Four New Sudanonautes Species of Freshwater Crabs (Crustacea: Decapoda: Potamonautidae) from Cameroon, Central Africa †
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling
2.2. Morphological Analyses
2.3. Molecular Analysis
3. Systematic Account
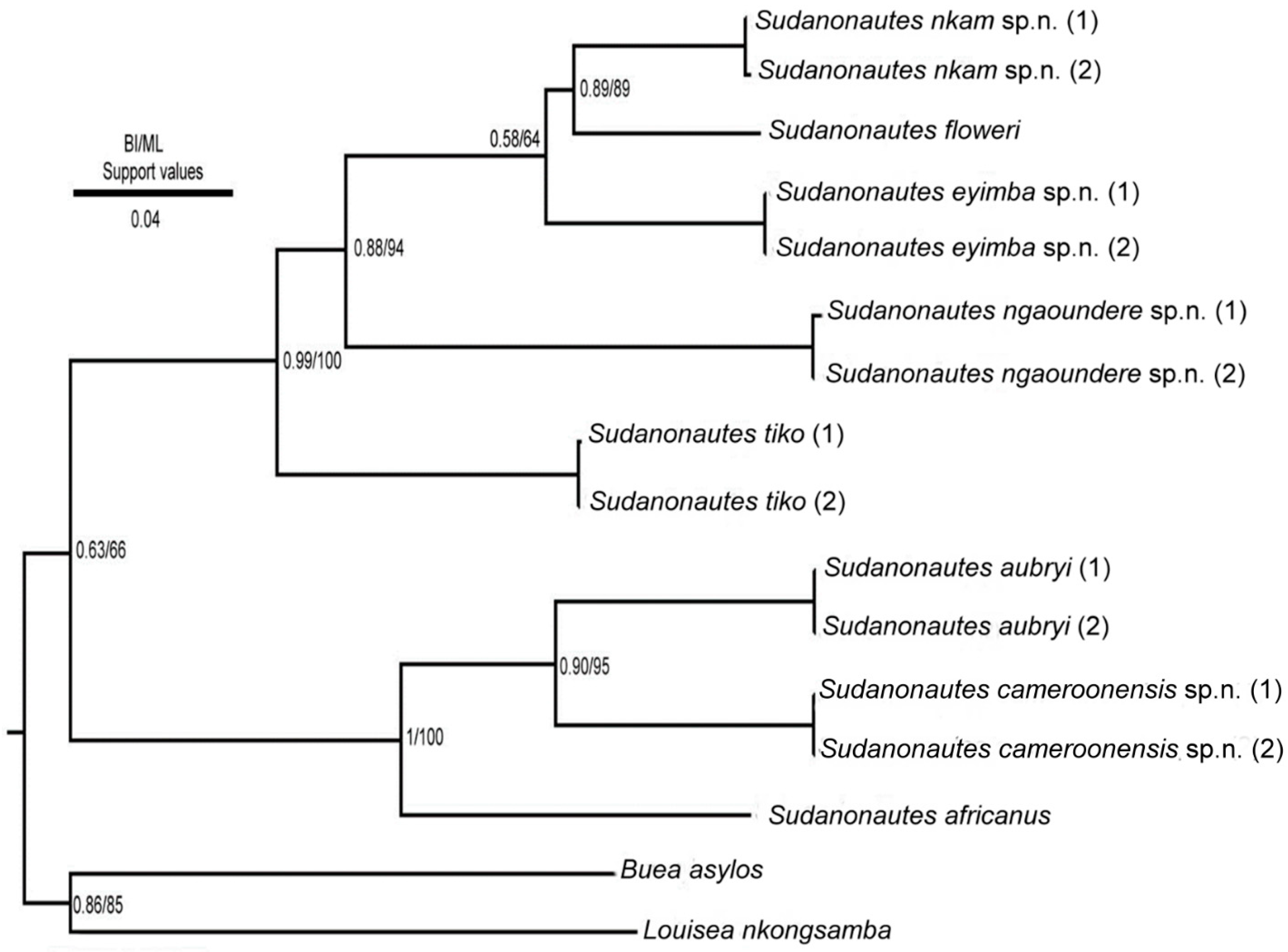
4. Molecular Results
5. Discussion
5.1. Morphology
5.2. Molecular Phylogeny
5.3. Threats, Impacts and Conservation
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cumberlidge, N. The Freshwater Crabs of West Africa, Family Potamonautidae; Faune et Flore Tropicales IRD; Institut de Recherche Pour le Développment: Paris, France, 1999; Volume 35, pp. 1–382. [Google Scholar]
- Balss, H. Potamonidae au Cameroon. In: Contribution à l’étude de la faune du Cameroun. Faune Colon. Françaises 1929, 3, 115–129. [Google Scholar]
- Cumberlidge, N. Redescription of Sudanonautes orthostylis Bott, 1955, a Freshwater Crab from Nigeria, Cameroon and Ghana with Notes on Its Ecology (Decapoda, Potamonautidae). Crustaceana 1989, 56, 230–245. [Google Scholar] [CrossRef]
- Cumberlidge, N. Redescription of Sudanonautes granulatus (Balss, 1929) (Potamoidea, Potamonautidae) from West Africa. J. Crust. Biol. 1993, 113, 805–816. [Google Scholar] [CrossRef]
- Cumberlidge, N. Further remarks on the identification of Sudanonautes orthostylis (Bott, 1955) with comparisons with other species from Nigeria and Cameroon. Proc. Biol. Soc. Wash. 1993, 106, 514–522. [Google Scholar]
- Cumberlidge, N. Two new species of Potamonemus Cumberlidge and Clark, 1992 (Brachyura, Potamoidea, Potamonautidae) from the rain forests of West Africa. J. Crust. Biol. 1993, 13, 571–584. [Google Scholar] [CrossRef]
- Cumberlidge, N. Identification of Sudanonautes aubryi (H. Milne-Edwards, 1853) (Brachyura: Potamoidea: Potamonautidae) from West and Central Africa. Z. Angew. Zool. 1994, 80, 225–241. [Google Scholar]
- Cumberlidge, N. Redescription of Sudanonautes floweri (de Man, 1901) (Brachyura: Potamoidea: Potamonautidae) from Nigeria and Central Africa. Bull. Brit. Mus. Nat. Hist. (Zool.) 1995, 61, 111–119. [Google Scholar]
- Cumberlidge, N. Redescription of the Central African fresh-water crab Sudanonautes faradjensis (Rathbun, 1921) (Brachyura: Potamoidea: Potamonautidae). Proc. Biol. Soc. Wash. 1995, 108, 629–636. [Google Scholar]
- Cumberlidge, N. Redescription of the African fresh-water crab Sudanonautes africanus (A. Milne-Edwards, 1869) (Brachyura: Potamoidea: Potamonautidae). J. Crust. Biol. 1995, 15, 588–598. [Google Scholar] [CrossRef]
- Cumberlidge, N. Remarks on the taxonomy of Sudanonautes chavanesii (A. Milne-Edwards, 1886) (Brachyura: Potamoidea: Potamonautidae) from Central Africa. Proc. Biol. Soc. Wash. 1995, 108, 238–246. [Google Scholar]
- Cumberlidge, N.; Boyko, C.B. Freshwater crabs (Brachyura: Potamoidea: Potamonautidae) from the rainforests of the Central African Republic. Proc. Biol. Soc. Wash. 2001, 3, 406–419. [Google Scholar]
- Mvogo Ndongo, P.A.; Schubart, C.D.; von Rintelen, T.; Tamesse, J.L.; Cumberlidge, N. Morphological and molecular evidence for a new species of freshwater crab of the genus Sudanonautes Bott, 1955 (Brachyura: Potamoidea: Potamonautidae) from Cameroon, with notes on its ecology. Zootaxa 2017, 4242, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Cumberlidge, N.; Clark, P.F.; Mvogo Ndongo, P.A. Disentangling the Sudanonautes granulatus (Balss, 1929) species complex (Potamoidea: Potamonautidae), with the description of two new freshwater crabs from Nigeria and Côte d’Ivoire, West Africa. Zootaxa 2021, 4948, 201–220. [Google Scholar] [CrossRef]
- Bott, R. Die Süßwasserkrabben von Afrika und ihre Stammesgeschichte. Annal. Musée R. Congo Belge (C Zool.) 1955, I, 209–352. [Google Scholar]
- Cumberlidge, N.; Daniels, S.R. A new multilocus phylogeny reveals overlooked diversity in African freshwater crabs (Potamonautidae Bott, 1970): A major revision with new higher taxa and genera. Zool. J. Linn. Soc. 2022, 194, 1268–1311. [Google Scholar] [CrossRef]
- Mvogo Ndongo, P.A.; von Rintelen, T.; Cumberlidge, N. Taxonomic revision of the endemic Cameroonian freshwater crab genus Louisea Cumberlidge, 1994 (Crustacea, Decapoda, Brachyura, Potamonautidae), with descriptions of two new species from Nkongsamba and Yabassi. ZooKeys 2019, 881, 135–164. [Google Scholar] [CrossRef] [PubMed]
- Mvogo Ndongo, P.A.; von Rintelen, T.; Tomedi-Tabi Eyango, M.; Cumberlidge, N. Morphological and molecular analyses reveal three new endemic species of the freshwater crab genus Buea Cumberlidge, Mvogo Ndongo, Clark & Daniels, 2019 (Crustacea: Brachyura: Potamonautidae) from a rainforest biodiversity hotspot in Cameroon, Central Africa. J. Crust. Biol. 2020, 40, 288–300. [Google Scholar] [CrossRef]
- Schubart, C.D. Mitochondrial DNA and decapod phylogenies; the importance of pseudogenes and primer optimization. In Decapod Crustacean Phylogenetics; Martin, J.W., Crandall, K.A., Felder, D.L., Eds.; Crust. Issues; Taylor & Francis/CRC Press: Boca Raton, FL, USA, 2009; Volume 18, pp. 47–65. [Google Scholar]
- Schubart, C.D.; Huber, M.G.J. Genetic comparisons of German populations of the stone crayfish, Austropotamobius torrentium (Crustacea: Astacidae). Bull. Français Pêche Pisc. 2006, 380–381, 1019–1028. [Google Scholar] [CrossRef]
- Palumbi, S.R. Nucleic Acids II: The Polymerase Chain Reaction. In Molecular Systematics, 2nd ed.; Hillis, D.M., Moritz, C., Mable, B.K., Eds.; Sinauer: Sunderland, MA, USA, 1996; pp. 205–247. [Google Scholar]
- Hall, T.A. BioEdit: A user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Villesen, P. FaBox: An online toolbox for Fasta sequences. Mol. Ecol. Notes 2007, 7, 965–968. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Posada, D.; Buckley, T.R. Model selection and model averaging in phylogenetics: Advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst. Biol. 2004, 53, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML-VI-HPC: Maximum likehood—Based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.R.; Ronquist, F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Daniels, S.R.; Phiri, E.E.; Klaus, S.; Albrecht, C.; Cumberlidge, N. Multilocus phylogeny of the Afrotropical freshwater crab fauna reveals historical drainage connectivity and transoceanic dispersal since the Eocene. Syst. Biol. 2015, 64, 549–567. [Google Scholar] [CrossRef]



| Species | Cameroon Locality | Museum Registration Number | DNA Extraction | References | GenBank Accession Number | |
|---|---|---|---|---|---|---|
| CO1 | 16S rRNA | |||||
| Sudanonautes africanus | Edea | LZUY-05 (CARC-18) | T263-4 | [13] | KY069942 | KY069948 |
| S. aubryi | Edea | LZUY-06 (CARC-19) | R1026-9 | [13] | KY069938 | KY069950 |
| S. aubryi | Edea | LZUY-07 (CARC-20) | T273-5 | [13] | KY069943 | KY069951 |
| S. cameroonensis sp. n. (1) | Mt. Nlonako Wildlife Reserve | CARC-16 | DNABer11 | present | OR887121 | OR892777 |
| S. cameroonensis sp. n. (2) | Bakossi National Park | CARC-17 | DNABer28 | present | OR887122 | OR892778 |
| S. eyimba sp. n. (1) | Eyimba, Mt. Nlonako Wildlife Reserve | ZMB Crust. 33108 | DNABerA9 | present | OR887120 | --- |
| S. eyimba sp. n. (2) | Eyimba, Mt. Nlonako Wildlife Reserve | ZMB Crust. 33109 | DNABer1x4 | present | OR887119 | OR892776 |
| S. ngaoundere sp. n. (1) | Near Lake Tison, Ngaoundéré | CARC-13 | DNABerB2 | present | OR887124 | --- |
| S. ngaoundere sp. n. (2) | Near Lake Mbakaou, Tibati | CARC-14 | DNABerA10 | present | OR887123 | --- |
| S. nkam sp. n. (1) | Near Yabassi | LZUY-10 | T262-3 | [13] | KY069939 | KY069952 |
| S. nkam sp. n. (2) | Near Yabassi | ZMB Crust. 33110 | DNABerA5 | present | OR887126 | --- |
| S. tiko | Tiko | ZMB Crust. 29628 | T262-11 | [13] | KY069941 | KY069954 |
| S. tiko | Tiko | RMNH.CRUST.57073 | T273-12 | [13] | KY069945 | KY069953 |
| Louisea nkongsamba | Mt. Nlonako Wildlife Reserve | ZMB Crust. 31618 | ---- | [19] | KP640480 | KP640444 |
| Buea asylos | Cameroon | NHM 1994.588-591 | ---- | [28] | KP640489 | KP640453 |
| Sudanonautes Species | Max. Distance within Species (%) | Distance Range between Species (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| S. eyimba sp. n. | S. nkam sp. n. | S. cameroonensis sp. n. | S. ngaoundere sp. n. | S. tiko | S. africanus | S. aubryi | S. floweri | ||
| S. eyimba sp. n. | 0.00 | --- | |||||||
| S. nkam sp. n. | 0.16 | 10.12–10.59 | --- | ||||||
| S. cameroonensis sp. n. | 0.00 | 19.39–20.19 | 19.29–20.23 | --- | |||||
| S. ngaoundere sp. n. | 0.69 | 13.56–15.17 | 15.56–16.89 | 16.48–18.62 | --- | ||||
| S. tiko | 0.17 | 14.65–15.76 | 13.52–14.06 | 16.75–17.44 | 16.30–17.24 | --- | |||
| S. africanus | --- | 17.45–18.47 | 17.01–17.09 | 13.90–15.63 | 17.00–21.37 | 18.27–18.31 | --- | ||
| S. aubryi | 0.00 | 20.06–21.42 | 18.51–18.85 | 13.76–14.91 | 17.92–20.00 | 17.77–18-18 | 13.63–13.88 | --- | |
| S. floweri | --- | 9.51–10.09 | 8.68–8.88 | 19.53–20.70 | 14.13–16.20 | 14.36–14.72 | 17.03 | 18.54–19.08 | --- |
| Character | S. cameroonensis sp. n. | S. eyimba sp. n. | S. ngaoundere sp. n. | S. nkam sp. n. |
|---|---|---|---|---|
| Adult size range | CW 36 to 47 mm | CW 34 to 38 mm | CW 46 to 53 mm | CW 42 to 45 mm |
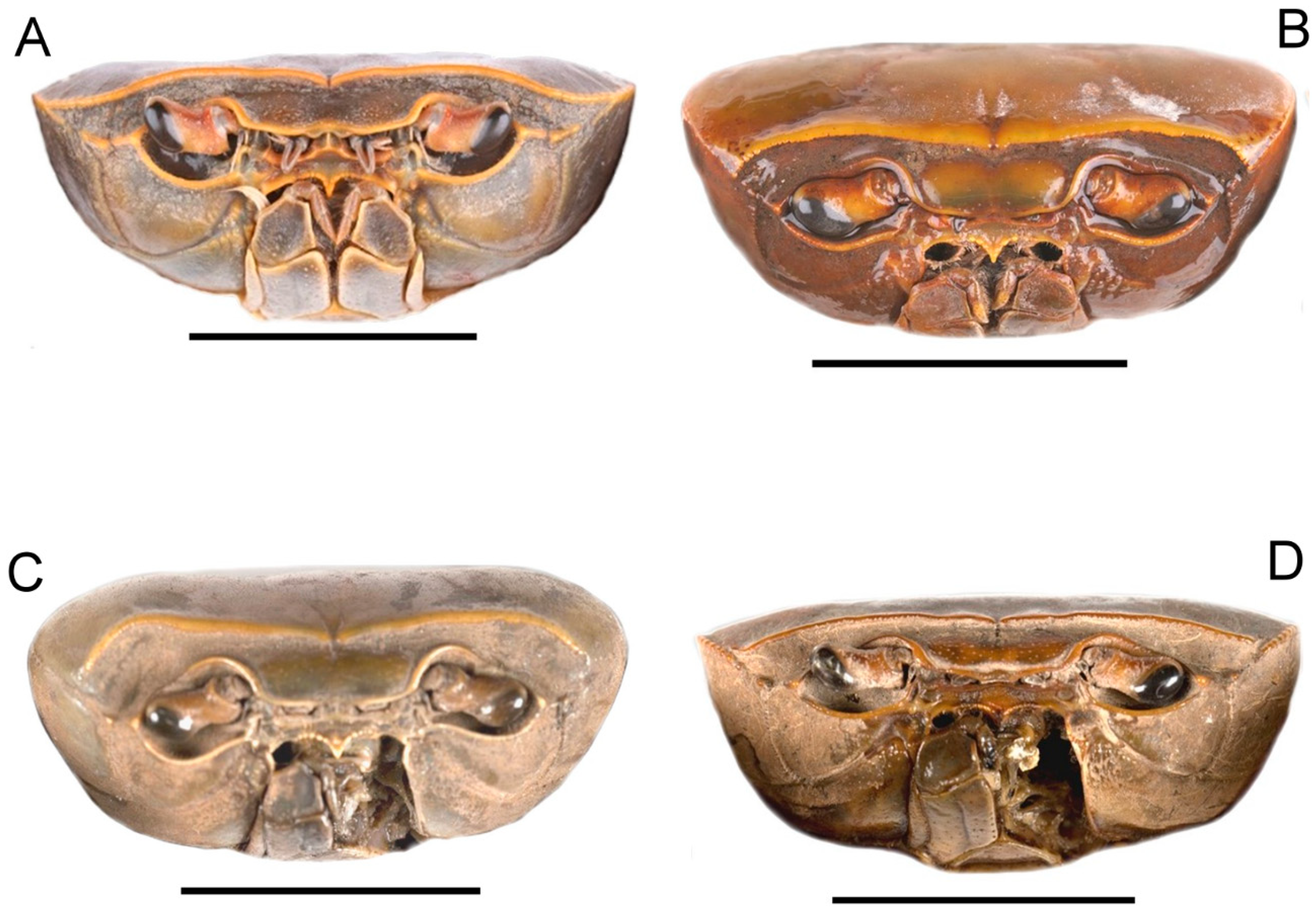
| Semi-circular, urogastric, cardiac, cervical, transverse branchial carapace grooves. | All shallow; Figure 1A and Figure 2A. | All deep; Figure 1B and Figure 2B. | All shallow; Figure 1C and Figure 2C. | All deep; Figure 1D and Figure 2D. |
| Postfrontal crest. | Complete, meeting carapace lateral margin behind epibranchial tooth; Figure 2A and Figure 3A. | Complete, meeting carapace lateral margin at epibranchial tooth; Figure 2B and Figure 3B. | Incomplete, ending just short of meeting lateral carapace margin at epibranchial tooth; Figure 2C and Figure 3C. | Complete, lateral end curving backward, not meeting either epibranchial tooth or carapace lateral margin; Figure 2D and Figure 3D. |
| Carapace lateral margin. | Granulated; Figure 1A and Figure 2A. | Smooth; Figure 1B and Figure 2B. | Smooth; Figure 1C and Figure 2C | Granulated; Figure 1D and Figure 2D. |
| Exorbital tooth. | Large, triangular, distinct; Figure 1A, Figure 2A and Figure 3A. | Small, low, distinct; Figure 1B, Figure 2B and Figure 3B. | Large, triangular, distinct; Figure 1C, Figure 2C and Figure 3C. | Small, pointed, distinct; Figure 1D, Figure 2D and Figure 3D. |
| Intermediate tooth on the anterolateral margin. | Small, triangular; Figure 1A, Figure 2A and Figure 3A. | Small, triangular; Figure 1B, Figure 2B and Figure 3B. | Small, pointed; Figure 1C, Figure 2C and Figure 3C. | Small, triangular; Figure 1D, Figure 2D and Figure 3D. |
| Epibranchial tooth. | Small granule; Figure 1A, Figure 2A and Figure 3A. | Small, triangular; Figure 1B, Figure 2B and Figure 3B. | Obscure; Figure 1C, Figure 2C and Figure 3C. | Large, triangular; Figure 1D, Figure 2D and Figure 3D. |
| Pterygostomial region of carapace sidewall. | Field of heavy granules medially, otherwise smooth; Figure 3A and Figure 4A. | Field of large granules medially, otherwise smooth; Figure 3B and Figure 4B. | Field of small but distinct granules medially, otherwise smooth; Figure 3C and Figure 4C. | Field of large granules medially, otherwise smooth; Figure 3D and Figure 4D. |
| Anterior thoracic sternum S3, S4 margins. | S3, S4 margins thin, not raised; Figure 4A. | S3, S4 margins thickened, raised; Figure 4B. | S3, S4 margins thickened, raised; Figure 4C. | S3, S4 margins thickened, raised; Figure 4D. |
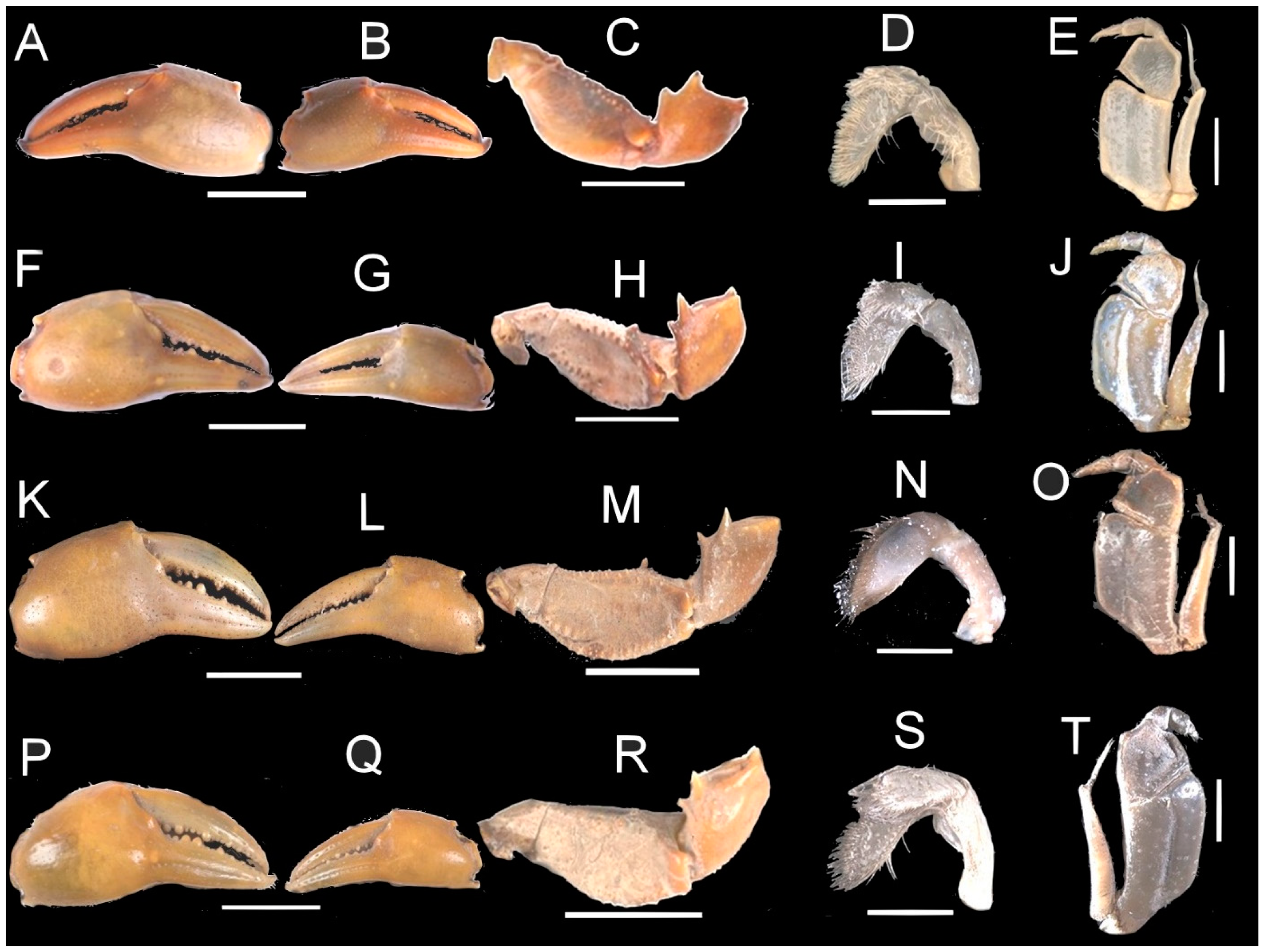
| Mandibular palp terminal article (TA). | Proximal superior margin of palp TA with small accessory lobe ca. ¼ length of TA; Figure 5E. | Proximal superior margin of palp TA with small accessory lobe ca. ¼ length of TA; Figure 5I. | Proximal superior margin of palp TA with small accessory lobe ca. ¼ length of TA; Figure 5N. | Proximal superior margin of palp TA with large rounded accessory lobe ca. ½ as length of TA; Figure 5S. |
| Major cheliped dactylus cutting edge. | With no large teeth; Figure 5A. | With two large proximal teeth, one large tooth at midpoint, one smaller tooth in between; Figure 5F. | With three large proximal teeth, one large tooth at midpoint; Figure 5K. | With two large proximal teeth, one large tooth at midpoint; Figure 5P. |
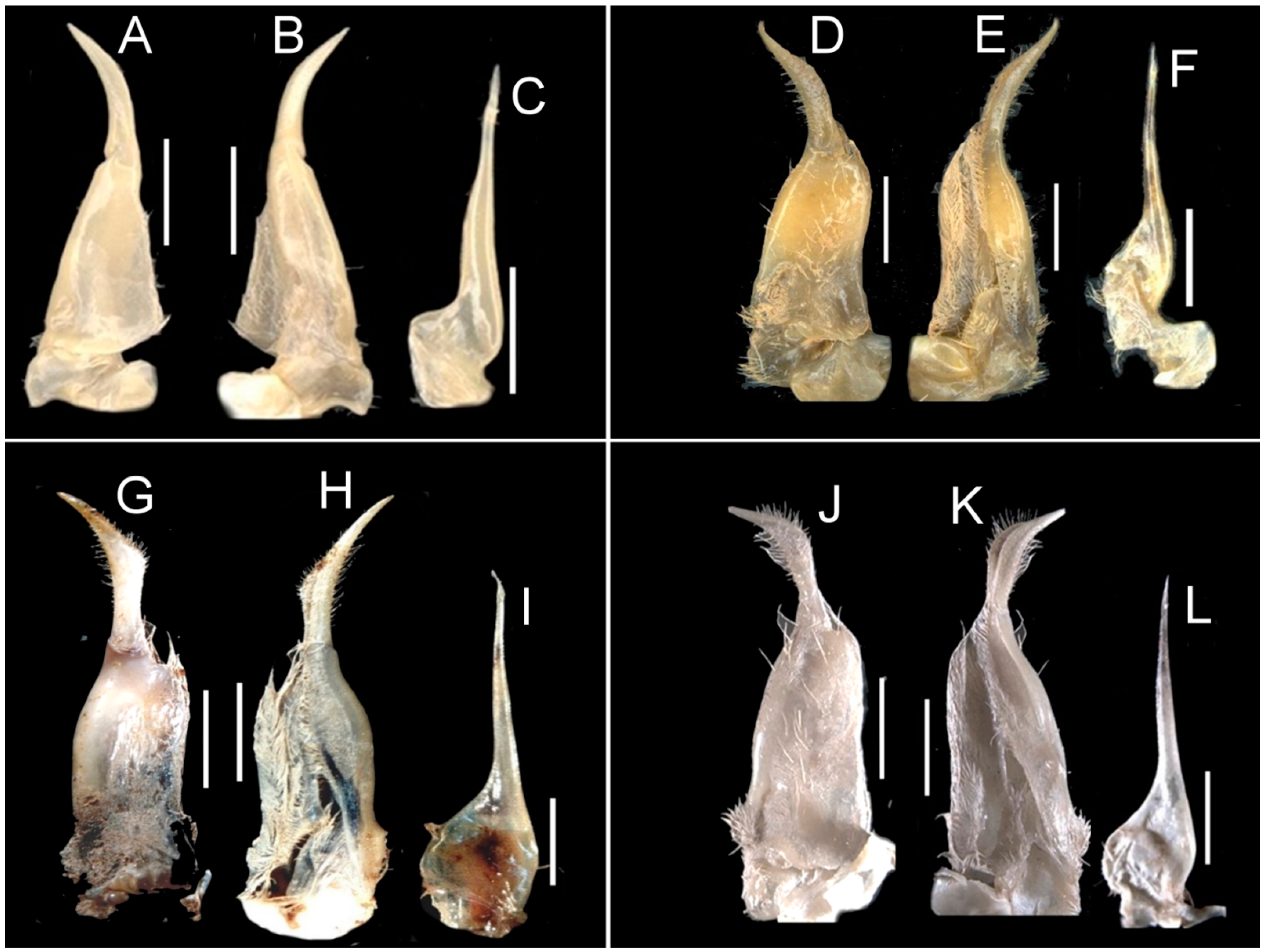
| G1SA shape near G1TA-G1SA junction. | Distal G1SA slim; Figure 6A,B. | Distal G1SA wide; Figure 6D,E. | Distal G1SA wide; Figure 6G,H. | Distal G1SA wide; Figure 6J,K. |
| G1TA margins. | Margins lacking setae, smooth; Figure 6A,B. | Margins lined by sparse short setae; Figure 6D,E. | Margins lined by short setae; Figure 6G,H. | Margins lined by dense long setae; Figure 6J,K. |
| G1TA relative length. | G1TA long (G1TA length 0.8–0.9 × G1SA length); Figure 6A,B. | G1TA medium length (G1TA length 0.6 × G1SA length); Figure 6D,E. | G1TA medium length (G1TA length 0.5 × G1SA length); Figure 6G,H. | G1TA medium length (G1TA length 0.6 × G1SA length); Figure 6J,K. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mvogo Ndongo, P.A.; Clark, P.F.; von Rintelen, T.; Cumberlidge, N. Four New Sudanonautes Species of Freshwater Crabs (Crustacea: Decapoda: Potamonautidae) from Cameroon, Central Africa. Diversity 2024, 16, 345. https://doi.org/10.3390/d16060345
Mvogo Ndongo PA, Clark PF, von Rintelen T, Cumberlidge N. Four New Sudanonautes Species of Freshwater Crabs (Crustacea: Decapoda: Potamonautidae) from Cameroon, Central Africa. Diversity. 2024; 16(6):345. https://doi.org/10.3390/d16060345
Chicago/Turabian StyleMvogo Ndongo, Pierre A., Paul F. Clark, Thomas von Rintelen, and Neil Cumberlidge. 2024. "Four New Sudanonautes Species of Freshwater Crabs (Crustacea: Decapoda: Potamonautidae) from Cameroon, Central Africa" Diversity 16, no. 6: 345. https://doi.org/10.3390/d16060345
APA StyleMvogo Ndongo, P. A., Clark, P. F., von Rintelen, T., & Cumberlidge, N. (2024). Four New Sudanonautes Species of Freshwater Crabs (Crustacea: Decapoda: Potamonautidae) from Cameroon, Central Africa. Diversity, 16(6), 345. https://doi.org/10.3390/d16060345






