Abstract
Mediterranean ecosystems are highly susceptible to wildfires, and shifts in fire patterns pose a threat to biodiversity, soil stability, and overall ecosystem health resilience. Implementing prescribed burning as a management strategy to lower wildfire risk has been proposed, but its ecological impacts in Greece are not well understood. This study examines the relationship between fireline intensity during prescribed burning and plant water potential, as well as its effects on soil properties and plant biodiversity on Chios Island, Greece. Field experiments were carried out in representative ecosystems, where we measured flame length to determine fireline intensity. In addition, we gathered soil samples before and after the prescribed burning and evaluated plant diversity. Measuring leaf water potential gave us a better understanding of how plants respond physiologically to different seasonal and site conditions. Our findings revealed that prescribed burning typically boosted plant diversity after the fire, with Fabaceae and Asteraceae playing a key role in regeneration. However, the soil responses differed from one site to another. Some sites saw a decline in organic carbon and nitrogen, while others showed an increase in exchangeable cations like calcium and magnesium, highlighting the importance of site-specific results. Studies on plant water potential revealed seasonal fluctuations in stress, underscoring the importance of accounting for seasonality in prescribed burn planning. Overall, prescribed burning has the potential to enhance biodiversity and ecosystem recovery, while also reducing fuel loads. These results highlight the importance of ongoing, site-specific monitoring for developing sustainable fire management strategies in Mediterranean ecosystems.
1. Introduction
Mediterranean ecosystems are globally recognized for their high biodiversity and the long-standing role of fire as a natural ecological factor shaping their structure and function [1,2]. Fire regimes, however, have been dramatically altered in recent decades due to land use changes, fire suppression policies, and climate change, resulting in more frequent, intense, and extensive wildfires [3].
Prescribed Burning (PB) is a fuel management method that offers multiple benefits [4], including wildfire hazard and risk reduction, enhanced forest ecosystem resilience, and biodiversity conservation [5,6]. The spatial and temporal scales of its implementation depend on fire management objectives, forest type, location, fire regime [7,8], and potential restrictions. Progress in adopting PB across Europe remains limited. In many regions, it is absent [9] while its use is mostly confined to fuel reduction in Portugal, Spain, and southern France, and to enhancing biodiversity in Sweden.
The need for an evidence-based and flexible approach to PB, emphasizing biology and habitat ecology, has been highlighted [10] along with a call to shift toward knowledge [11] that supports future research syntheses and fosters actionable science [12].
Unfortunately, planned PB experiments have been rare in Mediterranean ecosystems, despite their potential to provide valuable insights into fire impacts on soil properties and effects on trees. Eales et al. [13] noted that many studies on PB lack detailed reporting of fire behaviour metrics, such as fireline intensity (I, kW·m−1) [14,15,16] and flame length (FL, m). In subsequent years, however, researchers have attempted to correlate fire behaviour characteristics with biodiversity outcomes. Davies et al. [17] modelled fire behaviour during prescribed burns in Calluna heathlands, rather than measuring long-term biodiversity outcomes, while Zylstra [18] attempted to integrate detailed fire behaviour metrics, such as FL and I, with ecological outcomes and fire effects related to plant traits. Ramberg et al. [19] conducted a 16-year study on PB and fungal (polypore) diversity in boreal forests. They compared burned and unburned stands, assessing coarse woody debris, fungal species richness, and the presence of red-listed species, but did not report detailed metrics of FL or I.
Plant water potential serves as a key metric for assessing ecosystem health, particularly for evaluating plant responses to wildfire stress and tracking post-fire recovery [20]. Meanwhile, tracking biodiversity after prescribed burns offers valuable insights into whether ecosystems can preserve species diversity and ecological functions after disturbance [21]. It is worth noting that the severity of wildfires can affect both plant physiology and biodiversity patterns, but the link between these factors is not well understood in the Mediterranean region [22]. Furthermore, soil characteristics play a key role in determining how ecosystems respond to wildfires. Changes in soil characteristics caused by wildfires such as the amount of organic matter, nutrient availability, water infiltration, and microbial activity can significantly impact how plants recover after wildfires and biodiversity dynamics [23]. The intensity and severity of wildfires [24] directly affect how much soil is altered, with intense wildfires often causing soil to become water-repelling, nutrient loss, and damage to soil structure [25,26]. In Mediterranean environments, where soils are typically shallow and low in nutrients, understanding how prescribed burns impact soil properties is essential for predicting ecosystem resilience and developing sustainable fire management practices [27].
The first efforts to introduce and utilize the PB in Greece began in the 1970s [28], when members of the Greek forest scientific community and the Hellenic Forest Service applied PB experimentally, analyzed data and drew some preliminary conclusions [29]. They made some steps to document the use of fire and study its impacts, discussing also ecological and managerial issues as well as its potential combination with grazing and mechanical treatments [30].
Unfortunately, without sustained funding, legal support, logistics, ongoing scientific guidance, and clear objectives, the endeavour was soon abandoned. Almost half a century later, in 2021, a core team of researchers and practitioners started a pilot project on the implementation of the PB on the island of Chios aiming to introduce PB as a tool for forest fuel management, increase social–ecological resilience to wildfire and contribute to a climate-resilient future [31].
This study aims to address significant research gaps in the relationships among plant water potential, calculated Ι, post-burn vascular plant biodiversity and soil properties, to better understand fire effects associated with PB and, potentially, low-intensity wildfires. We utilized field recordings and FL measurements collected during PB on Chios island, Greece [31], to calculate and examine its correlation with post-burn vascular plant biodiversity. For the first time in Greece, PB are being used to simultaneously examine these factors, thereby bridging the gaps between ecological theory and fire management practice. The findings provide actionable knowledge for sustainable fire management and biodiversity conservation in a changing environment and fire regime.
2. Materials and Methods
2.1. Study Area
Chios, the fifth largest island in Greece (842 km2, coastline 229 km), is located in the central Aegean Sea and belongs to the North Aegean Region (Figure 1). Its relief is mainly mountainous-semi-mountainous, with steep slopes in the northern part and gentler ones in the southern part. The highest peak is Mount Pelinaio (1297 m), followed by other mountain peaks such as Mount (1126 m), Provatas (807 m) and Koklias (770 m), which create differentiated microclimatic conditions (Municipality of Chios, 2020).
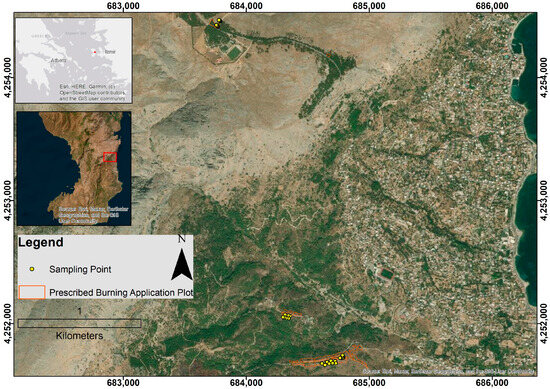
Figure 1.
The study area on the island of Chios, Greece, was projected using the Greek Grid system (EGSA87). The red point and red square in the two locator maps indicate the location and extent of the study area. The map was created using ArcGIS version 9.3 geographic information system software developed by ESRI.
The climate is typically Mediterranean (Csa, Köppen–Geiger), with hot and dry summers, mild and wet winters, a limited thermometric range and high summer drought [32].
The flora of Chios is of particular interest, as it is part of the thermo-Mediterranean vegetation zone (Oleo–Carpinetum). In the northern and mountainous parts, forests of Holm oak (Quercus ilex) and Calabrian pine (Pinus brutia) are found, while in the lower areas, maquis and phrygian vegetation dominate. Of particular importance is the endemic Fritillaria pelinaea, which was scientifically described from Mount Pelinae [33]. Furthermore, wild tulips (Tulipa agenensis, T. praecox, T. undulatifolia, T. clusiana) have been recorded on the island, which are typical elements of agro-pastoral ecosystems [34,35]. In particular, the cultivation of mastic (Pistacia lentiscus var. chia), unique worldwide, has been recognized as a Protected Designation of Origin (PDO) product and is a cultural and ecological landmark [36,37]. The fauna includes representative species of the Aegean. The presence of the otter (Lutra lutra) has been recorded in Chios, mainly in streams and coastal areas [38], while in the marine zones the habitat of the Mediterranean monk seal (Monachus monachus) is observed, although in limited numbers [39]. The bird fauna is rich, with the characteristic presence of the black-footed kestrel (Falco eleonorae) on microislets, such as the Venetian Islet (GR4130004) [40]. Significant parts of Chios and the surrounding islands are included in the Natura 2000 Network, which underlines their ecological value. Specifically, they include: GR4130001 “North Chios and Oinousses Islands and Coastal Marine Zone” (SAC), GR4130003 “North Chios” (SPA), GR4130004 “Venetiko Island (Chios)” (SPA), GR4130005 “Kalogeroi Rock Islands and Marine Zone” (SAC) [41]. These areas host habitats of community interest, such as Posidonia oceanica meadows (1120), reefs (1170), Sarcopoterium spinosum bryophytes (5420), riparian plane trees (92C0) and aloes (1410).
The main environmental threats include forest fires, which have caused significant ecological and economic losses (e.g., 2012, 2016, 2023, 2025) [42], uncontrolled construction, overgrazing, illegal fishing and hunting, as well as pressure from tourism development. Wildfires in particular pose a serious risk to the structure and functioning of ecosystems, as well as to mastic plantations.
2.2. Samplings
2.2.1. Vascular Plant Diversity
In the present study, vascular plant species were sampled at three designated sites: Resta, Aipos and Agios Stefanos (Figure 2). At each sampling site, plant species were recorded using four 1 m2 quadrats within each selected experimental area, of the research site, both before and after the application of PB, as well as in controls [43] (Figure 3). To determine the plant samples, the “Flora Europaea” [44,45], the “Flora Hellenica” [46], and the vascular plants of Greece: an annotated checklist [47] were used. The surface areas of the sites are as follows: 1727 m2 (I1, I2, I3 and I4), 1157 m2 (I5, I6, I7 and I8), 7396 m2 (I9, I10, I11 and I12), 1494 m2 (I13, I14, I15 and I16). Sampling events were conducted at annual intervals over a two-year period to capture post-fire vegetation dynamics. PB was implemented on the following dates for each site: Aipos (14 April 2022), Resta (17 April 2022 and 5 December 2022) and Agios Stefanos (6 December 2022).
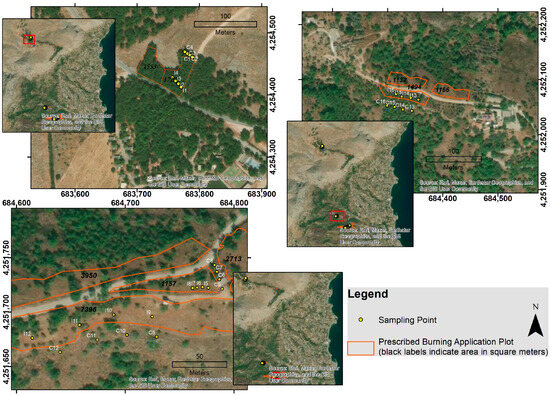
Figure 2.
The plant biodiversity sampling points (each one the centre of the 1 square metre plot). I: inside the prescribed burned area, C: control, outside the prescribed burned area). The map was created using ArcGIS version 9.3 geographic information system software developed by ESRI.

Figure 3.
Sampling of plant biodiversity in the experimental plots of the research area (photos by Dr. Alexandra Solomou and Elias Tziritis 2022).
Also, the coding used is as follows:
REAFTPBINS2: Resta-After prescribed burning_Inside Site_2023;
REBPBCONS2: Resta-Before prescribed burning_Control Site_2022;
REAFTPBCONS2: Resta-After prescribed burning_Control Site_2022;
REBPBINS2: Resta-Before prescribed burning_Inside Site_2022;
AIAFTPBINS1: Aipos-After prescribed burning_Inside site_2023;
AIBPBCONS1: Aipos-Before prescribed burning_Control Site_2022;
AIAFTPBCONS1: Aipos-After prescribed burning_Control site_2023;
AGBPBINS3: Agios Stefanos-Before prescribed burning_Inside Site_2022;
AGAFPBINS3: Agios Stefanos-After prescribed burning_Inside Site_2023;
AIBPBINS1: Aipos-Before prescribed burning_Inside site_2022;
AGAFPBCONS3: Agios Stefanos-After prescribed burning_Control Site_2023;
AGBPBCONS3: Agios Stefanos-Before prescribed burning_Control Site_2022.
2.2.2. Soil Sample Processing
In December 2022 and April 2023, soil samples were collected from three areas of Chios, Resta, Agios Stefanos and Aipos, before and after the application of PB. Three samplings were carried out in the Resta area and one in the other two. Three replicates were collected in each sampling. to a depth of 20 cm after the dry needles and dry leaves were removed from the soil surface. The areas and sampling dates are shown in Table A17, Table A18, Table A19, Table A20 and Table A21 (Appendix B).
The soils in the Resta and Agios Stefanos areas are classified as sandy loam, while those of the Aipos area are clayey to clayey loam. The parent material in the Aipos area is hard limestone, while in the Resta area it is likely to be shale. Calcium carbonate was detectable, with a maximum value of 3.5%, in the Aipos area and that of Agios Stefanos with a maximum value of 0.3%. The forest surface in the Resta area was phrygana which are low xeric and mostly spiny shrubs (Table A17 and Table A18 in Appendix B) as well as phrygana with Calabrian pine (Pinus brutia) litter with many gaps (Table A19 in Appendix B). The Agios Stefanos area was covered by pine litter, while the Aipos area was also covered by pine litter with gaps from rocky outcrops. Sampling was repeated in late autumn 2023 after the burning that took place immediately after sampling.
The soil samples were air-dried and passed through a 2 mm sieve. Some of them were ground in a ball mill to determine the concentrations of organic carbon, nitrogen and calcium carbonate. The pH of these soil samples was measured electrometrically in a soil-water suspension of 1:2.5 (w/v) and the mechanical composition was estimated by the hydrometer method [48]. Conductivity was measured in a soil-water suspension of 1:5 (w/v) [49]. Organic carbon was determined by wet oxidation with potassium dichromate (K2Cr2O7). Exchangeable cations Ca2+, Mg2+, Na+ and K+ were extracted with a 1 M ammonium acetate solution (NH4C2H3O2), pH 7 and their concentration was determined in an atomic absorption spectrophotometer. The concentration of organic + ammonium nitrogen (N) was determined by the Kjeldahl method. Free calcium carbonate (CaCO3) was determined with a Bernard calcimeter. Ammonium and nitrate ions were extracted with 1 M KCl solution [50]. Ammonium concentrations were measured with a Kjeldahl apparatus and nitrate with a visible–ultraviolet spectrophotometer (at 210 nm). The concentrations of all soil parameters in Table A17 and Table A18 (Appendix B) are expressed on an air-dried soil basis.
2.2.3. Fireline Intensity (Ι)
FL was recorded by following specific procedures [51,52,53,54,55,56,57,58,59] during PB on Chios Island, Greece. According to Gould et al. [60] (Table 1), the reliability of the methods and measurement techniques used to assess meteorological conditions describe forest fuels, and document fire rate of spread and behaviour was rated as 1. This rating was assigned because weather data were obtained from a nearby meteorological station and through direct field measurements, fuel characteristics were inferred using functions developed specifically for the fuel types, and fire spread was measured directly by the authors.

Table 1.
Reliability rating for weather, fuel and fire spread observations for wildfires in open eucalypt forests.
FL was measured using the following steps: Observer’s positions were recorded using a GPS device to obtain the geographic coordinates. Regarding ground-based photography, images of sequential locations of the fire perimeter were captured using a Canon EOS 70D DSLR camera manufactured by Canon Inc. (Tokyo, Japan). The horizontal azimuth for each photograph was recorded with a compass. The time each photo was captured was retrieved from the metadata automatically embedded in the digital image files by the camera. FL was then measured using basic geometric methods. Knowing the exact time of each FL observation allowed matching it with the corresponding fine live and/or dead fuel moisture content, which had been measured separately. Fire behaviour recording also included aerial photographs captured using a DJI Phantom 4 Pro unmanned aerial vehicle (UAV) manufactured by DJI Technology Co., Ltd. (Shenzhen, China) [29]. It carries a 1″ CMOS, 20 Mp sensor, with FOV 84 8.8 mm/24 mm (35 mm format equivalent) f/2.8–f/11 auto focus at 1 m-lens and a mechanical/electronic shutter speed of 8–1/2000 s. Images were created in JPEG format of 5472 × 3648 pixels.
Ι was then calculated from FL using the appropriate method, depending on fuel type, observed FL, and whether the fire spread was heading or backing. Specifically, we used the following equations: (a) Fernandes et al. [61] for litter in Mediterranean pine forests, (b) Fernandes et al. [62] for shrublands, namely Cistus spp. and Sarcopoterium spinosum, (c) Clark [63] for heading fire spread in grasslands; and (d) Nelson and Adkins [64] for heading fire spread in Mediterranean pine forest litter. Also, we used linear regression to assess the relationship between the difference in Shannon diversity and Fireline Intensity (I) values (kW·m−1).
2.2.4. Needle Water Potential
Predawn water potential (Ψleafpd) and pre-burn leaf water potential (ΨleafPB, measured near midday) were recorded for eight Pinus brutia trees at the Aipos site in spring 2022, 20 shrub and maquis species at the Resta site during winter 2022, 13 shrub and maquis species at the Resta site during spring 2022 and six Pinus brutia trees at the Agios Stefanos site during winter 2022. Measurements of ΨleafPB were taken between 11:00 and 14:00 using a portable pressure chamber (model PMS 1003, PMS Instruments, Corvallis, OR, USA). Ψleafpd served as an indicator of plant water stress, under the assumption of negligible night-time transpiration [65].
2.3. Statistical Analyses
The Kolmogorov–Smirnov and Shapiro–Wilk tests were used for the confirmation of the normal distribution of data. Plant diversity was assessed using the following biodiversity index [66,67]:
The Shannon diversity index (H): The index considers the number of species present in the sample and the relative number of individuals present for each species. It is used to quantify specific biodiversity. Values less than 1.5 are interpreted as sites with relatively low species diversity, while those greater than 2.0 are high. Mathematically, the Shannon index is calculated by the following expression:
where is the species diversity index, s is the number of species, and is the proportion of individuals of each species belonging to the ith species of the total number of individuals.
For the statistical comparison of the soil parameters, due to the high variability of the parameters, all values were transformed to logarithms, with the exception of pH, for a better distribution of the values (in relation to a normal distribution). The statistical comparison for each experimental surface was made with the analysis of variance (ANOVA). The comparison of the means was carried out with the Tukey test.
3. Results
3.1. Plant Composition and Diversity
In the studied area, 69 plant species belonging to 30 families were identified. The most numerous families were Fabaceae (16%), Asteraceae (101%), and Poaceae (8%) (Figure 4). The treatments with the greatest number of plant species were REAFTPBINS2 (38 species), REAFPBINS4 (31), REBPBINS2 (27), and REBPBCONS2 (26), while the treatment with the fewest plant species was AIAFTPBINS1 (8). Appendix A presents the plant species recorded at each site.
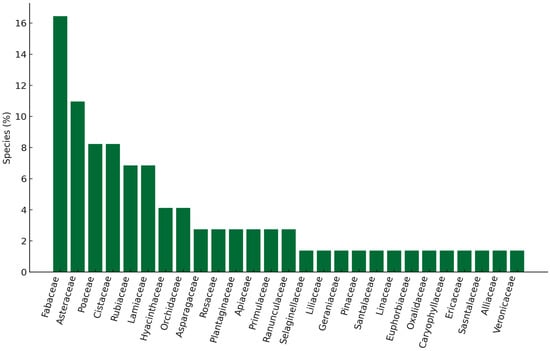
Figure 4.
Percentage distribution of plant species across plant families.
The Shannon diversity index was significantly different among treatments. Therandomization test indicated that the difference between sampling sites was statistically significant (p < 0.05). The highest diversity was reported in the treatment REAFTPBINS2 (2.14d) followed by REBPBCONS2 (1.97d), whereas intermediate values were observed for REAFTPBCONS2 and REBPBINS2 (1.78c and 1.61c, respectively) (Table 2).

Table 2.
Plant diversity index in sites.
3.2. Soil Parameters
Table A17, Table A18, Table A19, Table A20 and Table A21 (Appendix B) present the results of the soil analyses along with the statistical comparison of the parameters after and before the application of the PB. A characteristic feature is the variability of the chemical properties of the soils which is shown by the high values of the coefficient of variation in the tables. The elements most affected by the PB implementation were organic carbon and nitrogen. Regarding carbon, statistically significant differences (decrease in concentration after burning) were observed in Table A17 (Resta) and Table A20 (Agios Stefanos). In the area of Resta (Table A17) the C/N ratio also increased statistically significantly, which contributes to soil fertility. Total, organic and available nitrogen in the area of Agios s Stefanos (Table A20) followed organic carbon, i.e., it decreased significantly after burning. It should be noted in general that the percentage (%) of available N in relation to total N is very small (close to unity), but its importance is great given that it is the only form of N that plants can take up. A follow-up prescribed burn in the Agios Stefanos area should be conducted after a minimum of three years to assess whether available nitrogen levels return to pre-burn levels. Total and organic nitrogen in the Aipos area (Table A21) increased after burning. This finding is probably due to the variability of sampling. In Table A18 and Table A21 it appears that the concentrations of exchangeable Ca increased significantly. The same applies to Mg in the Aipos area (Table A21). It is known that burning increases the concentrations of exchangeable cations in the soil. However, the same did not happen with exchangeable K and Na in Table A20. Also, in all cases, the conductivity of the soil solution did not differ significantly.
The area of Resta and Agios Stefanos did not have any CaCO3 concentration. The Aipos had low concentrations of CaCO3 which ranged 0.79–1.64%.
3.3. Fire Behaviour and Plant Diversity
FL ranged from 0.5 to 1.8 m and I varied from 84 to 2695 kW·m−1, respectively. Measured FL, calculated I, and Shannon index values, are presented in Table 3.

Table 3.
Flame length (FL, m), fireline intensity (I, kW·m−1), post-burn Shannon diversity index and Shannon difference values.
We used linear regression to assess the relationship between the difference in Shannon diversity and I (Figure 5). The analysis revealed no statistically significant relationship (p = 0.856) and a poor model fit (adjusted R2 = −0.069), indicating that I does not linearly explain variation in Shannon diversity. This is consistent with the small unstandardized coefficient of I (0.00002921), suggesting a negligible effect size.
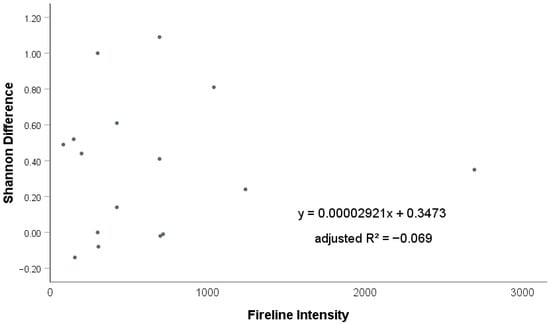
Figure 5.
Linear regression between Shannon difference and Fireline Intensity (I) values (kW·m−1).
3.4. Needle Water Potential
Ψleaf measurements across four Mediterranean sites, Aipos, Resta (Winter and Spring), and Agios Stefanos, show clear differences between Ψleafpd and ΨleafPB values. At all sites, Ψleafpd values were consistently higher (less negative), indicating better plant hydration during early morning. For example, at Aipos, the mean Ψleafpd was −1.00 MPa, which decreased to −1.94 MPa before the PB. Similarly, at Resta Winter, Ψleafpd averaged −1.54 MPa, dropping to −2.02 MPa pre-burn. Agios Stefanos showed a mean Ψleafpd of −1.45 MPa, which decreased substantially to −2.32 MPa before burning. At Resta Spring, however, the mean Ψleaf remained relatively stable between predawn and pre-burn measurements, around −1.11 MPa (Figure 6).
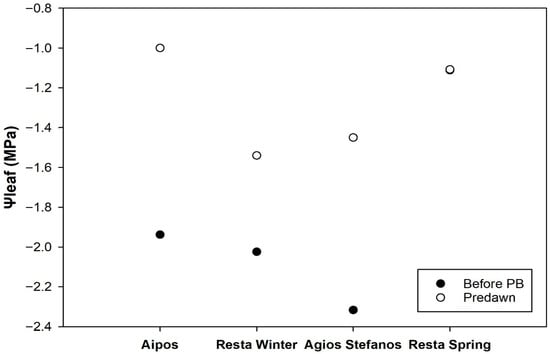
Figure 6.
Predawn and pre-burn Ψleaf measurements across four Mediterranean sites, Aipos, Resta (Winter and Spring), and Agios Stefanos on Chios Island.
Regarding the range of mean Ψleafpd across the four sites, they spanned from −1.00 to −1.54 MPa. Specifically, the sites Resta Winter and Agios Stefanos exhibited the lowest mean Ψleafpd at −1.54 and −1.45 MPa, respectively, reflecting the most negative (i.e., highest water stress) predawn leaf water potential among the locations measured. Resta Spring had intermediate values of −1.11 MPa, while Aipos recorded the highest mean Ψleafpd at −1.00, indicating comparatively better soil moisture conditions.
4. Discussion
4.1. Plant Composition and Diversity
The plant species of Mediterranean ecosystems, having been subjected to the action of fire for thousands of years, have developed mechanisms that ensure their survival as well as rapid regeneration and recovery [68]. The main mechanism is the vegetative regeneration (Resprouting) of burned individuals and the establishment of new individuals through the process of seed germination [69].
In the present study, it was observed that the most numerous families were Asteraceae, Poaceae and Fabaceae, a fact that reflects the prevailing situation in the Greek area, as these families are among the three most numerous families in Greece and the Mediterranean. The plant species belonging to the Asteraceae, Poaceae and Fabaceae families, in their majority, occur in ecosystems such as the research area and include plants with high ecological value [34,70,71].
Our results namely the dominance of Asteraceae, Fabaceae, Poaceae and the post-burn increase in α-diversity fit well within established Mediterranean post-fire patterns. At the community level, early post-fire assemblages in Mediterranean shrublands and open woodlands typically show a short-term surge in species richness and evenness driven by a pulse of annual forbs and graminoids together with the persistence of resprouting shrubs. This “early peak” has been documented across vegetation types and moisture settings in the Basin (e.g., maquis, garrigue/phrygana, and pine stands understoreys) and is especially evident after low to moderate intensity wildfires or prescribed burns that leave belowground organs and patches of live vegetation intact [72,73,74].
The family-level signal we observe is also expected for early post-fire stages in the eastern Mediterranean: Asteraceae and Poaceae contribute to many fast-cycling annuals, while Fabaceae are disproportionately represented among obligate seeders with physically dormant seeds. Heat pulses from fire and, in some taxa, smoke-derived compounds are well-known cues that break dormancy and synchronize emergence in Cistaceae and Fabaceae, thereby boosting short-term richness and altering composition [75,76,77]. Importantly, upper heat thresholds for breaking physical dormancy match fire-related temperatures, whereas lower thresholds are shaped by background summer temperatures together releasing Fabaceae-Cistaceae seed dormancy and synchronizing emergence after burns [75,76,77].
Plant diversity before the implementation of the PΒ was observed at satisfactory levels. The diversity of the topography of Chios, the altitudinal gradient from sea level to the highest peak, the variety of climatic conditions likely created a multitude of different habitats (stations), which support the above plant diversity. The geological substrate with the different types of soils creates another important parameter for plants. Specifically, the different rocks of Chios likely create various types of soils which affect the chemical composition, cohesion, pH and nutrients of the substrate, on which the plant species grow. Also, the existing neighbouring vegetation as well as the paleogeography, the colonization of the area by humans and the phylogeny that developed in the area over time are closely related to the significant number and diversity of plant species recorded in the research areas before the implementation of the PΒ.
From the analysis of the data, it emerged that the PΒ has a significant effect on the plant diversity (number of plant species, Shannon–Wiener and Simpson index) of the burned forest formations of Chios. This conclusion stems from the differentiation that the burned areas present compared to the unburned areas. In particular, high plant diversity values were observed after the implementation of the PΒ compared to those recorded before the implementation of the PΒ.
The increased diversity in the burned areas could be explained in various ways, such as, for example, by the entry of some precursor species, which disappear in the following period under conditions of competition. Furthermore, the increased plant diversity could be attributed to the many species of the Fabaceae family found in the samples, which show high abundance and coverage during the first post-fire years, as their germination is favoured by the action of fire [34]. A large part of the plant species of the Fabaceae family are therophytes, mainly annual herbs whose regeneration occurs by seed germination [78]. Also, another possible explanation of this result could be attributed to the fact that plants that die off from fire and regenerate by seed germination depend on this germination mechanism in order to exist in the specific area. For these plants, young shoots mature and produce seeds that feed the seed bank, thus ensuring the recovery of the plant species population [79].
From a functional perspective, our floristic shift reflects the coexistence of resprouting and seeding strategies under a mild fire regime. Low-intensity PB reduces above-ground competition and litter while preserving many resprouter rootstocks, creating establishment windows for obligate seeders; this mixture tends to elevate α-diversity and, at fine scales, β-diversity as well [80,81]. Trait syntheses for Mediterranean floras (BROT 2.0) show that the very species pools dominating our plots carry fire-linked traits (resprouting organs, serotiny in conifers where present, heat/smoke-cued germination), providing a mechanistic basis for the compositional patterns we report [82].
Patchiness also matters. Prescribed burns commonly generate a fine-grained mosaic of lightly charred, unburned, and more strongly heated microsites. Such pyrodiversity promotes niche complementarity resprouters reoccupy lightly affected patches while obligate seeders capitalize on hotter, more open microsites thereby maintaining higher local diversity in the short term [74,81]. In this context, our within-site heterogeneity in life forms and families is consistent with the notion that diversity benefits from a mixed-severity, patchy fire imprint.
In our sites, several species illustrate the typical Mediterranean fire-response spectrum. After a fire, the dwarf shrub Sarcopoterium spinosum grows quickly and can even grow from seed [83]. Smoke compounds can also help seeds germinate [84]. The obligate-seeding shrubs Cistus creticus and the Lavandula stoechas exhibit fire-induced germination through heat and/or smoke, and they also respond to post-fire light and temperature conditions [74,82,83,84,85,86]. Brachypodium retusum, a rhizomatous grass, usually grows back quickly after a fire, and fire can help it reproduce sexually in Mediterranean grasslands [87,88]. Annual legumes (Trifolium campestre, T. scabrum, T. stellatum) preserve physically latent, soil-stored seed banks and frequently germinate following disturbances such as fire [89,90]. The prickly legume Calicotome villosa establishes a lasting seed bank that facilitates post-fire recruitment and is also known to resprout, so integrating seeder and resprouter tactics [91]. Conversely, Pinus brutia is a non-resprouting obligate seeder that regenerates post-fire from canopy and soil seed storage [92]. Within the CSR framework [93,94], resprouting shrubs and perennial grasses in our plots tend to be CS-leaning, whereas obligate-seeding shrubs and annuals are more R-oriented; consequently, low-intensity burns at our sites were followed by short-term increases in annuals and other R-strategists, while CS-types recovered via resprouting consistent with the higher diversity observed in REAFTPBINS2 (H = 2.14) compared with unburned controls (Table 2). According to the research of Kazanis and Arianoutsou [95], the evolution of vegetation after fire follows the model of “auto-succession”, where the community that has burned, no matter how different it may seem from the unburned one, maintains its floristic identity. Thus, the geophytes, which pre-existed in the area and survived, benefit from the post-fire conditions of abundant nutrients, abundant sunlight and moisture, but also from the absence of competitors.
In addition to the application of PB, the systematic recording of environmental parameters and other factors (e.g., grazing) would enhance our understanding of how these variables interact with fire to influence biodiversity in the study areas.
Data from Mediterranean forests and grasslands indicate that diversification responses vary depending on post-fire management practices. A five-year study in southern Italy revealed that PB and mowing reduced the prevalence of tall perennial grasses due to abandonment, significantly improving plant diversity compared to unmanaged controls, which indicated a post-burn composition marked by an increase in short-lived forbs and legumes [96]. The initial regeneration of vegetation in Mediterranean forests after a fire was similarly affected by management approaches, showing increased species richness immediately post-fire before progressively returning to pre-fire composition [73]. Greek example studies confirm this timing pattern: in the Pinus halepensis forests of Attica, herbaceous plant species dominate during the first four years after a wildfire, with plant diversity peaking in the second year. Thereafter, their abundance declines as woody species gradually become more dominant [97,98]. Similarly, in Northern Achaia (Peloponnese), the first 1–2 years after wildfire were dominated by annual herbaceous species, primarily from the Leguminosae (Fabaceae) and Compositae (Asteraceae) families, whereas mature stands were characterized by a dense woody understory [99]. Ultimately, our observed species composition and functional strategies align with long-term successional patterns described in the literature. When fire-free intervals are prolonged or management practices promote the accumulation of dense litter and tall grasses, plant diversity may decline due to competitive exclusion. In contrast, regular low-intensity wildfires or targeted interventions can maintain a functional balance between resprouters and seeders, thereby supporting greater understory diversity [74,96]. These similarities reinforce the broader applicability of our findings and highlight a specific ecological mechanism linking the observed compositional shifts to well-established Mediterranean fire-response strategies.
4.2. Soil Parameters
The application of PB in the sites under study of Chios (Resta, Agios Stefanos, Aipos) revealed differentiated effects on the basic physicochemical parameters of the soil. The results demonstrate that organic matter and nitrogen are the most sensitive elements to fire, as has been documented in previous studies [95].
In the Resta area, a statistically significant decrease in organic carbon was observed after burning, which is attributed to the reduction in combustible material. At the same time, an increase in the C/N ratio is considered favourable for soil fertility, as it can enhance the long-term stability of organic matter [100]. On the contrary, in the Agios Stefanos area, burning led to a significant decrease in both organic carbon and available nitrogen. This finding is particularly important, as available nitrogen is the form readily available to plants and its reduction may have consequences for vegetation [50]. According to international literature, it takes approximately three to four years for available nitrogen levels to recover after burning [95], which confirms the need for long-term monitoring.
In the Aipos area, unlike the previous two ecosystems, an increase in total and organic nitrogen was recorded, probably due to spatial heterogeneity in sampling. At the same time, the concentrations of exchangeable Ca and Mg increased significantly after burning, a finding that is consistent with the observation that ash enriches the soil with base cations. The concentration of exchangeable Ca2+ also increased significantly in the Resta area which was covered by phrygana. Although exchangeable cations such as Ca2+, Mg2+, K+ have been reported in numerous studies to increase after forest fires [101,102], there can be variability. The existence of such variability was stressed by Arocena and Orio [103]. According to these authors, the main cause is the intensity of flame temperature. However, no statistically significant changes in electrical conductivity were observed, indicating that salt availability was not strongly affected by fire. The surface ash can cause problems in the selection of soil samples. When there are strong winds with different directions, the deposition and settlement of ash can be affected. This variability can be reduced by selecting similar topography for soil collection before and after the application of prescribed burning.
Overall, the results suggest that PB did not produce significant changes in most basic soil properties, at least in the short term. However, the variations recorded in carbon and nitrogen highlight the importance of burning as a management tool that can have both positive and negative impacts, depending on the ecosystem and the time frame. The continuation of experiments on a longer time scale is considered necessary to determine the long-term effects of this practice [49].
4.3. Fire Behaviour and Plant Diversity
The relationship between the plant diversity after the implementation of the PB method and the I during the prescribed burns is not linear (Figure 5). Since higher Shannon diversity values indicate more diverse and evenly distributed plant communities, a nonlinear pattern may better describe the relationship, where low-intensity wildfires promote diversity by reducing dominant species, while high-intensity wildfires reduce diversity by eliminating sensitive species [104]. The above-mentioned hypothesis aligns with the findings of Richter et al. [105] who reported that plant diversity peaks at intermediate wildfire severities, rather than under low or high severity conditions. It also supports the conclusions of Abedi et al. [106] who found that fire regimes, including I, significantly influence taxonomic diversity and that these effects can be mediated by landscape factors such as slope and aspect.
Future research may employ polynomial regression to capture potential nonlinear patterns and reveal ecological thresholds, bandwidths (e.g., peak diversity at intermediate fire intensity), or tipping points (e.g., a collapse in diversity beyond a certain intensity level).
4.4. Needle Water Potential
Measurements at the Resta Winter and Agios Stefanos sites showed the most negative Ψleafpd, both recorded during the winter period. This suggests that plants were still experiencing moderate water stress at that time, in contrast to the spring measurements from Aipos and Resta Spring, which indicated comparatively better soil moisture conditions. Such seasonal differences are consistent with precipitation patterns: between September 2021 and April 2022, when measurements at Aipos and Resta Spring were taken, cumulative rainfall was approximately 340 mm, whereas from May to December 2022, preceding the winter measurements, total rainfall was less than half that amount (around 150 mm) In both seasons, however, the vapour pressure deficit (VPD) remained very low (<0.3 kPa), suggesting minimal transpiration. Changes in plant water potential occur seasonally, in response to soil moisture (rainfall) and atmospheric demand (VPD). Several studies (e.g., [107,108,109]) have shown that following early autumn or spring rains, trees typically recover from drought stress. Similarly, in our study, spring rainfall led to higher (less negative) Ψleafpd, values, whereas during the drier winter period, slightly more negative Ψleafpd, values were recorded.
5. Conclusions
This study highlights the ecological effects of PB in Mediterranean vegetation on Chios island, Greece. The results showed that PB treatment influenced plant diversity, soil properties and plant water potential in ways that may support both ecosystem resilience and fire management measures. Overall, PB tended to increase plant diversity, indicating that fire promotes the regeneration and recruitment of post-fire specialist species. This finding supports the idea that PB can be an effective tool for maintaining biodiversity.
Soil analyses yielded disparate site responses, with organic carbon and nitrogen being the most sensitive to fire. While some cases showed a decrease in available nitrogen, the rise in exchangeable base cations (such as calcium and magnesium) suggests that burning can also temporarily enrich the soil. The differences between sites underscore the importance of local monitoring and long-term studies to determine if these changes are sustained.
Leaf water potential measurements were in line with the seasonal difference in plant water status, with higher stress conditions in winter compared to spring. This implies that prescribed burning interacts with weather conditions; thus, the seasonality should be taken into account when planning fire use.
These results demonstrate that well-planned PB can help mitigate fire danger while supporting biodiversity conservation and ecosystem functioning in Mediterranean ecosystems. Nevertheless, further long-term monitoring is needed to fully understand the cumulative fire effects and to establish suitable guidelines for sustainable fire regimes in Greece.
Author Contributions
Conceptualization, A.D.S., M.A., E.K. and P.M.; methodology, A.D.S., M.A., E.K. and P.M.; software, A.D.S., M.A. and E.K.; validation, A.D.S., M.A., E.K., P.M. and G.K.; formal analysis, A.D.S., M.A. and E.K.; investigation, A.D.S., M.A., E.K., P.M. and G.K.; resources, A.D.S., M.A. and E.K.; data curation, A.D.S., M.A. and E.K.; writing—original draft preparation, A.D.S., M.A., E.K. and P.M.; writing—review and editing, A.D.S. and M.A.; visualization, A.D.S. and M.A.; supervision, A.D.S. and M.A.; project administration, M.A.; funding acquisition, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by WWF Greece through a private sponsorship from Procter & Gamble Corporation.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the Municipality of Chios for providing water trucks and personnel during the burns. We also thank Georgios Rodakis and Nikolaos Vagianos, volunteer firefighters of Chios Voluntary Action Team—OMIKRON, for their valuable support during soil samplings. We thank all IMFE personnel for their support in the field and laboratory work.
Conflicts of Interest
The authors declare no conflicts of interest. This research was funded by Procter and Gamble, and all potential conflicts have been disclosed and managed according to standard ethical practices.
Appendix A

Table A1.
Vascular plant species at the AGAFPBCONS3 site.
Table A1.
Vascular plant species at the AGAFPBCONS3 site.
| Plant Species | Family |
|---|---|
| Aetheorhiza bulbosa | Asparagaceae |
| Anthyllis hermanniae | Fabaceae |
| Asparagus acutifolius | Fabaceae |
| Brachypodium retusum | Lamiaceae |
| Briza media | Poaceae |
| Galium aparine | Rubiaceae |
| Lavandula stoechas | Lamiaceae |
| Leontodon tuberosus | Asteraceae |
| Oxalis pes-caprae | Oxalidaceae |

Table A2.
Vascular plant species at the AGAFPBINS3 site.
Table A2.
Vascular plant species at the AGAFPBINS3 site.
| Plant Species | Family |
|---|---|
| Aetheorhiza bulbosa | Asteraceae |
| Anthyllis hermanniae | Fabaceae |
| Asparagus acutifolius | Asparagaceae |
| Asterolinon linum-stellatum | Primulaceae |
| Brachypodium retusum | Poaceae |
| Briza media | Poaceae |
| Himantoglossum robertianum | Orchidaceae |
| Lavandula stoechas | Lamiaceae |
| Urospermum picroides | Asteraceae |

Table A3.
Vascular plant species at the AGBPBCONS3 site.
Table A3.
Vascular plant species at the AGBPBCONS3 site.
| Plant Species | Family |
|---|---|
| Aetheorhiza bulbosa | Asparagaceae |
| Anthyllis hermanniae | Fabaceae |
| Asparagus acutifolius | Fabaceae |
| Brachypodium retusum | Lamiaceae |
| Briza media | Poaceae |
| Galium aparine | Rubiaceae |
| Lavandula stoechas | Lamiaceae |
| Leontodon tuberosus | Asteraceae |
| Oxalis pes-caprae | Oxalidaceae |

Table A4.
Vascular plant species at the AGBPBINS3 site.
Table A4.
Vascular plant species at the AGBPBINS3 site.
| Plant Species | Family |
|---|---|
| Aetheorhiza bulbosa | Asteraceae |
| Anthyllis hermanniae | Fabaceae |
| Asparagus acutifolius | Asparagaceae |
| Asterolinon linum-stellatum | Primulaceae |
| Brachypodium retusum | Poaceae |
| Briza media | Poaceae |
| Himantoglossum robertianum | Orchidaceae |
| Lavandula stoechas | Lamiaceae |
| Urospermum picroides | Asteraceae |

Table A5.
Vascular plant species at the AIAFTPBCONS1 site.
Table A5.
Vascular plant species at the AIAFTPBCONS1 site.
| Plant Species | Family |
|---|---|
| Aetheorhiza bulbosa | Asteraceae |
| Brachypodium retusum | Poaceae |
| Galium murale | Rubiaceae |
| Geranium robertianum | Geraniaceae |
| Lamium amplexicaule | Lamiaceae |
| Muscari neglectum | Hyacinthaceae |
| Silene sedoides | Caryophyllaceae |
| Taraxacum officinale | Asteraceae |
| Theligonum cynocrambe | Rubiaceae |

Table A6.
Vascular plant species at the AIAFTPBINS1 site.
Table A6.
Vascular plant species at the AIAFTPBINS1 site.
| Plant Species | Family |
|---|---|
| Aetheorhiza bulbosa | Asteraceae |
| Brachypodium retusum | Poaceae |
| Drimia maritima | Hyacinthaceae |
| Geranium robertianum | Geraniaceae |
| Lamium amplexicaule | Lamiaceae |
| Muscari comosum | Hyacinthaceae |
| Muscari neglectum | Hyacinthaceae |
| Theligonum cynocrambe | Rubiaceae |

Table A7.
Vascular plant species at the AIBPBCONS1site.
Table A7.
Vascular plant species at the AIBPBCONS1site.
| Plant Species | Family |
|---|---|
| Aetheorhiza bulbosa | Asteraceae |
| Allium nigrum | Alliaceae |
| Anemone coronaria | Ranunculaceae |
| Brachypodium retusum | Poaceae |
| Cynodon dactylon | Poaceae |
| Galium murale | Rubiaceae |
| Muscari neglectum | Hyacinthaceae |
| Ranunculus paludosus | Ranunculaceae |
| Urospermum picroides | Asteraceae |

Table A8.
Vascular plant species at the AIBPBINS1 site.
Table A8.
Vascular plant species at the AIBPBINS1 site.
| Plant Species | Family |
|---|---|
| Aetheorhiza bulbosa | Asteraceae |
| Brachypodium retusum | Poaceae |
| Euphorbia peplus | Euphorbiaceae |
| Galium murale | Rubiaceae |
| Geranium robertianum | Geraniaceae |
| Lamium amplexicaule | Lamiaceae |
| Muscari neglectum | Hyacinthaceae |
| Sherardia arvensis | Rubiaceae |
| Theligonum cynocrambe | Rubiaceae |
| Urospermum picroides | Asteraceae |

Table A9.
Vascular plant species at the REAFPBCONS4 site.
Table A9.
Vascular plant species at the REAFPBCONS4 site.
| Plant Species | Family |
|---|---|
| Aetheorhiza bulbosa | Asteraceae |
| Aira elegans | Poaceae |
| Anagallis arvensis | Primulaceae |
| Asparagus acutifolius | Asparagaceae |
| Asterolinon linum-stellatum | Primulaceae |
| Brachypodium retusum | Poaceae |
| Erica manipuliflora | Ericaceae |
| Fumana arabica | Cistaceae |
| Galium divaricatum | Rubiaceae |
| Hypochaeris achyrophorus | Asteraceae |
| Leontodon tuberosus | Asteraceae |
| Pinus brutia | Pinaceae |
| Psoralea bituminosa | Cistaceae |
| Sarcopoterium spinosum | Rosaceae |
| Selaginella denticulata | Selaginellaceae |

Table A10.
Vascular plant species at the REAFPBINS4 site.
Table A10.
Vascular plant species at the REAFPBINS4 site.
| Plant Species | Family |
|---|---|
| Aetheorhiza bulbosa | Asteraceae |
| Aira elegans | Poaceae |
| Anagallis arvensis | Primulaceae |
| Asterolinon linum-stellatum | Primulaceae |
| Brachypodium distachyon | Poaceae |
| Brachypodium retusum | Poaceae |
| Briza media | Poaceae |
| Calicotome villosa | Fabaceae |
| Cistus creticus | Cistaceae |
| Fumana arabica | Cistaceae |
| Gagea graeca | Liliaceae |
| Hypochaeris achyrophorus | Asteraceae |
| Lavandula stoechas | Lamiaceae |
| Leontodon tuberosus | Asteraceae |
| Ornithopus compressus | Fabaceae |
| Phagnalon rupestre | Asteraceae |
| Pinus brutia | Pinaceae |
| Plantago bellardii | Plantaginaceae |
| Ranunculus paludosus | Ranunculaceae |
| Sarcopoterium spinosum | Rosaceae |
| Selaginella denticulata | Selaginellaceae |
| Serapias bergonii | Orchidaceae |
| Sherardia arvensis | Rubiaceae |
| Teucrium capitatum | Lamiaceae |
| Tordylium apulum | Apiaceae |
| Trifolium campestre | Fabaceae |
| Trifolium lappaceum | Fabaceae |
| Trifolium scabrum | Fabaceae |
| Trifolium stellatum | Fabaceae |
| Tuberaria guttata | Cistaceae |
| Vicia pubescens | Fabaceae |

Table A11.
Vascular plant species at the REAFTPBCONS2 site.
Table A11.
Vascular plant species at the REAFTPBCONS2 site.
| Plant Species | Family |
|---|---|
| Aetheorhiza bulbosa | Asteraceae |
| Aira elegans | Poaceae |
| Anagallis arvensis | Primulaceae |
| Asparagus acutifolius | Asparagaceae |
| Asterolinon linum-stellatum | Primulaceae |
| Brachypodium retusum | Poaceae |
| Briza media | Poaceae |
| Erica manipuliflora | Ericaceae |
| Fumana arabica | Cistaceae |
| Galium divaricatum | Rubiaceae |
| Hypochaeris achyrophorus | Asteraceae |
| Lavandula stoechas | Lamiaceae |
| Leontodon tuberosus | Asteraceae |
| Osyris alba L. | Sasntalaceae |
| Pinus brutia | Pinaceae |
| Psoralea bituminosa | Cistaceae |
| Pyrus spinosa | Rosaceae |
| Sarcopoterium spinosum | Rosaceae |
| Selaginella denticulata | Selaginellaceae |
| Serapias bergonii | Orchidaceae |

Table A12.
Vascular plant species at the REAFTPBINS2 site.
Table A12.
Vascular plant species at the REAFTPBINS2 site.
| Plant Species | Family |
|---|---|
| Aetheorhiza bulbosa | Asteraceae |
| Aira elegans | Poaceae |
| Anagallis arvensis | Primulaceae |
| Asterolinon linum-stellatum | Primulaceae |
| Brachypodium distachyon | Poaceae |
| Brachypodium retusum | Poaceae |
| Briza media | Poaceae |
| Bromus rubens | Poaceae |
| Calicotome villosa | Fabaceae |
| Cistus creticus | Cistaceae |
| Euphorbia peplus | Euphorbiaceae |
| Fumana arabica | Cistaceae |
| Gagea graeca | Liliaceae |
| Galium divaricatum | Rubiaceae |
| Hypochaeris achyrophorus | Asteraceae |
| Lavandula stoechas | Lamiaceae |
| Leontodon tuberosus | Asteraceae |
| Linaria pelisseriana | Veronicaceae |
| Ornithopus compressus | Fabaceae |
| Phagnalon rupestre | Asteraceae |
| Pinus brutia | Pinaceae |
| Plantago bellardii | Plantaginaceae |
| Plantago lagopus | Plantaginaceae |
| Ranunculus paludosus | Ranunculaceae |
| Sarcopoterium spinosum | Rosaceae |
| Selaginella denticulata | Selaginellaceae |
| Serapias bergonii | Orchidaceae |
| Sherardia arvensis | Rubiaceae |
| Teucrium capitatum | Lamiaceae |
| Tolpis barbata | Asteraceae |
| Tordylium apulum | Apiaceae |
| Torilis nodosa | Apiaceae |
| Trifolium campestre | Fabaceae |
| Trifolium lappaceum | Fabaceae |
| Trifolium scabrum | Fabaceae |
| Trifolium stellatum | Fabaceae |
| Tuberaria guttata | Cistaceae |
| Vicia pubescens | Fabaceae |

Table A13.
Vascular plant species at the REBPBCONS2 site.
Table A13.
Vascular plant species at the REBPBCONS2 site.
| Plant Species | Family |
|---|---|
| Aetheorhiza bulbosa | Asteraceae |
| Aira elegans | Poaceae |
| Anagallis arvensis | Primulaceae |
| Anthyllis hermanniae | Fabaceae |
| Asterolinon linum-stellatum | Primulaceae |
| Brachypodium retusum | Poaceae |
| Briza media | Poaceae |
| Bromus rubens | Poaceae |
| Cistus parviflorus | Cistaceae |
| Euphorbia peplus | Euphorbiaceae |
| Fumana arabica | Cistaceae |
| Galium murale | Rubiaceae |
| Helianthemum apenninum | Cistaceae |
| Hypochaeris achyrophorus | Asteraceae |
| Lavandula stoechas | Lamiaceae |
| Leontodon tuberosus | Asteraceae |
| Linum strictum | Linaceae |
| Osyris alba | Santalaceae |
| Phagnalon rupestre | Asteraceae |
| Pinus brutia | Pinaceae |
| Ranunculus paludosus | Ranunculaceae |
| Sarcopoterium spinosum | Rosaceae |
| Selaginella denticulata | Selaginellaceae |
| Serapias bergonii | Orchidaceae |
| Sherardia arvensis | Rubiaceae |
| Trifolium campestre | Fabaceae |

Table A14.
Vascular plant species at the REBPBCONS4 site.
Table A14.
Vascular plant species at the REBPBCONS4 site.
| Plant Species | Family |
|---|---|
| Aetheorhiza bulbosa | Asteraceae |
| Aira elegans | Poaceae |
| Anagallis arvensis | Primulaceae |
| Anthyllis hermanniae | Fabaceae |
| Asterolinon linum-stellatum | Primulaceae |
| Brachypodium retusum | Poaceae |
| Briza media | Poaceae |
| Bromus rubens | Poaceae |
| Cistus parviflorus | Cistaceae |
| Euphorbia peplus | Euphorbiaceae |
| Fumana arabica | Cistaceae |
| Galium murale | Rubiaceae |
| Hypochaeris achyrophorus | Asteraceae |
| Lavandula stoechas | Lamiaceae |
| Leontodon tuberosus | Asteraceae |
| Osyris alba | Santalaceae |
| Phagnalon rupestre | Asteraceae |
| Sarcopoterium spinosum | Rosaceae |
| Selaginella denticulata | Selaginellaceae |
| Serapias bergonii | Orchidaceae |
| Sherardia arvensis | Rubiaceae |
| Trifolium campestre | Fabaceae |

Table A15.
Vascular plant species at the REBPBINS2 site.
Table A15.
Vascular plant species at the REBPBINS2 site.
| Plant Species | Family |
|---|---|
| Aetheorhiza bulbosa | Asteraceae |
| Anagallis arvensis | Primulaceae |
| Anthyllis hermanniae | Fabaceae |
| Anthyllis vulneraria | Fabaceae |
| Asterolinon linum-stellatum | Primulaceae |
| Brachypodium distachyon | Poaceae |
| Brachypodium retusum | Poaceae |
| Briza media | Poaceae |
| Calicotome villosa | Fabaceae |
| Cistus parviflorus | Cistaceae |
| Dorycnium hirsutum | Fabaceae |
| Euphorbia peplus | Euphorbiaceae |
| Fumana arabica | Cistaceae |
| Gagea graeca | Liliaceae |
| Lavandula stoechas | Lamiaceae |
| Leontodon tuberosus | Asteraceae |
| Ophrys lutea | Orchidaceae |
| Phagnalon rupestre | Asteraceae |
| Pinus brutia | Pinaceae |
| Plantago lagopus | Plantaginaceae |
| Ranunculus paludosus | Ranunculaceae |
| Sarcopoterium spinosum | Rosaceae |
| Selaginella denticulata | Selaginellaceae |
| Serapias bergonii | Orchidaceae |
| Sherardia arvensis | Rubiaceae |
| Trifolium angustifolium | Fabaceae |
| Trifolium campestre | Fabaceae |

Table A16.
Vascular plant species at the REBPBINS4 site.
Table A16.
Vascular plant species at the REBPBINS4 site.
| Plant Species | Family |
|---|---|
| Aetheorhiza bulbosa | Asteraceae |
| Anthyllis vulneraria | Fabaceae |
| Asterolinon linum-stellatum | Primulaceae |
| Brachypodium retusum | Poaceae |
| Briza media | Poaceae |
| Calicotome villosa | Fabaceae |
| Cistus parviflorus | Cistaceae |
| Dorycnium hirsutum | Fabaceae |
| Fumana arabica | Cistaceae |
| Gagea graeca | Liliaceae |
| Lavandula stoechas | Lamiaceae |
| Leontodon tuberosus | Asteraceae |
| Phagnalon rupestre | Asteraceae |
| Pinus brutia | Pinaceae |
| Sarcopoterium spinosum | Rosaceae |
| Selaginella denticulata | Selaginellaceae |
| Serapias bergonii | Orchidaceae |
| Trifolium angustifolium | Fabaceae |
| Trifolium campestre | Fabaceae |
Appendix B

Table A17.
Soil properties of the Resta area after (8 April 2023) and before (13 April 2022) the use of prescribed burning (PB). Organic carbon (Org. C) and clay are expressed in percentages (%), total and organic nitrogen are expressed in g/kg, ammonium (Am. N) and nitrate nitrogen (Nit. N) in mg/kg. Conductivity (Cond.) is expressed in μS/cm and exchangeable Ca, Mg, K and Na in meq/100 g of soil. Variability was calculated in percentages (%) of the standard deviation of the mean. Different letters in the same column mean a statistical difference for at least the 0.05 probability level. Absence of letters means non-statistical significance.
Table A17.
Soil properties of the Resta area after (8 April 2023) and before (13 April 2022) the use of prescribed burning (PB). Organic carbon (Org. C) and clay are expressed in percentages (%), total and organic nitrogen are expressed in g/kg, ammonium (Am. N) and nitrate nitrogen (Nit. N) in mg/kg. Conductivity (Cond.) is expressed in μS/cm and exchangeable Ca, Mg, K and Na in meq/100 g of soil. Variability was calculated in percentages (%) of the standard deviation of the mean. Different letters in the same column mean a statistical difference for at least the 0.05 probability level. Absence of letters means non-statistical significance.
| pH | Org. C | Tot. N | Org. Ν | Am. Ν | Νit. Ν | C/N | Cond. | Clay | Ca | Mg | K | Na | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After | |||||||||||||
| Average | 7.5 | 1.27 a | 2.06 | 2.05 | 5.92 | 10.5 | 6.2 a | 443 | 14.4 | 9.42 | 1.44 | 0.38 | 0.10 |
| Variability | (6) | (24) | (24) | (12) | (42) | (60) | (24) | (25) | (8) | (35) | (14) | (12) | (7) |
| Before | |||||||||||||
| Average | 8.0 | 3.31 b | 1.75 | 1.72 | 6.99 | 21.7 | 20 b | 529 | 12.1 | 11.4 | 1.31 | (0.26) | 0.10 |
| Variability | (3) | (20) | (19) | (10) | (10) | (47) | (35) | (50) | (21) | (57) | (13) | (38) | (18) |

Table A18.
Soil properties of the Resta area (with Phrygana) after (8 April 2023) and before (5 December 2022) the use of PB. Organic carbon (Org. C) and clay are expressed in percentages (%), total and organic nitrogen are expressed in g/kg, ammonium (Am. N) and nitrate nitrogen (Nit. N) in mg/kg. Conductivity (Cond.) is expressed in μS/cm and exchangeable Ca, Mg, K and Na in meq/100 g of soil. Variability was calculated in percentages (%) of the standard deviation of the mean. Different letters in the same column mean a statistical difference for at least the 0.05 probability level. Absence of letters means non-statistical significance.
Table A18.
Soil properties of the Resta area (with Phrygana) after (8 April 2023) and before (5 December 2022) the use of PB. Organic carbon (Org. C) and clay are expressed in percentages (%), total and organic nitrogen are expressed in g/kg, ammonium (Am. N) and nitrate nitrogen (Nit. N) in mg/kg. Conductivity (Cond.) is expressed in μS/cm and exchangeable Ca, Mg, K and Na in meq/100 g of soil. Variability was calculated in percentages (%) of the standard deviation of the mean. Different letters in the same column mean a statistical difference for at least the 0.05 probability level. Absence of letters means non-statistical significance.
| pH | Org.C | Tot. N | Org. Ν | Am. Ν | Νit. Ν | C/N | Cond. | Clay | Ca | Mg | K | Na | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After | |||||||||||||
| Average | 7.32 a | 1.95 | 1.60 | 1.58 | 4.42 | 9.07 | 11 | 452 | 15.1 | 8.40 a | 1.72 | 0.32 | 0.10 |
| Variability | (5) | (50) | (20) | (20) | (40) | (52) | (30) | (37) | (0) | (29) | (31) | (12) | (28) |
| Before | |||||||||||||
| Average | 6.45 b | 1.19 | 8.87 | 8.64 | 14.2 | 8.87 | 22 | 368 | 12.7 | 4.51 b | 1.67 | 0.39 | 0.18 |
| Variability | (4) | (32) | (58) | (59) | (65) | (28) | (103) | (11) | (15) | (17) | (11) | (11) | (46) |

Table A19.
Soil properties of the Resta area (with Trachea) after (8 April 2023) and before (7 December 2022) the use of PB. Organic carbon (Org. C) and clay are expressed in percentages (%), total and organic nitrogen are expressed in g/kg, ammonium (Am. N) and nitrate nitrogen (Nit. N) in mg/kg. Conductivity (Cond.) is expressed in μS/cm and exchangeable Ca, Mg, K and Na in meq/100 g of soil. Variability was calculated in percentages (%) of the standard deviation of the mean. Absence of letters means non-statistical significance.
Table A19.
Soil properties of the Resta area (with Trachea) after (8 April 2023) and before (7 December 2022) the use of PB. Organic carbon (Org. C) and clay are expressed in percentages (%), total and organic nitrogen are expressed in g/kg, ammonium (Am. N) and nitrate nitrogen (Nit. N) in mg/kg. Conductivity (Cond.) is expressed in μS/cm and exchangeable Ca, Mg, K and Na in meq/100 g of soil. Variability was calculated in percentages (%) of the standard deviation of the mean. Absence of letters means non-statistical significance.
| pH | Org.C | Tot. N | Org. Ν | Am. Ν | Νit. Ν | C/N | Cond. | Clay | Ca | Mg | K | Na | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After | |||||||||||||
| Average | 7.16 | 2.87 | 2.16 | 2.14 | 1.97 | 9.03 | 14 | 422 | 14.4 | 7.62 | 1.75 | 0.36 | 0.14 |
| Variability | (4) | (4) | (28) | (28) | (30) | (59) | (27) | (14) | (29) | (32) | (30) | (13) | 26 |
| Before | |||||||||||||
| Average | 7.19 | 2.80 | 1.97 | 1.96 | 2.80 | 11.0 | (12) | 456 | 13.1 | 6.50 | 1.75 | 0.36 | 0.17 |
| Variability | (5) | (13) | (30) | (35) | (10) | (21) | (32) | (11) | (24) | (24) | (14) | (22) | (18) |

Table A20.
Soil properties of the Agios Stefanos area after (8 April 2024) and before (6 December 2023) the use of PB. Organic carbon (Org. C) and clay are expressed in percentages (%), total and organic nitrogen are expressed in g/kg, ammonium (Am. N) and nitrate nitrogen (Nit. N) in mg/kg. Conductivity (Cond.) is expressed in μS/cm and exchangeable Ca, Mg, K and Na in meq/100 g of soil. Variability was calculated in percentages (%) of the standard deviation of the mean. Different letters in the same column mean a statistical difference for at least the 0.05 probability level. Absence of letters means non-statistical significance.
Table A20.
Soil properties of the Agios Stefanos area after (8 April 2024) and before (6 December 2023) the use of PB. Organic carbon (Org. C) and clay are expressed in percentages (%), total and organic nitrogen are expressed in g/kg, ammonium (Am. N) and nitrate nitrogen (Nit. N) in mg/kg. Conductivity (Cond.) is expressed in μS/cm and exchangeable Ca, Mg, K and Na in meq/100 g of soil. Variability was calculated in percentages (%) of the standard deviation of the mean. Different letters in the same column mean a statistical difference for at least the 0.05 probability level. Absence of letters means non-statistical significance.
| pH | Org.C | Tot. N | Org. Ν | Am. Ν | Νit. Ν | C/N | Cond. | Clay | Ca | Mg | K | Na | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After | |||||||||||||
| Average | 7.54 | 1.20 a | 1.23 a | 1.22 a | 3.76 a | 11.9 a | 11 a | 625 | 13.1 | 13.5 | 1.14 | 0.27 a | 0.21 a |
| Variability | (6) | (58) | (18) | (18) | (62) | (10) | (25) | (11) | (0) | (33) | (60) | (19) | (86) |
| Before | |||||||||||||
| Average | 7.32 | 6.0 b | 2.78 b | 2.73 b | 15.1 b | 27.9 b | 22 b | 785 | 10.1 | 17.0 | 2.38 | 0.44 b | 0.35 b |
| Variability | (3) | (43) | (31) | (31) | (56) | (16) | (56) | (18) | (11) | (9) | (33) | (22) | (4) |

Table A21.
Soil properties of the Aipou area after (8 April 2023) and before (13 December 2022) the use of PB. Organic carbon (Org. C) and clay are expressed in percentages (%), total and organic nitrogen are expressed in g/kg, ammonium (Am. N) and nitrate nitrogen (Nit. N) in mg/kg. Conductivity (Cond.) is expressed in μS/cm and exchangeable Ca, Mg, K and Na in meq/100 g of soil. Variability was calculated in percentages (%) of the standard deviation of the mean. Different letters in the same column mean statistical difference for at least the 0.05 probability level. Absence of letters means non-statistical significance.
Table A21.
Soil properties of the Aipou area after (8 April 2023) and before (13 December 2022) the use of PB. Organic carbon (Org. C) and clay are expressed in percentages (%), total and organic nitrogen are expressed in g/kg, ammonium (Am. N) and nitrate nitrogen (Nit. N) in mg/kg. Conductivity (Cond.) is expressed in μS/cm and exchangeable Ca, Mg, K and Na in meq/100 g of soil. Variability was calculated in percentages (%) of the standard deviation of the mean. Different letters in the same column mean statistical difference for at least the 0.05 probability level. Absence of letters means non-statistical significance.
| pH | Org.C | Tot. N | Org. Ν | Am. Ν | Νit. Ν | C/N | Cond. | Clay | Ca | Mg | K | Na | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After | |||||||||||||
| Average | 7.66 | 7.00 | 3.87 a | 3.84 a | 5.72 | 22.2 | 18 | 1076 | 31.1 | 34.0 a | 4.30 a | 1.25 | 0.29 |
| Variability | (4) | (43) | (28) | (27) | (82) | (67) | (22) | (27) | (34) | (20) | (4) | (11) | (27) |
| Before | |||||||||||||
| Average | 7.53 | 4.65 | 1.76 b | 1.74 b | 10.2 | 10.3 | 26 | 705 | 43.1 | 23.0 b | 3.04 b | 1.83 | 0.34 |
| Variability | (4) | (41) | (22) | (22) | (20) | (34) | (29) | (28) | (20) | (13) | (6) | (44) | (12) |
References
- Keeley, J.E.; Pausas, J.G. Distinguishing disturbance from perturbations in fire-prone ecosystems. Int. J. Wildland Fire 2019, 28, 282–287. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Evolutionary ecology of resprouting and seeding in fire-prone ecosystems. New Phytol. 2014, 204, 55–65. [Google Scholar] [CrossRef]
- Moreira, F.; Ascoli, D.; Safford, H.; Adams, M.A.; Moreno, J.M. Wildfire management in Mediterranean-type regions: Paradigm change needed. Environ. Res. Lett. 2020, 15, 011001. [Google Scholar] [CrossRef]
- Stephens, S.L.; McIver, J.D.; Boerner, R.E.; Fettig, C.J.; Fontaine, J.B.; Hartsough, B.R.; Schwilk, D.W. The effects of forest fuel-reduction treatments in the United States. BioScience 2012, 62, 549–560. [Google Scholar] [CrossRef]
- Boer, M.M.; de Dios, V.R.; Bradstock, R.A. Unprecedented burn area of Australian mega forest fires. Nat. Clim. Change 2020, 10, 171–172. [Google Scholar] [CrossRef]
- Varner, J.M.; Hiers, J.K.; Wheeler, S.B.; McGuire, J.; Quinn-Davidson, L.; Palmer, W.E.; Fowler, L. Increasing pace and scale of prescribed fire via catastrophe funds for liability relief. Fire 2021, 4, 77. [Google Scholar] [CrossRef]
- Jain, T.B.; Battaglia, M.A.; Han, H.S.; Graham, R.T.; Keyes, C.R.; Fried, J.S.; Sandquist, J.E. A Comprehensive Guide to Fuel Management Practices for Dry Mixed Conifer Forests in the North-Western United States; USDA Forest Service: Washington, DC, USA, 2012. [Google Scholar]
- Moghaddas, J.J.; Collins, B.M.; Menning, K.; Moghaddas, E.E.; Stephens, S.L. Fuel treatment effects on modeled landscape-level fire behavior in the northern Sierra Nevada. Can. J. For. Res. 2010, 40, 1751–1765. [Google Scholar] [CrossRef]
- Rego, F.C.; Silva, J.S.; Fernandes, P.; Rigolot, E. Solving the fire paradox–Regulating the wildfire problem by the wise use of fire. In Towards Integrated Fire Management: Outcomes of the European Project Fire Paradox; European Forest Institute: Joensuu, Finland, 2010; p. 219. [Google Scholar]
- Fernandes, P.M.; Davies, G.M.; Ascoli, D.; Fernandez, C.; Moreira, F.; Rigolot, E. Prescribed burning in southern Europe: Developing fire management in a dynamic landscape. Front. Ecol. Environ. 2013, 11, e4–e14. [Google Scholar] [CrossRef]
- Castellnou, M.; Kraus, D.; Miralles, M. Prescribed burning and suppression fire techniques: From fuel to landscape management. In Best Practices of Fire Use—Prescribed Burning and Suppression Fire Programmes in Selected Case-Study Regions in Europe; Montiel, C., Kraus, D., Eds.; European Forest Institute Research Report 24; European Forest Institute: Joensuu, Finland, 2010; pp. 3–16. [Google Scholar]
- Bonner, S.R.; Hoffman, C.M.; Kane, J.M.; Varner, J.M.; Hiers, J.K.; O’Brien, J.J.; Rickard, H.D.; Tinkham, W.T.; Linn, R.R.; Skowronski, N.; et al. Invigorating prescribed fire science through improved reporting practices. Front. For. Glob. Change 2021, 4, 750699. [Google Scholar] [CrossRef]
- Eales, J.; Haddaway, N.R.; Bernes, C.; Cooke, S.J.; Jonsson, B.G.; Kouki, J.; Taylor, J.J. What is the effect of prescribed burning in temperate and boreal forest on biodiversity, beyond pyrophilous and saproxylic species? A systematic review. Environ. Evid. 2018, 7, 19. [Google Scholar] [CrossRef]
- Byram, G.M. Combustion of forest fuels; Forest fire behavior. In Forest Fire: Control and Use; Davis, K.P., Ed.; McGraw-Hill: New York, NY, USA, 1959; pp. 61–123. [Google Scholar]
- Brown, A.A.; Davis, K.P. Forest Fire: Control and Use, 2nd ed.; McGraw-Hill: New York, NY, USA, 1973. [Google Scholar]
- Alexander, M.E. Calculating and interpreting forest fire intensities. Can. J. Bot. 1982, 60, 349–357. [Google Scholar] [CrossRef]
- Davies, G.M.; Legg, C.J.; Smith, A.A.; MacDonald, A. Development and participatory evaluation of fireline intensity and flame property models for managed burns on Calluna-dominated heathlands. Fire Ecol. 2019, 15, 30. [Google Scholar] [CrossRef]
- Zylstra, P. Linking fire behaviour and its ecological effects to plant traits, using FRaME in R. Methods Ecol. Evol. 2021, 12, 1365–1378. [Google Scholar] [CrossRef]
- Ramberg, E.; Berglund, H.; Penttilä, R.; Strengbom, J.; Jönsson, M. Prescribed fire is an effective restoration measure for increasing boreal fungal diversity. Ecol. Appl. 2023, 33, e2892. [Google Scholar] [CrossRef]
- West, A.G.; Hultine, K.R.; Sperry, J.S.; Bush, S.E.; Ehleringer, J.R. Transpiration and hydraulic strategies in plants following a wildfire in a Mediterranean woodland. Plant Cell Environ. 2008, 31, 901–913. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Wildfires as an ecosystem service. Front. Ecol. Environ. 2019, 17, 289–295. [Google Scholar] [CrossRef]
- Santana, V.M.; Alday, J.G.; Lee, H.; Allen, K.A. Fire intensity effects on soil properties and plant regeneration: A meta-analysis. J. Ecol. 2020, 108, 1632–1643. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Keeley, J.E. Fire intensity, fire severity and burn severity: A brief review and suggested usage. Int. J. Wildland Fire 2009, 18, 116–126. [Google Scholar] [CrossRef]
- DeBano, L.F. The role of fire and soil heating on water repellency in wildland environments: A review. J. Hydrol. 2000, 231–232, 195–206. [Google Scholar] [CrossRef]
- Shakesby, R.A.; Doerr, S.H. Wildfire as a hydrological and geomorphological agent. Earth-Sci. Rev. 2006, 74, 269–307. [Google Scholar] [CrossRef]
- Vallejo, V.R.; Aronson, J.; Pausas, J.G.; Cortina, J. Restoration of Mediterranean woodlands. In Restoration Ecology: The New Frontier; van Andel, J., Aronson, J., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 193–207. [Google Scholar]
- Papanastasis, V.P. Report on the seminar results, held in Thasos, for the improvement of Pinus brutia timber stands, by using the prescribed burning method. For. Chron. 1977, 19, 98–102. [Google Scholar]
- Athanasiou, M.; Bouchounas, T.; Korakaki, E.; Tziritis, E.; Xanthopoulos, G.; Sitara, S. Pilot project aims to change policy. Wildfire 2022, 4, 26–28. [Google Scholar]
- Nastis, A. Control of Forest Fires in Mediterranean Region by Use of Prescribed Burning and Grazing: Effect on Forest Ecosystem; CEE EV4V 0095-GR (TT); Progress Report; Commission of the European Communities (CEC): Brussels, Belgium, 1989. [Google Scholar]
- Athanasiou, M.; Bouchounas, T.; Korakaki, E.; Tziritis, E.; Xanthopoulos, G.; Sitara, S. Introducing the use of fire for wildfire prevention in Greece: Pilot application of prescribed burning in Chios island. In Proceedings of the 9th International Conference on Forest Fire Research and 17th International Wildland Fire Safety Summit, Coimbra, Portugal, 11–18 November 2022. [Google Scholar]
- Kitsara, G.; van der Schriek, T.; Varotsos, K.V.; Giannakopoulos, C. Future Changes in Climate Indices Relevant to Agriculture in the Aegean Islands (Greece). Eur. Mediterr. J. Environ. Integr. 2021, 6, 34. [Google Scholar] [CrossRef]
- Tzanoudakis, D. The genus Fritillaria in Greece. Ann. Mus. Goulandris 1999, 10, 145–180. [Google Scholar]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist; Englera 31; Hellenic Botanical Society: Athens, Greece, 2013. [Google Scholar]
- Krigas, N.; Lykas, C.; Ipsilantis, I.; Matsi, T.; Weststrand, S.; Havström, M.; Tsoktouridis, G. Greek Tulips: Worldwide Electronic Trade over the Internet, Global Ex Situ Conservation and Current Sustainable Exploitation Challenges. Plants 2021, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- UNESCO Chios Mastiha cultivation. In UNESCO Intangible Cultural Heritage Lists; UNESCO: Paris, France, 2014.
- European Commission. Mastiha Chiou—Protected Designation of Origin; PDO/PGI Register, European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Giannatos, G.; Marinos, Y.; Maragou, P.; Catsadorakis, G. The status of the otter (Lutra lutra) in Greece. Belg. J. Zool. 2005, 135, 183–188. [Google Scholar]
- Karamanlidis, A.A.; Dendrinos, P. Monachus monachus. In The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2015. [Google Scholar]
- Important Bird Areas Factsheet: Venetiko Islet (Chios); BirdLife Data Zone; BirdLife International: Cambridge, UK, 2021.
- Natura 2000 Standard Data Forms—Sites in Greece; European Environment Agency (EEA): Copenhagen, Denmark, 2023.
- WWF Greece. Annual Report on the Environment and Climate Change in Greece; WWF Greece: Athens, Greece, 2019. (In Greek) [Google Scholar]
- Dombois, D.M.; Ellenberg, H. Aims and Methods of Vegetation Ecology; John Wiley & Sons: New York, NY, USA; Sydney, Australia; Toronto, ON, Canada, 1974; pp. 1–547. [Google Scholar]
- Tutin, T.G.; Burges, N.A.; Chater, A.O.; Edmondson, J.R.; Heywood, V.H.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1968. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993; Volume 1. [Google Scholar]
- Strid, A.; Tan, K. Flora Hellenica; Koeltz Scientific Books: Koenigstein, Germany, 2002. [Google Scholar]
- Flora of Greece Web. Vascular Plants of Greece: An Annotated Checklist. 2022. Available online: https://portal.cybertaxonomy.org/flora-greece/content (accessed on 26 June 2023).
- Bouyoucos, G.J. A recalibration of the hydrometer method for making mechanical analysis of soils. Agron. J. 1951, 43, 434–437. [Google Scholar] [CrossRef]
- Booker Agriculture International (B.A.I.). Tropical Soil Manual; Booker Agriculture International Ltd.: London, UK, 1984. [Google Scholar]
- Jones, J.B., Jr. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: London, UK, 2001. [Google Scholar]
- Pickford, S.G.; Sandberg, D.A. Using Motion Pictures for Data Collection on Prescribed Burning Experiments; USDA Forest Service Research Note PNW-259; Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1975; p. 7. [Google Scholar]
- Rothermel, R.C. How to Predict the Spread and Intensity of Forest and Range Fires; General Technical Report INT-143; USDA Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1983; p. 161. [Google Scholar]
- Rothermel, R.C.; Rinehart, G.C. Field Procedures for Verification and Adjustment of Fire Behavior Predictions; General Technical Report INT-142; USDA Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1983. [Google Scholar]
- Clements, H.; Ward, D.; Adkins, C. Measuring fire behaviour with photography. Photogramm. Eng. Remote Sens. 1983, 49, 213–217. [Google Scholar]
- Norum, R.A.; Miller, M. Measuring Fuel Moisture Content in Alaska: Standard Methods and Procedures; General Technical Report PNW-171; USDA Forest Service, Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1984; 34p. [Google Scholar]
- McMahon, C.K.; Adkins, C.W.; Rodgers, S.L. A video image analysis system for measuring fire behavior. USDA For. Serv. 1986, 47, 10–15. [Google Scholar]
- Alexander, M.E.; Thomas, D.A. Wildland fire behavior case studies and analyses: Other examples, methods, reporting standards, and some practical advice. Fire Manag. Today 2003, 63, 4–12. [Google Scholar]
- Alexander, M.E.; Cruz, M.G. Limitations on the accuracy in model predictions of wildland fire behavior: A state-of-the-knowledge overview. For. Chron. 2013, 89, 372–383. [Google Scholar] [CrossRef]
- Lawson, B.; Armitage, O. Weather Guide for the Canadian Forest Fire Danger Rating System: A User Guide to National Standards and Practices; Information Report BC-X-177; Fisheries and Environment Canada, Canadian Forest Service, Pacific Forest Research Centre: Victoria, BC, Canada, 2008; 40p. [Google Scholar]
- Gould, J.; McCaw, L.; Cruz, M.; Anderson, W. How good are fire behaviour models? Validation of eucalypt forest fire spread. In Proceedings of the 5th International Wildland Fire Conference (WILDFIRE 2011), Sun City, South Africa, 9–13 May 2011; Available online: https://www.researchgate.net/profile/Miguel-Cruz-19/publication/267405282_How_good_are_fire_behaviour_models_Validation_of_eucalypt_forest_fire_spread_model/links/5878189008ae6eb871d190fb/How-good-are-fire-behaviour-models-Validation-of-eucalypt-forest-fire-spread-model.pdf?utm_source=chatgpt.com (accessed on 19 September 2025).
- Fernandes, P.M.; Botelho, H.S.; Rego, F.C.; Loureiro, C. Empirical modelling of surface fire behaviour in maritime pine stands. Int. J. Wildland Fire 2009, 18, 698–710. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Catchpole, W.R.; Rego, F.C. Shrubland fire behaviour modelling with microplot data. Can. J. For. Res. 2000, 30, 889–897. [Google Scholar] [CrossRef][Green Version]
- Clark, R.G. Threshold Requirements for Fire Spread in Grassland Fuels. Ph.D. Dissertation, Texas Tech University, Lubbock, TX, USA, 1983. [Google Scholar][Green Version]
- Nelson, R.M., Jr.; Adkins, C.W. Flame characteristics of wind-driven surface fires. Can. J. For. Res. 1986, 16, 1293–1300. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups, 4th ed.; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 2003. [Google Scholar] [CrossRef]
- Ibanez, J.J.; De-Alba, S.; Bermudez, F.F.; Garcia-Alvarez, A. Pedodiversity: Concepts and measures. Catena 1995, 24, 215–232. [Google Scholar] [CrossRef]
- Nur, N. A Statistical Guide to Data Analysis of Avian Monitoring Programs; BTP-R6001; U.S. Department of the Interior, Fish and Wildlife Service: Washington, DC, USA, 1999. [Google Scholar]
- Trabaud, L. Post-fire plant community dynamics in the Mediterranean basin. In The Role of Fire in Mediterranean-Type Ecosystems; Moreno, J.M., Oechel, W.C., Eds.; Springer: New York, NY, USA, 1994; pp. 1–15. [Google Scholar]
- Keeley, J.E.; Bond, W.J.; Bradstock, R.A.; Pausas, J.G.; Rundel, P.W. Fire in Mediterranean Ecosystems: Ecology, Evolution and Management; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Blondel, J.; Aronson, J. Biodiversity and ecosystem function in the Mediterranean basin. In Mediterranean-Type Ecosystems; The Function of Biodiversity; Davis, G.W., Richardson, D.M., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 1995; pp. 43–119. [Google Scholar]
- Maxted, N.; Bennett, S.J. Legume diversity in the Mediterranean region. In Plant Genetic Resources of Legumes in the Mediterranean; Maxted, N., Bennett, S.J., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2001; pp. 51–78. [Google Scholar]
- Arnan, X.; Rodrigo, A.; Retana, J. Post-fire regeneration of Mediterranean plant communities at a regional scale is dependent on vegetation type and dryness. J. Veg. Sci. 2007, 18, 111–122. [Google Scholar] [CrossRef]
- Carrari, E.; Biagini, P.; Selvi, F. Early vegetation recovery of a burned Mediterranean forest in relation to post-fire management strategies. Forestry 2022, 95, 548–561. [Google Scholar]
- Capitanio, R.; Carcaillet, C. Post-fire Mediterranean vegetation dynamics and diversity: A discussion of succession models. For. Ecol. Manag. 2008, 255, 431–439. [Google Scholar] [CrossRef]
- Moreira, B.; Pausas, J. Tanned or burned: The role of fire in shaping physical seed dor-mancy. PLoS ONE 2012, 7, e51523. [Google Scholar] [CrossRef]
- Moreira, B.; Pausas, J. Shedding light through the smoke on the germination of Mediterranean Basin flora. S. Afr. J. Bot. 2018, 115, 244–250. [Google Scholar] [CrossRef]
- Zomer, M.; Moreira, B.; Pausas, J. Fire and summer temperatures interact to shape seed dormancy thresholds. Ann. Bot. 2022, 129, 809–816. [Google Scholar] [CrossRef]
- Papavassiliou, S. The Importance of Legumes in the Post-Fire Regeneration of Mediterranean Forest Ecosystems. Ph.D. Thesis, National and Kapodistrian University of Athens, Athens, Greece, 2001; p. 235. (In Greek). [Google Scholar]
- Arianoutsou, M. Legume Post-Fire Flora and Its Contribution in the Regeneration of Mediterranean Ecosystems After Fire; Project 91 ED 944 Final Report; General Secretariat of Research and Technology: Athens, Greece, 1995. [Google Scholar]
- Rundel, P.W.; Arroyo, M.T.; Cowling, R.M.; Keeley, J.E.; Lamont, B.B.; Pausas, J.G.; Vargas, P. Fire and plant diversification in Mediterranean-climate regions. Front. Plant Sci. 2018, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Lamont, B.; Pausas, J. Fire as a key driver of Earth’s biodiversity. Biol. Rev. 2019, 94, 1983–2010. [Google Scholar] [CrossRef] [PubMed]
- Tavşanoğlu, Ç.; Çatav, Ş. Fire-related cues (heat shock and smoke) and seed germination in a Cistus creticus population in southwestern Turkey. Ekoloji 2011, 20, 99–104. [Google Scholar]
- Seligman, N.; Henkin, Z. Regeneration of a dominant Mediterranean dwarf-shrub after fire. J. Veg. Sci. 2000, 11, 893–902. [Google Scholar] [CrossRef]
- Golan, S.; Waitz, Y.; Ziffer-Berger, J.; Barzilai, M.; Hanin, N.; Henkin, Z.; Barazani, O. Germination behavior of Sarcopoterium spinosum in response to smoke chemicals. Isr. J. Plant Sci. 2019, 66, 103–112. [Google Scholar] [CrossRef]
- Tilki, F. Seed germination of Cistus creticus and C. laurifolius as influenced by dry heat, soaking in water and GA3. J. Environ. Biol. 2008, 29, 237–240. [Google Scholar]
- Ghaderi-Far, F.; Tavili, A.; Sadeghifar, A. Effects of light and temperature on seed ger-mination of eight Cistus species and associated aromatics including Lavandula stoechas. Seed Sci. Res. 2021, 31, 246–257. [Google Scholar]
- Caturla, R.; Raventós, J.; Vallejo, V. Early post-fire regeneration dynamics of Brachy-podium retusum Pers. Beauv. in old fields of the Valencia region (eastern Spain). Acta Oecologica 2000, 21, 1–12. [Google Scholar]
- Vidaller, C.; Dutoit, T.; Ramone, H.; Bischoff, A. Fire increases the reproduction of Brachy-podium retusum. Appl. Veg. Sci. 2019, 22, 127–137. [Google Scholar] [CrossRef]
- Arianoutsou, M.; Thanos, C. Legumes in the fire-prone Mediterranean region: An example from Greece. Int. J. Wildland Fire 1996, 6, 77–82. [Google Scholar] [CrossRef]
- Moreira, B.; Pausas, J.; Tavşanoğlu, Ç. Fire-released seed dormancy—A global synthesis. Biol. Rev. 2022, 97, 139–165. [Google Scholar]
- Galié, M.; Biscotti, N.; Polito, M. Post-fire regeneration of Calicotome villosa and the vegetation it dominates: An ecological and syntaxonomic study in the Mediterranean basin. Plant Sociol. 2018, 55, 95–121. [Google Scholar]
- Thanos, C.A.; Doussi, M.A. Post-fire regeneration of Pinus brutia forests. In Ecology, Biogeography and Management of Pinus halepensis and P. brutia Forest Ecosystems in the Mediterranean Basin; Backhuys: Kerkwerve, The Netherlands, 2000; pp. 291–301. [Google Scholar]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2001. [Google Scholar]
- Pierce, S.; Negishi, K.; Cerabolini, B.E.; Kattge, J.; Díaz, S.; Kleyer, M.; Shipley, B.; Wright, S.J.; Soudzilovskaia, N.A.; Onipchenko, V.G.; et al. A global method for calculating plant CSR strategies from traits (StrateFy). Funct. Ecol. 2017, 31, 444–457. [Google Scholar] [CrossRef]
- Kazanis, D.; Arianoutsou, M. Long term post-fire dynamics of Pinus halepensis forests of Central Greece: Plant community patterns. In Proceedings of the IV International Forest Fire Research Conference, Coimbra, Portugal, 18–23 November 2002; electronic edition. Millpress: Roterdam, The Netherlands, 2002. [Google Scholar]
- Bonanomi, G.; Idbella, M.; Abd-ElGawad, A.; Motti, R.; Ippolito, F.; Santorufo, L. Impact of prescribed burning, mowing and abandonment on a Mediterranean grassland: A 5-year multi-kingdom comparison. Sci. Total Environ. 2022, 834, 155442. [Google Scholar] [CrossRef]
- Kazanis, D.; Arianoutsou, M. Vegetation composition in a post-fire successional gradient of Pinus halepensis forests in Attica, Greece. Int. J. Wildland Fire 1996, 6, 83–91. [Google Scholar] [CrossRef]
- Kazanis, D.; Arianoutsou, M. Long-term post-fire vegetation dynamics in Pinus halepensis forests of Central Greece: A functional group approach. Plant Ecol. 2004, 171, 101–121. [Google Scholar] [CrossRef]
- Verroios, G.; Georgiadis, T. Post-fire vegetation succession: The case of Aleppo pine (Pinus halepensis Miller) forests of Northern Achaia (Greece). Fresenius Environ. Bull. 2002, 11, 186–193. [Google Scholar]
- Muqaddas, B.; Chen, C.; Lewis, T.; Wild, C. Temporal dynamics of carbon and nitrogen in the surface soil and forest floor under different prescribed burning regimes. Soil Res. 2016, 54, 129–140. [Google Scholar] [CrossRef]
- Alexakis, D.; Kokmotos, I.; Gamvroula, D.; Varelidis, G. Wildfire effects on soil quality: Application on a suburban area of West Attica (Greece). Geosci. J. 2021, 25, 243–253. [Google Scholar] [CrossRef]
- Rahimi, S.; Sharifi, Z.; Mastrolonardo, G. Comparative study of the effects of wildfire and culti-vation on topsoil properties in the Zagros forest. Iran Eurasian Soil Sci. 2020, 53, 1655–1668. [Google Scholar] [CrossRef]
- Arocena, J.M.; Opio, C. Prescribed fire-induced changes in properties of sub-boreal forest soils. Geoderma 2003, 113, 1–16. [Google Scholar] [CrossRef]
- Weeks, J.; Miller, J.; Steel, Z.; Batzer, E.; Safford, H. High-severity fire drives persistent floristic homogenization in human-altered forests. Ecosphere 2023, 14, e4409. [Google Scholar] [CrossRef]
- Richter, C.; Rejmánek, M.; Miller, J.; Welch, K.; Weeks, J.; Safford, H. The species diversity × fire severity relationship is hump-shaped in semiarid yellow pine and mixed conifer forests. Ecosphere 2019, 10, e02882. [Google Scholar] [CrossRef]
- Abedi, M.; Omidipour, R.; Hosseini, S.; Bahalkeh, K.; Gross, N. Fire disturbance effects on plant taxonomic and functional β-diversity mediated by topographic exposure. Ecol. Evol. 2022, 12, e8552. [Google Scholar] [CrossRef]
- Rhizopoulou, S.; Mitrakos, K. Water relations of evergreen sclerophylls, I. Seasonal changes in the water relations of eleven species from the same environment. Ann. Bot. 1990, 65, 171–178. [Google Scholar] [CrossRef]
- Tognetti, R.; Raschi, A.; Jones, M.B. Seasonal patterns of tissue water relations in three Mediterranean shrubs co-occurring at a natural CO2 spring. Plant Cell Environ. 2000, 23, 1341–1351. [Google Scholar] [CrossRef]
- Zunzunegui, M.; Díaz Barradas, M.C.; Ain-Lhout, F.; Alvarez-Cansino, L.; Esquivias, M.P.; García Novo, F. Seasonal physiological plasticity and recovery capacity after summer stress in Mediterranean scrub communities. Plant Ecol. 2011, 212, 127–142. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).