Feeding Ecology of Reintroduced Golden Parakeets (Guaruba guarouba, Psittacidae) in a Protected Area in the Amazon Forest
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matuzak, G.D.; Bezy, M.B.; Brightsmith, D.J. Foraging Ecology of Parrots in A Modified Landscape: Seasonal Trends and Introduced Species. Wilson J. Ornithol. 2008, 120, 353–365. [Google Scholar] [CrossRef]
- Selman, R.; Hunter, M.; Perrin, M. Rüppell’s Parrot: Status, ecology and conservation biology. Ostrich J. Afr. Ornithol. 2000, 71, 347–348. [Google Scholar] [CrossRef]
- Taylor, G.; Canessa, S.; Clarke, R.H.; Ingwersen, D.; Armstrong, D.P.; Seddon, P.J.; Ewen, J.G. Is Reintroduction Biology an Effective Applied Science? Trends Ecol. Evol. 2017, 32, 873–880. [Google Scholar] [CrossRef] [PubMed]
- White, T.H., Jr.; Abreu, W.; Benitez, G.; Jhonson, A.; Lopez, M.; Ramirez, L.; Rodriguez, I.; Toledo, M.; Torres, P.; Velez, J. Minimizing Potential Allee Effects in Psittacine Reintroductions: An Example from Puerto Rico. Diversity 2021, 13, 13. [Google Scholar] [CrossRef]
- Mathews, F.; Orros, M.; McLaren, G.; Gelling, M.; Foster, R. Keeping fit on the ark: Assessing the suitability of captive-bred animals for release. Biol. Conser. 2005, 121, 569–577. [Google Scholar] [CrossRef]
- Roberts, J.L.; Luther, D. An exploratory analysis of behavior-based and other management techniques to improve avian conservation translocations. Biol. Conser. 2023, 279, 109941. [Google Scholar] [CrossRef]
- White, T.H., Jr.; Collar, N.J.; Moorhouse, R.J.; Sans, V.; Stolen, E.D.; Brightsmith, D.J. Psittacine reintroductions: Common denominators of success. Biol. Conserv. 2012, 148, 106–115. [Google Scholar] [CrossRef]
- Birdlife International IUCN Red List of Threatened Species. 2023. Available online: https://www.birdlife.org/projects/iucn-red-list (accessed on 11 December 2023).
- Vergara-Tabares, D.L.; Cordier, J.M.; Landi, M.A.; Olah, G.; Nori, J. Global trends of habitat destruction and consequences for parrot conservation. Glob. Chan. Biol. 2020, 26, 4251–4262. [Google Scholar] [CrossRef] [PubMed]
- Agazzi Migotto, A.; Bocalini, F.; Francisco, M.R.; Reillo, P.; Silveira, L.F. Genetic Variability and Kinship Analyses of Seized Red-Browed Amazon, Amazona rhodocorytha (Aves, Psittacidae). Diversity 2023, 15, 923. [Google Scholar] [CrossRef]
- Rodrigues, V.P.; Caetano, M.A.L. The impacts of political activity on fires and deforestation in the Brazilian Amazon rainforest: An analysis of social media and satellite data. Heliyon 2023, 9, e22670. [Google Scholar] [CrossRef]
- Santos, Y.L.; Yanai, A.M.; Ramos, C.J.; Graça, P.M.; Veiga, J.A.; Correia, F.W.; Fearnside, P.M. Amazon deforestation and urban expansion: Simulating future growth in the Manaus Metropolitan Region, Brazil. Brazil. J. Environ. Manag. 2022, 304, 114279. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, R.; Leite, A.B.; Garbeloto, M.C.; Silveira, L.F.; Francisco, M.R. A critical assessment of ex situ conservation based on the Brazilian avifauna: Are we focusing on what is easier? Perspect. Ecol. Conserv. 2023, 21, 62–70. [Google Scholar] [CrossRef]
- Fraga, R.E.; Schiavetti, A.; Santos, C.M.R.; Silva, R.S.; Teixeira, R.E.R.; Tomazi, L.; dos Santos, C.Z. Reintroduction and monitoring of the bird Amazona aestiva (Psittaciformes: Psittacidae) in Brazil. Rev. Biol. Trop. 2021, 71, 1–15. [Google Scholar] [CrossRef]
- Kanaan, V. Reintroduction of the vinaceous-breasted Amazon at the Araucárias National Park, Santa Catarina, Brazil. Glob. Reintrod. Perspect. Case Stud. Around Globe 2016, 106, 106–110. [Google Scholar]
- Pacífico, E.C.; Filadelfo, T.; Paschotto, F.R.; Favoreto, G.R.; Andrade, T.S.; Denés, F.V. Results of the ongoing Lear’s Macaw release and monitoring project recovering a functionally extinct population. In Proceedings of the II Ornithological Congress of the Americas, Gramado, RS, Brazil, 1–4 August 2023; Ed. dos Autores: Porto Alegre, Brazil, 2023; Volume 1, pp. 9–370. [Google Scholar]
- Purchase, C.; Lugarini, C.; Purchase, C.; Ferreira, A.; Vercillo, U.E.; Stafford, M.L.; White, T.H., Jr. Reintroduction of the Extinct-in-theWild Spix’s Macaw (Cyanopsitta spixii) in the Caatinga Forest Domain of Brazil. Diversity 2024, 16, 80. [Google Scholar] [CrossRef]
- Sick, H. Ornitologia Brasileira, 3rd ed.; Nova Fronteira: Rio de Janeiro, Brazil, 1997; pp. 369–370. [Google Scholar]
- Laranjeiras, T.O.; Cohn-Haft, M. Where is the symbol of Brazilian Ornithology? The geographic distribution of the Golden Parakeet (Guarouba guarouba—Psittacidae). Rev. Bras. Ornitol. 2009, 17, 1–19. [Google Scholar]
- Oren, D.C.; Novaes, F.C. Observations on the golden parakeet Aratinga guarouba in Northern Brazil. Biol. Conserv. 1986, 36, 329–337. [Google Scholar] [CrossRef]
- Silveira, L.F.; Belmonte, F.J. Comportamento reprodutivo e hábitos da Ararajuba, Guarouba guarouba, no município de Tailândia, Pará. Ararajuba 2005, 13, 89–93. [Google Scholar]
- Laranjeiras, T.O. Biology and population size of the Golden Parakeet (Guaruba guarouba) in western Pará, Brazil, with recommendations for conservation. Rev. Bras. Ornitol. 2011, 19, 303–331. [Google Scholar]
- Moura, N.G.; Lees, A.C.; Aleixo, A.; Barlow, J.; Dantas, S.M.; Ferreira, J.; Lima, M.D.F.C.; Gardner, T.A. Two Hundred Years of Local Avian Extinctions in Eastern Amazonia. Conserv. Biol. 2014, 28, 1271–1281. [Google Scholar] [CrossRef]
- Vilarta, M.R.; Wittkoff, W.; Lobato, C.; Oliveira, R.d.A.; Pereira, N.G.P.; Silveira, L.F. Reintroduction of the Golden Conure (Guaruba guarouba) in Northern Brazil: Establishing a Population in a Protected Area. Diversity 2021, 13, 198. [Google Scholar] [CrossRef]
- Ferreira, L.V.; Maia, A.P.M.; Sarmento, P.S.M.; Jardim., M.A.G. Florística e estrutura da floresta de terra firme como instrumento de gestão ambiental do Parque Estadual do Utinga, Belém, Pará, Brasil. Rev. Bras. De Geogr. Física 2023, 16, 1419–1435. [Google Scholar] [CrossRef]
- Plano de Manejo do Parque Estadual do Utinga. Available online: http://ideflorbio.pa.gov.br/utinga/wp-content/uploads/2018/03/PMUtinga_26out2013.pdf (accessed on 20 December 2023).
- Renton, K.; Salinas-Melgoza, A.; De Labra-Hernández, M.Á.; de la Parra-Martínez, S.M. Resource requirements of parrots: Nest site selectivity and dietary plasticity of Psittaciformes. J. Ornithol. 2015, 156, 73–90. [Google Scholar] [CrossRef]
- Soares, C.S.; Barnett, A.A.; Scudeller, V.V.; Borges, S.H. Searching for food in a concrete jungle: Feeding ecology of a Psittacine assemblage (Aves, Psittacidae) in a major Amazonian city. An. Acad. Bras. Cienc. 2023, 95, e20220606. [Google Scholar] [CrossRef] [PubMed]
- Carrete, M.; Hiraldo, F.; Romero-Vidal, P.; Blanco, G.; Hernández-Brito, D.; Sebastián-González, E.; Díaz-Luque, J.A.; Tella, J.L. Worldwide Distribution of Antagonistic-Mutualistic Relationships Between Parrots and Palms. Front. Ecol. Evol. 2022, 10, 790883. [Google Scholar] [CrossRef]
- Tella, J.L.; Hiraldo, F.; Pacífico, E.; Díaz-Luque, J.A.; Dénes, F.V.; Fontoura, F.M.; Guedes, N.; Blanco, G. Conserving the Diversity of Ecological Interactions: The Role of Two Threatened Macaw Species as Legitimate Dispersers of “Megafaunal” Fruits. Diversity 2020, 12, 45. [Google Scholar] [CrossRef]
- Sazima, I. The parakeet Brotogeris tirica feeds on and disperses the fruits of the palm Syagrus romanzoffiana in Southeastern Brazil. Biota Neotrop. 2008, 8, 231–234. [Google Scholar] [CrossRef]
- Silva, L.B.; Pereira, G.A.; Passos, P.B.; Almeida, N.M. Seed dispersal of the palm Acrocomia aculeata by the blue-and-yellow macaw (Ara ararauna). Braz. J. Biol. 2021, 83, e244697. [Google Scholar] [CrossRef]
- Martens, J.; Hoppe, D.; Woog, F. Diet and feeding behaviour of naturalised Amazon Parrots in a European city. Ardea 2013, 101, 71–76. [Google Scholar] [CrossRef][Green Version]
- Sebastián-González, E.; Hiraldo, F.; Blanco, G. The extent, frequency and ecological functions of food wasting by parrots. Sci. Rep. 2019, 9, 15280. [Google Scholar] [CrossRef]
- Péron, F.; Thornberg, L.; Gross, B.; Gray, S.; Pepperberg, I.M. Human–Grey parrot (Psittacus erithacus) reciprocity: A follow-up study. Anim. Cogni. 2014, 17, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, C. Field observations and comments on the Indigo macaw (Anodorhynchus leari), a highly endangered species from northeastern Brazil. Wils. Bull. 1987, 99, 280–282. [Google Scholar]
- Reynolds, G. Golden Conure research will aid its survival. PsittaScene 2003, 15, 10–13. [Google Scholar]
- Amaya-Villarreal, A.M.; Estrada, A.; Vargas-Ramírez, N. Use of wild foods during the rainy season by a reintroduced population of scarlet macaws (Ara macao cyanoptera) in Palenque, Mexico. Trop. Conser. Scie. 2015, 8, 455–478. [Google Scholar] [CrossRef]
- Volpe, N.; Thalinger, B.; Vilacoba, E.; Braukmann, T.; Di Giacomo, A.; Berkunsky, I.; Lijtmaer, D.; Steinke, D.; Kopuchian, C. Diet composition of reintroduced Red-and-Green Macaws reflects gradual adaptation to life in the wild. Ornithol. Appl. 2021, 124, duab059. [Google Scholar] [CrossRef]



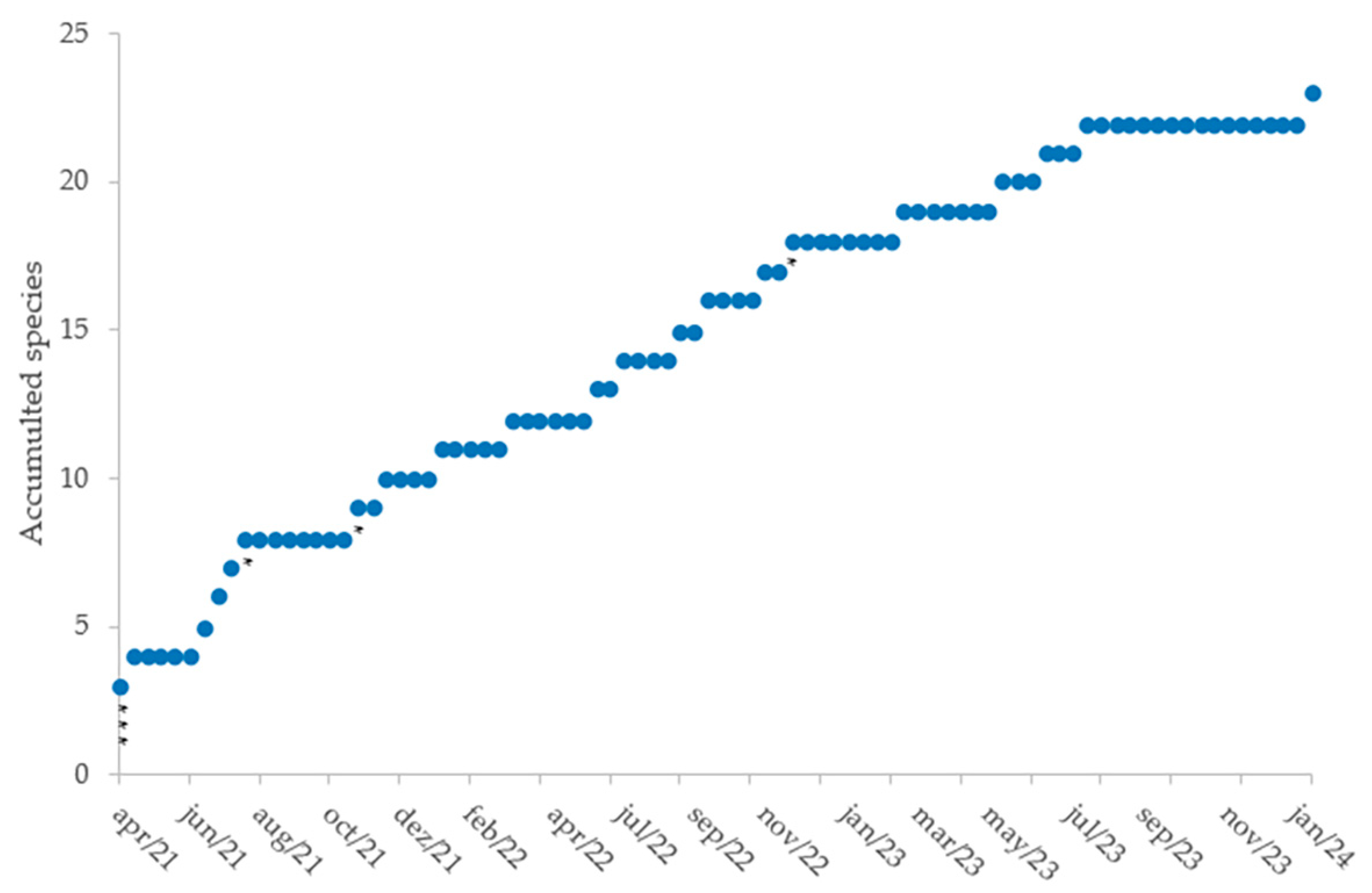
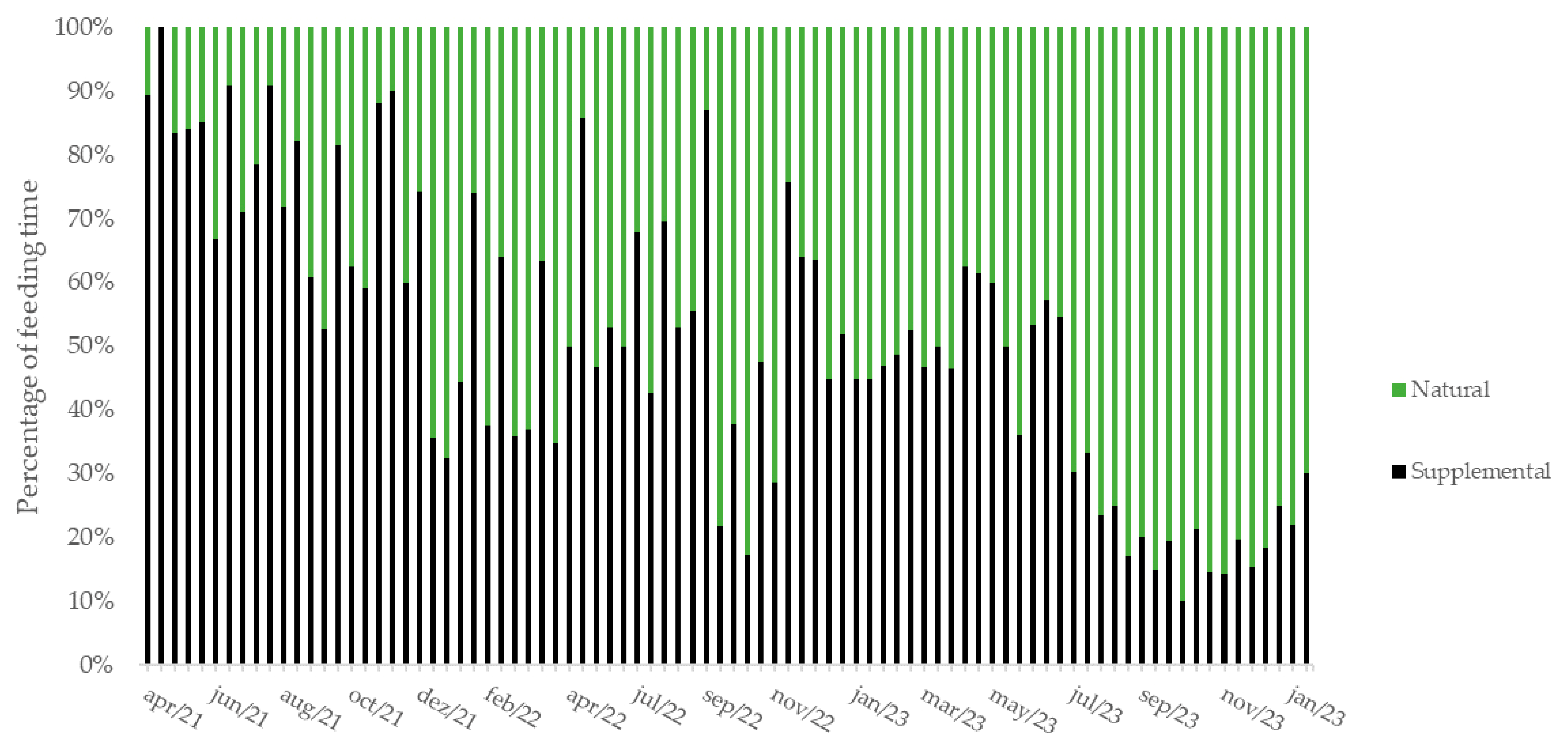
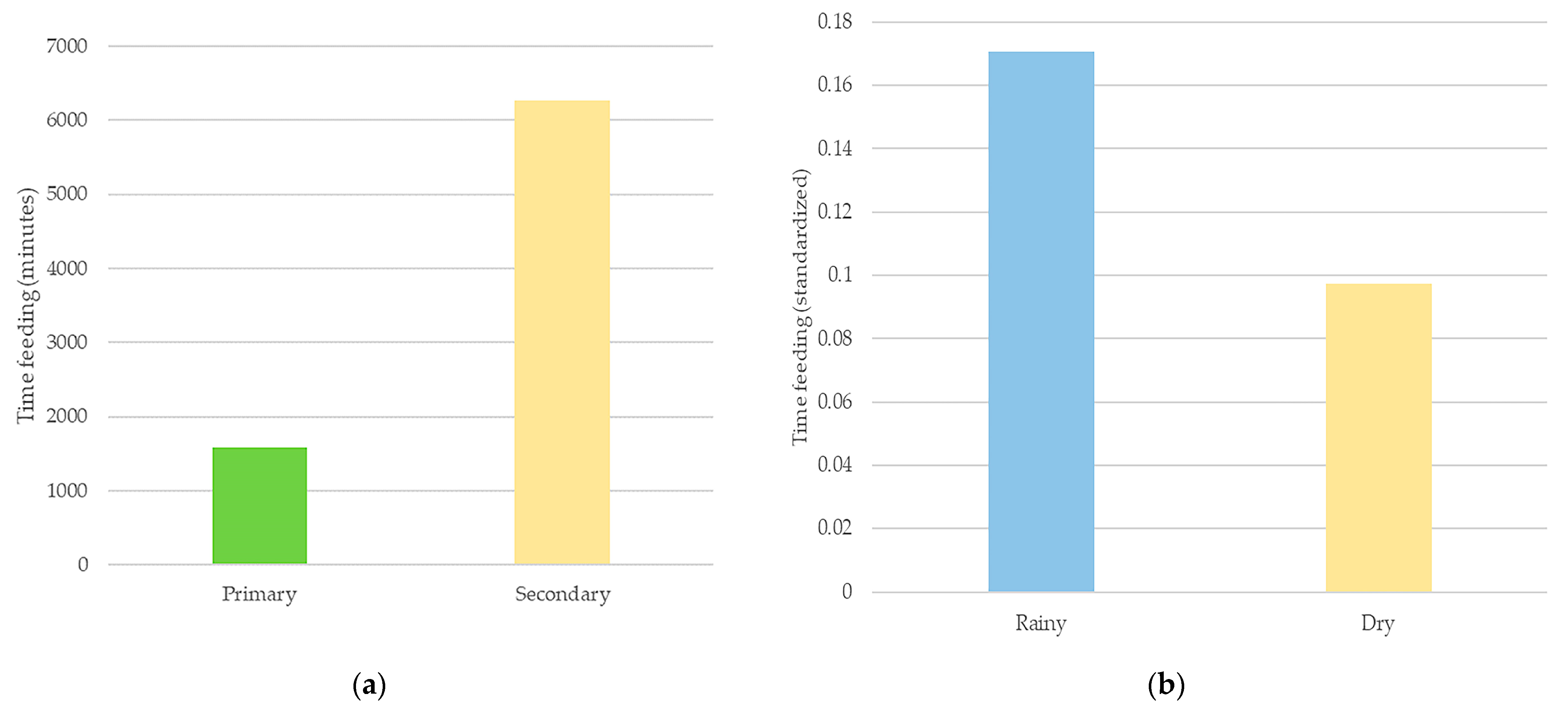
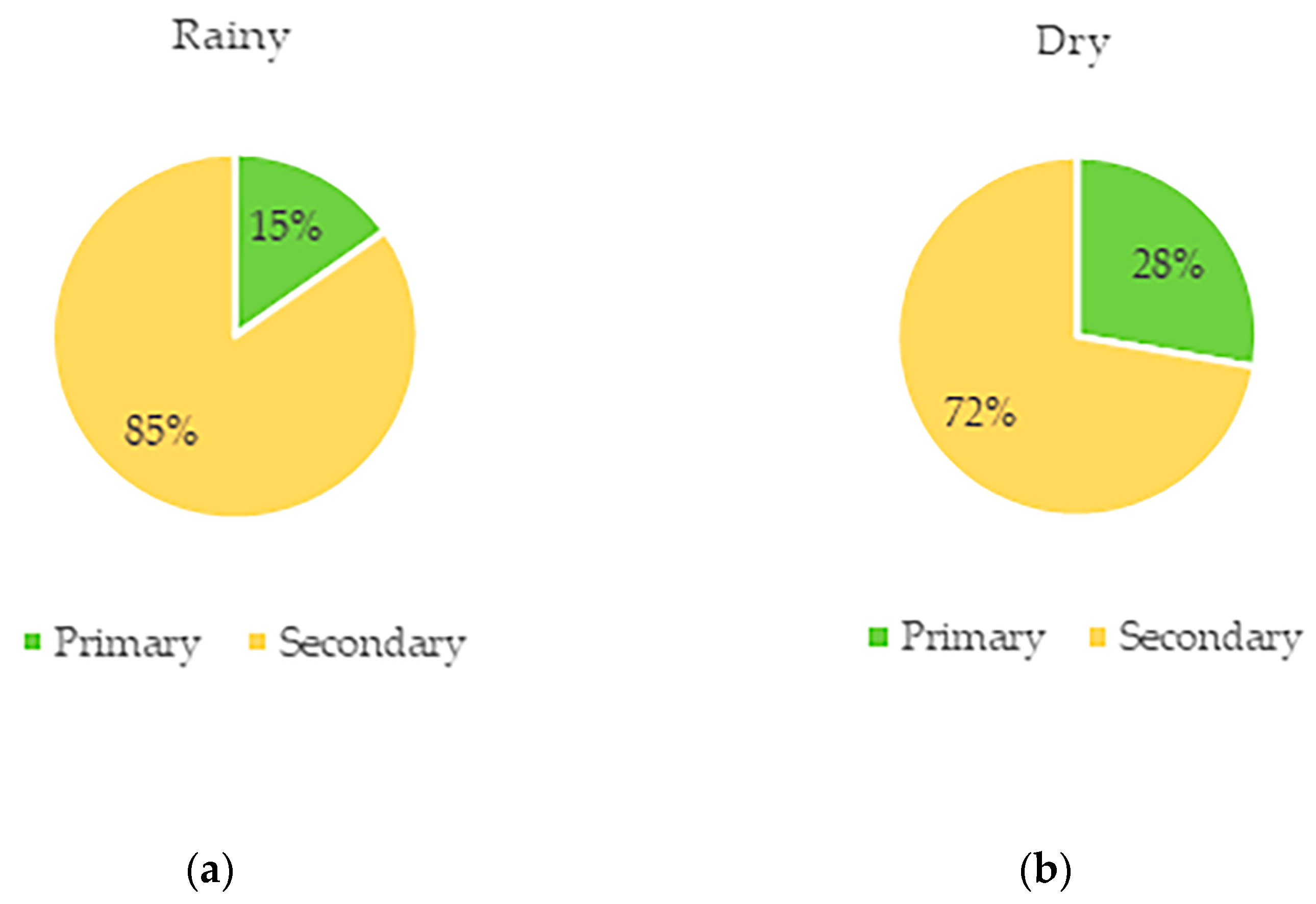
| Family | Species | Part | Bouts | Time (min) | % | J | F | M | A | M | J | J | A | S | O | N | D |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anacardiaceae | Tapirira guianensis | Fr, sd | 57 | 1510 | 18.8 | X | X | X | X | X | X | X | |||||
| Mangifera indica | Fr | 2 | 28 | 0.3 | X | X | |||||||||||
| Anacardium occidentale | Fw, fr | 2 | 15 | 0.2 | X | X | |||||||||||
| Araliaceae | * Scheflera morototoni | Fr, sd | 3 | 40 | 0.5 | X | X | ||||||||||
| Arecaceae | Euterpe oleracea | Fr, sd | 79 | 1590 | 19.8 | X | X | X | X | X | X | X | X | X | X | X | X |
| * Elaeis guineensis | Fr | 16 | 320 | 4.0 | X | X | X | X | X | ||||||||
| * Mauritiella armata | Fr, sd | 8 | 145 | 1.8 | X | X | X | X | |||||||||
| * Socratea exorrhiza | Fr, sd | 4 | 80 | 1.0 | X | X | |||||||||||
| Oenocarpus bacaba | Fw, fr | 5 | 60 | 0.7 | X | ||||||||||||
| * Mauritia flexuosa | Fw, fr, sd | 7 | 150 | 1.9 | X | X | |||||||||||
| Burseraceae | * Trattnnickia sp. | Fr, sd | 3 | 25 | 0.3 | X | X | ||||||||||
| Chrysobalanaceae | * Chrisobalanus icaco | Fw, fr | 32 | 240 | 3.0 | X | X | X | X | X | X | ||||||
| Clusiaceae | Symphonia globulifera | Fw, fr, sd | 8 | 124 | 1.5 | X | X | ||||||||||
| Cyperaceae | *Rhynchospora cephalotes | Fr, sd | 9 | 40 | 0.5 | X | X | X | X | ||||||||
| Dilleniaceae | * Tetracera sp. | Fr, sd | 2 | 14 | 0.2 | X | |||||||||||
| Erythroxilaceae | * Erythroxilum sp. | Fr, sd | 2 | 25 | 0.3 | X | X | ||||||||||
| Fabaceae | Ingá edulis | Fr, sd | 2 | 24 | 0.3 | X | |||||||||||
| Hypericaceae | * Vismia guianensis | Fw, fr, sd | 5 | 31 | 0.4 | X | X | X | X | ||||||||
| Malpighiaceae | Byrsonima crassifolia | Fw, fr, sd | 140 | 3115 | 38.7 | X | X | X | X | X | X | X | X | ||||
| Melastomataceae | Miconia cuspidata | Fr, sd | 14 | 190 | 2.4 | X | X | X | X | X | |||||||
| Miconia prasina | Fr, sd | 13 | 60 | 0.7 | X | X | X | X | X | ||||||||
| Myristicaceae | * Virola surinamensis | Sd | 8 | 175 | 2.2 | X | X | X | |||||||||
| Onagraceae | * Ludwigia decurrens | Fw | 8 | 42 | 0.5 | X | X | X |
| Family | Species | Vegetation Type | Forest Formation | Class | Common Name |
|---|---|---|---|---|---|
| Anacardiaceae | Tapirira guianensis | Secondary | Terra firma | Arboreal | Pau pombo |
| * Mangifera indica | Secondary | Terra firma | Arboreal | Manga | |
| * Anacardium occidentale | Secondary | Terra firma | Arboreal | Cajú | |
| Araliaceae | Scheflera morototoni | Primary | Terra firma | Arboreal | Morototó/mandiocão |
| Arecaceae | * Euterpe oleracea | Secondary | Terra firma and flooded | Palm | Açaí |
| Elaeis guineensis | Primary | Terra firma | Palm | Dênde | |
| Mauritiella armata | Primary | Terra firma and flooded | Palm | Miriti/Buritirana | |
| Socratea exorrhiza | Primary | Terra firma | Palm | Paxiubinha/Palmeira andante | |
| Oenocarpus bacaba | Primary | Terra firma | Palm | Bacaba | |
| Mauritia flexuosa | Primary | Terra firma | Palm | Buriti | |
| Burseraceae | Trattinichia sp. | Secondary | Terra firma | Arboreal | - |
| Chrysobalanaceae | * Chrisobalanus icaco | Secondary | Terra firma and campinarana | Shrub | Ajirú |
| Clusiaceae | Symphonia globulifera | Primary | Terra firma | Arboreal | Anani |
| Cyperaceae | Rhynchospora cephalotes | Secondary | Terra firma | Herb | - |
| Dilleniaceae | Tetracera sp. | Secondary | Terra firma | Shrub | - |
| Erythroxilaceae | Erythroxilum sp. | Secondary | Terra firma | Shrub | - |
| Fabaceae | * Inga edulis | Secondary | Terra firma | Arboreal | Ingá |
| Hypericacea | Vismia guianensis | Secondary | Terra firma and capinarana | Arboreal | Lacre |
| Malpighiaceae | * Byrsonima crassifolia | Secondary | Terra firma and campinarana | Arboreal | Muruci |
| Melastomataceae | Miconia cuspidate | Secondary | Terra firma | Arboreal | Pixiricão |
| Miconia prasine | Secondary | Terra firma | Shrub | Pixirico | |
| Myristicaceae | Virola surinamensis | Primary | Terra firma | Arboreal | Ucuuba |
| Onagraceae | Ludwigia decurrens | Secondary | Terra firma | Herb | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilarta, M.R.; De Moraes, T.T.; Gondim, M.F.N.; Lobato, C.; Da Costa, M.N.R.F.; Oliveira, R.d.A.; Silveira, L.F. Feeding Ecology of Reintroduced Golden Parakeets (Guaruba guarouba, Psittacidae) in a Protected Area in the Amazon Forest. Diversity 2024, 16, 188. https://doi.org/10.3390/d16030188
Vilarta MR, De Moraes TT, Gondim MFN, Lobato C, Da Costa MNRF, Oliveira RdA, Silveira LF. Feeding Ecology of Reintroduced Golden Parakeets (Guaruba guarouba, Psittacidae) in a Protected Area in the Amazon Forest. Diversity. 2024; 16(3):188. https://doi.org/10.3390/d16030188
Chicago/Turabian StyleVilarta, Marcelo Rodrigues, Thaís Tamamoto De Moraes, Maria Fernanda Naegeli Gondim, Crisomar Lobato, Mônica Nazaré Rodrigues Furtado Da Costa, Rubens de Aquino Oliveira, and Luís Fábio Silveira. 2024. "Feeding Ecology of Reintroduced Golden Parakeets (Guaruba guarouba, Psittacidae) in a Protected Area in the Amazon Forest" Diversity 16, no. 3: 188. https://doi.org/10.3390/d16030188
APA StyleVilarta, M. R., De Moraes, T. T., Gondim, M. F. N., Lobato, C., Da Costa, M. N. R. F., Oliveira, R. d. A., & Silveira, L. F. (2024). Feeding Ecology of Reintroduced Golden Parakeets (Guaruba guarouba, Psittacidae) in a Protected Area in the Amazon Forest. Diversity, 16(3), 188. https://doi.org/10.3390/d16030188






