Influence of Lunar Periodicity on Medusae (Cnidaria) Composition in a Western Caribbean Reef: Community Structure Before Sargassum Blooms
Abstract
1. Introduction
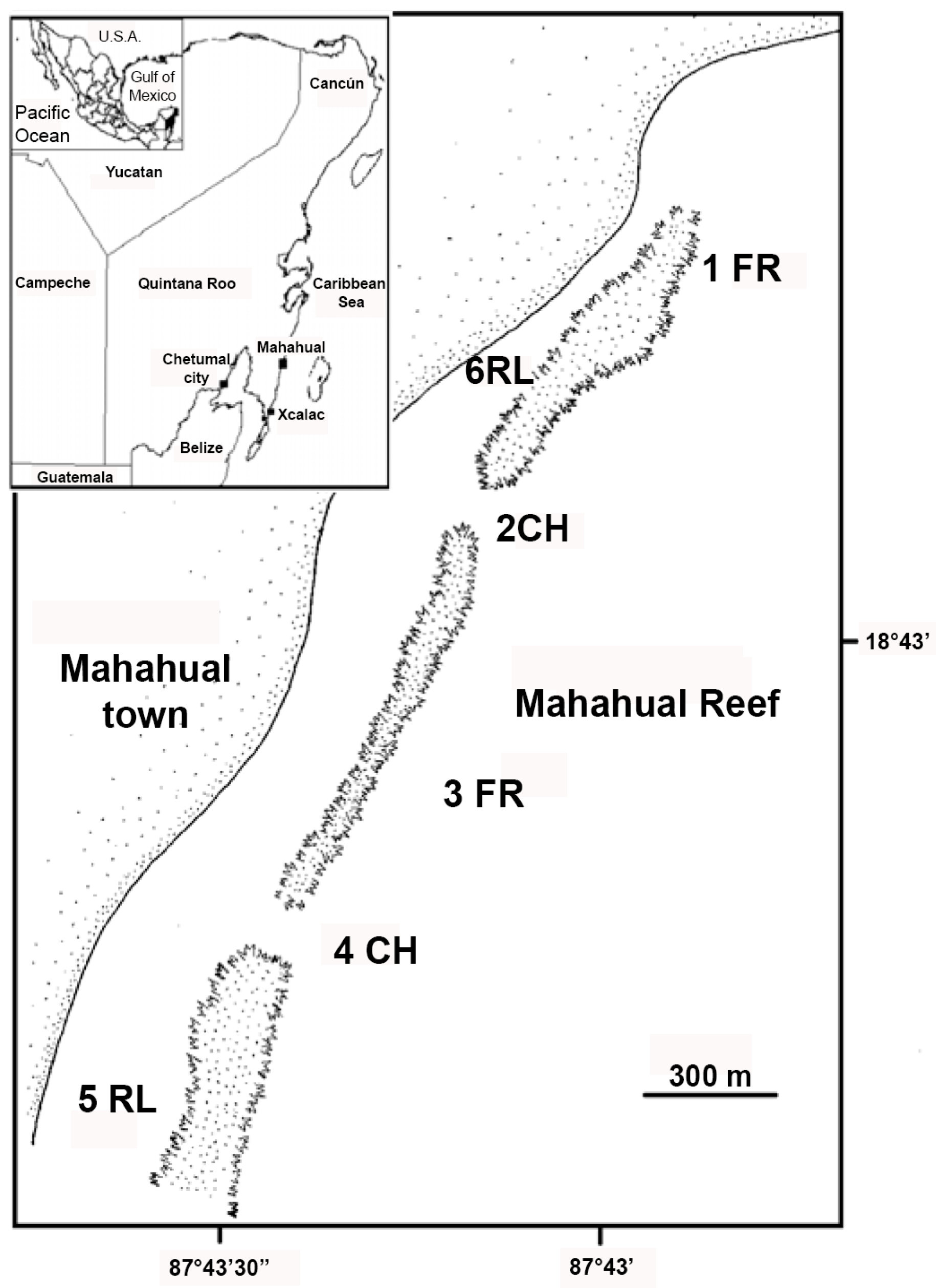
2. Materials and Methods
- S(t) being the expected value of species;
- z is curvature or saturation parameter;
- a is the initial species discovery rate parameter;
- t is the observed accumulated species number.
3. Results
3.1. Environmental Data
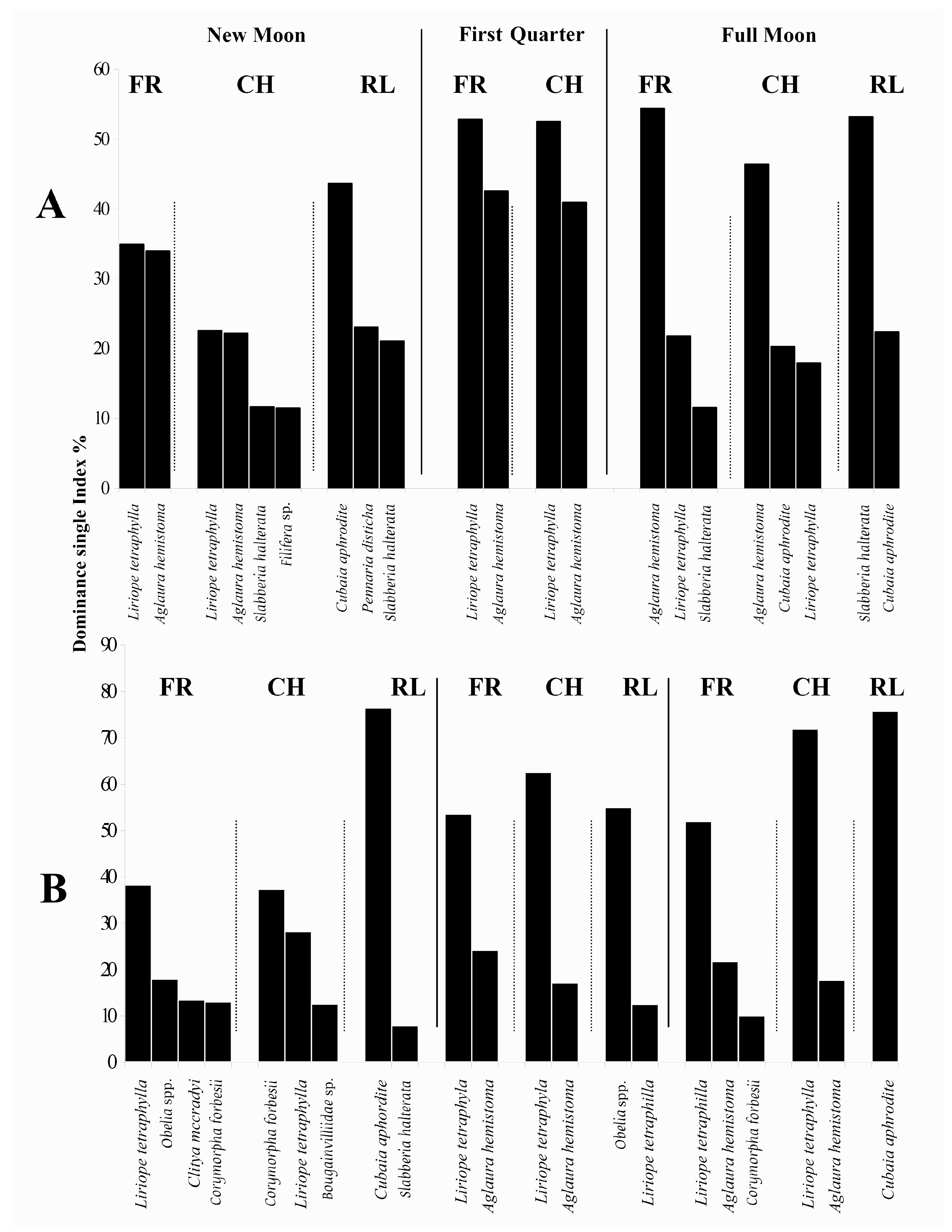
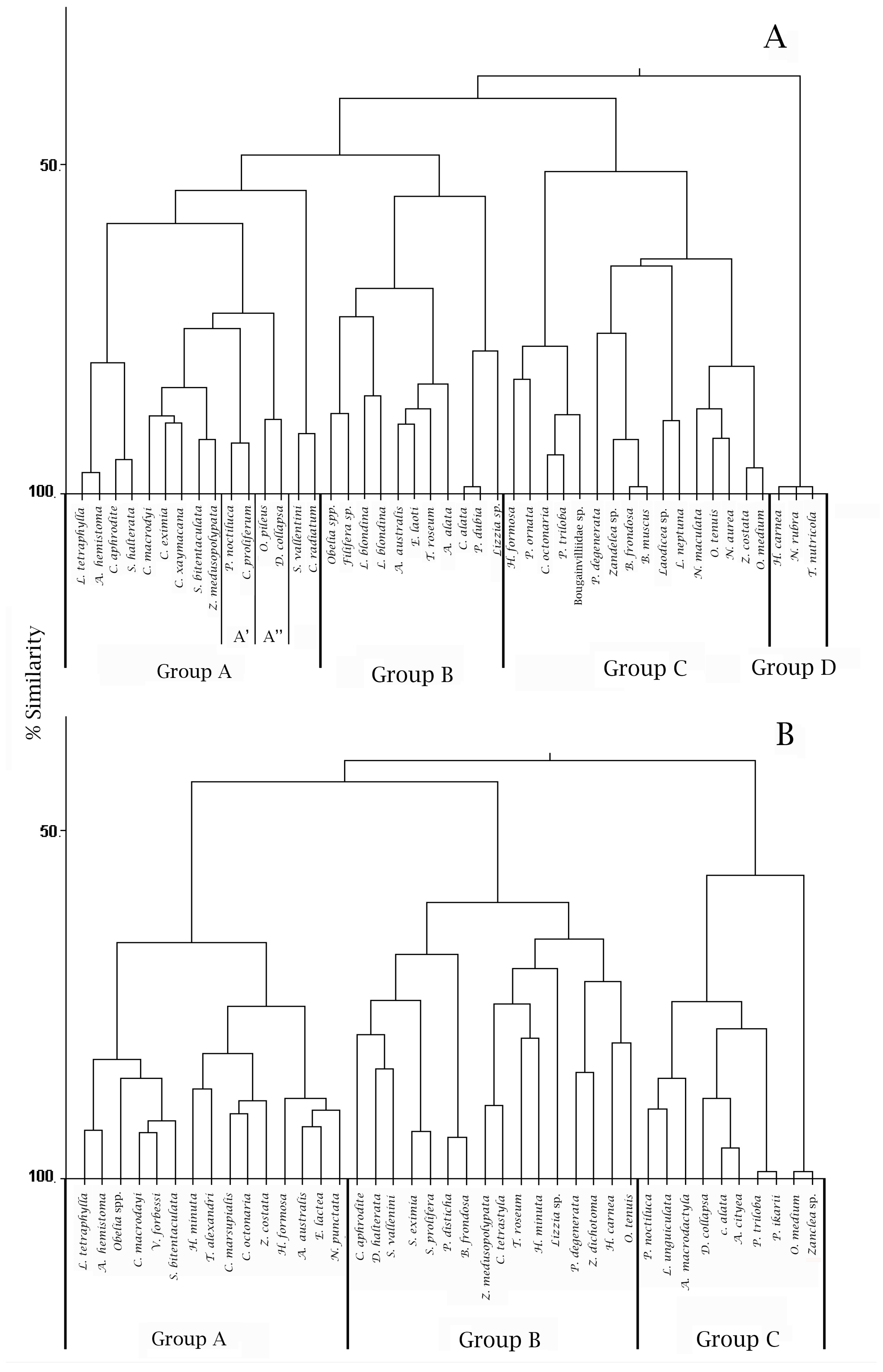
3.2. Composition and Assemblage Structure
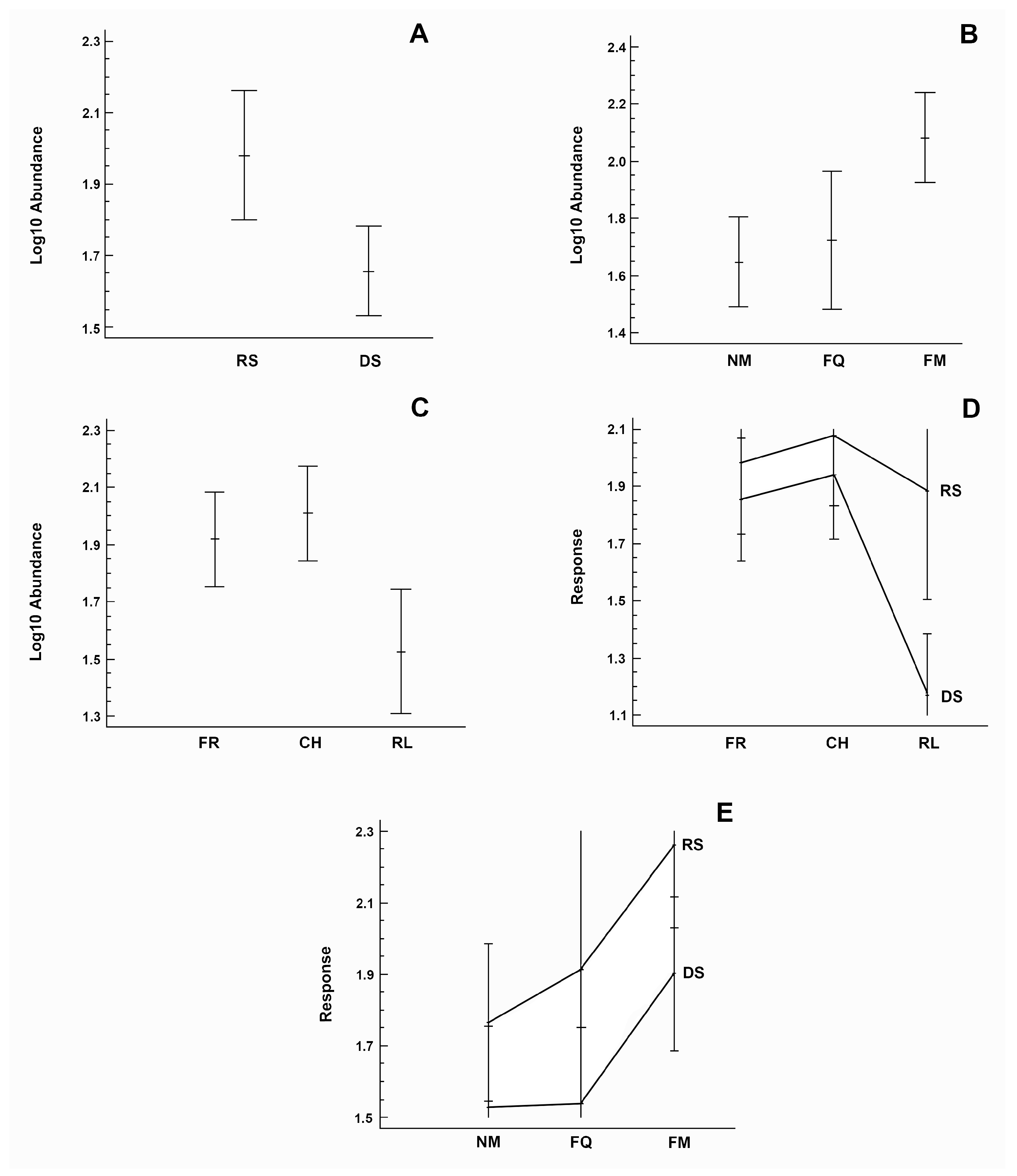
3.3. Biomass
3.4. Abundance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CH | Channel |
| DS | Dry season |
| FM | Full moon |
| FQ | First quarter moon |
| FR | Fore reef |
| NM | New moon |
| RL | Reef lagoon |
| RS | Rainy season |
References
- Gliwicz, Z.M. A lunar cycle in zooplankton. Ecology 1986, 67, 883–897. [Google Scholar] [CrossRef]
- Rios-Jara, E.; González, J. Effects of lunar periodicity on the emergence behavior of the demersal copepod Pseudodiaptomus cokeri in Phosphorescent Bay, Puerto Rico. Bull. Mar. Sci. 2000, 67, 887–901. [Google Scholar]
- Hernández-León, S.; Almeida, C.; Yebra, L.; Arístegui, J.; De Puelles, M.; Garcíia-Braun, J. Zooplankton abundance in subtropical waters: Is there a lunar cycle? Sci. Mar. 2001, 65, 59–63. [Google Scholar] [CrossRef]
- Huntsman, A. Odontosyllis at Bermuda and lunar periodicity. J. Fish. Res. Board. Can. 1948, 7, 363–369. [Google Scholar] [CrossRef]
- Korringa, P. Lunar periodicity. Mem. Geol. Soc. Am. 1957, 67, 917–934. [Google Scholar]
- Hernández-León, S.; Almeida, C.; Yebra, L.; Arístegui, J. Lunar cycle of zooplankton biomass in subtropical waters: Biogeochemical implications. J. Plankton Res. 2002, 24, 935–939. [Google Scholar] [CrossRef]
- Alldredge, A.L.; King, J.M. Effects of moonlight on the vertical migration patterns of demersal zooplankton. J. Exp. Mar. Biol. Ecol. 1980, 44, 133–156. [Google Scholar] [CrossRef]
- Segura-Puertas, L. Morfología, sistemática y zoogeografía de las medusas (Cnidaria: Hydrozoa y Scyphozoa) del Pacífico tropical oriental. An. Del. Inst. De Cienc. Del. Mar. Y Limnol. 1984, 8, 1–320. [Google Scholar]
- Costello, J.; Colin, S. Prey resource use by coexistent hydromedusae from Friday Harbor, Washington. Limnol. Oceanogr. 2002, 47, 934–942. [Google Scholar] [CrossRef]
- Moller, H. Reduction of a larval herring population by jellyfish predator. Science 1984, 224, 621–622. [Google Scholar] [CrossRef]
- Bamstedt, U. Trophodynamics of the scyphomedusae Aurelia aurita predation rate in relation to abundance, size and type of prey organism. J. Plankton Res. 1990, 12, 215–229. [Google Scholar] [CrossRef]
- Morejón-Arrojo, R.; Lüskow, F.; Fernández-Alías, A.; Ramírez, H.; Cróquer, A. First record of a cannonball jellyfish bloom (Stomolophus sp.) in Venezuelan Waters. J. Mar. Sci. Eng. 2025, 13, 689. [Google Scholar] [CrossRef]
- Brock, M.A. Circannual rhythms—I. Free-running rhythms in growth and development of marine cnidarian, Campanularia flexuosa. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 1975, 51, 377–383. [Google Scholar] [CrossRef]
- Pruski, S.; Miglietta, M. Fluctuation and diversity of Hydromedusae (Hydrozoa, Cnidaria) in a highly productive region of the Gulf of Mexico inferred from high frequency plankton sampling. Peerj 2019, 7, 24. [Google Scholar] [CrossRef]
- Elmhirst, R. Lunar Periodicity in Obelia. Nature 1925, 116, 358–359. [Google Scholar] [CrossRef]
- Phillips, P.J. The Pelagic Cnidaria of the Gulf of Mexico: Zoogeography, Ecology and Systematics; Texas A&M University: College Station, TX, USA, 1972. [Google Scholar]
- Zamponi, M.O.; Suárez-Morales, E. Algunas hidromedusas del Mar Caribe mexicano, con la descripción de Tetraotoporpa siankaanensis. Spheniscus 1991, 9, 41–46. [Google Scholar]
- Segura-Puertas, L. Medusae (Cnidaria) from the Yucatan Shelf and Mexican Caribbean. Bull. Mar. Sci. 1992, 51, 353–359. [Google Scholar]
- Segura-Puertas, L.; Ordóñez-López, U. Análisis de la comunidad de medusas (Cnidaria) de la región oriental del Banco de Campeche y Caribe mexicano. Caribb. J. Sci. 1994, 30, 104–115. [Google Scholar]
- Suárez-Morales, E.; Segura-Puertas, L.; Gasca, R. Medusas (Cnidaria: Hydrozoa) de la Bahía de Chetumal, Quintana Roo, México (1990–1991). Caribb. J. Sci. 1995, 31, 23–31. [Google Scholar]
- Gasca, R.; Castellanos-Osorio, I. Zooplancton de la Bahía de Chetumal, Mar Caribe, México. Rev. De Biol. Trop. 1993, 41, 619–625. [Google Scholar]
- Segura-Puertas, L.; Damas-Romero, M. Variación estacional de la comunidad de medusas (Cnidaria) en la laguna Bojórquez, Cancún, México. Hidrobiologica 1997, 7, 59–64. [Google Scholar]
- Suárez-Morales, E.; Zamponi, M.O.; Gasca, R. Hydromedusae (Cnidaria: Hydrozoa) of Bahia de la Ascension, Caribbean coast of Mexico: A seasonal survey. In Proceedings of the 6th International Conference on Coelenterate Biology, Noordwijkerhout, The Netherlands, 16–21 July 1995; pp. 465–472. [Google Scholar]
- Suárez-Morales, E.; Segura-Puertas, L.; Gasca, R. Medusan (Cnidaria) assemblages off the Caribbean coast of Mexico. J. Coast. Res. 1999, 15, 140–147. [Google Scholar]
- Suárez-Morales, E.; Segura-Puertas, L.; Gasca, R. A survey of the reef-related medusa (Cnidaria) community in the western Caribbean Sea. Gulf Res. Rep. 1999, 11, 23–31. [Google Scholar] [CrossRef]
- Larson, R. Medusae (Cnidaria) from Carrie Bow Cay, Belize. In The Atlantic Barrier Reef Ecosystem at Carrie Bow Cay, Belize; Rützler, K., Macintyre, I.G., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1982; pp. 253–269. [Google Scholar]
- Mayer, A.G. Some medusae from the Tortugas, Florida. Bull. Mus. Comp. Zool. Harv. 1900, 37, 13–82. [Google Scholar]
- Bourmaud, C.A.F.; Slobodov, S.; Guilhaumon, F.; Goy, J.; BGravier-Bonnet, N. Medusae (Cnidaria) of Reunion Island (South West Indian Ocean): Diversity, Abundance and Distribution. Diversity 2025, 17, 694. [Google Scholar] [CrossRef]
- Miglietta, M.; Rossi, M.; Collin, R. Hydromedusa blooms and upwelling events in the Bay of Panama, Tropical East Pacific. J. Plankton Res. 2008, 30, 783–793. [Google Scholar] [CrossRef]
- Pérez-Posada, I.; Cabanillas-Terán, N.; Carrera-Parra, L.; Lizcano, D.; Sánchez, A. Trophic ecology of Caribbean polychaetes: Responses to environmental changes driven by massive Sargassum arrivals. Food Webs 2025, 44, 12. [Google Scholar] [CrossRef]
- Rodríguez-Muñoz, R.; Muñiz-Castillo, A.; Euán-Avila, J.; Hernández-Núñez, H.; Valdés-Lozano, D.; Collí-Dulá, R.; Arias-González, J. Assessing temporal dynamics on pelagic Sargassum influx and its relationship with water quality parameters in the Mexican Caribbean. Reg. Stud. Mar. Sci. 2021, 48, 11. [Google Scholar] [CrossRef]
- Merino, M.; Otero-Dávalos, L. Atlas Ambiental Costero, Puerto Morelos-Quintana Roo; CIQRO: Chetumal, México, 1991; p. 80. [Google Scholar]
- Sanvicente-Añorve, L.; Sánchez-Ramírez, M.; Ocana-Luna, A.; Flores-Coto, C.; Ordóñez-López, U. Metacommunity structure of estuarine fish larvae: The role of regional and local processes. J. Plankton Res. 2011, 33, 179–194. [Google Scholar] [CrossRef]
- Spalding, M.D.; Brown, B.E. The Mesoamerican Reef. In Mesophotic Coral Ecosystems; Loya, Y., Puglise, K.A., Bridge, T.C.L., Eds.; Springer: Cham, Switzerland, 2019; pp. 119–132. [Google Scholar]
- Sullivan, K.; Bustamante, G. Setting Geographic Priorities for Marine Conservation in Latin America and the Caribbean; The Nature Conservancy: Arlington, VA, USA, 1999; p. 125. [Google Scholar]
- Kjerfve, B. Tides of the Caribbean Sea. J. Geophys. Res.-Ocean. 1981, 86, 4243–4247. [Google Scholar] [CrossRef]
- Smith, P.E.; Richardson, R.S. Técnicas Modelo Para Prospecciones de Huevos y Larvas de Peces Pelágicos. F.A.O.; Organización de las Naciones Unidas para la Agricultura y la Alimentación (FAO): Roma, Italy, 1979; p. 107. [Google Scholar]
- Russell, F. The Medusae of the British Isles: Anthomedusae, Leptomedusae, Limnomedusae, Trachymedusae, and Narcomedusae; Cambridge University Press: London, UK, 1953; p. 530. [Google Scholar]
- Kramp, P.L. Synopsis of the medusae of the world. J. Mar. Biol. Assoc. U. K. 1961, 40, 7–382. [Google Scholar] [CrossRef]
- Mayer, A.G. Medusae of the World; Carnegie Institution Washington: Washington, DC, USA, 1910; p. 382. [Google Scholar]
- Bouillon, J.; Gravili, C.; Pagès, F.; Gili, J.M.; Boero, F. An introduction to Hydrozoa. Mémoires Du. Muséum Natl. D’histoire Nat. 2006, 194, 1–591. [Google Scholar]
- Beers, J.R. Determinación de la biomasa del zooplancton. In Atlas del Zooplancton del Atlantico Sudoccidental y Métodos de Trabajo con el Zooplancton Marino; Boltovskoy, D., Ed.; INIDEP: Mar del Plata, Argentina, 1981; pp. 133–141. [Google Scholar]
- Rosenzweig, M.L. Species Diversity in Space and Time; Cambridge University Press: Cambridge, UK, 1995; p. 436. [Google Scholar]
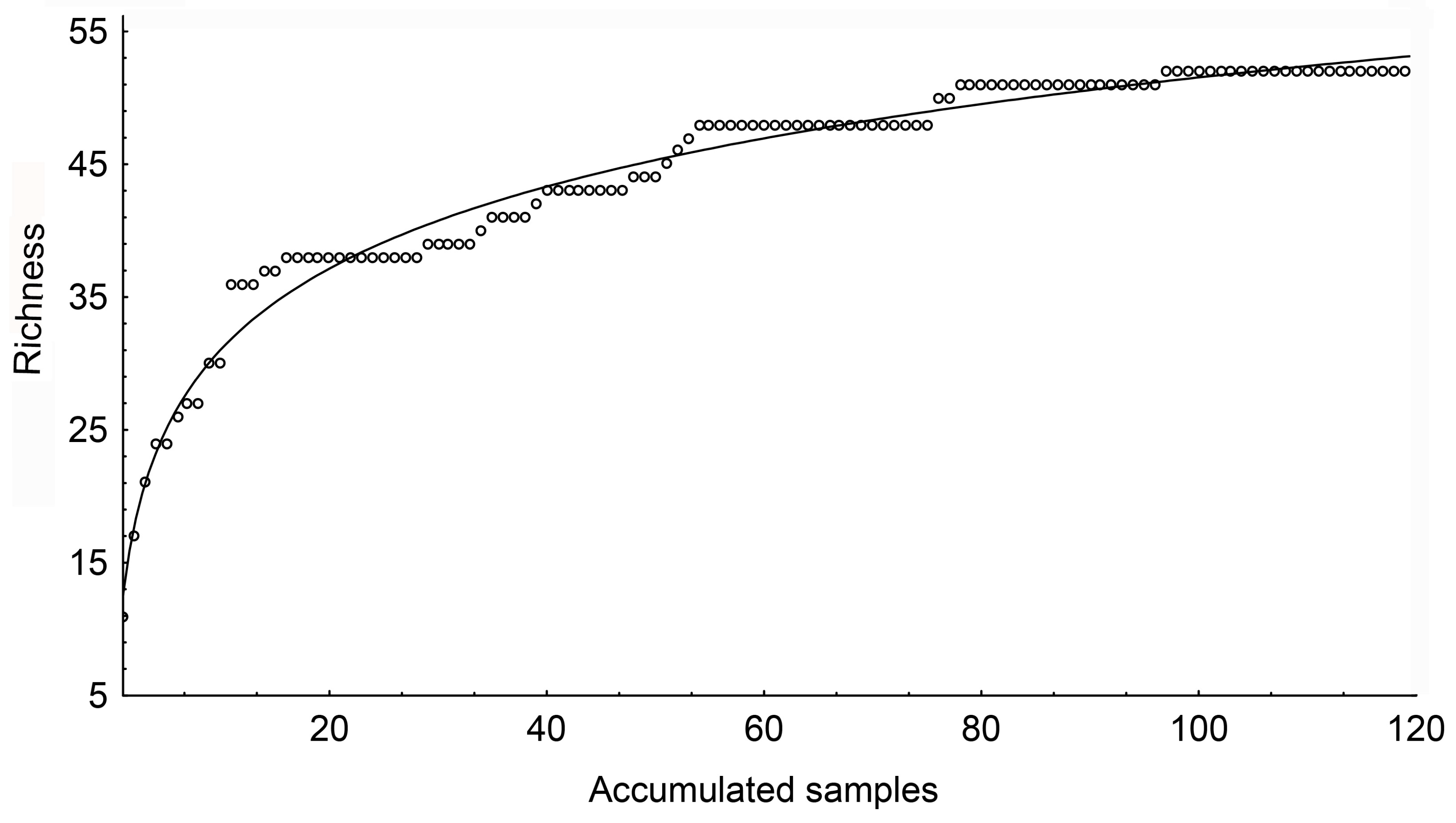
- Soberón, J.; Llorente, J. The use of species accumulation functions for the prediction of species richness. Conserv. Biol. 1993, 7, 480–488. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry; W. H. Freeman: New York, NY, USA, 1995; p. 887. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- De la Cruz, G. ANACOM: Sistema para el Análisis de las Comunidades, Version 3.0; CINVESTAV-IPN: Mérida, México, 1994. [Google Scholar]
- Schuchert, P.; Collins, R. Hydromedusae observed during night dives in the Gulf Stream. Rev. Suisse De Zool. 2021, 128, 237–356. [Google Scholar] [CrossRef]
- Schuchert, P. Hydrozoa (Cnidaria) of Iceland collected by the BIOICE programme. Sarsia 2000, 85, 411–438. [Google Scholar] [CrossRef]
- Segura-Puertas, L. Las medusas (Cnidaria) del Caribe Mexicano. In Planctología Mexicana; Barreiro-Güemes, M.T., Meave del Castillo, M.E., Signoret-Poillon, M., Torres-Figueroa, M.G., Eds.; Sociedad Mexicana de Planctología A.C.: Mexico City, México, 2003; Volume 11, pp. 213–228. [Google Scholar]
- Morejón-Arrojo, R.; Rodriguez-Viera, L. Medusozoans of Cuba (Cnidaria): An update. J. Mar. Biol. Assoc. U. K. 2025, 105, 27. [Google Scholar] [CrossRef]
- Jarms, G.; Morandini, A. Phylogeny and systematics. In World Atlas of Jellyfish: Scyphozoa Except Stauromedusae; Jarms, G., Morandini, A.C., Schmidt Rhaesa, A., Giere, O., StraehlerPohl, I., Eds.; Abhandlungen des Naturwissenschaftlichen Vereins in Hamburg; Dolling Und Galitz Verlag: Munich, Germany, 2019; Volume SPEC, pp. 41–45. [Google Scholar]
- Ramos, G.; Segura-Puertas, L. Seasonal occurrence of reef-related medusae (Cnidaria) in the Western Caribbean Sea. Gulf Caribb. Res. 2004, 16, 1–9. [Google Scholar] [CrossRef]
- Gasca, R.; Segura-Puertas, L.; Suárez-Morales, E. A survey of the medusan (Cnidaria) community of Banco Chinchorro, Western Caribbean Sea. Bull. Mar. Sci. 2003, 73, 37–46. [Google Scholar]
- Segura-Puertas, L.; Suárez-Morales, E.; Celis, L. A checklist of the medusae (Hydrozoa, Scyphozoa and Cubozoa) of Mexico. Zootaxa 2003, 194, 1–15. [Google Scholar] [CrossRef]
- Puente-Tapia, F.; Espinosa-Fuentes, M.; Zavala-García, F.; Olguín-Jacobson, C.; Flores-Coto, C. Spatial distribution of medusae (Cnidaria) assemblages in the southern Gulf of Mexico (dry season). Community Ecol. 2022, 23, 137–162. [Google Scholar] [CrossRef]
- Mills, C.E. Vertical migration and diel activity patterns of hydromedusae—Studies in a large tank. J. Plankton Res. 1983, 5, 619–635. [Google Scholar] [CrossRef]
- Martin, V.J. Photoreceptors of cnidarians. Can. J. Zool. 2002, 80, 1703–1722. [Google Scholar] [CrossRef]
- Wüst, G. Stratification and Circulation in the Antillean-Caribbean Basins: Spreading and Mixing of the Water Types with an Oceanographic Atlas; Columbia University Press: New York, NY, USA, 1964. [Google Scholar]
- Kjerfve, B.; Rützler, K.; Kierspe, G.H. Tides at Carrie Bow Cay, Belize. In The Atlantic Barrier Reef Ecosystem at Carrie Bow Cay, Belize; Rützler, K., Macintyre, I.G., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1982; pp. 47–51. [Google Scholar]
- Suárez-Morales, E.; Rivera-Arriaga, E. Zooplancton e hidrodinámica en zonas litorales y arrecifales de Quintana Roo, Mexico. Hidrobiologica 1988, 8, 19–32. [Google Scholar]
- Gili, J.M.; Pages, F.; Sabates, A.; Ros, J.D. Small-scale distribution of a cnidarian population in the Western Mediterranean. J. Plankton Res. 1988, 10, 385–401. [Google Scholar] [CrossRef]
- Yanagihara, A.; McManus, M.; Sevadjian, J.; Walker, G.; Wilcox, C.; Hurwitz, K.; Lee, A.; Kadler, R.; Powell, B.; Thompson, K. Alatina alata box jellyfish monthly migrations in Hawaii: Lunar and physical oceanographic triggers. Reg. Stud. Mar. Sci. 2022, 53, 11. [Google Scholar] [CrossRef]
- Lüskow, F.; Polgári, B.; Stibor, H.; Schachtl, K.; Abonyi, A. Light increases surface occurrence of the freshwater jellyfish Craspedacusta sowerbii via positive phototaxis. Hydrobiologia 2025, 10. [Google Scholar] [CrossRef]
- Ríos-Jara, E. Effects of lunar cycle and substratum preference on zooplankton emergence in a tropical, shallow-water embayment, in southwestern Puerto Rico. Caribb. J. Sci. 2005, 41, 108–123. [Google Scholar]
- Ohata, R.; Masuda, R.; Yamashita, Y. Ontogeny of antipredator performance in hatchery-reared Japanese anchovy Engraulis japonicus larvae exposed to visual or tactile predators in relation to turbidity. J. Fish. Biol. 2011, 79, 2007–2018. [Google Scholar] [CrossRef]
- Carral-Murrieta, C.; Serviere-Zaragoza, E.; Rivero, F.; Marques, A.; Mendoza-Becerril, M. Sargassum species as hydrozoans substrates: Key patterns of association or just availability? Aquat. Bot. 2024, 191, 10. [Google Scholar] [CrossRef]
- van Tussenbroek, B.; Arana, H.; Rodríguez-Martínez, R.; Espinoza-Avalos, J.; Canizales-Flores, H.; González-Godoy, C.; Barba-Santos, M.; Vega-Zepeda, A.; Collado-Vides, L. Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Mar. Pollut. Bull. 2017, 122, 272–281. [Google Scholar] [CrossRef]
| NM | FQ | FM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FR | CH | RL | FR | CH | RL | FR | CH | RL | ||
| RS | Temp. | 27.3 | 28.6 | 26.5 | 28.0 | 28.0 | --- | 29.1 | 28.7 | 27.6 |
| Salinity | 36.5 | 36.0 | 36.0 | 39.0 | 39.0 | --- | 36.7 | 36.5 | 36.5 | |
| Biomass | 11.6 | 15.3 | 15.1 | 8.1 | 8.0 | --- | 7.7 | 8.7 | 13.0 | |
| DS | Temp | 29.0 | 28.7 | 29.2 | 29.0 | 29.0 | 29.5 | 29.5 | 29.5 | 28.5 |
| Salinity | 34.5 | 34.5 | 34.5 | 38.0 | 38.0 | 35.0 | 38.0 | 38.0 | 32.0 | |
| Biomass | 5.1 | 7.9 | 17.1 | 4.6 | 5.3 | 4.5 | 3.6 | 5.4 | 5.2 | |
| Subphylum Medusozoa | Taxa | RS (Oct–Nov) | % | DS (Apr–May) | % | % Total |
|---|---|---|---|---|---|---|
| Class Cubozoa Werner, 1973 Order Carybdeida Lesson, 1843 | ||||||
| Alatinidae Gershwin, 2005 | Alatina alata (Reynaud, 1830) | 1 | 0.01 | 5 | 0.06 | 0.03 |
| Carybdeidae Lesson, 1843 | Carybdea xaymacana Conant, 1897 | 54 | 0.54 | 43 | 0.49 | 0.52 |
| Class Scyphozoa Goette, 1887 | ||||||
| Subclass Coronamedusae Calder, 2009 | ||||||
| Order Coronatae Vanhöffen, 1892 | ||||||
| Linuchidae Haeckel, 1880 | Linuche unguiculata (Swartz, 1788) | 8 | 0.09 | 0.04 | ||
| Nausithoidae Haeckel, 1880 | Nausithoe maculata Jarms, 1990 * | 4 | 0.04 | 0.02 | ||
| Nausithoe punctata Kölliker, 1853 | 9 | 0.09 | 54 | 0.62 | 0.34 | |
| Nausithoe rubra Vanhöffen, 1902 | 1 | 0.01 | 0.01 | |||
| Subclass Discomedusae Haeckel, 1880 | ||||||
| Order Semaeostomeae Agassiz, 1862 | ||||||
| Pelagiidae Gegenbaur, 1856 | Pelagia noctiluca (Forsskål, 1775) | 28 | 0.28 | 19 | 0.22 | 0.25 |
| Class Hydrozoa Owen, 1843 | ||||||
| Subclass Trachylinae Haeckel, 1879 | ||||||
| Order Limnomedusae Kramp, 1938 | ||||||
| Geryoniidae Eschscholtz, 1829 | Liriope tetraphylla (Chamisso & Eysenhardt, 1821) | 1997 | 20.06 | 4551 | 52.29 | 35.10 |
| Olindiidae Haeckel, 1879 | Cubaia aphrodite Mayer, 1894 | 1510 | 15.17 | 336 | 3.86 | 9.89 |
| Olindias tenuis (Fewkes, 1882) | 4 | 0.04 | 2 | 0.02 | 0.03 | |
| Order Narcomedusae Haeckel, 1879 | ||||||
| Aeginidae Gegenbaur, 1857 | Aegina citrea Eschscholtz, 1829 | 4 | 0.05 | 0.02 | ||
| Cuninidae Bigelow, 1913 | Cunina octonaria McCrady, 1859 | 3 | 0.03 | 26 | 0.30 | 0.16 |
| Solmarisidae Haeckel, 1879 | Pegantha triloba Haeckel, 1879 | 2 | 0.02 | 2 | 0.02 | 0.02 |
| Solmundaeginidae Lindsay, Bentlage & Collins, 2017 | Solmundella bitentaculata (Quoy & Gaimard, 1833) | 125 | 1.26 | 255 | 2.93 | 2.04 |
| Rhopalonematidae Russell, 1953 | Aglaura hemistoma Péron & Lesueur, 1810 | 3599 | 36.16 | 1516 | 17.42 | 27.42 |
| Rhopalonema velatum Gegenbaur, 1857 | 5 | 0.05 | 0.03 | |||
| Subclass Hydroidolina Marques in Collins, 2000 | ||||||
| Order Aplanulata Collins, Winkelman, Hadrys & Schierwater, 2005 | ||||||
| Corymorpha forbesii (Mayer, 1894) | 442 | 5.08 | 2.37 | |||
| Order Capitata Kühn, 1913 | ||||||
| Cladonematidae Gegenbaur, 1857 | Cladonema radiatum Dujardin, 1843 | 2 | 0.02 | 0.01 | ||
| Staurocladia vallentini (Browne, 1902) * | 4 | 0.04 | 9 | 0.10 | 0.07 | |
| Corynidae Johnston, 1836 | Codonium proliferum (Forbes, 1848) | 23 | 0.23 | 16 | 0.18 | 0.21 |
| Coryne eximia Allman, 1859 | 168 | 1.69 | 15 | 0.17 | 0.98 | |
| Slabberia halterata Forbes, 1846 | 1278 | 12.84 | 41 | 0.47 | 7.07 | |
| Sphaerocorynidae Prévot, 1959 | Euphysilla pyramidata Kramp, 1955 * | 2 | 0.02 | 0.00 | 0.01 | |
| Pennariidae McCrady, 1859 | Pennaria disticha Goldfuss, 1820 | 394 | 3.96 | 3 | 0.03 | 2.13 |
| Zancleidae Russell, 1953 | Zanclea costata Gegenbaur, 1857 | 5 | 0.05 | 23 | 0.26 | 0.15 |
| Zanclea medusopolypata Boero, Bouillon & Gravili, 2000 * | 175 | 1.76 | 9 | 0.10 | 0.99 | |
| Zanclea sp. | 3 | 0.03 | 1 | 0.01 | 0.02 | |
| Superorder Anthoathecata Cornelius, 1992 | ||||||
| Order Filifera Kühn, 1913 | ||||||
| Zancleopsidae Bouillon, 1978 | Zancleopsis dichotoma (Mayer, 1900) | 24 | 0.28 | 0.13 | ||
| Bougainvilliidae Lütken, 1850 | Bougainvillia frondosa Mayer, 1900 * | 2 | 0.02 | 2 | 0.02 | 0.02 |
| Bougainvillia muscus (Allman, 1863) | 2 | 0.02 | 0.01 | |||
| Bougainvilliidae sp. | 12 | 0.12 | 65 | 0.75 | 0.41 | |
| Cytaeididae L. Agassiz, 1862 | Cytaeis tetrastyla Eschscholtz, 1829 | 7 | 0.08 | 0.04 | ||
| Filifera incertae sedis | Filifera sp. | 90 | 0.90 | 54 | 0.62 | 0.77 |
| Hydractiniidae L. Agassiz, 1862 | Podocoryna carnea M. Sars, 1846 | 1 | 0.01 | 5 | 0.06 | 0.03 |
| Podocoryna dubia (Mayer, 1900) * | 1 | 0.01 | 0.01 | |||
| Podocorynoides minima (Trinci, 1903) | 2 | 0.02 | 0.01 | |||
| Pandeidae Haeckel, 1879 | Amphinema sp. | 18 | 0.18 | 75 | 0.86 | 0.50 |
| Pandeopsis ikarii (Uchida, 1927) * | 2 | 0.02 | 0.01 | |||
| Proboscidactylidae Hand & Hendrickson, 1950 | Proboscidactyla ornata (McCrady, 1859) * | 12 | 0.12 | 0.06 | ||
| Protiaridae Haeckel, 1879 | Halitiara formosa Fewkes, 1882 | 14 | 0.14 | 114 | 1.31 | 0.69 |
| Oceaniidae Eschscholtz, 1829 | Turritopsis nutricula McCrady, 1857 * | 1 | 0.01 | 0.01 | ||
| Rathkeidae Russell, 1953 | Lizzia blondina Forbes, 1848 | 120 | 1.21 | 73 | 0.84 | 1.03 |
| Lizzia sp. | 4 | 0.04 | 1 | 0.01 | 0.03 | |
| Superorder Leptothecata Cornelius, 1992 | ||||||
| Order Statocysta Leclère, Schuchert, Cruaud, Couloux & Manuel, 2009 | ||||||
| Aequoreidae Eschscholtz, 1829 | Aequorea macrodactyla (Brandt, 1835) | 38 | 0.38 | 6 | 0.07 | 0.24 |
| Eirenidae Haeckel, 1879 | Eirene lactea (Mayer, 1900) | 31 | 0.31 | 53 | 0.61 | 0.45 |
| Malagazziidae Bouillon, 1984 | Octophialucium medium Kramp, 1955 | 6 | 0.06 | 3 | 0.03 | 0.05 |
| Clytiidae Cockerell, 1911 | Clytia mccradyi (Brooks, 1888) | 99 | 0.99 | 371 | 4.26 | 2.52 |
| Obeliidae Haeckel, 1879 | Obelia spp. | 61 | 0.61 | 450 | 5.17 | 2.74 |
| Order Laodiceida Maronna, Miranda, Peña Cantero, Barbeitos & Marques, 2016 | ||||||
| Laodiceidae Agassiz, 1862 | Laodicea marama Agassiz & Mayer, 1899 * | 8 | 0.08 | 0.04 | ||
| Laodicea sp. | 1 | 0.01 | 0.01 | |||
| Incertae sedis | ||||||
| Orchistomatidae Bouillon, 1984 | Orchistoma pileus (Lesson, 1843) | 22 | 0.22 | 3 | 0.03 | 0.13 |
| Tiaropsidae Boero, Bouillon & Danovaro, 1987 | Tiaropsidium roseum (Agassiz & Mayer, 1899, sensu Maas, 1905) * | 14 | 0.14 | 14 | 0.16 | 0.15 |
| Totals | 9953 | 100 | 8704 | 100 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tovar-Juárez, E.; Elías-Gutiérrez, M.; Segura-Puertas, L.; Mendoza-Becerril, M.A. Influence of Lunar Periodicity on Medusae (Cnidaria) Composition in a Western Caribbean Reef: Community Structure Before Sargassum Blooms. Diversity 2025, 17, 769. https://doi.org/10.3390/d17110769
Tovar-Juárez E, Elías-Gutiérrez M, Segura-Puertas L, Mendoza-Becerril MA. Influence of Lunar Periodicity on Medusae (Cnidaria) Composition in a Western Caribbean Reef: Community Structure Before Sargassum Blooms. Diversity. 2025; 17(11):769. https://doi.org/10.3390/d17110769
Chicago/Turabian StyleTovar-Juárez, Edgar, Manuel Elías-Gutiérrez, Lourdes Segura-Puertas, and María A. Mendoza-Becerril. 2025. "Influence of Lunar Periodicity on Medusae (Cnidaria) Composition in a Western Caribbean Reef: Community Structure Before Sargassum Blooms" Diversity 17, no. 11: 769. https://doi.org/10.3390/d17110769
APA StyleTovar-Juárez, E., Elías-Gutiérrez, M., Segura-Puertas, L., & Mendoza-Becerril, M. A. (2025). Influence of Lunar Periodicity on Medusae (Cnidaria) Composition in a Western Caribbean Reef: Community Structure Before Sargassum Blooms. Diversity, 17(11), 769. https://doi.org/10.3390/d17110769






