The Role of Experience in the Visual and Non-Visual Prey Recognition of Fire Salamander Populations from Caves and Streams
Abstract
1. Introduction
2. Materials and Methods
2.1. Larvae Collection and Rearing
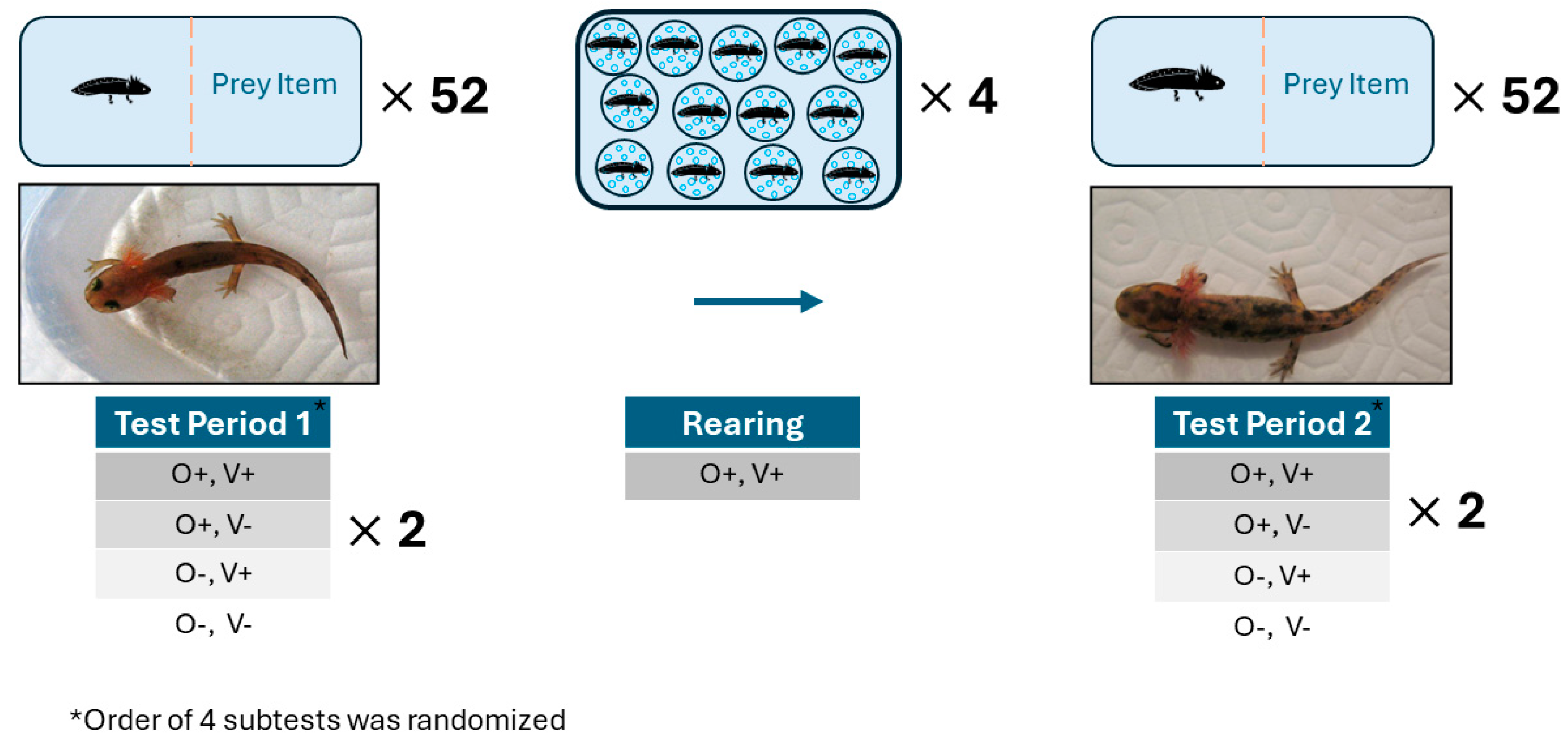
2.2. Behavioural Tests
- (a)
- Concurrent occurrence of chemical and visual stimuli (O+V+): A defrosted Chironomus sp. larva (red) was placed into the water in the area of prey introduction using shaking tweezers. Tweezer shaking continued throughout the trial. The defrosted larva released chemical cues into the water, and shaking gave visual cues.
- (b)
- Chemical stimulus only (O+V−): after dividing the arena from the area of prey introduction, a defrosted Chironomus sp. larva was quickly introduced by gravity.
- (c)
- Visual stimulus only (O−V+): A decoy (not coloured) constituting an odourless plastic string was placed into the area of prey introduction using shaking tweezers. Tweezer shaking continued throughout the trial.
- (d)
- Test control conditions, with the absence of both visual and chemical stimuli (O−V−): after dividing the arena from the area of prey introduction, a decoy constituting an odourless plastic string was placed by gravity.
2.3. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carey, M.P.; Wahl, D.H. Interactions of multiple predators with different foraging modes in an aquatic food web. Oecologia 2010, 162, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Barraquand, F.; Inchausti, P.; Bretagnolle, V. Cognitive abilities of a central place forager interact with prey spatial aggregation in their effect on intake rate. Anim. Behav. 2009, 78, 505–514. [Google Scholar] [CrossRef]
- Hosie, C.A.; Smith, T.E. Behavioral Biology of Amphibians. In Behavioral Biology of Laboratory Animals; Coleman, K., Schapiro, S.J., Eds.; Taylor & Francis: New York, NY, USA, 2021; pp. 36–61. [Google Scholar]
- Burghardt, G.M. Environmental enrichment and cognitive complexity in reptiles and amphibians: Concepts, review, and implications for captive populations. Appl. Anim. Behav. Sci. 2013, 147, 286–298. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Manenti, R.; De Bernardi, F.; Padoa-Schioppa, E. Can patterns of spatial autocorrelation reveal population processes? An analysis with the fire salamander. Ecography 2012, 35, 693–703. [Google Scholar] [CrossRef]
- Vignoli, L.; Caldera, F.; Bologna, M.A. Spatial niche of the Italian cave salamander, Speleomantes italicus (Dunn, 1923) (Plethodontidae, Amphibia), in a subterranean system of Central Italy. Ital. J. Zool. 2008, 75, 59–65. [Google Scholar] [CrossRef]
- Walker, D.M.; Murray, C.M.; Talbert, D.; Tinker, P.; Graham, S.P.; Crowther, T.W. A salamander’s top down effect on fungal communities in a detritivore ecosystem. FEMS Microbiol. Ecol. 2018, 94, fiy168. [Google Scholar] [CrossRef]
- Lima, S.L.; Dill, L.M. Behavioral decisions made under the risk of predation—A review and prospectus. Can J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Schlegel, P.A.; Steinfartz, S.; Bulog, B. Non-visual sensory physiology and magnetic orientation in the Blind Cave Salamander, Proteus anguinus (and some other cave-dwelling urodele species). Review and new results on light-sensitivity and non-visual orientation in subterranean urodeles (Amphibia). Anim. Biol. 2009, 59, 351–384. [Google Scholar] [CrossRef]
- Wells, K.D. The Ecology and Behaviour of Amphibians; The University of Chicago Press: Chicago, IL, USA, 2007; p. 1148. [Google Scholar]
- Bradley, T.M.; Goodding, D.D.; Ford, N.B.; Placyk, J.S. Sensory mediation of foraging behavior in the Western lesser siren (Siren intermedia nettingi). J. Herpetol. 2013, 47, 75–77. [Google Scholar]
- Dreiss, A.N.; Guillaume, O.; Clobert, J. Diverging Cave- and River-Dwelling Newts Exert the Same Mate Preference in their Native Light Conditions. Ethology 2009, 115, 1036–1045. [Google Scholar] [CrossRef]
- Uiblein, F.; Durand, J.P.; Juberthie, C.; Parzefall, J. Predation in caves—The effects of prey immobility and darkness on the foraging behavior of two salamanders, Euproctus asper and Proteus anguinus. Behav. Process. 1992, 28, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, S.B.; Bachmann, M.D. The role of visual and olfactory cues in the prey catching behavior of the tiger salamander, Ambystoma tigrinum. Copeia 1982, 1, 81–90. [Google Scholar] [CrossRef]
- Schülert, N.; Dicke, U. The effect of stimulus features on the visual orienting behaviour of the salamander Plethodon jordani. J. Exp. Biol. 2002, 205, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Pough, F.H.; Andrews, R.M.; Cadle, J.E.; Crump, M.L.; Savitky, A.H.; Wells, K.D. Herpetology, 3rd ed.; Benjamin Cummings: New York, NY, USA, 2003. [Google Scholar]
- Lanza, B.; Pastorelli, C.; Laghi, P.; Cimmaruta, R. A review of systematics, taxonomy, genetics, biogeography and natural history of the genus Speleomantes Dubois, 1984 (Amphibia Caudata Plethodontidae). Atti Mus. Civ. Stor. Nat. Trieste 2006, 52, 5–135. [Google Scholar]
- Lashley, K.S. Experimental analysis of instinctive behavior. Psychol. Rev. 1938, 45, 445–471. [Google Scholar] [CrossRef]
- Rabb, G.B.; Rabb, M.S. On the behavior and breeding biology of the African pipid frog: Hymenochirus boettgeri. Ethology 1963, 20, 215–241. [Google Scholar] [CrossRef]
- Crane, A.L.; Demuth, B.S.; Ferrari, M.C. Experience with predators shapes learning rules in larval amphibians. Behav. Ecol. 2016, 28, 312–318. [Google Scholar] [CrossRef]
- Stancher, G.; Rugani, R.; Regolin, L.; Vallortigara, G. Numerical discrimination by frogs (Bombina orientalis). Anim. Cogn. 2015, 18, 219–229. [Google Scholar] [CrossRef]
- David, R.S.; Jaeger, R.G. Prey location through chemical cues by a terrestrial salamander. Copeia 1981, 2, 435–440. [Google Scholar] [CrossRef]
- Kundey, S.M.A.; Millar, R.; McPherson, J.; Gonzalez, M.; Fitz, A.; Chadbourne, A. Tiger salamanders’ (Ambystoma tigrinum) response learning and usage of visual cues. Anim. Cogn. 2016, 19, 533–541. [Google Scholar] [CrossRef]
- Manenti, R.; Kristensen, N.; Cogliati, P.; Barzaghi, B.; Melotto, A.; Ficetola, G.F. Larval development and poor trophic resource availability: Local adaptations and plasticity in a widespread amphibian species. J. Evol. Biol. 2023, 36, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Manenti, R.; Ficetola, G.F.; Bianchi, B.; De Bernardi, F. Habitat features and distribution of Salamandra salamandra in underground springs. Acta Herpetol. 2009, 4, 143–151. [Google Scholar]
- Manenti, R.; Galbiati, M.; Lapadula, S.; Forlani, M.; Barzaghi, B.; Melotto, A.; Ficetola, G.F. Behavioural drivers of ecotone exploitation: Activity of groundwater animals in spring. Behav. Ecol. Sociobiol. 2023, 77, 23. [Google Scholar] [CrossRef]
- Manenti, R.; Vinci, L.; Barzaghi, B.; Lombardi, B.; Teodoro, C.; Baglioni, L.; Melotto, A.; Ficetola, G.F. Sit-and-wait foraging is not enough in food-deprived environments: Evidence from groundwater and salamanders. Anim. Behav. 2024, 207, 191–200. [Google Scholar] [CrossRef]
- Romero, A. Hypogean Communities as Cybernetic Systems. Diversity 2020, 12, 413. [Google Scholar] [CrossRef]
- Barzaghi, B.; Ficetola, G.F.; Pennati, R.; Manenti, R. Biphasic predators provide biomass subsidies in small freshwater habitats: A case study of spring and cave pools. Freshw. Biol. 2017, 62, 1637–1644. [Google Scholar] [CrossRef]
- Limongi, L.; Ficetola, G.F.; Romeo, G.; Manenti, R. Environmental factors determining growth of salamander larvae: A field study. Curr. Zool. 2015, 61, 421–427. [Google Scholar] [CrossRef]
- Dumas, P.; Fontanini, G. Sampling fauna in aquifers: A comparison of net-sampling and pumping. Archiv. Fur. Hydrobiol. 2001, 150, 661–676. [Google Scholar] [CrossRef]
- Friedrich, M. Biological Clocks and Visual Systems in Cave-Adapted Animals at the Dawn of Speleogenomics. Integr. Comp. Biol. 2013, 53, 50–67. [Google Scholar] [CrossRef]
- Guillaume, O. The importance of chemical communication in the social behaviour of cave salamanders. Comparison between a strict (Proteus anguinus L., Proteidae) and a facultative (Euproctus asper D., Salamandridae) cave dweller. Bull. Soc. Zool. Fr. 2002, 127, 263–272. [Google Scholar]
- Uiblein, F.; Engelke, S.; Parzefall, J. Trade-off between visual detectability and nutrient content in the patch choice of the Pyrenean salamander Euproctus asper. Ethology 1995, 101, 39–45. [Google Scholar] [CrossRef]
- Manenti, R.; Denoël, M.; Ficetola, G.F. Foraging plasticity favours adaptation to new habitats in fire salamanders. Anim. Behav. 2013, 86, 375–382. [Google Scholar] [CrossRef]
- Luthardt-Laimer, G. Ontogeny of preferences to visual prey stimulus parameters in salamanders. J. Herpetol. 1983, 17, 221–227. [Google Scholar] [CrossRef]
- Reilly, S.M. The ontogeny of aquatic feeding behavior in Salamandra salamandra: Stereotypy and isometry in feeding kinematics. J. Exp. Biol. 1995, 198, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Jusczcyk, W.; Zakrzewski, M. External morphology of larval stages of the spotted salamander Salamandra salamandra (L.). Acta Biol. Cracoviensa 1981, 23, 127–135. [Google Scholar]
- Manenti, R.; Pennati, R.; Ficetola, G.F. Role of density and resource competition in determining aggressive behaviour in salamanders. J. Zool. 2015, 296, 270–277. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2008, 24, 127–135. [Google Scholar] [CrossRef]
- R Development Core Team. R: A language and Environment for Statistical Computin; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Chivers, D.P.; Al-Batati, F.; Brown, G.E.; Ferrari, M.C.O. The effect of turbidity on recognition and generalization of predators and non-predators in aquatic ecosystems. Ecol. Evol. 2013, 3, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Turesson, H.; Bronmark, C. Predator-prey encounter rates in freshwater piscivores: Effects of prey density and water transparency. Oecologia 2007, 153, 281–290. [Google Scholar] [CrossRef]
- Manenti, R.; Melotto, A.; Denoël, M.; Ficetola, G.F. Amphibians breeding in refuge habitats have larvae with stronger antipredator responses. Anim. Behav. 2016, 118, 115–121. [Google Scholar] [CrossRef]
- Yovanovich, C.A.M.; Koskela, S.M.; Nevala, N.; Kondrashev, S.L.; Kelber, A.; Donner, K. The dual rod system of amphibians supports colour discrimination at the absolute visual threshold. Philos. Trans. R. Soc. Ser. B 2017, 372, 20160066. [Google Scholar] [CrossRef]
- Ibáñez, A.; Caspers, B.A.; López, P.; Martín, J.; Krause, E.T. Is the reaction to chemical cues of predators affected by age or experience in fire salamanders (Salamandra salamandra)? Amphib. Reptil. 2014, 35, 189–196. [Google Scholar] [CrossRef]
- Luthardt, G.; Roth, G. The Influence of Prey Experience on Movement Pattern Preference in Salamandra salamandra (L.). Z. Tierpsychol. 1979, 51, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Placyk, J.S., Jr.; Graves, B.M. Foraging behavior of the red-backed salamander (Plethodon cinereus) under various lighting conditions. J. Herpetol. 2001, 35, 521–524. [Google Scholar]
- Manenti, R.; Di Nicola, M.R.; Zampieri, V.; Grassi, G.; Creanza, T.; Mauri, E.; Ficetola, G.F.; Barzaghi, B. Wandering outside of the Styx: Surface activity of an iconic subterranean vertebrate, the olm (Proteus anguinus). Ecology 2024, 105, e4252. [Google Scholar] [CrossRef] [PubMed]
- Joly, P. La reproduction de la salamandre terrestre. In Traité de Zoologie; Grassé, P.P., Ed.; Masson: Paris, France, 1986; Volume XIV, pp. 471–486. [Google Scholar]
- Lönnstedt, O.M.; McCormick, M.I.; Meekan, M.G.; Ferrari, M.C.O.; Chivers, D.P. Learn and live: Predator experience and feeding history determines prey behaviour and survival. Proc. R. Soc. B-Biol. Sci. 2012, 279, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Hickman, C.R.; Stone, M.D.; Mathis, A. Priority use of chemical over visual cues for detection of predators by neotenic graybelly salamanders, Eurycea multiplicata griseogaster. Herpetologica 2004, 60, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Davenport, J.M.; Chalcraft, D.R. Nonconsumptive effects in a multiple predator system reduce the foraging efficiency of a keystone predator. Ecol. Evol. 2013, 3, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Barrio, I.C.; Bueno, C.G.; Banks, P.B.; Tortosa, F.S. Prey naivete in an introduced prey species: The wild rabbit in Australia. Behav. Ecol. 2010, 21, 986–991. [Google Scholar] [CrossRef]
- Peckarsky, B.L.; Abrams, P.A.; Bolnick, D.I.; Dill, L.M.; Grabowski, J.H.; Luttbeg, B.; Orrock, J.L.; Peacor, S.D.; Preisser, E.L.; Schmitz, O.J.; et al. Revisiting the classics: Considering nonconsumptive effects in textbook examples of predator-prey interactions. Ecology 2008, 89, 2416–2425. [Google Scholar] [CrossRef]
- Petranka, J.W.; Kats, L.B.; Sih, A. Predator Prey Interactions among Fish and Larval Amphibians—Use of Chemical Cues to Detect Predatory Fish. Anim. Behav. 1987, 35, 420–425. [Google Scholar] [CrossRef]
- Regester, K.J.; Whiles, M.R.; Lips, K.R. Variation in the trophic basis of production and energy flow associated with emergence of larval salamander assemblages from forest ponds. Freshw. Biol. 2008, 53, 1754–1767. [Google Scholar] [CrossRef]
- Chávez-Solís, E.M.; Mejía-Ortíz, L.M.; Simões, N. Predatory behavior of the cave shrimp Creaseria morleyi (Creaser, 1936) (Caridea: Palaemonidae), the blind hunter of the Yucatán cenotes, Mexico. J. Crustac. Biol. 2018, 38, 1–7. [Google Scholar] [CrossRef]
- Gaynor, K.M.; Brown, J.S.; Middleton, A.D.; Power, M.E.; Brashares, J.S. Landscapes of Fear: Spatial Patterns of Risk Perception and Response. Trends Ecol. Evol. 2019, 34, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, O. Surface newt Calotriton asper acclimation to cave conditions improved their foraging ability in darkness. Front. Ecol. Evol. 2022, 10, 1057023. [Google Scholar] [CrossRef]
- Manenti, R.; Forlani, M.; Lapadula, S.; Galbiati, M.; Barzaghi, B.; Ficetola, G.F.; Melotto, A. Landscape of fear in freshwater ecotones: How predation risk and light conditions affect mesopredator activity and foraging in springs. Freshw. Biol. 2023, 68, 1716–1725. [Google Scholar] [CrossRef]

| Variables | B | NumDF | DenDF | F. Value | p |
|---|---|---|---|---|---|
| Cave vs. stream | −0.01 | 1 | 65.06 | 0.03 | 0.85 |
| Test period | −0.021 | 1 | 636.76 | 0.52 | 0.47 |
| Visual cue | −0.47 | 1 | 605.63 | 271.76 | <0.001 |
| Chemical cue | −0.17 | 1 | 605.63 | 38.22 | <0.001 |
| Test period x visual cue | −0.93 | 1 | 605.49 | 475.52 | <0.001 |
| Test period x chemical cue | 0.12 | 1 | 605.45 | 7.86 | <0.01 |
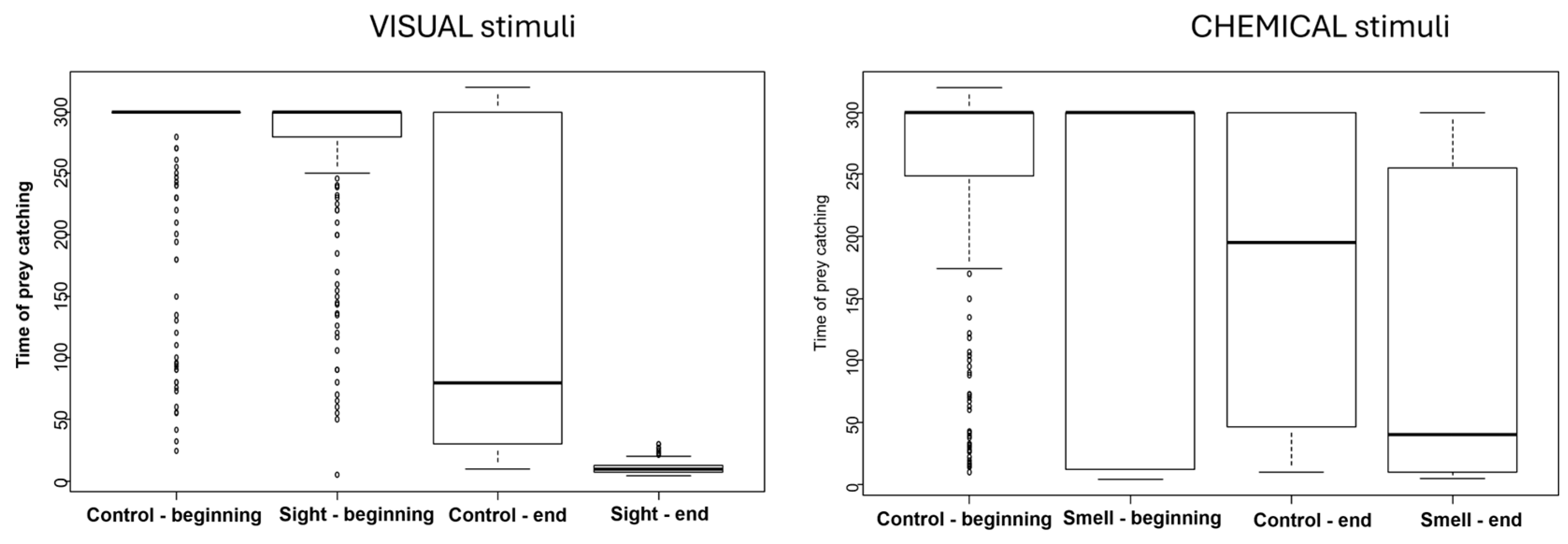
| O+V+ | O−V+ | O+V− | O−V− | |
|---|---|---|---|---|
| Before conditioning | 87 ± 13.06 s | 183 ± 17.11 s | 250 ± 12.64 s | 276 ± 8.2 s |
| After conditioning | 11 ± 0.69 s | 10 ± 0.74 s | 217 ± 11.83 s | 279 ± 7.81 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoover, H.; Manenti, R.; Melotto, A. The Role of Experience in the Visual and Non-Visual Prey Recognition of Fire Salamander Populations from Caves and Streams. Diversity 2024, 16, 312. https://doi.org/10.3390/d16060312
Hoover H, Manenti R, Melotto A. The Role of Experience in the Visual and Non-Visual Prey Recognition of Fire Salamander Populations from Caves and Streams. Diversity. 2024; 16(6):312. https://doi.org/10.3390/d16060312
Chicago/Turabian StyleHoover, Hayes, Raoul Manenti, and Andrea Melotto. 2024. "The Role of Experience in the Visual and Non-Visual Prey Recognition of Fire Salamander Populations from Caves and Streams" Diversity 16, no. 6: 312. https://doi.org/10.3390/d16060312
APA StyleHoover, H., Manenti, R., & Melotto, A. (2024). The Role of Experience in the Visual and Non-Visual Prey Recognition of Fire Salamander Populations from Caves and Streams. Diversity, 16(6), 312. https://doi.org/10.3390/d16060312







