The Mosquitoes of Morelos, Mexico: DNA Barcodes, Distribution, Ecology and the Resurrection of the Name Culiseta dugesi Dyar and Knab (Diptera: Culicidae) †
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
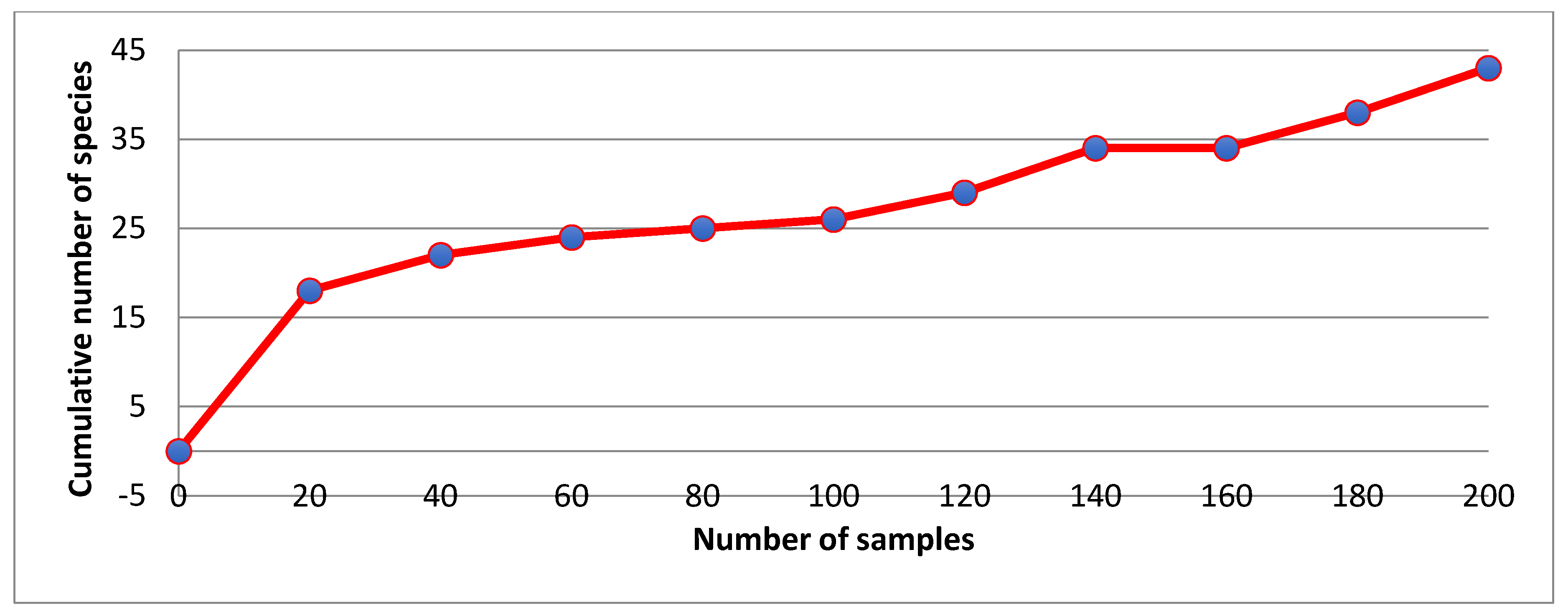
2.2. Mosquito Collection and Identification
2.3. Genomic DNA Extraction
2.4. Polymerase Chain Reaction and DNA Sequencing
2.5. Genetic Data Analysis
2.6. Ethical Consideration
3. Results
3.1. Mosquitoes and New Records
3.2. Species Originally Described in Morelos
3.3. Biological and Ecological Notes for the New State Records
3.3.1. Genus Aedes
3.3.2. Genus Psorophora
3.3.3. Genus Culex
3.3.4. Genus Toxorhynchites
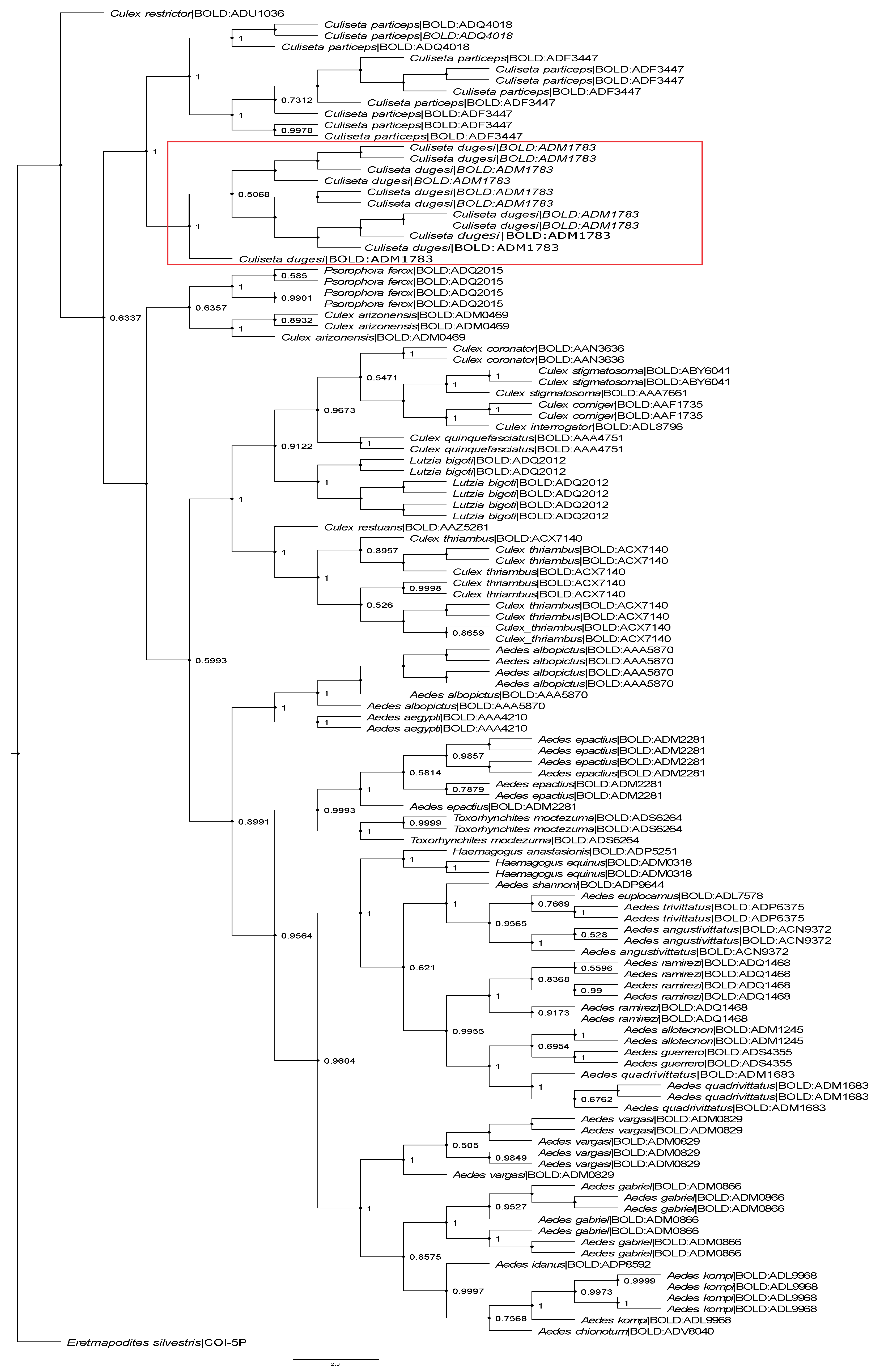
3.4. Molecular Analysis, COI-Barcoding Identification and Cryptic Diversity
3.5. Re-Description of Culiseta dugesi Dyar and Knab
3.6. Keys to Species of Larvae and Females of Culiseta in Mexico
| 1. Siphon with row of 8–14 setae along mid-ventral line…………………….Cs. melanura Distr. Canada, Mexico, USA [43]; Distr. Mex.: Nuevo León [68]. -Siphon with setae otherwise distributed, no mid ventral row……………………………2 2(1). Setae 5,6-C similar in size and number of branches………………………Cs. impatiens Distr. Canada, Mexico, USA [43]; Distr. Mex.: Nuevo León, Sonora [14]. -Setae 6-C with fewer branches and usually somewhat longer than 5-C……………………3 3(2). Saddle with coarse dorsoposterior aciculae …………………………………………4 -Saddle not aciculate dorsoposteriorly………………………………………………………5 4(3). Setae 4-C branched, nearly equal in size to setae 5-6C………………….Cs. particeps Distr. Costa Rica, El Salvador, Guatemala, Mexico, Panama, USA [43]; Distr. Mexico: Baja California, Baja California Sur, Chiapas, Coahuila, Durango, Guanajuato, Guerrero, Hidalgo, Mexico City Mexico, State, Michoacán, Morelos, Nuevo León, Oaxaca, Puebla, Sonora, Tamaulipas, Tlaxcala, Veracruz [31,56,60,69,70,71]. -Setae 4-C double, sometimes single……………………………………………Cs. dugesi Distr. Mexico; Distr. Mex: Guanajuato, Mexico City, Morelos, Querétaro. 5(3). Setae 1-X with branches equal to length of saddle or longer………………Cs. inornata Distr. Canada, Cuba, Mexico, USA [43]; Distr. Mex.: Aguascalientes, Baja California, Baja California Sur, Coahuila, Durango, Hidalgo, Mexico City, Mexico State, Nuevo León, Sonora, Tamaulipas, Tlaxcala [31,56,71]. -Setae 1-X with fine branches, shorter than saddle…….…………………….…Cs. incidens Distr. Canada, Mexico, USA [43]; Distr. Mex.: Baja California, Baja California Sur, Coahuila, Mexico State, Sonora, Tlaxcala [31,35,56]. |
| 1. Abdominal terga without basal pale bands……………………………………Cs. melanura -Abdominal terga with distinct basal pale bands…………………………………………….2 2(1). Hindtarsomeres with pale scale bands on some segments…………………….………3 -Hindtarsomeres unbanded………………………………………………………………….5 3(2). Hindtarsomeres with narrow pale bands, covering less than 0.1 of hind tarsomere 2; cross veins of wing without scales…………………………….….Cs. incidens Hindtarsomeres with broad pale bands, covering 0.25–0.33 of hind tarsomere 2; cross veins of wing with scales……………………………….……………………………………4 4(3). Integument of scutum and mesokatepisternum dark-brown………………Cs. particeps -Integument of scutum and mesokatepisternum yellowish or light-brown……….Cs. dugesi 5(2). Wing with dark and pale scales intermixed on anterior veins; hind tarsomeres 1,2 with dark and pale scales…………………………………………….………Cs. inornata -Wing and hind tarsomeres all dark-scaled………………………………………Cs. impatiens |
3.7. Medical Importance of the Mosquitoes of Morelos
4. Discussion and Conclusions
4.1. Mosquito Species-Groups Reaching Their Distributional Limits in Morelos
4.1.1. Group 1
4.1.2. Group 2
4.2. Species Not Collected during Our Collection Trips
4.3. Species from Adjacent Regions That May Occur in Morelos
4.4. Species Names Removed from Morelos
4.5. Mosquito Diversity in Morelos and Mexico
4.6. DNA Barcoding
Author Contributions
Funding
Institution Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harbach, R.E. Mosquito Taxonomic Inventory. 2013. Available online: https://mosquito-taxonomic-inventory.myspecies.info (accessed on 15 April 2023).
- Becker, N.; Petrić, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Medlock, J.K.; Snow, K.R.; Leach, S.A. Possible ecology and epidemiology of medically important mosquito-borne arboviruses in Great Britain. Epidemiol. Infect. 2007, 135, 466–482. [Google Scholar] [CrossRef]
- Hernández-Triana, L.M.; Brugman, V.A.; Prosser, S.W.J.; Weland, C.; Nikilova, N.; Thorne, L.; de Marco, M.F.; Fooks, A.R.; Johnson, N. Molecular approaches for blood meal analysis and species identification of mosquitoes (Insecta: Diptera: Culicidae) in rural locations in southern England, United Kingdom. Zootaxa 2017, 4250, 67–76. [Google Scholar] [CrossRef]
- Dorvillé, L.F.M. Mosquitoes as bioindicators of forest degradation in southern Brazil, a statical evaluation of published data in the literature. Stud. Neotrop. Fauna Environ. 1996, 31, 68–78. [Google Scholar] [CrossRef]
- Guedes, M.L.P.; Navarro-Silva, M.A. Mosquito community composition in dynamic landscapes from the Atlantic forest biome (Diptera, Culicidae). Rev. Bras. Entomol. 2014, 58, 88–94. [Google Scholar] [CrossRef]
- Montagner, F.R.G.; Silva, O.S.; Jahnke, S.M. Mosquito species occurrence in association with landscape composition in green urban areas. Braz. J. Biol. 2018, 78, 233–239. [Google Scholar] [CrossRef]
- Cywinska, A.; Hunter, F.F.; Hebert, P.D.N. Identifying Canadian mosquitoes species through DNA barcodes. Med. Vet. Entomol. 2006, 20, 413–424. [Google Scholar] [CrossRef]
- Linton, Y.; Lee, A.; Curtis, C. Discovery of a third member of the Maculipennis group in SW England. Eur. Mosq. Bull. 2005, 19, 5–9. [Google Scholar]
- Packer, L.; Gibbs, J.; Sheffields, C.; Hanner, R. DNA barcoding and the mediocrity of morphology. Mol. Ecol. Resour. 2009, 1, 42–50. [Google Scholar] [CrossRef]
- Versteirt, V.; Nagy, Z.T.; Roelants, P.; Denis, L.; Breman, F.C.; Damiens, D.; Dekomick, W.; Backelijau, T.; Coosemans, M.; Van Bortel, W. Identification of Belgian mosquito species (Diptera: Culicidae) by DNA barcoding. Mol. Ecol. Resour. 2015, 15, 449–457. [Google Scholar] [CrossRef]
- Hebert, P.N.D.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identification through DNA barcodes. Proc. R. Soc. B Biol. Sci. 2003, 270, 213–321. [Google Scholar] [CrossRef]
- Hebert, P.N.D.; Ratnasingham, S.; deWaard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. Biol. Sci. 2003, 270, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Morales, A.I.; León-Espinosa, G.A.; Rodríguez-Rojas, J.J. Updated checklist of the mosquitoes (Diptera: Culicidae) of Mexico. J. Vector Ecol. 2023, 49, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Bernal, S.; Mendoza-Palmero, F.S.; Hernández-Xoliot, R.A. Mosquitos (Insecta: Diptera: Culicidae). In La Biodiversidad de Veracruz Estudio de Estado, Vol. II.; Cruz-Angón, A., Lorca-Hernández, F.G., Hernández-Ortiz, V., Morales-Mavil., J.E., Eds.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad CONABIO: Mexico City, México, 2011; pp. 399–403. [Google Scholar]
- Mendez-Andrade, A.; Rivera-García, K.D.; Ibáñez-Bernal, S. Notes on the Toxorhynchites of Mexico: Redescription of Tx. moctezuma (Dyar & Knab) and new record for Tx. grandiosus (Williston) in Veracruz (Diptera: Culicidae). Zootaxa 2019, 4576, 140–150. [Google Scholar]
- Rivera-García, K.D.; Méndez-Andrade, A.; Ibáñez-Bernal, S. Trichoprosopon mixtli sp. nov., a new sabethini species (Diptera: Culicidae) from a Mexican cloud forest, with an assessment of the genus and keys for the identification pk known species. Zootaxa 2023, 5254, 94–116. [Google Scholar] [CrossRef]
- Torres-Chable, O.M.; Baak-Baak, C.M.; Cigarroa-Toledo, N.; Zaragoza-Vera, C.V.; Arjona-Jiménez, G.; Moreno-Perez, L.G.; Machain-Williams, C.; García-Rejon, J. Mosquito fauna un home environments of Tabasco, Mexico. Southwest. Entomol. 2017, 42, 969–982. [Google Scholar] [CrossRef]
- Ortega-Morales, A.I.; Méndez-López, R.; Garza-Hernández, J.A.; González-Álvarez, V.H.; Ruiz-Arrondo, I.; Huerta-Jiménez, H.; Rodríguez-Martínez, L.M.; Rodríguez-Pérez, M.A. The mosquitoes (Diptera: Culicidae) of Tabasco, Mexico. J. Vector Ecol. 2019, 44, 57–67. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, L.M.; Yzquierdo-Gómez, P.; González-Acosta, C.; Correa-Morales, F. First record of Aedes (Ochlerotatus) fulvus in Tabasco and distribution notes of other Aedes in Mexico. Southwest. Entomol. 2020, 45, 263–268. [Google Scholar] [CrossRef]
- Ortega-Morales, A.I.; Rodríguez-Martínez, L.M.; Méndez-Alvarado, W.; Garza-Hernández, J.A.; López-Hernández, I.; Medrano-Santillana, M.; González-Acosta, C.; Correa-Morales, F. The distribution of Uranotaenia sapphirina and Ur. socialis in Tabasco, southern Mexico. J. Am. Mosq. Control Assoc. 2022, 38, 141–147. [Google Scholar] [CrossRef]
- Ortega-Morales, A.I.; Mis-Ávila, P.C.; Elizondo-Quiroga, A.; Harbach, R.E.; Siller-Rodríguez, Q.K.; Fernández-Salas, I. The mosquitoes of Quintana Roo state, Mexico (Diptera: Culicidae). Acta Zool. Mex. (N.S.) 2010, 26, 33–46. [Google Scholar] [CrossRef]
- Ordóñez-Sánchez, F.; Sánchez-Trinidad, A.; Mis-Ávila, P.; Canul-Amaro, G.; Fernández-Salas, I.; Ortega-Morales, A.I. Nuevos registros de mosquitos (Diptera: Culicidae) en algunas localidades de Campeche y Quintana Roo. Entomol. Mex. 2013, 12, 850–854. [Google Scholar]
- Chan-Chablé, R.J.; Ortega-Morales, A.I.; Martínez-Arce, A. First record of Psorophora albipes in Quintana Roo, Mexico. J. Am. Mosq. Control Assoc. 2016, 32, 237–239. [Google Scholar] [CrossRef]
- Ortega-Morales, A.I.; Casas-Martínez, M.; Bond, G.; Harbach, R.E. First records of Psorophora cilipes and Culex theobaldi in Quintana Roo, Mexico. J. Am. Mosq Control Assoc. 2018, 34, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Chan-Chablé, R.J.; Martínez-Arce, A.; Mis-Ávila, P.C.; Ortega-Morales, A.I. DNA barcodes and evidence of cryptic diversity of anthropophagous mosquitoes in Quintana Roo, Mexico. Ecol. Evol. 2019, 9, 4692–4705. [Google Scholar] [CrossRef] [PubMed]
- Chan-Chablé, R.J.; Martínez-Arce, A.; Ortega-Morales, A.I.; Mis-Ávila, P.C. New records and updated checklist of mosquito species in Quintana Roo, Mexico, using DNA-Barcoding. J. Am. Mosq Control Assoc. 2020, 36, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Canto-Mis, K.L.; Chan-Chablé, R.J.; Gómez-Rivera, A.S.; López-Sosa, X.Y.; González-Acosta, C.; Correa-Morales, F.; Mis-Ávila, P.C. Nuevos registros de distribución para Uranotaenia sapphirina (Osten Sacken, 1868) (Diptera: Culicidae) en Quintana Roo, México. Rev. Chilena Entomol. 2021, 47, 613–617. [Google Scholar] [CrossRef]
- Canto-Mis, K.L.; Gómez-Rivera, A.S.; Chan-Chablé, R.J.; González-Acosta, C.; Correa-Morales, F.; Mis-Ávila, P.C. Primer registro de distribución para Psorophora varipes (Coquillett, 1904) (Diptera: Culicidae) en Quintana Roo, México. Rev. Chilena Entomol. 2022, 48, 293–297. [Google Scholar] [CrossRef]
- Ortega-Morales, A.I.; Zavortink, T.J.; Huerta-Jiménez, H.; Sánchez-Rámos, F.J.; Valdés-Perezgasga, M.T.; Reyes-Villanueva, F.; Siller-Rodríguez, Q.; Fernández-Salas, I. Mosquito records from Mexico: The mosquitoes (Diptera: Culicidae) of Tamaulipas state. J. Med. Entomol. 2015, 52, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cabrera, L.O.; Ibáñez-Bernal, S.; Corona-Vargas, M.C. Los mosquitos (Diptera: Culicidae) de Tlaxcala, México. I: Lista comentada de especies. Folia Entomol. Mex. 2006, 45, 223–271. [Google Scholar]
- Dampf, A. Distribución y ciclo annual de Uranotaenia syntheta Dyar & Shannon en México y descripción del hipopigio masculine (Insecta, Diptera). Rev. Soc. Mex. Hist. Nat. 1943, 4, 147–170. [Google Scholar]
- Dávalos-Becerril, E.; Correa-Morales, F.; González-Acosta, C.; Santos-Luna, R.; Peralta-Rodríguez, J.; Pérez-Rentería, C.; Ordóñez-Álvarez, R.; Huerta, H.; Carmona-Perez, M.; Díaz-Quiñonez, J.A.; et al. Urban and semi-urban mosquitoes of Mexico City: A risk for endemic mosquito borne-disease transmission. PLoS ONE 2019, 14, e0212987. [Google Scholar] [CrossRef]
- Ortega-Morales, A.I.; Hernández-Triana, L.M.; Siller-Rodríguez, Q.K. The mosquitoes of Querétaro, Mexico: Distribution, ecology, and the discovery of Shannoniana huasteca n. sp. (Diptera: Culicidae). Diversity 2023, 15, 697. [Google Scholar] [CrossRef]
- Adeniran, A.A.; Hernández-Triana, L.M.; Ortega-Morales, A.I.; Garza-Hernández, J.A.; de la Cruz-Ramos, J.; Chan-Chablé, R.J.; Vázquez-Marroquín, R.; Huerta-Jiménez, H.; Nikolova, N.I.; Fooks, A.R.; et al. Identification of mosquitoes (Diptera: Culicidae) from Mexico State, Mexico using morphology and COI DNA barcoding. Acta Trop. 2021, 213, 105730. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Morales, A.I.; Zavortink, T.J.; Huerta-Jiménez, H.; Ibáñez-Bernal, S.; Siller-Rodríguez, Q.K. The mosquitoes (Diptera: Culicidae) of Hidalgo state, Mexico. Acta Trop. 2019, 189, 94–103. [Google Scholar] [CrossRef]
- Ortega-Morales, A.I.; Pérez-Paredes, M.G.; Siller-Rodríguez, Q.K.; Moreno-García, M.; González-Acosta, C.; Correa-Morales, F. First record of Aedes gabriel in Hidalgo state, Mexico. J. Am. Mosq Control Assoc. 2019, 35, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Morales, A.I.; Zavortink, T.J.; Garza-Hernández, J.A.; Siller-Rodríguez, Q.K.; Fernández-Salas, I. The mosquitoes (Diptera: Culicidae) of Nuevo León, Mexico, with descriptions of two new species. PLoS ONE 2019, 14, e0217694. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Ramírez, H.M.; Ortega-Morales, A.I.; Flores-Suárez, A.; Fernández-Salas, I.; Ponce-García, G. First record of Aedes podographicus in Nuevo León state, Mexico. J. Am. Mosq Control Assoc. 2021, 37, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Morales, A.I.; Morillón-Borjón, G.; Morales-Avitia, I.J.; Sánchez-García, A.A.; Ayala-Sulca, Y.O.; Sánchez-Rámos, F.J. First record of Wyeomyia mitchellii in Nuevo León, Mexico. J. Am. Mosq Control Assoc. 2022, 38, 216–218. [Google Scholar] [CrossRef] [PubMed]
- INEGI. Instituto Nacional de Estadística y Geografía. 2018. Available online: https://cuentame.inegi.org.mx/monografias/informacion/mor/territorio/relieve.aspx?tema=me&e=17 (accessed on 15 September 2023).
- Harbach, R.E.; Knight, K.L. Taxonomist´s Glossary of Mosquito Anatomy; Plexus Publishing, Inc.: Marlton, NJ, USA, 1980; ISBN 9780937548004. [Google Scholar]
- Wilkerson, R.C.; Linton, T.-M.; Strickman, D. Mosquitoes of the World. Vol. 2; Johns Hopkins University Press: Baltimore, MD, USA, 2021. [Google Scholar]
- Montero-Pau, J.; Gómez, A.; Muñoz, J. Application of an inexpresive and high throughput genomic DNA extraction method for the molecular ecology of zooplanktonic diapausing eggs. Limnol. Oceanogr. 2008, 6, 218–222. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, T.; Vrijenhoek, R. DNA Primers for amplification of mitochondrial Cytochrome c Oxidase Subunit I from diverse Metazoan Invertebrtes. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Hernández-Triana, L.M.; Crainey, J.L.; Hall, A.; Faith, F.; Mackenzie-Dodds, J.; Shelley, A.J.; Zhou, X.; Post, R.G.; Gregory, R.T.; Hebert, P.N.D. The utility of DNA barcoding for species identification within the blackfly subgenus Trichodagmia Enderlein (Diptera: Simuliidae: Simulium) and related taxa in the New World. Zootaxa 2012, 3154, 43–69. [Google Scholar]
- Hernández-Triana, L.M.; Prosser, S.W.; Rodríguez-Pérez, M.A.; Chaverri, L.G.; Hebert, P.N.D.; Gregory, T.R. Recovery of DNA barcodes from blackfly museum specimens (Diptera: Simuliidae) using primer sets that target a variety of sequence lengths. Mol. Ecol. Resour. 2014, 4, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stoecher, G.; Nei, M.; Kumar, S. MEGA 5. 2007. Available online: http://www.megasoftware.net (accessed on 15 April 2020).
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kuhnet, D.; De Maio, N.; et al. BEAST 2.5: An advanced softwere platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Ratnasingham, S.; Hebert, P.N.D. A DNA-Based registry for all animal species: The barcode index number (BIN) system. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef]
- Martini, E.W. Los Mosquitos de México; Boletín técnico, Departamento de Salubridad: Mexico City, Mexico, 1935. [Google Scholar]
- Vargas, L.; Downs, W.G. Tres especies nuevas de Aedes (Diptera, Culicidae). Rev. Soc. Mex. Hist. Nat. 1950, 11, 160–177. [Google Scholar]
- Vargas, L.; Martínez-Palacios, A. Anofelinos Mexicanos, Taxonomía y Distribución; Secretaría de Salud: Ciudad de México, México, 1956; p. 181. [Google Scholar]
- Vargas, L. Especies y distribución de mosquitos mexicanos no Anofelinos (Insecta, Diptera). Rev. Inst. Salubr. Enf. Trop. 1956, 1, 19–36. [Google Scholar]
- Díaz-Nájera, A.; Vargas, L. Mosquitos mexicanos: Distribución geográfica actualizada. Rev. Inv. Salud Pública. 1973, 33, 111–125. [Google Scholar]
- Schick, R.X. Mosquito studies (Diptera, Culicidae) XX. The Terrens group of Aedes (Finlaya). Contrib. Am. Entomol. Inst. 1970, 5, 1–158. [Google Scholar]
- Zavortink, T.J. Mosquito studies (Diptera, Culicidae) XXVIII. The New World species formerly placed in Aedes (Finlaya). Contrib. Am. Entomol. Inst. 1972, 8, 1–206. [Google Scholar]
- Heinemann, S.J.; Belkin, J.N. Collection records of the project “Mosquitoes of Middle America” Mexico (MEX, MF, MT, MX). Mosq. Syst. 1977, 9, 483–535. [Google Scholar]
- Ibáñez-Bernal, S.; Martínez-Campos, C. Clave para la identificación de larvas de mosquitos comunes en las áreas urbanas y suburbanas de la República Mexicana (Diptera: Culicidae). Folia Entomol. Mex. 1994, 92, 43–73. [Google Scholar]
- Villegas-Trejo, A.; Manrique-Saide, P.; Che-Mendoza, A.; Cruz-Canto, W.; González-Fernández, M.; González-Acosta, C.; Dzul-Manzanilla, F.; Huerta, H.; Arredondo-Jiménez, J.A. First report of Aedes albopictus and other mosquito species in Morelos, Mexico. J. Am. Mosq. Control Assoc. 2010, 26, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Morales, A.I.; Moreno-García, M.; González-Acosta, C.; Correa-Morales, F. Mosquito surveillance in Mexico: The use of ovitraps for Aedes aegypti, Ae. albopictus, and non-target species. Fla. Entomol. 2018, 101, 623–626. [Google Scholar] [CrossRef]
- Hernández-Guevara, L.F.; Sánchez-Ramos, F.J.; Chan-Chable, R.J.; Hernández-Triana, L.M.; Valdés-Perezgasga, M.T.; González-Acosta, C.; Correa-Morales, F. First record of Mansonia dyari in the state of Morelos, Mexico, based on morphology and COI DNA Barcoding. J. Am. Mosq. Control Assoc. 2020, 36, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Anrell, J.H. Mosquito studies (Diptera, Culicidae) XXXIII. A revision of the Scapularis group of Aedes (Ochlerotatus). Contrib. Am. Entomol. Inst. 1976, 13, 1–144. [Google Scholar]
- Stone, A.; Knight, K. Type specimens of moquitoes in the United States National Museum: VI, Miscellaneous genera, addenda, and summary. J. Wash. Acad. Sci. 1957, 47, 196–202. [Google Scholar]
- Stone, A. Types of mosquitoes described by C. F. Adams in 1903 (Diptera, Culicidae). J. Kans. Entomol. Soc. 1958, 31, 235–237. [Google Scholar]
- Darsie, R.F.; Ward, R.A. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. University Press of Florida: Gainesville, FL, USA, 2005. [Google Scholar]
- Ortega-Morales, A.I.; Elizondo-Quiroga, A.; González-Villarreal, D.A.; Siller-Rodríguez, Q.K.; Reyes-Villanueva, F.; Fernández-Salas, I. First record of Culiseta melanura in Mexico, with additional Mexican records for Aedes sollicitans. J. Am. Mosq. Control Assoc. 2009, 25, 100–102. [Google Scholar] [CrossRef]
- Dzul-Manzanilla, F.A.; Manrique-Saide, P.; Huerta-Jiménez, H.; Morales-Rios, E.; Ventura-Juarez, O.; Hernández-Herrera, L. Mosquitos (Diptera: Culicidae) del estado de Guerrero, México. Entomol. Mex. 2013, 12, 659–861. [Google Scholar]
- Viveros-Santos, V.; Zumaquero-Rios, J.L.; Sandoval-Ruiz, C.A. Ensamble de mosquitos (Diptera: Culicidae) en la región central del estado de Puebla, México. Entomol. Mex. 2015, 2, 655–661. [Google Scholar]
- Pérez-Santiago, G.; Hernández-Amparan, S.; Ibáñez-Bernal, S.; Correa-Ramírez, M.M. First report of mosquitoes of the urban area of Durango City, Durango. Rev. Lat. Amb. Cienc. 2018, 9, 805–820. [Google Scholar]
- Berlin, O.G.W. Mosquito studies (Diptera, Culicidae) XVIII. The subgenus Micraedes of Culex. Contrib. Am. Entomol. Inst. 1969, 5, 21–63. [Google Scholar]
- Ibáñez-Bernal, S. Los mosquitos del estado de Hidalgo, México (Diptera: Culicidae). In Investigaciones Recientes Sobre Flora y Fauna de Hidalgo, México; Villaviciencio, M.A., Marmolejo, Y., Pérez, B.E., Eds.; Universidad Autónoma de Hidalgo: Pachuca, Hidalgo, Mexico, 1993; pp. 233–337. [Google Scholar]
- Beltrán-Aguilar, A.; Ibáñez-Bernal, S.; Mendoza-Palmero, F.; Sandoval-Ruiz, C.A.; Hernández-Xoliot, R.A. Taxonomía y distribución de los anofelinos en el estado de Veracruz, México (Diptera: Culicidae, Anophelinae). Acta Zool. Mex. 2011, 27, 601–755. [Google Scholar] [CrossRef]
- Sánchez-Trinidad, A.; Ordóñez-Sánez, F.; Valdés-Perezgasga, M.T.; Sánchez-Ramos, F.J.; Zavortink, T.; Cortés-Guzmán, A.J.; Ortega-Morales, A.I. Geographical distribution of the Aedes Triseriatus group (Diptera: Culicidae) in Mexico. J. Vector Ecol. 2014, 39, 134–137. [Google Scholar] [CrossRef] [PubMed]
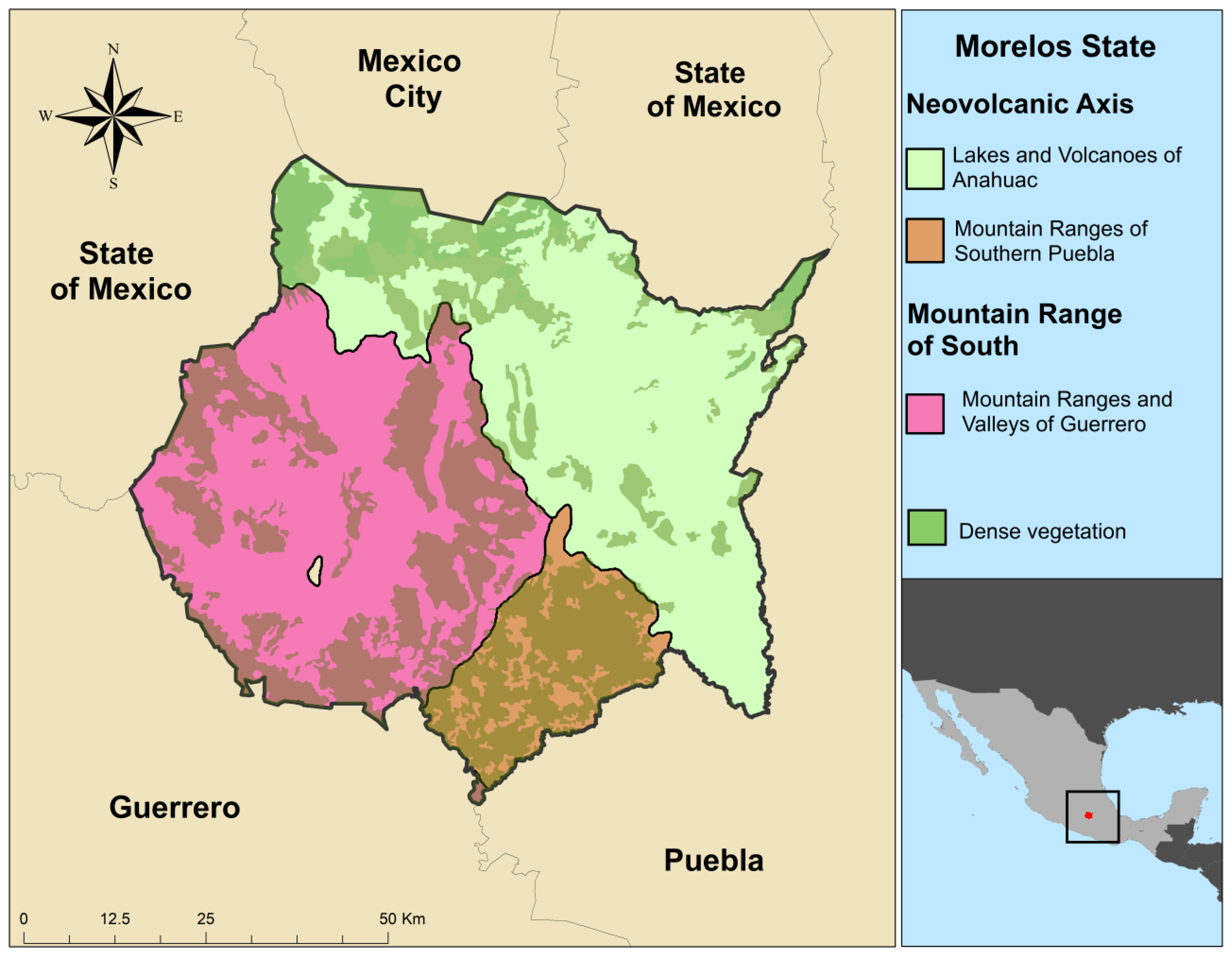






| Region (Sub-Region) | Municipalities Sampled | Description of Sub-Regions |
|---|---|---|
| Neo-volcanic Axis (Lakes and Volcanoes of Anahuac) | Axochiapan, Ayala, Cuautla, Cuernavaca, Huitzilac, Jantetelco, Jonacatepec, Temoac, Tepalcingo, Tepoztlán, Tetela del Volcán, Tlanepantla, Tlayacapan, Yecapixtla, Zacualpan | This sub-region has a relief with hills and mountains with elevations above 3000 m., the climate is temperate humid, cool in summer. Extensive regions of pine and oak forest, as well as shrub lands and grasslands. |
| Neo-volcanic Axis (Mountain Ranges of Southern Puebla) | Amacuzac, Jojutla, Tepalcingo, Tlaquiltenango | This sub-region is composed of volcanic rocks and hills, some mountains with elevations above 1500 m, the climate is temperate humid in winter and warm humid in summer. Extensive areas of sub-tropical forest and grasslands. |
| South Mountain Range (Mountain Ranges and Valleys of Guerrero) | Mazatepec, Miacatlán, Puente de Ixtla, Xoxocotla, Yautepec, | This sub-region is made up of small canyons and lower mountains, the climate is warm and humid, with rains during the summer. The vegetation is composed of grasslands, shrubs, copal and amate forests |
| Taxa | F.R. | Taxa | F.R. |
|---|---|---|---|
| Anopheles (Anopheles) | Culex (Anoedioporpa) | ||
| 1. An. eiseni Coquillett | VM | 30. Cx. restrictor Dyar and Knab | NSR |
| 2. An. parapunctipennis Martini | VM | Culex (Culex) | |
| 3. An. pseudopunctipennis Theobald | VM | 31. Cx. bidens Dyar and Knab | IM |
| Aedes(Aedimorphus) | 32. Cx. coronator Dyar and Knab | Va | |
| 4. Ae. vexans (Meigen) | NSR | 33. Cx. declarator Dyar and Knab | DV |
| Aedes (Aztecaedes) | 34. Cx. interrogator Dyar and Knab | NSR | |
| 5. Ae. ramirezi Vargas and Downs | VD | 35. Cx. nigripalpus Theobald | NSR |
| Aedes (Georgecriagius) | 36. Cx. quinquefasciatus Say | Ma | |
| 6. Ae. epactius Dyar and Knab | DV | 37. Cx. restuans Theobald | Vi |
| Aedes (Howardina) | 38. Cx. salinarius Coquillett | Va | |
| 7. Ae. allotecnon Kumm, Komp, and Ruiz | DV | 39. Cx. stigmatosoma Dyar | Ma |
| 8. Ae. guerrero Berlin | HB | 40. Cx. tarsalis Coquillett | NSR |
| 9. Ae. quadrivittatus (Coquillett) | Va | 41. Cx. thriambus Dyar | Va |
| 10. Ae. sexlineatus (Theobald) | DV | Culex (Melanoconion) | |
| Aedes (Ochlerotatus) | 42. Cx. conspirator Dyar and Knab | Va | |
| 11. Ae. angustivittatus Dyar and Knab | Va | 43. Cx. erraticus (Dyar and Knab) | Va |
| 12. Ae. shannoni Vargas and Downs | VD | 44. Cx. iolambdis Dyar | Va |
| 13. Ae. euplocamus Dyar and Knab | NSR | 45. Cx. limacifer Komp | Va |
| 14. Ae. taeniorhynchus (Wiedemann) | DV | 46. Cx. pilosus (Dyar and Knab) | Va |
| 15. Ae. trivittatus (Coquillett) | HB | Culex (Micraedes) | |
| Aedes (Protomacleaya) | 47. Cx. sandrae Berlin | NSR | |
| 16. Ae. chionotum Zavortink | Za | Culex (Neoculex) | |
| 17. Ae. gabriel Schick | Sc | 48. Cx. apicalis Adams | Va |
| 18. Ae. idanus Schick | HB | 49. Cx. arizonensis Bohart | Va |
| 19. Ae. kompi Vargas and Downs | VD | Culex (Phenacomyia) | |
| 20. Ae. vargasi Schick | NSR | 50. Cx. corniger Theobald | Vi |
| Aedes (Stegomyia) | 51. Cx. lactator Dyar and Knab | NSR | |
| 21. Ae. aegypti (Linneaus) | Va | Lutzia (Lutzia) | |
| 22. Ae. albopictus (Skuse) | Vi | 52. Lt. bigoti (Bellardi) | Va |
| Haemagogus (Haemagogus) | Culiseta (Culiseta) | ||
| 23. Hg. anastasionis Dyar | DV | 53. Cs. dugesi Dyar and Knab | NSR |
| 24. Hg. equinus Theobald | Or | 54. Cs. particeps (Adams) | DV |
| 25. Hg. mesodentatus Komp and Kumm | DV | Mansonia (Mansonia) | |
| Psorophora (Grabhamia) | 55. Ma. dyari Belkin, Heinemann, and Page | He | |
| 26. Ps. columbiae (Dyar and Knab) | DV | Toxorhynchites (Lynchiella) | |
| Psorophora (Janthinosoma) | 56. Tx. moctezuma (Dyar and Knab) | NSR | |
| 27. Ps. ferox (von Humboldt) Psorophora (Psorophora) 28. Ps. cilipes (Fabricius) 29. Ps. lineata (von Humboldt) | HB NSR NSR | Uranotaenia (Uranotaenia) 57. Ur. coatzacoalcos Dyar and Knab 58. Ur. sapphirina (Osten Sacken) | Va Va |
| Species | Habitat/Parameters | Environment | Location, County | Coordinate/Elevation | Collection Date | Associated spp. |
|---|---|---|---|---|---|---|
| Aedes vexans | Human-biting | Shrub region | Miacatlán | 18°46′44″ N–99°21′50″ W, 1004 m. | 12 September 2017 | No associated spp. |
| Aedes euplocamus | Temporal ground pool with clear water (pH 7.9, 23 PPM, 27 °C) | Mountain region | Quilamula, Tlaquiltenango | 18°30′52.61″ N–99°0′44.5″ W, 1098 m. | 7 June 2017 | Ae. shannoni, Ae. trivittatus, Cx. stigmatosoma |
| Aedes vargasi | Tree hole, colored water, (pH 9.8, >2000 PPM, 27 °C) | Mountain region | Quilamula, Tlaquiltenango | 18°30′16″ N–99°0′50.94″ W, 1118 m. | 8 June 2017 | Ae. gabriel, Tx. moctezuma |
| Tree hole (Licania arborea Seeman), colored water, (pH 8.5, 470 PPM, 27 °C) | Mountain region | Quilamula, Tlaquiltenango | 18°30′26.44″ N–99°1′5.75″ W, 1125 m. | 8 June 2017 | Hg. equinus | |
| Tree hole (Ruprechtia fusca Meyer), colored water, (pH 8.9, 950 PPM, 27 °C) | Mountain region | Quilamula, Tlaquiltenango | 18°30′26.44″ N–99°1′5.75″ W, 1125 m. | 8 June 2017 | Ae. gabriel, Hg. equinus | |
| Tree hole (Bursera lancifolia Schltdl.,), colored water, (pH 8.10, 418 PPM, 27 °C) | Mountain region | Quilamula, Tlaquiltenango | 18°30′26.44″ N–99°1′5.75″ W, 1125 m. | 8 June 2017 | Ae. gabriel, Hg. mesodentatus, Cx. lactator | |
| Tree hole (Licania arborea Seeman), colored water, (pH 8.17, 218 PPM, 27 °C) | Mountain region | Quilamula, Tlaquiltenango | 18°30′52.23″ N–99°0′50.04″ W, 1121 m. | 8 June 2017 | Ae. gabriel, Cx. stigmatosoma, Cx. corniger | |
| Discarded tire, clean water, (pH 8, 145 PPM, 29 °C) | Mountain region | Quilamula, Tlaquiltenango | 18°30′32.33″ N–99°1′7.97″ W, 1089 m. | 7 June 2017 | Ae. gabriel | |
| Plastic bottle, colored water, (pH 7.18, 3.75 PPM, 27 °C) | Mountain region | Quilamula, Tlaquiltenango | 18°30′39.82″ N–99°0′53.12″ W, 1087 m. | 8 June 2017 | Ae. albopictus | |
| Adults resting intra-domiciliary | Mountain region | Quilamula, Tlaquiltenango | 18°30′40.8″ N–99°1′10.47″ W, 1087 m. | 7 June 2017 | Hg. mesodentatus | |
| Psorophora cilipes | Adults resting intra-domiciliary | Shrub region | Miacatlán | 18°46′44″ N–99°21′50″ W, 1004 m. | 12 September 2017 | No associated spp. |
| Psorophora lineata | Artificial pool with clean water without vegetation | Urban area | Tepoztlán | 18°58.908′ N–99°6.66″ W, 1793 m. | 12 July 2009 | No associated spp. |
| Culex restrictor | Tree hole, colored water with abundant leaves | Mountain region | San Andrés de la Cal, Tepoztlán | 18°57′21″ N–99°6′46″ W, 1487 m. | 2 July 2017 | Ae. albopictus |
| Culex interrogator | Temporal ground pool with clean water and abundant emergent vegetation (pH 9.38, 798 PPM) | Hills region | Tehuixtla, Jojutla | 18°33′55″ N–99°16′7″ W, 884 m. | 13 June 2017 | Ae. epactius, Cx. coronator |
| Temporal ground pool with colored water (pH 9.96, 187 PPM) | Hills region | Puente de Ixtla | 18°36′47″ N–99°18′45″ W, 898 m. | 14 June 2017 | Ae. epactius, Cx. coronator, Cx. stigmatosoma | |
| Temporal ground pool with clean water and abundant vegetation (pH 9.53, 246 PPM) | Hills region | Puente de Ixtla | 18°36′47″ N–99°18′45″ W, 898 m. | 14 June 2017 | Cx. coronator, Cx. stigmatosoma, Cx. quinquefasciatus | |
| Temporal ground pool with colored water and abundant vegetation (pH 9.53, 246 PPM) | Hills region | Puente de Ixtla | 18°37′20″ N–99°19′7″ W, 902 m. | 14 June 2017 | Ae. epactius, Ae. shannoni, Cx. coronator | |
| Temporal ground pool with clean water and abundant vegetation | Mountain region | Mazatepec | 18°43′21.78″ N–99°21′24.03″ W, 968 m. | 14 June 2017 | Cx. coronator, Cx. quinquefasciatus, Cx. stigmatosoma | |
| Temporal ground pool with clean water and abundant vegetation | Hills region | Xoxocotla, Puente de Ixtla | 18°39′42″ N–99°13′33″ W, 940 m. | 14 June 2017 | Cx. coronator, Cx. stigmatosoma | |
| Adults resting in vegetation, total shade | Hills region | Jojutla | 18°36′27″ N–99°11′1″ W, 880 m. | 14 June 2017 | Cx. coronator | |
| Culex nigripalpus | Temporal ground pool with clean water, abundant vegetation and green algae (pH 8.1, 266 PPM, 29 °C) | Mountain region | Quilamula, Tlaquiltenango | 18°30′52.61″ N–99°0′44.5″ W, 1098 m. | 7 June 2017 | An. pseudopunctipennis, Ae. shannoni, Cx. coronator, Cx. declarator |
| Culex tarsalis | Adults resting in vegetation, total shade | Hills region | Jojutla | 18°36′27″ N–99°11′1″ W, 880 m. | 14 June 2017 | No associated spp. |
| Culex sandrae | Axils of bromeliads with clean water, 2 mts. from ground level | Mountain region | Los Venados, Tetela del Volcán | 18°53′59″ N–98°42′26″ W, 2182 m. | 30 June 2017 | Ae. quadrivittatus |
| Axils of bromeliads with clean water, 3 mts. from ground level | Mountain region | Tetela del Volcán | 18°54′33.01″ N–98°42′9.46″ W, 2314 m. | 1 July 2017 | No associated spp. | |
| Culex lactator | Tree hole (Bursera lancifolia Schltdl.,), colored water, (pH 8.10, 418 PPM, 27 °C) | Mountain region | Quilamula, Tlaquiltenango | 18°30′26.44″ N–99°1′5.75″ W, 1125 m. | 8 June 2017 | Ae. gabriel, Ae. vargasi, Hg. mesodentatus |
| Culiseta dugesi | Adults approaching humans | Mountain region | CEDIF, Huitzilac | 19°1′58.11′ N″–99°15′18.21″ W, 2600 m. | 15 June 2017 | Ae. kompi, Cx. sp. |
| Discarded tire with clean water and total shade (pH 7.2, 110 PPM, 20.4 °C) | Mountain region | La Encantada, Huitzilac | 19°0′59′ N″–99°13′13″ W, 2450 m. | 18 September 2017 | Cx. arizonensis, Cs. particeps | |
| Adults resting inside artificial container | Mountain region | La Encantada, Huitzilac | 19°0′59′ N″–99°13′13″ W, 2450 m. | 18 September 2017 | No associated spp. | |
| Toxorhynchites moctezuma | Tree hole, colored water, (pH 9.8, >2000 PPM, 27 °C) | Mountain region | Quilamula, Tlaquiltenango | 18°30′16″ N–99°0′50.94″ W, 1118 m. | 8 June 2017 | Ae. gabriel, Ae. vargasi |
| Discarded tire with clean water and total shade | Hills region | Yautepec | 18°54′13″ N–99°3′51″ W, 1223 m. | 27 June 2017 | No associated spp. |
| Taxa | Mal | DI | DENV | ZIKV | CHKV | YF | SLE | WNV | VEEV | EEEV | WEEV | LCV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| An. pseudopunctipennis * | ✓ | |||||||||||
| Aedes vexans * | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Ae. ramirezi * | ||||||||||||
| Ae. epactius * | ||||||||||||
| Ae. guerrero * | ||||||||||||
| Ae. quadrivittatus * | ||||||||||||
| Ae. angustivittatus * | ✓ | |||||||||||
| Ae. shannoni * | ||||||||||||
| Ae. taeniorhynchus | ✓ | ✓ | ✓ | |||||||||
| Ae. trivittatus | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Ae. gabriel * | ||||||||||||
| Ae. kompi * | ||||||||||||
| Ae. aegypti * | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Ae. albopictus * | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Haemagogus equinus | ✓ | |||||||||||
| Hg. mesodentatus * | ✓ | ✓ | ✓ | ✓ | ||||||||
| Ps. columbiae | ✓ | ✓ | ||||||||||
| Ps. ferox * | ✓ | ✓ | ||||||||||
| Culex nigripalpus | ✓ | ✓ | ✓ | ✓ | ||||||||
| Cx. quinquefasciatus | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Cx. restuans | ✓ | ✓ | ✓ | |||||||||
| Cx. salinarius | ✓ | ✓ | ✓ | |||||||||
| Cx. tarsalis | ✓ | ✓ | ✓ | |||||||||
| Cx. erraticus | ✓ | |||||||||||
| Cs. dugesi * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Morales, A.I.; Hernández-Triana, L.M.; Garza-Hernández, J.A.; Ramírez-Huicochea, C.M.; Martínez-Gaona, A.J.; Quijano-Barraza, J.M.; González-Acosta, C.; Correa-Morales, F. The Mosquitoes of Morelos, Mexico: DNA Barcodes, Distribution, Ecology and the Resurrection of the Name Culiseta dugesi Dyar and Knab (Diptera: Culicidae). Diversity 2024, 16, 261. https://doi.org/10.3390/d16050261
Ortega-Morales AI, Hernández-Triana LM, Garza-Hernández JA, Ramírez-Huicochea CM, Martínez-Gaona AJ, Quijano-Barraza JM, González-Acosta C, Correa-Morales F. The Mosquitoes of Morelos, Mexico: DNA Barcodes, Distribution, Ecology and the Resurrection of the Name Culiseta dugesi Dyar and Knab (Diptera: Culicidae). Diversity. 2024; 16(5):261. https://doi.org/10.3390/d16050261
Chicago/Turabian StyleOrtega-Morales, Aldo I., Luis M. Hernández-Triana, Javier A. Garza-Hernández, Carlos M. Ramírez-Huicochea, Andrés J. Martínez-Gaona, J. Manuel Quijano-Barraza, Cassandra González-Acosta, and Fabián Correa-Morales. 2024. "The Mosquitoes of Morelos, Mexico: DNA Barcodes, Distribution, Ecology and the Resurrection of the Name Culiseta dugesi Dyar and Knab (Diptera: Culicidae)" Diversity 16, no. 5: 261. https://doi.org/10.3390/d16050261
APA StyleOrtega-Morales, A. I., Hernández-Triana, L. M., Garza-Hernández, J. A., Ramírez-Huicochea, C. M., Martínez-Gaona, A. J., Quijano-Barraza, J. M., González-Acosta, C., & Correa-Morales, F. (2024). The Mosquitoes of Morelos, Mexico: DNA Barcodes, Distribution, Ecology and the Resurrection of the Name Culiseta dugesi Dyar and Knab (Diptera: Culicidae). Diversity, 16(5), 261. https://doi.org/10.3390/d16050261








