Abstract
Anacondas, genus Eunectes, are a group of aquatic snakes with a wide distribution in South America. The taxonomic status of several species has been uncertain and/or controversial. Using genetic data from four recognized anaconda species across nine countries, this study investigates the phylogenetic relationships within the genus Eunectes. A key finding was the identification of two distinct clades within Eunectes murinus, revealing two species as cryptic yet genetically deeply divergent. This has led to the recognition of the Northern Green Anaconda as a separate species (Eunectes akayima sp. nov), distinct from its southern counterpart (E. murinus), the Southern Green Anaconda. Additionally, our data challenge the current understanding of Yellow Anaconda species by proposing the unification of Eunectes deschauenseei and Eunectes beniensis into a single species with Eunectes notaeus. This reclassification is based on comprehensive genetic and phylogeographic analyses, suggesting closer relationships than previously recognized and the realization that our understanding of their geographic ranges is insufficient to justify its use as a separation criterion. We also present a phylogeographic hypothesis that traces the Miocene diversification of anacondas in western South America. Beyond its academic significance, this study has vital implications for the conservation of these iconic reptile species, highlighting our lack of knowledge about the diversity of the South American fauna and the need for revised strategies to conserve the newly identified and reclassified species.
Keywords:
cryptic diversity; Boidae; South America; Llanos; Pebas system; Orinoco basin; redundant species 1. Introduction
South America is the most biologically diverse landmass in the world, with the highest diversity of species of multiple taxa compared to any other continent [1,2,3,4,5]. As such, South America is a natural laboratory for studying diversity and speciation, as well as a hotspot for conservation efforts. One problem that hinders our understanding of diversity in general is our ability to determine how many species there are in an area. In addition to the inherent difficulties of thorough sampling and fieldwork across multiple countries, understanding species diversity is complicated by the presence of both cryptic species—species that appear morphologically identical but are genetically different (e.g., Astraptes spp. (Lepidoptera: Hesperiidae))—and populations that look superficially distinct but lack the genetic divergence to infer reproductive isolation and be considered separate species [6,7,8]. Despite relatively low human population densities, the economies of most South American countries are largely dependent on extractive industries [9]. As a result, habitat degradation is an increasing problem due to land fragmentation caused by industrialized agriculture [10,11] and heavy metal pollution associated with mining activities [12,13]. These problems are exacerbated by the effect of climate change (particularly drought), the increase in fires [14,15], and the volatile politics of the region, resulting in drastic and frequent changes in environmental policy [16,17,18].
Eunectes (anacondas; Boidae) is a genus of large-bodied aquatic snakes endemic to the east of the Andes in South America [19]. Anacondas inhabit lowland rivers and wetlands. These snakes have the typical adaptations for an aquatic lifestyle, such as nostrils and eyes located dorsally on the head, and displaying a dorsal coloration and pattern that blend well with the aquatic vegetation [20,21,22]. Currently, four species are recognized in this genus, with E. murinus (Linnaeus 1758) representing a sister lineage to a clade composed of E. beniensis (Dirksen 2002), E. deschauenseei (Dunn and Conant 1936), and E. notaeus (Cope 1862) [23,24,25]. The largest of these species, Eunectes murinus (or Green Anaconda), occurs in most of the tropical regions of the continent, including the basins of the Amazon, Esequibo, and Orinoco rivers, and several smaller watersheds [22,24]. The other three species are smaller than E. murinus and are distributed within or adjacent to the distribution of E. murinus. The recently described species Eunectes beniensis, or Beni Anaconda, has a distribution restricted to the Beni region of Bolivia [25,26]. Eunectes deschauenseei, or Dark Spotted Anaconda, is distributed in the northeast of the continent [25,27]. It is found from the Amazon River delta in Brazil to French Guiana and possibly Suriname [28] (Figure 1). Eunectes notaeus, or Yellow Anaconda, has a distribution to the south of E. murinus including the Pantanal, Chaco, and other hyper-seasonal areas of tropical and subtropical South America including Brazil, Bolivia, Paraguay, Argentina, and Uruguay [29].
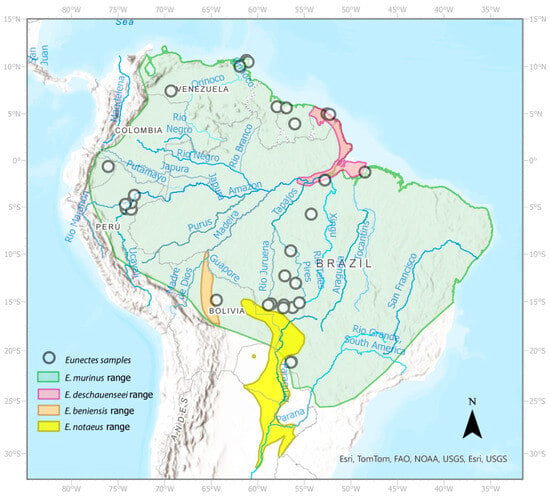
Figure 1.
Sampling location of samples used in this study. The green area is the known distribution of the Green Anaconda (Eunectes murinus). The yellow area is the distribution of the Yellow Anaconda (E. notaeus). The orange area is the reported distribution of E. beniensis and the red area is the distribution of E. deschauenseei.
Both E. deschauenseei and E. beniensis overlap strongly with E. murinus in their respective distributions and habitats [30,31]. The sympatry of E. murinus and E. notaeus is less certain (Figure 1). There is no obvious biogeographic barrier separating both species. In regions of sympatry such as the Pantanal, the extent of their syntopy is unclear; it is thought that E. murinus may venture into the deeper rivers and ponds, while E. notaeus prefers hyper-seasonally flooded habitats and appears to avoid the deeper river [32]. We know that some of these species can interbreed [24] and anecdotal reports from the pet trade suggest that their offspring may be fertile.
There have been comprehensive studies on the general natural history of the genus Eunectes [22,33,34,35] including diet [36,37,38,39,40,41,42,43,44,45], diseases [46,47], habitat use and mobility [22,30,31,32,33,44,45], allometric growth [48,49], and demography [22,50]. On the other hand, the conservation status of anacondas throughout their range is largely unexplored, although Eunectes species are protected from international trade by CITES’s Appendix 2 [51,52,53]. Eunectes species are often persecuted by humans and used for commercial trade [51,54,55,56,57,58]. All anaconda species are potentially collected locally, nationally, and internationally for medicinal and clothing purposes [51,59]. The IUCN Redlist categorizes all four Eunectes species as “least concern”. E. murinus is listed as “least concern” due to its wide range across eleven countries. However, population trends are unknown. E. notaeus is listed as “least concern” overall and is estimated to have stable populations, although it is listed as vulnerable in Argentina [60,61] and as a priority species for conservation in Uruguay [62]. Eunectes notaeus is locally threatened by agricultural development and hydroelectric dams. It is also collected for the pet trade and harvested for its skin [58,63]. Eunectes beniensis is also listed as “least concern” throughout its range [64]. This species has unknown population trends and faces the same threats as other Eunectes species. Eunectes deschauenseei is the least known species within the genus. It has a still unclear distribution range unknown population trends, and faces habitat loss due to agricultural encroachment throughout its known range.
The systematics of the group have been studied, but patterns of morphological and genetic divergence within the genus are still unclear. Dirksen [24], in a morphological revision of the genus, found that Green Anacondas from Perú had fewer but larger and rounder black spots on the dorsum than specimens from Brazil. This difference was attributed to clinal variation. The author suggested that there might be different lineages within E. murinus associated with the different drainages of its distribution. Preliminary data, using mitochondrial DNA on their phylogenetic relationships, showed that there may be different clades within E. murinus, while the differences between the clades of smaller anacondas might not be strong enough to support there being different species [65,66]. A later study using molecular and morphological data found similar results [23]. However, the phylogenetic relationships within the Eunectes complex remain unclear due to sparse and incomplete sampling, forcing inferences across vast expanses of forest and swamps with few representative samples. Also, because the distribution of E. murinus is so extensive, encompassing so many aquatic habitats, ecosystems, and different major watersheds, it is difficult to determine what biogeographic barrier may be acting to isolate different lineages today. Furthermore, due to the dynamic paleo-history of the continent, these isolation mechanisms may not even exist currently. A detailed knowledge of the natural history of the anaconda is essential to formulate well-founded hypotheses about the biogeographic barriers for each taxon, not to mention a good understanding of their divergence time in order to understand how South America’s paleo-history may have shaped them.
In this study, we use representative samples of all Eunectes species across their distribution, including nine countries, to disentangle the phylogenetic relationships of anacondas. We propose new candidate species and explore the conservation implications of our findings. We use our current knowledge of the paleo-history of South America and the distribution of other taxa with similar, or complementary, habitats and evolutionary histories to speculate on the speciation events that led to the diversification of this group.
2. Materials and Methods
2.1. Study Site and Sampling
We surveyed anacondas from various locations throughout the range of Eunectes species in South America (Figure 1). While collecting demographic and ecological data, we also collected tissue and/or blood from each specimen. In the field, we collected blood and tissue samples from E. murinus in the Venezuelan Llanos at Hato El Cedral and Hato El Frio (Table S1 for details of each sampled individual); the Brazilian states of Mato Grosso, Mato Grosso do Sul, and Para; and the Bameno region of the Baihuaeri Waorani Territory in the Ecuadorian Amazon. We collected E. beniensis and E. murinus samples in the Bolivian Beni in the Sirionó Indigenous Territory. Additional samples were donated by the Bronx Zoo (NY), Miami Metro Zoo, National Museum of Natural History, Smithsonian Museum of Natural History, Museu Emilio Goeldi, Muséum de Toulouse, The Naturalis Biodiversity Center, Universidade Federal do Mato Grosso, private collectors, and Colección Boliviana de Fauna, Bolivia (see Figure 1 for locations and Table S1 for a specimen list). Blood samples were stored in Queen’s lysis buffer [67], and scales were either stored in 80% ethanol or dried at −20 °C.
2.2. DNA Isolation and Sequencing
Genetic work was carried out at New Mexico Highlands University in the US, Instituto Federal do Mato Grosso, Naturalis Biodiversity Center in the Netherlands, and Universidad Indoamérica in Ecuador. Genomic DNA was extracted from blood and scale tissue with the Qiagen DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA). A standard protocol was used for blood samples, while scales needed to initially be lysed with the kit’s ATL buffer and proteinase K with a 12 h digest at 55 °C. We used the polymerase chain reaction (PCR) to amplify portions of mitochondrial genes cytochrome b (Cytb), NADH dehydrogenase subunit 2 (ND2), and NADH dehydrogenase subunit 4 (ND4) using both published and newly designed primers (Table 1). We modified published primers and created new primers using the E. notaeus mitochondrial genome [68] (Genbank accession AM236347.1) and the NCBI primer-BLAST implementation of Primer 3 [69]. We ran 25 μL PCR reactions using NEB Hot Start Taq 2X Master Mix (NEB, Ipswich, Maine, USA), 50–100 ng of genomic DNA, 0.4 μM of each primer. Thermocycling consisted in 5 min of initial denaturation at 94 °C followed by 36 cycles of a 40 s denaturation at 94 °C, 30 s annealing at 55 °C, and a 45 s elongation at 72 °C. A final extension step at 72 °C for 7 min was specified. PCR products were cleaned using the Monarch® PCR & DNA Cleanup Kit (NEB, Ipswich, MA, USA) using the standard protocol. Cleaned PCR products were quantified with Nanodrop One (Thermofisher, Waltham, MA, USA). Sanger sequencing was carried outin both directions using the same PCR forward and reverse primers (Psomagen (Rockville, MD, USA).

Table 1.
Summary of oligonucleotide primers and their location in the mitochondrial genome, used for PCR and Sanger sequencing of mitochondrial genes in this study.
Additional samples belonging to outgroup taxa were amplified and sequenced from specimens collected in the field, including Epicrates cenchria (Venezuela), Boa constrictor (Venezuelan origin), Corallus ruschenbergerii (Venezuela), and Corallus hortulanus (Bolivia). We also added published mtDNA sequences of Eunectes notaeus to the phylogenetic analysis (Table S1).
Base calls were verified and contigs were assembled in Sequencher® version 5.3 (Gene Codes Corporation, Ann Arbor, MI, USA). We used MEGA XI: Molecular Evolutionary Genetics Analysis version 11.0.13 [73] to align each set of gene sequences with ClustalW v.2.1 (default parameters). The mitochondrial gene sequences were concatenated into a partitioned nexus file. Sequences were deposited in Genbank under accession numbers PP273560-PP273621 (Cytb), PP334792-PP334847 (ND2), and PP334848-PP334905 (ND4) (Table S1).
Nuclear markers CMOS, RAG1, BDNF, ODC, and NT3 were also amplified with primers from [74] and TBP [75] and sequenced but did not provide sufficient numbers of variable sites within the Eunectes genus to distinguish lineages and were not included in phylogenetic analyses (Figure S2).
2.3. Phylogenetic Analysis and Genetic Divergence
Phylogenetic analyses were conducted using Bayesian inference (BI) and Maximum Likelihood (ML) methods with the concatenated matrix of ND2, ND4, and CytB genes. For both methods, we rooted the resulting trees using Boa constrictor as the outgroup. We used ModelTest-NG [76] implemented in the raxmlGUI 2.0 [77,78] to determine the best-fit model of nucleotide substitution for each gene sequence matrix using Akaike’s Information Criterion. The BI analysis was conducted in MrBayes v3.2.7 [79] using the three-gene matrix partitioned by gene and with the GTR+I+Γ model selected as the nearest overparameterized model associated with the AIC-selected best-fit model (TrN+I for ND2, TPM2uf+I+G4 for ND4, and GTR+I for CytB). MrBayes rates were estimated under a GTR model and parameter estimation was allowed to vary in each partition (ratepr = variable). We conducted two independent runs of four-chain MCMC for 100 million generations with 25% burn-in and an automatic average standard deviation of split frequencies (ASDSF) stop value of 0.01. Summary node and branch parameter estimates were examined to assess convergence (PSRF converging on 1.0). The BI 50% majority-rule consensus tree is reported with Bayesian posterior probability tree node support values. The ML analysis was executed with the three-gene matrix partitioned by gene and run in RaxML v.8.2.12 [77] using a general time-reservable (GTR) model of evolution with a gamma distribution (GAMMA) and the thorough bootstrapping algorithm with 1000 bootstrap replicates and 100 independent searches. Genetic divergence was calculated using the three-gene matrix, and also separately for each gene, as mean uncorrected genetic pairwise distances between and within lineages that were identified in our phylogenetic analyses.
2.4. Divergence Time Estimation
To estimate divergence times within the genus Eunectes, we conducted multiple molecular clock analyses using the software BEAST v.2.7.6 [80]. We implemented a GTR+I+Γ nucleotide substitution model for each gene partition (CytB, ND2, ND4). All analyses were performed under the assumptions of a Birth–Death model to infer macroevolutionary patterns and an Optimized Relaxed Clock (ORC), which was calculated uniformly across the three gene partitions. The complete dataset for this analysis comprised a total of 78 sequences. The homologous sequences from Sanzinia madagascariensis, Acrantophis dumerili (Boidae: Sanziniinae), Ungaliophis panamensis (Boidae: Ungaliophiinae), Charina bottae, Lichanura trivirgata (Boidae: Charininae), Boa constrictor, Corallus hortulana, Corallus ruschenbergerii, and Chilabothrus argentum (Boidae: Boinae) were added to the three-gene matrix to allow the use of additional calibration points. Sequences from these species were obtained from NCBI Genbank (Table 1 and Table S1). Five independent analyses were run for 40 million generations, and 15% burn-in values were chosen based on the output from Tracer (v.1.7.2). Tracer was also used to assess convergence by comparing Effective Sample Sizes (ESSs). In addition, posterior distribution plots were examined for further evidence of convergence.
We used four different approaches to estimate divergence times. These approaches make similar use of fossil evidence to impose ‘hard’ minimum age constraints on several nodes in the Boidae [81,82] but differ in their consideration of Late Cretaceous land bridges between East Gondwanan land masses to explain the divergence of Madagascan Sanziniinae from other boids [74,83,84] (Table 2).

Table 2.
Calibration points used in this study. Maximum ages were not applied for the fossil evidence; n.a stands for “not applied”.
In our first approach, we constrained the node marking the split between Sanziniinae and the rest of Boidae (a mostly Neotropical group) to be at least 80 Mya. The hard minimum used for this approach takes into account the possibility of dispersal across Gondwanan landmasses through the Gunnerus Ridge, a hypothetical land bridge that may have directly connected Madagascar to Antarctica, by 80 Mya [74,84]. A soft maximum was applied by setting a lognormal prior for this node, implying a <5% probability that its age exceeds 98.32 Mya. This age roughly corresponds to the Early/Late Cretaceous transition and matches the age of Coniophis, the oldest known crown-group fossil of snakes [79].
In a second approach, we constrained the split between Sanziniinae and other Boidae to be at least 88 Mya. This hard minimum disproves the existence of the Gunnerus Ridge [84] but allows for the possibility of dispersal from western Gondwana to Madagascar via the Indian subcontinent (which separated from Madagascar at 88 Mya [83]) and the Kerguelen Plateau (a land bridge connecting Antarctica to the Indian Continent; [84]). The same soft maximum of 98.32 Mya was applied to this node.
Third, we set the split between Sanziniinae and other Boidae to be at least 120.4 Mya. This minimum denies the existence of any land bridge to Indo-Madagascar and assumes that intercontinental dispersal across southern oceans was unlikely [84]. As a soft maximum, we used 145 Mya, which roughly corresponds to the Jurassic/Cretaceous boundary and corresponds to the oldest fossils marking the split between snakes and Anguimorph lizards [79].
Our fourth, and final, approach dates the splits within Boidae through the aid of fossil evidence only. Despite the limitations of dating from paleontological information alone, especially given the poor fossil record from Central–South America [5,85,86,87], this approach serves to test the impact of deep-time paleogeographic patterns on the resulting Boidae timeline. Therefore, it provides an alternative perspective to the other approaches used in this study. The timelines for the fossil calibration points were adapted from the other methods to include soft maximum limits (based on [79]), which are essential for reliable dating in the analysis.
2.5. Morphological Comparison of E. murinus between North and South
Comparisons were made with museum specimens from Venezuela (Museo de Ciencias Naturales de Guanare: MCNG 1042, Museo de Biología Universidad Central de Venezuela: MBUCV 1836, MBUCV 7193, MBUCV 7189), Surinam (Naturalis Biodiversity Center; RMNH.RENA.20768), as well as Brazil (Museu Emilio Goeldi MPEG 27428), and information from the literature on animals from Venezuela [88], Guyana [89], Peru, Bolivia, and Brazil [23,24]. We collected the following meristic information: the number of dorsal scales at the mid-body, number of subcaudal scales, number of ventral scales, number of ocular scales (average of the number of scales around each eye), number of supralabial scales (average from both sides), number of infralabial scales (average from both sides), number of suborbitalia scales (scales simultaneously in contact with supralabials and infra-orbitals; often called lorilabials), number of dorsal blotches from the neck to the tail (excluding the head) in the back and sides (blotches in contact were counted as different blotches), and number of blotches in contact with other blotches.
3. Results
3.1. Phylogenetics
3.1.1. Eunectes Overview
The three-gene matrix counted 2939 bp, charset ND4 = 1–848; charset ND2 = 849–1863; and charset Cytb = 1864–2939, including 1015 bp of ND2, 848 bp of ND4, and 1076 bp of cytochrome B for 71 individuals (including outgroups Boa, Corallus, Epicrates; Table S1). The concatenated alignment had 24.5% missing data, reflecting the inclusion of published sequence data for some but not all genes in favor of increasing phylogenetic accuracy by increasing taxonomic coverage [90] (coverage by taxon and gene summarized in Table S1). The BI consensus tree and ML tree had very similar topologies and therefore the BI tree is presented here (Figure 2; ML tree is presented in Figure S1). Both methods confirm a sister-clade relationship between Green Anacondas, and a clade composed of the other three anaconda species as proposed by Dirksen [24].
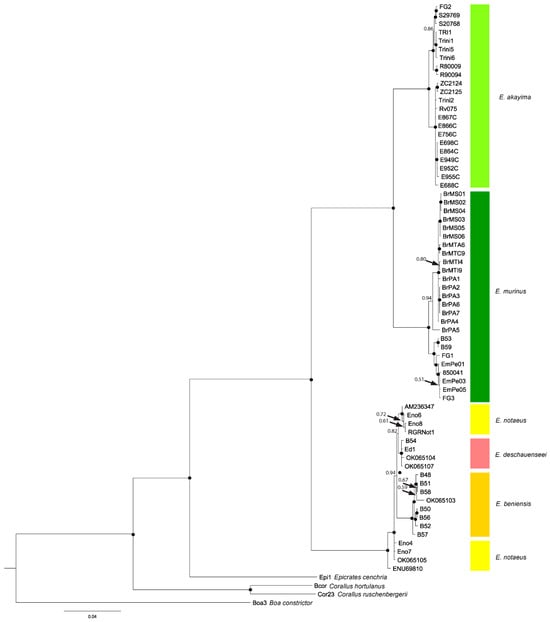
Figure 2.
Bayesian consensus phylogram for Eunectes species (50% majority-rule consensus tree) using the mtDNA gene sequence dataset (ND2, ND4, Cytb). Bayesian posterior probability node support values > 0.95 are indicated with black circles and distal values are not shown. Refer to Table S1 for details on tip labels.
3.1.2. Yellow Anaconda Phylogenetics and Taxonomy
Our analyses indicate a poorly defined phylogenetic structure in the clade composed of E. notaeus, E. deschauenseei, and E. beniensis. Although specimens identified as E. deschauenseei or E. beniensis are found in well-supported clades, those identified as E. notaeus are not, and instead represent a paraphyletic clade with E. deschauenseei and E. beniensis (Figure 2).
Comparisons were made to relate morphology to genetic placement. One of the snakes captured in the Bolivian Beni (B54) has markings that best fit the description of E. deschauenseei, as indicated by the height of the lateral flecks, which do not reach half the height of the snake, and the dorsal blotches separated by two or three scales (Figure 3). This specimen is recovered with other E. deschauenseei from French Guiana and Marajo Island in the phylogenetic analysis (Figure 2 and Figure S1). In addition, other anacondas caught in Beni (B52 and B58) also had markings that best fit the description of E. deschauenseei, but these specimens were recovered with E. beniensis in the phylogenetic analysis (Figure 2 and Figure 3). For comparison, Figure 3 also shows E. beniensis with a characteristic pattern of these lineages (larger lateral flecks), caught in Beni, that is recovered as E. beniensis in the tree (Figure 2). Therefore, our results challenge the validity of E. beniensis and E. deschauenseei as distinct species from E. notaeus.

Figure 3.
(a) E. deschauenseei caught in Beni, Bolivia (B54). (b,c) Anacondas caught in Beni that had markings of E. deschauenseei but were recovered as E. beniensis in the phylogenetic analysis (B52 and B58). (d) E. beniensis recovered as E. beniensis in the phylogenetic analysis (Photo: Paola de La Quintana).
Consistent with a previous study [23], we recovered the Yellow Anacondas as paraphyletic, with E. beniensis and E. deschauenseei nested within E. notaeus (Figure 2) and with shallow levels of divergence between the clades (Table 3). Our sampled taxa included one from the Bolivian Beni that was both genetically and morphologically E. deschauenseei, despite being outside the known range of this species. In addition, two other anacondas from the Bolivian Beni had markings that would classify them as E. deschauenseei, while the phylogenetic analysis placed them within E. beniensis. Therefore, our results challenge the validity of the Yellow Anaconda being split into species.

Table 3.
Mean pairwise genetic distances between and within known and candidate species of the Eunectes species complex. E. notaeus 1 refers to samples Eno6, Eno8, RGRnot1, and AM236347. E. notaeus 2 refers to samples Eno4, Eno7, and ENU69810. Individual gene pairwise distant matrix is presented in Table S2.
3.1.3. Green Anaconda Phylogenetics and Taxonomy
Our analyses further identify two deeply divergent, highly supported sister clades of the Green Anaconda. One clade is composed of specimens sampled in the northern part of the E. murinus range; we find this clade in Ecuador, Venezuela, Trinidad, Guyana, Suriname, and French Guiana. It can be assumed that it is also present in Colombia. The other clade includes specimens from the southern part of South America, including Perú, Bolivia, French Guiana, and Brazil. Specimens of both clades are found in French Guiana, suggesting that this country may be a contact zone for these two groups (Figure 2). The northern and southern clades have levels of divergences much higher than those for the Yellow Anaconda variants (Figure 2, Table 3). Our morphological data show that specimens from the northern and southern clades are indistinguishable morphologically (Table 4). Irrespective of crypsis, our genetic data show that these two distinct lineages within E. murinus form well-supported deep clades, allowing the separation into two species based on their genetic divergence (Table 3 and Table S2, Figure 2), temporal divergence (Figure 4 and Table 5), and branch length in both the Bayesian analysis and Maximum Likelihood trees (Figure 2 and Figure S1). The high level of genetic divergence and geographic separation justifies the recognition of the northern population as a distinct species. Therefore, we propose the scientific name Eunectes akayima sp. Nov. (see Table 6 for holotype details, and Discussion Section 4.2 for the etymology and more in-depth considerations) and the common name Northern Green Anaconda.

Table 4.
Morphological and meristic comparison of different species of Green Anaconda. The specimen listed as Linnaeus’s is the combination of type series 319 described in Systema Naturae [91] and Gronovious [92]. The other morphological info reported is from NRM-9 from Stockholm Museum. In addition, Roze [88] reports 242–262 ventral, 63–73 subcaudal, and 57–64 dorsal scales at mid-body for Venezuela. Gorzula and Pilgrim [89] scale count is within these ranges for Guyana. Data from E. akayima from this study come from Venezuela (n = 3). Other data from the literature come from Dirksen [24], reported by Tarkhnishvili et al. [23]. Suborbitalia are also called lorilabials in other references. Here, we followed Dirksen (2002) for consistency.
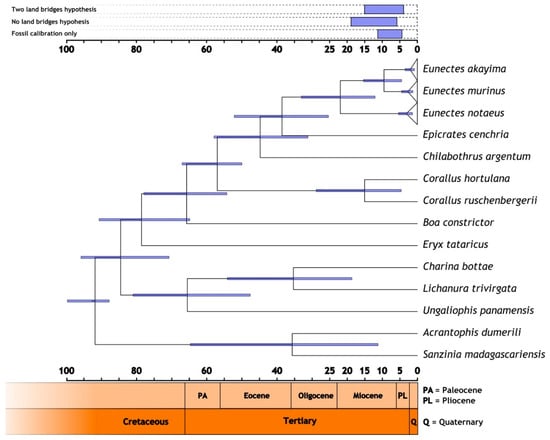
Figure 4.
Calibrated species tree depicting inferred lineage splits, assuming the scenario of one land bridge. Node bars on the tree represent the 95% highest posterior density (HPD95%) divergence interval of each node. Legend at the top shows the split of the E. akayima and E. murinus under the three other scenarios that we tested for.

Table 5.
Calculated median ages for the split of the different lineages under different evolutionary scenarios. HPD95% confidence intervals are listed in parentheses.

Table 6.
Morphological comparison of the holotype and paratype of E. akayima, and the lectotype of E. murinus.
Intriguingly, within both E. akayima and E. murinus, there are well-supported subclades with divergences at or above the level of the structures within the Yellow Anaconda clade. For the Southern Green Anaconda clade, further geographic structure is evident: one subclade is restricted to the east, around the Xingu river basin (eastern Brazil, in the states of Para, Mato Grosso, and Mato Grosso do Sul), and the other subclade extends from Peru and Bolivia to French Guiana, probably including the main channel of the Amazon River.
3.2. Divergence Time Estimation
The results of our multiple molecular clock analyses showed that the analysis based on paleontological information alone yielded slightly more recent splits than the other setups. On the other hand, the approach excluding the possibility of dispersal across Cretaceous land bridges gave older divergence times. Despite these minor differences, the ranges obtained from the different analyses were found to overlap to a large extent (Figure 4, Table 5). Our estimate for the divergence of Eunectes from its sister lineage Epicrates is approximately 46–35 Mya (95% HPD: 66.58–28.40 to 45.41–23.98; Paleocene/Eocene), depending on the approach.
4. Discussion
Our phylogenetic analyses reveal two major clades within what we currently recognize as E. murinus, one distributed in the northern part of South America and another one distributed toward the central and southern parts of the continent. The genetic distances inferred here, as well as the molecular clock analyses, suggest that these clades are divergent enough to justify their separation into two distinct species (E. akayima and E. murinus). In contrast, our analyses reveal much lower genetic divergence among three smaller-bodied species and fail to recover the monophyly of E. notaeus. These results, together with the lack of reliable diagnostic morphological characters, cast doubt on E. notaeus, E. beniensis, and E. deschauenseei as separate species.
4.1. Taxonomic Implications for Yellow Anacondas
Tarkhnishvili et al. [23] found similarly small genetic distances, and paraphyly, within the Yellow Anaconda clade and concluded that the involved species had not reached lineage sorting, with both E. beniensis and E. deschauenseei appearing as sister taxa but both nested within E. notaeus. Despite their genetic proximity and paraphyletic nesting position within E. notaeus, Tarkhnishvili et al. [23] suggested that they should be kept as separate species due to the physical geographic distance separating their known distributions. Our data show a similar genetic proximity between these groups and the same paraphyletic pattern within E. notaeus. However, we identified one individual who is morphologically and genetically similar to E. deschauenseei in the Bolivian Beni, more than 1700 Km from its known distribution. This finding calls into question the currently known geographic distributions of these species (Figure 1 and Figure 5), and even whether they are different species at all. At least two anacondas from the Bolivian Beni have markings that classify them as E. deschauenseei, while the phylogenetic analysis places them as clustered with E. beniensis samples. Taken together, this calls into question both the morphological differences found by Dirksen and Böhme [25] as well as the differences in their geographic distribution.
A crucial aspect considering E. beniensis and E. deschauenseei as valid species or even subspecies is that in biological taxonomy, a clade is a group of organisms that includes a common ancestor and all its descendants. If we were to recognize either of these as subspecies of E. notaeus, this would make E. notaeus a paraphyletic species, which is not desirable in modern taxonomy. Thus, despite the fact that E. beniensis and E. deschauenseei each form monophyletic groups, they do so by rendering E. notaeus paraphyletic (Figure 2 in this study, which is consistent with previous work [23]). Therefore, by adhering to phylogenetic principles, we do not recognize them as either species or subspecies. It may well be that there are other subspecies within E. notaeus that our dataset is insufficient to detect but with the data available, we prefer to be conservative. We propose that E. beniensis and E. deschauenseei be grouped together within E. notaeus until more detailed studies using nuclear DNA and more complete geographic sampling can determine their relationships, if any.
One possibility is that E. beniensis and E. deschauenseei are forest-dwelling ecotypes of E. notaeus, rather than different species. The darker coloration and similarities that they share with E. murinus could be homoplasy as a result of adaptation to the same habitat. It has been proposed that the preference of E. notaeus for open habitats keeps its distribution separate from that of E. murinus [23]. However, while it is true that some studies show that E. notaeus and E. beniensis may prefer open habitats, the same studies also show that both species can be found in closed-canopy forests [30,32], so closed-canopy forests would not be a barrier for their distribution. Furthermore, we have found evidence that E. notaeus does occur in closed-canopy forest (see below). Alternatively, Dirksen and Böhme [93] proposed that E. beniensis was a hybrid between E. notaeus and E. murinus but later revised this interpretation and described it as separate species [24,25]. This possibility could be re-examined. A more complete study, including nuclear patterns of lineage sorting and introgression, is needed to answer this question.
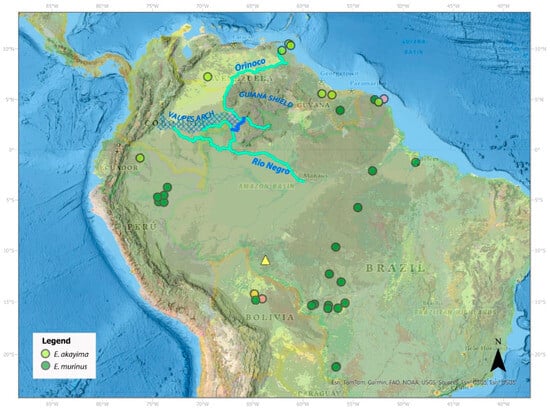
Figure 5.
Distribution of E. akayima and E. murinus samples in this study. The light-yellow dot represents E. notaeus of the Beni ecotype (formerly E. beniensis). The orange dots represent E. notaeus of the Dark Spotted Anaconda ecotypes (formerly E. deschauenseei). Notice the substantial distance between the mouth of the Amazon where the Dark Spotted Anaconda general distribution is and one of our samples found in the Bolivian Beni. The yellow triangle shows the location of a recent Yellow Anaconda reported in Rondonia, Brazil [94]. The Casiquiare river is presented in dark blue, connecting the Orinoco river (turquoise) with the Rio Negro (turquoise), which is a tributary of the Amazon. The Vaupes arch indicates where waters from the north and the south were divided in geological time.
Either scenario, hybridization or a forest ecotype of E. notaeus, would imply the existence of a population of E. notaeus living in Beni (Bolivia) and northeastern Brazil, or throughout the Amazon basin, for which there is no evidence. Unfortunately, because the distribution of each species is taken for granted, collected specimens are generally not systematically keyed. Instead, the criterion used to identify a species is where it is found [25]. For example, a recent paper on snakes from Rondonia (Brazil) includes an image of a Yellow Anaconda (E. notaeus) that is misidentified as a Green Anaconda (Figure 5) [94]. Also, a recent paper on Beni Anacondas also includes a picture of a specimen with small lateral flecks that best fits the description of E. deschauenseei [26]. We also made this mistake when working in Beni. We assumed that all anacondas that were not E. murinus were E. beniensis without properly keying all specimens. Therefore, the presence of a low-density population of E. notaeus in the Amazon basin that escaped detection cannot be ruled out.
4.2. A New Species of Green Anaconda
Our data show that two distinct lineages within the former E. murinus form well-supported deep clades, allowing the separation of two species based on their genetic divergence, time divergence, and branch length in both the Bayesian analysis and Maximum Likelihood trees: E. akayima sp. nov. and E. murinus. Although we are aware that our data come only from mitochondrial DNA, the divergence of these clades is substantial. Male and female anacondas have comparable dispersal patterns, showing strong philopatry in both sexes [22]; therefore, it is unlikely that the structure found in mDNA is the result of differential dispersal of males and females within the same species. We believe that the lack of support from nuclear genes for the separation of these clades is due to the low rate of variation at these loci, rather than a lack of separation between taxa. We also examined TATA-binding protein (TBP) and intron data, which also failed to distinguish the northern and southern clades. It separates E. murinus from E. notaeus with an extremely short branch length and a difference of one pair of bases. If two clades that separated at 24 Mya (Table 5, Figure 4) show such a small difference (Figure S2), it stands to reason that this marker would not be able to detect a split that occurred at 10 Mya. Thus, the lack of nuclear support is more likely to be related to inappropriate markers than to a lack of difference. The mitochondrial support for the separation of these two clades is superior to that found in other vertebrates in the recent literature [95,96].
In addition to the strong mitochondrial DNA support, there is a well-established pattern of the presence of sister species with northern and southern distributions on the continent. These include lizards of the genus Tupinambis, with T. cryptus occupying a distribution similar to the northern species and other species in the south [97]; Dracaena, with D. guianensis in the north and D. paraguayensis in the south [98,99]; matamata turtles with Chelus orinocensis in the north and C. fimbriata in the south [100]; Red-headed Amazon River turtles (Podocnemis erythrocephala) [101]; the arboreal boas Corallus with C. ruschenbergerii in the north and others to the south [102,103]; boas of the genus Epicrates with E. maurus in the north and the other lineages in the south [104]. While there may not be a clear barrier separating the northern clades from the southern species today, these patterns likely speak to paleogeographic events that produced this split at the continental scale in a variety of taxa (see below). Thus, the separation of E. akayima sp. nov. from E. murinus is not unique and is likely part of this continent-wide biogeographic pattern.
The first challenge in describing this new species of Green Anaconda is to determine which is the new species. In his 1758 Systema Naturae, Linnaeus gave only “America” as the place of origin [91]. The Adolphi Friderici Museum has a specimen labelled NRM9, identified as Boa murina, which could be the specimen described by Linnaeus. The record of this specimen is unclear and there is no provenance for it, but it appears to be a specimen in Linnaeus’ collection and its scale number matches that of #319 in Linnaeus’ Systema Naturae. Attempts to obtain tissue samples from this specimen were unsuccessful, which is to be expected given the low probability of obtaining usable DNA from such an old, formalin-fixed specimen. It is likely that the specimen described by Linnaeus was from Suriname, as much of the trade to Europe came from this area (E. Åhlander pers. comm.). However, our data show that French Guiana is probably a contact zone where both species can be found, and Suriname may also be a contact zone. Therefore, even if we knew for sure that specimen #319 in Linnaeus’ collection was from Suriname, we would still not know which species it was, because both species are truly cryptic, and there is no way to tell from morphological data which species the type belongs to, as far as anyone can tell. When Linnaeus described the Boa murina, he provided reference to other specimens from Seba [105] and Gronovious [92], who in turn cited two specimens by Seba and added his description of a third specimen. The plate 29 specimen cited by Linnaeus has no source in Seba’s catalogue. Gronovius refers to this specimen in one of his entries, entry 44, and also to specimen 1 from plate 23 of Seba’s catalogue. This snake, whose drawing resembles that of E. deschauenseei, has a source, Guianensis, which probably refers to present-day French Guiana. As there is no scale number on either of Seba’s specimens, it is uncertain whether Linnaeus ever examined the specimens himself, or whether he simply based his inclusion of these specimens on the drawings in Seba’s catalogue. Seba’s second collection was sold at auction after his death [106] and is probably lost. Gronovious also provides a description of a specimen of his own and alludes to specimen A in plate 606 of Physica Sacra [107]. This specimen is doubtlessly an anaconda that was in the collection of Johann Heinrich Linck, Leipzig. However, there is no type specimen of anacondas in this collection today (Bauer, per com).
Since the provenance of specimen NRM9 is unknown, and given that both Green Anaconda species are truly cryptic, there is no way to determine which clade the syntypes belong to other than genotyping, which is not possible with such an old specimen. We propose to name E. murinus as the southern species because of its larger distribution and for historical reasons. We believe that naming the new species as the one with the smallest distribution will contribute to the stability of the nomenclature code, as it will result in less geographical change. In addition, although E. akayima sp. nov. is found in French Guiana and Suriname, it is a species of the Orinoco Basin, which was not explored by European naturalists until later. The lectotype for E. murinus is a specimen from the Xingu River in Para, Brazil, labelled MPEG 27,428 in the Museu Paraense Emílio Goeldi. It was found in Altamira, State of Para, Brazil (3°9′16″ S, 52°14′11″ W) in October 2011 by Emil Hernández. Since most of the distribution of E. murinus is in Brazil, we consider it appropriate that the lectotype is in that country, even though the original specimen may have been collected in Suriname. We also designate specimen MCNG 1042 from UNELLEZ Museo de Ciencias Naturales, Venezuela, as the holotype for E. akayima sp. nov. This specimen was collected by Jesús Rivas in March 1993 at Hato El Cedral, Apure Estate, Venezuela (7°25′0.4″ N, 69°19′51″ W). The diagnostic features of this species, which are morphologically cryptic, required DNA sequencing. We also designate a paratype for E. akayima sp. nov. specimen RMNH.RENA.20768 deposited in the Naturalis Biodiversity Center in the Netherlands and MBUCV 7189 located at the Museo the Biología de la Universidad Central de Venezuela. Information for other type specimens can be found in Table S3.
Etymology
We propose the common name, Northern Green Anaconda, for Eunectes akayima sp. nov. Before the arrival of the Spaniards, northern Venezuela was occupied by various Indigenous nations, among which the Caribs were an important group. Several Carib nations remain including the Kariña, Panare, Yekuana, Pemones, and Akawaio. The word for anaconda in various Cariban languages is a variant of akayima/okoyimo/okoimo, in which akayi/okoyi/okoi means “snake” and the suffix -ima/-imo means “large”. The suffix -ima/-imo does not necessarily mean ‘large’ in a physical sense. Rather, it is used to denote the kind of largeness that indicates a different category of being. The literal translation of akayima is “The Great Snake” (S. Gildea pers. Communication [52]). The species name akayima is pronounced as follows: əkəyimə in standard dictionary pronunciation font; ŭkŭyēmŭ using the phonics; and uh-kuh-yee-muh using the Plotkin method for English-like writing to capture Cariban language pronunciations [108]. The word akayima is also used to refer to the rainbow, probably associated with a feathered serpent in their belief system that came out after rains to dry its feathers [109]. We, therefore, acknowledge the culture of these Indigenous people who share their territories with this species by adopting their word for anaconda as the specific epithet for this new species. We propose the common name for E. murinus as Southern Green Anaconda, to promote taxonomic stability for the most widely distributed species and avoid confusion. Table 6 provides a comparison between the E. akayima sp. nov. holotype, one of its paratypes, and the E. murinus lectotype.
Previous work had identified other candidate species and subspecies of the anaconda in the Orinoco basin with somewhat similar distribution to E. akayima [110]. However, all of these differences have been found to be inconsistent [24,27,111]; therefore, these synonyms are all invalid. In addition, the word “akayima” has been indigenously used to designate this species for at least hundreds (and perhaps even thousands) of years before the use of any of the other synonyms. It was certainly in use in 1758 when the Code started counting names as valid; so, akayima is clearly the senior synonym. This is, admittedly, an unorthodox position regarding the International Code of Zoological Nomenclature [112], which prefers the names that have been published in Western science as “valid”. However, it is well due time that Western science starts recognizing the ancestral knowledge and cultural legacy of non-Westernized society. If we respect and honor the culture of these original nations, accepting akayima as the senior synonym is unavoidable.
4.3. Paleogeographic Events Triggered the Origin of Large-Bodied Aquatic Snakes
Our estimate for the divergence of Eunectes from its sister lineage Epicrates is approx. 46–35 Mya (Paleocene/Eocene) depending on the approach (Table 5). Previous studies have claimed this divergence to be more recent [74,113], but this estimate has been subject to great variability [103]. The differences we found are likely the result of the impossibility to apply soft maximum limits to approximately half of our fossil-based calibration points in our analysis. Therefore, those were only treated as hard minima. While the problem of the underestimation of divergence dates due to fossil calibration featuring hard maxima may be a common issue in molecular clocks, this might be particularly impactful for regions with notoriously poor fossil records, like South America. During much of the Cenozoic large extensions of the Amazon basin were flooded forest and flooded habitats covered by black water systems with a very low pH [5,85,86]. This low pH would have dissolved the calcium phosphate from the bones in a short time, making fossilization substantially less likely than it normally is.
A Paleocene/Eocene date for the origin of Eunectes as an aquatic lineage seems plausible because it matches relevant paleogeographic events and similar origins of other South American aquatic taxa. As the Nazca plate subsided under South America, circa 90 Mya [114], it would have made the mouth of the Proto-Orinoco/Amazon shallower, preventing it from draining all its volume and flooding extensive parts of the continent. This was a process of general flooding of the western part of the continent at geological speed. It likely started with the river backing up and permanently flooding its flood plains [5,87]. The proportion of flooded forest, Varzea and Igapo, increased over time, gaining surface over Terra Firme forest, with Varzea forest expanding from west to east into the continent [115]. Because of the slow nature of this process, it would have allowed for natural selection to develop aquatic lineages as the aquatic habitat became more abundant and aquatic niches became available [87].
Previous studies have estimated the appearance of Chelus, an aquatic turtle specializing in small forest creeks around 70 Mya [116]. Around 60 Mya, South American side-necked turtles (Podocnemididae) diversified into new lineages [117]. At approximately the same time, alligatorids split into two lineages [118]: a larger one, Caiman, that prefers rivers and lagoons, and Paleosuchus, a smaller forest specialist living in small creeks inside the forest [119]. Around 40 Mya, a lineage of very large caimans, Melanosuchus, also split from the same lineages [118]. In addition, approximately 40–35 Mya, Teiidae produced two aquatic linages, Crocodilurus and Dracaena, from terrestrial ancestors [120]. Lastly, approximately 49 Mya, we see the appearance of a strictly arboreal lineage of boids: Corallus [103,113]. Specialization to living on the trees could be an evolutionary response to a flooded understory that was unavailable. Taken together, this scenario speaks of a generalized increase in habitats for aquatic lineages throughout the continent, and throughout the Cenozoic, that supports the notion that Eunectes split from Epicrates earlier than other studies have estimated.
We hypothesize that Eunectes diverged from Epicrates due to the increased occurrence of flooded forest resulting from the initial damming of the Proto-Orinoco/Amazon. This process would have opened extensive habitats for aquatic snakes to exploit. The evolution of the dorsal eye and nostril placement and cryptic coloration would have been beneficito hunt terrestrial prey in the flooded forest. Surprisingly, despite Eunectes being an aquatic lineage, and the abundance of variety of fish in South America, fish are not an important part of the diet of any extant lineages of anacondas. While E. notaeus has been reported scavenging on fish dead in droughts [40], the importance of fish in anacondas’ diets is negligible [33,39,40,121]. The shallow water of the flooded forest likely became quickly dysoxic or anoxic with the warmth of the area [122,123], not allowing for fish as reliable prey. Thus, as anacondas adapted to their new aquatic habitats, they continued preying on terrestrial prey in the flooded forest, rather than undergoing a dietary shift to fish [52].
It is likely that the last common ancestor of all Eunectes species was not much larger than a regular Epicrates. The large size of today’s Eunectes probably evolved later, as living in water released them from the constraints of gravity and the aquatic vegetation allowed them to hide from their prey despite their large size. The split between the large- and the small-bodied clades occurred an estimated 26 (95% HPD: 40.97–14.67)-20 (95% HPD: 29.61–12.24) Mya (Oligocene), a time when the western Amazon was covered by a mega wetland [124,125]. At this point, the Andes had completely blocked the passage of the Proto-Orinoco/Amazon and the river was diverted to the north, forming the Pebas system [5,87,114,115,126]. While the permanent waters of the Pebas system may have allowed the evolution of large-bodied aquatic specialists, the specific topology of the adjacent area might be the reason for the evolutionary preservation of smaller-bodied relatives. Due to the extremely flat relief of the area (1.5 cm/km; [127]), a small change in the water level would have resulted in a substantial displacement of the water edge [5]. The Eunectes populations living at the edges of the hyper-seasonal Pebas system would, therefore, need to travel long distances on dry land to track the receding waters in every dry season. The need to move across dry land might have constrained their growth, thus maintaining a lineage of small-bodied anacondas (E. notaeus). Since Pebas drained toward the north, there would have been a constant volume of water in this direction, causing only large-bodied anacondas (E. akayima) to be found toward the north of the Pebas system. This would explain today’s lack of small-bodied Eunectes to the north of Pebas, even today with part of the area possessing developed hyper-seasonal Savannahs. These are no older than ten thousand years [128,129].
4.4. Miocene Divergence of Northern and Southern Green Anacondas
Our molecular clock analyses indicate that E. akayima and E. murinus diverged in the Miocene at the same time that other South American taxa were undergoing similar-aged north–south divergences (Table 5, Figure 4). The vicariant event splitting these lineages might have been associated with the uplift of the Vaupés arch, an elevation that connected the Andes with the Guyana shield on the southern end (Figure 5). The rise of this arch separated the north from the south, in what is now the Venezuelan and Colombian Llanos. This was the result of the continent-wide readjustment of the landscape that resulted in tilting the continent to the east and the separation of the Proto-Orinoco and Proto-Amazon River into their current descendants [114,126]. The rise of this arch occurred almost synchronously to the split of these clades. So, this was likely the vicariant event that separated these two species. However, the current distribution of E. akayima is far south of the Vaupés arch, all the way to the Yasuni National Park, in the Ecuadorian Amazon, as sedimentation has changed the current topology of the region [127]. Looking at the big picture, combined, the presence of the Pebas system as a barrier for dispersal of shallow water organisms [130] as well as the Vaupés arch splitting the watersheds likely explain the separation between the north and south of much of the aquatic fauna in South America including not only anacondas but also caimans [131,132], matamata turtles [100], stingrays [127], and lizards [97,133].
Eunectes murinus is composed of an eastern clade associated with the Xingu river, which diverged approximately 3.5-2.53 Mya from the one found in the west, associated with the Beni drainage. It is possible that rivers Tapajos and Madeiras might also have independent lineages since they have similar topologies. The similarities between anacondas in the western Amazon and those in French Guiana speak of the Amazon River as a waterway connecting these areas.
Unfortunately, before drawing more precise conclusions about the distribution ranges of both species of Green Anaconda and potential interaction zones, a more comprehensive sampling of the intervening areas will be needed. One problem hindering our understanding of these distribution patterns is the difficulty of identifying what would constitute a biogeographic barrier for anacondas. At first glance, the whole basin seems like fair ground for anaconda dispersal. It seems like they should be able to disperse more broadly into their landscape given today’s homogeneity of many habitats. Indeed, there does not seem to be any barrier preventing E. akayima from moving into the southern part of the continent or preventing E. murinus from dispersing north. The Casiquiare river flows from the Orinoco to the Rio Negro, which in turn flows into the Amazon (Figure 5) [114,127]. Consequently, E. akayima could disperse down to the northern bank of the Amazon. At its broadest section, and perhaps starting at Manaus, the Amazon is a formidable water body that anacondas might not be inclined to swim across, since they much prefer shallow water bodies with aquatic coverage [22], but the presence of E. murinus in French Guiana and the ecotype of E. notaeus known as the Dark Spotted Anaconda north and south of the lower Amazon suggests that the river is not a definite barrier for anaconda dispersal. Our Peruvian samples come from the Iquitos region. The Amazon in this region is narrow enough not to constitute a barrier against anaconda dispersal. In addition, E. akayima is also found in Yasuni, Ecuador, where the Napo River flows into the Amazon from eastern Ecuador, so there would be a clear path for this species to colonize the rest of the Amazon basin. Clearly, more sampling is needed to determine possible contact zones between E. akayima and E. murinus.
4.5. The Arrival of the Pleistocene
It is intriguing that circa 3 Mya, all lineages underwent further splits. We see this in the E. akayima splitting in Venezuela with the watersheds to the south of the mouth of the Orinoco. We find the same split between the Peru/Bolivia clade and the eastern one in E. murinus. It is noteworthy to mention that at this same time, E. notaeus was splitting into the three lineages we find today. While these splits are not deep enough to grant separation into multiple species, they do tell us about the dynamics in the continent. They coincide with the start of the Pleistocene at its glaciations. As the South American continent was rearranging its drainage, the Amazon and the Orinoco had their current location. There were still extensive wetlands and marshes covering a good part of the continent [134] where likely anacondas thrived. During the glaciations, the icecaps sequestered a lot of the global water, producing the expansion of forest [135] as the marshes were receding. This would have led to the separation of aquatic habitats, likely resulting in the synchronous split among these aquatic lineages.
4.6. Conservation Assessments
The proposed division of distinctive Green Anaconda lineages into two species may prompt further conservation assessment in their respective ranges and countries. This study stresses the lack of knowledge on the distribution of species representing large top predators. As top predators, anacondas are especially vulnerable to habitat degradation: not only do they suffer from the damage to the habitat, they are also heavily impacted by the damage to their prey base [136,137]. In addition, conflict with humans also threatens predators, since they may accidentally prey upon livestock, as well as being generally perceived as a danger to humans [22,51,52,138]. The reason behind E. murinus being considered of least concern is its large distribution [52,53]. Evidence pointing toward two species in its original range may change that conclusion. Our study highlights our lack of knowledge on the distribution of different populations and their possible connectivity; we do not know which populations may be under stress due to inbreeding. As habitat fragmentation and other forms of habitat degradation continue, the conservation status of these top predators may change.
Further surveys should aim to decipher the status of the different lineages in E. notaeus. The newly documented distribution of E. notaeus makes us wonder about its true conservation status. The forest-dwelling ecotype seems to have very small densities and would represent a very vulnerable lineage. Alternatively, E. notaeus may be more common in the Amazon basin but misidentified in the field, demonstrating the need to further clarify morphological distinctions of these subspecies.
Introgression among Eunectes species is undocumented, but possible. Future studies using nuclear markers may help to identify the geographic areas and extent of potential introgression and comment on the potential conservation concerns of hybridization zone expansion with climate change and threats of outbreeding depression for any of our proposed species or subspecies groups.
The deforestation of the Amazon basin due to agricultural expansion has resulted in an estimated 20 to 31% habitat loss [11,15], which might impact up to 40% of its forests by 2050 [15]. Anaconda species are likely to be threatened by deforestation processes driven by agriculture, forest fires, climate change, and drought [52]. These phenomena affect their prey base and habitat in general. This emphasizes the need to further delineate Eunectes species ranges and population trends.
5. Conclusions
This study provides the most extensive sampling of anacondas to date and raises new questions about the distinctive lineages, geological history, and conservation status of the Eunectes group. Historical, geographic, and landscape-scale events may have shaped the current distribution and composition of the species. Looking at the ecology of present-day anacondas, it would seem that the entire Amazon/Orinoco basin would be an area of free dispersal for anacondas. However, the presence of a new cryptic species in the north and the E. murinus in the south tells us that we still know very little about the gene flow dynamics of a large vertebrate in the world’s most diverse terrestrial ecosystem. The idea that there could be a population of E. notaeus living throughout the Amazon basin that has managed to evade detection thanks to a coloration that superficially resembles that of E. murinus is puzzling, and speaks loudly to the need for thorough sampling to better document the diversity we still have.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16020127/s1: File S1: Sequences analyzed in this study. Figure S1: Phylogeny constructed from mitochondrial three-gene concatenated partitioned Maximum Likelihood analysis; Figure S2: Maximum Likelihood using TBP showing lack of resolution between the clades; Table S1: Samples analyzed in this study; Table S2: Mean pairwise genetic distances using individual genes; Table S3: Location of the types designated in this study for this study.
Author Contributions
Conceptualization, J.A.R. and S.C.-R.; methodology, S.C.-R. and B.G.F.; software, S.C.-R. and M.M.; validation, B.G.F. and F.J.V.; formal analysis, S.C.-R., D.S.-V. and S.M.; investigation, J.A.R. and S.C.-R.; resources, M.T.B. and P.B. (capture of E. akayima anacondas in Baihuaeri Waorani Territory), F.J.V., L.F.P., P.D.L.Q. and E.H.; data curation, S.C.-R.; writing—original draft preparation, J.A.R. and S.C.-R.; writing—review and editing, J.A.R., P.D.L.Q., M.M., L.F.P., G.A.R., S.M., D.S.-V., M.T.B., P.B., G.M.B., F.J.V., E.H., J.E.G.-P., B.G.F. and S.C.-R.; visualization, J.A.R., S.C.-R., M.M., M.T.B. and P.B.; supervision, J.A.R. and B.G.F.; funding acquisition, J.A.R., S.C.-R., B.G.F. and F.J.V. All authors have read and agreed to the published version of the manuscript.
Funding
Partial Sequencing charges were under-written by National Geographic, NM Inbre, NMHU FRC, and Disney. BGF was funded as a National Geographic Explorer and the Waorani expedition was documented by National Geographic for their upcoming series Pole to Pole.
Institutional Review Board Statement
Animal handling was carried out and tissues were obtained under IACUC protocol at New Mexico Highlands University, Approval 2012/7-12-2012, and University of Queensland, Animal Ethics Approval 15 March 2021/AE000075. Registration of E. akayima in zoobank: A58A262E-2E07-48D3-B712-209CCDFFD038. Permit for working on Indigenous land in Ecuador: MAATE-DBI-CM-2022-0259.
Data Availability Statement
All Genbank accession numbers of gene sequences are available in the Supplementary Materials.
Acknowledgments
We thank The Wildlife Conservation Society, The National Geographic Society, the Doue de le Fountain Zoological Park, Miami Metro Zoo, Bronx Zoo, American Museum of Natural History, and private collectors. We are very grateful to the following four people from the Naturalis Biodiversity Center for their great help in sequencing the DNA of two (museum) Northern Green Anaconda specimens (RMNH.RENA.20768 and RMNH.RENA.29769): Elza Duijm, Esther Dondorp, Stacey Dubbeldam, and Dick Groenenberg. We would also like to express our gratitude toward the Naturalis Biodiversity Center for swiftly providing us with the necessary permission to perform the invasive sampling in these two anaconda specimens. We also thank COVEGAN, Estación Biologica Hato El Frío, for logistic assistance and for permission to perform this study on their property. We thank New Mexico Highlands University FRC and NM INBRE for partial funding. We also thank the governments of Bolivia, Brazil, Ecuador, France, Perú, Venezuela, Suriname, and Trinidad and Tobago and the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) for research permits. We also thank Erik Åhlander and Claudia Samuelsson for information on NRM9 from the museum Adolphi Friderici. We thank the staff of the Museo de Biología of the Universidad Central de Venezuela (MBUCV) for providing access to their collection of amphibians and reptiles, especially Professors Mercedes Salazar and Hedelvy J. Guada. We are in debt to Anthony Fouquet for early comments on the ms and providing tissue samples. We thank Nico Lormand for use of E. notaeus photo. We thank Christine Strussmann and Amalia Espinoza for help and collaboration during fieldwork in Brazil and Ecuador. We also thank Kim Roelants for early comments on the ms and assistance in the phylogenetic dating analysis. We thank Andi Wolfe for help, encouragement, and assistance in the beginning of the molecular study. We thank E. Lavilla for advice and help navigating taxonomic questions. In addition, we thank Spike Gildea for help with Carib language and its pronunciation. Finally, we thank Will Smith for his help in collecting the E. akayima samples from the Bameno region of the Baihuaeri Waorani Territory in Ecuador.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rull, V. Speciation Timing and Neotropical Biodiversity: The Tertiary-Quaternary Debate in the Light of Molecular Phylogenetic Evidence. Mol. Ecol. 2008, 17, 2722–2729. [Google Scholar] [CrossRef]
- Haffer, J. Hypotheses to Explain the Origin of Species in Amazonia. Braz. Biogeogr. 2008, 68, 917–947. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The Biodiversity of Species and Their Rates of Extinction, Distribution, and Protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef] [PubMed]
- Claramunt, S.; Cracraft, J. A New Time Tree Reveals Earth History’s Imprint on the Evolution of Modern Birds. Sci. Adv. 2015, 1, e1501005. [Google Scholar] [CrossRef]
- Rivas, J.A. Climate Changes and Speciation Pulses in a Nearly Flooded Continent: Tackling the Riddle of South America’s High Diversity. Ecotrópicos 2020, 32, 1–21. [Google Scholar] [CrossRef]
- Alroy, J. How Many Named Species Are Valid? Proc. Natl. Acad. Sci. USA 2002, 99, 3706–3711. [Google Scholar] [CrossRef] [PubMed]
- Bickford, D.; Lohman, D.J.; Sodhi, N.S.; Ng, P.K.L.; Meier, R.; Winker, K.; Ingram, K.K.; Das, I. Cryptic Species as a Window on Diversity and Conservation. Trends Ecol. Evol. 2007, 22, 148–155. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten Species in One: DNA Barcoding Reveals Cryptic Species in the Neotropical Skipper Butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.; Schmink, M.; Abers, R.; Assad, E.; Humphreys Bebbington, D.; Eduaro, B.; Costa, F.; Durán Calisto, A.M.; Fearnside, P.M.; Garrett, R.; et al. The Amazon in Motion: Changing Politics, Development Strategies, Peoples, Landscapes, and Livehoods. In Amazon Assessment Report 2021, Part II; United Nations Sustainable Development Solutions Network: New York, NY, USA, 2021; pp. 14.1–14.65. [Google Scholar]
- Alencar, A.A.; Brando, P.M.; Asner, G.P.; Putz, F.E.; Alencar, A.A.; Brando, P.M.; Asner, G.P.; Putz, F.E. Landscape Fragmentation, Severe Drought, and the New Amazon Forest Fire Regime. Ecol. Appl. 2015, 25, 1493–1505. [Google Scholar] [CrossRef]
- Albert, J.S.; Carnaval, A.C.; Flantua, S.G.A.; Lohmann, L.G.; Ribas, C.C.; Riff, D.; Carrillo, J.D.; Fan, Y.; Figueiredo, J.J.P.; Guayasamin, J.M.; et al. Human Impacts Outpace Natural Processes in the Amazon. Science 2023, 379, eabo5003. [Google Scholar] [CrossRef]
- Velásquez Ramírez, M.G.; Barrantes, J.A.G.; Thomas, E.; Gamarra Miranda, L.A.; Pillaca, M.; Tello Peramas, L.D.; Bazán Tapia, L.R. Heavy Metals in Alluvial Gold Mine Spoils in the Peruvian Amazon. Catena 2020, 189, 104454. [Google Scholar] [CrossRef]
- Lages, A.S.; Miranda SA, F.; Ferreira SJ, F.; Albuquerque, S.D.; Cetauro, A.; Lopes, A.; Silva, M.L.D. Dynamics of Heavy Metals in the Waters of Igarape Do Quarenta: The Water Body That Crosses the Industrial Hub in the Brazilian Amazon. Open Sci. J. 2022, 7, 1–13. [Google Scholar]
- Laurance, W.F.; Vasconcelos, H.L.; Lovejoy, T.E. Forest Loss and Fragmentation in the Amazon: Implications for Wildlife Conservation. Oryx 2000, 34, 39–45. [Google Scholar] [CrossRef]
- Feng, X.; Merow, C.; Liu, Z.; Park, D.S.; Roehrdanz, P.R.; Maitner, B.; Newman, E.A.; Boyle, B.L.; Lien, A.; Burger, J.R.; et al. How Deregulation, Drought and Increasing Fire Impact Amazonian Biodiversity. Nature 2021, 597, 516–521. [Google Scholar] [CrossRef]
- Raftopoulos, M.; Morley, J. Ecocide in the Amazon: The Contested Politics of Environmental Rights in Brazil. Int. J. Hum. Rights 2020, 24, 1616–1641. [Google Scholar] [CrossRef]
- De Oliveira, G.M.; Sellare, J.; Börner, J. Mind Your Language: Political Discourse Affects Deforestation in the Brazilian Amazon; ZEF Discussion Papers on Development Policy N. 326; ZEF: Bonn, Germany, 2023. [Google Scholar]
- Zuluaga, S.; Vargas, F.H.; Kohn, S.; Grande, J.M. Top-down Local Management, Perceived Contribution to People, and Actual Detriments Influence a Rampant Human—top Predator Conflict in the Neotropics. Perspect. Ecol. Conserv. 2022, 20, 91–102. [Google Scholar] [CrossRef]
- Nogueira, C.C.; Argôlo, A.J.S.; Arzamendia, V.; Azevedo, J.A.; Barbo, F.E.; Bérnils, R.S.; Bolochio, B.E.; Borges-Martins, M.; Brasil-Godinho, M.; Braz, H.; et al. Atlas of Brazilian Snakes: Verified Point-Locality Maps to Mitigate the Wallacean Shortfall in a Megadiverse Snake Fauna. South Am. J. Herpetol. 2019, 14, 1–274. [Google Scholar] [CrossRef]
- Wagler, J.G. Natürliches System der Amphibien: Mit Vorangehender Classification der Säugethiere und Vögel: Ein Beitrag zur Vergleichenden Zoologie; der J. G. Cotta’schen Buchhandlung: München, Germany, 1830. [Google Scholar]
- Lopez, C.G. Fauna Legendaria; Editorial Arte: Caracas, Venezuela, 1984. [Google Scholar]
- Rivas, J.A. Anaconda: The Secret Life of the Wolrd’s Largest Snake; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Tarkhnishvili, D.; Hille, A.; Waller, T.; Todua, M.; Murtskhvaladze, M.; Böhme, W. Morphological Trends and Genetic Divergence in Anacondas, Genus Eunectes Wagler, 1830 (Serpentes: Boidae). Amphibia-Reptilia 2022, 43, 379–393. [Google Scholar] [CrossRef]
- Dirksen, L. Anakondas: Monographische Revision der Gattung Eunectes Wagler, 1830 (Serpentes, Boidae); Natur und Tier—Verlag: Münster, Germany, 2002. [Google Scholar]
- Dirksen, L.; Böhme, W. Studies on Anacondas III. A Reappraisal of Eunectes beniensis Dirksen, 2002, from Bolivia, and a Key to the Species of the Genus Eunectes Wagler, 1830 (Serpentes: Boidae). Russ. J. Herpetol. 2005, 12, 223–229. [Google Scholar]
- Powell, R.L.; Eversole, C.B.; Rivas, L.R.; Crocker, A.V.; De la Quintana, P. Feliz cumpleaños, 21 years for the Beni Anaconda, Eunectes beniensis (Dirksen, 2002) (Serpentes, Boidae): An update of voucher specimens, species’ distribution, and clarification of locality data of type specimens. Check List 2023, 19, 847–854. [Google Scholar] [CrossRef]
- Dirksen, L.; Henderson, R.W. Eunectes deschauenseei Dunn and Conant de Schauensee’s Anacondaz. Cat. Am. Amphib. Reptiles 2002, 755, 1–3. [Google Scholar]
- Dirksen, L.; Oubotar, P. Eunectes deschauenseei. In The IUCN Red List of Threatened Species 2021; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2021. [Google Scholar] [CrossRef]
- Santos, G.S.; de Lema, T.; Winck, G.R.; Cechin, S.Z.; Boelter, R.A. Distribution Extension of the Yellow Anaconda Eunectes notaeus Cope, 1862 (Squamata: Boidae) in the state of Rio Grande do Sul, Brazil. Check List. 2013, 9, 660–662. [Google Scholar] [CrossRef][Green Version]
- De La Quintana, P.; Rivas, J.A.J.A.; Valdivia, F.; Pacheco, L.F.L.F. Home Range and Habitat Use of Beni Anacondas (Eunectes beniensis) in Bolivia. Amphib. Reptil. 2017, 38, 547–553. [Google Scholar] [CrossRef]
- De la Quintana, P.; Rivas, J.A.; Valdivia, F. Eunectes murinus (Green Anaconda): Dry Season Home Range. Herpetol. Rev. 2018, 49, 546–547. [Google Scholar]
- Smaniotto, N.P.; Moreira, L.F.B.; Rivas, J.A.; Strüssmann, C. Home Range Size, Movement, and Habitat Use of Yellow Anacondas (Eunectes notaeus). Salamandra 2020, 56, 159–167. [Google Scholar]
- Rivas, J.A. Natural History of the Green Anaconda: With Emphasis on Its Reproductive Biology; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2015. [Google Scholar]
- Rivas, J.A.; del Muñoz, M.C.; Thorbjarnarson, J.B.; Burghardt, G.M.; Holmstrom, W.; Calle, P. Natural History of the Green Anacondas in the Venezuelan Llanos. In Biology of Boas, Pythons, and Related Taxa; Henderson, R.W., Powell, R., Eds.; Eagle Mountain Publishing Company: Eagle Mountain, UT, USA, 2007; pp. 128–138. [Google Scholar]
- Rivas, J.A.; Molina, C.R.; Corey-Rivas, S.J.; Burghardt, G.M. Natural History of Neonatal Green Anacondas (Eunectes murinus): A Chip off the Old Block. Copeia 2016, 104, 402–410. [Google Scholar] [CrossRef]
- Rivas, J.A. Predatory Attacks of Green Anacondas (Eunectes murinus) on Adult Human Beings. Herpetol. Nat. Hist. 1998, 6, 157–159. [Google Scholar]
- Rivas, J.A. Eunectes murinus (Green Anaconda): Subduing Behavior. Herpetol. Rev. 2004, 35, 66–67. [Google Scholar]
- Rivas, J.A.; Ascanio, R.E.; Munoz, M.D. What Is the Length of a Snake? Contemp. Herpetol. 2008, 2008, 1–3. [Google Scholar] [CrossRef]
- Miranda, E.B.P.; Ribeiro-Jr, R.P.; Camera, B.F.; Barros, M.; Draque, J.; Micucci, P.; Waller, T.; Strüssmann, C. Penny and Penny Laid Up Will Be Many: Large Yellow Anacondas Do Not Disregard Small Prey. J. Zool. 2017, 301, 301–309. [Google Scholar] [CrossRef]
- Strüssman, C. Hábitos Alimantares Da Sucuri-Amarela, Eunectes notaeus Cope, 1862, No Pantanal Matogrossense. Biociencias 1997, 1, 35–52. [Google Scholar]
- Valderrama, X.; Thorbjarnarson, J.B. Eunectes murinus: Predation. Herpetol. Rev. 2001, 32, 46–47. [Google Scholar]
- Rivas, J.A.; Thorbjarnarson, J.B.; Owens, R.Y.; Muñoz, M.d.C. Eunectes murinus: Caiman Predation. Herpetol. Rev. 1999, 30, 101. [Google Scholar]
- Rivas, J.A.; Owens, R.Y.; Calle, P.P. Eunectes murinus: Juvenile Predation. Herpetol. Rev. 2001, 32, 107–108. [Google Scholar]
- Terra, J.D.S. Ecologia, Nicho Climático e Efeito Das Mudanças Climáticas Sobre a Distribuição Potencial Das Espécies Do Gênero Eunectes (Squamata, Serpente). Ph.D. Thesis, Universidade de São Paulo, Sao Paulo, Brazil, 2018. [Google Scholar]
- Champagne, P. Stephane. Conservation Ecology of Eunectes murinus (Green Anaconda) in the Madre de Dios Region of Southeastern Peru Using Remote Sensing Techniques and Machine Learning Driven Geospatial Modeling. Ph.D. Thesis, Acadia University, Wolfville, NS, Canada, 2022. [Google Scholar]
- Calle, P.P.; Rivas, J.A.; Munoz, M.d.C.; Thorbjarnarson, J.; Dierenfeld, E.S.; Holmstrom, W.; Karesh, W.E.B.W.B. Health Assessment of Free Ranging Anacondas (Eunectes murinus) in Venezuela. J. Zoo Wildl. Med. 1994, 25, 53–61. [Google Scholar]
- Calle, P.P.P.; Rivas, J.A.; Muñoz, M.; Thorbjarnarson, J.B.; Holmstrom, W.; Karesh, W.B.W.B. Infectious Disease Serologic Survey in Free-Ranging Venezuelan Anacondas (Eunectes murinus). J. Zoo Wildl. Med. 2001, 32, 320–323. [Google Scholar] [CrossRef]
- Rivas, J.A. Determining Breeding Status of Green Anacondas (Eunectes murinus): A Condition Index Assuming Isometry. South Am. J. Herpetol. 2023, 28, 89–94. [Google Scholar] [CrossRef]
- Rivas, J.A. What Can Studying Anacondas Tell Us about Titanoboa cerrejonensis? Exploring the Life of an Extinct Giant Snake Using an Extant Pretty Big Snake. Herpetol. J. 2023, 33, 68–75. [Google Scholar] [CrossRef]
- Rivas, J.A.; Corey-Rivas, S.J. Eunectes murinus (Green Anaconda), Longevity. Herpetol. Rev. 2008, 39, 469. [Google Scholar]
- Rivas, J.A. Conservation of Green Anacondas: How Tylenol Conservation and Macroeconomics Threaten the Survival of the World’s Largest Snake. Iguana 2007, 14, 10–21. [Google Scholar]
- Rivas, J.A.; del Munoz, M.C.; Thorbjarnarson, J.B.; Burghardt, G.M. Eunectes murinus. In Boas of the World; Reynolds, R.G., Robert, W.H., Eds.; In Revision; Comstock Publishing Associates: Ithaca, NY, USA.
- Calderón, M.; Ortega, A.; Scott, N.; Cacciali, P.; de Nogueira, C.C.; Gagliardi, G.; Catenazzi, A.; Cisneros-Heredia, D.F.; Hoogmoed, M.S.; Schargel, W.; et al. Eunectes murinus. In The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2001; Available online: https://www.iucnredlist.org/species/44580041/44580052 (accessed on 15 February 2024).
- Waller, T.; Micucci, P.A.; Alvarenga, E. Conservation Biology of the Yellow Anaconda (Eunectes notaeus) in Northeastern Argentina. In Biology of Boas, Pythons, and Related Taxa; Henderson, R.W., Powell, R., Eds.; Eagle Mountain Publishing Company: Eagle Mountain, UT, USA, 2007; pp. 340–362. [Google Scholar]
- Micucci, P.A.; Waller, T.; Alvarenga, E. Programa Curiyú. In Manejo de Fauna Silvestre en la Argentina: Programa de Uso Sustentable; Bolkovic, M.L., Ramadori, D., Eds.; Ministerio de Salud y Ambiente de la Nación: Buenos Aires, Argentina, 2006; pp. 77–92. [Google Scholar]
- Waller, T.; Micucci, P.A. Anaconda Conservation: A Reply to Rivas. Iguana 2008, 15, 51–53. [Google Scholar]
- Rivas, J.A. Is Wildlife Management Business or Conservation—A Question of Ideology. Reptiles Amphib. 2010, 17, 112–115. [Google Scholar] [CrossRef]
- Camera, B.F.; Quintana, I.; Strussmann, C.; Waller, T.; Barros, M.; Draque, J.; Micucci, P.A.; Miranda, E.B.P. Assessing the Sustainability of Yellow Anaconda (Eunectes notaeus) Harvest. PLoS ONE 2023, 18, e0277629. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.R.N.; Pereira Filho, G.A. Commercialization and Use of Snakes in North and Northeastern Brazil: Implications for Conservation and Management. Biodivers. Conserv. 2007, 16, 969–985. [Google Scholar] [CrossRef]
- Giraudo, A.R.; Arzamendia, V.; Bellini, G.P.; Bessa, C.A.; Calamante, C.C.; Cardozo, G.; Chiaraviglio, M.; Constanzo, M.B.; Etchepare, E.G.; Di Cola, V.; et al. Categorización Del Estado de Conservación de Las Serpientes de La República Argentina. Cuad. Herpetol. 2012, 26, 303–326. [Google Scholar]
- Waller, T.; Camera, B.; Cacciali, P.; Miranda, E.; Buongermini, E.; Micucci, P.; Smaniotto, N.; Strüssmann, C.; Barros, M.; Draque, J. Eunectes notaeus. In The IUCN Red List of Threatened Species; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2021. [Google Scholar] [CrossRef]
- Carreira, S.; Estrades, A. Reptil. In Especies Prioritarias para la Conservación en Uruguay: Vertebrados, Moluscos Continentales y Plantas Vasculares; Soutullo, A., Clavijo, C., Martínez-Lanfranco, J.A., Eds.; SNAP/DINAMA/MVOTMA y DICYT/MEC: Montevideo, Uruguay, 2013; pp. 129–147. [Google Scholar]
- Micucci, P.A.; Waller, T. The Management of Yellow Anacondas (Eunectes notaeus) in Argentina: From Historical Misuse to Resource Appreciation. Iguana 2007, 14, 160–171. [Google Scholar]
- Muñoz, A.; Gonzales, L.; Embert, D.; Aparicio, J.; Aguayo, R. Eunectes beniensis . Available online: https://www.iucnredlist.org/species/174126/18978378 (accessed on 15 February 2024).
- Rivas, J.A. ¿Cuantas Anacondas Hay? In Proceedings of the XI Latin American Congress of Herpetology, Quito, Ecuador, 24–28 July 2017. [Google Scholar]
- Rivas, J.A.; Corey-Rivas, S.J. How Many Green Anacondas Are There?: Evidence of Multiple Lineages of Green Anaconda. In Proceedings of the Joint Meeting of Ichthyologist and Herpetologist 2017, Austin, TX, USA, 12–17 July 2017. [Google Scholar]
- Seutin, G.; White, B.N.; Boag, P.T. Preservation of Avian Blood and Tissue Samples for DNA Analyses. Can. J. Zool. 1991, 69, 82–90. [Google Scholar] [CrossRef]
- Douglas, D.A.; Janke, A.; Arnason, U. A Mitogenomic Study on the Phylogenetic Position of Snakes. Zool. Scr. 2006, 35, 545–558. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- de Queiroz, A.; Lawson, R.; Lemos-Espinal, J. A Phylogenetic Relationships of North American Garter Snakes (Thamnophis) Based on Four Mitochondrial Genes: How Much DNA Sequence Is Enough? Mol. Phylogenet. Evol. 2002, 22, 315–329. [Google Scholar] [CrossRef]
- Burbrink, F.T.; Lawson, R.; Slowinski, J.B. Mitochondrial DNA Phylogeography of the Polytypic North American Rat Snake (Elaphe Obsoleta): A Critique of the Subspecies Concept. Evolution 2000, 54, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, E.; Davis, S.K.; Sites, J.W. Mitochondrial DNA Sequence Divergence and Phylogenetic Relationships among Eight Chromosome Races of the Sceloporus grammicus Complex (Phrynosomatidae) in Central Mexico. Soc. Syst. Biol. 1994, 43, 387–418. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Noonan, B.P.; Chippindale, P.T. Dispersal and Vicariance: The Complex Evolutionary History of Boid Snakes. Mol. Phylogenet. Evol. 2006, 40, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, H.L.; Diaz, J. Identification of Single Copy Nuclear DNA Markers for North American Pit Vipers. Mol. Ecol. Resour. 2010, 10, 177–180. [Google Scholar] [CrossRef]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: A New and Scalable Tool for the Selection of DNA and Protein Evolutionary Models. Mol. Biol. Evol. 2019, 37, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. RaxmlGUI 2.0: A Graphical Interface and Toolkit for Phylogenetic Analyses Using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.M.; Teslenko, P.; van der Mark, D.L.; Ayres, A.; Darling, S.; Höhna, B.; Larget, L.; Liu, M.A.S.; Huelsenbeck, J.P. MRBAYES 3.2: Efficient Bayesian Phylo-Genetic Inference and Model Selection across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian Evolutionary Analysis by Sampling Trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef]
- Smith, K.T.; Scanferla, A. A Nearly Complete Skeleton of the Oldest Definitive Erycine Boid (Messel, Germany). Geodiversitas 2021, 43, 1–24. [Google Scholar] [CrossRef]
- Head, J.J. Fossil Calibration Dates for Molecular Phylogenetic Analysis of Snakes 1: Serpentes, Alethinophidia, Boidae, Pythonidae. Palaeontol. Electron. 2015, 18, 1–17. [Google Scholar] [CrossRef]
- Storey, M.; Mahoney, J.J.; Saunders, A.D.; Duncan, R.A.; Kelley, S.P.; Coffin, M.F. Timing of Hot Spot—Related Volcanism and the Breakup of Madagascar and India. Science 1995, 267, 852–855. [Google Scholar] [CrossRef]
- Ali, J.R.; Krause, D.W. Late Cretaceous Bioconnections between Indo-Madagascar and Antarctica: Refutation of the Gunnerus Ridge Causeway Hypothesis. J. Biogeogr. 2011, 38, 1855–1872. [Google Scholar] [CrossRef]
- Prance, G.T. Biological Diversification in the Tropics; Columbia University Press: New York, NY, USA, 1982. [Google Scholar]
- Junk, W.J.; Wittmann, F.; Schöngart, J.; Piedade, M.T.F. A Classification of the Major Habitats of Amazonian Black-Water River Floodplains and a Comparison with Their White-Water Counterparts. Wetl. Ecol. Manag. 2015, 23, 677–693. [Google Scholar] [CrossRef]
- Rivas, J.A. The Missing River. Front. Earth Sci. 2023, 11, 1203667. [Google Scholar] [CrossRef]
- Roze, J.A. La Taxonomía y Zoogeografía de Los Ofidios de Venezuela; Universidad Central de Venezuela, Ediciones de la Biblioteca: Caracas, Venezuela, 1966. [Google Scholar]
- Gorzula, S.; Pilgrim, K. Snakes of the Family Boidae in the Cooperative Republic of Guayana: An Assessment of the Trade and Status of Sixe Species; Report to the Secretariat of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES): Lausanne, Switzerland, 1992. [Google Scholar]
- Wiens, J.J.; Tiu, J. Highly Incomplete Taxa Can Rescue Phylogenetic Analyses from the Negative Impacts of Limited Taxon Sampling. PLoS ONE 2012, 7, e42925. [Google Scholar] [CrossRef] [PubMed]
- von Linnaeus, C. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Differentiis, Synonymis, Locis; Salvii, L., Ed.; Impensis Direct: Holmiae, Sweden, 1758; Volume 1. [Google Scholar]
- Gronovius, L.T. Museum Ichthyologicum, Sistens Piscium Indigenorum & Quorumdam Exoticorum; Haak, T., Ed.; Lugduni-Batavorum: Leiden, The Netherlands, 1756; Volume 3. [Google Scholar] [CrossRef]
- Dirksen, L.; Bohme, W. Studien an Anakondas. I: Indizien Filr Natiirliche Bastardierung Zwischen Der Groben Anakonda Eunectes murinus Und Der Paraguayanakon da Eunectes notaeus in Bolivien, Mit Bemerkungen Zur Taxonomie der Gattung Eunectes. Zool. Abh. Staatl. Mus. Dresden 1998, 4, 45–58. [Google Scholar]
- Turci, L.C.B.; Bernarde, P.S. Composition and Natural History of Snakes from Zona Da Mata in Rondônia, Southwestern Brazilian Amazon. J. Neotrop. Biol. 2022, 19, 111–126. [Google Scholar] [CrossRef]
- Shankar, P.G.; Swamy, P.; Williams, R.C.; Ganesh, S.R.; Moss, M.; Höglund, J.; Das, I.; Sahoo, G.; Vijayakumar, S.P.; Shanker, K.; et al. King or Royal Family? Testing for Species Boundaries in the King Cobra, Ophiophagus hannah (Cantor, 1836), Using Morphology and Multilocus DNA Analyses. Mol. Phylogenet. Evol. 2021, 165, 107300. [Google Scholar] [CrossRef]
- Brandt, A.L.; Ishida, Y.; Georgiadis, N.J.; Roca, A.L. Forest Elephant Mitochondrial Genomes Reveal That Elephantid Diversification in Africa Tracked Climate Transitions. Mol. Ecol. 2012, 21, 1175–1189. [Google Scholar] [CrossRef]
- Murphy, J.C.; Jowers, M.J.; Lehtinen, R.M.; Charles, S.P.; Colli, G.R.; Peres, A.K.; Hendry, C.R.; Pyron, R.A. Cryptic, Sympatric Diversity in Tegu Lizards of the Tupinambis teguixin Group (Squamata, Sauria, Teiidae) and the Description of Three New Species. PLoS ONE 2016, 11, e0158542. [Google Scholar] [CrossRef]
- Avila, I.; Bauer, F.; Boungermini, E.; Martinez, N. Dracaena paraguayensis: Aportes Sobre El Conocimiento De Su Biología, Ecología y distribución. Kempffiana 2016, 12, 122–130. [Google Scholar]
- Avila-pires, T.C.S. Lizards of Brazilian Amazonia (Reptilia: Squamata). Zool. Verh. 1995, 599, 1–706. [Google Scholar]
- Vargas-Ramírez, M.; Caballero, S.; Morales-Betancourt, M.A.; Lasso, C.A.; Amaya, L.; Martínez, J.G.; das Neves Silva Viana, M.; Vogt, R.C.; Farias, I.P.; Hrbek, T.; et al. Genomic Analyses Reveal Two Species of the Matamata (Testudines: Chelidae: Chelus spp.) and Clarify Their Phylogeography. Mol. Phylogenet. Evol. 2020, 148, 106823. [Google Scholar] [CrossRef] [PubMed]
- Michels, J.; Vargas-Ramírez, M. Red-Headed Amazon River Turtles in Venezuela and Colombia: Population Separation and Connection along the Famous Route of Alexander von Humboldt. Zoology 2018, 130, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.; Henderson, R.W.; Delmas, A.-S.S.; Hedges, S.B. A Phylogenetic Study of the Emerald Treeboa (Corallus caninus). J. Herpetol. 2005, 39, 500–503. [Google Scholar] [CrossRef]
- Colston, T.J.; Grazziotin, F.G.; Shepard, D.B.; Vitt, L.J.; Colli, G.R.; Henderson, R.W.; Hedges, B.; Zaher, H.; Burbrink, F.T. Molecular Systematics and Historical Biogeography of Tree Boas (Corallus spp.). Mol. Phylogenet. Evol. 2013, 66, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Passos, P.; Fernandes, R. Revision of the Epicrates cenchria Complex (Serpentes: Boidae). Herpetol. Monogr. 2008, 22, 1–30. [Google Scholar] [CrossRef]
- Seba, A. Locupletissimi Rerum Naturalium Thesauri Accurata Descriptio, et Iconibus Artificiosissimis Expressio, per Universam Physices Historiam: Opus, Cui, in Hoc Rerum Genere, Nullum Par Exstitit. Tomus II; Wetstenium, J., Smith, G., Waesberg, J., Eds.; Amstelaedami: Amsterdam, The Netherlands, 1735; ISBN 9788527729833. [Google Scholar]
- Bauer, A.M.; Wahlgren, R. On the Linck Collection and Specimens of Snakes Figured by Johann Jakob Scheuchzer (1735)—The Oldest Fluid-Preserved Herpetological Collection in the World? Bonn Zool. Bull. 2013, 62, 220–252. [Google Scholar]
- Scheuchzer, J.J. Physica Sacra; Wagner, C.U., Ed.; Christian Ulrich Wagner: Augsburg and Ulm, Germany, 1735; Volume 4, ISBN 2013206534. [Google Scholar]
- Plotkin, M.J. Tales of a Shaman’s Apprentice: An Ethnobotanist Searches for New Medicines in the Rain Forest; Penguin: New York, NY, USA, 1994. [Google Scholar]
- Gumilla, J. El Orinoco Ilustrado y Defendido; Editorial Arte: Madrid/Caracas, Spain, 1740. [Google Scholar]
- Dunn, E.R.; Conant, R. Notes on Anacondas, with Descriptions of Two New Species. Proc. Acad. Nat. Sci. Phila. 1936, 88, 503–506. [Google Scholar]
- Dirksen, L.; Bohme, W. Studien an Anakondas. II: Zurn Taxonomischen Status von Eunectes murinus gigas (Latreille, 1801) (Serpentes: Boidae), Mit Neuen Ergebnis-Sen Zur Gattung Eunectes Wagler, 1830. Salamandra 1998, 34, 359–374. [Google Scholar]
- International Commission on Zoological Nomenclature. International Code of Zoological Nomenclature, 4th ed.; International Trust for Zoological Nomenclature: London, UK, 1999. [Google Scholar]
- Reynolds, R.G.; Niemiller, M.L.; Hedges, S.B.; Dornburg, A.; Puente-Rolón, A.R.; Revell, L.J. Molecular Phylogeny and Historical Biogeography of West Indian Boid Snakes (Chilabothrus). Mol. Phylogenet. Evol. 2013, 68, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.G.; Marshall, L.G.; Guerrero, J.; Horton, B.; Malabarba, M.C.S.L.; Wesselingh, F. The Stage for Neotropical Fish Diversification: A History of Tropical South American Rivers. In Phylogeny and Classification of Neotropical Fishes; Malabarba, L.R., Reis, R.E., Vari, R.P., Lucena, Z.M.S., Lucena, C.A.S., Eds.; Edipucrs: Porto Alegre, Brazil, 1998; pp. 13–48. [Google Scholar]
- Bicudo, T.C.; Sacek, V.; de Almeida, R.P.; Bates, J.M.; Ribas, C.C. Andean Tectonics and Mantle Dynamics as a Pervasive Influence on Amazonian Ecosystem. Sci. Rep. 2019, 9, 16879. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, X.; Nie, L.; Xia, X.; Liu, L.; Jiang, Y.; Huang, Z.; Jing, W. The Complete Mitochondrial Genome Sequences of Chelodina rugosa and Chelus fimbriata (Pleurodira: Chelidae): Implications of a Common Absence of Initiation Sites (OL) in Pleurodiran Turtles. Mol. Biol. Rep. 2012, 39, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.S.; Bronzati, M.; Langer, M.C.; Sterli, J. Phylogeny, Biogeography and Diversification Patterns of Side-Necked Turtles (Testudines: Pleurodira). R. Soc. Open Sci. 2018, 5, 171773. [Google Scholar] [CrossRef] [PubMed]
- Roberto, I.J.; Bittencourt, P.S.; Muniz, F.L.; Hernández-Rangel, S.M.; Nóbrega, Y.C.; Ávila, R.W.; Souza, B.C.; Alvarez, G.; Miranda-Chumacero, G.; Campos, Z.; et al. Unexpected but Unsurprising Lineage Diversity within the Most Widespread Neotropical Crocodilian Genus Caiman (Crocodylia, Alligatoridae). Syst. Biodivers. 2020, 18, 377–395. [Google Scholar] [CrossRef]
- Villamarín, F.; Escobedo-Galván, A.H.; Siroski, P.; Magnusson, W.E. Geographic Distribution, Habitat, Reproduction, and Conservation Status of Crocodilians in the Americas. In Conservation Genetics of New World Crocodilians; Zucoloto, R.B., Amavet, P.S., Verdade, L.M., Farias, I.P., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Giugliano, L.G.; Collevatti, R.G.; Colli, G.R. Molecular Dating and Phylogenetic Relationships among Teiidae (Squamata) Inferred by Molecular and Morphological Data. Mol. Phylogenet. Evol. 2007, 45, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Waller, T.; Buongermini, E.; Micucci, P.A. Eunectes notaeus. Diet. Herpetol. Rev. 2001, 32, 47. [Google Scholar]
- Crawford, R.M.M. Oxygen availability as an ecological limit to plant distribution. In Advances in Ecological Research; Begon, M., Fitter, A.H., Eds.; Academic Press: Cambridge, MA, USA, 1992; Volume 23, pp. 93–185. [Google Scholar]
- Wittmann, F.; Householder, J.E.; Piedade, M.T.F.; Schöngart, J.; Demarchi, L.O.; Quaresma, A.C.; Junk, W.J. A Review of the Ecological and Biogeographic Differences of Amazonian Floodplain Forests. Water 2022, 14, 3360. [Google Scholar] [CrossRef]
- Hoorn, C.; Wesselingh, F.P.; Steege, H.T.; Bermudez, M.A.; Mora, A.; Sevink, J.; Sanmartín, I.; Sanchez-Meseguer, A.; Anderson, C.L.; Figueiredo, J.P.; et al. Amazonia Through Time: Andean Uplift, Climate Change, Landscape Evolution, and Biodiversity. Science 2010, 330, 927. [Google Scholar] [CrossRef]
- Wesselingh, F.P.; Rasanen, M.E.; Irion, G.; Vonhof, H.G.; Kaandorp, R.J.G.; Renema, W.; Romero-Pittman, L.; Gingras, M. Lake Pebas: Palaeoecological Reconstruction of a Miocene Long-Lived Lake Complex in Western Amazonia. Cainozoic Res. 2002, 2001, 35–81. [Google Scholar]
- Hoorn, C.; Guerrero, J.; Sarmiento, G.; Lorente, M.A. Andean Tectonics as a Cause for Changing Drainage Patterns in Miocene Northern South America. Geology 1995, 23, 237–240. [Google Scholar] [CrossRef]
- Winemiller, K.O.; Willis, S.C. The Vaupes Arch and Casiquiare Canal: Barriers and Passages; Albert, J.S., Reis, R.E., Eds.; University of California Press: Berkeley, CA, USA, 2011; ISBN 0520268685. [Google Scholar]
- Rivas, J.A.; Rodriguez, J.V.; Mittermeier, C.G. The Llanos. In Wilderness: Earth’s Last Wildl Places; Mittermeier, R., Mittermeier, C.G., Robles Gil, P., Pilgrim, J., Konstant, W.R., Fonseca, G.A.B., Brooks, T.M., Eds.; Cemex: San Pedro Garza García, Mexico, 2002; Volume 53, pp. 1689–1699. ISBN 9788578110796. [Google Scholar]
- Sarmiento, G. The Savannas of Tropical America. In Ecosystems of the World: Tropical Savannas; Bourliere, F., Ed.; Elsevier Scientifc Publishing Co.: New York, NY, USA, 1983; Volume 13, pp. 245–288. [Google Scholar]
- Hoorn, C.; Boschman, L.M.; Kukla, T.; Sciumbata, M.; Val, P. The Miocene Wetland of Western Amazonia and Its Role in Neotropical Biogeography. Bot. J. Linn. Soc. 2022, 199, boab098. [Google Scholar] [CrossRef]
- Antoine, P.O.; Abello, M.A.; Adnet, S.; Altamirano Sierra, A.J.; Baby, P.; Billet, G.; Boivin, M.; Calderón, Y.; Candela, A.; Chabain, J.; et al. A 60-Million-Year Cenozoic History of Western Amazonian Ecosystems in Contamana, Eastern Peru. Gondwana Res. 2016, 31, 30–59. [Google Scholar] [CrossRef]
- Salas-Gismondi, R.; Flynn, J.J.; Baby, P.; Tejada-Lara, J.V.; Wesselingh, F.P.; Antoine, P.O. A Miocene Hyperdiverse Crocodylian Community Reveals Peculiar Trophic Dynamics in Proto-Amazonian Mega-Wetlands. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142490. [Google Scholar] [CrossRef] [PubMed]
- Tucker, D.B. Molecular Studies of South American Teiid Lizards (Teiidae: Squamata) from Deep Time to Shallow Divergences from Deep Time to Shallow Divergences. Ph.D. Thesis, Brigham University, Provo, UT, USA, 2016. [Google Scholar]
- Kirschner, J.A.; Hoorn, C. The Onset of Grasses in the Amazon Drainage Basin, Evidence from the Fossil Record. Front. Biogeogr. 2020, 12, e44827. [Google Scholar] [CrossRef]
- Leite, Y.L.R.; Costa, L.P.; Loss, A.C.; Rocha, R.G.; Batalha-Filho, H.; Bastos, A.C.; Quaresma, V.S.; Fagundes, V.; Paresque, R.; Passamani, M.; et al. Neotropical Forest Expansion during the Last Glacial Period Challenges Refuge Hypothesis. Proc. Natl. Acad. Sci. USA 2016, 113, 1008–1013. [Google Scholar] [CrossRef]
- Natsukawa, H.; Sergio, F. Top Predators as Biodiversity Indicators: A Meta-Analysis. Ecol. Lett. 2022, 25, 2062–2075. [Google Scholar] [CrossRef]
- Sergio, F.; Newton, I.; Marchesi, L. Top Predators and Biodiversity. Nature 2005, 436, 192. [Google Scholar] [CrossRef]
- Ojasti, J. Manejo de Fauna Silvestre Neotropical; Dallmeier, F., Ed.; Smithsonian Institution Press: Washington, DC, USA, 2000; ISBN 189391206X. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).