Abstract
In times of global insect decline, agricultural ecosystems need to be designed in an as insect-friendly manner as possible to halt the progressive loss of biodiversity. This is particularly important for steep-slope viticulture being established on sites with high biodiversity potential. Therefore, we compared different vineyard types (cross-slope with greened embankments vs. down-slope or other types without greened embankments), using wild bees and butterflies as indicators for biodiversity in the lower Moselle region (SW Germany). The numbers of species and individuals in both groups were significantly higher in cross-slope vineyards with greened embankments. This also held true for the number of specialised and endangered species. The communities of wild bees and butterflies differed remarkably between the vineyard types. Three wild bee and five butterfly species were identified as indicator species and hence can be used as such for further monitoring. Our results underline that the structure of steep-slope vineyards has tremendous importance for biodiversity conservation. Since the cultivation of cross-slope vineyards on steep slopes is easier than that of down-slope vineyards, we assume the great synergistic potential to reconcile agricultural use and biodiversity conservation and, in addition, to preserve steep-slope viticulture as a structural element in landscape planning.
1. Introduction
The decline in biological diversity is a severe global challenge. So far, we have not been able to stop or even slow it down []. This also applies to insects, which are of paramount importance for the functioning of all terrestrial ecosystems [,]. Consequently, mitigating insect decline is a fundamental aspect of nature conservation, especially in reconciling the maintenance of biodiversity and human land-use. A high proportion of the land area is used for agricultural production, e.g., 40% of the total surface of the European Union []. Globally, agriculture is considered the main driver of biodiversity loss []. The effects of climate change, conversion of natural habitats into farmland, intensification of agricultural methods, and the use of agro-chemicals are important factors [] causing the degradation, fragmentation, and loss of habitats [,]. Particularly troublesome are the increasing losses of pollinators, which are responsible for the reproduction of more than 80% of the world’s flowering plants, including a large number of crop species necessary for human nutrition [,]. Taking this into consideration, environmental policies are increasingly addressing this problem, e.g., the Green Deal of the European Community [], which attempts to reconcile economy and ecology in agriculture.
In Europe, insect decline has particularly accelerated over the last decades, also affecting sites of high biodiversity and hence conservation value [,]. This underlines the urgent necessity of reconciling economy and ecology in agriculture, with a special focus on the most valuable habitats in terms of biodiversity within regions. One frequent agricultural use at sites of regionally important biodiversity is viticulture, performed on steep slopes []. These sites are highly important habitats for xerothermophilic plants and animals, particularly in the northern winegrowing regions. Many of these species are considered vulnerable on different spatial scales []. In the past, plot realignments implemented to improve the economic viability of steep-slope viticulture have caused losses of two important determinants of biodiversity, namely, structural diversity and habitat heterogeneity [], which have been further aggravated by the intensive application of a large variety of different agro-chemicals []. However, in viticulture, the pioneering adoption of integrated production strategies using alternatives to chemical pesticides [] has led to a drastic reduction in the use of acaricides and insecticides in temperate European winegrowing regions []. This is a prerequisite for the conservation of biodiversity through the adaptation of vineyard management regimes. Still, the cultivation of steep-slope vineyards is highly labour- and cost-intensive, often resulting in abandonment []. The subsequent succession of fallow land results in the loss of structural diversity, which is accompanied by a loss of habitat quality for many of the typical and endangered species [].
Consequently, it will be necessary to move towards a structure of vineyards which can reconcile economic efficiency with the needs of biodiversity conservation. So far, the most frequently used vineyard type is down-slope rows, i.e., the rows of vine plants running strictly cross-contour-wise (Figure 1a), hereafter called down-slope vineyards. Although this vineyard type maximises the density of crop plants and hence the quantity of wine produced, it suffers from increasingly severe problems on particularly steep slopes [], which in turn often feature the vineyards potentially supporting valuable plant and animal communities. Thus, the economic reward of managing such vineyards is often severely reduced by the high costs caused by inaccessibility to machinery and the consequent reliance on a large amount of manual labour. This results in particularly high rates of abandonment of such extreme sites []. Alternatively, these steep slopes can be planted in a contour-wise manner (Figure 1b), hereafter called cross-slope vineyards. This vineyard design allows much easier cultivation, especially if terraces are sufficiently broad for the use of standard machinery, thus compensating for the reduced quantities of wine produced. The establishment of such cross-slope vineyards results in the creation of within-vineyard embankments, which reduce soil erosion in comparison with that of down-slope vineyards, and are also assumed to be highly beneficial for the maintenance of biodiversity, if they are equipped with a cover of flowering plants (hereafter called cross-slope vineyards with greened embankments; Figure 1b).
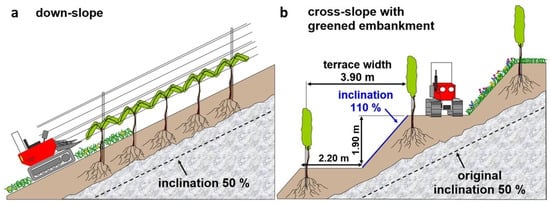
Figure 1.
Comparison of different vineyard types. Cross-contour-wise vineyards with rows running down-slope (a) in comparison to contour-wise vineyards with rows running cross-slope and with greened embankments (b).
To evaluate the potential positive effects of cross-slope vineyard types, a detailed comparison of their biodiversity with the traditional down-slope vineyards is urgently needed. In addressing this research aspect, we studied these two vineyard types in the Moselle region (southwestern Germany), which is one of the most important winegrowing regions of central Europe, particularly famous for its large number of steep-slope vineyards. As study organisms, we selected wild bees and butterflies, which fulfil an important role in maintaining functioning ecosystems as pollinators []. Furthermore, both of these insect groups are highly sensitive ecological indicators often used for the evaluation of environmental quality. In central Europe, the important criteria for such ecological indicators are rarity, endangerment, mobility, colonisation potential, the contribution of species to the natural balance, and the diversity of ecological requirements [,,,,]. In addition, they respond quickly to environmental changes due to high reproductive rates (more so in butterflies), short life cycles, and their low trophic level [].
In this context, we address the following research questions: (i) Do differences exist between the insect communities of cross-slope vineyards with greened embankments and vineyards without greened embankments? If so, (ii) do greened cross-slope vineyards harbour more species of conservation concern than vineyards without greened embankments? And, more generally, (iii) does the restructuring of steep-slope vineyards in the Moselle region (and beyond) have the potential to reconcile economically viable wine production with the maintenance of high biodiversity? Finally, we discuss the consequences for the preservation of a traditional cultural landscape, which is economically important, also beyond wine production, e.g., for regional tourism.
2. Materials and Methods
2.1. Study Area
The study was conducted in the vicinity of the village of Pommern (N 50°10′12, E 7°16′25; Moselle valley, Rhineland-Palatinate, southwestern Germany; Supplementary Figure S1). This region is characterised by steep slopes used for viticulture. The determining steep relief results in a highly heterogeneous landscape consisting of small cultivated and uncultivated plots, ecotone habitats, and structural elements such as dry stone walls [,], featuring a highly diverse bee fauna [].
The cultivated vineyards can be differentiated into two types: vineyards including greened embankments (GEVs) and vineyards with no such embankments (NEVs). GEVs are characterised by cross-slopes management techniques, enabled by drivable terraces about 2.20 m wide and separated by greened embankments up to 2 m high, with fractions of open soil on terraces between each row of vines. These transverse terraces allow the use of standard machinery in steep terrain, even at gradients of more than 60% []. A time-shifted mosaic mulching of the greened embankments is conducted only once or twice a year. Hence, the vegetation on the embankments shows an extended flowering period.
NEVs are characterised by down-slope management practices using narrow-track tractors, operated cross-contour along the steepest gradient. Such vineyards can normally be found up to a slope gradient of 40% []. Soil conditions, tillage (2 events per vegetation period), and vegetation management (2–4 mulching events per vegetation period) commonly lead to only sparse vegetation between the rows of vines. Furthermore, NEVs are represented by narrow cross-slope vineyards without terraces, which do not allow the use of machinery. Here, neither greened embankments nor other rich vegetation exist.
GEVs and NEVs were represented in the study by three vineyards each. Two of the three NEVs were down-slope vineyards; one was a cross-slope vineyard without terraces. All sampled vineyards had similar orientation and inclination and were managed conventionally. Both vineyard types were tilled 2–3 times per vegetation period. NEV inter-rows were mulched 2–4 times per vegetation period, and GEV inter-rows were mulched 1–2 times. Greened embankments were mulched with a combination of bar mower and flail mulcher only once a year at the end of June, and no tillage was undertaken here at all. Pesticide applications were standard local practice in all vineyards, and there were no applications of pesticides known to pose unacceptable risks to bees or the natural environment, as determined by state authorities in the pesticide registration process.
2.2. Sampling
For sampling wild bees (Hymenoptera: Apiformes excluding Apis mellifera) and butterflies (Lepidoptera: Papilionoidea), we selected a total of 24 fixed transects (4 within each of 6 vineyards; Supplementary Figure S1), each 50 m long. During each sampling event, every transect was sampled once. The sampling sequence of transects was randomised for each sampling day, so that the individual transects were sampled at different times of the day, with the restriction that sampling along shaded transects was avoided. All sampling was undertaken by the same observers (wild bees: A. Krahner; butterflies: L. Böhm; plant species: D. Braun).
Bees were sampled by targeted hand netting in 2012 (21 sampling events from April to September) and 2013 (4 sampling events from May to August) under favourable weather conditions (no or little wind, cloud cover 50% or less, temperatures ≥ 15 °C), from 10:00 to 17:00, around midsummer from 9:00 to 18:00. All bee individuals occurring in a strip 2 m wide, centred on the transect line, as well as up to 2 m above the ground and in front of the observer, were targeted for sampling. Transect walks were standardised to 10 min of sampling time per transect (excluding time for handling of caught individuals), and sampling motivation was to sample as many species and individuals as possible within the given time. In case of high bee abundances, for example, on highly attractive flower patches, the sampling protocol prioritised the collection of as many morphospecies as possible over the collection of all sighted individuals, in order to achieve an overall biodiversity assessment representative for the whole transect, within the fixed time window of 10 min. All sampled individuals were killed with ethyl acetate and taken to the laboratory for species identification. Sixteen species belonging to five closely related species groups were difficult to distinguish morphologically (e.g., no clear morphological differences in females) and were therefore pooled as in earlier studies [] (i.e., Andrena ovatula agg., comprising Andrena ovatula, A. wilkella, A. intermedia, A. similis and A. gelriae; Bombus terrestris agg., comprising Bombus cryptarum, B. lucorum, B. magnus and B. terrestris; Bombus hortorum agg., comprising Bombus hortorum and B. ruderatus; Lasioglossum smeathmanellum agg., comprising Lasioglossum nitidulum and L. smeathmanellum; Halictus simplex agg., comprising Halictus eurygnathus, H. langobardicus and H. simplex).
The survey of butterflies was conducted in 2014 (14 sampling events from March to September) following a standardised butterfly transect method []. All butterflies (species, number of individuals) occurring in the area 2.5 m to each side of the transect as well as 5 m above and in front of the counting person were recorded [,]. To prevent double counting, butterflies behind the counting person were ignored []. If necessary, butterflies were netted for determination and released afterwards. Surveys were only undertaken under suitable weather conditions (wind speed < 4 on the Beaufort scale, sunny, temperatures ≥ 17 °C) []. If the sampling on all transects could not be completed within one day, it was continued as soon as possible thereafter.
The species richness of insect-pollinated plants in flower was surveyed in 2012 (12 sampling events per transect from April to September) and 2013 (6 sampling events from May to September), recording all plant species in flower along the transects.
2.3. Statistical Analyses
Statistical analyses were performed in the R environment []. To compare insect diversity between GEVs and NEVs, the data of all records including the total numbers of individuals per species were used. For nonmetric multidimensional scaling (NMDS) analyses of wild bees, data of 2012 and 2013 were pooled by combining all sampled individuals and species in one data set, because sampling effort was markedly reduced in the second year of sampling. Mantel tests were performed to analyse spatial autocorrelation of transect data within each vineyard type, measuring the correlation between the spatial distance matrix and the distance matrix of species communities along transects (99,999 permutations; R packages: vegan 2.5.6 [], ade4 1.7-16 []). The spatial distance matrix of the transects was calculated using the transect centres in QGIS []. The Bray–Curtis dissimilarity was used for calculating the distance matrix of species communities. Abundance differences were downweighted via transformation (ln+1) of individual numbers.
For bees and butterflies, numbers of individuals and species as well as different diversity parameters were compared between GEVs and NEVs, while, for plants in flower, only number of species was compared. Exponential Shannon diversity (H′) and evenness (J′) as well as Hill numbers were computed using the R package vegan 2.5.6 []. Exponential Shannon diversity was used, as it facilitates interpreting differences in diversity compared to Shannon entropy []. Rényi diversity profiles, which are used for ranking communities from low to high diversity, were created using Hill numbers on different scale parameters []. Rényi diversity profiles allowed a general statement about the wild bee and butterfly diversity of the vineyard types, which provides more information than the single diversity indices [], because it allows for evenness to be taken into account with different weightings via varying scale parameters (alpha). Differences between the vineyard types were then tested by fitting LMMs or GLMMs (abundance, species richness, exponential Shannon diversity, evenness, Hill numbers). For the GLMM approach, full models were created including vineyard type as the fixed effect. Random-intercept effects included vineyard (butterflies) or vineyard nested in sampling year (bees and plants in flower), in order to take the nested structure of the sampled data into account. The choice of the GLMM family and model reduction was based on the AICc. Overdispersion of Poisson-GLMMs was assessed using R-package blmeco 1.4 []. GLMMs were fitted with R-package glmmTMB 1.0.2.1 []. For automated model selection, the ‘dredge’ function from the ‘MuMIn’ package version 1.43.17 [] was used. Models were validated through visual inspection of residual plots, using R-package DHARMa 0.4.1 []. For post hoc tests, using the Tukey method for p value correction, the R-package emmeans 1.6.0 was used [].
To investigate differences in the composition of the wild bee and butterfly communities between the GEV and NEV types, NMDS was performed. All analyses were based on Bray–Curtis distances after transformation (ln+1) of the individual numbers. R2 values (nonmetric fit) were calculated in order to obtain the combined portion of variance explained by the axes [] and stress values to assess the interpretability of two-dimensional projections (stress values 0.05–0.2) []. A combination of permutation-based multivariate analysis of variance (PERMANOVA) [] and permutation-based test for homogeneity of multivariate dispersions (PERMDISP) [] was used to analyse the differences between the vineyard types. NMDS, PERMANOVA, and PERMDISP (999 permutations) were performed using the R package vegan 2.5.6 []. Indicator species analyses were used to detect species potentially characteristic of GEVs and NEVs (R package indicspecies 1.7.9 []). Permutation-based significance tests (999 permutations) determined p-values. All graphics were created using the R package ggplot2 [].
3. Results
3.1. Monitoring Data
The monitoring resulted in data on 110 wild bee species represented by 1013 individuals in the years 2012 and 2013 and 31 butterfly species represented by 448 individuals in the year 2014 (Supplementary Table S1). A total of 96 plant species in flower were observed in 2012 and 2013. The recorded wild bee species belonged to seven families and the butterfly species to six families. Most bee individuals belonged to the Halictidae, followed by the Apidae and Andrenidae (Table 1). The majority of the butterflies belonged to a few species of the family Pieridae, followed by Nymphalidae and Satyrinae (Table 1). Among the total of 110 recorded bee species, 34 (i.e., 31%) are listed in the Red List of Germany: 21 species (i.e., 19%) in categories 2–3 corresponding to the IUCN Red List categories EN and VU, 11 species on the prewarning list (10%), 2 species with deficient data (2%) [], and 24 species (22%) in the Red List of Rhineland-Palatinate (categories 2–3) []. Of the 31 butterfly species recorded in total, 6 (i.e., 19%) are listed in the Red List of Germany: 3 species (i.e., 10%) in the categories 1–3, and 3 species (10%) on the prewarning list []. Overall, 13 species (38%) are listed in the Red List of Rhineland-Palatinate: 6 species (19%) in categories 1–3 and 7 species (23%) on the prewarning list [] (Table 1).

Table 1.
Total detected species and individuals of wild bees (2012, 2013) and butterflies (2014), subdivided into their families, and proportion of endangered species according to the Red List of Germany (GER) and Rhineland-Palatinate (RLP) [,,,] (category 1: threatened with extinction; category 2: critically endangered; category 3: endangered; see Supplementary Table S1 for a detailed species list).
Mantel tests (99,999 permutations) indicated the spatial autocorrelation of the data of wild bees within the GEV type (r = 0.591, p = 0.001). Reducing the number of transects to the two transects most distant from each other within the same vineyard resolved the problem of spatial autocorrelation (GEV: r = 0.053, p = 0.336, NEV: r = 0.400, p = 0.07). Accordingly, statistical analyses using NMDS for wild bees were conducted using this reduced data set comprising 568 individuals belonging to 88 species; however, the proportions of the seven families did not change for the composition of species and individuals (Supplementary Table S2). Transect data of butterflies did not indicate any spatial autocorrelation (GEV: r = 0.005, p = 0.436; NEV: r = 0.183, p = 0.071); hence, further statistical analyses using NMDS were based on the entire data set.
3.2. Species Richness and Diversity Differ between Different Vineyard Types
Numbers of recorded species of wild bees, butterflies, and plants in flower, as well as numbers of individuals of wild bees and butterflies, were significantly higher in cross-slope vineyards with greened embankments (GEVs) for all species as well as for single considered groups (Poisson and negative binomial GLMM: p < 0.05, Table 2, Supplementary Table S3) in comparison with vineyards without greened embankments (NEVs) (Figure 2). Numbers of species were two to more than three times higher in GEVs, while numbers of individuals were two to more than five times higher. Especially, species and individuals of endangered species as well as oligolectic wild bees and xerothermophilic butterflies were observed in higher numbers in GEVs.

Table 2.
Number of species and individuals of wild bees, butterflies, and insect-pollinated plants in flower, sampled in cross-slope vineyards with greened embankments (GEVs; n = 24 for wild bees and plants, n = 12 for butterflies) and in vineyards without greened embankments (NEVs; n = 24 for wild bees and plants, n = 12 for butterflies; model estimates, means, and standard errors). Significant differences: * (p < 0.05), ** (p < 0.01), *** (p < 0.001) (negative binomial/Poisson GLMM, Tukey’s test, Supplementary Table S3). NA: not assessed. Data on endangered species refers to the Red List of Germany (GER) and Rhineland-Palatinate (RLP) [,,,].
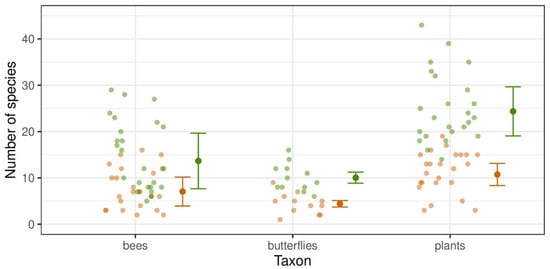
Figure 2.
Comparison of species richness of bees, butterflies, and insect-pollinated plants in flower between vineyard types. Pale smaller points represent transect observations, while larger points with error bars represent model estimates (means and standard errors). In cross-slope vineyards with greened embankments (GEVs, green), significantly higher species numbers were recorded compared with vineyards without greened embankments (NEVs, brown; Poisson/negative binomial GLMM, Tukey’s test: p < 0.05, Table 2, Supplementary Table S3).
Furthermore, the exponential Shannon diversity of wild bees and butterflies was higher for species in GEVs. These differences were significant for all species (butterflies and wild bees) and all considered groups of wild bees (LMM and Tweedie GLMM: p < 0.05, Table 3, Supplementary Table S3). Wild bees did not show any significant differences in evenness between GEVs and NEVs (LMM: p > 0.05, Table 3, Supplementary Table S3). For butterflies, however, evenness was significantly higher in GEVs than in NEVs. This applied to the analyses of all species as well as endangered species according to the Red List of Rhineland-Palatinate and Germany (beta GLMM and LMM: p < 0.05, Table 3, Supplementary Table S3) but not to monophagous and xerothermophilic species (Supplementary Table S3).

Table 3.
Exponential Shannon diversity (H′) and evenness (J′) (mean ± standard error) of the wild bee and butterfly communities sampled in cross-slope vineyards with greened embankments (GEVs; n = 24 for wild bees, n = 12 for butterflies) and in vineyards without greened embankments (NEVs; n = 24 for wild bees, n = 12 for butterflies). Significant differences between GEVs and NEVs: * (p < 0.05), ** (p < 0.01), *** (p < 0.001) (LMM/Beta/Tweedie GLMM, Tukey test, Supplementary Table S3). NA: vineyard type not included in model selection. Data on endangered species refers to the Red List of Germany (GER) and Rhineland-Palatinate (RLP) [,,,].
3.3. Insect Communities Differ between Vineyard Types
The communities of wild bees and butterflies recorded in GEVs were characterised by a higher diversity than those recorded in NEVs. This applied independent of how strongly the species represented in large numbers were downweighted in comparison to the rarer species. The difference in diversity was significant for all scale parameters regarding wild bees and to scale parameters α < 4 regarding butterflies (LMM, Poisson/Tweedie GLMM: p < 0.05, Figure 3, Supplementary Table S3).
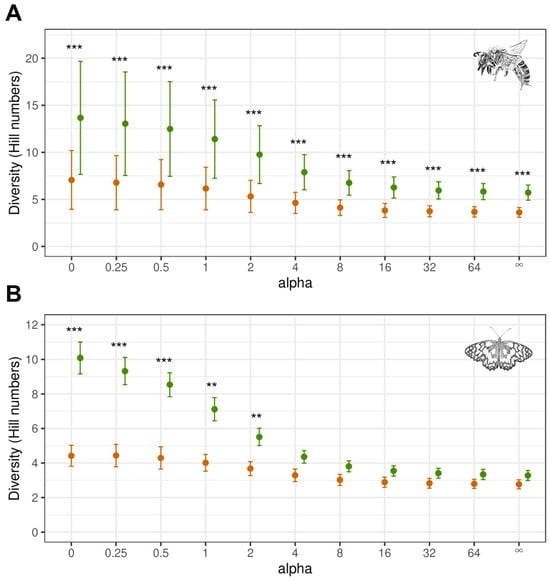
Figure 3.
Diversity profile of the wild bee (A) and butterfly communities (B). Diversity profiles are illustrated using Hill numbers (estimated means and standard errors) calculated with different scale parameters (alpha). Green: cross-slope vineyards with greened embankments (GEVs; n = 24 for wild bees, n = 12 for butterflies). Brown: vineyards with no greened embankments (NEVs; n = 24 for wild bees, n = 12 for butterflies). Significant differences between GEVs and NEVs: ** (p < 0.01), *** (p < 0.001) (LMM/Poisson/Tweedie GLMM, Tukey test, Supplementary Table S3). In the calculation of Hill numbers, weighting of evenness increased with the increase in alpha.
Differences in the similarity of wild bee and butterfly communities between GEVs and NEVs were observed (NMDS, Figure 4). These differences were highly significant for both insect groups (PERMANOVA, 999 permutations: (A) wild bees F = 3.142; df = 1; p = 0.003; (B) butterflies F = 3.142; df = 1; p = 0.003). Furthermore, the variability in the species composition of both wild bees and butterflies was significantly higher in NEVs (PERMDISP, 999 permutations: (A) wild bees F = 6.094; df = 1; p = 0.047; (B) butterflies F = 5.696; df = 1; p = 0.018).
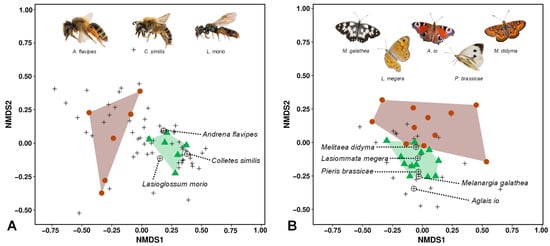
Figure 4.
Delineation of insect communities among different vineyard types. Nonmetric multidimensional scaling (NMDS) of wild bee (A) and butterfly communities (B) sampled in two different structural types of vineyards (Bray–Curtis distance, ln+1 transformed data). (A) Stress (3 runs) = 0.137; R2 = 0.981 (nonmetric fit); (B) stress (14 runs) = 0.160; R2 = 0.884 (nonmetric fit). Cross-slope vineyards with greened embankments (GEVs; green) and vineyards without greened embankments (NEVs; brown) were significantly different regarding community composition: PERMANOVA (A) F = 3.142, df = 1, 999 permutations, p = 0.003; (B) F = 4.506, df = 1, 999 permutations, p = 0.001. Furthermore, the variability in community composition significantly differed between GEVs and NEVs: PERMDISP (A) F = 6.094, df = 1, 999 permutations, p = 0.047; (B) F = 5.696, df = 1, 999 permutations, p = 0.018. Black crosses: species (indicator species, see Table 4 are highlighted); convex hulls: insect communities in GEVs (green triangles: transects in GEV plots) and NEVs (brown circles: transects in NEV plots). For the wild bees, a reduced data set was used to solve the problem of spatial autocorrelation.
3.4. Indicator Species of Cross-Slope Vineyards with Greened Embankments
Characteristic wild bee and butterfly species for the GEV type were identified using indicator species analyses (Figure 4, Table 4). With regard to bee indicator species, Andrena flavipes and Lasioglossum morio were observed in all GEV plots (maximum sensitivity), while Colletes similis was observed only in GEV plots (maximum specificity). Regarding butterfly indicator species, Lasiommata megera occurred in all GEV plots; Aglais io and Melanargia galathea appeared only in GEV plots. Melitaea didyma has a particular role as indicator species, as this species is critically endangered in Rhineland-Palatinate and Germany [,].

Table 4.
Analysis of indicator species, i.e., species of wild bees and butterflies, that were significantly associated with cross-slope vineyards with greened embankments (GEVs; α = 0.05, 999 permutations): * (p < 0.05), ** (p < 0.01), *** (p < 0.001). Maximum specificity (1.0) = species observed only in GEV plots; maximum sensitivity (1.0) =species observed in all GEV plots. No significant indicator species were found for vineyards without greened embankments (NEVs).
4. Discussion
The findings of this study are based on a relatively low number of sampled vineyards within a single study region. The small-scale and sometimes narrow nature of the Moselle valley, as well as the fact that only a very small number of NEVs have been cultivated to date, limited the selection of our sampled vineyards. Therefore, it may be difficult to generalise and transfer our results to other larger wine-growing regions, and the present results have to be regarded as applicable to the studied vineyards. This has to be kept in mind when interpreting the results and transferring them to other wine-growing regions. Given the rarity of vineyards with greened embankments (GEVs) as a novel vineyard design at present, the pool of GEVs from which we selected our sample was very limited, as was the number of suitable study regions. However, focusing on a single landscape in this case study, we were able to minimise landscape-scale effects on bee and butterfly communities. Moreover, we consider the selected study region to be representative for many landscapes with steep-slope viticulture in Germany and other parts of central Europe. We therefore argue that the findings of this study are relevant beyond the investigated study region. Still, with increases in the number and distribution of GEVs expected in the near future, further studies investigating GEVs in multiple regions will be necessary to corroborate that the present findings apply to additional landscapes and regions.
We found significantly more species and individuals and generally a higher diversity of wild bees and butterflies in the GEVs than in the vineyards without these structures (NEVs; Table 2 and Table 3, Figure 2 and Figure 3). A large part of the recorded species in the GEVs were categorised as endangered according to the Red Lists of Rhineland-Palatinate [,] and Germany [,]. Overall, the GEV communities of both insect groups were rich, hence differing remarkably from the NEV communities (Figure 4). This suggests the general conservation value of GEVs, whose specific benefits are discussed below.
Our analyses demonstrated that, embedded within the studied vineyards under cultivation, greened embankments are seminatural habitats that have a positive influence on the diversity of our indicator groups. Such effects of seminatural habitats on wild bee diversity have been shown for other agro-ecosystems [,]. In addition to the structurally rich vegetation on the embankments, the higher light exposure and air circulation in GEVs result in a more diverse microclimate [], which increases heterogeneity and thus general habitat quality. In GEVs, insolation is higher due to the larger distance between vine rows compared with the down-slope structures of NEVs. In particular, the rather steep greened embankments collect more solar energy over the year than all other parts of the vineyards, offering more suitable conditions for the development of many thermophilic species.
Dover et al. [] drew comparable conclusions for arable fields, emphasising in particular the favourable microclimate of green lanes in combination with embankments, and pointed out their importance for the thermoregulation of butterflies. In general, the width and plant species composition of the vegetated linear elements are crucial for their conservation value []. In this context, green lanes adjacent to arable land are known to support a high abundance of bumblebees [] and butterflies [,], highlighting their importance as linear landscape elements with high biodiversity value for nectar-feeding insects. Similarly, flower strips in the agricultural landscape are well known to promote wild bee and butterfly populations by providing a continuous supply of floral resources [,,]. In contrast to our study, Wersebeckmann et al. [] found no differences in bee abundance between vertically oriented vineyards (comparable to NEVs) and terraced vineyards (comparable to GEVs). However, this study was based on pan trap sampling, which has been cautioned against for providing unreliable abundance measures, especially when there is a contrast in flower cover []. Corroborating our results, Wersebeckmann et al. [] observed a trend of higher bee species diversity in terraced compared with vertically oriented vineyards using a sampling method complementary to our method with regard to representativeness of the sampled bee community [].
The microclimatically favourable conditions of narrow river valleys like the Moselle, combined with the species-rich flora and the structural richness of the GEVs, enhance the preservation of high-conservation-value habitats, especially for xerothermophilic species. Structural diversity, which is indispensable for a rich biodiversity, e.g., in the form of structural elements such as dry-stone walls or rock outcrops, can be maintained or established within GEVs. Accordingly, several publications have noted the importance of a mosaic-like distribution of semi-natural habitats in vineyard landscapes for the conservation of wild bees and biodiversity in general [,]. Additionally, it was emphasised that a dense network of suitable habitats is crucial for maintaining wild bee diversity []. Consequently, the conservation value of GEVs is clearly superior to that of NEVs because heterogeneous habitats on different spatial scales are key elements for improving biodiversity in viticulture []. Butterfly species recorded in the NEVs were mainly dispersive species (e.g., Pieris rapae, Aglais urticae or the highly dispersive Gonepteryx rhamni []). According to their characteristics, they are assumed to be not using the NEVs as habitats but are merely transient. Furthermore, the rare and endangered species present in the GEVs (e.g., Satyrium acaciae, S. spini) were not detected in the NEVs. Consequently, GEVs are diverse habitats for insects and should be so for other rare and endangered species. They represent a high-quality alternative to the traditional and more widespread, often monotonous, down-slope vineyards. With wild bees commuting between partial habitats and being influenced by landscape factors and with butterflies moving primarily at habitat level, this combination of indicator species shows very sensitive results regarding the benefits of GEVs for biodiversity.
The greened embankments of the vineyards, which, from the wine grower’s point of view, protect against erosion, provide a rich and almost permanent plant cover. This vegetation richness provides necessary resources in abundance for the two investigated indicator groups [,,]. Oviposition sites for butterflies, host plants of their caterpillars, pollen plants for bee larvae, and nectar plants for the imagines of both groups are provided through plant species diversity in the greened embankments, explaining the high community diversity found in these vineyards in our study. In contrast, the NEVs showed a low degree of floral richness and accordingly less-diverse nutritional resources. Corroborating our results, a clear correlation between species richness of wild bees and butterflies on the one hand and the diversity of flowers available on the other has frequently been reported [,,]. The diversity of vascular plants is of particular importance due to their function as food resources. Furthermore, species-rich spontaneous vegetation has an augmentative effect on the biological control of grapevine pests by predators []. We attribute the greater variability in the species composition of wild bee and butterfly communities in the NEVs to the lower structural and resource diversity in these vineyards: because the NEVs offer suitable resources for a smaller fraction of the communities compared to the GEV, species turnover in the sampled communities was higher in the NEVs than in the GEVs due to greater vineyard-to-vineyard variation in the limited resources in the NEVs compared to the GEVs.
In addition, regular mowing or mulching events occur in NEVs, simultaneously reducing nutritional resources on large scales. On the contrary, in our study area, only single mulching events in the inter-rows as well as only an annual time-shifted mosaic mulching of the greened embankments was performed in the GEVs. This practice is not only a viticultural necessity: it is also important for the preservation of floral diversity. This underlines the importance of managing such human-made landscape features to optimise their wildlife potential and thus conservation value, e.g., by cutting vegetation to prevent scrub invasion as well as to support annual and perennial flowering plants [,]. As competition for water between vines and weeds can be problematic [], removal of flowers within the NEVs is more often performed later in the summer season when floral resources in the landscape are decreasing []. These differences in vineyard types and management are reflected in the higher abundance of bees and butterflies observed in the GEVs than in the NEVs. However, in order to isolate the effects of inter-row vegetation management on plant, butterfly, and bee communities in GEVs, further studies are necessary.
Furthermore, other disturbance events such as tillage and frequent passage of heavy equipment along both down-slope and cross-slope inter-rows do not occur within greened embankments. Consequently, NEVs are much more affected by such disturbances and represent less suitable habitats for insects, since the required resources are not available in sufficient quantity and quality, and not for all developmental stages. Ground nesting bees, which represent the majority of the native bee species and are characterised by a high site fidelity, are likely to avoid nesting habitats that are subjected to such excessive disturbance []. Tillage is especially associated with the destruction of nests of bees built in the ground in agricultural areas [,], which very well explains the low abundance of wild bees in the NEVs. Furthermore, it was pointed out that frequent soil tillage is responsible for the destruction of floral resources, thus resulting in indirect negative impacts on wild bees []. However, species-rich green cover in alternate rows of NEVs is gaining importance due to improved steep-slope mechanisation tools [].
We recognised several indicator species for the GEVs in both studied insect groups but not for the NEVs (Figure 4, Table 4). All bee indicator species are ground-nesting, with Andrena flavipes and Lasioglossum morio being ubiquitous but often observed on embankments; only the xerothermophilic Colletes similis, specialised in its use of pollen sources, is included in the prewarning list for Germany []. Furthermore, A. flavipes and C. similis are typical species in vineyard fallows [], which might play a role similar to the linear structures of greened embankments. In this context, the spatial configuration of habitats has only a minor influence on the conservation of wild bees [].
Most of the butterfly indicator species prefer warm and dry habitats (Lasiommata megera, Melitaea didyma, Melanargia galathea) and are often observed basking as well as searching for nectar or oviposition sites on embankments (L. megera, M. galathea, Pieris brassicae) []. Furthermore, L. megera and P. brassicae prefer stone walls for basking and pupation, respectively []. These structures are often in the immediate vicinity of cross-slope vineyards or can even be integrated in them, like in our study area. Particularly noteworthy is the highly abundant occurrence of the xerothermophilic butterfly species M. didyma, with its strong preference for mosaic structures [] and status as critically endangered at the national and regional scales [,]. This strongly underlines the conservation value of greened embankment structures in the studied vineyards.
As one of our important findings, we show that nature conservation is not necessarily in conflict with agricultural use, particularly in the case of steep-slope vineyards harbouring a high biodiversity potential, even more so when seminatural elements are integrated as in the GEVs. Landscape diversification caused by the alternation between cross-slope and down-slope vineyards breaks structural monotony, thus having a positive effect on disease and pest control []. Furthermore, GEVs allow mechanisation even on steep slopes, and consequently wine production can be more cost-efficient than in NEVs [], which on steep slopes is much more labour-intensive because it requires a lot of cultivation by hand. In addition, GEV cultivation requires larger areas than NEVs, as fewer vines grow per unit of surface. This higher area requirement of GEVs in locations with generally high land abandonment has the desirable side effect of reducing the proportion of fallow land in succession on steep slopes.
From 1999 to 2020, the number of stocked vineyards in Rhineland-Palatinate decreased by 25% []. Steep-slope viticulture is disproportionately often affected by this decrease in stocked area, well known for high-quality wine, but also for high production costs []. As it is particularly these sites which possess the highest biodiversity potential, counteracting the processes of abandonment is crucial for nature conservation and in line with the concept of “vinecology”, which proposes the integration of ecological and viticultural practices as a win-win solution [].
Furthermore, like in other European wine-growing regions [], viticulture in the Moselle valley is important not only for wine production and nature conservation but also for the maintenance of the traditional landscape scenery. The aesthetics of these picturesque landscapes are of great significance for another important regional source of income: tourism. Steep-slope viticulture is one of the image carriers and an important part of eco-tourism in the region []. Tourists expect and appreciate a bio-diverse landscape covered with vineyards on steep slopes and not large stretches of fallow land in natural succession. The preservation of steep-slope vineyards therefore has mutual benefits, hence also achieving one of the important objectives of the National Biodiversity Strategy. Although the results of this study require confirmation by a more extensive analysis as soon as more terraced vineyards become available, they are confirmed by a similar study from another wine-growing region []. Consequently, the establishment of biodiversity-friendly GEVs should be of vital interest to winegrowers, conservationists, and landscape planners. The establishment of GEVs, which requires costly soil modifications, should therefore be increasingly supported through financial subsidies in order to preserve steep-slope viticulture and the associated biodiversity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16010044/s1, Figure S1: Map of the study area; Table S1: Taxa of wild bees and butterflies recorded in the sampled vineyards; Table S2: Detected species and individuals of wild bees for the total and reduced data set; Table S3: Model statistics and effects for mixed models.
Author Contributions
A.K., M.P., M.M. and T.S., conceived and designed the study. M.P., M.M. and T.S. acquired the funding of the study. L.B. and A.K. sampled and analysed the data. All authors discussed the results. L.B., A.K., J.S. and T.S. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the German Federal Ministry of Food and Agriculture (BMEL) based on a decision of the Parliament of the Federal Republic of Germany, granted by the Federal Office for Agriculture and Food (BLE; grant number 2811HS003). The APC was funded by Julius Kühn Institute (JKI)–Federal Research Centre for Cultivated Plants.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and supplementary material, further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank D. Braun for surveying the flowering vegetation; A.W. Ebmer, O. Diestelhorst, G. Reder, H.H. Dathe, and J. Esser for supporting bee species identification; the Moselle valley wine growers for giving access to their vineyards; E. Kohl for providing us with a schematic figure of the vineyard designs for further modification; and A. Liston for improving the manuscript language. The permission required for the collection of bees and butterflies was granted by the responsible authority (Struktur- und Genehmigungsdirektion Nord, Koblenz).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wagner, D.L. Insect Declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Samways, M.J. Insect Conservation: A Global Synthesis; CABI Publishing: Wallingford, UK, 2020; ISBN 978-1-78924-167-9. [Google Scholar]
- Habel, J.C.; Samways, M.J.; Schmitt, T. Mitigating the precipitous decline of terrestrial European insects: Requirements for a new strategy. Biodivers. Conserv. 2019, 28, 1343–1360. [Google Scholar] [CrossRef]
- European Union. Agriculture, Forestry and Fishery Statistics: 2018 Edition; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-94758-2. [Google Scholar]
- Dudley, N.; Alexander, S. Agriculture and biodiversity: A review. Biodiversity 2017, 18, 45–49. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2012, 12, e0185809. [Google Scholar] [CrossRef] [PubMed]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; The Insect Pollinators Initiative. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef] [PubMed]
- European Comission. The European Green Deal. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:b828d165-1c22-11ea-8c1f-01aa75ed71a1.0002.02/DOC_1&format=PDF (accessed on 12 December 2023).
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Košulič, O.; Michalko, R.; Hula, V. Recent artificial vineyard terraces as a refuge for rare and endangered spiders in a modern agricultural landscape. Ecol. Eng. 2014, 68, 133–142. [Google Scholar] [CrossRef]
- Paiola, A.; Assandri, G.; Brambilla, M.; Zottini, M.; Pedrini, P.; Nascimbene, J. Exploring the potential of vineyards for biodiversity conservation and delivery of biodiversity-mediated ecosystem services: A global-scale systematic review. Sci. Total Environ. 2020, 706, 135839. [Google Scholar] [CrossRef]
- Oussama, M.; Kamel, E.; Le Philippe, G.; Elisabeth, M.; Jacques, F.; Habiba, A.; Jean-Paul, B. Assessing plant protection practices using pressure indicator and toxicity risk indicators: Analysis of therelationship between these indicators for improved risk management, application in viticulture. Environ. Sci. Pollut. Res. Int. 2015, 22, 8058–8074. [Google Scholar] [CrossRef]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Viret, O.; Spring, J.-L.; Zufferey, V.; Gindro, K.; Linder, C.; Gaume, A.; Murisier, F. Past and future of sustainable viticulture in Switzerland. BIO Web Conf. 2019, 15, 1013. [Google Scholar] [CrossRef]
- Porten, M.; Treis, F.J. Querterrassierung: Die Rettung der Steillagen? Das Deutsche Weinmagazin 2006, 11, 22–29. [Google Scholar]
- Caraveli, H. A comparative analysis on intensification and extensification in mediterranean agriculture: Dilemmas for LFAs policy. J. Rural Stud. 2000, 16, 231–242. [Google Scholar] [CrossRef]
- IBES. Summary for Policymakers of the Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2016; ISBN 978-92-807-3568-0. [Google Scholar]
- Dylewski, Ł.; Maćkowiak, Ł.; Banaszak-Cibicka, W. Are all urban green spaces a favourable habitat for pollinator communities? Bees, butterflies and hoverflies in different urban green areas. Ecol. Entomol. 2019, 44, 678–689. [Google Scholar] [CrossRef]
- Holland, J.M.; Smith, B.M.; Storkey, J.; Lutman, P.J.; Aebischer, N.J. Managing habitats on English farmland for insect pollinator conservation. Biol. Conserv. 2015, 182, 215–222. [Google Scholar] [CrossRef]
- Krahner, A.; Schmidt, J.; Maixner, M.; Porten, M.; Schmitt, T. Evaluation of four different methods for assessing bee diversity as ecological indicators of agro-ecosystems. Ecol. Indic. 2021, 125, 107573. [Google Scholar] [CrossRef]
- Kudrna, O. Butterflies of Europe; Aula-Verl.: Wiesbaden, Germany, 1986; ISBN 9783891040393. [Google Scholar]
- Rákosy, L.; Schmitt, T. Are butterflies and moths suitable ecological indicator systems for restoration measures of semi-natural calcareous grassland habitats? Ecol. Indic. 2011, 11, 1040–1045. [Google Scholar] [CrossRef]
- Thomas, J.A. Monitoring change in the abundance and distribution of insects using butterflies and other indicator groups. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 339–357. [Google Scholar] [CrossRef]
- Obermann, H.-W.; Gruschwitz, M. Ökologische Untersuchungen zur Fauna von Trockenmauern in Weinanbaugebieten, dargestellt am Beispiel einer Weinbergslage an der Mosel. Fauna Und Flora Rheinl.-Pfalz. 1992, 6, 1085–1139. [Google Scholar]
- Schmitt, T. Xerothermvegetation an der Unteren Mosel: Schutzwürdigkeit und Naturschutzplanung von Trockenbiotopen auf Landschaftsökologischer Grundlage; Selbstverlag des Geographischen Instituts der Justus Liebig-Universität Giessen: Giessen, Germany, 1989. [Google Scholar]
- Krahner, A.; Dathe, H.H.; Schmitt, T. Wildbienen (Hymenoptera, Aculeata: Apiformes) des Mittleren Moseltals: Die Weinbausteillagen im Klotten-Treiser Moseltal. Contrib. Entomol. 2018, 68, 107–131. [Google Scholar] [CrossRef]
- Pollard, E.; Yates, T.J. Monitoring Butterflies for Ecology and Conservation: The British Butterfly Monitoring Scheme, 1st ed.; Chapman & Hall: London, UK, 1993; ISBN 978-0-412-40220-3. [Google Scholar]
- Kühn, E.; Musche, M.; Harpke, A.; Feldmann, R.; Metzler, B.; Wiemers, M.; Hirneisen, N.; Settele, J. Tagfalter-Monitoring Deutschland. Oedippus 2014, 27, 1–47. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- Oskanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Package ‘vegan’: Community Ecology Package. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 20 January 2021).
- Dray, S.; Dufour, A.-B. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Soft. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- QGIS Geographic Information System; QGIS Development Team: Beaverton, OR, USA, 2014.
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Kindt, R.; van Damme, P.; Simons, A.J. Tree Diversity in Western Kenya: Using Profiles to Characterise Richness and Evenness. Biodivers. Conserv. 2006, 15, 1253–1270. [Google Scholar] [CrossRef]
- Korner-Nievergelt, F.; Roth, T.; von Felten, S.; Guelat, J.; Almasi, B.; Korner-Nievergelt, P. Bayesian Data Analysis in Ecology Using Linear Models with R, BUGS, and STAN; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780128013700. [Google Scholar]
- Brooks, M.; Kristensen, K.; Benthem, K.; Magnusson, A.; Berg, C.; Nielsen, A.; Skaug, H.; Mächler, M.; Bolker, B. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference: R Package Version 1.43.17. Available online: https://cran.r-project.org/package=DHARMahttps://cran.r-project.org/package=DHARMa (accessed on 14 February 2022).
- Hartig, F.; Lohse, L. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models: R Package Version 0.4.1. Available online: https://cran.r-project.org/package=DHARMa (accessed on 14 February 2022).
- Lenth, R.V.; Bolker, B.; Burkner, P.; Giné-Vázquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Riebl, H.; Singmann, H. emmeans: Estimated Marginal Means, aka Least-Squares Means: R Package Version 1.6.0. Available online: https://cran.r-project.org/package=emmeans (accessed on 14 February 2022).
- Anderson, M.J.; Walsh, D.C.I. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. (Eds.) Analysing Ecological Data; Springer: New York, NY, USA, 2007. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef]
- Cáceres, M.D.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; ISBN 978-0-387-98141-3. [Google Scholar]
- Westrich, P. Rote Liste und Gesamtartenliste der Bienen (Hymenoptera, Apidae) Deutschlands. In Rote Liste Gefährdeter Tiere, Pflanzen und Pilze Deutschlands: Band 3: Wirbellose Tiere (Teil 1); Bundesamt für Naturschutz, Ed.; Bundesamt für Naturschutz: Bonn, Germany, 2011; pp. 373–417. [Google Scholar]
- Schmid-Egger, C.; Risch, S.; Niehuis, O. Die Wildbienen und Wespen in Rheinland-Pfalz (Hymenoptera, Aculeata): Verbreitung, Ökologie und Gefährdungssituation. Fauna Flora Rheinl.-Pfalz. Beih. 1995, 16, 1–296. [Google Scholar]
- Reinhardt, R.; Bolz, R. Rote Liste und Gesamtartenliste der Tagfalter (Rhopalocera) (Lepidoptera: Papilionoidea et Hesperioidea) Deutschlands. In Rote Liste Gefährdeter Tiere, Pflanzen und Pilze Deutschlands: Band 3: Wirbellose Tiere (Teil 1); Bundesamt für Naturschutz, Ed.; Bundesamt für Naturschutz: Bonn, Germany, 2011; pp. 167–194. [Google Scholar]
- Schmidt, A. Rote Liste der Großschmetterlinge in Rheinland-Pfalz; Ministerium für Umwelt, Landwirtschaft, Ernährung, Weinbau und Forsten Rheinland-Pfalz: Mainz, Germany, 2013. [Google Scholar]
- Carré, G.; Roche, P.; Chifflet, R.; Morison, N.; Bommarco, R.; Harrison-Cripps, J.; Krewenka, K.; Potts, S.G.; Roberts, S.P.; Rodet, G.; et al. Landscape context and habitat type as drivers of bee diversity in European annual crops. Agric. Ecosyst. Environ. 2009, 133, 40–47. [Google Scholar] [CrossRef]
- Lentini, P.E.; Martin, T.G.; Gibbons, P.; Fischer, J.; Cunningham, S.A. Supporting wild pollinators in a temperate agricultural landscape: Maintaining mosaics of natural features and production. Biol. Conserv. 2012, 149, 84–92. [Google Scholar] [CrossRef]
- Dover, J.; Sparks, T.; Clarke, S.; Gobbett, K.; Glossop, S. Linear features and butterflies: The importance of green lanes. Agric. Ecosyst. Environ. 2000, 80, 227–242. [Google Scholar] [CrossRef]
- Croxton, P.J.; Carvell, C.; Mountford, J.O.; Sparks, T.H. A comparison of green lanes and field margins as bumblebee habitat in an arable landscape. Biol. Conserv. 2002, 107, 365–374. [Google Scholar] [CrossRef]
- Croxton, P.J.; Hann, J.P.; Greatorex-Davies, J.N.; Sparks, T.H. Linear hotspots? The floral and butterfly diversity of green lanes. Biol. Conserv. 2005, 121, 579–584. [Google Scholar] [CrossRef]
- Wix, N.; Reich, M.; Schaarschmidt, F. Butterfly richness and abundance in flower strips and field margins: The role of local habitat quality and landscape context. Heliyon 2019, 5, e01636. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R. Flower plantings increase wild bee abundance and the pollination services provided to a pollination-dependent crop. J. Appl. Ecol. 2014, 51, 890–898. [Google Scholar] [CrossRef]
- Wersebeckmann, V.; Warzecha, D.; Entling, M.H.; Leyer, I. Contrasting effects of vineyard type, soil and landscape factors on ground-versus above-ground-nesting bees. J. Appl. Ecol. 2023, 60, 601–613. [Google Scholar] [CrossRef]
- Portman, Z.M.; Bruninga-Socolar, B.; Cariveau, D.P. The State of Bee Monitoring in the United States: A Call to Refocus Away From Bowl Traps and Towards More Effective Methods. Ann. Entomol. Soc. Am. 2020, 113, 337–342. [Google Scholar] [CrossRef]
- Kehinde, T.; Samways, M.J. Management defines species turnover of bees and flowering plants in vineyards. Agric. For. Entomol. 2014, 16, 95–101. [Google Scholar] [CrossRef]
- Gathmann, A.; Tscharntke, T. Foraging ranges of solitary bees. J. Anim. Ecol. 2002, 71, 757–764. [Google Scholar] [CrossRef]
- Bink, F.A. Ecologische Atlas van de Dagvlinders van Noordwest-Europa; Schuyt: Haarlem, The Netherlands, 1992; ISBN 9789060973189. [Google Scholar]
- Garbuzov, M.; Balfour, N.J.; Shackleton, K.; Al Toufailia, H.; Scandian, L.; Ratnieks, F.L.W. Multiple methods of assessing nectar foraging conditions indicate peak foraging difficulty in late season. Insect Conserv. Divers. 2020, 13, 532–542. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Tscharntke, T. Early succession of butterfly and plant communities on set-aside fields. Oecologia 1997, 109, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Möth, S.; Walzer, A.; Redl, M.; Petrović, B.; Hoffmann, C.; Winter, S. Unexpected Effects of Local Management and Landscape Composition on Predatory Mites and Their Food Resources in Vineyards. Insects 2021, 12, 180. [Google Scholar] [CrossRef]
- Pardini, A.; Faiello, C.; Longhi, F.; Mancuso, S.; Snowball, R. Cover crop species and their management in vineyards and olive groves. Adv. Hortic. Sci. 2002, 16, 225–234. [Google Scholar]
- Corbet, S.A. Insects, plants and succession: Advantages of long-term set-aside. Agric. Ecosyst. Environ. 1995, 53, 201–217. [Google Scholar] [CrossRef]
- Shuler, R.E.; Roulston, T.H.; Farris, G.E. Farming practices influence wild pollinator populations on squash and pumpkin. J. Econ. Entomol. 2005, 98, 790–795. [Google Scholar] [CrossRef]
- Ullmann, K.S.; Meisner, M.H.; Williams, N.M. Impact of tillage on the crop pollinating, ground-nesting bee, Peponapis pruinosa in California. Agric. Ecosyst. Environ. 2016, 232, 240–246. [Google Scholar] [CrossRef]
- Kratschmer, S.; Pachinger, B.; Schwantzer, M.; Paredes, D.; Guzmán, G.; Goméz, J.A.; Entrenas, J.A.; Guernion, M.; Burel, F.; Nicolai, A.; et al. Response of wild bee diversity, abundance, and functional traits to vineyard inter-row management intensity and landscape diversity across Europe. Ecol. Evol. 2019, 9, 4103–4115. [Google Scholar] [CrossRef] [PubMed]
- Kohl, E.; Porten, M. Alternative für den Steillagenweinbau? Wildkräuterbegrünung im Versuch, Teil 1. Das Dtsch. Weinmagazin 2017, 18, 26–29. [Google Scholar]
- Westrich, P. Die Wildbienen Deutschlands; 2., aktualisierte Auflage; Ulmer: Stuttgart, Germany, 2019; ISBN 978-3-8186-0880-4. [Google Scholar]
- Feldmann, R.; Reinhardt, R.; Settele, J. Bestimmung und Kurzcharakterisierung der außeralpinen Tagfalter Deutschlands. In Die Tagfalter Deutschlands; Settele, J., Ed.; Ulmer: Stuttgart, Germany, 2000; pp. 247–369. [Google Scholar]
- Altieri, M.A.; Nicholls, C.I. The simplification of traditional vineyard based agroforests in northwestern Portugal: Some ecological implications. Agrofor. Syst. 2002, 56, 185–191. [Google Scholar] [CrossRef]
- Huber, E.C. Vergleich von Steillagen-Mechanisierungsformen im Weinbau. Master’s Thesis, Hochschule Mainz, Mainz, Germany, 2015. [Google Scholar]
- Statistisches Landesamt Rheinland-Pfalz. Statistische Berichte. Bestockte Rebflächen. 2020. Available online: https://www.statistik.rlp.de/fileadmin/dokumente/berichte/C/1073/C1073_202000_1j_Bereich.pdf (accessed on 12 December 2023).
- MWVLW Ministerium für Wirtschaft, Verkehr, Landwirtschaft und Weinbau Rheinland-Pfalz. WeinKulturLandschaftsProgramm Steillagen. 2005. Available online: https://www.edoweb-rlp.de/resource/edoweb:4369798/data (accessed on 6 June 2021).
- Viers, J.H.; Williams, J.N.; Nicholas, K.A.; Barbosa, O.; Kotzé, I.; Spence, L.; Webb, L.B.; Merenlender, A.; Reynolds, M. Vinecology: Pairing wine with nature. Conserv. Lett. 2013, 6, 287–299. [Google Scholar] [CrossRef]
- Šmid Hribar, M.; Geršič, M.; Pipan, P.; Repolusk, P.; Tiran, J.; Topole, M.; Ciglič, R. Cultivated terraces in Slovenian landscapes. Acta Geogr. Slov. 2017, 57, 83–97. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).