Helminths of Cats (Felis catus Linnaeus, 1758) and Their Larval Stages in Reptiles in Dubai, United Arab Emirates
Abstract
1. Introduction
2. Materials and Methods
2.1. Cat Helminths
2.2. Reptile Helminths
2.3. Statistical Analysis
3. Results
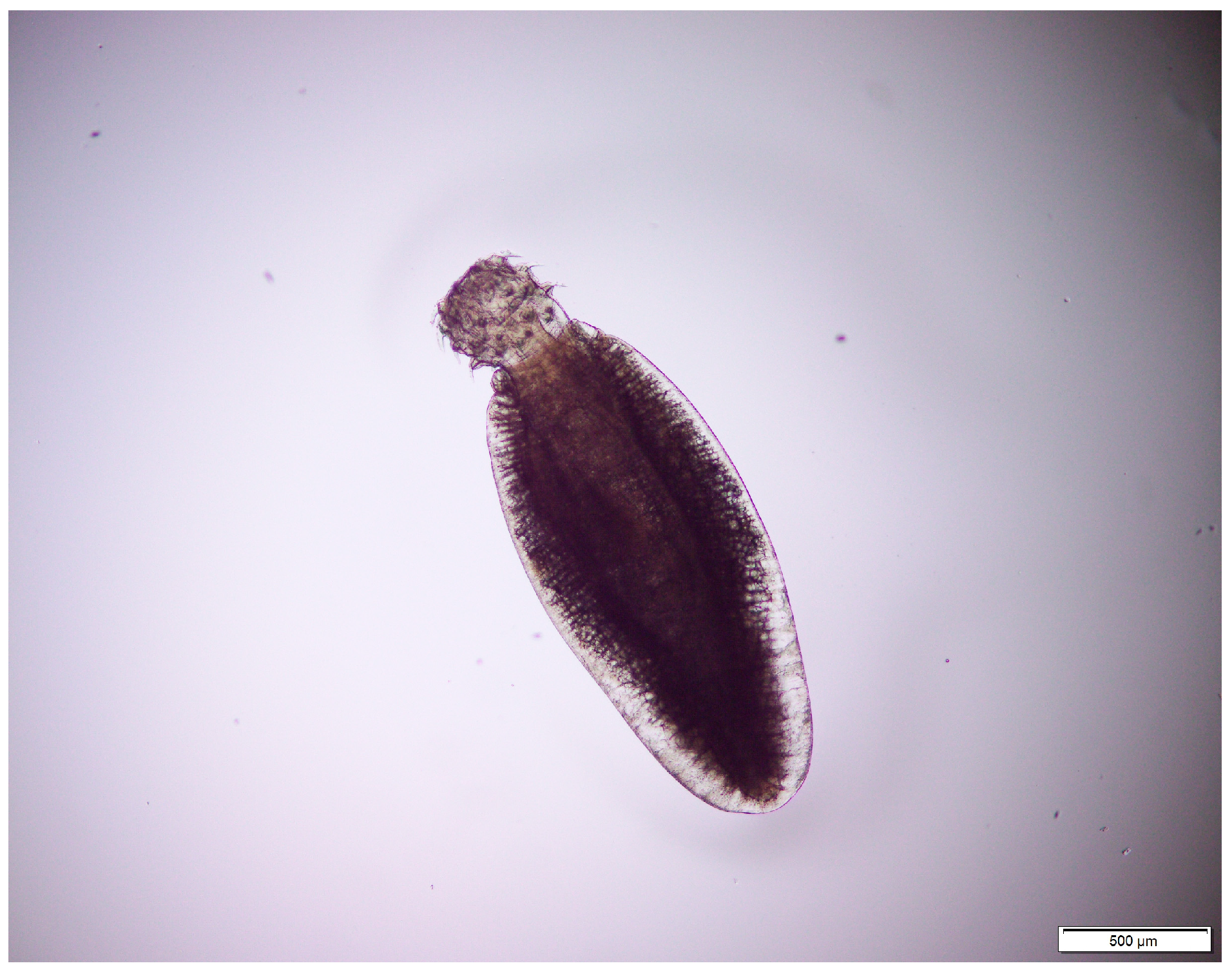
3.1. Helminths of Cats
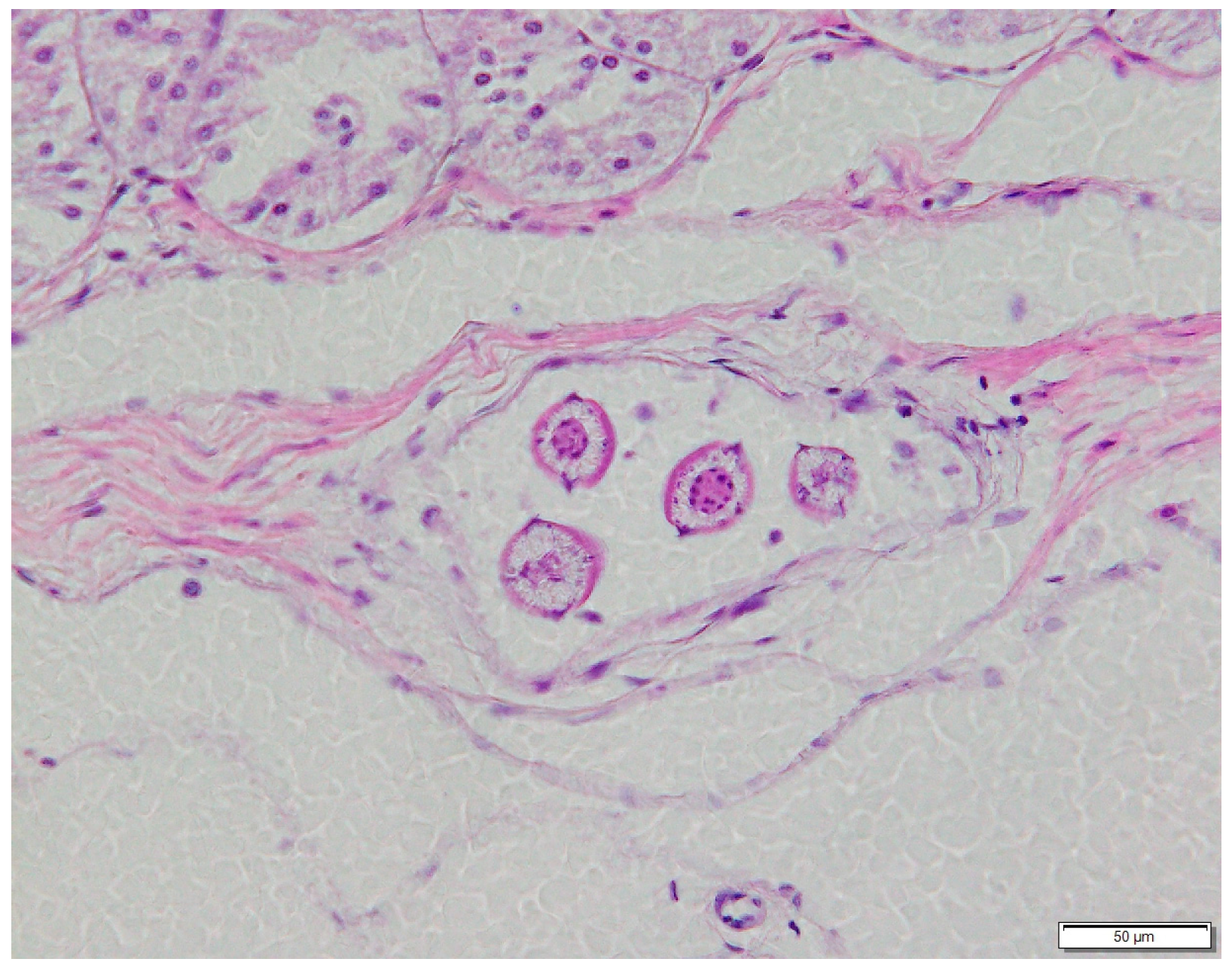
3.2. Larval Helminths of Reptiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schuster, R.K. Cestodes of the genera Diplopylidium and Joyeuxiella (Eucestoda: Dipylidiidae—A review of historical data, species inventory and geographical distribution. Sci. Parasitol. 2020, 21, 1–17. [Google Scholar]
- Witenberg, G. On the cestodes subfamily Dipylidiinae Stiles. Z. Parasitenk. 1932, 4, 542–584. [Google Scholar] [CrossRef]
- Marchi, M. Sopra una nuovo cestode trovato nell’ Ascalabotes mauritanicus. Atti Soc. Ital. Sci. Nat. 1872, 15, 505–506. [Google Scholar]
- Parona, C. Elmintologia sarda. Contribuzione allo studio dei vermi parassiti in animali di Sardegna. Ann. Mus. Civ. Genova 1886, 24, 275–384. [Google Scholar]
- Diamare, V. Bemerkungen ueber Dipylidienlarven. Zentralbl. Bact. Parasitenk. 1894, 14, 565–566. [Google Scholar]
- Parrot, L.; Joyeux, C. Les cysticercoids de Tarentola mauritanica L. et les tenias du chat. Bull. Soc. Pathol. Exot. 1920, 13, 687–695. [Google Scholar]
- Gardner, A.S. The Amphibians and Reptiles of Oman and the UAE; Edition Chimaira: Frankfurt am Main, Germany, 2013; 480p. [Google Scholar]
- Schuster, R.K.; Thomas, K.; Sivakumar, S.; O’Donovan, D. The parasite fauna of stray domestic cats (Felis catus) in Dubai, United Arab Emirates. Parasitol. Res. 2009, 105, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.K.; Mustafa, M.B.; Chester, S.T.; Knaus, M. Efficacy of Broadline® (Merial) against naturally acquired infections with cestodes of the genus Joyeuxiella. Parasitol. Res. 2016, 115, 2679–2684. [Google Scholar] [CrossRef]
- Reiczigel, J.; Marozzi, M.; Fabian, I.; Rozsa, L. Biostatistics for parasitologists—A primer to Quantitative Parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef]
- Schuster, R.K.; Mehmood, N.; Varcasia, A.V.; Veneziano, V. Redescription and resurrection of the status of Joyeuxiella gervaisi (Setti, 1895) (Eucestoda, Dipylidiidae). Helminthologia 2023, 60, 166–174. [Google Scholar] [CrossRef]
- Abu-Madi, M.A.; Al-Ahbabi, D.A.; Al-Mashhadani, M.M.; Al-Ibrahim, R.; Pal, P.; Lewis, J.W. Patterns of parasitic infections in faecal samples from stray cat populations in Qatar. J. Helminthol. 2007, 81, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Abu-Madi, M.A.; Behnke, J.M.; Prabhaker, K.S.; Al-Ibrahim, R.; Lewis, J. Intestinal helminths of feral cat populations from urban and suburban districts of Qatar. Vet. Parasitol. 2010, 168, 284–292. [Google Scholar] [CrossRef]
- Abu-Madi, M.A.; Pal, P.; Al-Thani, A.; Lewis, J.W. Descriptive epidemiology of intestinal helminth parasites from stray cat populations in Qatar. J. Helminthol. 2008, 82, 59–68. [Google Scholar] [CrossRef]
- Abdul-Salam, J.; Baker, K. Prevalence of intestinal helminths in stray cats in Kuwait. Pakistan Vet. J. 1990, 10, 17–21. [Google Scholar]
- El-Azazy, O.M.E.; Abdou, N.-E.; Khalil, A.I.; Al-Batel, M.K.; Majeed, Q.A.; Henedi, A.A.M.; Tahrani, L.M. Potential Zoonotic Trematodes Recovered in Stray Cats from Kuwait Municipality, Kuwait. Korean J. Parasitol. 2015, 53, 279–287. [Google Scholar] [CrossRef] [PubMed]
- El-Azazy, O.M.E.; Abdou, N.-E.; Khalil, A.I.; Al-Batel, M.K.; Majeed, Q.A.; Henedi, A.A.M.; Tahrani, L.M.A. Cestodes and nematodes recorded in stray cats in Kuwait. Glob. Vet. 2016, 16, 111–118. [Google Scholar]
- Morsy, T.A.; Michael, S.A.; El-Disi, A.M. Cats as reservoir hosts of human parasites in Amman, Jordan. J. Egypt. Soc. Parasitol. 1980, 10, 5–18. [Google Scholar]
- Morsy, T.A.; Sadek, M.S.M.; Al-Hamid, M.Y. Intestinal parasites in stray cats in Cairo, Egypt. J. Egypt. Soc. Parasitol. 1981, 11, 331–345. [Google Scholar]
- Khalafalla, R.E. A survey study on gastrointestinal parasites of stray cats in northern region of Nile Delta, Egypt. PLoS ONE 2011, 6, e20283. [Google Scholar] [CrossRef]
- Ayoub, M.B. Parasitic infection in stray cats and dogs with special reference to ultrastructure of the recovered worms. Animal Health Res. J. 2014, 2, 165–178. [Google Scholar]
- Nihad, W.; Al-Khalidi, M.; Tafiq, I.; Al-Alousi, M.; Subber, A.H. Internal and external parasites in cats in Mosul, Iraq. J. Vet. Parasitol. 1988, 2, 137–138. [Google Scholar]
- Al-Obaidi, Q.T. Prevalence of internal helminthes in stray cats (Felis catus) in Mosul city, Mosul-Iraq. J. Anim. Vet. Adv. 2023, 11, 2732–2736. [Google Scholar] [CrossRef]
- Hadi, A.M.; Faraj, A.A. Role of domestic cats Felis catus as reservoir hosts of internal parasites and protozoa in Baghdad. Bull. Iraq Nat. Hist. Mus. 2014, 13, 89–94. [Google Scholar]
- Al-Aredhi, H.S. Prevalence of gastrointestinal parasites in domestic cats (Felis catus) in Al-Diwaniya province/Iraq. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 166–171. [Google Scholar]
- Al-Tae, A.-R.A.; Al Rubaie, A.-R.L. Checklist of parasites of stray cats Felis catus of Iraq. Ibn Al-Haitham J. Pure Appl. Sci. 2017, 1782, 143–152. [Google Scholar] [CrossRef]
- Changizi, E.; Mobedi, I.; Salimi-Bejestani, M.R.; Rezaei-Doust, A. Gastrointestinal helmintic parasites in stray cats (Felis catus) from north of Iran. Iran. J. Parasitol. 2007, 2, 25–29. [Google Scholar]
- Esmaeilzadeh, M.; Shamsfard, M.; Kazemi, A.; Khalafi, S.A.; Altome, S.A. Prevalence of protozoa and gastrointestinal helminthes in stray cats in Zanjan Province, north-west of Iran. Iran. J. Parasitol. 2009, 4, 71–75. [Google Scholar]
- Arbabi, M.; Hoshyar, H. Gastrointestinal parasites of stray cats in Kashan, Iran. Trop. Biomed. 2009, 26, 16–22. [Google Scholar]
- Garedaghi, Y.; Firouzivand, Y. Prevalence of gastrointestinal parasites of domestic cats and its zoonotic importance in Tabriz city, Iran. Cibtech J. Zool. 2014, 3, 87–92. [Google Scholar]
- Hajipour, N.; Baran, A.I.; Yakhchali, M.; Banan Khojasteh, S.M.; Hesari, F.S.; Esmaeinejad, B.; Arimand, J. A survey study on gastrointestinal parasites of stray cats in Azarshahr, (East Azerbaijan province, Iran). J. Parasit. Dis. 2016, 40, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Yakhchali, M.; Hajipour, N.; Malekzadeh-Viayeh, R.; Esmaeilnejad, B.; Nemati-Haravani, T.; Fathollahzadeh, M.; Jafari, R. Gastrointestinal helminths and ectoparasites in the stray cats (Felidae: Felis catus) of Ahar Municipality, Northwestern Iran. Iran. J. Parasitol. 2017, 12, 298–304. [Google Scholar]
- Soroushianfar, M.; Sadr, A.; Sazmand, A.; Dianaty, S.; Khedi, J.; Schuster, R.K.; Borji, H. Gastrointestinal parasites of cats in the Middle East (2000–2023): A literature review. Parasitol. Int. 2024, 102, 102919. [Google Scholar] [CrossRef]
- Ahmadi, A.; Oryyan, A.; Alidadi, S. Parasites of stray cats in Iran: A parasitological and histopathological study. Acta Parasitol. 2024, 69, 664–674. [Google Scholar] [CrossRef]
- Barih, Ö; Tuygun, T.; Gençay Topçu, E.B.; Umur, Ş. The parasites of cats in Türkiye. Turkiye Parazitol. Derg. 2023, 47, 190–199. [Google Scholar] [CrossRef]
- Diamare, V. Note su cestodi. Boll. Soc. Natur. Napoli 1893, 7, 9–13. [Google Scholar]
- Sonsino, P. Noticie elmintologiche. Atti della Societa Toscana di Scienze Naturali Residente in Piesa. Process. Verbali 1889, 6, 191–194. [Google Scholar]
- Chochlova, I.G. Acanthocephala of Terrestrical Animals of the Fauna of the USSR [Akantocefaly Nazemnych Zivotnych Fauny SSSR]; Izdatel’stvo Nauka: Moscow, Russia, 1986; 275p. (In Russian) [Google Scholar]
- Schuster, R.K.; Sivakumar, S.; Kinne, J. Parasite findings in the MacQueen’s bustard, Chlamydotis macqueenii (Grey, 1832), and considerations on the parasite fauna of bustards and the systematic position of some of the parasites. Vet. Parasitol. Reg. Stud. Rep. 2025, 57, 101146. [Google Scholar] [CrossRef] [PubMed]
- Popov, P. Sur le development de Diplopylidium skrjabini n. sp. Ann. Parasitol. 1935, 13, 322–326. [Google Scholar] [CrossRef]
- Lopez-Neyra, C.R. Sobre la evolucion de la Joyeuxia chyceri v. Ratz. Infeccion experimental de la salamanquesas. Bol. Real. Soc. Espan. Hist. Nat. 1927, 27, 398–399. [Google Scholar]
- Bezerra-Santos, M.A.; Mendoza-Roldan, J.A.; Lia, R.P.; Annoscia, G.; Schuster, R.; Varcasia, A.; Sgroi, G.; Modry, D.; Otranto, D. Description of Joyeuxiella pasqualei (Cestoda: Dipylidiidae) from an Italian domestic dog, with a call for further research on its first intermediate host. Parasitology 2022, 149, 1769–1774. [Google Scholar] [CrossRef]

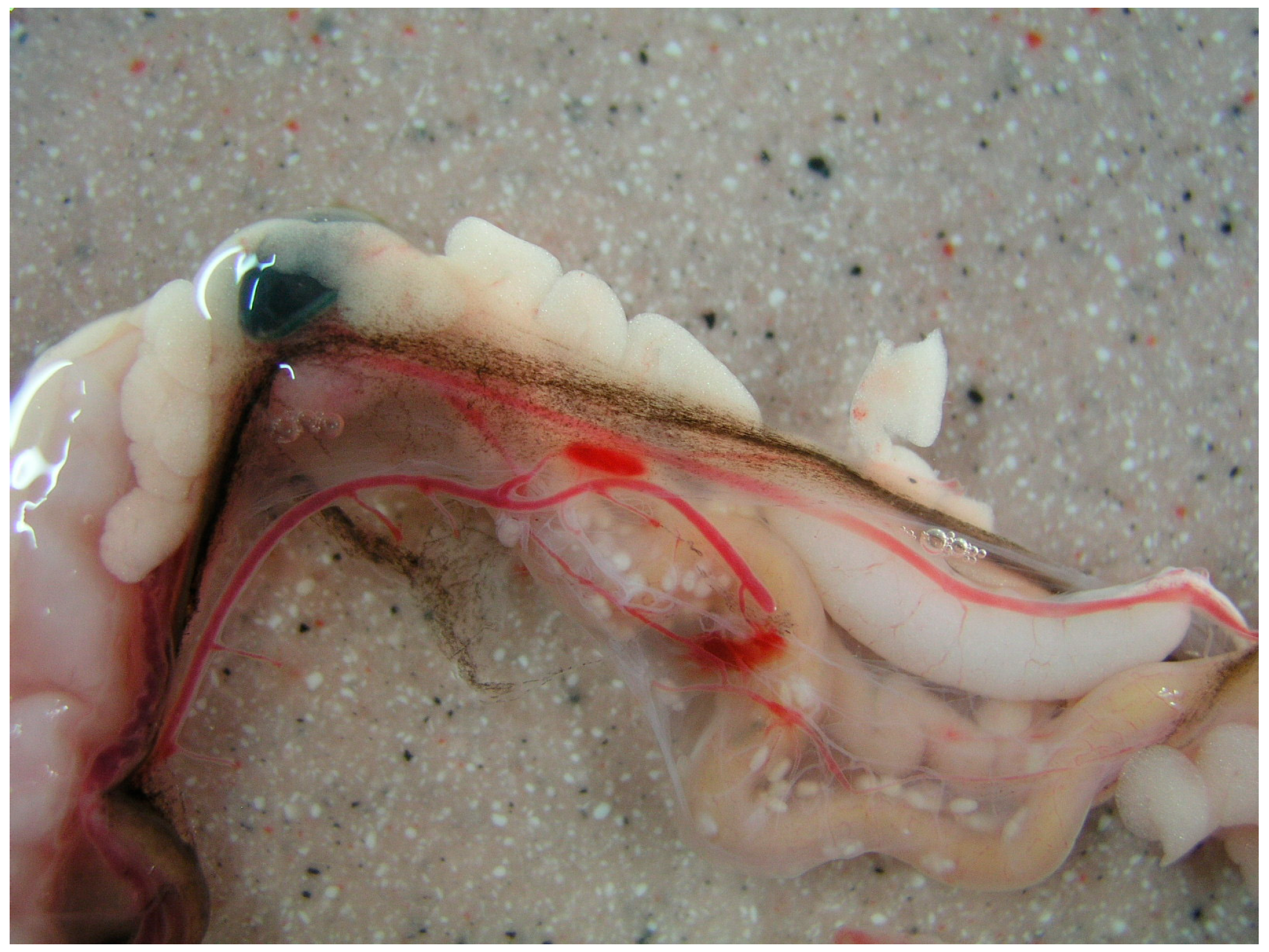

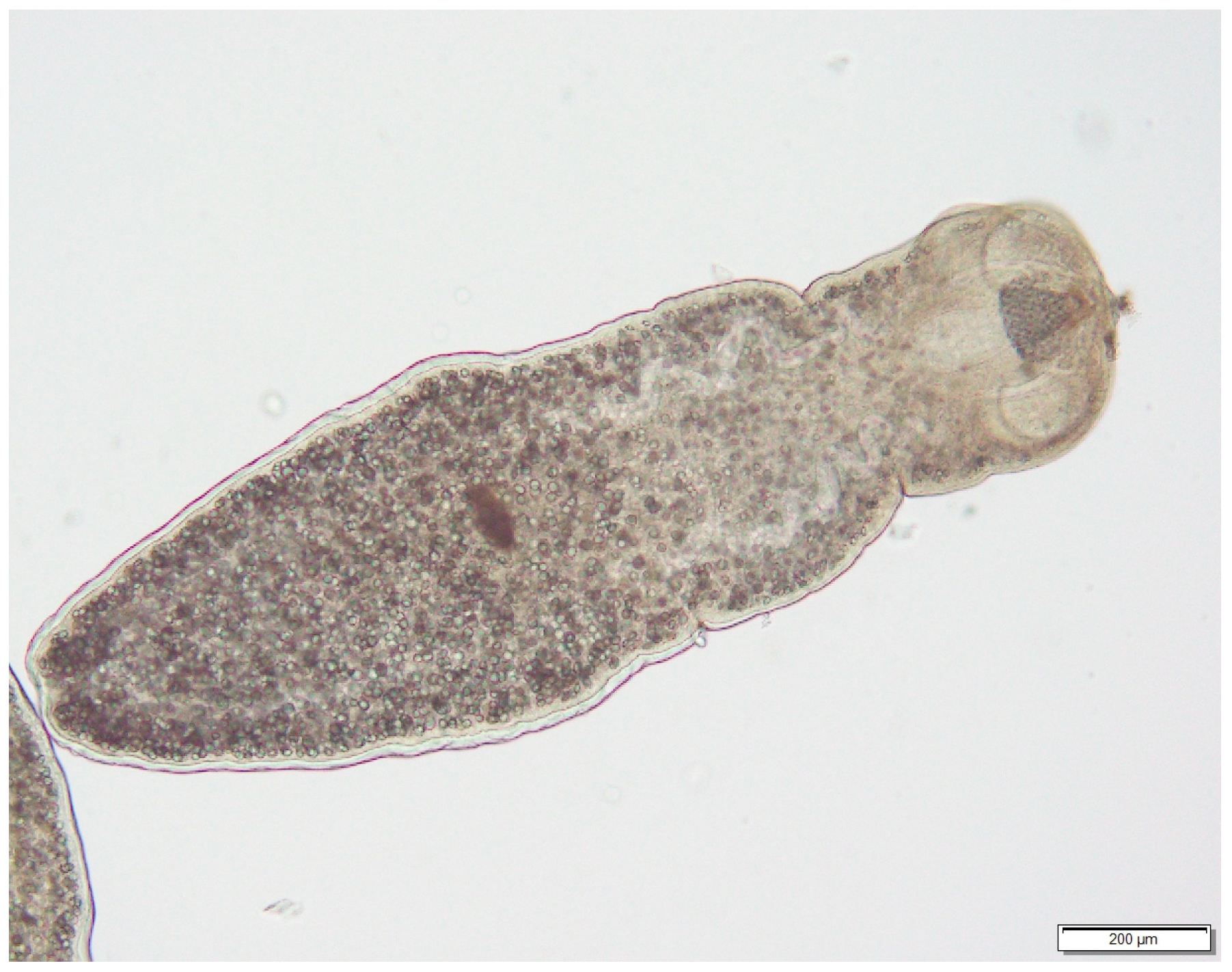
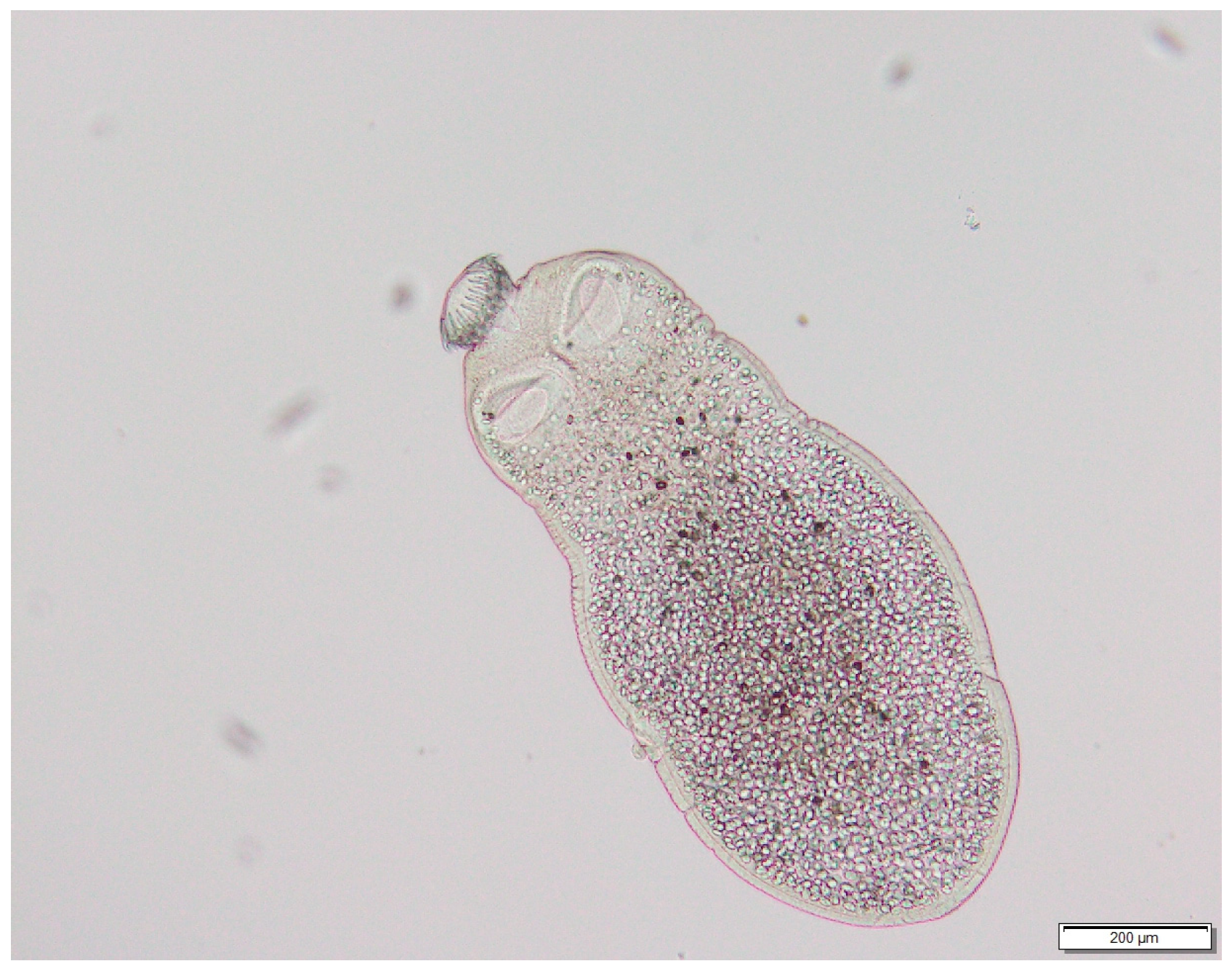
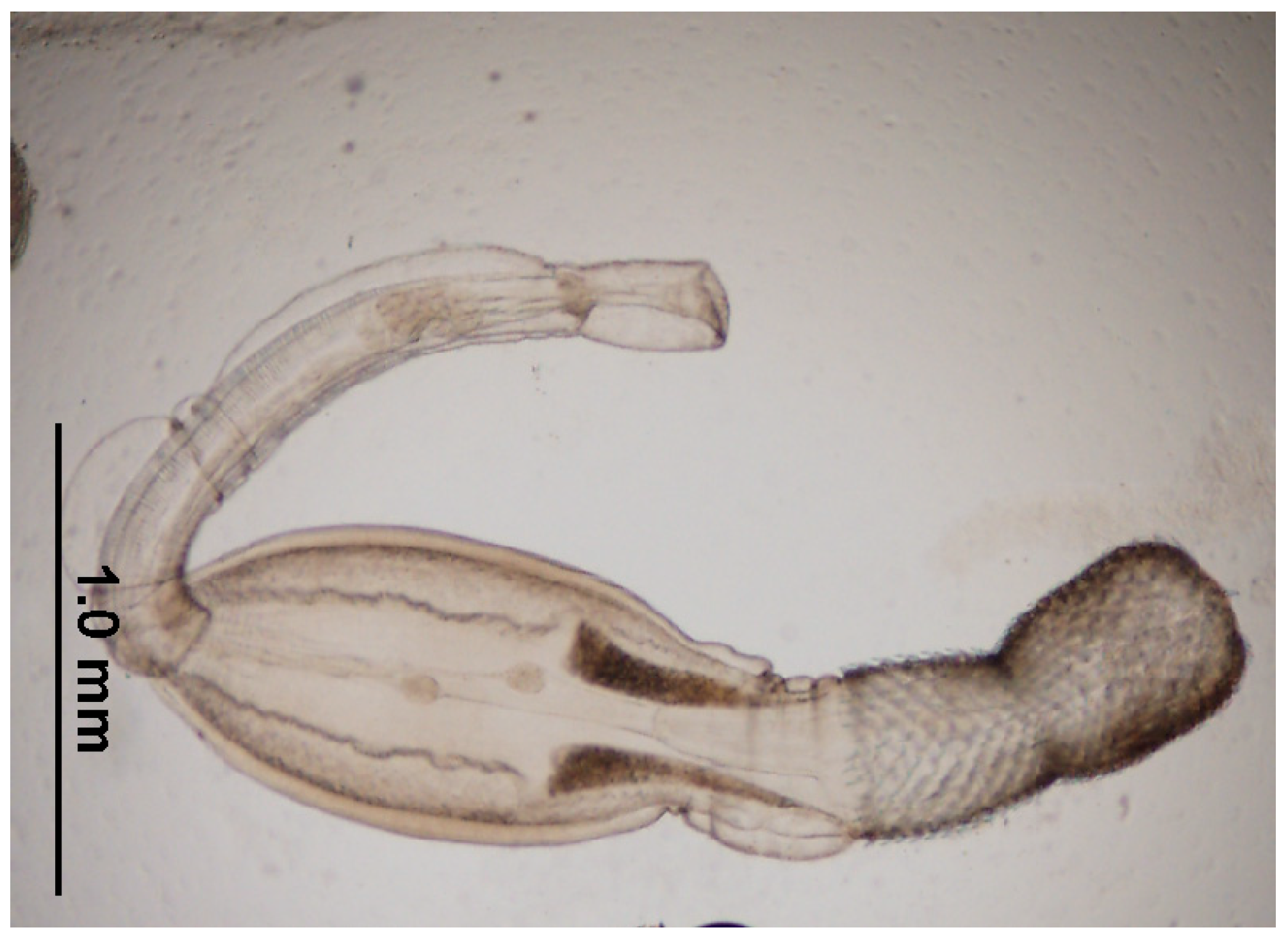

| Species | Habitat | Number Positives | Prevalence | Confidence Intervals | Average Burden | Confidence Intervals | ||
|---|---|---|---|---|---|---|---|---|
| (%) | Upper | Lower | Upper | Lower | ||||
| Joyeuxiella pasqualei | city center | 55 | 63.2 | 52.2 | 73.3 | |||
| outskirts | 74 | 52.5 | 43.9 | 60.9 | ||||
| desert | 102 | 77.3 | 69.2 | 84.1 | ||||
| all | 231 | 64.2 | 59.0 | 69.1 | ||||
| Joyeuxiella gervaisi | city center | 2 | 2.3 | 0.3 | 8.1 | |||
| outskirts | 23 | 16.3 | 10.3 | 23.5 | ||||
| desert | 55 | 41.7 | 33.2 | 50.6 | ||||
| all | 80 | 22.2 | 18.0 | 26.9 | ||||
| Joyeuxiella spp. | city center | 56 | 64.4 | 53.4 | 74.4 | 59.3 | 39.3 | 99 |
| outskirts | 81 | 57.4 | 48.8 | 65.7 | 58.3 | 42.5 | 85.7 | |
| desert | 106 | 80.3 | 72.5 | 86.7 | 62.5 | 46.5 | 86.1 | |
| all | 243 | 67.5 | 62.4 | 72.3 | 60.4 | 49.1 | 74.5 | |
| Diplopylidium noelleri | city center | 28 | 32.2 | 22.6 | 43.1 | 73.4 | 31.0 | 148.0 |
| outskirts | 32 | 22.7 | 16.1 | 30.5 | 48.2 | 19.6 | 151.0 | |
| desert | 73 | 55.3 | 46.4 | 64.4 | 51.0 | 33.1 | 80.2 | |
| all | 133 | 36.9 | 31.9 | 42.2 | 55.2 | 38.4 | 82.4 | |
| Pterygodermatites cahirensis | city center | 40 | 46.0 | 35.2 | 57.0 | 28.0 | 14.3 | 51.9 |
| outskirts | 36 | 25.5 | 18.6 | 33.6 | 13.2 | 8.5 | 22.1 | |
| desert | 45 | 34.1 | 26.1 | 42.8 | 7.6 | 4.6 | 14.1 | |
| all | 121 | 33.6 | 28.7 | 38.7 | 16 | 11.1 | 25.1 | |
| Parasite Species | Age Group of Cats | Number Positives | Prevalence | Confidence Intervals | Burden | Confidence Intervals | ||
|---|---|---|---|---|---|---|---|---|
| (%) | Lower | Upper | Average | Lower | Upper | |||
| Joyeuxiella pasqualei | adult | 203 | 72.8 | 67.1 | 77.9 | |||
| juvenile | 28 | 34.6 | 24.3 | 46.0 | ||||
| Joyeuxiella gervaisi | adult | 78 | 28.0 | 22.8 | 33.6 | |||
| juvenile | 2 | 2.5 | 0.3 | 8.6 | ||||
| Joyeuxiella spp. | adult | 214 | 76.7 | 71.3 | 81.5 | 60.3 | 48.6 | 77.8 |
| juvenile | 29 | 35.8 | 25.4 | 47.2 | 60.9 | 38.4 | 103 | |
| Diplopylidium noelleri | adult | 118 | 42.3 | 36.4 | 48.3 | 55.9 | 37.7 | 83.5 |
| juvenile | 15 | 18.5 | 10.8 | 28.7 | 49.2 | 10.3 | 192 | |
| Pterygodermatites cahierensis | adult | 97 | 34.8 | 29.2 | 40.7 | 12.3 | 8.7 | 19.5 |
| juvenile | 24 | 29.6 | 20.0 | 40.8 | 30.8 | 13.7 | 66.2 | |
| Reptile Hosts | No of Examined | Number of Hosts Infected with Larval Stages of | ||||
|---|---|---|---|---|---|---|
| Joyeuxiella spp. | Diplopylidium noelleri | Spirurata spp. | Centrorhynchus sp. | Macracanthorhynchus sp. | ||
| ||||||
| Eryx jayakari | 15 | 0 | 0 | 4 | 0 | 0 |
| Rhagerhis moilensis | 1 | 1 | 1 | 0 | 1 | 0 |
| Platyceps rhodorachis | 3 | 1 | 0 | 1 | 2 | 1 |
| Psammophis schokari | 13 | 6 | 5 | 3 | 6 | 0 |
| Cerastes gasperetti | 5 | 1 | 2 | 0 | 2 | 1 |
| Echis carinatus | 26 | 20 | 11 | 3 | 8 | 1 |
| Echis omanensis | 1 | 1 | 1 | 1 | 0 | 1 |
| Dolichophis jugularis | 2 | 2 | 2 | 1 | 1 | 0 |
| Total: | 66 | 32 | 22 | 13 | 19 | 4 |
| ||||||
| Hemidactylus flaviviridis | 32 | 13 | 0 | 3 | 0 | 0 |
| Hemidactylus robustus | 9 | 3 | 0 | 1 | 0 | 0 |
| Chalcides ocellatus | 22 | 0 | 0 | 0 | 0 | 0 |
| Diplometopon zarudnyi | 5 | 0 | 0 | 2 | 0 | 0 |
| Total: | 68 | 16 | 0 | 6 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuster, R.K.; Sivakumar, S. Helminths of Cats (Felis catus Linnaeus, 1758) and Their Larval Stages in Reptiles in Dubai, United Arab Emirates. Diversity 2025, 17, 578. https://doi.org/10.3390/d17080578
Schuster RK, Sivakumar S. Helminths of Cats (Felis catus Linnaeus, 1758) and Their Larval Stages in Reptiles in Dubai, United Arab Emirates. Diversity. 2025; 17(8):578. https://doi.org/10.3390/d17080578
Chicago/Turabian StyleSchuster, Rolf K., and Saritha Sivakumar. 2025. "Helminths of Cats (Felis catus Linnaeus, 1758) and Their Larval Stages in Reptiles in Dubai, United Arab Emirates" Diversity 17, no. 8: 578. https://doi.org/10.3390/d17080578
APA StyleSchuster, R. K., & Sivakumar, S. (2025). Helminths of Cats (Felis catus Linnaeus, 1758) and Their Larval Stages in Reptiles in Dubai, United Arab Emirates. Diversity, 17(8), 578. https://doi.org/10.3390/d17080578






