Characteristics of Rhizosphere Soil Fungal Communities of Cypripedium macranthos Sw. at Different Latitudes in Heilongjiang Province
Abstract
1. Introduction
2. Materials and Methods
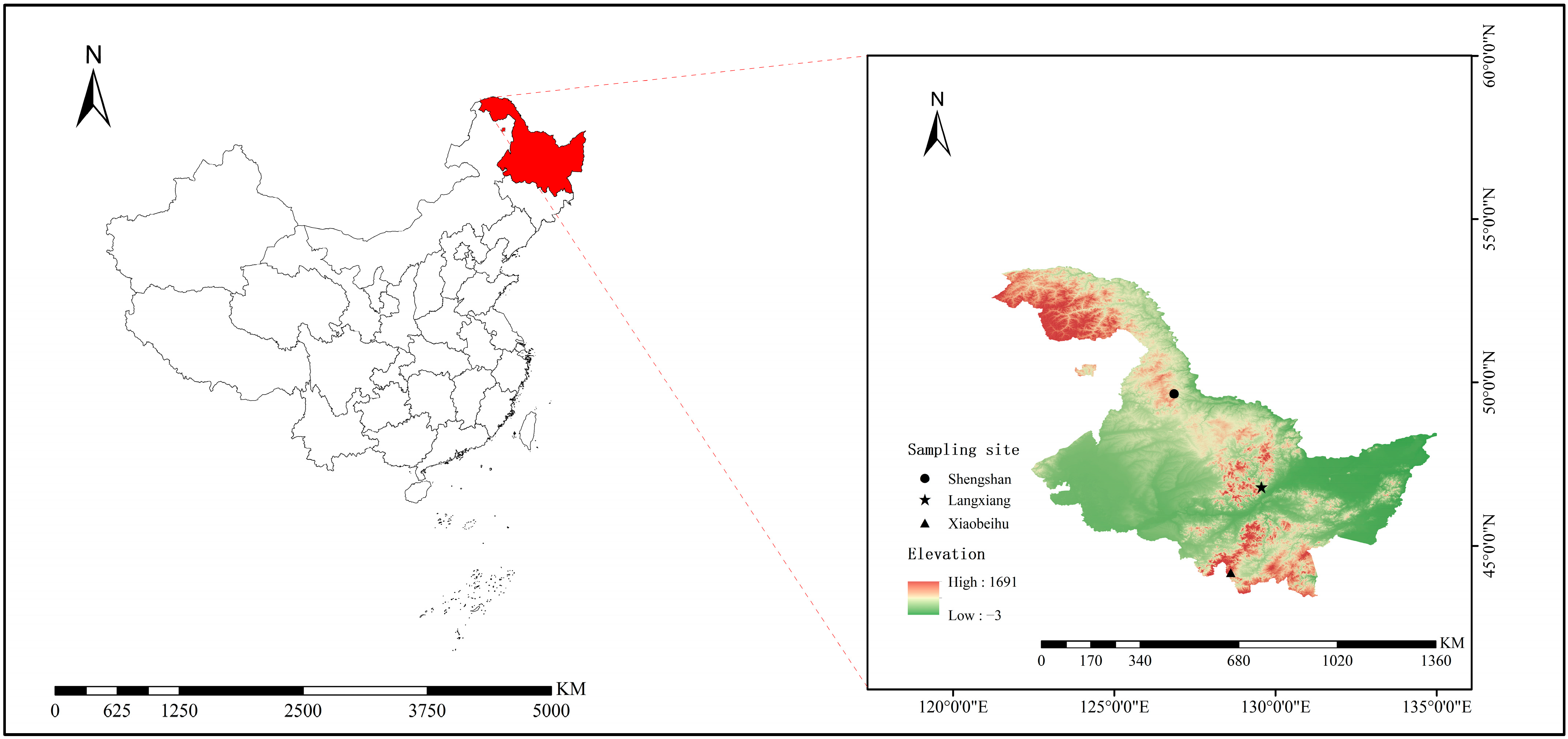
2.1. Study Area and Sampling Locations
2.2. Sample Collection
2.3. Analysis of Soil Chemical Properties
2.4. Soil DNA Extraction, Amplicon Generation, and Sequencing
2.5. Sequencing Data Processing
2.6. Statistical Analysis
3. Results
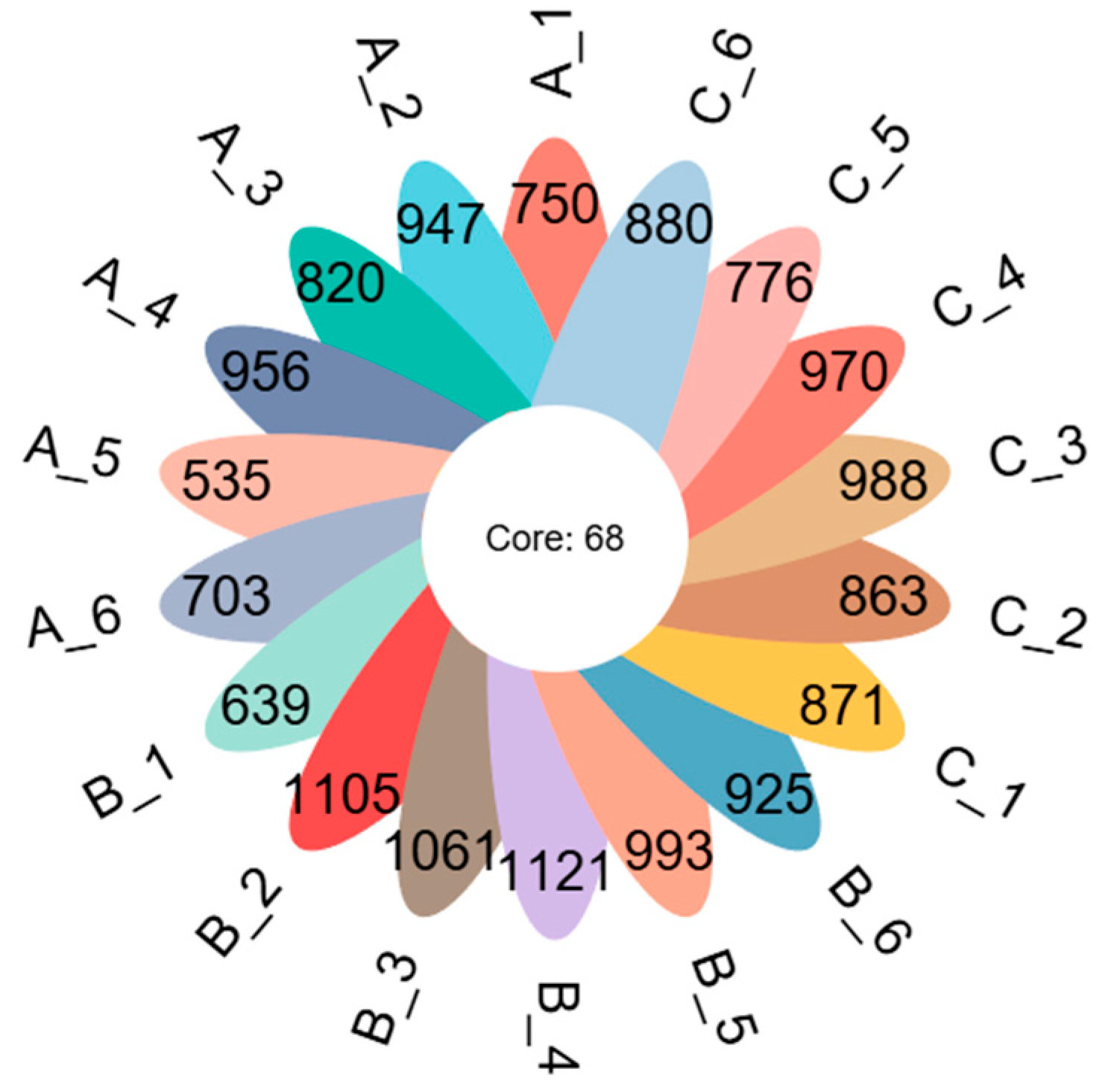
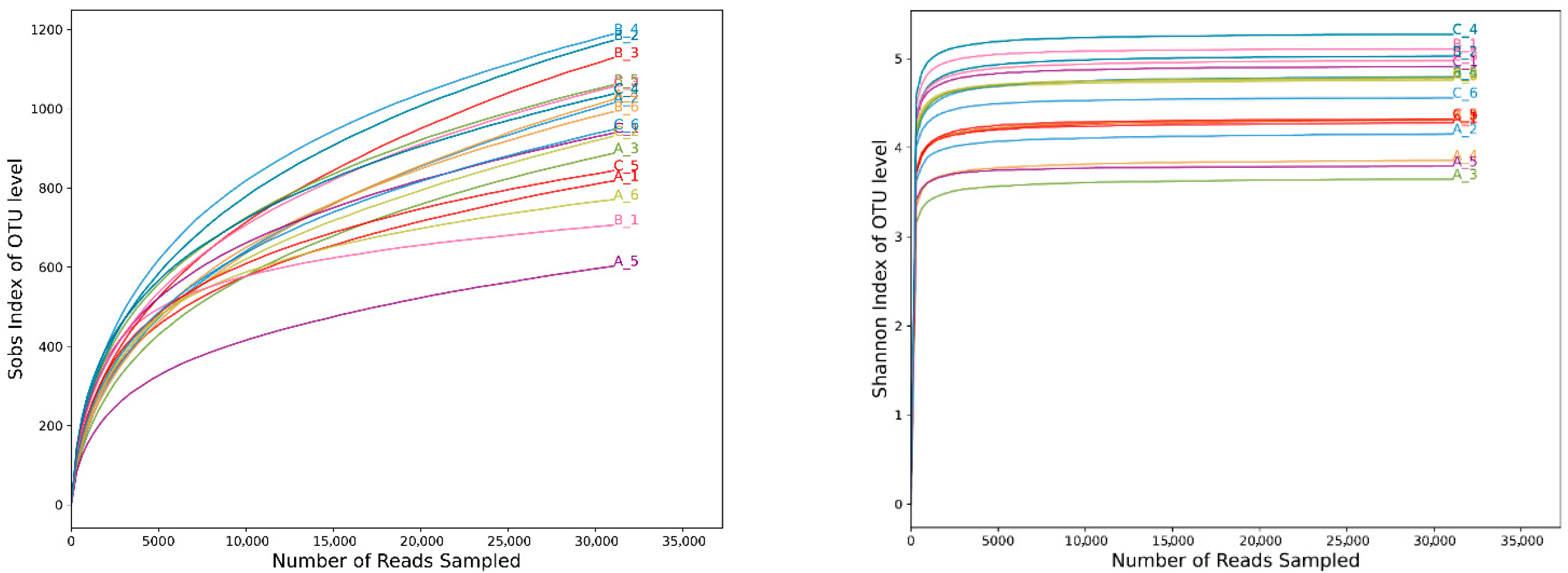
3.1. Quality Analysis of Rhizosphere Soil Fungi Sequencing of C. macranthos
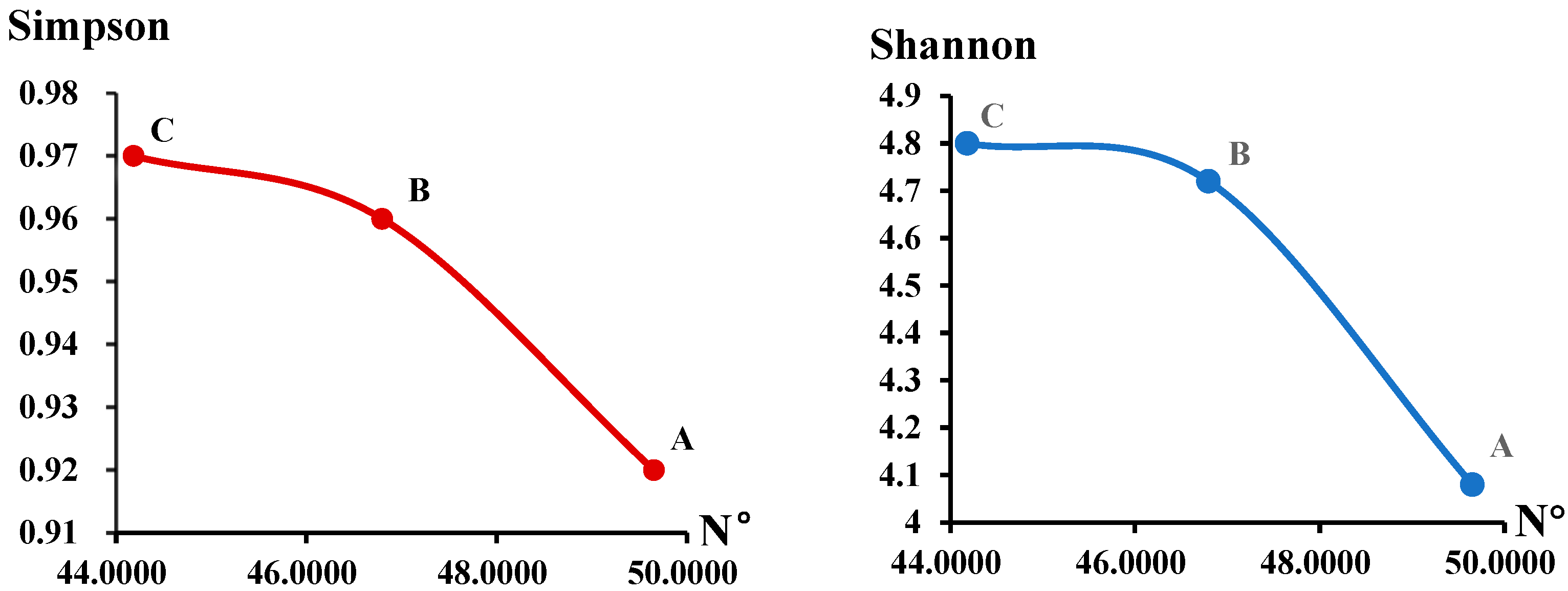
3.2. Rhizosphere Soil Fungal Community Diversity of C. macranthos at Different Latitudes
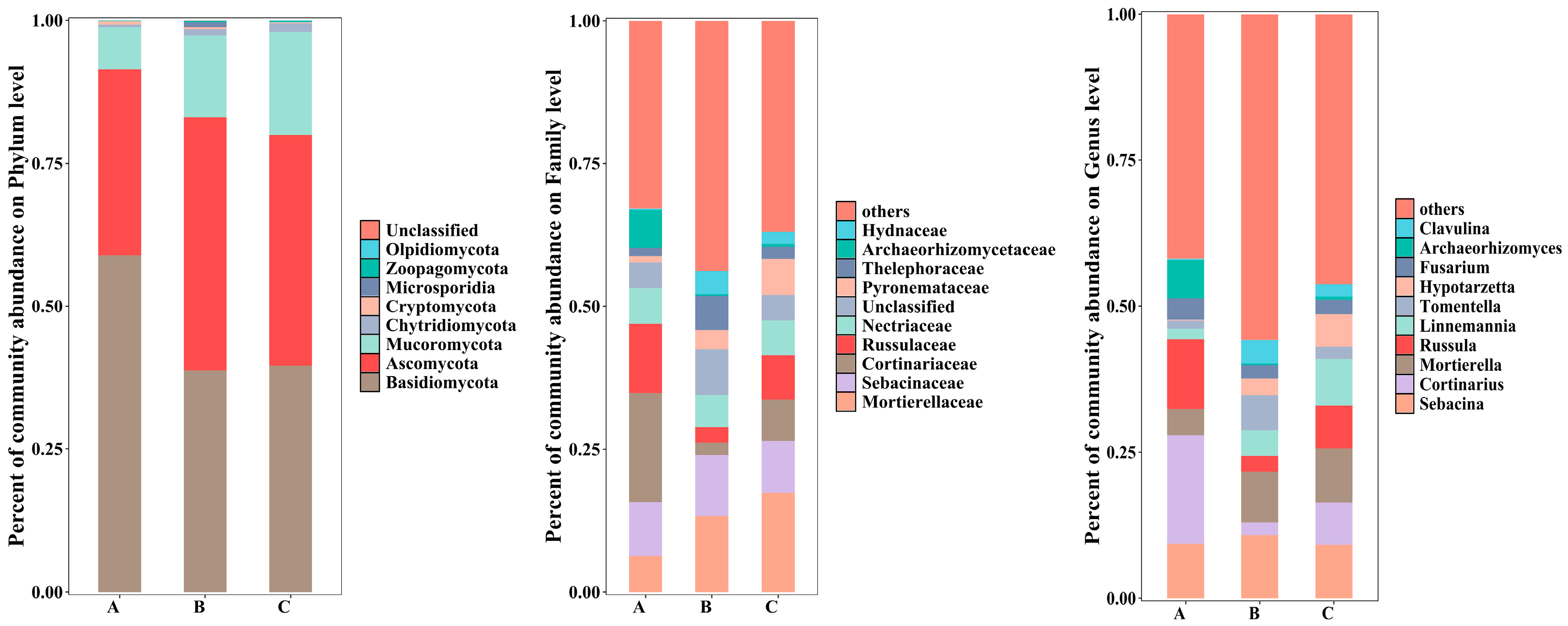
3.3. Community Composition of Rhizosphere Soil Fungi of C. macranthos
Analysis of Rhizosphere Soil Fungal Composition of C. macranthos at Different Taxonomic Levels
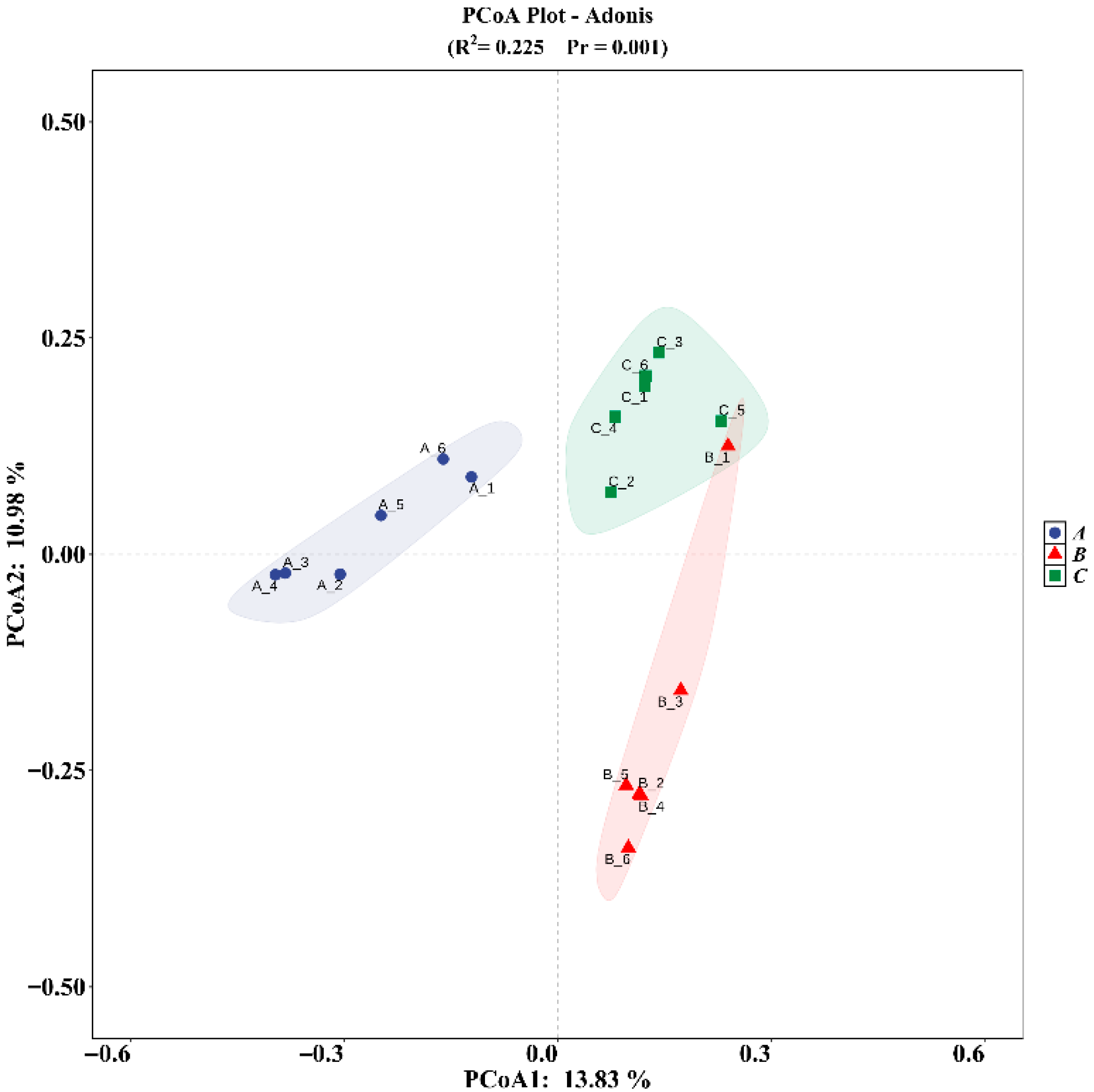
3.4. Differences in Rhizosphere Soil Community Structure of C. macranthos at Different Latitudes
3.5. Soil Properties and Their Effects on the Rhizosphere Soil Fungal Communities of C. macranthos
3.5.1. Soil Properties
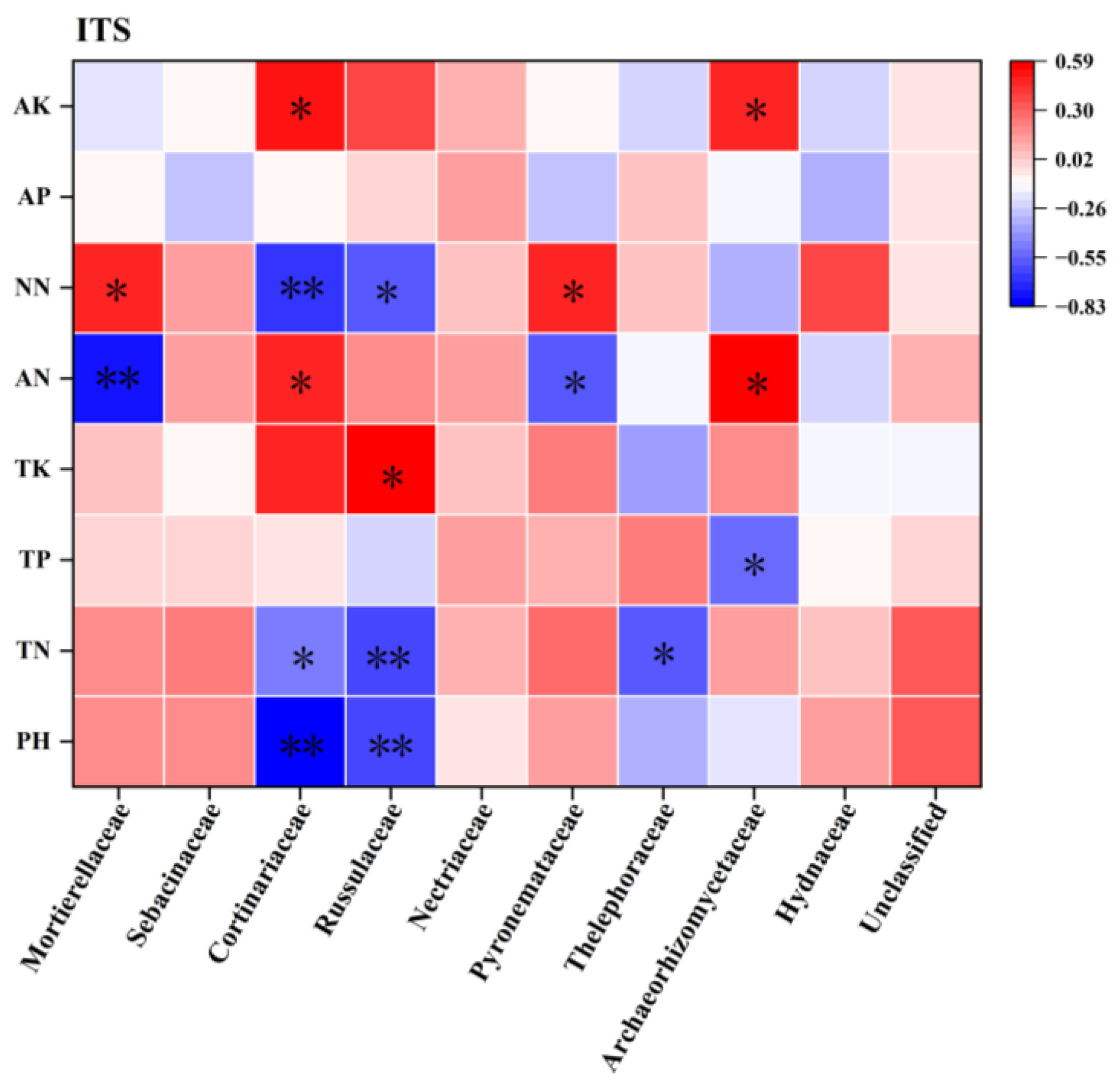
3.5.2. Correlation Analysis of Soil Properties and Rhizosphere Soil Fungal Communities of C. macranthos
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kloepper, J.; Schroth, M.N. Plant growth-promoting rhizobacteria on radishes. In Proceedings of the IV International Conference on Plant Pathogenic Bacteria, Angers, France, 27 August–2 September 1978; Volume 2, pp. 879–882. [Google Scholar]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.K.; Lin, X.M.; Lin, W.X. Research progress and prospect of plant-soil-microbe interactions mediated by root exudates. Chin. J. Plant Ecol. 2014, 38, 298–310. [Google Scholar]
- Wang, B.; Na, X.; Huang, S.; Li, Z.; Zhou, Z.; Huang, J.; Pu, M.; Cheng, Z.; He, X. Contrasting fungal community assembly mechanisms in bulk soil and rhizosphere of Torreya grandis across a 900-year age gradient. Plant Soil 2025, 1–18. [Google Scholar] [CrossRef]
- Baldrian, P.; Kolařík, M.; Štursová, M.; Kopecký, J.; Valášková, V.; Větrovský, T.; Žifčáková, L.; Šnajdr, J.; Rídl, J.; Vlček, Č.; et al. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J. 2012, 6, 248–258. [Google Scholar] [CrossRef]
- Van Wees, S.C.M.; Van der Ent, S.; Pieterse, C.M.J. Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 2008, 11, 443–448. [Google Scholar] [CrossRef]
- Kyaschenko, J.; EClemmensen, K.; Hagenbo, A.; Karltun, E.; Lindahl, B.D. Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. ISME J. 2017, 11, 863–874. [Google Scholar] [CrossRef]
- Bödeker, I.T.M.; Lindahl, B.D.; Olson, Å.; Clemmensen, K.E.; Treseder, K. Mycorrhizal and saprotrophic fungal guilds compete for the same organic substrates but affect decomposition differently. Funct. Ecol. 2016, 30, 1967–1978. [Google Scholar] [CrossRef]
- Chu, Q.Q. Study on Characteristics of Rhizosphere Soil Fungal Communities in Gannan Navel Orange. Master’s Thesis, East China University of Technology, Nanchang, China, 2023. [Google Scholar]
- Fontana, A.; Reichelt, M.; Hempel, S.; Gershenzon, J.; Unsicker, S.B. The Effects of Arbuscular Mycorrhizal Fungi on Direct and Indirect Defense Metabolites of Plantago lanceolata L. J. Chem. Ecol. 2009, 35, 833–843. [Google Scholar] [CrossRef]
- Guo, C.G.; Zhang, L.; Shen, R.Q.; Xu, B.L. Study on fungal diversity in rhizosphere soil of sand-fixing plants in Tengger Desert of Ningxia. Mycosystema 2017, 36, 552–562. [Google Scholar]
- Li, Z.Y. Geographical Variation and Conservation of Functional Traits in Cypripedium Populations in Northeast China. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2020. [Google Scholar]
- Fu, Y.J.; Zhang, J.L.; Hou, X.Q. High-throughput sequencing analysis of fungal diversity in rhizosphere and non-rhizosphere soil of Cypripedium macranthos Sw. Acta Agric. Boreali-Occident. Sin. 2019, 28, 253–259. [Google Scholar]
- Guo, X.L.; Zhao, J.C.; Peng, X.J. Research on resources of rare and endangered medicinal plants in Hebei Province. J. Arid. Land Resour. Environ. 2010, 24, 144–149. [Google Scholar]
- Chen, L.; Liu, W.; Jiang, N.; Xiao, Y.; Shan, Y.; Wang, S.; Wu, S.; Wang, Q.; Yu, J.; Zhang, Y.; et al. Population Dynamics of Cypripedium macranthos Sw. and Its Interactions with Environmental Factors in the Changbai Mountains. Agronomy 2024, 15, 68. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Lu, X.; Li, S.; Li, Y.; Shan, Y.; Wang, S.; Zhou, Y.; Chen, L. Effects of different light conditions on morphological, anatomical, photosynthetic and biochemical parameters of Cypripedium macranthos Sw. Photosynth. Res. 2024, 160, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H. Quantitative Variability of Flower Color and Outer Morphological Traits in Cypripedium macranthos Sw. lat. (Orchidaceae) on Rebun Island, Hokkaido, Japan. Acta Phytotaxon. Geobot. 2023, 74, 105–120. [Google Scholar]
- Kota, K.; Kaien, F.; Hanako, S. Construction of a de novo assembly pipeline using multiple transcriptome data sets from Cypripedium macranthos (Orchidaceae). PLoS ONE 2023, 18, e0286804. [Google Scholar]
- Chung, J.; Park, K.W.; Park, C.S.; Lee, S.H.; Chung, M.G.; Chung, M.Y. Contrasting levels of genetic diversity between the historically rare orchid Cypripedium japonicum and the historically common orchid Cypripedium macranthos in South Korea. Bot. J. Linn. Soc. 2009, 160, 119–129. [Google Scholar] [CrossRef]
- Wu, Q.; Dong, S.; Zhao, Y.; Yang, L.; Qi, X.; Ren, Z.; Dong, S.; Cheng, J. Genetic diversity, population genetic structure and gene flow in the rare and endangered wild plant Cypripedium macranthos revealed by genotyping-by-sequencing. BMC Plant Biol. 2023, 23, 254. [Google Scholar] [CrossRef]
- Lee, J.K.; Kwon, Y.H.; Kim, H.K.; Kim, K.O.; Park, J.S.; Jeong, M.J.; Son, S.W.; Suh, G.U. Analysis of factors on the asymbiotic germination of white lady’s slipper orchid (Cypripedium macranthos Sw. albiflorum). In Proceedings of the Plant Resources Society of Korea Conference, Chungbuk, Republic of Korea, 25–26 April 2019; p. 53. [Google Scholar]
- Huh, Y.S.; Lee, J.K.; Paek, K.Y.; Park, S.Y.; Son, S.W.; Suh, G.U. Effect of seed maturity on germination and proliferation of Cypripedium macranthos Sw. during asymbiotic seed culture. Acta Hortic. 2019, 1262, 53–62. [Google Scholar] [CrossRef]
- Huh, Y.S.; Lee, J.K.; Nam, S.Y.; Paek, K.Y.; Suh, G.U. Improvement of asymbiotic seed germination and seedling development of Cypripedium macranthos Sw. with organic additives. J. Plant Biotechnol. 2016, 43, 138–145. [Google Scholar] [CrossRef]
- Sugiura, N. Consistent pollination services to Cypripedium macranthos var. rebunense (Orchidaceae) by Bombus pseudobaicalensis. Plant Species Biol. 2019, 34, 38–42. [Google Scholar] [CrossRef]
- Wang, Q.; An, J.; Wang, Y.; Zheng, B. The complete chloroplast genome sequences of three Cypripedium species and their phylogenetic analysis. Sci. Rep. 2025, 15, 13461. [Google Scholar] [CrossRef]
- National Key Protected Wild Plants (2021 Edition). Available online: https://www.iplant.cn/redbook/splist#EN (accessed on 23 May 2024).
- McCormick, M.K.; Jacquemyn, H. What constrains the distribution of orchid populations? New Phytol. 2013, 202, 392–400. [Google Scholar] [CrossRef]
- Bunch, W.D.; Cowden, C.C.; Wurzburger, N.; Shefferson, R.P. Geography and soil chemistry drive the distribution of fungal associations in lady’s slipper orchid, Cypripedium acaule. Botany 2013, 91, 850–856. [Google Scholar] [CrossRef]
- Hou, X.; Fu, Y.; Yuan, J.; Wang, L.; Li, W. Study on the diversity of endophytic fungi in Cypripedium macranthos Sw. Hubei Agric. Sci. 2015, 54, 1357–1360. [Google Scholar]
- Zhang, Y.P. Diversity of Endophytic Fungi in Three Cypripedium Species in Northern China and Their Effects on Protocorm Growth. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2013. [Google Scholar]
- Fan, J.; Li, C.C.; Qi, X.C.; Huang, Q.; Ren, Y.; Liu, J.; Du, C. Analysis of fungal community composition in roots and rhizosphere soil of Cypripedium macranthos Sw. Chin. Wild Plant Resour. 2024, 43, 59–67. [Google Scholar]
- He, P.; Liu, Y.; Chen, Y.; Liu, Y.; Tian, S.; Qi, F. Spatiotemporal pattern of vegetation NPP and its influencing factors in Heilongjiang Province. Environ. Ecol. 2024, 6, 19–29+36. [Google Scholar]
- Li, Y.; Wu, X.; Yuan, Y.; Dong, L. Spatiotemporal evolution and driving mechanisms of vegetation carbon-water use efficiency in Heilongjiang Province. Chin. J. Appl. Ecol. 2024, 35, 3349–3358. [Google Scholar]
- Wang, Z.; Sun, C.; Liu, Y.; Jiang, Q.; Zhu, T. Spatiotemporal variation of vegetation index and its response to climatic factors in Heilongjiang Province. South-North Water Transf. Water Sci. Technol. 2022, 20, 737–747. [Google Scholar]
- Zhu, H.; Zhang, H.; Liu, H.; Yin, C. Study on the variation characteristics of climate comfort in Heilongjiang Province. Environ. Sci. Manag. 2025, 50, 28–32+47. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Feng, K.; He, Q.; Peng, X.; Yang, X.; Du, X.; Wei, Z.; Wang, S.; Zou, X.; Zhang, Y.; Deng, Y. Temperature and Biodiversity Regulate the Robustness of Plant-Microbe Networks in Natural Forests at Large Scale. Glob. Change Biol. 2025, 31, e70335. [Google Scholar] [CrossRef] [PubMed]
- Lombard, N.; Prestat, E.; van Elsas, J.D.; Simonet, P. Soil-specific limitations for access and analysis of soil microbial communities by metagenomics. FEMS Microbiol. Ecol. 2011, 78, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, H. On the generality of the latitudinal diversity gradient. Am. Nat. 2004, 163, 192–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jacquemyn, H.; Yu, S.; Chen, W.; He, X.; Huang, Y. Mycorrhizal diversity and community composition in co-occurring Cypripedium species. Mycorrhiza 2022, 33, 107–118. [Google Scholar] [CrossRef]
| Sampling Site | Chao1 | Shannon | Simpson | goods_coverage |
|---|---|---|---|---|
| A | 1215.90 ± 251.45 a | 4.08 ± 0.40 b | 0.92 ± 0.04 b | 0.99 |
| B | 1439.27 ± 312.24 a | 4.72 ± 0.34 a | 0.96 ± 0.02 ab | 0.99 |
| C | 1367.88 ± 124.99 a | 4.80 ± 0.33 a | 0.97 ± 0.03 a | 0.99 |
| Taxonomy | Number of Microbial Species in Each Sampling Site | Total | ||
|---|---|---|---|---|
| A | B | C | ||
| Phylum | 8 | 8 | 8 | 8 |
| Class | 34 | 35 | 34 | 48 |
| Order | 96 | 101 | 111 | 152 |
| Family | 198 | 225 | 223 | 376 |
| Genus | 395 | 465 | 415 | 1008 |
| Species | 625 | 751 | 652 | 2039 |
| Location | pH | Total Nitrogen TN (g/kg) | Total Phosphorus TP (g/kg) | Total Potassium TK (g/kg) | Ammonium Nitrogen AN (mg/kg) | Nitrate Nitrogen NN (mg/kg) | Available Phosphorus AP (mg/kg) | Quick-Acting Potassium AK (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| A | 6.3 ± 0.3 b | 0.9 ± 0.3 a | 1.5 ± 0.5 a | 27.2 ± 1.6 a | 18.1 ± 4.7 a | 0.3 ± 0.2 c | 15.9 ± 6.2 a | 118.2 ± 25.2 a |
| B | 6.8 ± 0.4 a | 1.1 ± 0.4 a | 1.7 ± 0.7 a | 24.1 ± 1.2 b | 9.2 ± 2.2 b | 0.9 ± 0.2 a | 12.4 ± 8.2 a | 43.7 ± 19.1 b |
| C | 6.5 ± 0.1 ab | 0.8 ± 0.1 a | 1.4 ± 0.4 a | 28.9 ± 1.8 a | 6.3 ± 2.2 b | 0.6 ± 0.1 b | 13.6 ± 5.2 a | 99.1 ± 40.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, J.; Dong, S.; Liu, J.; Li, M.; Mu, L. Characteristics of Rhizosphere Soil Fungal Communities of Cypripedium macranthos Sw. at Different Latitudes in Heilongjiang Province. Diversity 2025, 17, 577. https://doi.org/10.3390/d17080577
Qian J, Dong S, Liu J, Li M, Mu L. Characteristics of Rhizosphere Soil Fungal Communities of Cypripedium macranthos Sw. at Different Latitudes in Heilongjiang Province. Diversity. 2025; 17(8):577. https://doi.org/10.3390/d17080577
Chicago/Turabian StyleQian, Jiawei, Shang Dong, Jiale Liu, Mengsha Li, and Liqiang Mu. 2025. "Characteristics of Rhizosphere Soil Fungal Communities of Cypripedium macranthos Sw. at Different Latitudes in Heilongjiang Province" Diversity 17, no. 8: 577. https://doi.org/10.3390/d17080577
APA StyleQian, J., Dong, S., Liu, J., Li, M., & Mu, L. (2025). Characteristics of Rhizosphere Soil Fungal Communities of Cypripedium macranthos Sw. at Different Latitudes in Heilongjiang Province. Diversity, 17(8), 577. https://doi.org/10.3390/d17080577





