Abstract
The widespread perception of New Zealand is of a group of remote islands dominated by reptiles and birds, with no native mammals except a few bats. In fact, the islands themselves are only part of a wider New Zealand Region which includes a large section of Antarctica. In total, the New Zealand Region has at least 63 recognised taxa (species, subspecies and distinguishable clades) of living native mammals, only six of which are bats. The rest comprise a large and vigorous assemblage of 57 native marine mammals (9 pinnipeds and 48 cetaceans), protected from human knowledge until only a few centuries ago by their extreme isolation in the southwestern Pacific Ocean. Even after humans first began to colonise the New Zealand archipelago in about 1280 AD, most of the native marine mammals remained unfamiliar because they are seldom seen from the shore. This paper describes the huge contrast between the history and biogeography of the tiny fauna of New Zealand’s native land mammals versus the richly diverse and little-known assemblage of marine mammals.
1. Introduction
Modern New Zealand is the only emergent part of a large fragment of continental crust known as Zealandia (Figure 1), which began to split off from the rest of the ancient continent of Gondwanaland about 82 million years ago, at the time when Australia and Antarctica were still joined [1]. Zealandia is big enough to qualify as Earth’s eighth continent, and the parts of it that have been at various times above sea level were always thickly forested. They were at first inhabited by terrestrial representatives of the ancient Gondwanaland fauna and flora [2], supplemented over time by immigrants and filtered by repeated changes in sea level and climate [3].
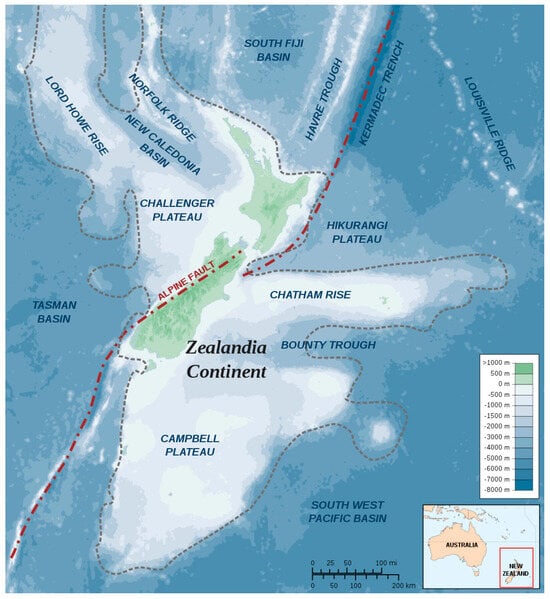
Figure 1.
Position and extent of Zealandia, Earth’s hidden eighth continent. Sourced from Wikipedia Commons, 2008, https://en.m.wikipedia.org/wiki/File:Zealandia-Continent_map_en.svg (accessed on 5 December 2023).
On the islands, a long history of geological upheavals has sifted through a series of variable subsets of terrestrial wildlife species, part legacy, part immigrant, thrown together in combinations unknown elsewhere [3]. The one constant theme has always been the complete absence of natural invasion and colonisation by any living species of land mammals unable to travel from distant continental centres of evolution. In such severe solitude, priceless remnants of early experiments in evolutionary adaptation of frogs, lizards and invertebrates, which have been extinguished everywhere else, have been preserved alongside new lineages arising from scattered populations stranded by millions of years of varying sea levels or tectonic uplifts, as reviewed by Tennyson [4]. All land mammals other than bats have been introduced since the mid-18th century [5]. In the surrounding oceans, and in the Antarctic, native faunas evolved independently, far outside human view.
2. The Benefits of Splendid Isolation
Like the Galapagos, the islands of modern New Zealand comprise an archipelago greatly favouring the evolution of new species surrounded by rich feeding grounds for marine mammals (Figure 2A). Their total land area of 270,000 km2, with a collective coastline of about 15,000 km, is scattered across a broad swathe of the southwest Pacific Ocean.
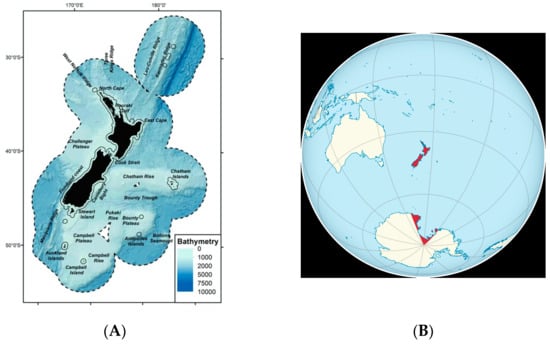
Figure 2.
(A) Map showing New Zealand’s Exclusive Economic Zone (EEZ, black dashed line) extending to 200 nautical miles (370.4 km) from land, showing water depth and island names. From F. Stephenson et al., 2022, DOI: 10.3389/fmars.2021.792712. (B) The position on the globe of the New Zealand Region, including both the island archipelago and the Ross Dependency in Antarctica. From:Wikipedia Commons, 2011. https://commons.wikimedia.org/wiki/File:New_Zealand_on_the_globe_%28New_Zealand_centered%29.svg (accessed on 5 December 2023).
Unlike the Galapagos, the islands are regarded, in biogeographic terms, as only part of a wider New Zealand Region, which includes a triangular chunk of Antarctica and the Southern Ocean (Figure 2B). This area, the Ross Dependency (450,000 km2), is formally defined as a sector of Antarctica originating at the South Pole, passing along longitudes 160° East to 150° West, and terminating at latitude 60° South. The native mammal faunas of the habitable land areas versus the uninhabited polar seas could not be more different.
2.1. Native Land Mammals
The North, South and Stewart Islands (113,729 km2, 150,437 km2 and 1748 km2 respectively), collectively known as the “mainland”, support a human population of 5.2 million spread over 1430 km between latitudes 34°and 47° South. The three main islands are only the largest of 735 inshore and outlying islands of various sizes larger than 1 ha. They range from fragments separated from the mainland coast by narrow channels to scattered larger chunks of land in distant, splendid isolation. Major outlying groups range from the subtropical Kermadec group (Raoul and Macauley Islands, at 29 degrees South), via the Chatham Islands (800 km east of Christchurch) to the subantarctic groups (Snares, Auckland, Antipodes Bounty and Campbell Islands, at 52° S) (Figure 2A). All islands are fringed with huge expanses of mostly wild coasts and surrounded by vast areas of pristine ocean.
The gap that opened up as Zealandia drifted away eastwards is now called the Tasman Sea. Around the present New Zealand islands, open ocean stretches in all directions; 2388 km north to New Caledonia, 1500 km west to Australia, 6252 km south to Antarctica and 9625 km east to Chile. Ocean currents bring warm waters from the tropical north, and cold ones from the Antarctic; the prevailing westerly winds bring storms, heavy rain and the occasional stray from Australia; cyclones forming in the Pacific can devastate the north and east of the North Island.
For decades, biogeographers have assumed that the reason there are no living land mammals (or snakes) in modern New Zealand is that their ancestors all “missed the boat”. In fact, the earliest members of all of these groups (and their archaic predecessors) were already well established in Australia long before Zealandia began to separate away [6]. So the alternative explanation is that there once were pre-modern land mammals in New Zealand, but they have no living descendants, and we have not yet found any fossils showing that they were ever here.
In 2006, fragments of a hitherto unknown terrestrial mouse-sized mammal (not an ordinary mouse, or a marsupial), were found in fossil deposits at St Bathans, in Otago, 19–16 million years old [7]. The implication is that tiny land animals belonging to some ancient, mystery Gondwanan lineage were indeed living on Zealandia at some point since it separated from the rest of the world. Even more intriguing, if they were as ancient as other known relicts which survived on small fragments of land throughout the Oligocene period around 25 million years ago, when most of Zealandia’s previously emergent surface was under water [8], their previously unsuspected existence would be globally significant. All the recognisably modern mammals, round the world or introduced to New Zealand, are very recent by comparison.
Those few fragments found by lucky chance in Otago are our only evidence that any such animal once existed. On the other hand, no-one has been able firmly to identify them, despite all efforts to find more material. So there still is not enough to say who these enigmatic little creatures might have been or how they fitted into the story of New Zealand’s mammal history. Meanwhile, apart from them, the history of the mammals of Zealandia includes no four-footed species at all.
The first human voyagers to settle in the land they called Aotearoa arrived from eastern Polynesia in about 1280 AD [9], and the only living land mammals they found were those whose ancestors had been able to travel across vast ocean barriers. These early Polynesian colonists found great populations of bats pouring out of caves and tree roosts, plus seasonal assemblages of breeding fur seals and sea lions gathered on rocky shores and beaches.
At that time, bats were the only mammalian residents of an extensive forested archipelago which had become, alone among all places on earth, the place where birds and reptiles had adapted to fill all available niches normally dominated by terrestrial mammals. In the rest of the world, the evolution of non-volant mammals proceeded along other paths, long after all feasible cross-Tasman land connections were lost.
2.2. Native Marine Mammals
By contrast, the vast majority of mammals native to the New Zealand Region are marine species. The wide shallow seas of Zealandia’s continental shelves have provided rich feeding for a long succession of coastal species, illustrating the importance of coastal Zealandia to breeding marine species. In the past, at least three species of huge penguins (and others known but still undescribed) evolved in this area, including some that were much larger than any known today [10]. Among the few endemic cetaceans described below are the two subspecies of New Zealand dolphins, both confined to coastal waters.
Further out, the rich oceans of the New Zealand Region swarm with marine mammals, comprising one of the world’s most important and least known biodiversity hotspots. They were protected from human exploitation until James Cook and other early explorers commented on their huge abundance throughout the Southern Ocean and its uninhabited islands [11]. Most are relatively unknown even today.
Definitions
In this paper, ‘Native’ means any species whose ancestors evolved in the New Zealand Region (including a section of Antarctica) or arrived without human help, and which now maintains independent populations in the wild. All of New Zealand’s indigenous species are native under that definition, and all are strictly protected. ‘Taxa’ includes species, subspecies and recognisable clades, not all fully determined.
DOC stands for the New Zealand Department of Conservation, which is responsible for protecting all native species. DOC’s categories of conservation status are listed in Table 1.

Table 1.
DOC’s categories of conservation status for native mammal taxa [12,13].
2.3. Why the Mammals of New Zealand Are Unique
The net result of New Zealand’s peculiar history and geography is that the combination of known native mammalian species in the New Zealand Region is unique in the world, for several reasons. (Macquarie, Lord Howe and Norfolk Islands are part of the same biogeographic assemblage but administered from Australia, so are not included in the New Zealand Region).
First, the New Zealand Region offers a greater range of habitats and climates than any other biogeographical region in the world. It includes not only a sprawling archipelago of tropical, temperate and subantarctic islands, but also the Ross Ice Shelf and two important Antarctic research stations.
Second, New Zealand is the only country in which the non-native terrestrial species are completely dominant (in numbers both of species and of individuals) over the native species [14]. The only exceptions are the bats, of which four of the six known lineages are threatened or at risk (Table 2). Yet, off shore, the opposite is true: all of the 57 marine species or subspecies are native, and only two pinnipeds and five cetaceans are threatened or at risk (Table 3)—although some are only just recovering from historic human exploitation. This imbalance tends to strike overseas observers as astonishing, so one of the purposes of this paper is to explain how it came about.

Table 2.
Species and subspecies of bats in New Zealand.

Table 3.
Living species and subspecies of marine mammals of the New Zealand Region [12]. * Endemic; NR: Non-resident.
Third, because New Zealand’s native species are all by definition good travellers, few are classed as endemic. Only the longest-resident and most special group of all, the Mystacinidae (short-tailed bats), are endemic at the family level. Only the New Zealand sea lion (Phocarctos hookeri) is endemic at the genus level, because its isolated main breeding areas lie within New Zealand’s section of the subantarctic region. The most recently established residents, the New Zealand long-tailed bat Chalinolobus tuberculatus, the New Zealand fur seal Arctocephalus forsteri and the two lineages of New Zealand dolphin Cephalorhynchus hectori, are endemic at the species or subspecies level. No other bats, and very few other marine mammals, are confined to the New Zealand Region, so the vast majority of the 63 taxa recognised by DOC as belonging to the New Zealand native mammal fauna (Table 1) are not endemic at any level.
Fourth, New Zealand was the most recent large land mass to be settled by people. It is the largest by far of the Pacific islands to be colonised by the Polynesians, but also the coldest. Organised settlement by Europeans began only in 1840, a full 50 years after the first convict ships landed their reluctant passengers in Australia [15].
This late start did at least mean that successive New Zealand governments could avoid some of the mistakes made by other colonial powers, including the deliberate importation of at least the worst exotic mammalian species that have caused havoc elsewhere. Our acutely vulnerable native bats, birds, lizards, and land-breeding pinnipeds would have been even worse off but for the absence of foxes, squirrels and mink, but they have not escaped many other imported pests including rats, mice, cats, rabbits, mustelids and hedgehogs [5]. The once enormously abundant marine mammals (especially fur seals and commercially important whales), which were once the backbone of early colonial economics, have suffered decades of ruthless human exploitation.
3. History and Evolution of New Zealand’s Chiroptera
The Australian mainland is an island continent with an ancient and diverse fauna, and Aotearoalies downwind of it across the Tasman Sea. The gap between the two land masses eventually spread southwards to reach >2000 km wide. The last connection, via the Lord Howe Rise, was cut some 52 million years ago [1].
Ever since then, the turbulent Southern Ocean has generated many westerly storms that rage around the bottom of the world. They have occasionally caught up stray birds and bats from Australian airspace and carried them eastwards. A lucky few wind-blown stragglers found landfall in time to establish new populations. At least three Australian bat species survived a 500 km trip to Lord Howe Island; another two survived being blown a full 1300 km to Norfolk Island [14].
Of about 1000 species of bats in the world, Australia has at least 90. New Zealand has only a handful of living or extinct resident species, all derived from Australian strays. No doubt many others have also been making the trip for tens of millions of years, but we know of only four long-established members of one ancient Australasian family, the Mystacindae, of which only one survives, and one much more recent arrival representing a different and widely dispersed family, the Vespertilionidae, as described below and in Table 2.
The only vagrant (or, more likely, a storm-driven waif) we know of that lived long enough to be observed was a small fruit bat, a little red flying fox, Pteropus scapulatus (Family Pteropodidae), which died on landing in about 1926 [16]. It is rated by DOC as a Vagrant, and it could not have survived for long, because New Zealand forest does not supply the foods that fruit bats live on. It is worth mentioning here only because it shows the continuing potential for windblown bats to cross the Tasman Sea unaided.
Advances in field technology have enabled a huge increase in research on New Zealand bats since the 1990s. All are adapted to hunting and roosting in forests, which is why historic deforestation has affected them so badly. Radio-transmitters small enough (0.4–0.7 g) to attach to bats weighing <15 g are providing a wealth of information on the natural lives of wild bats for up to a month at a time. Small hand-held automated heterodyne Batbox III bat detectors, which record the ultrasonic sounds emitted by bats measured in kHz, have been used throughout the country to study distribution and habitat use. Flying bats can be distinguished with detectors set at different values of kHz. There is some overlap between the species, but they can be distinguished because they have different peak amplitudes [17].
3.1. The Mystacinids, New Zealand’s Ancient Bats
The Mystacinidae comprise an ancient family of short-tailed bats originating in Australia at least 40 million years ago [18]. Of those members of the family whose ancestors were windblown across the Tasman Sea by around 35 million years ago, we know of four species. Evolution in such prolonged isolation has made the New Zealand mystacinids different from any other bats in the world, so they are all are counted as truly endemic species at the family level.
The lesser short-tailed bat (Mystacina tuberculata) is the only one that still survives [19], although a close relative (the greater short-tailed bat, M. robusta) has declined to probable extinction within living memory [13]. In addition, there are two described and several undescribed extinct mystacinid species found as fossils. The Australian lineage (known only from fossils, the oldest dated to 26 million years ago) is now extinct.
All mystacinids are semi-terrestrial, that is, they are described as ‘walking bats’, adapted to save some of the huge energy costs of hunting in flight by spending a lot of time foraging on the ground with their wings tucked into protective pouches [20]. In Aotearoa’s ancient forests, free from snakes and four-footed mammalian hunters, walking was a safe hunting strategy for the mystacinids, one that is not open to bats native to other countries. The short wingspan and rounded wingtips allow short-tail bats to take off from the ground, and enough manoeuvrability to navigate between branches through cluttered forest interiors, yet they are still capable of fast flight in the open.
We did not know how much more diverse our bat fauna once was until palaeontologists began excavating New Zealand’s oldest mammal-bearing sediments in the South Island. Near the village of St Bathans is a rich palaeoecological site formed from the ancient bed of Lake Manuherikia, once a vast expanse of fresh water extending across nearly 6000 km2 of Otago [21]. The sediments have yielded the remains of a huge variety of animals and plants that once lived in and around the lake in Miocene times. This complex community included ancient species such as tuatara (Sphenodon punctatus), frogs (Leiopelma spp.), wrens (Family Acanthisittidae), kiwi (Apteryx spp.) and early moa (Order Dinornithiformes). Fossils recovered from that treasure trove have enabled detailed reconstructions of how different life in New Zealand was around 19–16 million years ago, when global temperatures averaged up to 12 °C warmer than today.
Mystacina miocenalis [18] is a large extinct bat, both semi-terrestrial and omnivorous, weighing around 39–40 g. The structure of the skeleton suggests that these bats spent much of their foraging time on the ground searching for insects, spiders, weta (native Orthoptera), fruit, flowers and nectar.
Vulcanops jennyworthyae [22] is different enough from the other mystacinids to warrant its own separate genus. It is the largest of the four known species of walking bats. Its teeth suggest that it fed on a wide range of food resources, up to and including small vertebrates. It lived on the shores of Lake Manuherikia among a diverse range of other warm-climate Miocene-era species, but had disappeared from the fossil record by the end of the Pleistocene period.
3.2. The Most Recent Successful Colonist
The only other species of living bat in New Zealand is the very different longtailed bat Chalinolobus tuberculatus [17]. Its ancestors arrived much more recently than did those of the mystacinids, and it represents an entirely different bat family, the Vespertilionidae. It evolved in New Zealand from an Australian species of Chalinolobus that arrived unaided sometime during the early Pleistocene, within the last million years. It is distinct from its Australian relatives, so is endemic to New Zealand, but only at the species level.
4. History and Evolution of New Zealand’s Marine Mammals
The New Zealand archipelago is just an array of isolated chunks of land surrounded by shallow seas covering the broad continental shelf of Zealandia. During the Oligocene period, almost all of Zealandia sank below sea level [8], so that much of what is now dry land was once covered by sunlit water thronged with penguins, seals, whales and dolphins—not to mention sharks and extinct marine reptiles. Their fossils are preserved in the accumulated sediments deposited then, until earth movements associated with the building of the Southern Alps exposed them. The emergent part of Zealandia still amounts to only about 6% of the total 49 million km2 continental shelf, but its peculiar geography has made it one of the best places in the world to study the evolutionary history of marine mammals.
The Diversity of New Zealand’s Native Marine Mammals
The living taxa of New Zealand marine mammals are listed in Table 3 by their conservation status, as determined by DOC in 2019. Most are scarcely known, but more details about some of particular interest are added below. The Marine Mammals Protection Act of 1978 provides for the complete protection of all marine mammals, whether dead or alive, within New Zealand fisheries waters—i.e., within 200 nautical miles (370.4 km) of land.
5. Pinnipeds
5.1. Fossil History of Southern Pinnipeds
The Otariidae (fur seals and sea lions) and the Phocidae (true seals) are both derived from a common caniform ancestor that lived in the early Miocene era, 28–20 million years ago, and are collectively known as the pinnipeds.
The earliest ancestors of the modern pinnipeds appeared in the Southern Ocean much later than the cetaceans, and their fossils are rare. The lack of older fossils was once thought to mean that seals and sea lions evolved in the Northern Hemisphere and were slow to migrate southwards, but several important fossils found only recently have changed our understanding of the origins of the pinnipeds.
The earliest New Zealand fossil phocid was excavated from a rich deposit of marine fossils of Pliocene age dated to 3.0–3.4 million years ago, found on the coast of Taranaki (western North Island). From an extensive phylogenetic analysis, and on that fossil, Rule et al. [23] suggested that it lived in southern waters about 3 million years ago and later crossed the equator northwards. It was named Eomonachus belegaerensis—the dawn monk seal from Belegaer (the western “Great Sea” from Tolkien’s Middle Earth, whose unofficial home is also in New Zealand). It shows that the ancestors of monarchid seals evolved in the Southern Hemisphere.
The earliest New Zealand fossil otariid known so far is Neophoca palatina, found as a nearly complete adult male skull collected on Ōhope Beach on the North Island in 1937 [24]. It is similar to but distinguishable from the Australian seal lion N. cinerea, and is of Pleistocene age.
5.2. Living Otariids
Fur seals are still found on or near suitable rocky shorelines around both New Zealand and Australia. Genetic analyses suggest three non-exclusive breeding groups, based around the coasts of New Zealand, Australia and the subantarctic islands [25]. Since the end of commercial sealing, recolonisation of the depleted New Zealand populations has come mainly from remote western colonies.
Sea lions once ranged right up to the northern North Island, and pre-historic remains up to 10,000 years old have been reported from at least 47 sites, but intense harvesting limited their distributions further and further southwards over time [26]. Now the main breeding populations are confined to the Auckland and Campbell Islands. Wandering males often visit the southern coasts of Otago and Stewart Island. A thriving new breeding colony in southern Otago comprises the descendants of one female, born on Auckland Island in the late 1980s, who produced 11 pups before disappearing in 2010.
5.3. Living Phocids
New Zealand has five species of true seals, all members of the subgroup Monachinae. Two of them often visit the New Zealand mainland, but the other three are confined to the Antarctic, not only to the Ross Dependency (Figure 2B). All use delayed implantation to synchronize parturition among females.
The Southern elephant seal Mirounga leonina has four main genetically independent breeding stocks spread around the Antarctic, one of which has rookeries on Campbell and Antipodes Islands. [27]. Some individuals visit Auckland, Snares, Stewart, Chatham and eastern South Islands and occasionally produce pups there. Males are much larger than females because they have stable breeding colonies and vigorous competition between males for mating rights. Intensive harvesting for blubber oil ended in the New Zealand Region in 1830. The surviving breeding population on Campbell Island was numbered at 417 in the late 1940s, and has since declined by 97%. The South Atlantic population is large (>460,000) and growing [28].
The Weddell seal Leptonychotes weddellii lives on the Ross ice shelf, McMurdo Sound, Ross and White Islands and throughout the Ross Sea of Antarctica. Adults haul out on the fast ice surface alongside the spaces between deep, perennial tide cracks, and range to the edge of the pack ice. Weddell seals were once killed for dog meat to support polar expeditions and Antarctic bases, but killing them ceased in 1996, and dogs are no longer allowed in Antarctica. The present world population is estimated at ca. 1,000,000 [29].
The leopard seal Hydrurga leptonyx ranges throughout the Ross Dependency, and individuals occasionally visit New Zealand waters and cause alarm by hauling out on popular beaches. They are the only seals that frequently hunt warm-blooded prey, and can impose significant mortality on penguin [30] and fur seal [31] populations. They have never been commercially exploited, but are hard to count, so their present population numbers are uncertain. The International Pack Ice Seal Program surveys estimated about 15,000 leopard seals in the Ross and Amundsen Seas alone [32].
The crabeater seal Lobodon carcinophagus is found on pack ice throughout the Ross Dependency, and occasionally, for unknown reasons, far inland. They are hugely abundant—numbering about 1.7 million in in the Ross and Amundsen Seas [32]—but have never been exploited.
The Ross seal Ommatophoca rossii is the smallest of the Southern Ocean seals. They live mainly in the Ross Sea, widely dispersed on circumpolar pack ice, but are seldom seen except when breeding or moulting; at other times of year, they spend long periods in open water. They have never been exploited, but are vulnerable to attack by orcas (killer whales) when at sea far from the ice. There are about 22,600 of them in the Ross and Amundsen Seas [32].
6. Cetacea
The cetaceans (whales and dolphins) are descended from terrestrial mammals belonging to an early hoofed lineage. Over time their descendants evolved amphibious forms, gradually adapting to living more and more of their lives at sea. Most living species of cetaceans still retain tiny vestigial hind limbs, visible only in skeletons. Unlike seals and sea lions, the cetaceans do not have to return to land in the breeding season. They are the only mammals to have fully adapted to mating, birthing and suckling their young entirely under water.
About 35 million years ago, the cetaceans began to separate into two quite different lineages. In one, the Mysticeti (baleen whales), the teeth were lost and replaced by baleen plates suspended from an enlarged upper jaw, an efficient system of filtering swarms of invertebrates out of the water. In the other lineage, the Odontoceti (toothed whales and dolphins), the teeth were retained but simplified into rows of sharp pegs.
The two lineages of cetaceans have been evolving separately since about 34 million years ago [33]. World-wide, there are about 66 species of toothed whales, and 10 species of baleen whales, depending on the authority cited, of which a great majority are recorded from the New Zealand Region (Table 3).
The exceptionally diverse fauna of native cetaceans of the Southern Hemisphere can be traced back to the late Eocene, some 30 million years ago, when the opening of the Drake Passage cut the last link between the southern tip of South America and the Antarctic Peninsula, and established the circum-polar current [34,35]. At that time, there was no land to stand in the way of the near-continuous westerly wind and cold currents roaring clockwise around Antarctica, cutting off the rapidly freezing southern continent from all contact with warmer waters from the north. This in turn stimulated huge upwellings of nutrients supporting the plankton and invertebrates that feed on them, especially krill. Hence the Southern Ocean has been a rich feeding ground for cold-adapted marine mammals for much longer than has the Arctic, which began to freeze less than 5 million years ago. The evolution of huge baleen whales has been continuing in their “cold southern cradle” for at least 20 million years [36].
6.1. Fossil History of Southern Cetaceans
The history of southern cetaceans is well documented by a series of amphibious intermediates, including many important extinct species preserved as fossils found in New Zealand [37]. The earliest known whales (the Archaeoceti) still showed evidence of their terrestrial origins, and New Zealand has a good example of that stage represented by the fossil teeth and skull fragments of Kekenodon onamata. It lived 42.6 million years ago, and had enormous long jaws studded with complex teeth similar to those of land animals. The patterns of wear on these teeth suggest that it had already become an apex marine predator comparable with the modern orca, hunting hard-boned prey such as penguins and other marine mammals [38].
The New Zealand fossil record supplies early examples of both modern cetacean lineages [39]. Among the first Mysticeti was the dawn baleen whale Tokarahia kauaeroa, 26 million years old, found in the Otekaieke limestone deposits in Otago. Early Odontoceti are represented by two important fossils of extinct New Zealand dolphins excavated from the Otekaieke limestone, both are estimated to be about 25–26 million years old. The many species of Squalodon, the shark-toothed dolphins, were distributed worldwide as successful near-top predators, but they had to compete with the very large sharks and with other advancing lineages of predatory cetaceans inhabiting the oceans of their time. Waipatia maerewhenua was a small archaic dolphin, with skull features showing that it was already capable of echolocation. It represents a new family, related to but distinct from the Squalodon linages, and is of critical importance in piecing together the evolution of the toothed whales generally [40].
The sequence of richly fossiliferous rocks exposed in the limestone escarpments of the Waitaki valley, north Otago, is so important for studies of New Zealand marine palaeontology that the entire area has been recognised as New Zealand’s first UNESCO Global Geopark, the Waitaki Whitestone Geopark. Many important fossils have been recovered, especially of whales and dolphins that lived in the shallow seas that covered the area during the Oligocene period. Some 13 species of fossil whales have been recovered from the Waitaki area alone. In the middle of it is the village of Duntroon, and the Vanished World Centre. Casts of all the fossils listed above can be seen in the Centre’s Museum.
6.2. Living Odontoceti
Table 3 lists the total number of cetacean species recognised as members of the native mammal fauna of New Zealand as of 2019. Most are summarised and described in more detail elsewhere [41]. The great majority (30 of 48) are classed as deficient in data or not threatened, leaving only three as Nationally Critical, one as Nationally Endangered and one as Nationally Vulnerable. Only the two subspecies of the New Zealand dolphin Cephalorhynchus hectori are at serious risk of extinction [42]. Here I mention a selected few of the best-known species.
The odontocetes (toothed whales) are fast swimmers, hunting fish and squid by echo-location, often diving to great depths. They all have single blow-holes, and range in size from the 1.4 m New Zealand dolphins (small by cetacean standards) to the 16 m or longer sperm whale Physeter macrocephalus. The earliest species were known only from isolated teeth until the first full skulls were found.
The toothed whales are all active hunters. They use a variety of clicks generated by their single blow-hole to create a stream of sound signals to serve for communication and navigation by echolocation. They have large brains in relation to their body mass, and a ‘melon’ in the head, used to focus sound waves. Some have more than 100 simple peg-like teeth, and others have few, more bizarrely shaped teeth, more for display than for hunting.
The three species of sperm whales (Physeteroidea) all have a spermaceti organ and teeth only in the lower jaw, and use echolocation for hunting and social communication. In pre-whaling days, sperm whales ranged around the southern oceans in huge numbers, up to a million strong. The meat and teeth of stranded sperm whales were a very important resource for Māori. European and American whalers took advantage of their strong social bonding to kill thousands a year, ruthlessly targeting them for their valuable sperm oil. Now they are reduced to less than 400,000, and (since females give birth only every 4–6 years) are only slowly recovering from historic slaughter [43], but they still live year-round in New Zealand waters.
Much less known are the beaked whales (Ziphidae), which are medium sized, with a pointed beak and few teeth, living in deep water far offshore. Most are very difficult to tell apart, and typically seen only as (uncommon) strandings.
The dolphins (Family Delphinidae) are a world-wide group of 37 species of small (most <4 m), agile, fast-swimming cetaceans with slender, streamlined bodies, large dorsal fins and pointed snouts. Table 3 lists the species known to frequent New Zealand waters, or visiting as rare vagrants. The best known New Zealand dolphins are the two subspecies of Cephalorhynchus hectori [42]: C. h. hectori found only off the coast of the South Island and C. h. maui off the western North Island. Both are listed among the small number of most threatened marine species (Table 3) and are particularly important because they are among our very few endemic cetaceans. They inhabit shallow coastal waters where they are very vulnerable to entanglement in fishing nets.
Some individual dolphins seem to enjoy contact with humans, and their activities become temporarily famous. “Pelorus Jack” was a Risso’s dolphin (Grampus griseus) which, over a period of at least 24 years from 1888 to 1912, routinely accompanied steam ships passing through Cook Strait on a fixed route between Wellington and Nelson [44]. “Opo” was a young female bottlenose dolphin (Tursiops truncatus) that regularly mingled with swimmers at Opononi beach in the southern summer of 1955–56. She attracted crowds of visitors, especially many fascinated children, and a great deal of publicity that benefited the local tourist industry [45].
Less friendly is the orca, also known as the killer whale (Orcinus orca). Orca are commonly seen in New Zealand coastal waters, but they are very mobile, so frequent sightings do not necessarily equate to more than a few hundred individuals in fast-moving groups. The Southern Ocean population comes in four distinct types, A, B, C and D [46]. Most orca in New Zealand are of the A type and live in three distinguishable sub-populations—one each in North and South Island waters and a third less settled. The total resident population in New Zealand is estimated at fewer than 250, of which at least 117 individuals are identified and named. The taxonomic status of these sub-groups is still undetermined [41].
6.3. Living Mysticeti
The baleen whales have no teeth, but their gigantic upper jaws are hung with fringed baleen plates made of keratin. They swim slowly near the surface, searching the wind for the scent of aggregations of krill when coming up for air, then, when finding one, taking in huge volumes of water into their expandable mouths. They then use their massive tongue to force water out sideways past the baleen plates that strain out small food items. This filtering system is very energy-efficient, and allows the baleen whales to reach enormous sizes. They have paired blow-holes, and range in size from the 5 m pygmy right whale (Caperea marginata) to the 30 m Antarctic blue whale (Balaeonoptera musculus intermedia).
Baleen whales spend the southern winter gorging on krill in the rich subantarctic waters, but regularly move north from their cold Antarctic feeding areas past New Zealand to the warmer subtropical waters more suitable for birthing their calves. On their return journey southwards, many females would be accompanied by their calves. There is also a population of pygmy blue whales (Balaenoptera musculus brevicauda) that lives permanently in the South Taranaki Bight [47].
The known species of baleen whales that have been sighted in New Zealand waters are listed in Table 3, of which the most significant in New Zealand’s history are the Southern right whale Eubalaena australis and the humpback whale Megaptera novaeangliae.
Southern right whales prefer shallow water, and are well adapted to it, so they rarely strand. Adults and calves are often sighted off the east coast of the main islands and off Auckland and Campbell Islands in winter and spring. The slow swimming speed of the southern right whales made them easy targets for the early whalers, and hence were hunted for their oil and baleen almost to extinction. The estimated total pre-whaling population was between 60,000 to 120,000 worldwide. By 1920, only about 60 breeding females were left. In New Zealand waters, the estimated pre-whaling population of between 22,000 and 32,000 was practically wiped out by the 1850s, leaving only about 14–52 individuals by 1923 [41]. The total southern hemisphere population is now around 12,000 to 15,000. Recovery has been slow [48].
Humpback whales’ regular migration routes made their seasonal locations easy for whalers to predict. There were once some 50,000–60,000 in the South Pacific, of which about 20,000–30,000 migrated back and forth along the eastern Australian coast and another 20,000 past New Zealand [41]. Between 1959 and 1961, more than 25,000 humpback whales were killed in New Zealand waters, virtually wiping out the breeding stock [49]. By 2015 there were only about 4300 humpbacks breeding around the South Pacific islands, of which fewer than 300 migrated past New Zealand.
Now, passive monitoring of singing males by automated acoustic recorders is tracking increasing numbers of whales passing northbound through New Zealand waters during the migration period, as they recover post-whaling. Australian waters host another 15,000 or so [41].
7. The Consequences of Early Human Colonisation
7.1. Impacts of Māori, 1280 to 1769 AD
7.1.1. Pinnipeds
From ca. 3500 years BP, the last wave of prehistoric human migration transported Polynesian colonists across the SW Pacific from SE Asia to the Society Islands, and thence to Aotearoa by about AD 1280 [50]. The enormous seasonal aggregations of fur seals and sea lions assembled on their mainland haul-out sites and breeding rookeries offered unprecedented sources of abundant meat to the newly arrived Polynesians, the very first humans to set foot on the last major uninhabited landmass on Earth. Moreover, the forest also teemed with large, unwary birds, including nine species of moa (Dinornithiformes). These huge supplies of high-quality protein help to explain the rapid population growth of the first settlers [51].
The culture of their Pacific island homeland offered no tradition of sustainable management of such apparently boundless resources. Early hunters harvested all accessible colonies of fur seals and sea lions for meat and clothing over the next 150 years. Both fur seals and sea lions were at first easy to hunt, especially the juveniles and pups, so were drastically reduced in numbers and distribution. Archaeological excavations and genetic analyses of material from early settlement sites in the far south of the South Island document numerous remains of pinnipeds deposited in early middens (dated to 1280–1450 AD), that are absent from later ones [52]. Within two hundred years, all the moa, plus many other large flightless birds, were extinct [53], and the surviving seals and sea lions had retreated to breeding colonies on distant offshore islands.
For example, excavations of the lowest levels of South Island middens, dated to the early to mid-1300s, found that the estimated weight of pinniped meat represented was, briefly, the same or more than that from moa. In the village at the mouth of the Shag River occupied in the mid-1300s, seals provided almost 40% of the meat eaten there, falling to 12% within 20 years [54]. At Papatowai, the bones recovered represented 12 seals and 10 moa; at Pounawea, 25 seals and 10 moa, and at Tiwai Point, 31 seals and 11 moa [55]. At any one site, this unsustainable harvest would not last long before the community moved on.
This early reliance on seals, and the eventual transition to other resources, is clear from the details of timing and location of food remains, strongly suggesting that all local and accessible colonies of pinnipeds were eradicated in turn by Māori hunters. The bones and teeth of fur seals and (less often) sea lions were important resources for carvers, until the supply ran out at around the same time as did other larger and meaty prey, including moa and penguins. By the mid-1500s, midden sites in the far south were dominated by sea shells, and in due course were overgrown by trees dating from the 1600s. For more details of the distribution of the last southern fur seal colonies, see Richards [56].
The cultural evolution of the skilled Polynesian hunter-colonists rapidly shifted from that of their Pacific island ancestors to a new, indigenous Māori system. It was based largely on local adaptations of traditional horticulture and fishing, and reached its classic form as described by the first European explorers in the mid-18th century [57]. Fortunately, the lost mainland colonies of pinnipeds and penguins (but not moa) could be partially re-established from related populations that had survived on the southern islands, out of reach of Māori hunting technology [58].
7.1.2. Cetaceans
Stranded whales and dolphins were regarded by Māori as taonga (sacred) species, representing the abundance and richness of gifts to the local tribe from Tangaroa, the god of the oceans. These great creatures appeared to come ashore of their own accord, presenting their finders with enormous quantities of meat, oil and whalebone [59].
Weapons, fish hooks, harpoon heads and tools were made from whale bone [60]. Auckland Museum’s Māori Natural History gallery has a case displaying combs, ornaments and weapons carved from whale bone, plus a video presenting a traditional story recounting a conversation between a whale and a kauri tree.
Some tribes depicted stylised carvings of whales on their elaborately adorned storehouses or marae, surmounted by the figure of an important ancestor. A carving atop the marae at Whangara, Gisborne District, celebrates the story of Tohora (the Māori name for southern right whales and humpback whales) who saved legendary hero Paikea from drowning and carried him to land. A carving in a meeting house at Manutuke, south of Gisborne, probably depicts Tinirau, lord of all the fishes, with his pet whale, Tutunui (clearly a baleen whale) [59].
Teeth from smaller species were used as necklaces and pendants. The huge teeth of the sperm whale were especially impressive, and its great strength symbolised the prestige of a powerful chief. A sperm whale stranded on the beach was likened to a chief killed in battle. The national museum of New Zealand in Wellington, Te Papa (Māori for ‘the treasure box’), displays a magnificent example of carving made by Rangi Kip, from the Taranaki iwi Te Ati Awa, on a tooth from a sperm whale that had been stranded on the Wairarapa coast in 1995.
7.1.3. The Kiore, an Honorary Native
The Pacific rat, or kiore, Rattus exulans cannot be included among the true natives because it could not have reached Aotearoa unaided, but it is important to Māori colonisation history and genealogy because its genetics have proven crucial in documenting the history of Polynesian migration across the Pacific [61].
The kiore is widely distributed on most Pacific islands. It was transported with migrating people around the Pacific islands in their ocean-going canoes, long before any European rat species reached the Pacific Ocean. Evidence of the earliest presence of people in New Zealand dated to the late 13th century AD agrees with the earliest archaeological evidence for first human arrival, the onset of widespread megafaunal extinctions, and the pre-European decline of marine mammal populations [62,63]. Human settlers and their two companions (the kiore, and the kuri, or Polynesian domestic dog Canis familiaris) made an immediate and detectable imprint on New Zealand’s native biota from the early 1300s onwards [50].
8. Impacts of Europeans and Americans, after 1769
8.1. Pinnipeds
During the peak of industrial sealing (1810–1830), both species of otariids were practically exterminated from all known haul-out sites by European and American sealers. They operated ruthless, uncontrolled, indiscriminate slaughtering expeditions from ocean-going ships capable of reaching all known mainland and accessible subantarctic island populations that had previously been safe from Māori hunters. Historian Ray Richards [64] estimated that at least 7 million fur seals were killed by 1833, but commercial competition ensured that many sealing locations were kept secret and/or their records falsified. Eventually, the increasingly poor economics of seal harvesting, plus closed seasons imposed from 1875, saved both species from extinction. All sealing was stopped by 1916 [65].
8.2. Cetaceans
Human activities that could harm cetaceans living far offshore are very recent. The huge diversity and abundance of cetaceans in the southwestern Pacific had benefitted from their longer isolation, and longer protection from human activities, than anywhere else in the world. Long after whaling on an industrial scale was well established in the north Atlantic by 1725, the earliest European explorers venturing into the Southern Ocean had never seen anything like what they found in southern waters. James Cook and many of those who followed him after 1769 often commented on the huge numbers of whales and fur seals they saw. Better still, in those lawless days, these rich, untouched resources were open to anyone to harvest, free of regulations or taxes. The first whalers to break into this Pacific bounty rounded the Horn in 1788, and many others followed from the 1790s onwards.
The business of setting up a commercial expedition for pelagic whaling was expensive, in ships, equipment and crew. So, from the beginning, this trade was dominated by established British and American interests. The earliest whalers hunted whales on their breeding grounds north and east of New Zealand. Harvesting of whales so far offshore was difficult and dangerous, and the early whale-chasing boats launched from modest motherships under sail were slow and vulnerable to being flipped—chase boat, oars and men—by a wounded whale.
Skilled Māori sailors welcomed the opportunity to join whaling ships as crew. Their familiarity with use of spears translated readily into great expertise as harpooners [65]. Less welcome were the supplies of muskets (traded by European and American whalers in exchange for pork and potatoes grown by Māori) that caused huge disruptions of Māori society in pre-colonial days. The inevitable arrival of Norway rats and cats escaping from whaling ships began the assault by invasive species on the native taonga that Māori loved [14].
Despite the risks, the early crews targeted the huge, challenging sperm whales. The spermaceti oil stored in the head of the sperm whale had especially desirable qualities that made hunting for them a primary target. Spermaceti oil was a very high-quality fuel that did not smoke or burn when heated, or solidify when frozen, so was ideal for lighting, cooking and lubrication of sensitive machinery in extreme conditions. Spermaceti oil was in huge demand in European and American markets, and could be bailed out of a dead sperm whale’s head by the bucketful. Also, the large teeth in their lower jaws were a valuable source of ivory. They were extracted for carving and for scrimshaw (the art of engraving or carving on teeth or bone from marine mammals).
When the captains of pelagic whaling ships needed to replenish stocks of fresh food and water, or find a temporary base for repairs, they made for the nearest friendly shore, often in northern New Zealand. In 1830, there were 30 whaling ships anchored off Kororareka, carrying crews totalling up to 1000 men [66]. The visits of whaling crews to the Bay of Islands changed the material culture of the northern Māori tribes. By the 1840s there were more than 700 American whalers working worldwide, most in the Pacific. Inevitably, pelagic whale stocks and profits were rapidly declining by the 1850s.
A less expensive method available to whaling crews based in New Zealand was to establish a whaling station on a shore overlooking a regular whale migration route. In earlier times, huge numbers of baleen whales passed the coasts of New Zealand and eastern Australia, easily spotted by lookouts stationed on cliff-tops. The chase boats would be launched, and if a kill was made, the crew would tow the carcase back for processing on land. In either case, harpoons and chase boats were at first powered by human effort, which meant that most of the largest and faster-moving whale species were out of reach.
Shore whaling stations often developed into local farming communities providing employment, education, tools and provisions during the off season. Local Māori permitted their presence, and benefitted from the opportunities arising from mutual cooperation. The last operational shore whaling station in New Zealand closed in 1964. The remains of at least 87 shore whaling stations are known and protected as archaeological sites [67]. The era of European whaling provided Māori living near shore whaling stations with unregulated access to the remains of whale carcases for their own uses. In many districts, shore whalers formed the first European community, where Māori gained access to goods from the outside world and to new commercial opportunities relating to northern hemisphere industrial economics.
Bay whaling from ships anchored in sheltered bays started in about 1827 as the first phase of pelagic whaling was slowing down. Bay whalers targeted the slower-moving right and humpback whales for their black oil, boiled down from their blubber, and for their baleen, which was often more valuable than the oil. Between them, deep-sea and shore-based whaling, unregulated throughout the 1800s, devastated all populations of large whales throughout New Zealand waters. By the middle of the 19th century, right whales were almost gone, and it began to look as if the whaling era was over. Then the development of the harpoon gun and the steam-powered chaser made whaling operations from shore stations or anchored ships much more efficient, and enabled crews to target whale species which had until then been safe.
Until 1904, whales were not pursued into the freezing waters of the far Southern Ocean. Then, when other species became uneconomic, attention shifted to the Antarctic. Over the years 1923–1933, a Norwegian company ran a series of nine expeditions that set off the last major phase of unregulated and destructive whaling. The Norwegians had been granted a licence by the British government to hunt for whales in the Ross Sea [68]. They came equipped with powerful factory ships that could be independent of shore for long periods, each with a fleet of steam-driven chasers armed with massive harpoon guns with explosive heads. They concentrated first on the biggest species, the blue, sperm and humpback whales. As those stocks dwindled, the whalers moved on in turn to sei, fin and minke whales. The total catch over the decade of the Norwegians’ annual visits amounted to 18,610 whales; the largest of the Norwegian ships, the Kosmos, and its chasers alone took 1822 whales in the 1929–1930 season, and 2431 in 1930–1931, before the slaughter was stopped in 1933 [69].
The International Whaling Commission (IWC, established 1946) now regulates commercial operations, and in 1994 established a circumpolar Southern Ocean whale sanctuary extending from the Antarctic coast to 40° S, which includes New Zealand waters south of the southern North Island. In addition, New Zealand’s Marine Mammals Act of 1978 strictly protects all marine mammals throughout New Zealand territorial waters, although they all remain vulnerable to accidental drowning in fishing nets.
Funding
This research received no external funding.
Data Availability Statement
This paper summarises published information into an original narrative. Greatly extended accounts of the land-breeding marine mammal species (bats and pinnipeds) can be found in [14], and of cetaceans in [41]. The primary data can be found in the references cited.
Acknowledgments
Readers looking for illustrations of the living species mentioned here can find them in my Native Mammals of New Zealand, a visually rich but minimally referenced guide book to be published for the tourist market in 2024. This paper is a companion publication for readers wanting the bibliographic details. Thanks to Upstart Press, Auckland, for permission to make this unusual arrangement possible. I acknowledge with profound thanks the diligent work of two anonymous referees, who provided careful and detailed comments that have greatly improved the draft MS. Any faults that remain are my own.
Conflicts of Interest
The author declares no conflicts of interests.
References
- Campbell, H.; Hutching, G. In Search of Ancient New Zealand; Penguin and GNS Science: Auckland, New Zealand, 2011. [Google Scholar]
- Gibbs, G.W. Ghosts of Gondwana; Craig Potton Publishing: Nelson, New Zealand, 2006. [Google Scholar]
- Worthy, T.H.; de Pietri, V.L.; Schofield, R.P. Recent advances in avian palaeobiology in New Zealand with implications for understanding New Zealand’s geological, climatic and evolutionary histories. N. Z. J. Zool. 2017, 44, 177–211. [Google Scholar] [CrossRef]
- Tennyson, A.J.D. The origin and history of New Zealand’s terrestrial vertebrates. N. Z. J. Ecol. 2010, 34, 6–27. [Google Scholar]
- King, C. Abundance and Dynamics of Small Mammals in New Zealand: Sequential Invasions into an Island Ecosystem Like No Other. Life 2023, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Archer, M.; Hand, S.J.; Godthelp, H. Riversleigh: The Story of Animals in Ancient Rainforests of Australia; Reed Books Pty.: Balgowiah, NSW, Australia, 1991. [Google Scholar]
- Worthy, T.H.; Tennyson, A.J.D.; Archer, M.; Musser, A.M.; Hand, S.J.; Jones, C.; Douglas, B.J.; McNamara, J.A.; Beck, R.M.D. Miocene mammal reveals a Mesozoic ghost lineage on insular New Zealand, southwest Pacific. Proc. Natl. Acad. Sci. USA 2006, 103, 19419–19423. [Google Scholar] [CrossRef]
- Campbell, H. The Zealandia Drowning Debate: Did New Zealand Sink beneath the Waves? BWB Texts: Wellington, New Zealand, 2013. [Google Scholar]
- Wilmshurst, J.M.; Hunt, T.L.; Lipo, C.P.; Anderson, A. High-precision radiocarbon dating shows recent and rapid initial human colonization of East Polynesia. Proc. Natl. Acad. Sci. USA 2011, 108, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Mayr, G.; Scofield, P.; De Pietri, V.; Tennyson, A.J.D. A Paleocene penguin from New Zealand substantiates multiple origins of gigantism in fossil Sphenisciformes. Nat. Commun. 2017, 8, 1927. [Google Scholar] [CrossRef] [PubMed]
- Beaglehole, J.C. (Ed.) The Journals of Captain James Cook on His Voyages of Discovery: Volume I: The Voyage of the Endeavour 1768–1771; Cambridge University Press for the Hakluyt Society: Cambridge, UK, 1955. [Google Scholar]
- Baker, C.; Boren, L.; Childerhouse, S.; Constantine, R.; van Helden, A.; Lundquist, D.; Rayment, W.; Rolfe, J.R. Conservation Status of New Zealand Marine Mammals, 2019; New Zealand Threat Classification Series 29; Department of Conservation: Wellington, New Zealand, 2019. [Google Scholar]
- O’Donnell, C.F.J.; Borkin, K.M.; Christie, J.; Davidson-Watts, I.; Dennis, G.; Pryde, M.; Michel, P. Conservation Status of Bats in Aotearoa New Zealand, 2022; New Zealand Threat Classification Series 41; Department of Conservation: Wellington, New Zealand, 2023. [Google Scholar]
- King, C.M.; Forsyth, D.M. (Eds.) The Handbook of New Zealand Mammals, 3rd ed.; CSIRO Publications: Melbourne, Australia, 2021. [Google Scholar]
- King, M. The Penguin History of New Zealand; Penguin Books: Auckland, New Zealand, 2003. [Google Scholar]
- Daniel, M.J. First record of an Australian fruit bat (Megachiroptera: Pteropodidae) reaching New Zealand. N. Z. J. Zool. 1975, 2, 227–231. [Google Scholar] [CrossRef]
- O’Donnell, C.F.J.; Borkin, K.M. Chalinolobus tuberculatus. In The Handbook of New Zealand Mammals, 3rd ed.; Families Vespertilionidae and Mysticinidae; King, C.M., Forsyth, D.M., Eds.; CSIRO Publishing: Melbourne, Australia, 2021; pp. 95–130. [Google Scholar]
- Hand, S.J.; Lee, D.E.; Worthy, T.H.; Archer, M.; Worthy, J.P.; Tennyson, A.J.D.; Salisbury, S.W.; Schofield, R.P.; Mildenhall, D.C.; Kennedy, E.M.; et al. Miocene fossils reveal ancient roots for New Zealand’s endemic Mystacina (Chiroptera) and its rainforest habitat. PLoS ONE 2015, 10, e0128871. [Google Scholar] [CrossRef]
- Parsons, S.; Toth, C.A. Mystacina tuberculate. In The Handbook of New Zealand Mammals, 3rd ed.; Families Vespertilionidae and Mysticinidae; King, C.M., Forsyth, D.M., Eds.; CSIRO Publishing: Melbourne, Australia, 2021; pp. 95–130. [Google Scholar]
- Hand, S.J.; Weisbecker, V.; Beck, R.M.D.; Archer, M.; Godhelp, H.; Tennyson, A.J.D.; Worthy, T.H. Bats that walk: A new evolutionary hypothesis for the terrestrial behaviour of New Zealand’s endemic mystacinids. BMC Evol. Biol. 2009, 9, 169. [Google Scholar] [CrossRef]
- Reichgelt, T.; Kennedy, E.M.; Conran, J.G.; Mildenhall, D.C.; Lee, D.E. The early Miocene paleolake Manuherikia: Vegetation heterogeneity and warm-temperate to subtropical climate in southern New Zealand. J. Paleolimnol. 2015, 53, 349–365. [Google Scholar] [CrossRef]
- Hand, S.J.; Beck, R.M.D.; Archer, M.; Simmons, N.B.; Gunnell, G.F.; Schofield, R.P.; Tennyson, A.J.D.; De Pietri, V.L.; Salisbury, S.W.; Worthy, T.H. A new, large-bodied omnivorous bat (Noctilionoidea: Mystacinidae) reveals lost morphological and ecological diversity since the Miocene in New Zealand. Sci. Rep. 2018, 8, 235. [Google Scholar] [CrossRef]
- Rule, J.P.; Adams, J.W.; Marx, F.G.; Evans, A.R.; Tennyson, A.J.D.; Scofield, R.P.; Fitzgerald, E.M.J. First monk seal from the Southern Hemisphere rewrites the evolutionary history of true seals. Proc. R. Soc. B 2020, 287, 20202318. [Google Scholar] [CrossRef] [PubMed]
- King, J.E. The Ohope skull—A new species of Pleistocene sealion from New Zealand. N. Z. J. Geol. Geophys. 1983, 26, 105–120. [Google Scholar] [CrossRef]
- Dussex, N.; Robertson, B.C.; Salis, A.T.; Kalinin, A.; Best, H.; Gemmell, N.J. Low spatial genetic differentiation associated with rapid recolonisation in the New Zealand fur seal Arctocephalus forsteri. J. Hered. 2016, 107, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Childerhouse, S.; Gales, N. Historical and modern distribution and abundance of the New Zealand sea lion Phocarctos hookeri. N. Z. J. Zool. 1998, 25, 1–16. [Google Scholar] [CrossRef]
- Hoelzel, A.R.; Campagna, C.; Arnbom, T. Genetic and morphometric differentiation between island and mainland southern elephant seal populations. Proc. R. Soc. Lond. 2001, 268B, 325–332. [Google Scholar] [CrossRef]
- Laws, R.M. History and present status of southern elephant seal populations. In Elephant Seals: Population Ecology, Behavior, and Physiology; Le Boeuf, B.J., Laws, R.M., Eds.; University of California: Berkeley, CA, USA, 1994; pp. 49–65. [Google Scholar]
- Kooyman, G.L. Weddell Seal: Consummate Diver; Cambridge University Press: Cambridge, UK, 1981. [Google Scholar]
- Penney, R.L.; Lowry, G. Leopard seal predation on Adèlie penguins. Ecology 1967, 48, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Boveng, P.L.; Hiruki, L.M.; Schwartz, M.K.; Bengtson, J.L. Population growth of Antarctic fur seals: Limitation by a top predator, the leopard seal? Ecology 1998, 79, 2863–2877. [Google Scholar] [CrossRef]
- Bengston, J.L.; Laake, J.L.; Boveng, P.L.; Cameron, M.F.; Hanson, M.B.; Stewart, B.S. Distribution, density and abundance of pack-ice seals in the Amundsen and Ross Seas, Antarctica. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 1261–1276. [Google Scholar]
- Pyenson, N.D. The Ecological Rise of Whales Chronicled by the Fossil Record. Curr. Biol. 2017, 27, R558–R564. [Google Scholar] [CrossRef]
- Barker, P.F.; Burrell, J. The opening of Drake Passage. Mar. Geol. 1977, 25, 15–34. [Google Scholar] [CrossRef]
- Fordyce, R.E. The development of the Circum-Antarctic Current and the evolution of the Mysticeti (Mammalia: Cetacea). Palaeogeog. Palaeoclimatol. Palaeoecol. 1977, 21, 265–271. [Google Scholar] [CrossRef]
- Rule, J.P.; Duncan, R.J.; Marx, F.G.; Pollock, T.I.; Evans, A.R.; Fitzgerald, E.M.G. Giant baleen whales emerged from a cold southern cradle. Proc. R. Soc. B 2023, 290, 20232177. [Google Scholar] [CrossRef]
- Whitmore, F.C.J.; Sanders, A.E. Review of the Oligocene Cetacea. Syst. Biol. 1976, 24, 304–320. [Google Scholar] [CrossRef]
- Corrie, J.E.; Fordyce, R.E. A redescription and re-evaluation of Kekenodon onamata (Mammalia: Cetacea), a late-surviving archaeocete from the Late Oligocene of New Zealand. Zool. J. Linn. Soc. 2022, 196, 1637–1670. [Google Scholar] [CrossRef]
- Marx, F.G.; Lambert, O.; Uhen, M.D. Cetacean Paleobiology; Wiley Blackwell: Oxford, UK, 2016. [Google Scholar]
- Fordyce, R.E. Waipatia maerewhenua: New Genus and New Species (Waipatiidae, New Family), an Archaic Late Oligocene Dolphin (Cetacea:Odontoceti: Platanistoidea) from New Zealand. Proc. San Diego Soc. Nat. Hist. 1994, 29, 147–176. [Google Scholar] [CrossRef]
- Todd, B. Whales and Dolphins of Aotearoa New Zealand; Te Papa Press: Wellington, New Zealand, 2014. [Google Scholar]
- Slooten, L.; Dawson, S. Dolphins Down Under: Understanding the New Zealand Dolphin; Otago University Press: Dunedin, New Zealand, 2013. [Google Scholar]
- Whitehead, H.; Shin, M. Current global population size, post-whaling trend and historical trajectory of sperm whales. Sci. Rep. 2022, 12, 19468. [Google Scholar] [CrossRef]
- Cowan, J. Pelorus Jack: The White Dolphin of French Pass, New Zealand; Whitcombe & Tombs Ltd.: Christchurch, New Zealand, 1911. [Google Scholar]
- Lee-Johnson, E. Opo: The Hokianga Dolphin; David Ling Publishing Ltd.: Auckland. New Zealand, 1994. [Google Scholar]
- Pitman, R.L.; Ensor, P. Three forms of killer whales (Orcinus orca) in Antarctic waters. J. Cetacea Res. Manag. 2003, 5, 131–139. [Google Scholar] [CrossRef]
- Barlow, D.R.; Torres, L.G.; Hodge, K.B.; Steel, D.; Baker, C.S.; Chandler, T.E.; Bott, N.; Constantine, R.; Double, M.C.; Gill, P.; et al. Documentation of a New Zealand blue whale population based on multiple lines of evidence. Endanger. Species Res. 2018, 36, 27–40. [Google Scholar] [CrossRef]
- Carroll, E.L.; Patenaude, N.J.; Childerhouse, S.J.; Kraus, S.D.; Fewster, R.M.; Baker, C.S. Abundance of the New Zealand subantarctic southern right whale population estimated from photo-identification and genotype mark-recapture. Mar. Biol. 2011, 158, 2565–2575. [Google Scholar] [CrossRef]
- Constantine, R.; Jackson, J.A.; Steel, D.; Baker, C.S.; Brooks, L.; Burns, D.; Clapham, P.; Hauser, N.; Madan, B.; Mattila, D.; et al. Abundance of humpback whales in Oceania using photo-identification and microsatellite genotyping. Mar. Ecol. Prog. Ser. 2012, 453, 249–261. [Google Scholar] [CrossRef]
- Matisoo-Smith, E. The Human Landscape: Population Origins, Settlement and Impact of Human Arrival in Aotearoa/New Zealand. In Landscape and Quaternary Environmental Change in New Zealand; Atlantis Advances in Quaternary Science; Shulmeister, J., Ed.; Atlantis Press: Paris, France, 2017; Volume 3. [Google Scholar] [CrossRef]
- Brown, A.A.; Crema, E.R. Maori Population Growth in Pre-contact New Zealand: Regional Population Dynamics Inferred from Summed Probability Distributions of Radiocarbon Dates. J. Isl. Coast. Archaeol. 2021, 16, 572–590. [Google Scholar] [CrossRef]
- Seersholm, F.V.; Cole, T.L.; Grealy, A.; Rawlence, N.L.; Greig, K.; Knappe, M.; Stat, M.; Hansen, A.J.; Easton, L.J.; Shepherd, L.; et al. Subsistence practices, past biodiversity, and anthropogenic impacts revealed by New Zealand-wide ancient DNA survey. Proc. Natl. Acad. Sci. USA 2018, 115, 7771–7776. [Google Scholar] [CrossRef] [PubMed]
- Holdaway, R.N.; Allentoft, M.E.; Jacomb, C.; Oksam, C.L.; Beaven, N.R.; Bunce, M. An extremely low-density human population exterminated New Zealand moa. Nat. Commun. 2014, 5, 5436. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, L. Using diversity indices to measure changes in prey choice at the Shag River Mouth site, southern New Zealand. Int. J. Osteoarchaeol. 2001, 11, 101–111. [Google Scholar] [CrossRef]
- Anderson, A.; Smith, I. The Papatowai site: New evidence and interpretations. J. Polyn. Soc. 1992, 101, 129–158. [Google Scholar]
- Richards, R. Sealing in the Southern Oceans, 1788–1833; Paremata Press: Paremata, New Zealand, 2010. [Google Scholar]
- Salmond, A. Two Worlds. First Meetings between Maori and Europeans 1642–1772; Viking Press: Auckland, New Zealand, 1991. [Google Scholar]
- Collins, C.J.; Rawlence, N.J.; Prost, S.; Anderson, C.N.K.; Knapp, M.; Scofield, R.P.; Robertson, B.C.; Smith, I.; Matisoo-Smith, E.A.; Chilvers, B.; et al. Extinction and recolonization of coastal megafauna following human arrival in New Zealand. Proc. R. Soc. B 2014, 281, 20140097. [Google Scholar] [CrossRef] [PubMed]
- Orbell, M. The Natural World of the Maori; William Collins Publishers: Auckland, New Zealand, 1985. [Google Scholar]
- Cunliffe, E.A.; Brooks, E. Prehistoric Whale Bone Technology in Southern New Zealand. Int. J. Osteoarch. 2014, 26, 384–396. [Google Scholar] [CrossRef]
- Matisoo-Smith, E.; Roberts, R.M.; Irwin, G.J.; Allen, J.S.; Penny, D.; Lambert, D.M. Patterns of prehistoric human mobility in Polynesia indicated by mtDNA from the Pacific rat. Proc. Natl. Acad. Sci. USA 1998, 95, 15145–15150. [Google Scholar] [CrossRef]
- Davidson, J. The Prehistory of New Zealand; Longman Paul: Auckland, New Zealand, 1984. [Google Scholar]
- Wilmshurst, J.; Anderson, A.J.; Higham, T.F.G.; Worthy, T.H. Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. Proc. Natl. Acad. Sci. USA 2008, 105, 7676–7680. [Google Scholar] [CrossRef]
- Richards, R. New market evidence on the depletion of southern fur seals: 1788–1833. N. Z. J. Zool. 2003, 30, 1–9. [Google Scholar] [CrossRef]
- McNab, R. Murihiku: A History of the South Island of New Zealand and the Islands Adjacent and Lying to the South, from 1642 to 1835; Whitcombe & Tombs Ltd.: Wellington, New Zealand, 1909; (reprinted by Cambridge University Press: Cambridge, UK, 2011). [Google Scholar]
- Wolfe, R. Kororareka: Hell-Hole of the Pacific; Penguin: Auckland, New Zealand, 2005. [Google Scholar]
- Prickett, N. The Archaeology of New Zealand Shore Whaling; Department of Conservation, National Historic Heritage Workshop: Wellington, New Zealand, 2002. [Google Scholar]
- Tønnessen, J.N. Norwegian Antarctic whaling, 1905–1968: An historical appraisal. Polar Rec. 2009, 15, 283–290. [Google Scholar] [CrossRef]
- Esler, L. Whaling and Sealing in Southern New Zealand; Lloyd Esler: Invercargill, New Zealand, 2014. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).