Abstract
The bivalve mollusc Pinna nobilis, endemic to the Mediterranean Sea, has been vanishing since 2016 from the whole basin because of an infection by multiple pathogens that caused mass mortality events. In the Eastern Mediterranean, some small populations seem to be resistant to the infection. These individuals could represent the only possibility for the species to recolonize desert habitats. Thus, according to the recommendations of IUCN, looking for living specimens of P. nobilis is a priority. With this goal in mind, we carried out surveys in different areas of Southern Italy, and in 2018, we launched a Citizen Science campaign to involve recreational and professional divers in this challenge. As a result of a monitoring activity carried out in 2022–2024, along the Ionian coast of Apulia, in Southern Italy, we can say that there are no more living specimens there but only empty shells. Concurrent to the vanishing of the queen P. nobilis, its congeneric P. rudis, resistant to the infection, seems to be taking advantage, becoming more common and colonizing habitats once exclusive to P. nobilis. In fact, from different areas of the Mediterranean, sightings of the new possible queen, P. rudis, are increasing, together with the discovery of individuals exhibiting morphological traits that are a mixture of the two species. In some cases, these morphological features are not easy to detect; nevertheless, the presence of these hybrids, resistant to the infection, is important for the conservation of the species.
Pinna nobilis (Linnaeus, 1758), the pen shell, and the congeneric P. rudis (Linnaeus, 1758), the spiny/rough fan mussel, are two molluscan species of the family Pinnidae that inhabit the Mediterranean Sea [1]. A third species of this family, Atrina fragilis (Pennant, 1777), is rarer in the Mediterranean, preferring detritic bottoms at depths of up to 600 m [2].
P. nobilis is endemic to the Mediterranean [3], while P. rudis has a subtropical affinity, and it is mainly present along the Atlantic African coasts [4]. They are sibling species whose distribution in the Mediterranean partially overlaps [5]; in fact, although the priority habitats are different, their bathymetric distribution is widely shared [6]. In particular, P. nobilis preferentially inhabits seagrass meadows, including dead matte of Posidonia oceanica, macroalgal canopies, muddy and sandy bottoms and detritic patches in rocky bottoms, at depths of up to 60 m [3,7]; P. rudis is thermophilic and colonizes gravel and rocky bottoms even though its presence has also been reported in P. oceanica meadows [8].
These species are both strictly protected by EC directives and national laws, and are listed in the Annex II of the Barcelona Convention as threatened or endangered. Since 2016, dramatic mass mortality events (MMEs), spreading from the Mediterranean coast of Spain eastward, caused, in a few years, the almost complete vanishing of living specimens of P. nobilis from many areas of Spain, Italy and Greece, leading the species to the brink of extinction [9]. Initially, the newly described Haplosporidium pinnae [10] was considered to be responsible for the mortality events. This etiology was confirmed by several studies, but in some of them, other pathogens or opportunist microorganisms were detected [11,12,13] like Mycobacterium spp. and Vibrio spp. Now, we are aware that an even more complex etiology is the cause of the MMEs; even though Haplosporidium and Mycobacterium are mostly involved, multifactorial diseases that include also Vibrio spp., Perkinsus spp. together with infections by picornaviruses [14], concur to establish an immunodeficiency status that favors the onset of an inflammatory syndrome and the consequential death of fan mussels.
Now, the status of the species, assessed by the red list of the International Union for the Conservation of Nature (IUCN), is critically endangered [15]. According to the recommendations of the IUCN, the search for living individuals is of paramount importance for the survival of the species. With this goal in mind, we carried out surveys in different areas of Southern Italy (Figure 1), and in 2018, we launched a Citizen Science campaign to involve recreational and professional divers in this challenge [16].
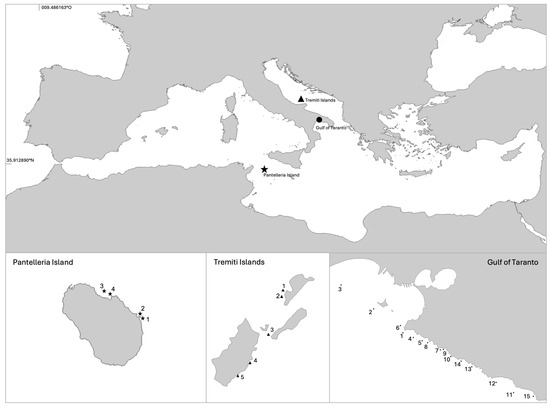
Figure 1.
Map of the areas investigated in the Mediterranean Sea, searching for living specimens of Pinna nobilis. Pantelleria Island—1 Faraglione, 2 Cala Tramontana, 3 Secca di Campobello, 4 Baia di Campobello. Tremiti Islands—1 Secca della Vedova West side, 2 Secca della Vedova East side, 3 Canale Travicello, 4 Cala del Sale, 5 Scoglio dell’Elefante. Gulf of Taranto—1 San Vito, 2 San Pietro Island, 3 Secca dell’Armeleia, 4 Lido Bruno, 5 Lama, 6 San Vito, 7 Mon Reve, 8 Saint Bon, 9 Lido Gandoli, 10 Saturo, 11 Le Conche, 12 Torre Zozzoli, 13 Monte d’Arena, 14 Baia d’Argento, 15 Torre Ovo.
Along the coasts of Apulia, in Southern Italy, P. nobilis was historically largely distributed. In the Taranto marine area [17,18], until 2018, this species was very abundant, with more than 10,000 individuals present in the two basins of the Mar Piccolo and in the Mar Grande (Figure 2). In 2016, an important translocation activity was carried out to move nearly 2000 specimens from an area outside the Mar Grande, where the Port Authority of Taranto realized a “sediment tank” deputed to host sediments dredged in an area of the port. The entire population was transferred into an area of the Mar Grande where a large natural population was already present, settled over an extensive dead matte of P. oceanica [19] (Figure 3).
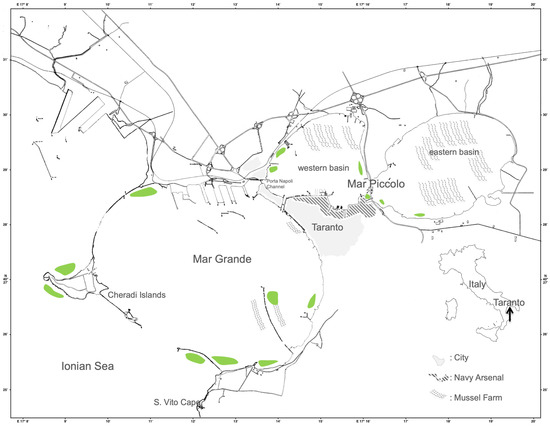
Figure 2.
Map of the Taranto marine area. The green blocks indicate the distribution of living Pinna nobilis populations until 2018.
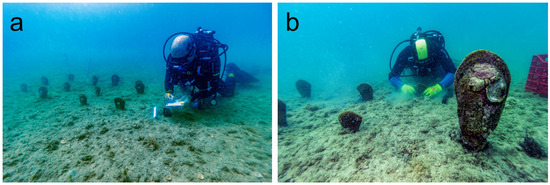
Figure 3.
Two moments of the relocation activities of a population of Pinna nobilis in the Mar Grande of Taranto: (a) Data collection of the newly placed specimens. (b) Placement of a specimen in the new site.
Starting from the spring of 2018, in a very short time, thousands of specimens died both in the Mar Piccolo and Mar Grande, and in 2019, fewer than 10 living specimens were observed during the monitoring activities carried out by the SCUBA divers of the CNR IRSA of Taranto (Figure 4). The cause of this mass mortality was ascribed to Haplosporidium pinnae [13,20], as had already been reported for the same events in the Western Mediterranean [21].

Figure 4.
In 2019 in the Mar Piccolo of Taranto a few living individuals were surrounded by many empty shells: (a) One living specimen in the eastern basin of the Mar Piccolo (−4 m) with the posterior margin clean, i.e., not colonized by epibionts. (b) Many dead specimens in the western basin of the Mar Piccolo (−3.5 m).
We found a very similar situation at the Archipelago of Tremiti Islands, in the Southern Adriatic Sea (Figure 1), where the local P. nobilis population was reported in a good health until the summer of 2019 [22]. Notwithstanding, in September 2019, during a survey organized with the cooperation of the Coast Guard Maritime Directorate of Pescara, a diffuse mortality was observed with very few living individuals at sites with depths of over 20 m and a water temperature of 13–15 °C (Figure 5). The mantle biopsies of some moribund specimens revealed the presence of Mycobacterium sp. and Haplosporidium sp. [16].

Figure 5.
In 2019 at Tremiti Islands, a widespread mortality of Pinna nobilis was observed with a few survivors: (a) Capraia Island, loc. Secca della Vedova; living specimen (−27 m). (b) Canale Travicello, moribund specimen in Posidonia oceanica meadow (−6 m).
In this very frustrating scenario, the empty shells maintain the important ecological function of living fan mussels as ecosystem engineers [23]. According to Jones et al. [24], an ecosystem engineer has the capacity of modifying, maintaining and/or creating habitats. The fan mussel’s shells, remaining erect and anchored to the substratum, act like isles of biodiversity on sandy or muddy bottoms, attracting many benthic sessile and vagile organisms (Video S1). When the individual is alive, only the posterior margin remains clean, due to the activity of the mantle; instead, the empty shells are also completely colonized in their internal side (Figure 6). That is why the collection of empty shells is still prohibited and subject to heavy fines.

Figure 6.
An empty shell of Pinna nobilis in the Mar Piccolo of Taranto (February 2019) is colonized by many species of algae and invertebrates and offers refuge to a small fish.
Concurrent to the quite complete vanishing of P. nobilis, the sightings of P. rudis are increasing, mentioned both in the scientific literature [8,25,26] and on social media, suggesting that this species could benefit from the disappearance of its sister species, also colonizing habitats that were originally exclusive to P. nobilis.
We observed the first signs of this trend at Pantelleria Island (Sicily Channel, Mediterranean Sea), in October 2022 (Figure 1). We have never seen specimens of P. rudis in all our dives in Taranto, Tremiti and many other Apulian sites, and only sporadic observations are reported from these localities, as evidence of its rarity. Considering the few dives made on the sea beds of the island, the finding of 11 living specimens of P. rudis, plus 1 dead one, in our opinion, is a relevant number (Figure 7).

Figure 7.
A notable number of living individuals of Pinna rudis was observed at Pantelleria: (a) Secca Campobello (−25 m). (b) Large size individual at Baia di Campobello (−18 m).
Furthermore, for a long time, we did not see any healthy individuals of P. nobilis either. Living specimens of both species were present there, together with a few empty shells, and all of them were found on rocky or coralligenous bottoms. After a more careful observation, the individuals initially recognized as P. nobilis, despite the fact that they exhibited the shells and size typical of the adults of such species, showed a different coloration of the mantle; it was not the typical pink [27] (Figure 8a), but iridescent with dark belts, more similar to that of P. rudis (Figure 8b). During the dives, we have observed other individuals exhibiting morphological traits, like shell ornamentations, their size, and the coloration of the mantle, that seemed to be a mixture of the two species (Figure 9). According to Vázquez-Luis et al. [28], it is possible that they could be hybrids. We did not perform mantle biopsies to confirm this hypothesis, and even though the putative hybrids exhibited morphological traits quite different from the hybrids showed in Vázquez-Luis et al. [28], these findings are very interesting and deserve more in-depth investigations.

Figure 8.
(a) A juvenile specimen in the Mar Grande of Taranto in 2016 with the typical coloration of the mantle (−4.5 m). (b) The posterior margin of a putative hybrid of Pinna nobilis x Pinna rudis. The peculiar coloration of the mantle is well visible. Pantelleria, Secca Campobello (−28 m).
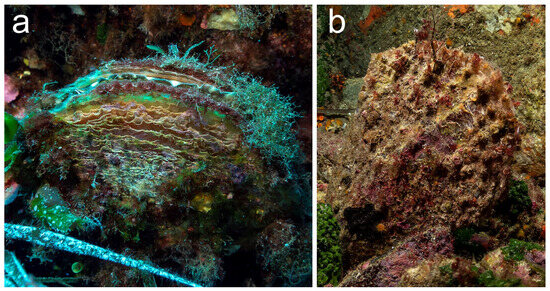
Figure 9.
Putative hybrids of Pinna nobilis and P. rudis at Pantelleria: (a) Secca Campobello (−28 m). (b) Cala Tramontana (−22 m).
To our knowledge, the first documented reports of P. rudis in Apulia—and in the Province of Taranto in particular—are dated to summer 2023. Two specimens were reported on a social media forum from a locality 20 km away from Taranto [8], while we discovered a third at San Vito, a locality near the city of Taranto, thanks to notification from the Diving Center “Taras Sub” that was involved in our Citizen Science campaign. This specimen was very difficult to see, settled into a little hole among the coralligenous rocks, and the shell completely covered by organisms (Figure 10).

Figure 10.
Pinna rudis at San Vito, Taranto, hardly visible in the coralligenous bottom (−27 m).
The first reports of P. rudis follow the consistent vanishing of its sister species from the sea beds along the Apulian coast of the Ionian Sea in the Gulf of Taranto. In 2022–2024 we carried out a monitoring activity aimed to discover any survivors of P. nobilis in this area by investigating 15 sites along the coast in the province of Taranto (Figure 1). We carried out dives both in the meadows of Posidonia oceanica and underwater linear transects of 200–300 m at three depths: 5, 15 and 25 m. The utilization of underwater scooters allowed us to survey more than 15 km of sea bottom. No living individuals were observed, and 67 empty shells were registered, most of them at 15 m of depth and outside the P. oceanica meadows (Figure 11). In a similar study, extended to the entire south-eastern coast of Apulia [29], the same situation is reported, confirming the total disappearing of the species from the Apulian sea beds.

Figure 11.
One of the 67 empty shells of Pinna nobilis observed during the monitoring activity along the Apulian coast of the Ionian Sea.
At present, this situation is widespread in the whole Mediterranean. Recent studies reported that in areas considered safe environments for the species, after a period of resistance, the survivors were also impacted by a mass mortality event [30]. Small, unaffected populations remain, especially in the Eastern Mediterranean [31,32,33]. These individuals could play a crucial role in the natural recolonization of the impacted areas, but, at the same time, according to Vázquez-Luis et al. [28], these survivors could be hybrids, not purebred individuals of P. nobilis, and incorrect identification may cause problems regarding the conservation of the species. This issue must be better understood together with the plausible rise in P. rudis populations that opens new perspectives of study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16060341/s1, Video S1: Empty shell of Pinna nobilis observed in 2019 in the Mar Piccolo of Taranto.
Author Contributions
Conceptualization, F.R.; methodology, F.R., G.F. and G.D.; validation, F.R. and G.D.; formal analysis, F.R. and G.D.; investigation, F.R., G.F. and G.D.; resources, F.R.; data curation, G.D.; writing—original draft preparation, F.R.; writing—review and editing, F.R., G.F. and G.D.; funding acquisition, F.R. and G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by: Regione Puglia, POR Puglia 2014/2020—Asse VI—Az. 6.5—6.5.a, Project title “M.I.A. Rete Natura 2000 Innovative Environmental Monitoring”, CUP B35F21002450001. The activities at Taranto, Tremiti and Pantelleria were carried out in the framework of the Project “RiPinTa—Relocation of Pinna nobilis in Taranto”, funded by Port Authority of Taranto, Contract Number n. 515787 (2016) and the Citizen Science campaign “SOS Pinna: la Subacquea aiuta la Ricerca”.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data are reported in the present publication.
Acknowledgments
This research was carried out in the mark of the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4—Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union—NextGenerationEU, Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP B83C22002930006 Project title “National Biodiversity Future Center—NBFC”. Giovanni Squitieri (www.officinadellimmagine.eu) is acknowledged for his participation in all the underwater activities and for all the photo/video documentation produced. The comments and remarks of two Reviewers helped us to improve the quality of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lemer, S.; Buge, B.; Bemis, A.; Giribet, G. First molecular phylogeny of the circumtropical bivalve family Pinnidae (Mollusca, Bivalvia): Evidence for high levels of cryptic species diversity. Mol. Phylogenet. Evol. 2014, 75, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Fryganiotis, K.; Antoniadou, c.; Chintiroglou, C. Comparative distribution of the fan mussel Atrina fragilis (Bivalvia, Pinnidae) in protected and trawled areas of the north Aegean Sea (Thermaikos Gulf). Mediterr. Mar. Sci. 2013, 14, 119–124. [Google Scholar] [CrossRef]
- Basso, L.; Vázquez-Luis, M.; García-March, J.R.; Deudero, S.; Alvarez, E.; Vicente, N.; Duarte, C.M.; Hendriks, I.E. The pen shell Pinna nobilis: A review of populations status and recommended research priorities in the Mediterranean Sea. Adv. Mar. Biol. 2015, 71, 109–160. [Google Scholar] [CrossRef]
- Gvozdenovic, S.; Macic, V.; Pesic, V.; Nikolic, M.; Peras, I.; Mandic, M. Review on Pinna rudis (Linnaeus, 1758) (Bivalvia: Pinnida) presence in the Mediterranean. J. Agric. For. 2019, 65, 115–126. [Google Scholar] [CrossRef]
- Kersting, D.K.; Ballesteros, E. Is the local extinction of Pinna nobilis facilitating Pinna rudis recruitment? Mediterr. Mar. Sci. 2021, 22, 623–626. [Google Scholar] [CrossRef]
- Vázquez-Luis, M.; March, D.; Alvarez, E. Spatial distribution modelling of the endangered bivalve Pinna nobilis in a Marine Protected Area. Mediterr. Mar. Sci. 2014, 15, 626–634. [Google Scholar] [CrossRef]
- Kersting, D.K.; García-March, J.R. Long-term assessment of recruitment, early stages and population dynamics of the endangered Mediterranean fan mussel Pinna nobilis in the Columbretes Islands (NW Mediterranean). Mar. Environ. Res. 2017, 130, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Zotou, M.; Papadakis, O.; Catanese, G.; Stranga, Y.; Ragkousis, M.; Kampouris, T.E.; Naasan Aga-Spyridopoulou, R.; Papadimitriou, E.; Koutsoubas, D.; Katsanevakis, S. New kid in town: Pinna rudis spreads in the eastern Mediterranean. Mediterr. Mar. Sci. 2023, 24, 709–721. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Carella, F.; Çinar, M.E.; Čizmek, H.; Jimenez, C.; Kersting, D.K.; Moreno, D.; Rabaoui, L.; Vicente, N. The fan mussel Pinna nobilis on the brink of extinction in the Mediterranean. In Imperilled: The Encyclopaedia of Conservation; Della Sala, D.A., Goldstein, M.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 2, pp. 700–709. [Google Scholar] [CrossRef]
- Catanese, G.; Grau, A.; Valencia, J.M.; March, J.R.G.; Vázquez-Luis, M.; Álvarez, E.; Deudero, S.; Darriba, S.; Carballal, M.J.; Villalba, A. Haplosporidium pinnae sp. nov., a haplosporidan parasite associated with mass mortalities of the fan mussel, Pinna nobilis, in the Western Mediterranean Sea. J. Invertebr. Pathol. 2018, 157, 9–24. [Google Scholar] [CrossRef]
- Carella, F.; Antuofermo, E.; Farina, S.; Salati, F.; Mandas, D.; Prado, P.; Panarese, R.; Marino, F.; Fiocchi, E.; Pretto, T.; et al. In the wake of the ongoing mass mortality events: Co-occurrence of Mycobacterium, Haplosporidium and other pathogens in Pinna nobilis collected in Italy and Spain (Mediterranean Sea). Front. Mar. Sci. 2020, 7, 48. [Google Scholar] [CrossRef]
- Scarpa, F.; Sanna, D.; Azzena, I.; Mugetti, D.; Cerruti, F.; Hosseini, S.; Cossu, P.; Pinna, S.; Grech, D.; Cabana, D.; et al. Multiple non-species-specific pathogens possibly triggered the mass mortality in Pinna nobilis. Life 2020, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Tiscar, P.G.; Rubino, F.; Paoletti, B.; Francesco, C.E.D.; Mosca, F.; Salda, L.D.; Hattab, J.; Smoglica, C.; Morelli, S.; Fanelli, G. New insights about Haplosporidium pinnae and the pen shell Pinna nobilis mass mortality events. J. Invertebr. Pathol. 2022, 190, 107735. [Google Scholar] [CrossRef] [PubMed]
- Carella, F.; Prado, P.; Vico, G.D.; Palić, d.; Villari, G.; García-March, J.R.; Tena-Medialdea, J.; Melendreras, E.C.; Giménez-Casalduero, F.; Sigovini, M.; et al. A widespread picornavirus affects the hemocytes of the noble pen shell (Pinna nobilis), leading to its immunosuppression. Front. Vet. Sci. 2023, 10, 1273521. [Google Scholar] [CrossRef] [PubMed]
- Kersting, D.; Benabdi, M.; Čizmek, H.; Grau, A.; Jimenez, C.; Katsanevakis, S.; Öztürk, B.; Tuncer, S.; Tunesi, L.; Vázquez-Luis, M.; et al. Pinna nobilis. The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2019; p. e.T160075998A160081499. [Google Scholar] [CrossRef]
- Denti, G.; Fanelli, G.; Tiscar, P.G.; Paoletti, B.; Hattab, J.; Mastrototaro, F.; Carella, F.; Villari, G.; Montesanto, F.; Chimienti, G.; et al. Mass mortality of Pinna nobilis Linnaeus, 1758 (Mollusca, Bivalvia) in the Taranto and Tremiti Islands populations: Citizen science to help scientists. Biol. Mar. Medit. 2023, 27, 21–24. [Google Scholar]
- Centoducati, G.; Tarsitano, E.; Bottalico, A.; Marvulli, M.; Lai, O.R.; Crescenzo, G. Monitoring of the endangered Pinna nobilis Linné, 1758 in the Mar Grande of Taranto (Ionian Sea, Italy). Environ. Monit. Assess. 2007, 131, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Cecere, E.; Petrocelli, A.; Casale, A.; Casale, V.; Passarelli, S. Recent observations of Pinna nobilis (Mollusca. Bivalvia) in the Mar Piccolo basin (Gulf of Taranto, Mediterranean Sea). Biol. Mar. Medit. 2015, 22, 107–708. [Google Scholar]
- Fanelli, G.; Rubino, F. Relocation of Pinna nobilis (Mollusca, Bivalvia) in the port of Taranto. Biol. Mar. Medit. 2017, 24, 142–143. [Google Scholar]
- Panarese, R.; Tedesco, P.; Chimienti, G.; Latrofa, M.S.; Quaglio, F.; Passantino, G.; Buonavoglia, C.; Gustinelli, A.; Tursi, A.; Otranto, D. Haplosporidium pinnae associated with mass mortality in endangered Pinna nobilis (Linnaeus 1758) fan mussels. J. Invertebr. Pathol. 2019, 164, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Luis, M.; Alvarez, E.; Barrajón, A.; García-March, J.R.; Grau, A.; Hendriks, I.E.; Jimenez, S.; Kersting, D.; Perez, M.; Ruiz, J.M.; et al. Pinna nobilis: A mass mortality event in western Mediterranean Sea. Front. Mar. Sci. 2017, 4, 220. [Google Scholar] [CrossRef]
- Mastrototaro, F.; (University “Aldo Moro”, Bari, Italy). Personal communication, 2019.
- Iannucci, S.; Auriemma, R.; Davanzo, A.; Ciriaco, S.; Segarich, M.; Del Negro, P. Can the empty shells of Pinna nobilis maintain the ecological role of the species? A structural and functional analysis of the associated mollusc fauna. Diversity 2023, 15, 956. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Donato, G.; Vázquez-Luis, M.; Nebot-Colomer, E.; Lunetta, A.; Giacobbe, S. Noble fan-shell, Pinna nobilis, in Lake Faro (Sicily, Italy): Ineluctable decline or extreme opportunity? Estuar. Coast. Shelf Sci. 2021, 261, 107536. [Google Scholar] [CrossRef]
- Oprandi, A.; Aicardi, S.; Azzola, A.; Benelli, F.; Bertolino, M.; Bianchi, C.N.; Chiantore, M.; Ferranti, M.P.; Mancini, I.; Morri, C.; et al. A tale of two sisters: The southerner Pinna rudis is getting North after the regional extinction of the congeneric P. nobilis (Mollusca: Bivalvia). Diversity 2024, 16, 120. [Google Scholar] [CrossRef]
- García-March, J.R.; Vicente, N. Protocol to Study and Monitor Pinna nobilis Populations within Marine Protected Areas; Malta Environmental and Planning Authority: Gozo, Italy, 2006; MedPAN Project; 78p. [Google Scholar]
- Vázquez-Luis, M.; Nebot-Colomer, E.; Deudero, S.; Planes, S.; Boissin, E. Natural hybridization between pen shell species: Pinna rudis and the critically endangered Pinna nobilis may explain parasite resistance in P. nobilis. Mol. Biol. Rep. 2021, 48, 997–1004. [Google Scholar] [CrossRef]
- Pensa, D.; Fianchini, A.; Grosso, L.; Ventura, D.; Cataudella, S.; Scardi, M.; Rakaj, A. Population status, distribution and trophic implications of Pinna nobilis along the South-eastern Italian coast. npj Biodivers. 2022, 1, 3. [Google Scholar] [CrossRef]
- Donato, G.; Lunetta, A.; Spinelli, A.; Catanese, G.; Giacobbe, S. Sanctuaries are not inviolable: Haplosporidium pinnae as responsible for the collapse of the Pinna nobilis population in Lake Faro (central Mediterranean). J. Invertebr. Pathol. 2023, 201, 108014. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, O.; Mamoutos, I.; Ramfos, A.; Catanese, G.; Papadimitriou, E.; Theodorou, J.A.; Batargias, C.; Papaioannou, C.; Kamilari, M.; Tragou, E.; et al. Status, distribution, and threats of the last surviving fan mussel populations in Greece. Mediterr. Mar. Sci. 2023, 24, 679–708. [Google Scholar] [CrossRef]
- Karadurmus, U.; Benli, T.; Sari, M. Discovering new living Pinna nobilis populations in the Sea of Marmara. Mar. Biol. 2024, 171, 90. [Google Scholar] [CrossRef]
- Zotou, M.; Gkrantounis, P.; Karadimou, E.; Tsirantinis, K.; Sini, M.; Poursanidis, D.; Azzolin, M.; Dailianis, T.; Kytinou, E.; Issaris, Y.; et al. Pinna nobilis in the Greek seas (NE Mediterranean): On the brink of extinction? Mediterr. Mar. Sci. 2020, 21, 575–591. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).