. The diameter at breast height (DBH) of woody plants with ≥1 cm was measured per tree. Three 1 m × 1 m herbaceous quadrats were randomly set up in each quadrat. We recorded the species name, number of trees, average height, and canopy cover of trees, shrubs and herbs [13]. The plant species diversity index is calculated as follows:
where pi is the relative abundance of the i-th species.
where pi is the relative abundance of the i-th species.
where H′ is the Shannon index, and S is the number of species.
where S is the number of species and N is the total number of individuals.
where D is the Simpson index and S is the number of species.
Species richness: S = Total species
Shannon–Wiener index: H′ = −∑ (pi × log(pi))
Simpson diversity index: D = 1 − ∑ (pi2)
Pielou’s evenness index: J = H′/log (S)
Margalef richness index: Dm = (S − 1)/log (N)
Simpson evenness: E = D/log (S)
When the seed experiment was completed, in June 2017, five transects were randomly selected for rodent species identification and population density surveys in L. principis-rupprechtii forest and Q. wutaishanica shrub. Ten live traps (25 × 12 × 12 cm steel cages) were placed along each transect, spaced at least 5 m apart. Using peanuts as bait, the cages were deployed in the evening and checked the following morning. Data including rodent counts, body weight, body length, tail length, and ear height were recorded, with specimens photographed for species identification. This monitoring protocol was conducted continuously for six days.
2.3. Seed Processing
Q. wutaishanica seeds were sampled from shrub plants in the vicinity of the study site. The fresh masses of small and large seeds were 1.46 ± 0.27 g (n = 100) and 3.05 ± 0.38 g (mean ± standard deviation, n = 100), respectively, showing a significant difference in fresh weight between different-sized seeds (p < 0.05) [31,37]. We drilled a small hole at the base of the seed with an electric drill of 0.5 mm in diameter. Then, a copper wire with a length of 7.5 cm was put through a small hole. The opposite end of the copper wire was attached to a red plastic tag measuring 2.5 cm × 1.2 cm (the total mass including the copper wire was 0.17 ± 0.002 g, n = 100).
2.4. Seed Experiment
In April 2017, we established seven sample transects in the Q. wutaishanica shrub (dominated by Q. wutaishanica with diverse shrub species) and L. principis-rupprechtii forest on the west side of Longtan Forest Farm in Liupanshan Nature Reserve (Figure 1C). Each sample transect was about 90 m long and 25 m wide, and three seed release sites were established along the sample transect from its top to the bottom, with each release point 20 m apart (Figure 1D). Each seed release point had 40 large seeds and 40 small seeds. The total number of seeds was 80 × 3 (slope repeat) × 7 (transect repeat) × 2 (forest type) = 3360 (Figure 1D).
We recorded the count of seeds subjected to predation and dispersal on the 1st, 2nd, 3rd, 5th, 7th, 10th, 20th, 30th and 35th days from release plots and within a radius of 30 m from each plot. The label records the information of the transect, release point and location coordinates [20,32,37,38]. Once small mammals consume the seeds, the attached labels are discarded at the feeding sites; meanwhile, labels on seeds that are either cached or discarded again post-dispersal are typically exposed on the surface. Consequently, the fate categories of the seeds can be identified by searching for these labels during field surveys [20]. Here, we recorded seed fates [9,30]: predation in situ (PIS), predation after dispersal (PAD) and hoarding after dispersal (HAD). For seeds that were missed (possibly located in burrows, not visible, or outside the 30 m boundary), no further analysis was carried out, as no significant differences were found across seed sizes and forest types. Additionally, two dispersal distances were recorded: predation distance after dispersal (PDAD) and hoarding distance after dispersal (HDAD) [30].
2.5. Data Analysis
The proportion of seeds in each fate category was calculated relative to the total number of seeds released. A mixed effects model was used to test the effects of seed size and forest type on predation and dispersal behavior in small mammals. We used seed size and forest type as explanatory variables, Block as a random effect, and predation in situ (PIS), predation after dispersal (PAD), hoarding after dispersal (HAD), predation distance after dispersal (PDAD) and hoarding distance after dispersal (HDAD) as dependent variables. We fitted generalized linear mixed-effects models for Gaussian variables (family = Gaussian, link = identity) and count variables (family = Poisson, link = log) in the “lmer4” package [39]. Furthermore, we used generalized linear mixed-effects models to test the pairwise interaction between seed size and forest type on seed fate and dispersal distance. All statistical analyses were conducted using R software (version 4.2.2, R Core Team, New Zealand) [40]. Then, we used two-way ANOVA to analyze the percentage of seeds with different fates and dispersal distance in different forest types. We also used two-way ANOVA to test the differences in different seed sizes in the same forest type. A square root transformation was applied if the data did not satisfy a normal distribution. We used the “vegan” package to analyze the plant diversity of different forest types. Data are expressed as mean ± standard deviation (SD) with a significant level of p < 0.05. Figures and tables were made with the assistance of Excel 2016 and SigmaPlot 12.5.
3. Results
3.1. Plant and Animal Survey
The plant species richness, Shannon–Wiener index, Simpson diversity index, Pielou evenness and Margalef index of the Q. wutaishanica shrub were significantly higher than that in L. principis-rupprechtii forest (p < 0.05) (Figure 2A–E) (Table S1). There was no significant difference in the Simpson evenness between the two forest types (Figure 2F) (Table S1). This evidence all indicates that the plant diversity of the Q. wutaishanica shrub is higher than that in L. principis-rupprechtii forest.
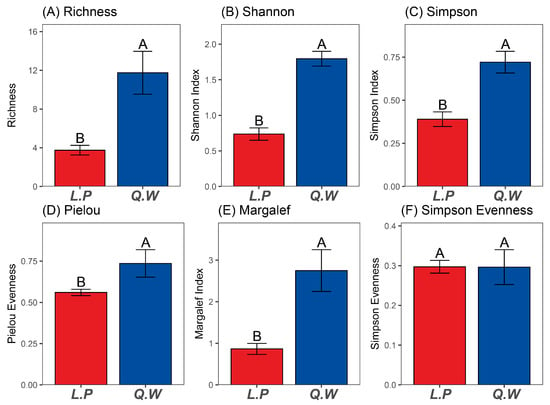
Figure 2.
Diversity index of two forest types. Note: Q.W: Quercus wutaishanica shrub; L.P: Larix principis-rupprechtii forest. The capital letters A and B indicate that there is a significant difference in species diversity between the Q.W and the L.P forest.
The L. principis-rupprechtii forest recorded five rodent species, while the Q. wutaishanica shrub recorded six species (Table 1 and Table S2). Capture rates of rodents for the Q. wutaishanica shrub were higher than for the L. principis-rupprechtii forest, implying a higher rodent density in the Q. wutaishanica shrub (Table 1 and Table S2).

Table 1.
Species composition of small rodent communities in Dadaogou in the Liupan mountains.
3.2. Effects of Seed Size and Forest Type on Seed Fates
Seed size had a significant effect on the predation in situ rate, which was significantly higher for small seeds than large seeds (F = 17.790, p < 0.001) (Figure 3A). The hoarding after dispersal rate of large seeds was significantly higher than small seeds (F = 9.487, p = 0.007) (Figure 3C). Different forest types had a significant impact on hoarding after dispersal rate, and the hoarding after dispersal of seeds in L. principis-rupprechtii forest was significantly higher than that in the Q. wutaishanica shrub (F = 6.553, p = 0.019) (Figure 3C). The interaction of seed size and forest type had no significant effect on seed fates (Table S3).
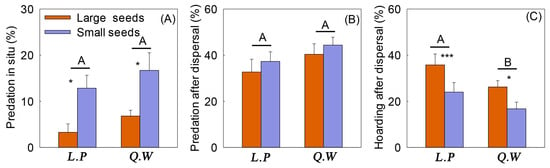
Figure 3.
Effect of seed size and forest type on seed predation and dispersal in small mammals. Note: L.P: Larix principis-rupprechtii forest; Q.W: Quercus wutaishanica shrub. Different capital letters indicate difference in different forest types, * indicates difference in seed size in same forest type, * p < 0.05, *** p < 0.001. (A): Predation in situ rates of rodents on large and small seeds under different forest types. (B): Predation after dispersal rates of rodents on large and small seeds under different forest types. (C): Hoarding after dispersal rates of rodents on large and small seeds under different forest types.
3.3. Effects of Seed Size and Forest Type on Seed Dispersal Distance
Both seed size and forest type significantly affected seed dispersal distance (Figure 4) (Table S3). The predation distance after dispersal (F = 94.029, p < 0.001) and hoarding distance after dispersal (F = 16.998, p < 0.001) of the large seeds were significantly higher than small seeds (Figure 4). The predation distance after dispersal (F = 21.753, p <0.001) and hoarding distance after dispersal (F = 7.522, p = 0.013) in the L. principis-rupprechtii forest were significantly higher than those in the Q. wutaishanica shrub (Figure 4). Seed size and forest type interaction had no significant effect on the distance of predation after seed dispersal (Figure 4).
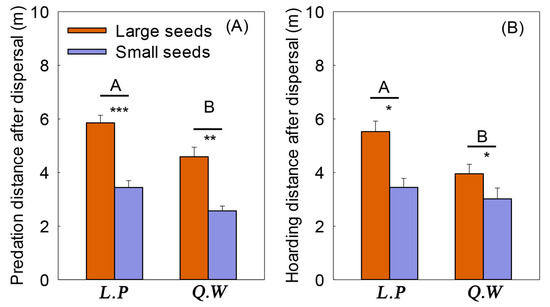
Figure 4.
Effect of seed size and forest type on predation and hoarding distance in small mammals. Note: L.P: Larix principis-rupprechtii forest; Q.W: Quercus wutaishanica shrub. Different capital letters indicate difference in different forest types, * indicates difference in seed sizes in the same forest type, * p < 0.05, ** p < 0.01, *** p < 0.001. (A): Predation distance after dispersal of rodents on large and small seeds under different forest types. (B): Hoarding distance after dispersal of rodents on large and small seeds under different forest types.
4. Discussion
We conducted field seed experiments and found that seed size and forest type affect seed consumption and dispersal by small mammals. Specifically, both seed size and forest type affect seed fates and dispersal distance, which is important for the regeneration of plant populations and the stability of forest ecosystems. Furthermore, we did not find an interaction of seed size and forest type in shaping seed consumption and dispersal in small mammals. This may be due to the strong influence of a single factor on hoarding behavior, masking potential interactive effects, or because the experiment scale was too small [9]. However, this study still has great significance. We proved that seed size and forest type can affect seed consumption and dispersal in small mammals, providing a scientific basis for understanding the role of animal hoarding behavior in seed dispersal and plant population regeneration.
4.1. Seed Size Affects Seed Consumption and Dispersal in Small Mammals
Small mammals select seeds according to a trade-off mechanism related to size. Seed size is related to nutrient content. Generally, the larger the seeds, the more nutrients they contain, and the more likely they are to be dispersed and hoarded [6,30,34,41]. In this study, we found that the selection of seeds of different sizes by rodents was consistent with the results of previous studies [24,30,31]. Small mammals prefer to eat small seeds in situ, disperse and cache large seeds, and disperse large seeds across longer distances [9,42]. This behavior suggests trade-offs in predation and dispersal related to seed size. Small mammals need to expend energy when foraging or dispersing seeds and may therefore consume more small seeds to meet immediate energy demands [27,30,34,42]. Small seeds are also easier to handle and can be quickly opened to access cotyledons [43].
We also found that the hoarding after dispersal of large seeds was significantly higher than that of small seeds, consistent with the findings of Wang et al. [23,44]. By hoarding larger seeds, small mammals can achieve equal or greater nutritional gains while having to search for fewer caching points [11,45]. Small animals take some time and energy to remove a large or small seed at the same distance, but hoarding large seeds provides higher returns in the future [9,14]. The results showed that the predation distance after dispersal and hoarding distance after dispersal of large seeds were significantly higher than those of small seeds, supporting the observations of Xiao et al. [29] and Jansen et al. [27]. The further away from the mother tree, the fewer competitors, the smaller the risk of seed theft [45]. To minimize the risk of food loss at cache sites, small mammals preferentially scatter-hoard large, nutrient-rich seeds at more distant locations [10].
4.2. Selection of Seeds in Small Mammals Under Different Forest Types
Forest type influenced seed dispersal by small mammals. Forest type differences are generally related to the distribution of animals and the safety of cache points [9]. Although some rodents unrelated to seed dispersal (e.g., Scaptochirus moschatus, Alexandromys fortis and Ellobius talpinus) were present, the rodent density in Q. wutaishanica shrub was still higher than that in L. principis-rupprechtii forest. In this study, the hoarding after dispersal in L. principis-rupprechtii forest was higher than in Q. wutaishanica shrub. According to our survey of animals and plants in the field experimental sites, the high shrub density and small mammal abundance in Q. wutaishanica shrub may intensify intraspecific competition [46]. To reduce the risk of food loss, small mammals may increase the larder hoarding of seeds and transport more seeds to the larder hoarding sites or nests [11,12]. This may explain the reduced probability of recovering cached seeds in shrub habitats. Forest type can affect seed dispersal distance. Our results showed that small mammals showed no significant difference in predation in situ or after dispersal in L. principis-rupprechtii forest or Q. wutaishanica shrub; this may be because the forest environment is safer compared to bare land and abandoned arable land, so it does not affect the seed predation of small mammals [47].
Our study found that both the predation distance after dispersal and hoarding distance after dispersal in L. principis-rupprechtii forest were larger than in Q. wutaishanica shrub, consistent with findings by Zhang and Zhang [48] and Lu and Zhang [7,8]. Forest types with dense vegetation conditions may have more hidden points and suitable cache points, while in forest types with poor vegetation conditions, small mammals need to disperse food to find safer cache points [14,48,49,50]. In this study, L. principis-rupprechtii forest shrubs had low shrub density and fewer hidden and suitable cache points than Q. wutaishanica shrub, forcing small mammals to hoard seeds at greater distances. Moreover, we should also consider that intraspecific personality differences among rodents may influence the selection of seeds of different sizes under different forest types [20,43,51,52].
The interaction between forest type and seed size has no significant impact on rodent-mediated seed dispersal. This may indicate that the influence of seed size or forest type on seed dispersal is greater than their interaction. It suggests that rodents tend to have consistent preferences for seed size in different secondary forests. However, rapid environmental changes caused by human activities (such as deforestation and urbanization) might alter how rodents disperse seeds [2,53]. For example, large-scale deforestation may degrade rodent habitats, strongly affecting seed size selection and dispersal outcomes, ultimately influencing plant population dynamics [5,20]. In the future, we should continue to investigate how rodents change their seed dispersal patterns when forest communities undergo varying degrees of change.
5. Conclusions
In conclusion, small mammals exhibited a preference for larger seeds, dispersing them over longer distances, possibly as a strategy to optimize future food resources. The dispersal of large seeds to greater distances may offer advantages for seed germination, seedling establishment, and the overall regeneration of plant populations. In forest types with poor vegetation conditions, the scarcity of suitable cache sites forces small mammals to disperse seeds further to secure safer storage. Conversely, in dense forests, heightened small mammal activity may intensify competition, potentially leading to greater hoarding but also to negative consequences for plant regeneration. Importantly, no significant interaction between seed size and forest type was detected in seed predation or dispersal. Further investigations are needed to clarify the combined effects of seed size and forest type to achieve a more comprehensive understanding of seed dispersal dynamics.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d17090643/s1, Table S1: Species composition of plant communities in Dadaogou in the Liupan mountains; Table S2: Species composition of small rodents in Dadaogou in the Liupan mountains; Table S3: Seed fate statistics across blocks, forest types, and seed sizes.
Author Contributions
Conceptualization: J.C. and Y.F.; methodology: Y.F. and N.W.; formal analysis and investigation: J.C., C.Z., J.L. and X.C.; writing—original draft preparation: J.C. and Y.F.; writing—review and editing: N.W., C.Z., J.L., X.C., X.Y., and Y.L.; funding acquisition: J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Special Fund for Basic Scientific Research Expenses of Central Universities of North Minzu University (Grant No. 2025QNPY12) and the National Natural Science Foundation of China (Grant No. 32560127).
Institutional Review Board Statement
The animal study protocol was approved by the Experimental Animal Welfare and Ethics Review Committee of North Minzu University, Yinchuan, in accordance with the university’s guidelines for good scientific practice (2015-03, 16 January 2015).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to in fluence the findings reported in this paper.
References
- Qiao, X.; Lamy, T.; Wang, S.P.; Hautier, Y.; Geng, Y.Y.; White, H.J.; Zhang, N.L.; Zhang, Z.H.; Zhang, C.Y.; Zhao, X.H.; et al. Latitudinal patterns of forest ecosystem stability across spatial scales as affected by biodiversity and environmental heterogeneity. Glob. Change Biol. 2023, 29, 2242–2255. [Google Scholar] [CrossRef] [PubMed]
- Potapov, A.M.; Drescher, J.; Darras, K.; Wenzel, A.; Janotta, N.; Nazarreta, R.; Kasmiatun; Laurent, V.; Mawan, A.; Utari, E.H.; et al. Rainforest transformation reallocates energy from green to brown food webs. Nature 2024, 627, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Giljum, S.; Maus, V.; Sonter, L.; Luckeneder, S.; Werner, T.; Lutter, S.; Gershenzon, J.; Cole, M.J.; Siqueira-Gay, J.; Bebbington, A. Metal mining is a global driver of environmental change. Nat. Rev. Earth Environ. 2025, 6, 441–455. [Google Scholar] [CrossRef]
- Gómez, J.M. Spatial patterns in long-distance of Quercus ilex acorns by jays in a heterogeneus landscape. Ecography 2003, 26, 573–584. [Google Scholar] [CrossRef]
- Niu, H.Y.; Rehling, F.; Chen, Z.W.; Yue, X.C.; Zhao, H.Y.; Wang, X.R.; Zhang, H.M.; Schabo, D.G.; Farwig, N. Regeneration of urban forests as influenced by fragmentation, seed dispersal mode and the legacy effect of reforestation interventions. Landsc. Urban Plan. 2023, 233, 104712. [Google Scholar] [CrossRef]
- Fricke, E.C.; Bello, C.; Chaplin-Kramer, R.; Dent, D.H.; Feeley, K.J.; Galetti, M.; González-Varo, J.P.; Heleno, R.; Reid, J.L. Drivers and impacts of global seed disperser decline. Nat. Rev. Biodivers. 2025, 1, 386–400. [Google Scholar] [CrossRef]
- Lu, J.Q.; Zhang, Z.B. Effects of habitat and season on removal and hoarding of seeds of wild apricot (Prunus armeniaca) by small rodents. Acta Oecol. 2004, 26, 247–254. [Google Scholar]
- Lu, J.Q.; Zhang, Z.B. Seed-hoarding behavior of wild apricot and Liaodong oak by small rodents. Acta Theriol. Sin. 2004, 24, 132–138. [Google Scholar]
- Zhang, Z.B. Studies on the Rodent–Seed Interactions of Forest Ecosystems: Exploring the Secrets of Cooperation Between Antagonists; Science Press: Beijing, China, 2019. [Google Scholar]
- Brehm, A.M.; Mortelliti, A. Small mammal personalities generate context dependence in the seed dispersal mutualism. Proc. Natl. Acad. Sci. USA 2022, 119, e2113870119. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, Y.; Zhang, Z.B. Response of seed-hoarding behaviour to consepecific audiences in scatter- and/or larder-hoarding rodents. Behaviour 2011, 148, 825–842. [Google Scholar]
- Zhang, H.M.; Steele, M.A.; Zhang, Z.B.; Wang, W.; Wang, Y. Rapid sequestration and recaching by a scatter-hoarding rodent (Sciurotamias davidianus). J. Mammal. 2014, 95, 480–490. [Google Scholar] [CrossRef]
- Garcés-Pastor, S.; Heintzman, P.D.; Zetter, S.; Lammers, Y.; Yoccoz, N.G.; Theurillat, J.-P.; Schwörer, C.; Tribsch, A.; Walsh, K.; Vannière, B.; et al. Wild and domesticated animal abundance is associated with greater late-Holocene alpine plant diversity. Nat. Commun. 2025, 16, 3924. [Google Scholar] [CrossRef]
- Vander Wall, S.B. Food Hoarding in Animals; University of Chicago Press: Chicago, IL, USA, 1990. [Google Scholar]
- Howe, H.F.; Smallwood, J. Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 1982, 13, 201–228. [Google Scholar] [CrossRef]
- Zhang, H.M.; Niu, H.Y.; Steele, M.A.; Peng, L.Q.; He, H.M.; Li, A.Q.; Yi, X.F.; Li, H.J.; Zhang, Z.B. Masting promotes transformation from predation to mutualism in an oak-weevil-rodent system. Sci. China Life Sci. 2024, 67, 1514–1524. [Google Scholar] [CrossRef]
- Janzen, D.H. Herbivores and the number of tree species in tropical forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Connell, J.H. On the role of natural enemies in preventing competitive exclusion in some marine mammals and in rain forest trees. In Dynamics of Populations; Boer, P.J., Gradwell, G., Eds.; Center for Agricultural Publication and Documentation: Wageningen, The Netherlands, 1971. [Google Scholar]
- Loayza, A.P.; Luna, C.A.; Calviño-Cancela, M. Predators and dispersers: Context-dependent outcomes of the interactions between rodents and a megafaunal fruit plant. Sci. Rep. 2020, 10, 6106. [Google Scholar] [CrossRef]
- Cheng, J.M.; He, H.M.; Zheng, L.L.; Zhang, C.; Wang, X.R.; Hu, X.Y.; Niu, H.Y.; Zhang, H.M. Bold rats (Niviventer confucianus) are more effective in seed dispersal: Evidences both under enclosure conditions and in the field. Integr. Zool. 2025, 20, 740–754. [Google Scholar] [CrossRef]
- Lewis, A.R. Selection of nuts by gray squirrels and optimal foraging theory. Am. Midl. Nat. 1982, 107, 250–257. [Google Scholar] [CrossRef]
- Rizzuto, M.; Carbone, C.; Pawar, S. Foraging constraints reverse the scaling of activity time in carnivores. Nat. Ecol. Evol. 2018, 2, 247–253. [Google Scholar] [CrossRef]
- Wang, B.; Chen, J.; Corlett, R.T. Factors influencing repeated seed movements by scatter-hoarding rodents in an alpine forest. Sci. Rep. 2014, 4, 4786. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.M.; Zhang, M.; Yan, X.F. Effects of seed size and cache density on the seed fate of Quercus wutaishanica mediated by rodents. Life 2024, 14, 286. [Google Scholar] [CrossRef]
- Janzen, D.H. Seed predation by animals. Annu. Rev. Ecol. Syst. 1971, 2, 465–492. [Google Scholar] [CrossRef]
- Qiu, T.; Andrus, R.; Aravena, M.-C.; Ascoli, D.; Bergeron, Y.; Berretti, R.; Berveiller, D.; Bogdziewicz, M.; Boivin, T.; Bonal, R.; et al. Limits to reproduction and seed size-number trade-offs that shape forest dominance and future recovery. Nat. Commun. 2022, 13, 2381. [Google Scholar] [CrossRef]
- Jansen, P.A.; Bongers, F.; Hemerik, L. Seed mass and mast seeding enhance dispersal by a neotropical scatter-hoarding rodent. Ecol. Monogr. 2004, 74, 569–589. [Google Scholar] [CrossRef]
- Xiao, Z.S.; Zhang, Z.B.; Wang, Y.S. Dispersal and germination of big and small nuts of Quercus serrata in a subtropical broad-leaved evergreen forest. For. Ecol. Manag. 2004, 195, 141–150. [Google Scholar] [CrossRef]
- Xiao, Z.S.; Zhang, Z.B.; Wang, Y.S. The effects of seed abundance on seed predation and dispersal by rodents in Castanopsis fargesii (Fagaceae). Plant Ecol. 2005, 177, 249–257. [Google Scholar] [CrossRef]
- Zhang, H.M.; Chen, Y.; Zhang, Z.B. Differences of dispersal fitness of large and small acorns of Liaodong oak (Quercus liaotungensis) before and after seed caching by small rodents in a warm temperate forest, China. For. Ecol. Manag. 2008, 255, 1243–1250. [Google Scholar] [CrossRef]
- Cheng, J.M.; Zhang, M.; Yan, X.F.; Zhang, C.; Zhang, J.F.; Luo, Y.H. Effects of seed size and frequency on seed dispersal and predation by small mammals. Biology 2024, 13, 353. [Google Scholar] [CrossRef]
- Xiao, Z.S.; Jansen, P.A.; Zhang, Z.B. Using seed-tagging methods for assessing post-dispersal seed fate in rodent-dispersed trees. For. Ecol. Manag. 2006, 223, 18–23. [Google Scholar] [CrossRef]
- Liang, Z.L.; Ma, J.Z.; Rong, K. Animal scatter-hoarding behavior and its impact on the regeneration of plant populations. Acta Ecol. Sin. 2016, 36, 1162–1169. [Google Scholar] [CrossRef]
- Dylewski, L.; Ortega, Y.K.; Bogdziewicz, M.; Pearson, D.E. Seed size predicts global effects of small mammal seed predation on plant recruitment. Ecol. Lett. 2020, 23, 1024–1033. [Google Scholar] [CrossRef]
- Chen, S.C.; Antonelli, A.; Huang, X.; Wei, N.; Dai, C.A.; Wang, Q.F. Large seeds as a defensive strategy against partial granivory in the Fagaceae. J. Ecol. 2025, 113, 598–607. [Google Scholar] [CrossRef]
- Cheng, J.M.; Zhang, C.; Yan, X.F.; Feng, Y.Q.; Wang, J.Z.; Wei, S.H.; Jin, L.; Zhang, J.F.; Chen, J.; Zhang, J.B.; et al. The photosynthetic traits of dominant species drive the multifunctionality of Liaodong oak (Quercus wutaishanica) communities in northern China. Glob. Ecol. Conserv. 2024, 56, e03323. [Google Scholar] [CrossRef]
- Luo, Y.H.; Cheng, J.M.; Yan, X.F.; Yang, H.; Shen, Y.; Ge, J.R.; Zhang, M.; Zhang, J.F.; Xu, Z.W. Density-dependent seed predation of Quercus wutaishanica by rodents in response to different seed states. Animals 2023, 13, 1732. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Wang, Z.Y.; Chang, G.; Yi, X.F.; Lu, J.Q.; Xiao, Z.S.; Zhang, H.M.; Cao, L.; Wang, F.S.; Li, H.J.; et al. Trade-off between seed defensive traits and impacts on interaction patterns between seeds and rodents in forest ecosystems. Plant Ecol. 2016, 217, 253–265. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Henry, H.M. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2019, 24, 127–135. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Xiao, Z.S.; Zhang, Z.B. Nut predation and dispersal of Harland Tanoak Lithocarpus harlandii by scatter-hoarding rodents. Acta Oecol. 2006, 29, 205–213. [Google Scholar] [CrossRef]
- Vander Wall, S.B. On the relative contributions of wind vs. animals to seed dispersal of four Sierra Nevada pines. Ecology 2008, 89, 1837–1849. [Google Scholar] [CrossRef]
- Cheng, J.M.; He, H.M.; Niu, H.Y.; Zhang, H.M. Research progress on the effect of intraspecific personality differences on seed dispersal in rodents. Biodivers. Sci. 2023, 31, 171–185. [Google Scholar] [CrossRef]
- Wang, B.; Wang, G.; Chen, J. Scatter-hoarding rodents use different foraging strategies for seeds from different plant species. Plant Ecol. 2012, 213, 1329–1336. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Wang, Y.; Zhang, H.M.; Wu, F.Q.; Zhang, Z.B. Behavioural responses of sympatric rodents to complete pilferage. Anim. Behav. 2011, 81, 831–836. [Google Scholar] [CrossRef]
- Huang, W.; Traulsen, A.; Werner, B.; Hiltunen, T.; Becks, T. Dynamical trade-offs arise from antagonistic coevolution and decrease intraspecific diversity. Nat. Commun. 2017, 8, 2059. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, A.L.; Ensing, J.; Rahn, O.; Oliveira, F.M.P.; Burkiewicz, J.; Lafond, J.; Haeussler, S.; Byerley-Best, M.B.; Lazda, K.; Slinn, H.L.; et al. Latitudinal gradients in seed predation persist in urbanized environments. Nat. Ecol. Evol. 2024, 8, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Zhang, Z.B. Effects of soil depth, cache spacing and cache size of sunflower (Helianthus annuus) seeds on seed discovery by Siberian chipmunk (Tamias sibiricus senescens). Acta Theriol. Sin. 2006, 26, 398–402. [Google Scholar]
- Jansen, P.A.; Forget, P.M. Scatter-hoarding rodents and tree regeneration. In Dynamics and Plant-Animal Interaction in a Neotropical Rainforest; Bongers, F., Charles-Dominique, P., Forget, P.M., Théry, M., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2001; pp. 275–288. [Google Scholar]
- Yan, X.F.; Zhou, L.B.; Liu, J.L. Effects of different habitats and coverage treatments on the fates of Quercus wutaishanica seeds under the predation pressure of rodents. Acta Theriol. Sin. 2012, 32, 2778–2787. [Google Scholar]
- Cheng, J.M.; He, H.M.; Zhang, M.; Gan, B.Q.; Niu, H.Y.; Zhang, H.M. A review to the methods of personality measurements in wild animals. Wildl. Lett. 2023, 1, 131–142. [Google Scholar] [CrossRef]
- Cheng, J.M.; He, H.M.; Zheng, L.L.; Niu, H.Y.; Zhang, H.M. Are the personality measures for laboratory mice applicable for wild rats? Behav. Ecol. Sociobiol. 2025, 79, 62. [Google Scholar] [CrossRef]
- Guo, W.Y.; Serra-Diaz, J.M.; Eiserhardt, W.L.; Maitner, B.S.; Merow, C.; Violle, C.; Pound, M.J.; Sun, M.; Slik, F.; Blach-Overgaard, A.; et al. Climate change and land use threaten global hotspots of phylogenetic endemism for trees. Nat. Commun. 2023, 14, 6950. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).