Abstract
We revise the taxonomy of the frog genus Noblella on the basis of a molecular phylogeny. Previous studies recognized that Noblella is non-monophyletic, with one clade distributed from southeastern Peru to northeastern Bolivia and adjacent areas in Brazil and another clade distributed from northern Peru to Ecuador and southeastern Colombia. The lack of sequences from the type species Noblella peruviana prevented the investigation of its phylogenetic position and the status of related taxa. Our rediscovery after more than 115 years allowed for the inclusion of DNA sequences of Noblella peruviana obtained from specimens collected at the type locality in southeastern Peru. We inferred a phylogeny based on a concatenated dataset (three mitochondrial and two nuclear loci) using Bayesian and maximum likelihood methods. Our phylogeny corroborated the non-monophyly of Noblella and helped resolve the status of related taxa, including Psychrophrynella bagrecito, the type species of the genus Psychrophrynella (rediscovered after 42 years). We identified a clade containing N. peruviana, P. bagrecito, and other species of Noblella and Psychrophrynella distributed in southern Peru. Given that the name Noblella predates Psychrophrynella, we propose that Psychrophrynella should be considered a junior synonym of Noblella. The second clade contains species of Noblella distributed in Ecuador and northern Peru, including N. myrmecoides, which used to be the type species of the genus Phyllonastes. Consequently, we propose to reinstate the genus Phyllonastes to accommodate all species of Noblella distributed in Ecuador, northern Peru, southeastern Colombia, and adjacent areas in Brazil. We present an updated taxonomy including new combinations for 12 species and reinstatements for three species.
1. Introduction
Terrestrial-breeding frogs (Strabomantidae) living at high elevations in the Andes exhibit morphological traits associated with a predominantly terrestrial lifestyle and cold climate, including a robust body, relatively short limbs and head, absence of digital discs, and cryptic coloration [1]. The genus Noblella Barbour, 1930 [2] currently contains 17 species distributed in the Andes and Amazon region of Colombia, Ecuador, Peru, Bolivia, and adjacent areas in Brazil [3]. Many of these species are morphologically similar to those in closely related genera (e.g., Psychrophrynella Hedges, Duellman, and Heinicke, 2008 [4]) distributed in montane habitats. Previous phylogenetic analyses revealed that Noblella is non-monophyletic, with a “northern clade” distributed in Ecuador, northern Peru, southeastern Colombia, and adjacent areas in Brazil and a “southern clade” distributed from southeastern Peru to northeastern Bolivia and adjacent areas in Brazil [5,6,7]. These studies showed that the “northern clade” is closely related to Bahius bilineatus (Bokermann, 1975) [8] and Barycholos Heyer, 1969 [9], whilst the “southern clade” is closely related to Microkayla De la Riva, Chaparro, Castroviejo-Fisher, and Padial, 2017 [10] and the newly described genus Qosqophryne Catenazzi, Mamani, Lehr, and von May, 2020 [7].
Over the past few years, it became clear that DNA sequences for Noblella peruviana (Noble, 1921) [11], the type species for the genus, were needed to resolve the discrepancy in the distribution of species of Noblella [7]. Sequences for N. peruviana were not included in previous analyses because no tissues were available for DNA extraction from the old, and presumably formalin-fixed, specimens of the type series. These specimens are the only previously known specimens of N. peruviana, which H. H. Keays collected between December 1899 and August 1900 [12] in the Department of Puno. The verbatim location for the original shipment of specimens was Juliaca, in the cold and dry, high-Andean Peruvian Altiplano, certainly not a suitable location for Noblella [12]. Instead, the most likely type locality is the vicinity of the Inca Mine of Santo Domingo, in the Cordillera de Carabaya, where, according to Vaurie [13], Keays collected most of the specimens of vertebrates he shipped to the British Museum. Santo Domingo can only be reached by foot, descending the Limbani Valley and crossing the Inambari River, and it is unclear whether Keays collected the specimens at Santo Domingo or along the Limbani trail [12]
Recent studies have highlighted the taxonomic uncertainty surrounding the status of the genus Psychrophrynella, which appears to belong to the same lineage containing species of Noblella distributed in southern Peru and northern Bolivia [7]. Psychrophrynella bagrecito (Lynch, 1986) [14] was designated as the type species of the genus, but its DNA sequences were not included in the phylogeny of Hedges et al. [4] or in subsequent studies [10,15,16]. Similarly to N. peruviana, no specimens had been collected since 1974, when K.R. Thomas collected the holotype and some of the paratypes [14]. Additional specimens, also collected in 1974 and assigned as paratypes of P. bagrecito [14], belong to a different, undescribed taxon [17]. Thus, a revision of the taxonomy of Psychrophrynella has been pending since it was proposed [4].
Here, we examine the phylogenetic relationships among species of Noblella and Psychrophrynella using DNA sequences obtained from specimens collected during field expeditions in southern Peru. During separate expeditions to the type localities of both type species in 2016 and 2017, we were able to rediscover N. peruviana and P. bagrecito and obtain genetic sequences. Our analyses include these novel sequences, which are crucial to resolving the taxonomic conundrum of Noblella, as well as other previously unsampled species.
2. Materials and Methods
2.1. Field Surveys and Specimen Collection
The putative type locality of N. peruviana is the Cordillera de Carabaya, located in Región (Departamento) Puno, southern Peru [12]. Expeditions in 2016 and 2017 to the type locality near the abandoned Inca Mine of Santo Domingo, in the upper reach of a tributary of the Inambari River in the Cordillera de Carabaya, resulted in the rediscovery and collection of voucher specimens and tissues of N. peruviana at the type locality and along the Limbani trail in Oconeque near Aquele, in the upper Limbani Valley, at an airline distance of ~26 km from the type locality. Collection at both Santo Domingo and the Limbani trail is important because of the uncertainty regarding the precise location where Keays collected the holotype in 1899–1900 (see Section 1). The Limbani Valley runs from the opposite side of the Inambari River with respect to the Santo Domingo watershed and is generally drier than the montane forest at the type locality. The type locality of P. bagrecito is Marcapata, located in Región (Departamento) Cusco, southern Peru [14]. Expeditions in 2016 and 2017 to Marcapata allowed for the collection of specimens and tissues used in this study.
Research and use of vertebrate animals were approved by the Institutional Animal Care and Use Committees of Southern Illinois University Carbondale (IACUC protocol #16-006), Florida International University (IACUC protocol #18-009), and the University of California (ACUC #R278-0412, #R278-0413, and #R278-0314). The Dirección General Forestal y de Fauna Silvestre, Servicio Nacional Forestal y de Fauna Silvestre (SERFOR), Ministerio de Agricultura y Riego, Peru, issued research and collecting permits (R.D.G. N° 120-2012-AG-DGFFS-DGEFFS, N° 064-2013-AG-DGFFS-DGEFFS, N° 0146-2013-AG-DGFFS-DGEFFS, N° 292-2014-AG-DGFFS-DGEFFS, N° 029-2016-SERFOR-DGGSPFFS, N° 405-2016-SERFOR-DGGSPFFS, and Contrato de Acceso Marco a Recursos Genéticos N° 359-2013-MINAGRI-DGFFS-DGEFFS).
Museum abbreviations are CORBIDI—Centro para Ornitología y Biodiversidad, Lima; CBF—Colección Boliviana de Fauna; KU—Kansas University, Lawrence; LSUMZ—Louisiana Museum of Natural History, Baton Rouge; MNCN—Museo Nacional de Ciencias Naturales, Madrid; MNRJ—Museu Nacional, Rio de Janeiro; MUBI—Museo de Biodiversidad del Perú, Cusco; MUSA—Museo de Historia Natural, Universidad Nacional de San Agustín de Arequipa, Arequipa; MUSM—Museo de Historia Natural de la Universidad de San Marcos, Lima.
2.2. Taxonomic Scope
Our analyses focused on taxa in the subfamily Holoadeninae [4], which includes nine genera [18]: Bahius, Barycholos, Bryophryne, Euparkerella, Holoaden, Microkayla, Noblella, Psychrophrynella, and Qosqophryne. We used sequences from GenBank and newly obtained sequences from previously unsampled species, including N. lynchi, N. peruviana, P. bagrecito, and several undescribed species of Noblella and Psychrophrynella. Additionally, we included representative species of the genera Lynchius, Oreobates, Phrynopus, and Pristimantis, as they are closely related to Holoadeninae [16].
2.3. Laboratory Work
We followed laboratory protocols used in previous studies of terrestrial breeding frogs to extract, amplify, and sequence DNA [4,7,15,19,20]. Primers and thermocycling conditions to amplify DNA via PCR are described in [7,19,20]. We used forward and reverse primers to amplify five loci, including a fragment of the 16S rRNA mitochondrial gene (16S), a fragment of the 12S rRNA mitochondrial gene rRNA gene (12S), the protein-coding mitochondrial gene cytochrome c oxidase subunit I (COI), the nuclear protein-coding gene recombination-activating protein 1 (RAG1), and the tyrosinase precursor (Tyr). We completed the cycle sequencing reactions by using the corresponding PCR primers and the BigDye Terminator 3.1 (Applied Biosystems, Waltham, MA, USA) and obtained sequences by running the purified reaction products in an ABI 3730 Sequence Analyzer (Applied Biosystems). We deposited the newly obtained sequences in GenBank, and we updated the species names associated with four GenBank sequences (Appendix A; Table S1).
2.4. Molecular Phylogenetic Analyses
Our analyses included sequences from 153 specimens. We used Geneious R6, version 6.1.8 [21], to align the sequences using the Geneious multiple alignment program for nucleotide (consensus) sequences. We used PartitionFinder, version 1.1.1 [22], to select the appropriate models of nucleotide evolution, and we used the Bayesian information criterion (BIC) to determine the best partitioning schemes and substitution models. Our analyses included two outgroup taxa, Niceforonia philippi and Niceforonia brunnea. It is worth noting that sequences of N. philippi downloaded from GenBank were listed as N. dolops, which is a junior synonym of N. philippi [23]. The best partitioning scheme included six subsets (BIC value: 59,247.168). The first partition subset included both the 12S and 16S sequences and the best fitting substitution model was GTR + I + G. The remaining five subsets were partitioned according to codon positions as follows (substitution model in parenthesis): one set including the 1st codon position of COI (SYM + I + G), one set including the 2nd codon position of COI (F81 + I), one set including the 3rd codon position of COI (GTR + G), one set including the 1st and 2nd codon position of RAG1 and the 1st and 2nd codon position of Tyr (GTR + I + G), and one set including the 3rd codon position of both RAG1 and Tyr (K80 + G).
We used Bayesian and Maximum Likelihood (ML) methods to infer a phylogeny of the study species. A 2546-bp concatenated alignment, including the five loci, was used in both analyses. First, we used MrBayes, version 3.2.0 [24], to perform an MCMC Bayesian analysis consisting of two simultaneous runs of 10 million generations, and we set the sampling rate to be once every 1000 generations. Each run had three heated chains and one “cold” chain, and the burn-in was set to discard the first 25% of samples from the cold chain. At the end of the MCMC run, the average standard deviation of split frequencies was 0.004046.
Following the completion of the analysis, we used Tracer 1.6 [25] to verify convergence. Subsequently, we used FigTree (http://tree.bio.ed.ac.uk/software/figtree/; URL accessed on 15 January 2024) to visualize the majority-rule consensus tree and the posterior probability values to assess node support. We used IQ-TREE [26] to perform the ML analysis on the concatenated dataset. We ran the analysis using the default setting (ultrafast bootstrap method, 10,000 bootstrap alignments) and the partitioning scheme and substitution models determined by PartitionFinder. Then, we used FigTree to visualize the ML consensus tree.
3. Results
3.1. Molecular Phylogenetic Analysis
Both Bayesian and maximum likelihood analyses showed that Noblella is non-monophyletic, with a clade distributed in southern Peru and another clade distributed in Ecuador and northern Peru (Figure 1 and Figure S1). The first clade contains Noblella peruviana, Psychrophrynella bagrecito, and other species of Noblella and Psychrophrynella distributed in southern Peru; in turn, this clade is sister to the clade formed by Microkayla and Qosqophryne. The second clade contains species of Noblella distributed in Ecuador and northern Peru, including N. myrmecoides, which was previously placed in the genus Phyllonastes [27] (Figure 1 and Figure S1).
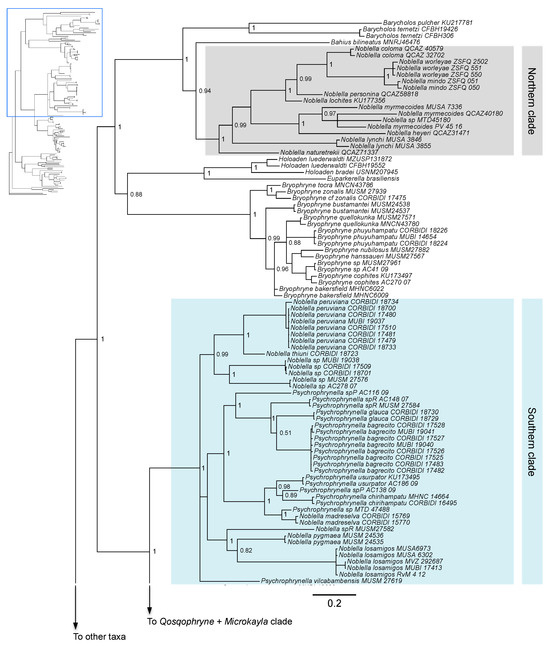
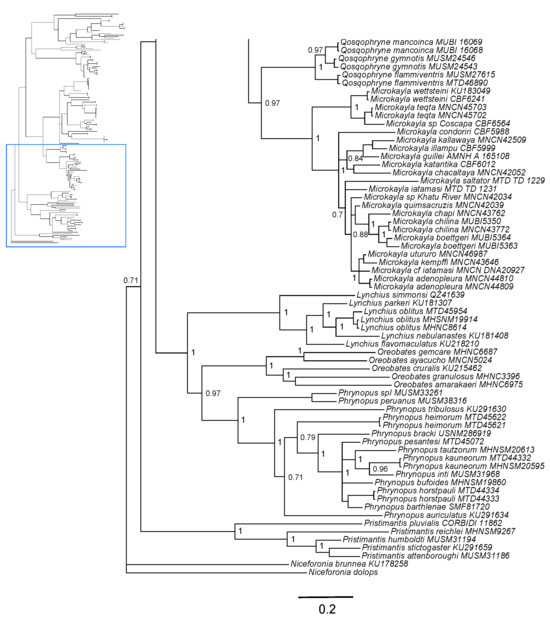
Figure 1.
(Top): Consensus Bayesian phylogeny based on a 2546-bp concatenated dataset (fragments of genes 16S, 12S, COI, RAG1, and Tyr) analyzed in MrBayes. Posterior probability values are indicated at each node. The area in gray indicates a clade with species distributed in northern Peru and Ecuador (“northern clade”), and the area in light blue indicates a clade with species distributed in southern Peru (“southern clade” containing the type species Noblella peruviana). Consensus Bayesian phylogeny based on a 2546-bp concatenated dataset (fragments of genes 16S, 12S, COI, RAG1, and Tyr) analyzed in MrBayes. Posterior probability values are indicated at each node. (Bottom): Part of the consensus Bayesian phylogeny showing closely related clades Qosqophryne and Microkayla, and more distantly related clades including Lynchius, Oreobates, and Phrynopus.
3.2. Taxonomy
In this section, we present an updated taxonomy of Noblella consistent with the molecular phylogeny results. Given that the name Noblella predates Psychrophrynella, we propose that Psychrophrynella should be considered a junior synonym of Noblella. Additionally, we propose to reinstate the genus Phyllonastes to accommodate all species of Noblella distributed in Ecuador, northern Peru, southeastern Colombia, and adjacent areas in Brazil. In addition to the updated list of species included under each genus, new combinations and reinstatements are included below.
- Noblella Barbour
- Noblella Barbour, 1930: 81 [2]. Type species Sminthillus peruvianus Noble, 1921: 1 [11], by original designation.
- Phyllonastes Heyer, 1977: 151 [27]. Type species Euparkella myrmecoides Mynch, 1976, by original designation.
- Content: Twelve species currently recognized in the genus (this paper): N. bagrecito, N. carrascoicola, N. chirihampatu, N. glauca, N. losamigos, N. madreselva, N. peruviana, N. pygmaea, N. ritarasquinae, N. thiuni, N. usurpator, and N. vilcabambensis.
- (Note: No sequences available for N. carrascoicola and N. ritarasquinae. Proposed inclusion in the genus is based on similarities in morphology and geographic distribution.)
- Noblella bagrecito new combination (Lynch, 1986)
- Phrynopus bagrecito Lynch, 1986 [14]
- Psychrophrynella bagrecito—Hedges, Duellman, and Heinicke, 2008: 103 [4].
- Psychrophrynella bagrecito—Duellman and Lehr, 2009: 260 [1].
- Holotype. KU 196512, adult female, from Río Marcapata below Marcapata, about 2740 m a.s.l., Provincia Quispicanchis, Región (Departamento) Cusco, Perú.
- Additional specimens. Paratopotypes KU 196512–26 and LSUMZ 32150–64. Four males CORBIDI 17482, 17483, 17525, MUBI 19040, one female CORBIDI 17527, and one juvenile CORBIDI 17526 from Cachihua (13.58975 S, 70.95609 W), near Marcapata, 2688 m a.s.l., Provincia Quispicanchis, Región (Departamento) Cusco, Perú; and one male CORBIDI 17528 and one juvenile MUBI 19041 from Limacpunko (13.55277 S, 70.90308 W), 2280 m a.s.l., Provincia Quispicanchis, Región (Departamento) Cusco, Perú, all collected on 9 August 2017 by A. Catenazzi and A. Ttito (Figure 2).
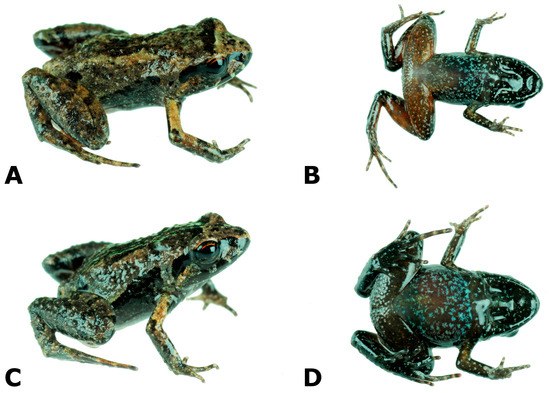
 Figure 2. Noblella bagrecito, adult specimens collected at the type locality (A–F) and at the nearby locality of Limacpunko (G,H). Snout-vent length (SVL) is given in mm. (A,B) male (CORBIDI 17482, SVL = 17.5 mm); (C,D) male (CORBIDI 17483, SVL = 12.5 mm); (E,F) female (CORBIDI 15727, SVL = 21.5 mm); (G,H) male (CORBIDI 15728, SVL = 12.5 mm).
Figure 2. Noblella bagrecito, adult specimens collected at the type locality (A–F) and at the nearby locality of Limacpunko (G,H). Snout-vent length (SVL) is given in mm. (A,B) male (CORBIDI 17482, SVL = 17.5 mm); (C,D) male (CORBIDI 17483, SVL = 12.5 mm); (E,F) female (CORBIDI 15727, SVL = 21.5 mm); (G,H) male (CORBIDI 15728, SVL = 12.5 mm). - Genus reallocation. We assign Psychrophrynella bagrecito to the genus Noblella based on molecular phylogenetic analyses that indicate that it belongs to a reciprocally monophyletic clade that includes Noblella peruviana, the type species of the genus Noblella.
- Remarks. Four paratypes (KU 196527–28 and LSUMZ 32167–68) from Huyro between Huayopata and Quillabamba [14] belong to a distinct, undescribed species, as confirmed by DNA sequences (unpublished data). All additional localities reported by Duellman and Lehr [1] (p. 319) also refer to other species of Noblella. Therefore, the known distribution of N. bagrecito is restricted to the Marcapata Valley from 2280–2740 m a.s.l.
- Noblella chirihampatu new combination (Catenazzi and Ttito, 2016)
- Psychrophrynella chirihampatu Catenazzi and Ttito 2016 [28]
- Holotype. CORBIDI 16495, an adult male from Área de Conservación Privada (ACP) Ukumari Llaqta, 2730 m, Comunidad Campesina de Japu, Distrito Paucartambo, Provincia Paucartambo, Región Cusco, Perú.
- Additional specimens. Ten paratopotypes: five adult males, CORBIDI 16496 and 16497 and MHNC 14658, 14664, and 14666, and five adult females, CORBIDI 16498–16499, 16696, and MHNC 14661–14662, collected at the type locality. Sixteen paratypes: all from ACP Ukumari Llakta: nine adult males, CORBIDI 16505–16509 and MHNC 14656 and 14670–14672, and one adult female, CORBIDI 16504, collected near Tambo, 3160 m; two adult males, CORBIDI 16503 and MHNC 14667, and four adult females, CORBIDI 16501–2 and MHNC 14668–69, collected at the Playa campsite, 2780 m [28].
- Genus reallocation. We assign Psychrophrynella chirihampatu to the genus Noblella based on molecular phylogenetic analyses that indicate that it belongs to a reciprocally monophyletic clade that includes Noblella peruviana, the type species of the genus Noblella.
- Noblella glauca new combination (Catenazzi and Ttito, 2018)
- Psychrophrynella glauca Catenazzi and Ttito 2018 [29]
- Holotype. CORBIDI 18729, an adult female from 13.67603 S, 70.46588 W (WGS84), 2225 m, near Thiuni, Distrito Ollachea, Provincia Carabaya, Región (Departamento) Puno, Peru, collected by A. Catenazzi and A. Ttito on 14 August 2017 [29].
- Additional specimens. Three paratopotypes, one adult male, CORBIDI 18730, one adult female, MUBI 16322, and one juvenile, MUBI 16323, were collected with the holotype [29].
- Genus reallocation. We assign Psychrophrynella glauca to the genus Noblella based on molecular phylogenetic analyses that indicate that it belongs to a reciprocally monophyletic clade that includes Noblella peruviana, the type species of the genus Noblella.
- Noblella peruviana (Noble, 1921)
- Sminthillus peruvianus Noble, 1921 [11]
- Noblella peruviana—Barbour, 1930 [2], Griffiths, 1959 [30], De la Riva, Chaparro, and Padial, 2008 [12]
- Eleutherodactylus peruvianus—Lynch, 1971 [31]
- Phrynopus peruvianus—Lynch, 1975 [32] (Based on misidentifications, in reference to Noblella usurpator.)
- Holotype. Holotype: AMNH 14526, “near Juliaca, Departamento Puno, Peru”, most likely in error, collected by H. H. Keays, date not known.
- Paratopotypes. AMNH 14527–28, UMMZ 56621; AMNH 88061 (cleared and stained).
- Newly referred specimens. Eight specimens: two females, CORBIDI 17510, CORBIDI 18700; one male, MUBI 10937, and one juvenile, CORBIDI 17480, collected at the type locality, along the trail from Santo Domingo to Punto 1 (13.83210 S, 69.63710 W), 1955 m, on 7 June 2016 by A. Catenazzi, A. Ttito, and J. C. Jahuanchi; and three males CORBIDI 18733, CORBIDI 17479, CORBIDI 17481 from the type locality, collected on 24 on July 2017 by A. Catenazzi, A. Ttito, I. Diaz, and J. C. Jahuanchi; and one male, CORBIDI 18734, from Oconeque, near Aquele, Limbani Valley (14.05853 S, 69.71528 W), 1982 m, collected on 4 August 2017 by A. Catenazzi, I. Diaz, A. Ttito and J. C. Jahuanchi (Figure 3).
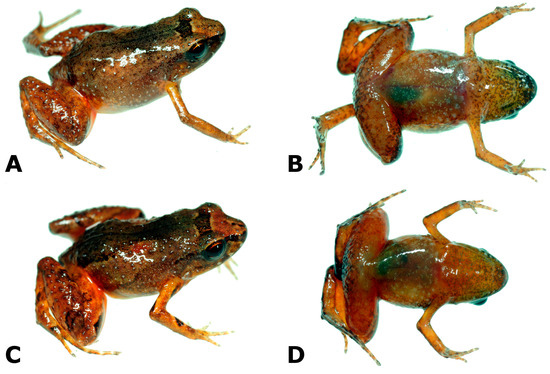
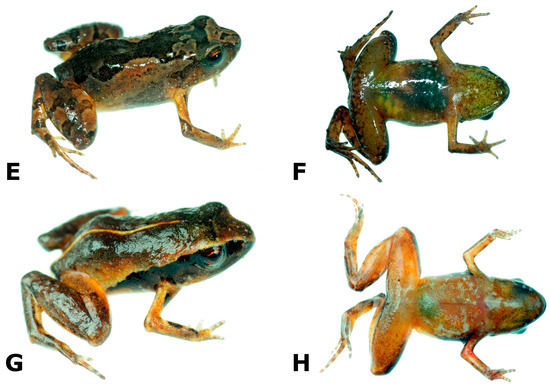 Figure 3. Noblella peruviana, adult specimens collected at the type locality (A–F) and at Oconeque, Aquele, Limbani, Puno (G,H). Snout-vent length (SVL) is given in mm. (A,B) female (CORBIDI 17510, SVL = 17.8 mm); (C,D) male (MUBI 19037, SVL = 13.3 mm); (E,F) female (CORBIDI 18700, SVL = 16.7 mm); (G,H) male (CORBIDI 18734, SVL = 11.0 mm).
Figure 3. Noblella peruviana, adult specimens collected at the type locality (A–F) and at Oconeque, Aquele, Limbani, Puno (G,H). Snout-vent length (SVL) is given in mm. (A,B) female (CORBIDI 17510, SVL = 17.8 mm); (C,D) male (MUBI 19037, SVL = 13.3 mm); (E,F) female (CORBIDI 18700, SVL = 16.7 mm); (G,H) male (CORBIDI 18734, SVL = 11.0 mm). - Generic placement. This is the type species of the genus.
- Remarks. Referred specimen CORBIDI 18734 is the first record outside of the type locality. The known distribution range of N. peruviana spans ~30 km by airline and includes localities from the upper Limbani Valley to Santo Domingo in the Cordillera de Carabaya.
- Noblella usurpator new combination (De la Riva, Chaparro, and Padial, 2008)
- Phrynopus peruvianus (part) Lynch, 1975 [32] (p. 36).
- “Phrynopus” peruvianus—Hedges, Duellman, and Heinicke, 2008 [4] (p. 103).
- Psychrophrynella usurpator—De la Riva, Chaparro, and Padial 2008 [12] (p. 44).
- Psychrophrynella usurpator—Duellman and Lehr, 2009 [1] (p. 261).
- Holotype. KU 138939, adult female, from the north slope of Abra Acjanacu, 3400 m a.s.l., 29 km NNE Paucartambo, Región (Departamento) Cusco, Perú.
- Genus reallocation. We assign Psychrophrynella usurpator to the genus Noblella based on molecular phylogenetic analyses that indicate that it belongs to a reciprocally monophyletic clade that includes Noblella peruviana, the type species of the genus Noblella.
- Noblella vilcabambensis new combination (Condori, Acevedo-Rincón, Mamani, Delgado C., and Chaparro, 2020)
- Psychrophrynella vilcabambensis—Condori, Acevedo-Rincón, Mamani, Delgado C., and Chaparro 2020 [33] (p. 129).
- Holotype. MUBI 13485, adult male, from Challcha, 3707 m a.s.l., Distrito Vilcabamba, Provincia La Convención, Región (Departamento) Cusco, Perú.
- Genus reallocation. We assign Psychrophrynella vilcabambensis to the genus Noblella based on molecular phylogenetic analyses that indicate that it belongs to a reciprocally monophyletic clade that includes Noblella peruviana, the type species of the genus Noblella.
- Phyllonastes Heyer
- Phyllonastes Heyer, 1977 [27] (p. 151). Type species Euparkerella myrmecoides Lynch, 1976 [34] (p. 50), by original designation.
- Content: Ten species currently recognized in the genus (this paper): P. coloma, P. duellmani, P. heyeri, P. lochites, P. lynchi, P. mindo, P. myrmecoides, P. naturetrekii, P. personina, and P. worleyae.
- (Note: No sequences available for P. duellmani. Proposed inclusion in Phyllonastes is based on similarities in morphology and geographic distribution.)
- Phyllonastes coloma new combination (Guayasamin and Terán-Valdez, 2009)
- Noblella coloma Guayasamin and Terán-Valdez, 2009 [35] (p. 48).
- Holotype. QCAZ 26307, from Reserva Florística Río Guajalito (0°14′ S, 78°49′ W), 1800–2000 m a.s.l., 3 km NW from Km 59 on the old road from Quito to Santo Domingo, Provincia Pichincha, Ecuador.
- Genus reallocation. We assign Noblella coloma to the genus Phyllonastes based on molecular phylogenetic analyses that indicate that it belongs to a reciprocally monophyletic clade that includes Phyllonastes myrmecoides, the type species of the genus Phyllonastes.
- Phyllonastes duellmani reinstatement (Lehr, Aguilar, and Lundberg, 2004)
- Phyllonastes duellmani Lehr, Aguilar, and Lundberg, 2004 [36] (p. 214).
- Noblella duellmani—De la Riva, Chaparro, and Padial, 2008 [12] (p. 68).
- Holotype. KU 196529, from Cillapata (approximately 1.5 km north-northeast of Auquimarca), approximately 10°43′52″ S, 75°42′48″ W, 2900 m a.s.l., Distrito Paucartambo, Provincia Pasco, Departamento Pasco, Peru.
- Genus reallocation. We reinstate Noblella duellmani to the genus Phyllonastes based on morphological similarity and relative geographic proximity to other species that belong to a reciprocally monophyletic clade that includes Phyllonastes myrmecoides, the type species of the genus Phyllonastes.
- Phyllonastes heyeri reinstatement (Lynch, 1986)
- Phyllonastes heyeri Lynch, 1986 [14] (p. 426).
- Noblella heyeri—De la Riva, Chaparro, and Padial, 2008 [12] (p. 68).
- Noblella heyeri—Duellman and Lehr, 2009 [1] (p. 92).
- Holotype. KU 196529, 33 km SW Huancabamba, Región (Departamento) Piura, Perú, 3100 m a.s.l.
- Genus reallocation. We reinstate Noblella heyeri to the genus Phyllonastes based on molecular phylogenetic analyses that indicate that it belongs to a reciprocally monophyletic clade that includes Phyllonastes myrmecoides, the type species of the genus Phyllonastes.
- Phyllonastes lochites new combination (Lynch, 1976)
- Euparkerella lochites Lynch, 1976 [34] (p. 49).
- Phyllonastes lochites—Heyer, 1977 [27] (p. 152).
- Phyllonastes lochites—Lynch, 1986 [24] (p. 426).
- Noblella lochites—De la Riva, Chaparro, and Padial, 2008 [12] (p. 68).
- Holotype. KU 147070, from Río Piuntza, on the northern end of the Cordillera del Condor, Morona-Santiago Province, Ecuador, 1550 m a.s.l. (approximately 3°15′ S, 78°20′ W).
- Genus reallocation. We assign Noblella lochites to the genus Phyllonastes based on molecular phylogenetic analyses that indicate that it belongs to a reciprocally monophyletic clade that includes Phyllonastes myrmecoides, the type species of the genus Phyllonastes.
- Phyllonastes lynchi reinstatement (Duellman, 1991)
- Phyllonastes lynchi Duellman, 1991 [37] (p. 10).
- Noblella lynchi—De la Riva, Chaparro, and Padial, 2008 [12] (p. 68).
- Noblella lynchi—Duellman and Lehr, 2009 [1] (p. 93).
- Holotype. KU 196529, from 33 km SW Huancabamba, Región (Departamento) Piura, Perú, 3100 m a.s.l.
- Genus reallocation. We reinstate Noblella heyeri to the genus Phyllonastes based on molecular phylogenetic analyses that indicate that it belongs to a reciprocally monophyletic clade that includes Phyllonastes myrmecoides, the type species of the genus Phyllonastes.
- Phyllonastes mindo new combination (Reyes-Puig, Guayasamin, Koch, Brito-Zapata, Hollanders, Costales, and Cisneros-Heredia, 2021)
- Noblella mindo Reyes-Puig, Guayasamin, Koch, Brito-Zapata, Hollanders, Costales, and Cisneros-Heredia, 2021 [38] (p. 66).
- Holotype. ZSFQ 050, from El Cinto, 11 Km E from Mindo town, Mindo (0.09022° S, 78.818581° W), 1673 m a.s.l., province of Pichincha, Ecuador.
- Genus reallocation. We assign Noblella mindo to the genus Phyllonastes based on molecular phylogenetic analyses that indicate that it belongs to a reciprocally monophyletic clade that includes Phyllonastes myrmecoides, the type species of the genus Phyllonastes.
- Phyllonastes myrmecoides new combination (Lynch, 1976)
- Euparkerella myrmecoides Lynch, 1976 [34] (p. 50).
- Phyllonastes myrmecoides—Heyer, 1977 [27] (p. 152).
- Noblella myrmecoides—De la Riva, Chaparro, and Padial, 2008 [12] (p. 68).
- Holotype. TCWC 41532, from Mishana (2 1/2 h by speedboat up the Río Nanay from the Navy dock 5 km NNE Iquitos), Departamento Loreto, Perú.
- Additional specimens. Two adult males from Manseriche, Datem del Marañon, Loreto (MUSA 7332, SVL 11.4 mm; MUSA 7333) (Figure S2).
- Genus reallocation. We assign Noblella myrmecoides to the genus Phyllonastes based on molecular phylogenetic analyses and because it is the type species of the genus Phyllonastes.
- Remarks. Many specimens of small Holoadeninae from the lowlands of southern Peru and Bolivia have been identified as Noblella myrmecoides, most likely in error (e.g., see recent checklist of amphibians of Madidi National Park in [39]). Several of these specimens were reassigned to Noblella losamigos [40], but it is unclear whether additional species of Noblella might be concealed under the former identification of Noblella myrmecoides.
- Phyllonastes naturetrekii new combination (Reyes-Puig, Reyes-Puig, Ron, Ortega, Guayasamin, Goodrum, Recalde, Vieira, Koch, and Yánez-Muñoz, 2019)
- Noblella naturetrekii Reyes-Puig, Reyes-Puig, Ron, Ortega, Guayasamin, Goodrum, Recalde, Vieira, Koch, and Yánez-Muñoz, 2019 [5] (p. 6).
- Holotype. DHMECN 13390, from Bosque Protector Cerro Candelaria (1.428722° S, -78.30421° W), 2000 m a.s.l., Naturetrek Reserve, Río Verde, Cantón Baños, Tungurahua Province, Ecuador.
- Genus reallocation. We assign Noblella naturetrekii to the genus Phyllonastes based on molecular phylogenetic analyses that indicate that it belongs to a reciprocally monophyletic clade that includes Phyllonastes myrmecoides, the type species of the genus Phyllonastes.
- Phyllonastes personina new combination (Harvey, Almendáriz C., Brito-M., and Batallas-Revelo, 2013)
- Noblella personina Harvey, Almendáriz C., Brito-M., and Batallas-Revelo, 2013 [41] (p. 3).
- Holotype. EPN 14324, from the forest at the Sardinayacu Lake Complex (2°03′48.4″ S, 78°14′11.3″ W), 1916 m a.s.l., Cantón Morona, Parroquia Sinaí, Morona Santiago, Ecuador.
- Genus reallocation. We assign Noblella personina to the genus Phyllonastes based on molecular phylogenetic analyses that indicate that it belongs to a reciprocally monophyletic clade that includes Phyllonastes myrmecoides, the type species of the genus Phyllonastes.
- Phyllonastes worleyae new combination (Reyes-Puig, Maynard, Trageser, Vieira, Hamilton, Lynch, Culebras, Kohn, Brito-M., and Guayasamin, 2020)
- Noblella worleyae Reyes-Puig, Maynard, Trageser, Vieira, Hamilton, Lynch, Culebras, Kohn, Brito-M., and Guayasamin, 2020 [42] (p. 164).
- Holotype. ZSFQ 551, from Río Manduriacu Reserve (0.312057° N, 78.854330° W), 1184 m a.s.l., Cantón Cotacachi, Imbabura Province, Ecuador.
- Genus reallocation. We assign Noblella worleyae to the genus Phyllonastes based on molecular phylogenetic analyses that indicate that it belongs to a reciprocally monophyletic clade that includes Phyllonastes myrmecoides, the type species of the genus Phyllonastes.
- DNA sequences: Incertae sedis
- The list included below represents DNA sequences that differ considerably from sequences from closely related species (in the same genus), likely derived from errors in assigning the tissue source or locality applied to the tissue source when uploading sequences to GenBank). We recommend not using these sequences of dubious origin. If used in the future, results should be interpreted with caution.
- MF186541. Bryophryne tocra RAG1 sequence. This sequence is very similar to RAG sequences from several species of Phrynopus rather than other Bryophryne.
- MF186583. Bryophryne tocra Tyr sequences. This sequence is very similar to Tyr sequences from several species of Phrynopus rather than other Bryophryne.
4. Discussion
Our study helps resolve the taxonomy and systematics of the genus Noblella, and it is the first to include DNA sequences from the type species (N. peruviana) in a phylogenetic analysis. As previously suggested [7,40], any taxonomic decision on the status of the “northern clade” of Noblella depended on the resolution of the “southern clade” as the latter is geographically closer to the type locality of N. peruviana (Figure 4). The non-monophyly of Noblella was recognized in previous studies, yet the lack of molecular data from the type species prevented clarifying the taxonomic status of members of both clades. Our study also clarifies the status of Psychrophrynella, which is here considered to be a junior synonym of Noblella.
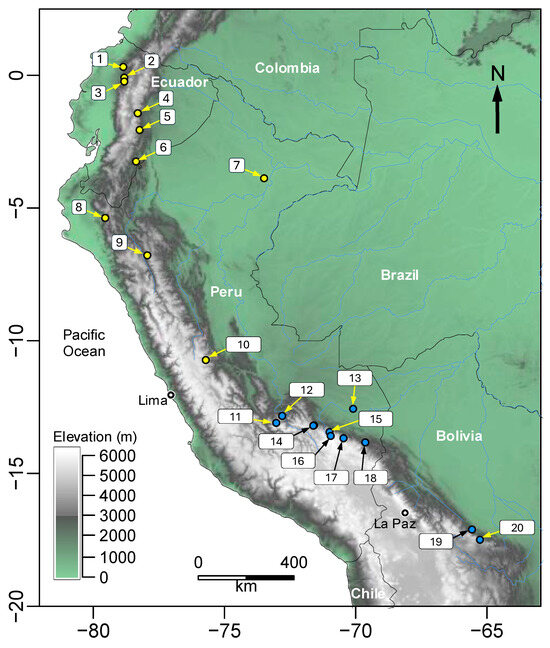
Figure 4.
Map of northwestern South America showing the location of the type localities of species in the genera Phyllonastes (yellow dots) and Noblella (blue dots). (1 = Phyllonastes worleyae, 2 = Phyllonastes mindo, 3 = Phyllonastes coloma, 4 = Phyllonastes naturetrekii, 5 = Phyllonastes personina, 6 = Phyllonastes lochites, 7 = Phyllonastes myrmecoides, 8 = Phyllonastes heyeri, 9 = Phyllonastes lynchi, 10 = Phyllonastes duellmani, 11 = Noblella vilcabambensis, 12 = Noblella madreselva, 13 = Noblella losamigos, 14 = Noblella pygmaea and Noblella usurpator, 15 = Noblella chirihampatu, 16 = Noblella bagrecito, 17 = Noblella glauca and Noblella thiuni, 18 = Noblella peruviana, 19 = Noblella ritarasquinae, 20 = Noblella carrascoicola.
Crucial to our findings was the rediscovery of the two type species, N. peruviana and N. bagrecito, and the inclusion (for the first time) of their DNA sequences in a phylogenetic analysis. We rediscovered N. peruviana 116–117 years after it was last collected and N. bagrecito 42 years after it was last collected, exemplifying the necessity of fieldwork when searching for ‘lost’ and missing species. A variety of reasons may explain amphibian rediscoveries [43], including hypothesized recoveries following disease-driven declines [44], but our rediscoveries espouse a trend common to many tropical, small, and endemic species of rediscovered amphibians [45]. Specifically, both N. peruviana and N. bagrecito are minute, inconspicuous inhabitants of the leaf litter or terrestrial moss layer and only known from their type localities, which in the case of N. peruviana is quite remote. These characteristics help explain the lack of observations during several decades.
According to several authors [4,12,35,37], the genus Noblella can be recognized by a combination of morphological characters, including a differentiated tympanic membrane (except in N. madreselva [46]); head narrower than body; cranial crests absent; dentigerous processes of vomers absent; Finger I shorter than, or equal in length to, Finger II; Toe III shorter than Toe V; tips of at least Toes III–IV pointed; subarticular tubercles not protruding; conspicuous tarsal tubercle; dark inguinal spots; small body size (SVL < 22 mm). However, some characters are ambiguous (e.g., terminal discs on fingers and toes not expanded or slightly expanded; discs and circumferential grooves present distally; terminal phalanges narrowly T-shaped). Moreover, most of these characters are present in species of Phyllonastes.
Further research on osteological, morphological, and life-history traits will help elucidate true synapomorphies in each genus. For example, only some species of Noblella (N. chirihampatu, N. bagrecito, and N. usurpator) bear a tarsal tubercle, varying in size and shape from large, elongate, and oblique (N. usurpator; [12]) to small and sickle-shaped (N. bagrecito; [12]). In contrast, members of Microkayla lack a tarsal tubercle [10]. Osteological studies will likely reveal important diagnostic and evolutionary informative traits in the future, and we recommend the use of computed tomography to examine osteological features in specimens of these minute and fragile frogs. Additional fieldwork is also needed to characterize the advertisement calls of Noblella species. For example, preliminary analyses suggest that the advertisement call of N. pygmaea has 6–8 notes, whereas the call of N. usurpator typically has 20–30 notes (A. Catenazzi, personal observation); the call of N. glauca has 26 notes [29]. Similar bioacoustic data from other localities will allow us to determine key differences between co-occurring species.
In light of our results, the geographic distribution of Phyllonastes myrmecoides needs to be updated. The currently available map includes a large region of lowland and montane forests in five countries [47]. However, it is likely that P. myrmecoides is restricted to a smaller region in northern Peru and Ecuador.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16100613/s1, Figure S1: Maximum likelihood (ML) phylogeny based on a 2546-bp concatenated dataset (fragments of genes 16S, 12S, COI, RAG1, and Tyr); Figure S2: Photographs of live specimens of (A) Phyllonastes myrmecoides (B) Phyllonastes cf. lynchi; (C) Noblella usurpator; (D) Noblella losamigos; Table S1: GenBank accession numbers for the taxa sampled in this study.
Author Contributions
Conceptualization, R.v.M. and A.C.; methodology, R.v.M. and A.C.; software, R.v.M.; validation, R.v.M., R.S.-C. and A.C.; formal analysis R.v.M.; investigation, R.v.M., M.I.D., A.T., R.S.-C. and A.C.; resources, R.v.M., R.S.-C. and A.C.; data curation, R.v.M., R.S.-C. and A.C.; writing—original draft preparation, R.v.M. and A.C.; writing—review and editing, R.v.M., M.I.D., A.T., R.S.-C. and A.C.; visualization, R.v.M. and A.C.; supervision, R.v.M. and A.C.; project administration, R.v.M. and A.C.; funding acquisition, R.v.M. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the U.S. National Science Foundation (BRC-BIO grant DBI-2218191 to R.v.M., PRFB award DBI-1103087 to R.v.M., DBE-2003497 to A.C.), the National Geographic Society Committee for Research and Exploration (Grant # 9191-12 to R.v.M.), the Amazon Conservation Association (to R.v.M. and A.C.), and a CSUCI Faculty Research & Development Mini-Grant (to R.v.M.). A.C. is supported with funds from the E.O. Wilson Biodiversity Foundation. Fieldwork in Cusco and Puno was funded by grants from The Eppley Foundation and the Chicago Board of Trade Endangered Species Fund to A.C.
Institutional Review Board Statement
The collection of specimens was approved by Institutional Animal Care and Use Committees as well as the wildlife service in Peru.
Data Availability Statement
Newly obtained DNA sequences are available on GenBank (see Table S1 for accession numbers).
Acknowledgments
We thank J.C. Jahuanchi, J.C. Cusi, and other colleagues who assisted in fieldwork. Jesús H. Córdova and César Aguilar provided a loan of specimens from the Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Peru (MUSM), and J.C. Chaparro a loan of specimens from the Museo de Biodiversidad del Perú, Cusco, Peru (MUBI).
Conflicts of Interest
The authors declare no conflict of interest. The funding organizations that provided support for this work had no role in the design of the study; data collection, analyses, or interpretation of data; writing of the manuscript, or decision to publish the results.
Appendix A
Note about species names associated with GenBank sequences. GenBank sequence with accession number EF493714 represents Noblella usurpator. This sequence was originally published as Phrynopus peruvianus on GenBank (Hedges et al. [4]). Some researchers erroneously used it as representative of Noblella peruviana in their analysis (e.g., EF493714. Noblella peruviana 12S and 16S sequences). De la Riva et al. [17] confirmed that it belongs to Noblella usurpator.
References
- Duellman, W.E.; Lehr, E. Terrestrial-Breeding Frogs (Strabomantidae) in Peru; Natur und Tier Verlag: Münster, Germany, 2009; p. 382. [Google Scholar]
- Barbour, T. A list of Antillean reptiles and amphibians. Zoologica 1930, 11, 61–116. [Google Scholar] [CrossRef]
- AmphibiaWeb. University of California, Berkeley, CA, USA. 2024. Available online: https://amphibiaweb.org (accessed on 14 January 2024).
- Hedges, S.B.; Duellman, W.E.; Heinicke, M.P. New World direct-developing frogs (Anura: Terrarana): Molecular phylogeny, classification, biogeography, and conservation. Zootaxa 2008, 1737, 1–182. [Google Scholar] [CrossRef]
- Reyes-Puig, J.P.; Reyes-Puig, C.; Ron, S.R.; Ortega, J.A.; Guayasamin, J.M.; Goodrum, M.; Recalde, F.; Vieira, J.; Koch, C.; Yánez-Muñoz, M.H. A new species of terrestrial frog of the genus Noblella Barbour, 1930 (Amphibia: Strabomantidae) from the Llanganates-Sangay Ecological Corridor, Tungurahua, Ecuador. PeerJ 2019, 7, e7405. [Google Scholar] [CrossRef] [PubMed]
- Catenazzi, A.; Ttito, A. Noblella thiuni sp. n. a new (singleton) species of minute terrestrial-breeding frog (Amphibia, Anura, Strabomantidae) from the montane forest of the Amazonian Andes of Puno, Peru. PeerJ 2019, 7, e6780. [Google Scholar] [CrossRef] [PubMed]
- Catenazzi, A.; Mamani, L.; Lehr, E.; von May, R. A new genus of terrestrial-breeding frogs (Holoadeninae, Strabomantidae, Terrarana) from southern Peru. Diversity 2020, 12, 184. [Google Scholar] [CrossRef]
- Bokermann, W.C.A. Três espécies novas de Eleutherodactylus do sudeste da Bahia, Brasil (Anura, Leptodactylidae). Rev. Bras. Biol. 1975, 34, 11–18. [Google Scholar]
- Heyer, W.R. Studies on the genus Leptodactylus (Amphibia, Leptodactylidae) III. A redefinition of the genus Leptodactylus and a description of a new genus of leptodactylid frogs. Contrib. Sci. 1969, 155, 1–14. [Google Scholar] [CrossRef]
- De La Riva, I.; Chaparro, J.C.; Castroviejo-Fisher, S.; Padial, J.M. Underestimated anuran radiations in the high Andes: Five new species and a new genus of Holoadeninae, and their phylogenetic relationships (Anura: Craugastoridae). Zool. J. Linn. Soc. 2018, 182, 129–172. [Google Scholar] [CrossRef]
- Noble, G.K. Five new species of Salientia from South America. Am. Mus. Novit. 1921, 29, 1–7. [Google Scholar]
- De la Riva, I.; Chaparro, J.C.; Padial, J.M. The taxonomic status of Phyllonastes Heyer and Phrynopus peruvianus (Noble) (Lissamphibia, Anura): Resurrection of Noblella Barbour. Zootaxa 2008, 1685, 67–68. [Google Scholar] [CrossRef]
- Vaurie, C. An ornithological gazetteer of Peru (based on information compiled by J. T. Zimmer). Am. Mus. Novit. 1972, 2491, 1–36. [Google Scholar]
- Lynch, J.D. New species of minute leptodactylid frogs from the Andes of Ecuador and Peru. J. Herpetol. 1986, 20, 423–431. [Google Scholar] [CrossRef]
- Padial, J.M.; Grant, T.; Frost, D.R. Molecular systematics of terraranas (Anura: Brachycephaloidea) with an assessment of the effects of alignment and optimality criteria. Zootaxa 2014, 3825, 1–132. [Google Scholar] [CrossRef]
- Heinicke, M.P.; Lemmon, A.R.; Lemmon, E.M.; McGrathc, K.; Hedges, S.B. Phylogenomic support for evolutionary relationships of New World direct-developing frogs (Anura: Terraranae). Mol. Phylogenet. Evol. 2018, 118, 145–155. [Google Scholar] [CrossRef]
- De la Riva, I.; Chaparro, J.C.; Padial, J.M. A new longstanding misidentified species of Psychrophrynella Hedges, Duellman & Heinicke from Departamento Cusco, Peru (Anura: Strabomantidae). Zootaxa 2008, 1823, 42–50. [Google Scholar]
- Frost, D.R. Amphibian Species of the World: An Online Reference. Version 6.2. Available online: https://amphibiansoftheworld.amnh.org/ (accessed on 23 October 2023).
- Lehr, E.; von May, R.; Moravec, J.; Cusi, J.C. A new species of Phrynopus (Amphibia, Anura, Craugastoridae) from upper montane forests and high Andean grasslands of the Pui Pui Protected Forest in central Peru. ZooKeys 2017, 713, 131–157. [Google Scholar] [CrossRef][Green Version]
- von May, R.; Lehr, E.; Rabosky, D.L. Evolutionary radiation of earless frogs in the Andes: Molecular phylogenetics and habitat shifts in high-elevation terrestrial breeding frogs. PeerJ 2018, 6, e4313. [Google Scholar] [CrossRef]
- Biomatters. Geneious R6, Version 6.1.5. 2013. Available online: http://www.geneious.com/ (accessed on 31 May 2019).
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined Selection of Partitioning Schemes and Substitution Models for Phylogenetic Analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Sánchez-Vialas, A.; Miñarro, M.; Padial, J.M.; De la Riva, I. Taxonomic reassessment and nomenclatural status of Niceforonia dolops and Hylodes philippi (Amphibia: Anura: Strabomantidae). Zootaxa 2023, 5330, 117–125. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A. Tracer. Version 1.5. 2007. Available online: http://tree.bio.ed.ac.uk/software/tracer (accessed on 30 October 2019).
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Heyer, W.R. Taxonomic notes on frogs from the Madeira and Purus rivers, Brasil. Pap. Avulsos Zool. São Paulo 1977, 31, 141–162. [Google Scholar] [CrossRef]
- Catenazzi, A.; Ttito, A. A new species of Psychrophrynella (Amphibia, Anura, Craugastoridae) from the humid montane forests of Cusco, eastern slopes of the Peruvian Andes. PeerJ 2016, 4, e1807. [Google Scholar] [CrossRef] [PubMed]
- Catenazzi, A.; Ttito, A. Psychrophrynella glauca sp. n., a new species of terrestrial-breeding frogs (Amphibia, Anura, Strabomantidae) from the montane forests of the Amazonian Andes of Puno, Peru. PeerJ 2018, 6, e4444. [Google Scholar] [CrossRef]
- Griffiths, I. The phylogeny of Smithillus limbatus and that status of the Brachycephalidae (Amphibia, Salientia). Proc. Zool. Soc. Lond. 1959, 132, 457–487. [Google Scholar] [CrossRef]
- Lynch, J.D. Evolutionary relationships, osteology, and zoogeography of leptodactyloid frogs. Misc. Publ. 1971, 53, 1–238. [Google Scholar]
- Lynch, J.D. A review of the Andean leptodactylid frog genus Phrynopus. Occas. Pap. 1975, 35, 1–51. [Google Scholar]
- Condori, F.P.; Acevedo, A.A.; Mamani, L.; Delgado, J.A.; Chaparro, J.C. A new species of terrestrial-breeding frog of the genus Psychrophrynella (Anura: Strabomantidae) from the Cordillera de Vilcabamba, southeastern Peru. Amphib. Reptile Conserv. 2020, 14, 127–137. [Google Scholar]
- Lynch, J.D. Two new species of frogs of the genus Euparkerella (Amphibia: Leptodactylidae) from Ecuador and Perú. Herpetologica 1976, 32, 48–53. [Google Scholar]
- Guayasamin, J.M.; Terán-Valdez, A. A new species of Noblella (Amphibia: Strabomantidae) from the western slopes of the Andes of Ecuador. Zootaxa 2009, 2161, 47–59. [Google Scholar] [CrossRef]
- Lehr, E.; Aguilar, C.; Lundberg, M. A new species of Phyllonastes from Peru (Amphibia, Anura, Leptodactylidae). J. Herpetol. 2004, 38, 214–218. [Google Scholar] [CrossRef]
- Duellman, W.E. A new species of leptodactylid frog, genus Phyllonastes, from Peru. Herpetologica 1991, 47, 9–13. [Google Scholar]
- Reyes-Puig, C.; Guayasamin, J.M.; Koch, C.; Brito-Zapata, D.; Hollanders, M.; Costales, M.; Cisneros-Heredia, D.F. A new species of the genus Noblella (Amphibia: Strabomantidae) from Ecuador, with new information for Noblella worleyae. Acta Herpetol. 2021, 16, 63–87. [Google Scholar] [CrossRef]
- Ocampo, M.; Aparicio, J.; Hoverud, N.B.; Domic, E.; Wallace, R.B. Amphibian diversity in Madidi National Park and Natural Integrated Management Area, Bolivia, one of the most diverse parks in South America. Herpetol. Notes 2024, 17, 371–389. [Google Scholar]
- Santa-Cruz, R.; von May, R.; Catenazzi, A.; Whitcher, C.; Tejeda, E.L.; Rabosky, D.L. A new species of terrestrial-breeding frog (Amphibia, Strabomantidae, Noblella) from the upper Madre de Dios watershed, Amazonian Andes and lowlands of southern Peru. Diversity 2019, 11, 145. [Google Scholar] [CrossRef]
- Harvey, M.B.; Almendáriz, C.A.; Brito, M.J.; Batallas-Revelo, D. A new species of Noblella (Anura: Craugastoridae) from the Amazonian slopes of the Ecuadorian Andes with comments on Noblella lochites (Lynch). Zootaxa 2013, 3635, 1–14. [Google Scholar] [CrossRef]
- Reyes-Puig, C.; Maynard, R.J.; Trageser, S.J.; Vieira, J.; Hamilton, P.S.; Lynch, R.L.; Culebras, J.; Kohn, S.; Brito, J.; Guayasamin, J.M. A new species of Noblella (Amphibia: Strabomantidae) from the Río Manduriacu Reserve on the Pacific slopes of the Ecuadorian Andes. Neotrop. Biodivers. 2020, 6, 162–171. [Google Scholar] [CrossRef]
- Lindken, T.; Anderson, C.V.; Ariano-Sánchez, D.; Barki, G.; Biggs, C.; Bowles, P.; Chaitanya, R.; Cronin, D.T.; Jähnig, S.C.; Jeschke, J.M.; et al. What factors influence the rediscovery of lost tetrapod species? Glob. Chang. Biol. 2024, 30, e17107. [Google Scholar] [CrossRef]
- Jaynes, K.E.; Páez-Vacas, M.I.; Salazar-Valenzuela, D.; Guayasamin, J.M.; Terán-Valdez, A.; Siavichay, F.R.; Fitzpatrick, S.W.; Coloma, L.A. Harlequin frog rediscoveries provide insights into species persistence in the face of drastic amphibian declines. Biol. Conserv. 2022, 276, 109784. [Google Scholar] [CrossRef]
- Scheffers, B.R.; Yong, D.L.; Harris, J.B.C.; Giam, X.; Sodhi, N.S. The world’s rediscovered species: Back from the brink? PLoS ONE 2011, 6, e22531. [Google Scholar] [CrossRef]
- Catenazzi, A.; Uscapi, V.; von May, R. A new species of Noblella (Amphibia, Anura, Craugastoridae) from the humid montane forests of Cusco, Peru. ZooKeys 2015, 516, 71–84. [Google Scholar] [CrossRef] [PubMed]
- IUCN SSC Amphibian Specialist Group. Noblella myrmecoides. The IUCN Red List of Threatened Species 2023, e.T57235A85895894. Available online: https://www.iucnredlist.org/species/57235/85895894 (accessed on 8 January 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).