Abstract
Indian jujube displays genetic diversity and does not prominently display minute morphometric variations, and this makes correct identification a difficult and long-term task. However, little work has been conducted to bring jujube cultivars into domestication. So, the present study aimed to evaluate eleven cultivars of Indian jujube in terms of the fruit’s morphometric characteristics, as well as molecular marker studies by plastidial megakaryocyte-associated tyrosine kinase (matK) barcoding and inter-simple sequence repeats (ISSR) markers for species differentiation, identification, and relationships among Indian jujube cultivars. The results of the morphometric characteristics showed that the mean geometric diameter, surface area, sphericity, sphericity ratio, shape index, fruit length, fruit diameter, fruit weight, and seed weight varied among cultivars. The results also showed that the color values of L*, a*, and b* for fruits differed in different cultivars. In addition, the results showed a discrepancy in the genetic diversity parameters related to the matK barcoding, ISSR markers, and relationships among Indian jujube cultivars. Substantially, hierarchical clustering by heatmap revealed that ‘Zytoni’ and ‘Um-Sulaem’ with spines seem to be mono-clades distinct from other cultivars, which related to variations in the expression levels of genes. Therefore, they should be relied upon together to distinguish and identify cultivars in order to maximize the effectiveness of local germplasm conservation and exploitation.
1. Introduction
Ziziphus mauritiana Lamk. is known as Indian jujube, ber, or Chinese apple, which belongs to the Rhamnaceae family and is native to tropical and subtropical areas of South Asia [1,2,3]. Indian jujube has been dubbed the “King of Arid Fruits” due to its ability to grow in waste, marginal, unproductive, or inferior soil and other diversified soil with pH as high as 9.0 in arid and semi-arid regions characterized by extreme variations in diurnal annual temperatures and high evaporation, combined with sparse and highly variable precipitation [4,5,6]. Its cultivation is important because of its xerophytic qualities, such as early age of maturity, tolerance of hard conditions, resistance to drought and salinity stress, cheap cost of cultivation, high nutritional value, and affordable pricing [7,8,9,10].
Indian ber trees’ wider adaptability to adverse soil and climate conditions has allowed the build-up of a rich gene pool [6]. It is widely known that Ziziphus mauritiana makes a large contribution to household income and offers small farmers a great opportunity to increase their income and transition to a sustainable cropping system because they are a part of the natural world [11,12]. Farmers are therefore more interested in domesticated Indian cultivars that yield early, large, and flavorful fruits. On the other hand, not much has been done to domesticate jujube cultivars and it could be achieved through a combination of approaches including morphometric properties, matK barcoding, and ISSR markers diversity. Given that genetic and environmental interactions also play a key part in the expression of qualitative and quantitative features in an individual, it is essential to choose a cultivar that is specifically adapted to different agroclimatic zones in order to produce Indian jujube that is high in yield and of high quality. As a result, description is crucial for recording the effectiveness of the researched cultivars to introduce, pick, and enhance the numerous Indian jujube cultivars already in existence within such a diversity of germplasm.
Fruit morphometric attributes are beneficial for finding noticeable variations and separating fruits since they are objective and reproducible. Furthermore, fruit identification is impossible without knowledge of morphological descriptors and color characteristics. Different fruit morphometric properties, such as perpendicular axes, color, and texture features are frequently taken into account during Indian jujube classification and identification [8,13,14,15]. However, some Ziziphus plants almost do not prominently display minute morphological variations due to some adaptive and/or commercially related forces, linguistic issues in folk taxonomy, or inconsistency in ethnobotanical nomenclature [16]. We therefore urgently need quick, accurate, and on-hand identification. This has raised a need for the use of more advanced and efficient molecular tools for functional identification models, which require a framework for the incorporation of new data. Overall, this makes correct identification an easy and short-term task.
The advancement of molecular genetics has resulted in a new approach termed “DNA barcoding” that is based on the polymorphism of nucleotide sequences across species [17,18]. A small uniform fragment of the genome that functions as a “barcode” pattern has been sparkled as a technique for accurate species identification [19]. Furthermore, DNA polymorphism is frequently found in noncoding ITS2 regions and is useful for identifying species [20]. It is typically rare in coding plastid genes like rbcL and matK. Two chloroplast genes are commonly used for the analysis; the matK and rbcL are the best gene loci for analyzing relationships in plants [21]. The locus of rbcL can be recognized at the genus and family levels, while matK is the variable portion of the coding for species confirmation [22,23,24]. They have been proposed as the primary plant barcode regions by the committee Barcode of Life (CBOL) [25] consortium [26]. The matK gene appears to have a high evolution rate as compared to the other DNA sequences. It has a high mutation rate, and its sequence is substantially more diverse than that of most other genes [27]. As a result, the matK gene appears to evolve swiftly allowing us to distinguish between species. Among the most effective marker systems currently in use are inter-simple sequence repeats (ISSR) markers. Genomic DNA is amplified by PCR using a primer that is usually attached at the 5′ or 3′ end and contains 1–4 random, frequently degenerate bases that extend into the flanking regions between two microsatellites [28]. They are widely utilized because they use random markers and may be generated without exact sequence information [29,30]. Because of their variance in polymorphism, resolving power (Rp), and informativeness of the bands (Ib), ISSR primers are powerful molecular markers that can discriminate between genotypes [31,32]. If the genome contains a sufficient number of ISSR motifs, any species of plant can be used [33].
Until recently, there were no studies that compared the morphometric characteristics of the Indian jujube cultivars chosen under similar conditions to matK barcoding and ISSR markers. In order to improve the efficiency of screening, conservation, promotion, and exploitation of Indian jujube genetic resources, this paper investigates the variation in fruit-related parameters and ascertains whether any relationships exist between morphometric characteristics and/or matK barcoding and ISSR for eleven Indian jujube (Ziziphus mauritiana Lamk.) cultivars grown in Saudi Arabia: ‘Kashmiri’, ‘Komethry’, ‘Um-Sulaem without spines’, ‘Um-Sulaem with spines’, ‘Toffahy’, ‘Abdel-Sattar’, ‘Pu-Pineau’, ‘Pu-Yun’, ‘Buddling Ber’, ‘Seedy Ber’, and ‘Zaytoni’.
2. Materials and Methods
2.1. Experimental Site and Plant Materials
The present study was conducted in the 2021/2022 and 2022/2023 seasons on the mature trees of 11 Indian jujubes (Ziziphus mauritiana) cultivars. Those are considered the most important cultivars grown successfully in Saudi Arabia under the same environmental conditions. They are ‘Zaytoni’ (Z1), ‘Kashmiri’ (Z2), ‘Komethry’ (Z3), ‘Um-Sulaem with spines’ (Z4), ‘Toffahy’ (Z5), ‘Um-Sulaem without spines’ (Z6), ‘Abdel-Sattar’ (Z7), ‘Pu-Yun’ (Z8), ‘Pu-Pineau’ (Z9), ‘Seedy Ber’ (Z10), and ‘Buddling Ber’ (Z11) (Figure 1). These ten-year-old trees were grown in sandy loam at the King Saud University agricultural research farm in the Dirab district of Riyadh, Saudi Arabia (GPS coordinates: 24°24′43.0″ N latitude, 46°39′30.7″ E). Except for Seedy Ber, all of the trees were budded on ber rootstock and spaced 3.5–5 m apart. From each cultivar, three identical trees were chosen, and each selected cultivar was reproduced in this study (11 cultivars, 3 replicates, 1 tree per replicate = 33 trees). Additionally, all cultivars were subject to standard cultural practices, such as fertilization, pest and disease control, and watering with a drip irrigation system.

Figure 1.
Eleven Indian jujubes (Ziziphus mauritiana) cultivars.
2.2. Morphometric Characteristics
In March 2022 and 2023, fruits were randomly selected when fully grown according to commercial practice. The physical characteristics and geometrical features of the fruit were used to assess morphometric traits; Fruit length (L), polar diameter (D1), equatorial diameter, weight, volume, and stone weight were the physical characteristics recorded from the fruit. In addition, the L*, a*, and b* color values of fruit peels were also noted. The pulp–stone ratio, fruit density, sphericity percentage, geometric mean diameter, arithmetic mean diameter, and surface area were taken into account as geometrical characteristics.
For each tree, one hundred and fifty mature fruits were randomly selected from each replicate (tree), and a total of four hundred and fifty fruits of each cultivar were assessed to determine the morphometric attributes of the fresh fruit. A digital Vernier caliper (Mitutoyo, Japan) was used to measure the length (L), polar diameter (D1), and equatorial diameter (D2) of a single fruit along three perpendicular axes with an accuracy of 0.01 mm. An electronic weighing scale (Metler Toledo; lowest count 0.001 g) was applied to measure the weight (in grams) of the fruit, stone, and flesh. By measuring displacement in a water-filled cylindrical tube, fruit volume was calculated. The L*, a*, and b* values of the fruit peel color, according to Itle and Kabelka [34], were measured using a Minolta Chroma Metre Model CR-2000 (Osaka, Japan) at a single location in the equatorial region of each fruit; L* denotes lightness (0 to 100, representing black to white), and a*denotes +red, +green, +yellow, and −blue.
The fruit density (g/cm3) of the fruit was calculated using the following formula:
The sphericity index (φ, %) of the fruit was calculated using the following formula [35]:
The geometric mean diameter (Dg, mm) of the fruit was calculated using the following formula [35]:
The arithmetic diameter (Da, mm) was calculated using the formula used by Sunmonu et al. [36]:
The surface area (Sa, mm2) was calculated as follows [37]:
2.3. Molecular Marker Studies
100 mg of Ziziphus leaves were completely powdered in liquid nitrogen. DNA was extracted using the TaqMan® Sample-to-SNP™ Kit (Catalog number: 4403319, Thermo Fisher Scientific Inc., Waltham, MA, USA) according to the instructions provided by the manufacturer. The quality and quantity of DNA were checked using UV spectroscopy and by electrophoresis in 1% agarose gel.
For barcoding, matK PCR the reverse and forward primers were, respectively, matK- 472F (5′-CCCRTYCATCTGGAAATCTTGGTTC-3′) and matK- 1248R (5′-GCTRTRATAATGAGAAAGATTTCTGC-3′) [38]. Five ISSR primers (Table 1) were tested in this experiment to amplify the templated DNA according to Alansi et al. [39]. Amplification was carried out in a Perkin-Elmer/Gene Amp® PCR System 9700 (PE Applied Bios stems) configured to complete 40 cycles. Each cycle was composed of a denaturation step lasting 30 s at 94 °C, an annealing step lasting 30 s at 45 °C, and an elongation step lasting 30 s at 72 °C. In the last cycle, the primer extension phase was prolonged to 7 min at 72 °C. PCR amplification was confirmed on 1.5% gel electrophoresis. The manufacturer’s instructions were followed using a 1500 bp DNA ladder as a marker (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA). Nucleotide sequences were matched using the National Centre for Biotechnology Information (NCBI) after sequencing. Using the Basic Local Alignment Search Tool (BLAST) program, the similarity between the cultivars was determined.

Table 1.
Five ISSR primer sequences.
2.4. Statistical Analysis
The statistical design, according to Snedecor and Cochran [40], used a one-way analysis of variance (ANOVA) technique with the least significant difference (LSD) at a probability level of 0.05. According to Gomez and Gomez [41], the combined analysis was performed across two seasons after testing the homogeneity of error variance. The statistical analysis was carried out using the XLSTAT Statistical Package Software [42] (ver. 2019.1, Excel Add-ins soft SARL, New York, NY, USA).
A dendrogram illustrating the genetic relationships between Indian Jujube genotypes based on fruit prosperities was built using the unweighted pair group method with arithmetic average (UPGMA) of NTSYS-pc version 2.2 software [43]. Documentation systems like GrabCut and gray-level co-occurrence matrix (GLCM) were applied to record fruit data. After that, scaling was created using fruit parameters, and fruit scales were converted to matrix format (1 for presence and 0 for absence; Supplementary Table S1). Finally, the clusters’ tree design was conducted by binary data. Gel documentation system (Geldoc-it, UVP, Cambridge, UK), was applied for molecular data analysis using Totallab analysis software, https://totallab.com/ (accessed on 3 March 2024), (Ver.1.0.1) to construct a dendrogram for ISSR and matK data based on the genetic distance, UPGMA. From the data of morphology and molecular analyses, a heatmap was generated using R software through the web tool Clustvis [44] to study the genetic diversity and relationships among cultivars.
3. Results
3.1. Fruit Morphometric Attributes
‘Toffahy’ and ‘Komethry’ had significantly higher averages of fruit length (L) than the other cultivars, followed by ‘Kashmiri’, whereas ‘Um-Sulaem with spines’ had significantly the lowest one (Figure 2). It was also found that the rest of the cultivars came behind ‘Kashmiri’ in descending order, as follows: ‘Pu-Yun’, ‘Pu-Pineau’, ‘Abdel-Sattar’, ‘Zaytoni’, ‘Buddling Ber’, ‘Seedy Ber’, and ‘Um-Sulaem without spines’, as compared to the ‘Um-Sulaem with spines’ cultivar. Moreover, no significant differences were found between the fruit length (L) of ‘Pu-Yun’ and ‘Pu-Pineau’ regarding this trait. ‘Toffahy’ had a significantly higher average of fruit polar diameter (D1) and equatorial diameter (D2) than the other cultivars, followed by ‘Pu-Pineau’, whereas ‘Um-Sulaem with spines’ had the significantly lowest one. It was obvious that the rest of the cultivars could be arranged in the following descending order with respect to the polar diameter (D1) and equatorial diameter (D2), as follows: ‘Pu-Yun’ > ‘Abdel-Sattar’ > ‘Kashmiri’ > ‘Buddling Ber’ >’Komethry’ > ‘Seedy Ber’> ‘Zaytoni’ > ‘Um-Sulaem without spines’, and differences among them were statistically, significant, except between ‘Abdel-Sattar’ and ‘Kashmiri’ and also between ‘Komethry’, ‘Seedy Ber’, as well as ‘Zaytoni’ and ‘Um-Sulaem without spines’.
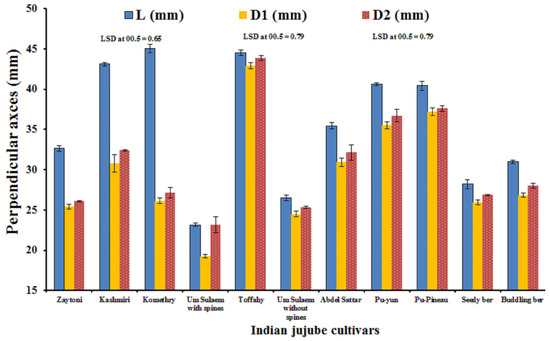
Figure 2.
Perpendicular axes values of eleven Indian jujube cultivars. Combined data represents the means 2022 and 2023 seasons.
‘Toffahy’ fruit followed by ‘Pu-Pineau’ was significantly heavier than the fruit of the other cultivars, followed by ‘Pu-Pineau’, whereas those of ‘Um-Sulaem without spines’ and ‘Seedy Ber’ showed significantly the lowest average fruit weight. Significant differences were found between the fruit weights of ‘Zaytoni’, ‘Kashmiri’, ‘Komethry’, ‘Um-Sulaem with spines’, ‘Abdel-Sattar’, ‘Pu-Yun’, and ‘Buddling Ber’ (Figure 3). ‘Toffahy’ fruit followed by ‘Pu-Pineau’ had significantly higher pulp weight than the other cultivars, whereas ‘Um-Sulaem with spines’ had a significantly lower one. The other cultivars could be arranged concerning pulp weight in the following descending order: ‘Pu-Yun’, ‘Kashmiri’, ‘Abdel-Sattar’, ‘Komethry’, ‘Buddling Ber’, ‘Zaytoni’, ‘Seedy Ber’, and ‘Um-Sulaem without spines’, and differences were statistically significant. ‘Um-Sulaem with spines’ and ‘Um-Sulaem without spines’, followed by ‘Pu-Pineau’ and ‘Kashmiri’, had significantly higher stone weights as compared with the other cultivars, whereas ‘Seedy Ber’ and ‘Zaytoni’, which did not significantly vary from those of ‘Buddling Ber’ and ‘Zaytoni’, showed the lowest values. On the contrary, no significant difference was found between the stone weights of ‘Abdel-Sattar’, ‘Pu-Yun’, and ‘Toffahy’, nor between ‘Komethry’, ‘Zaytoni’, and ‘Buddling Ber’.
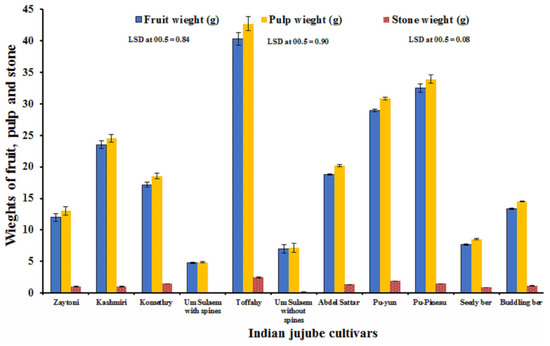
Figure 3.
Weight of fruit, stone, and pulp of 11 Indian jujube cultivars. Combined data represents the means of the 2022 and 2023 seasons.
Concerning fruit color, the results presented in Figure 4 show that ‘Kashmiri’, ‘Pu-Yun’, and ‘Buddling Ber’, followed by ‘Abdel-Sattar’, recorded a significantly lighter fruit color than the other cultivars, while a darker fruit color was obtained with ‘Toffahy’. No significant differences were noticed between the lighter fruit color of ‘Komethry’ and ‘Um-Sulaem with spines’, which did not significantly differ from ‘Um-Sulaem without spines’, ‘Zaytoni’ and ‘Seedy Ber’. ‘Um-Sulaem without spines’, followed by ‘Um-Sulaem with spines’ produced the most significantly redder fruits, but ‘Pu-Pineau’ recorded significantly less redness as compared with other cultivars. The differences were not significant between the a* values of ‘Toffahy’ and ‘Pu-Yun’ or between ‘Zaytoni’ and ‘Abdel-Sattar’. In addition, ‘Pu-Pineau’ followed by ‘Toffahy’ and ‘Pu-Yun’, recorded a significantly higher value (b*) than other cultivars, although the fruits from ‘Um-Sulaem’, both with and without spines, reported a noticeably lower value of (b*). No significant differences were found among the (b*) values of ‘Komethry’, ‘Abdel-Sattar’, and ‘Buddling Ber’.
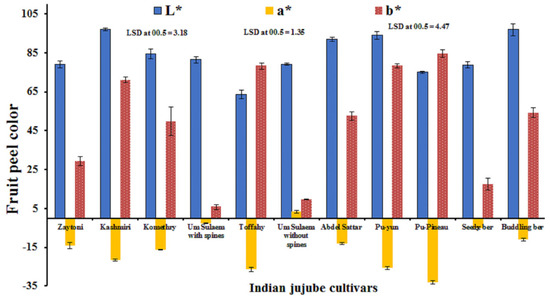
Figure 4.
The fruit peel color of 11 Indian jujube cultivars. Combined data represents the means of the 2022 and 2023 seasons.
The ‘Toffahy’ cultivar recorded the highest significant sphericity index of 98.34% as compared to the other cultivars, while ‘Komethry’ had the lowest values. ‘Buddling Ber’, ‘Um-Sulaem without spines’, and ‘Pu-Yun’ displayed no significant differences. ‘Um-Sulaem with spines’ had a significantly higher sphericity index compared to the other cultivars. ‘Abdel-Sattar’, ‘Pu-Yun’, and ‘Buddling Ber’ had a significantly higher sphericity index than the ‘Zaytoni’ and ‘Kashmiri’ cultivars (Table 2). Furthermore, ‘Toffahy’ had a higher fruit volume than the other cultivars, followed by ‘Pu-Pineau’, while ‘Um-Sulaem without spines’ had the lowest value. Moreover, no significant differences were found between the fruit volumes of ‘Buddling Ber’ and ‘Zaytoni’. Additionally, according to the data from both seasons, ‘Kashmiri’ and ‘Pu-Pineau’ had much higher pulp-to-stone ratios than the other cultivars, followed by ‘Um-Sulaem with and without spines’, whereas ‘Seedy Ber’ showed the lowest values. Furthermore, ‘Buddling Ber’ had a significantly higher fruit density as compared with that of the other cultivars, while ‘Seedy Ber’ and ‘Toffahy’ did not significantly vary from those of ‘Kashmiri’, ‘Zaytoni’, and ‘Pu-Yun’, which had the lowest fruit density values. No significant differences were observed between the fruit density of ‘Um-Sulaem with spines’, ‘Komethry’, ‘Um-Sulaem without spines’, ‘Pu-Pineau’, and ‘Abdel-Sattar’.

Table 2.
Fruit volume, pulp/stone ratio, fruit density, and sphericity index of 11 Indian jujube cultivars. Combined data represents the means of the 2022 and 2023 seasons.
Data in Figure 5 indicate that ‘Toffahy’ had the highest significance in the geometrical features [arithmetic diameter (Da), geometric diameter (Dg), surface area (Sa)] value compared with the other cultivars, followed by ‘Pu-Pineau’, while ‘Um-Sulaem with spines’ had the lowest value. It was also noticed that ‘Pu-Yun’ had a higher significance in the geometrical features than the rest of the cultivars, followed by ‘Kashmiri’. ‘Abdel-Sattar’ had significantly higher geometrical feature values than those of ‘Komethry’, ‘Buddling Ber’, ‘Seedy Ber’, ‘Zaytoni’, and ‘Um-Sulaem without spines’. Also, no significant difference was found between ‘Zaytoni’ and ‘Buddling Ber’ and between ‘Abdel-Sattar’ and ‘Komethry’ regarding arithmetic diameter. Likewise, no significant difference was found between ‘Zaytoni’ and ‘Buddling Ber’ regarding the surface area.
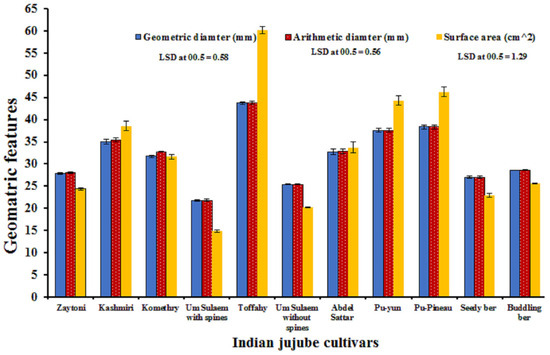
Figure 5.
Fruit geometrical attributes of 11 Indian jujube cultivars. Combined data represents the means 2022 and 2023 seasons.
3.2. Molecular Marker Studies
The DNA had an A260/280 ratio of 1.7, which is considered pure for DNA. The number of plant species that successfully produced a sequence for a given marker was used to assess the success of sequencing barcode markers. Plastidial matK amplicons were detected at nearly at nearly 896–1126 bp (Figure 6). This band was purified, sequenced, and aligned using an align sequence nucleotide BLAST (detailed sequencing result; Supplementary Figure S1). The percent identity matrix for all cultivars fluctuated near 99% and was identified as Ziziphus mauritiana.
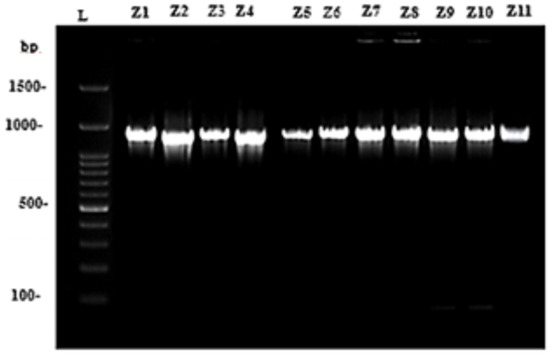
Figure 6.
Semi-quantitative expression of Plastidial matK amplicons for eleven Ziziphus mauritiana cultivars (Z1–Z11). L; ladder 1500 bp.
The successful five ISSR primers from the ten primers utilized in this study’s amplification yielded good, replicable, highly informative, and scorable patterns as fingerprints (Figure 7). All ISSR primers had a medium-to-high GC range of 44% to 50% and showed a clear banding profile. Herein, the five ISSR primers created 67 polymorphic bands out of 70 bands. The percentage of polymorphic bands ranged from 80% with ISSR5 to 100% with ISSR2, ISSR3, and ISSR4, with an overall average polymorphism percentage of 95%. The efficiency of the chosen five ISSR primers slightly differed and is shown in Table 3. The polymorphism information content (PIC) ranged from 0.424 for ISSR3 to 0.495 for ISSR5, with a mean moderate value of 0.47. ISSR1 and ISSR5 showed a higher value for the heterozygosity index (H), the arithmetic mean of H (H.av), the marker index (MI), and the discriminating power (D). Their values are H: 0.565, 0.576; H.av: 0.565, 0.576; MI: 0.565, 0.576; and D: 0.519, 0.586, respectively, with mean values ranging from 0.47 to 0.54.
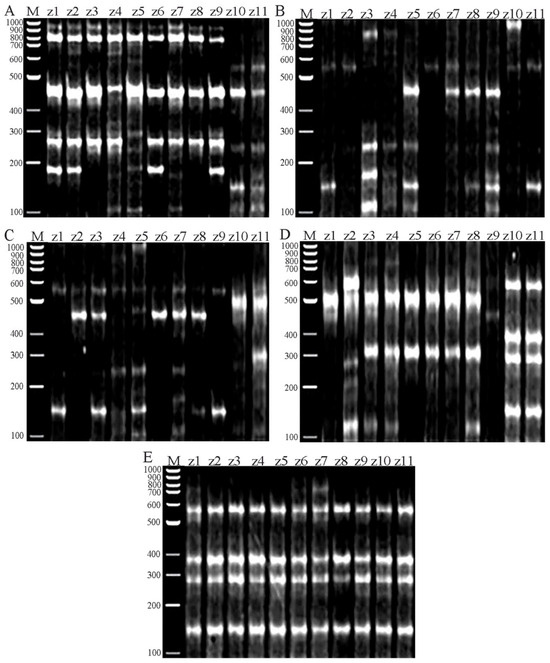
Figure 7.
Amplifications of the eleven Ziziphus cultivars using five ISSR primers: (A) ISSR1, (B) ISSR2, (C) ISSR3, (D) ISSR4, and (E) ISSR5.

Table 3.
Monomorphic bands, polymorphic bands, unique bands, percentage of polymorphism, and six marker indices of Ziziphus genotypes by five ISSR primers: 1 heterozygosity index, 2 polymorphic information content, 3 effective multiplex ratio, 4 arithmetic mean of H, 5 marker index, 6 discriminating power.
3.3. Relationships among Indian Jujube Cultivars
In order to assess the relatedness and genetic similarity among the assisted cultivars, the fruit morphometric characteristics were extracted to perform their clustering. At a 25% similarity level, the cultivars were divided into two groups (I and II, Figure 8). Group I separated seven cultivars at 50% into two clusters (A and B). Cluster (A) included ‘Komethry’ and ‘Kashmiri’ at 80%, while Cluster (B) collected ‘Toffahy’ and ‘Zytoni’ in one clade, and ‘Pu-Pineau’ and ‘Pu-Yun’ in another clade. The ‘Abdel-Sattar’ cultivar remained in a separate clade. Group II distinguished cultivar ‘Um-Sulaem without spines’ uniquely (cluster D) and separated the other cultivars (‘Um-Sulaem with spines’, ‘Buddling Ber’, and ‘Seedy Ber’) in another clade (cluster C).
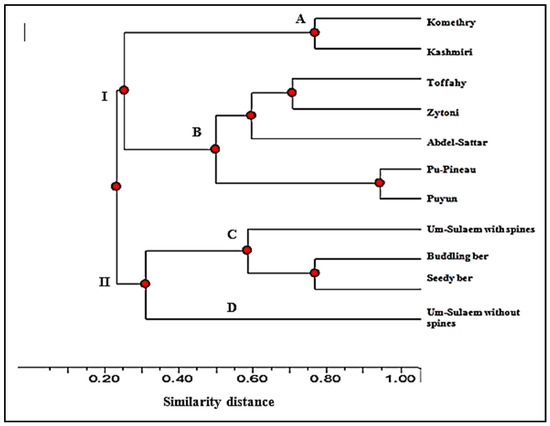
Figure 8.
Dendrogram for eleven Ziziphus cultivars based on fruit properties data.
Due to the high similarity between cultivars a high genetic similarity ranged from 57% to 99%, together, a dendrogram of matK and ISSR data was conducted using UPGMA and Euclidean Similarity index (Figure 9). All cultivars were divided into two main groups (I and II) at 55% similarity. The first group included ‘Um-Sulaem with spines’ and ‘Zaytoni’ in a separate clade. The second group subsequently divided into “A” and “B” clusters. Cluster “A” includes ‘Komethry’, ‘Abdel-Sattar’, ‘Buddling Ber’, ‘Toffahy’, and ‘Pu-Yun’. On the other hand, Group ‘B’ gathered the ‘Pu-Pineau’, ‘Seedy Ber’, and ‘Um-Sulaem without spines’ cultivars.
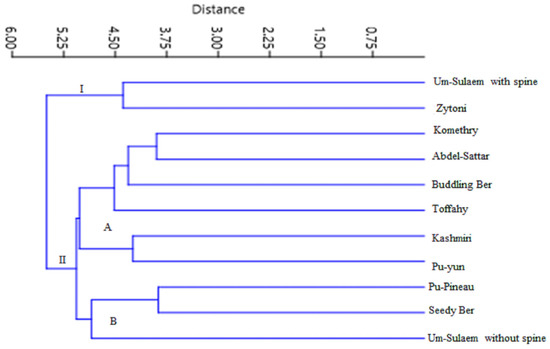
Figure 9.
Dendrogram for eleven Ziziphus cultivars based on ISSR and matK data. UPGMA and Euclidean Similarity index.
In order to complete the assessment of morphometric and genetic diversity between cultivars, hierarchical cluster analysis (HCA) and its associated heatmap were constructed (Figure 10). Columns correspond to the studied Ziziphus cultivars, whereas Rows correspond to different morphometric, matK, and ISSR markers. ‘Um-Sulaem with spines’ and ‘Zaytoni’ were grouped in the first group and the other cultivars in the second group. The intensity of colors visually reflects the high, intermediate, and low character values. Dark colors like red usually indicate higher data values for cultivars, such as ‘Um-Sulaem with spines’, ‘Zaytoni’, ‘Pu-Pineau’, ‘Abdel-Satter’, ‘Toffahy’, ‘Um-Sulaem without spines’, ‘Kashmiri’, ‘Pu-Yun’. Cooler colors like blue and brown represent medium and lower data values, which are mostly for the second group.
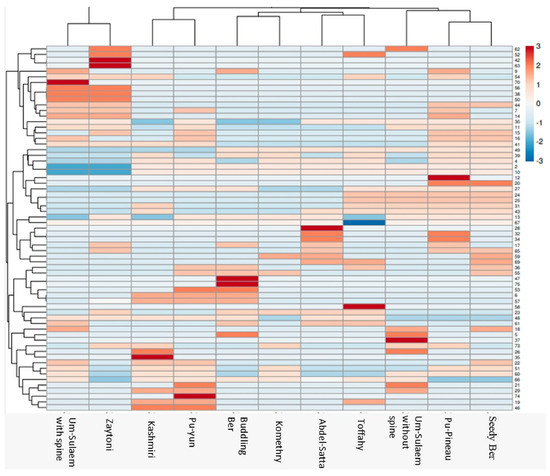
Figure 10.
Hierarchical clustering by heatmap of eleven Ziziphus cultivars with the bands (gene) regions for matK and ISSR analysis using Euclidean distance.
4. Discussion
In this work, we characterized an ample collection of 11 Indian jujube cultivars using different physical fruit features and geometric attributes. The study highlighted that the characteristics related to fruit morphometrics, such as fruit dimensions, shape, and fruit flesh color, are valuable traits for fast and easy discrimination of Indian jujube cultivars. Based on the results it is reported that significant variation was observed in morphometric characteristics of different ber genotypes, by which it can be concluded that the ber genotypes showed a wide genetic diversity with various quantitative morphological traits and geometric attributes in the existing population. These results accord with the earlier findings of Obeed et al. [45] and Abdel-Sattar et al. [8], who reported that the morphological and chemical characteristics of Indian jujube fruit vary greatly amongst the particular cultivars planted in Saudi Arabia. Morphometric features are important for cultivar characterization because they are technically simple, applicable in a variety of cultural contexts, and easily translatable into information that stakeholders can understand [16,46]. Different physical properties and geometric attributes, such as fruit length (L), fruit polar diameter (D1), fruit equatorial diameter, fruit weight stone weight, pulp weight, and fruit diameter, in Indian jujube cultivars showed substantial similarities and dissimilarities among different cultivars. Our findings also demonstrated the relationship between the particular cultivars and the geometric properties, including the surface area, geometric mean diameter, sphericity index, aspect ratio, length, and minor and major diameters, and these results are in agreement with Ghosh and Mathew [47], Obeed et al. [45], Razi et al. [48], and Abdel-Sattar et al. [8]. Furthermore, as jujube fruits can be classified based on their size and form, the geometric characteristics of the Indian jujube fruit cultivars are crucial in defining the quality of Indian jujube fruit. The L*a* and b* among the Indian jujube cultivars showed a definite shift. Changes in L*, a*, and b* are associated with an increase in carotenoid levels and a decrease in chlorophyll in the pericarp, as explained by Itle and Kabelka [34] and Cardenas-Perez et al. [49]. Interestingly, the phenotypes that mostly contribute to the discrimination of the analyzed Indian jujube cultivars were related to the color attributes of the fruits (color values of L*, a*, and b* of the peel of fruits). This indicates that these visual features are probably the main drivers of the variation of the cultivars at a regional scale [16]. Furthermore, genotype, innate traits, and climatic adaptability in a particular region may all contribute to the variation in cultivars’ fruit equatorial diameter, fruit polar diameter, fruit length, fruit weight stone weight, pulp weight, fruit volume, color values of L*, a*, and b* of the peel of fruits, as well as the pulp-stone ratio fruit density, surface area, sphericity percent, arithmetic mean diameter, and geometric mean diameter, which may serve as a crucial diagnostic trait for choosing the best cultivar for a given location [5,9,50,51]. The use of physical fruit characteristics and geometrical properties variables provided different and complementary information, sufficient to discriminate all the cultivars [45,47,48,52,53,54]. Therefore, our results were consistent with the past findings, as they mentioned significantly varied values for the fruit’s physical properties and geometric attributes among the Indian jujube cultivars. Morphometric characteristics allow a technically undemanding evaluation of the diversity and represent an easily adaptable classification approach, while molecular markers are an indispensable tool to assess genetic diversity, discriminate varieties, identify possible synonyms and homonyms, and genetically trace plant varieties in food chains [55].
Based on genetic makeup, one of the main goals of this work was the genetic diversity of Z. mauritiana and the identification of cultivars, which can be accomplished with the aid of ISSR markers, the matK gene, and morphometric characteristics. The matK primers used showed that the aligned matK sequence length was around 1000 bp, much longer, by 400 bp, than that used for Ziziphus by Alkaraki et al. [56] and shorter than earlier amplification by Yu et al. [38] in dicots (1515–1580 bp). Our findings for employing Plastidial matK gene sequences as a molecular barcoding tool for Ziziphus cultivars, which indicated increasing genetic similarity among Ziziphus mauritiana cultivars, were in agreement with those of Moudi et al. [57]. The latter applied two chloroplast genes (rbcL and matK) for barcoding 25 Jujube (Ziziphus jujuba Mill.) ecotypes from six different provinces in Iran and indicated that there is no significant genetic diversity among jujube ecotypes and morphological differences are caused by environmental factors.
To assess the genetic diversity between cultivars as the matK gene analysis showed great similarity, ten ISSR primers were used to produce DNA fingerprint profiles. ISSR primers can be used to target microsatellites found throughout the plant genome. Therefore, the markers have been found to be more repeatable than others, such as RAPD [58]. Five primers amplified 70 loci across 11 cultivars. The remaining primers were considered unsuitable due to poor amplification. Out of these 70 loci, 67 loci were polymorphic, reflecting rich allelic diversity between cultivars, with 94.88% average polymorphism. The size of the amplified bands ranged between 100 and 1042 bp. This highly detected polymorphism is in agreement with the findings of Singh et al. [59], who studied the genetic diversity of Z. mauritiana using ISSR markers, and Sareen et al. [60] using SSR markers. A marker’s capability to identify polymorphisms among members of a population is measured by its polymorphism information content (PIC), and the higher this capacity, the higher the marker’s value. In genetic investigations, it is one of the markers of marker quality indicators. According to Botstein et al. [61], markers with PIC values greater than 0.5 are considered to be very informative, values between 0.25 and 0.50 are somewhat informative, and values lower than 0.25 are not very informative. In the present study, the average value of PIC is 0.47 which indicates that ISSR markers are very informative and can be used for gene mapping, molecular breeding, and germplasm evaluation based on their PIC value [62].
According to Khodaee et al. [63], MI and D were the most relevant indices for measuring marker efficiency and in this study, the average of both indices is 0.5. These findings verified the utility of these ISSR markers for detecting genetic diversity between cultivars. In addition, ISSR primers yielded many distinct unique fragments in different cultivars; for instance, primer ISSR3 yielded three distinct unique bands in ‘Kashmiri’, ‘Um-Sulaem without spines’, and ‘Zaytoni’; ISSR4 displayed two bands for ‘Buddling Ber’ and ‘Toffahy’; and ISSR1, ISSR2, and ISSR5 showed only one band for ‘Abdel-Sattar’, ‘Zaytoni’, and ‘Pu-Pineau’, respectively. The analysis of the intra-cultivar genetic structure confirmed by the UPGMA results, that ‘Um-Sulaem with spines’ and ‘Zaytoni’ had a specific genetic makeup compared to other cultivars. Overall, the obtained results revealed that ISSR markers are suitable for use in genome analysis and genetic diversity studies as mentioned by Alansi et al. [39] for Z. spina-christi. The incompatibility in the dendrogram analysis between the two markers (matK and ISSR) is expected because each marker targets diverse genome sequences, the same as mentioned by other authors who used ISSR and SCoT markers and between RAPD and ISSR [63]. Matching the clustering of the fruit morphometric and the combined molecular markers trees revealed a coincidence in clustering ‘Um-Sulaem without spines’ with ‘Seedy Ber’ in the same cluster and ‘Komethry’, ‘Kashmiri’, ‘Abdel-Sattar’, ‘Toffahy’, and ‘Pu-Yun’ in another cluster, while the other four cultivars differ in clustering in both trees. Plants that present similarities in terms of agro-morphological characteristics may have significantly different molecular characteristics, and vice versa [64].
Plastidial matK gene sequences and ISSR markers that incorporate the fruit morphology yielded were applied to construct a promising heat map. The current study showed promising results and grouping owing to each marker’s ability to recreate distinct sections of the genome. Consequently, ‘Um-Sulaem with spine’ and ‘Zytoni’ appear to be mono-clade from other cultivars. Generally, differences were detected among constructed dendrogram topologies according to matK gene sequences, ISSR markers, and fruit properties for the investigated cultivars. Varied genetic dissimilarity among eleven Ziziphus mauritiana cultivars, based on molecular marker studies and fruit properties, could be explained in the light of different expression levels for fruit properties controlling genes as a response to biotic and abiotic stresses. As mentioned by Song et al. [65], different abiotic stresses may affect jujube’s primary and secondary metabolisms in different ways, thus altering the quality. This is in agreement with our hypothesis for integrating both fruit properties and plastidial matK gene sequence data and ISSR markers to obtain a better understanding of genetic relationships among cultivars.
Despite the fact that morphological and genetic divergences already exist in plants, they are not related; Cabrera-Toledo et al. [66] reported that seeds are protected from the effects of various conditions on plant growth since they develop in the same environment. As a result, genetics dominates the control of variation [67]. The outcomes of the morphometric cluster analysis and the molecular marker cluster analysis in this investigation differed significantly. Numerous genes that regulate the quantitative features of plants may be the cause of these differences. Phenotypic investigations and molecular marker studies do not necessarily correlate, according to research by Song et al. [68] and Nizar and Mulani [69]. The greater discriminatory capacity of molecular markers compared to morphological markers may be explained by their genetic determinism. Despite the fact that the number of genes involved in the morphometric characters is not known, some of them are qualitative and could therefore be under one or more gene controls. However, an exact assessment of the number of genes involved in the different marker systems is not known; certainly, their expression of them differs [70]. The poor match of morphometric traits with molecular data is realistic, since the number of markers used in this study is not so high compared with the total genome, only a small part of the genome has been investigated. Therefore, the possibility that these particular markers did not cover the coding genes of morphometric traits is also very high, resulting in the poor match between molecular data and morphometric traits [71].
5. Conclusions
Results revealed that eleven Indian jujube cultivars were found to be widely different in their fruit physical properties and geometric attributes. In addition, the study on matK barcoding and ISSR markers of Indian jujube (Z. mauritiana) showed that there are variations among the germplasms. The heterogeneity of the gathered genotypes may be used to generate a superior cultivar of Indian jujube. This allows for the selection of germplasm based on desirable characteristics to achieve the highest fruit yield and quality. Also, differences in the characteristics of Indian Jujube fruit assist customers in selecting the appropriate fruits for various uses, improving the post-harvest period, and preserving fruit quality to boost marketability and achieve the highest fruit yield. Comprehensive analyses using the combination of morphometric characteristics, ISSR markers, and matK barcode analysis allowed the differentiation of Indian jujube according to cultivar and production region. Consequently, these data generated precise genetic information to complement the passport data of this cultivar germplasm. Therefore, in order to preserve the genetic heritage, we should promote and conserve local and traditional Indian jujube cultivars that are preferred by consumers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16060313/s1, Figure S1: amplification of matK and its alignment; Table S1: presence and absence of matK and ISSR bands.
Author Contributions
Conceptualization, M.A.-S., H.Z.R. and G.E.E.-B.; data curation, M.A.-S. and R.S.A.-O.; formal analysis, M.A.-S., R.S.A.-O. and G.E.E.-B.; methodology, M.A.-S., R.S.A.-O. and G.E.E.-B.; investigation, M.A.-S. and G.E.E.-B.; resources, M.A.-S., R.S.A.-O., H.Z.R. and G.E.E.-B.; software, M.A.-S., R.S.A.-O. and G.E.E.-B.; validation, M.A.-S., H.Z.R. and R.S.A.-O.; visualization, M.A.-S. and H.Z.R.; writing—original draft preparation, M.A.-S., R.S.A.-O., H.Z.R. and G.E.E.-B.; writing—review and editing, M.A.-S., R.S.A.-O., H.Z.R. and G.E.E.-B.; supervision, M.A.-S.; funding acquisition, M.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Researchers Supporting Project (number: RSPD2024R707), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary File.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project (number: RSPD2024R707), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, S.K.; Midha, A.; Kumar, U.; Singh, S.K. Phytochemical study of aerial part of different species of Ziziphus genus. Asian J. Pharm. Res. Dev. 2017, 5, 1–5. [Google Scholar]
- Naveed, F.; Nawaz, A.; Ali, S.; Ejaz, S. Xanthan gum coating delays ripening and softening of jujube fruit by reducing oxidative stress and suppressing cell wall polysaccharides disassembly. Postharvest Biol. Technol. 2024, 209, 112689. [Google Scholar] [CrossRef]
- Meighani, H.; Sadat-Hosseini, M. Assessment of Quality and Biochemical Changes in Indian Jujube Fruits (Ziziphus mauritiana Lamk.): Effect of Chitosan Coating and Putrescine Treatments. Int. J. Hort. Sci. Technol. 2024, 11, 481–490. [Google Scholar]
- Pareek, O.P. Fruits for the Future 2: Ber; International Centre for Underutilized Crops: Southampton, UK, 2001; 290p. [Google Scholar]
- Kumari, S.; Bhat, D.J.; Wali, V.K.; Bakshi, P.; Jasrotia, A. Physico-chemical studies of different ber (Zizyphus mauritiana Lamk.) germplasm under rainfed conditions of Jammu. The Bioscan 2015, 10, 1427–1430. [Google Scholar]
- Kumar, P.; Tripathi, V.K. Correlation Studies in Ber (Ziziphus mauriatiana Lamk.) in Eastern Region of Uttar Pradesh, India. Int. J. Plant Soil Sci. 2024, 36, 135–141. [Google Scholar] [CrossRef]
- Anjum, M.A.; Rauf, A.; Bashi, M.A.; Ahmad, R. The evaluation of biodiversity in some indigenous Indian jujube (Zizyphus mauritiana) germplasm the physico–chemical analysis. Acta Sci. Pol. Hortorum Cultus 2018, 17, 39–52. [Google Scholar] [CrossRef]
- Abdel-Sattar, M.; Almutairi, K.F.; Al-Saif, A.M.; Ahmed, K.A. Fruit properties during the harvest period of eleven Indian jujube (Ziziphus mauritiana Lamk.) cultivars. Saudi J. Biol. Sci. 2021, 28, 3424–3432. [Google Scholar] [CrossRef] [PubMed]
- Al-Saif, A.M.; Abdel-Sattar, M.; Aboukarima, A.M.; Eshra, D.H. Identification of Indian jujube cultivars cultivated in Saudi Arabia using an artificial neural network. Saudi J. Biol. Sci. 2021, 28, 5765–5772. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Tripathi, V.K. Phenotypic and Quantative Characterization of Ber (Ziziphus mauritiana Lamk.) Germplasm under Eastern Region of Uttar Pradesh, India. Int. J. Environ. Clim. Chang. 2024, 14, 264–272. [Google Scholar] [CrossRef]
- Bâ, M.; Danthu, P.; Duponnois, R.; Soleviev, P. Domestication of jujube fruit trees (Zizyphus mauritiana Lam.). In Crop Management and Postharvest Handling of Horticultural Crops; Science Publishers, Inc.: Enfield, NH, USA, 2003; Volume 3, Chapter 9; pp. 255–279. [Google Scholar]
- Owolarafe, T.A.; Salawu, K.; Ihegboro, G.O.; Ononamadu, C.J.; Alhassan, A.J.; Wudil, A.M. Investigation of cytotoxicity potential of different extracts of Ziziphus mauritiana (Lam.) leaf Allium cepa model. Toxicol. Rep. 2020, 7, 816–821. [Google Scholar] [CrossRef]
- Muhammad, G. Date fruits classification using texture descriptors and shape-size features. Eng. Appl. Artif. Intel. 2015, 37, 361–367. [Google Scholar] [CrossRef]
- Bahri, M.H.; Rashid, M.; Farahmandfar, R. Kiwi fruit shape classification based on geometrical attributes analysis. Agric. Eng. Res. J. 2017, 7, 1–5. [Google Scholar] [CrossRef]
- Çetin, N.; Yaman, M.; Karaman, K.; Bünyamin, D. Determination of some physicomechanical and biochemical parameters of hazelnut (Corylus avellana L.) cultivars. Turki. J. Agric. For. 2020, 44, 1–12. [Google Scholar] [CrossRef]
- Basile, B.; Mataffo, A.; Forlani, M.; Corrado, G. Diversity in Morphometric, Pomological, and Fruit-Quality Traits of Apricot (Prunus armeniaca) Traditional Varieties: Implications for Landrace Differentiation at Regional Scale. Diversity 2022, 14, 608. [Google Scholar] [CrossRef]
- Barthet, M. Expression and Function of the Chloroplast-Encoded Gene Matk. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2006. [Google Scholar]
- Ali, A.N.A.; Al-Sokari, S.S.; Gushash, A.; Anwar, S.; Al-Karani, K.; Al-Khulaidi, A. Ethnopharmacological survey of medicinal plants in Albaha region, Saudi Arabia. Pharmacogn. Res. 2017, 9, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Chester, K.; Zahiruddin, S.; Ahmad, A.; Khan, W.; Paliwa, S.; Ahmad, S. Bioautography-based identification of antioxidant metabolites of Solanum nigrum L. and exploration its hepatoprotective potential against D-galactosamine-induced hepatic fibrosis in rats. Pharmacogn. Mag. 2017, 13, 179–188. [Google Scholar] [CrossRef]
- Parmentier, I.; Duminil, J.; Kuzmina, M.; Philippe, M.; Thomas, D.W.; Kenfack, D.; Chuyong, G.B.; Cruaud, C.; Hardy, O.J. How Effective Are DNA Barcodes in the Identification of African Rainforest Trees? PLoS ONE 2013, 8, e54921. [Google Scholar] [CrossRef]
- Parson, W.; Pegoraro, K.; Niederstätter, H.; Föger, M.; Steinlechner, M. Species identification by means of the cytochrome b gene. Int. J. Legal Med. 2000, 114, 23–28. [Google Scholar] [CrossRef]
- Hollingsworth, P.M.; Graham, S.W.; Little, D.P. Choosing and using a plant DNA barcode. PLoS ONE 2011, 6, e19254. [Google Scholar] [CrossRef]
- Coissac, E.; Hollingsworth, P.M.; Lavergne, S.; Taberlet, P. From barcodes to genomes: Extending the concept of DNA barcoding. Mol. Ecol. 2016, 25, 1423–1428. [Google Scholar] [CrossRef]
- Alasmari, A. DNA-Barcoding of Some Medicinal Plant Species in Saudi Arabia Using rbcL and matK Genes. Phyton 2020, 89, 1059–1081. [Google Scholar] [CrossRef]
- CBOL Plant Working Group; Hollingsworth, P.M.; Forrest, L.L.; Spouge, J.L.; Hajibabaei, M.; Ratnasingham, S.; van der Bank, M.; Chase, M.W.; Cowan, R.S.; Erickson, D.L.; et al. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA. 2009, 106, 12794–12797. [Google Scholar] [CrossRef] [PubMed]
- Cutarelli, G.G.; Capuano, F.C.F. Species identification by means of mitochondrial cytochrome b DNA sequencing in processed anchovy, sardine and tuna products. Food Nutr. Sci. 2018, 9, 369–375. [Google Scholar] [CrossRef][Green Version]
- Lucas, C.; Thangaradjou, T.; Papenbrock, J. Development of a DNA barcoding system for seagrasses: Successful but not simple. PLoS ONE 2012, 7, e29987. [Google Scholar] [CrossRef] [PubMed]
- Zietkiewicz, E.; Rafalski, A.; Labuda, D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 1994, 20, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Ramzan, F.; Ramzan, Y.; Zulfiqar, F.; Ahsan, M.; Lim, K.B. Molecular Markers Improve Abiotic Stress Tolerance in Crops: A Review. Plants 2020, 9, 1374. [Google Scholar] [CrossRef]
- Shaban, A.S.; Arab, S.A.; Basuoni, M.M.; Abozahra, M.S.; Abdelkawy, A.M.; Mohamed, M.M. SCoT, ISSR, and SDS-PAGE investigation of genetic diversity in several Egyptian wheat genotypes under normal and drought conditions. Int. J. Agron. 2022, 14, 7024028. [Google Scholar] [CrossRef]
- Vaishnav, K.; Tiwari, V.; Durgapal, A.; Meena, B.; Rana, T.S. Estimation of genetic diversity and population genetic structure in Gymnema sylvestre (Retz.) R. Br. ex Schult. populations using DAMD and ISSR markers. J. Genet. Eng. Biotechnol. 2023, 21, 42. [Google Scholar] [CrossRef]
- Wen, S.; Zhao, H.; Zhang, M.; Qiao, G.; Shen, X. IRAPs in Combination with Highly Informative ISSRs Confer Effective Potentials for Genetic Diversity and Fidelity Assessment in Rhododendron. Int. J. Mol. Sci. 2023, 24, 6902. [Google Scholar] [CrossRef] [PubMed]
- Khuntia, S.; Kar, B.; Sahoo, S. Molecular Marker Based Genetic Diversity Study of Curcuma Caesia Roxb.—A Mini Review. J. Microbiol. Biotechnol. 2023, 8, 2. [Google Scholar] [CrossRef]
- Itle, R.A.; Kabelka, E.A. Correlation between L*a*b* color space values and carotenoid content in pumpkins and squash (Cucurbita spp.). HortScience 2009, 44, 633–637. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C. Physicochemical characterization and mass modelling of Sohiong (Prunus nepalensis L.) fruit. J. Food Meas. Charact. 2018, 12, 923–936. [Google Scholar] [CrossRef]
- Sunmonu, M.O.; Iyanda, M.O.; Odewole, M.M.; Moshood, A.N. Determination of some mechanical properties of almond seed related to design of food processing machines. Niger. J. Technol. Dev. 2015, 12, 22–26. [Google Scholar] [CrossRef]
- Altuntas, E.; Saracoglu, O.; Polatci, H. Physico-mechanical, colour and chemical properties of selected cherry laurel genotypes of Turkey. Adv. Agric. Sci. 2018, 6, 61–76. [Google Scholar]
- Yu, J.; Xue, J.H.; Zhou, S.L. New universal matK primers for DNA barcoding angiosperms. J. Syst. Evol. 2011, 49, 176–181. [Google Scholar] [CrossRef]
- Alansi, S.; Tarroum, M.; Al-Qurainy, F.; Khan, S.; Nadeem, M. Use of ISSR markers to assess the genetic diversity in wild medicinal Ziziphus spina-christi (L.) Willd. collected from different regions of Saudi Arabia. Biotechnol. Biotechnol. Equip. 2016, 30, 942–947. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; The Iowa State University Press: Ames, IA, USA, 1990; p. 593. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- XLSTAT Statistical Package Software. The XLSTAT System for Windows; Version 2019.1; Excel Add-Ins Soft SARL: New York, NY, USA, 2019. [Google Scholar]
- Rohlf, F.J. NTSYS-pc: Numerical Taxonomy and Multivariate Analysis System; Exeter Publishing: Exeter, UK, 1988. [Google Scholar]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Obeed, R.S.; Harhash, M.M.; Abdel-Mawgood, A.L. Fruit properties and genetic diversity of five ber (Ziziphus mauritiana Lamk.) cultivars. Pak. J. Biol. Sci. 2008, 11, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, F.; Bellincontro, A.; Forniti, R.; Vizovitis, K.; Botondi, R.; Valentini, M.; Sequi, P.; DiNatale, C.; Basile, B.; Romano, R. Factors affecting the apricot quality for the consumer with special attention to the use of 1-MCP and of NDT for detection of bruising. Acta Hortcult. 2006, 717, 315–320. [Google Scholar] [CrossRef]
- Ghosh, S.N.; Mathew, B. Performance of nine ber cultivars (Zizyphus mauritiana Lamk.) on top working in semi-arid region of West Bengal. J. Appl. Hortic. 2002, 4, 49–51. [Google Scholar] [CrossRef]
- Razi, M.F.; Anwar, R.; Basra, S.M.A.; Khan, M.M.; Khan, I.A. Morphological characterization of leaves and fruit jujube (Ziziphus mauritiana Lamk.) Germplasm in Faisalabad, Pakistan. Pak. J. Agric. Sci. 2013, 50, 211–216. [Google Scholar]
- Cardenas-Perez, S.; Chanona-Perez, J.; Mendez-Mendez, J.V.; Calderon-Dominguez, G.; Lopez-Santiago, R.; Perea-Flores, M.J.; Arzate-Vazquez, I. Evaluation of the ripening stages of apple (Golden Delicious) by means of computer vision system. Biosyst. Eng. 2017, 159, 46–58. [Google Scholar] [CrossRef]
- Mahajan, B.V.C.; Dhillon, S.B. Evaluation of different cultivars of litchi (Litchi chinensis Sonn.) under sub-mountainous regions of Punjab. Haryana J. Hort. Sci. 2000, 29, 184. [Google Scholar]
- Kumar, H.; Katiyar, P.N.; Singh, A.K.; Rajkumar, B.V. Effect of different pruning severity on physico-chemical properties of ber (Zizyphus mauritiana Lamk.) cv. Banarasi Karaka. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 935–940. [Google Scholar]
- Bhargava, R.; Shukla, A.K.; Chauhan, N.; Vashishtha, B.B.; Dhandar, D.G. Impact of hybridity on flavonoid spectrum of ber (Ziziphus mauritiana Lamk.). Environ. Exp. Bot. 2005, 53, 135–138. [Google Scholar] [CrossRef]
- Shukla, A.K.; Awasthi, O.P.; Shukla, A.K.; Vashishtha, B.B.; Bhargava, R. Evaluation of ber (Ziziphus mauritiana L.) cultivars under hot arid ecosystem of Rajasthan. Progress. Hortic. 2007, 39, 22–27. [Google Scholar]
- Singh, A.; Singh, R.K.; Kumar, A.; Ashwani, K.; Kumar, R.; Kumar, N.; Sheoran, P.; Yadav, R.K.; Sharma, D.K. Adaptation to social-ecological stressors: A case study with Indian jujube (Ziziphus mauritiana Lam.) growers of north-western India. Environ. Dev. Sustain. 2021, 23, 3265–3288. [Google Scholar] [CrossRef]
- Corrado, G.; Forlani, M.; Rao, R.; Basile, B. Diversity and Relationships among Neglected Apricot (Prunus armeniaca L.) Landraces Using Morphological Traits and SSR Markers: Implications for Agro-Biodiversity Conservation. Plants 2021, 10, 1341. [Google Scholar] [CrossRef]
- Alkaraki, A.K.; Aldmoor, M.A.; Lahham, J.N.; Nusair, S.D. DNA Barcoding of Selected Medicinal Plant Species from Jordan Using matK, rbcL, and rpoC1 Genes. Int. J. Biol. Biomed. Eng. 2021, 15, 376–411. [Google Scholar] [CrossRef]
- Moudi, M.; Kouhi, S.M.M.; Mood, S.G.; Ayoobi, A. DNA barcoding of Ziziphus jujuba Mill. in Iran using chloroplast genes (rbcL and matK). J. Plant Prod. Resch. 2021, 28, 115–130. [Google Scholar] [CrossRef]
- El-Haggar, M.A.; Mahgoub, Y.A.; Aly, H.M.; Ghazy, N.M.; El-Fiky, F.K.; El-Hawiet, A.M. DNA barcodes, ISSR, RAPD and SCAR markers as potential quality control tools for molecular authentication of black and white mulberry. Food Control 2023, 152, 109821. [Google Scholar] [CrossRef]
- Singh, A.K.; Devanshi; Singh, R.; Singh, B.; Koundal, K.R.; Singh, N.K. Assessment of genetic diversity in Ziziphus mauritiana using inter-simple sequence repeat markers. J. Plant Biochem. Biotechnol. 2007, 16, 35–40. [Google Scholar] [CrossRef]
- Sareen, A.; Sharma, V.; Gupta, R.C. Assessment of genetic diversity and population structure in wild Ziziphus species from northwest India using SSR marker technique. J. Genet. Eng. Biotechnol. 2023, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Botstein, D.; White, R.L.; Skalnick, M.H.; Davies, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphism. Am. J. Hum. Gen. 1980, 32, 314–331. [Google Scholar]
- Khodaee, L.; Azizinezhad, R.; Etminan, A.R.; Khosroshahi, M. Assessment of genetic diversity among Iranian aegilops triuncialis accessions using ISSR, SCoT, and CBDP markers. J. Genet. Eng. Biotech. 2021, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Lateif, K.S.; Hewedy, O. Genetic diversity among Egyptian wheat cultivars using SCoT and ISSR markers. J. Breed. Genet. 2018, 50, 36–45. [Google Scholar]
- Cornea-Cipcigan, M.; Pamfil, D.; Sisea; Rodica Margaoan, C.R. Characterization of Cyclamen genotypes using morphological descriptors and DNA molecular markers in a multivariate analysis. Front. Plant Sci. 2023, 14, 1100099. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Meinhardt, L.W.; Bailley, B.; Zhan, D. Genetic improvement of Chinese gugube for disease resistances: Status, knowledge gaps and research needs. Crop Breed. Genet. Genom. 2019, 1, e190015. [Google Scholar] [CrossRef]
- Cabrera-Toledo, D.; Vargas-Ponce, O.; Ascencio-Ramírez, S.; Valadez-Sandoval, L.M.; Pérez-Alquicira, J.; Morales-Saavedra, J.; Huerta-Galván, O.F. Morphological and Genetic Variation in Monocultures, Forestry Systems and Wild Populations of Agave maximiliana of Western Mexico: Implications for Its Conservation. Front. Plant Sci. 2020, 11, 817. [Google Scholar] [CrossRef]
- Qiao, Q.; Ye, M.; Wu, C.; Wang, J.; Liu, Q.; Tao, J.; Zhang, L.; Feng, Z. Analysis of leaf morphology variation and genetic diversity via SRAP markers for near-threatened plant Acer truncatum. Glob. Ecol. Conserv. 2022, 33, e01980. [Google Scholar] [CrossRef]
- Song, Y.; Fan, L.; Chen, H.; Zhang, M.; Ma, Q.; Zhang, S.; Wu, J. Identifying genetic diversity and a preliminary core collection of Pyrus pyrifolia cultivars by a genome-wide set of SSR markers. Sci. Hortic. 2014, 167, 5–16. [Google Scholar] [CrossRef]
- Nizar, M.; Mulani, R. Genetic diversity in indigenous and exotic linseed germplasm (Linum usitatissimum L.). Elect. J. Plant Breed 2015, 6, 848–854. [Google Scholar]
- Pissard, A.; Arbizu, C.; Ghislain, M.; Faux, A.; Paulet, S.; Bertin, B. Congruence between morphological and molecular markers inferred from the analysis of the intra-morphotype genetic diversity and the spatial structure of Oxalis tuberosa Mol. Genetica 2007, 132, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Normohamadi, S.; Solouki, M.; Heidari, F. Diversity in Cucumber Genotypes Based on Morphological Traits and SSR Molecular Markers. Biosci. Biotechnol. Res. Asia 2017, 14, 775–782. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).