Abstract
Polychaetes play a vital role in the structure and functioning of benthic communities in mangrove ecosystems. Nevertheless, our understanding of the diversity and functional structure of polychaete assemblages across different habitats in the mangrove ecosystems along the coast of the Persian Gulf and Gulf of Oman is limited. In this study, we investigated the species and trait composition of polychaetes and environmental variables, in vegetated and mudflat habitats of three subtropical mangroves. The results showed that Neanthes glandicincta was widely distributed across all regions and habitats. The three-factor ANOVA showed that the abundance and taxonomic diversity of polychaetes differed significantly between two habitats and three mangrove ecosystems. The abundance of polychaetes was observed to be higher in mud habitats than in vegetated habitats. There was a significant difference in species and trait composition between different regions and habitats. Vegetated habitats had higher proportions of crawler predatory species that are longer lived (3–5 years), with larger body size (80–100 mm), and are upward conveyors, whereas mudflat habitats had higher proportions of mobile (burrower) omnivore species that are moderately lived (1–3 years), with larger body size (>100 mm), and are biodiffusers. The three-factor ANOVA showed a significant difference in the community weighted mean (CWM) index between two habitats and three mangrove ecosystems. Thus, the species and trait composition of polychaetes depend on the structural complexity of their respective habitats. The DistLM analysis showed that total organic carbon content of the sediment was the main predictor variable influencing species composition, while silt/clay content and salinity were the main predictor variables influencing the traits’ composition. The results showed how the composition of traits and the structure of polychaete communities change in mangrove ecosystems, which can be used for future studies on conservation strategies for mangrove ecosystems throughout the world.
1. Introduction
Mangrove ecosystems grow along the shallow coastlines of tropical and subtropical regions [1]. They are one of the most biologically diverse ecosystems, rich in organic matter and nutrients that support a large biomass of plants and animals [2]. There are a number of natural and anthropogenic disturbances that threaten mangroves, including changes in hydrodynamics, subsidence, pollution from industries, clearing of mangrove trees, and climate change [3]. Globally, mangrove areas decline by 0.22% per year [4,5].
The infauna play essential roles in the secondary production of a sediment, bioturbation, and nutrient cycling [6]. They display various nutritional behaviors in mangrove ecosystems, which can be used as bioindicators of the ecosystem’s structure and environmental conditions and disturbances [7]. Polychaetes constitute a macrobenthic group of great abundance and diversity in marine ecosystems, including mangroves. Additionally, they are eaten by seabirds, fish, and crustaceans with commercial value. As a result of their abundance, diverse feeding habits, occupation of niches, and the relationship to different sediment types, they play an important role in the structure and functioning of benthic communities [8]. Also, polychaetes are active participants in a variety of marine ecological processes, including bioturbation, nutrient cycling, secondary production, and energy flow [9,10].
The change in environmental conditions strongly affects the community structure (species composition and diversity) and consequently the overall functioning of ecosystems [11,12]. Increasing evidence suggests that species diversity and species richness alone are not sufficient to explain or predict ecosystem functions [10,13] and a species’ ability to cope with environmental disturbance is at least partly driven by its traits [9,14,15]. These traits include morphological, behavioral, and physiological characteristics that can explain the interaction between species and their environment, conspecifics, and individuals of other species [16,17]. A biological trait analysis (BTA) has been proposed as a useful approach for describing the ecological functioning of marine ecosystems. BTA was first used in freshwater ecosystems [18,19] and has progressively been applied to marine benthic communities [20,21,22,23,24,25]. As one of the trait-based indices, the community weighted mean value (CWM) is a value in a community that shows the number of species expressing a specific trait in a given community [12] weighted by the relative abundance of the species carrying each value in the community. It is increasingly used to evaluate the response of communities to disturbances that can manage “multiple” different traits [9,22]. The CWM approach can be used to analyze shifts in mean traits within communities as the result of the environmental selection for certain traits [22]. It presents the underlying qualitative features of the traits expressed and their potential implications for ecosystem function.
The Persian Gulf and Gulf of Oman experience extreme environmental pressures, including salinity and temperature fluctuations, which place organisms at the limits of their physiological tolerance to environmental conditions [26]. As a diverse and important coastal habitat, mangroves are threatened by natural and anthropogenic pressures, which makes further studies of their biodiversity and function imperative. The structural complexity of mangroves plays a crucial role in the biological organization of communities by promoting species coexistence by reducing niche overlap, mediating predation by providing refuge for smaller organisms, and altering the physical environment [27]. There have been some studies that have evaluated the influence of mangrove vegetation on the functional diversity of invertebrates and the consequent changes in ecosystem functioning [28,29,30,31,32]. Although some studies have also been conducted on the taxonomic and functional diversity of macrofaunal communities of mangrove forests along the coasts of the Persian Gulf and Gulf of Oman [22,23,24], no study has been conducted on the taxonomic diversity and functional trait composition of polychaete assemblages. The main objectives of this study are to (i) compare the species and functional trait composition between vegetated and mudflat habitats and (ii) investigate the effects of environmental conditions and habitat complexity driven by features associated with mangrove trees on species and trait composition in three subtropical mangrove ecosystems.
2. Materials and Methods
2.1. Study Area
There are 94.03 km2 of mangrove forests in Iran, located in the geographic range between 25°11′ and 27°52′ N [33]. Avicennia marina and Rhizophora mucronata are the two species of mangrove forest that grow in Iran, but the latter is found only in the estuaries of Sirik [34]. The Nayband estuary is in the geographic range of 52°24′58″ to 52°38′58″ E and 27°22′58″ to 27°39′58″ N. There are about 3.9 km2 of mangrove forest in Nayband Bay, which has a subtropical climate. During the winter months, the average temperature is 12–16 °C, while during the summer months, it reaches 42 °C. Through Hale Channel, tidal water exchanges with the open sea without major freshwater discharges into the forest [34]. The Sirik estuary with an area of over 270 km2 is located in-between 57°11′ and 57°20′ E and 26°10′ and 26°26′ N. Air temperature in winter and summer exceeds 10 and 50 °C, respectively. This forest is normally affected by freshwater carried by Gaz River [35]. A large portion of Gwadar Bay lies within Pakistan (about 69%) and about 31% in Iran. The Iranian part is located at 25°1′ and 25°12′ N and 61°34′ and 61°47′ E. The area of mangroves in the Gwadar estuary is 1.593 km2. The average air temperature was 42 °C in summer and 20 °C in winter. The Gwadar forest is normally affected by freshwater carried by Bahu Kalat River [36]. The mangrove forests of these three estuaries are listed in the Ramsar Convention as protected areas and are among the most important mangrove ecosystems in Iran.
2.2. Sampling of Polychaetes
Polychaetes were sampled from three mangrove regions of Nayband (Persian Gulf), Sirik, and Gwadar (Gulf of Oman), during the warmest (July) and coldest (January) seasons in 2019. Randomly, 5 plots were chosen in each region (Figure 1). Each plot consisted of vegetated (with trees and pneumatophores) and mudflat (without trees and pneumatophores) habitats. In each habitat, one random plot was centered at least 8 m from the forest fringe to avoid edge effects that could affect the community composition. Three random sediment samples were collected from each habitat using a metal 25 × 25 cm quadrat, with a depth of 25 cm.
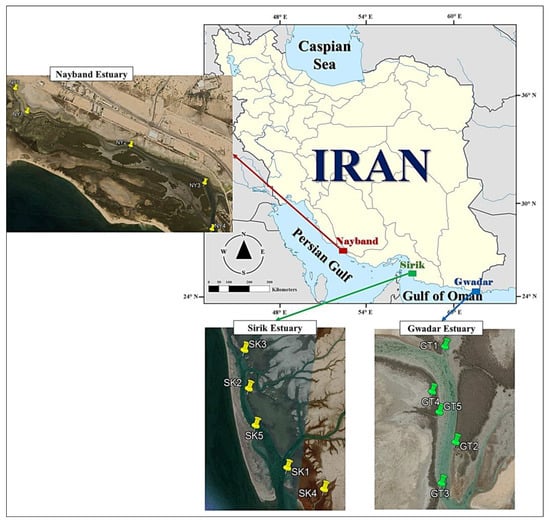
Figure 1.
Positions of the sampling sites of polychaetes in Hara Biosphere Reserve, Persian Gulf and Gulf of Oman.
The sediment samples were washed with sea water on a 0.5 mm sieve until clear water emerged. The residue containing polychaetes was preserved using 70% ethanol. In a laboratory, the polychaetes were collected, sorted, and identified to the lowest possible taxonomic group using identification sources [37,38,39,40,41] and a species–sites matrix was created.
2.3. Environmental Data
Temperature (°C) and salinity (PPT) of water were measured in situ using a HACH Multi-parameter device that had been recently calibrated. To measure total organic carbon (TOC), total organic nitrogen (TON), and sediment grain size, three replicates of the sediment were collected from each habitat using volumetric soil cores (internal diameter—5 cm, depth—10 cm). The Micro–Kjeldahl method [42] and the Walkley–Black method [43] were used to measure total organic nitrogen (TON) and total organic carbon (TOC), respectively. The particle size of the sediment was determined using the hydrometer technique [44].
2.4. Trait Data
Seven biological traits, composed of 34 modalities, were selected to investigate polychaete functional roles and patterns in the mangrove forests (Table 1). To quantify the affinity of each species to each modality, fuzzy coding was used [45], in which species are assigned to scores ranging from 0 to 3 for each modality (0 = no affinity, 1 = low affinity, 2 = moderate affinity, and 3 = high affinity). The classification of polychaetes was collected from online databases (e.g., http://www.marlin.ac.uk/biotic/; accessed on 25 March 2023, and http://polytraits.lifewatchgreece.eu/; accessed on 25 March 2023), the peer-reviewed literature, and an identification key [7,37,40,41,46]. A matrix was created listing each species’ affinity to the different trait modalities and showing the abundance of biological traits for each combination of a region, habitat, and season. CWM was calculated, using the ‘trait-by-station’ matrix, to analyze differences in the composition and expression of functional characteristics in the mangrove ecosystems, which may reflect the trait strategies exhibited by a region’s species pool and environmental conditions [47]. Every replicate sample was defined as a community for calculating CWM.

Table 1.
List of polychaete traits and trait modalities used for the biological trait analysis. A label is given for each category.
2.5. Data Analysis
For each multivariate analysis, the two matrices (i.e., species composition and CWM values) were square root transformed, and Bray–Curtis similarity was calculated [48].
The abundance data of the polychaetes were used in PRIMER 6.0 to calculate species diversity indices for each habitat in the regions, including the Margalef species richness index (d), the Pielou evenness index (J’), the Shannon–Wiener diversity index (H’), and the Simpson dominance index.
The non-metric multidimensional scaling analysis (nMDS) ordination model was applied to the taxa abundance matrix to analyze species and trait composition. After verifying normality and homoscedasticity, each analysis was performed. A SIMPER analysis was used to assess the dissimilarity between regions, habitats, and seasons regarding their species and trait modalities composition, and to identify which species and traits contributed most to these significant differences.
A distance-based linear model (DistLM) was used to evaluate the relative contribution of abiotic parameters to the variability observed in species and trait composition of each mangrove region. The BEST selection procedure was applied to the DistLM model in order to select the best combination of predictor variables using the AIC (Akaike’s Information Criteria). Subsequently, a distance-based redundancy analysis (dbRDA) was performed on the fitted values obtained from the given model built with DISTLM [49].
To assess differences in abundance, taxonomic diversity, and the CWM index between regions, habitats, and sampling seasons, we used a repeated measure three-factor ANOVA, where ‘region’ and ‘season’ were fixed factors and ‘habitat’ was a random factor. All tests were considered significant if the p-value was less than 0.05.
Multivariate analyses were conducted using PRIMER v6 with the PERMANOVA add-on package. The CWM index was calculated in R v4.1.0 software using the package FD, ade4, and ggplot2 for each sampling habitat and season [50]. Also, the three-factor ANOVA was performed with statistical software R [51].
3. Results
A total of 33 species belonging to 16 families were identified in the three mangrove regions. The most abundant species was Neanthes glandicincta in Gwadar (709.45 ± 105.9 ind.m−2) and Sirik (255.42 ± 35.12 ind.m−2) regions, while the most abundant species was Simplisetia erythraeensis in the Nayband region (75 ± 11.87 ind.m−2). Generally, the most abundant families were Nereididae, Capitellidae, and Paraonidae in the three studied regions (Table S1).
The three-factor ANOVA showed a significant difference in polychaete abundance in Gwadar, Sirik, and Nayband regions (Table 2). The lowest and highest abundances were observed in Nayband mudflats during winter (16 ± 0.64 ind.m−2) and Sirik mudflats during summer (640 ± 45 ind.m−2), respectively (Figure 2a). In the summer, no species were observed in the vegetated and mudflat habitats of Nayband. The abundance was significantly higher in mudflat than in vegetated habitats. The three-factor ANOVA also revealed significant differences in the polychaete abundance between seasons (p < 0.05).

Table 2.
Results of three-factor ANOVA for comparing the abundance, taxonomic diversity, and CWM index of polychaetes across regions, habitats, and seasons in mangrove ecosystems in the Persian Gulf and Gulf of Oman. Factors: region (levels: Gwadar, Sirik, and Nayband), habitat (levels: vegetated and mudflat), and season (levels: winter and summer); bold values indicate significant level.
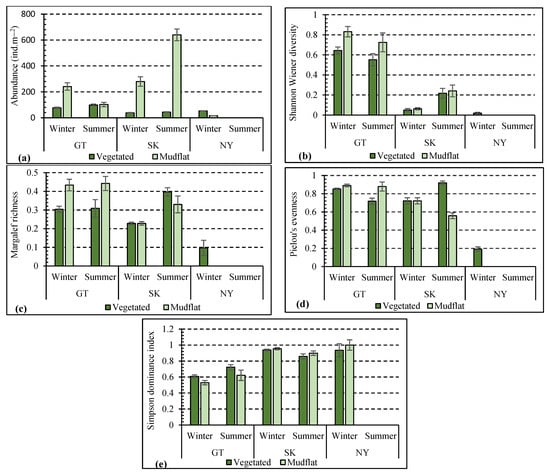
Figure 2.
Abundance and taxonomic diversity (mean ± SE) of polychaete assemblages in vegetated and mudflat habitats of different mangrove ecosystems (GT—Gwadar, SK—Sirik, and NY—Nayband) in the Persian Gulf and Gulf of Oman; (a) abundance, (b) Shannon Wiener diversity, (c) Margalef richness, (d) Pielou’s evenness, (e) Simpson dominance index.
In winter, the Shannon diversity index showed the lowest value in Nayband (0.02 ± 0.008) and the highest value in Gwadar (0.83 ± 0.38) (Figure 2b). Based on the three-factor ANOVA, Shannon’s index differed significantly between the three regions and two habitats (p < 0.05), being higher in mudflats than in vegetated habitats (Table 2 and Figure 2b). The Margalef richness index ranged from 0.1 ± 0.07 to 0.43 ± 0.12 (Figure 2c), and the three-factor ANOVA indicated that the polychaete community structure differed significantly between the regions and habitats (p < 0.05). The three-factor ANOVA also revealed a significant difference in Shannon diversity and Margalef richness between seasons (p < 0.05) (Table 2). The lowest and highest values of the evenness index were 0.19 ± 0.08 and 0.91 ± 0.32, for vegetated habitats of Nayband in winter and vegetated habitats of Sirik in summer, respectively (Figure 2d). The mean Simpson’s dominant index values varied between 0.52 ± 0.23 and 1 in the three studied regions, with the lowest and highest values calculated in the mudflats of Nayband and Gwadar in winter (Figure 2e). Pielou’s evenness and Simpson’s dominant indices differed significantly between regions (p < 0.05), whereas there was no significant difference between the seasons and habitats (p > 0.05).
Average values of CWM were plotted to visualize a functional trait composition for each habitat and season in each mangrove region (Figure 3). Gwadar’s dominant trait modalities were a size > 100, biodiffuser, omnivore, life span of 1–3 years, burrower, and AMBI-IV. The dominant trait modalities were a size > 100 mm, biodiffuser, omnivore, life span of 1–3 and 3–5 years, burrower, and AMBI-I in Sirik. The highest values of CWM were obtained with a size of 10–20 and > 100 mm, biodiffuser, omnivore–predator, life span of 1–3 and 3–5 years, crawler, and AMBI-IV in Nayband (Figure 3). The results of the three-factor ANOVA revealed significant differences in the community weighted mean (CWM) between regions and habitats, while there were no significant differences between seasons (Table 2).
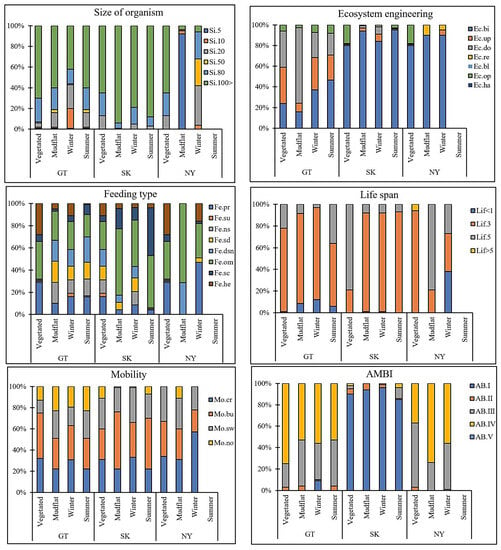
Figure 3.
Community weighted mean (CWM) of trait modalities of polychaetes from different regions (GT—Gwadar, SK—Sirik, and NY—Nayband), habitats, and seasons in mangrove ecosystems in the Persian Gulf and Gulf of Oman. Color codes represent the trait affiliation; individual bars represent the trait modality expression. For trait modalities’ labels, see Table 1.
The composition of trait modalities for each trait was almost the same in all habitats, but the percentage contribution varied, which explained the observed differences. Species inhabiting vegetated habitats tended to be dominated by crawling predators and moderately large (80–100 mm), relatively long-lived (3–5 years), and upward conveyors, whereas species inhabiting mudflat habitats tended to be dominated by omnivores, larger bodies (>100 mm), relatively moderate-lived (1–3 years), burrowers, and biodiffusers (Figure 3).
Non-metric multidimensional scaling (nMDS) based on polychaete species and trait composition highlighted a higher variability of assemblages between habitats and regions rather than seasons (Figure 4). Overall, the spatial dissimilarity between two habitats (vegetated and mudflat) and three regions (Gwadar, Sirik, and Nayband) was confirmed with the three-factor ANOVA analysis (p < 0.05, Table 2).
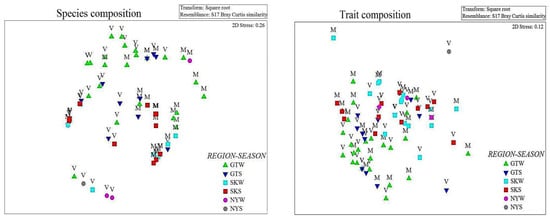
Figure 4.
nMDS ordination plots of species and trait composition of polychaetes across regions, habitats, and seasons in mangrove ecosystems in the Persian Gulf and Gulf of Oman (GT—Gwadar, SK—Sirik, NY—Nayband, W—Winter, S—Summer, V—Vegetated, and M—Mudflat).
Based on the SIMPER analysis of polychaete abundance, Gwadar and Sirik had a dissimilarity of 83.91%. The species Neanthes glandicincta contributed significantly to Gwadar and Sirik divisions (cont. = 27.61%). The dissimilarity between Gwadar and Nayband was 39.99%, mainly caused by Simplisetia erythraeensis (cont. = 15.16%). The dissimilarity between Sirik and Nayband (average dissimilarity = 100%) was mainly because of the N. gladicincta (cont. = 23.28), S. erythraeensis (cont. = 20.26), and Perinereis horsti (cont. = 19.92). The average dissimilarity between habitats (vegetated and mudflats) and seasons (winter and summer) was 83.83% and 79.38%, respectively. In particular, N. gladicincta contributed mainly to the dissimilarity between habitats (cont. = 33.02%) and seasons (cont. = 31.55%) (Table S2).
According to the SIMPER analysis, the average dissimilarity between Gwadar and Sirik (dissimi. = 30.41%) was mainly due to the upward conveyor (cont. = 4.39%) and deposit feeder (selective or non-selective) (cont. = 4.39%). There was an average dissimilarity of 52.95% between Gwadar and Nayband, mostly due to the modalities’ life span of ≤1 year’s contribution of 4.5% and the modalities’ AMBI IV’s contribution of 4.35%. Sirik and Nayband had an average dissimilarity of 36.79% and a modality life span of ≤1 year (cont. = 7.34%) and size of 21–50 (cont. = 6.21%) contributed the most to this region division. The average dissimilarity between habitats (vegetated and mudflats) was 33.44%, and the average dissimilarity between seasons was 32.71% (winter and summer). The average dissimilarity was mostly explained by the modalities’ size of 21–50 and scavenger between habitats (size = 21–50, contribution = 4.66%, and scavenger cont. = 4.36%) and seasons (size = 21–50, cont. = 4.37%, and scavenger cont. = 4.26%) (Table S3).
Several environmental variables were measured in different regions and seasons, including TOC, TON, and silt/clay content sediments, salinity, and temperature (Table 3).

Table 3.
Variations in environmental data (mean ± SD) between regions, habitats, and seasons in mangrove ecosystems in the Persian Gulf and Gulf of Oman (TOC = Total Organic Carbon; TON = Total Organic Nitrogen).
The contribution of environmental variables to variation in species and trait compositions of polychaetes for three regions was determined using the DistLM model (Table 4 and Table 5). Using the DISTLM routine, fitted values were then ordinated using a distance-based redundancy analysis (dbRDA) (Figure 5). The marginal test indicated that TOC significantly explained species variation in Sirik (p < 0.05), while environmental variables did not show significant differences in the other regions (p > 0.05). The Best model indicated that TOC (AIC = 314.1, R2 = 4.3531) in Gwadar, the combination of TON, TOC, and silt/clay (AIC = 238.73, R2 = 0.26977) in Sirik, and the combination of temperature and silt/clay (AIC = 31.688, R2 = 0.82016) in Nayband were the most powerful predictors of the variance in the polychaete community (Table 4).

Table 4.
Results of the DistLM analysis used to identify environmental variables influencing the species composition of polychaete assemblages in mangrove ecosystems (GT—Gwadar, SK—Sirik, and NY—Nayband) in the Persian Gulf and Gulf of Oman. p-Values were obtained from 9999 permutations of residuals under a reduced model. SS (trace) = portion of sum of squares related to the analyzed environmental variable; Pseudo-F = F value with permutation; bold values indicate significant level.

Table 5.
Results of the DistLM analysis used to identify environmental variables influencing the trait composition of polychaete assemblages in mangrove ecosystems (GT—Gwadar, SK—Sirik, and NY—Nayband) in the Persian Gulf and Gulf of Oman. p-Values were obtained from 9999 permutations of residuals under a reduced model. SS (trace) = portion of sum of squares related to the analyzed environmental variable; Pseudo-F = F value with permutation; bold values indicate significant level.
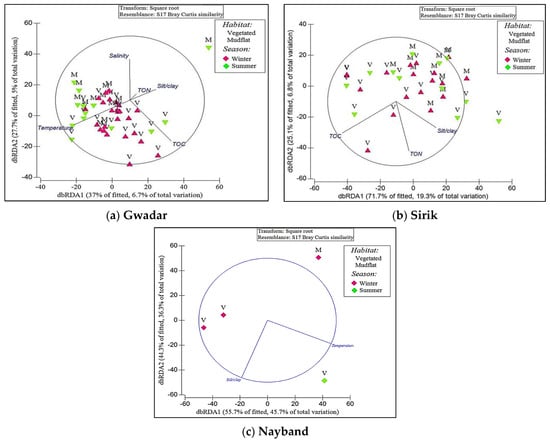
Figure 5.
Distance-based redundancy analysis (dbRDA) ordination based on the best set of abiotic variables (using BEST as selection procedure and AIC as selection criterion) and polychaete species composition data from the three mangrove ecosystems in the Persian Gulf and Oman Gulf; (a) Gwadar, (b) Sirik, and (c) Nayband. The length and direction of vectors indicate the strength and direction of the relationship, respectively.
The marginal test revealed that silt/clay in Sirik and salinity in Nayband each significantly explained the variation in the trait composition (p < 0.05). According to the Best model, the most important predictor variables of polychaete traits in mangrove ecosystems were the combination of temperature and TOC (AIC = 254.77, R2 = 0.15525) in Gwadar, the combination of TON, TOC, salinity, and temperature (AIC = 181.46, R2 = 0.39001) in Sirik, and the combination of temperature and salinity (AIC = 22.609, R2 = 0.89395) in Nayband (Table 5).
The first two axes of dbRDA of Gwadar explained 100% of the fitted variation and 4.4% of the total variation. The first dbRDA axis was mainly determined with TOC (dbRDA = 0.986), whereas the second axis was defined with TON and silt/clay (dbRDA = 0.856) (Figure 5a). In Sirik, the first two axes of dbRDA explained 96.8% of the total variation (dbRDA1 = 71.7% and dbRDA2 = 25.1%) (Figure 5b). The first axis was related to TOC (dbRDA = −0.786) and the second axis was defined with the combination of TON (dbRDA = 0.76) and silt/clay (dbRDA = −0.4) (Figure 5b). In Nayband, the first dbRDA axis was mainly determined with silt/clay (dbRDA = 0.926), while the second axis was defined with temperature (dbRDA = 0.926), which explained 100% of the fitted variation (dbRDA1 = 55.7% and dbRDA2 = 44.3%) (Figure 5c).
The dbRDA plots showed the contributors’ variables in the variation of the traits of the three regions. Temperature was the main predictor of the first axis (dbRDA = −0.775), while TOC was the main predictor of the second axis (dbRDA = 0.775), which explained 100% of the total variation in Gwadar (dbRDA = 88.9% and dbRDA = 11.1% dbRDA2) (Figure 6a). In Sirik, the first two dbRDA axes explained 97.6% of the total variation (dbRDA1 = 77.6% and dbRDA2 = 26%). The first axis was silt/clay (dbRDA = 0.785) and the second axis was the combination of TOC (dbRDA= 0.624), TON (dbRDA = 0.008), and temperature (dbRDA = −0.78) (Figure 6b). The first dbRDA axis of Nayband was mainly defined with salinity (dbRDA = 0.946) while the second axis was related to temperature (dbRDA = 0.946), which explained 100% of the total variation (dbRDA1 = 64.5% and dbRDA2 = 35.5%) (Figure 6c).
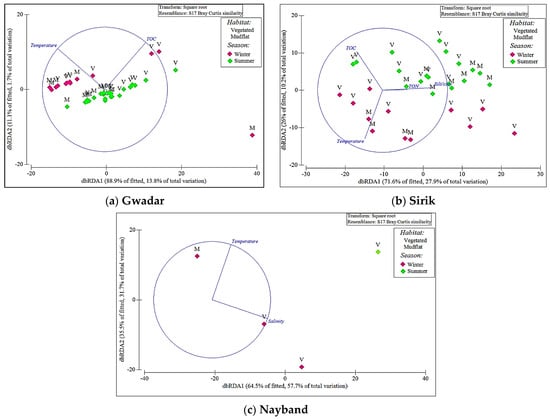
Figure 6.
Distance-based redundancy analysis (dbRDA) ordination based on the best set of abiotic variables (using BEST as selection procedure and AIC as selection criterion) and polychaete trait composition data from the three mangrove ecosystems in the Persian Gulf and Oman Gulf; (a) Gwadar, (b) Sirik, and (c) Nayband. The length and direction of vectors indicate the strength and direction of the relationship, respectively.
4. Discussion
In this study, the most abundant polychaete species belonged to the Nereididae, Capitellidae, and Paraonidae families in mangrove ecosystems (Table S1). Delfan et al. (2021) [23] reported that the most abundant polychaete families were Capitellidae and Nereididae, the latter being represented mostly by Perinereis horsti and Simplisetia qeshmensis in a mangrove ecosystem in the Persian Gulf. Hajalizadae et al. (2020) [22] stated that the most abundant polychaetes were Capitellidae and Nereididae in different mangrove habitats in the Persian Gulf. Nereididae are omnivores with jaws that can reduce the particle size of detritus [46]. Therefore, they can increase bioturbation and fragmentation in mangroves. Since capitellidae are deposit feeders, infunal, and burrowers, they play an essential role in cycling nutrients in estuaries [52].
The abundance and taxonomic diversity of polychaetes in our study were different in the three regions. The lowest richness and diversity indices were observed in the Nayband region. Mangroves of the Persian Gulf have less species richness and diversity due to higher salinity and temperature levels than tropical mangroves [23]. Therefore, the Nayband estuary is under stress from environmental variables, and the results of DistLM indicate that temperature is the most powerful predictor of the species composition of polychaete assemblages. In addition, the highest amount of organic carbon was obtained in the Nayband estuary. In this region, oil, gas, and petrochemical industries, as well as fishing activities and the movement of boats and barges, have led to the entry of a wide variety of pollutants. Additionally, the closure of a part of the Nayband estuary entrance has disrupted the tidal cycle and thus enriched organic matter [53]. Therefore, these stressors are limiting factors commonly known to restrict both the abundance and diversity of polychaete assemblages in the Nayband estuary.
The highest abundance of polychaetes was observed in Sirik, but it had less diversity compared to the Gowadar region. There are two tree species, viz. Avicennia marina and Rhizophora mucronata, that are in the Sirik region, whereas only A. marina is present in the other two regions. The presence of two species of trees enhances structural complexity, creating complex habitats [23,24,27]. The structural complexity of R. mucronata affects tidal currents, which ultimately affect sedimentation and sediment characteristics within mangrove forests. In addition, R. mucronata roots have a large volume and variety of interstitial spaces that might be advantageous for sheltering mobile organisms that can navigate through narrow gaps to avoid larger predators [34]. Therefore, these reasons may be the factors affecting the increase in the abundance of polychaetes in the Sirik region. Delfan et al. (2021) [23] proposed that the low richness of the macrofauna may be attributed to low mangrove tree diversity in the Persian Gulf. Overall, the difference in mangrove benthic communities depends on the latitude, mangrove species, estuary morphology, and presence or absence of fresh water in the region, evaporation, and other environmental variables, which can be the result of specific environmental and ecological forms of each community [36].
The abundance, Shannon diversity, and richness index showed seasonal variation, which is consistent with the results of Hajializadea et al. (2020) [22], in the mangroves of the Qeshm region in the Persian Gulf.
The results of our study showed that the abundance and taxonomic diversity of polychaetes increased from the mudflats to the vegetated habitats. Also, nMDS showed that species composition differed between habitats. We observed similar results to those of Checon et al. (2017) [29], Leung (2015) [31], and Pan et al. [5]. Many studies, including the present study, show that the presence of vegetation and structural components (such as roots, algal mats, and pneumatophores) significantly changed sediment characteristics. In the mangrove ecosystem, prop roots and pneumatophores helped trap detritus and litter, and also weakened wave action to increase sedimentation rates, which resulted in finer sediment and more organic matter [31,54]. The abundance and diversity indices decrease with the increase in root biomass, which may lead to inhibition and reduction in the burrowing behavior and feeding activity [5,31,55]. As reported by Leung (2015) [31] and Checon et al. (2017) [29], the density of polychaetes in the mangrove was lower than those in mudflats, and the density of polychaetes was negatively correlated with root biomass. There may be a difference in species composition of polychaete assemblages between vegetated and mudflats due to the distinct sedimentary characteristics, such as higher organic matter in a mudflat habitat [56], different temperatures, and the shelter provided by mangrove stand structures [32,57]. However, some studies showed that roots had positive effects on macrobenthos abundance and diversity because they enhanced sediment stability, the complexity of the habitat, and protected against predators [22,23,58,59].
There were several dominant trait modalities found in the three studied mangrove regions, including a large size, biodiffuser, burrower, crawler, life span of 1–3 years, and omnivore. Even though bioturbation is often regarded as a small-scale process, it plays an important role in the ecology of mangroves. For example, it can change the topography, increase oxygenation of deep sediment layers, affect community metabolism directly, alter organic content, rework sediments, reduce sediment density, and influence the fate of pollutants [52]. A bioturbation process is particularly relevant in areas with muddy sediments, low permeability, low oxygen concentrations, and high concentrations of contaminants (i.e., a reduced exchange of water between sediments and the water column). Polychaetes perform functions such as organic matter rotation, turbidity control, and stability through bioturbation [60]. In the studied mangrove forests, almost all polychaetes were classified as a biodiffuser, which is consistent with the results of Martins and Barros (2022) [52].
In this study, large-sized polychaetes were dominant in Gwadar and Sirik regions in both seasons, while small-sized polychaetes were dominant in Nayband and mudflat habitats. Body size is related to such circumstances as the food web structure, trophic levels, and energy flow in the ecosystem [61] and indicates disturbance, movement of organic matter, and biological interactions in a community [22]. The results of the present study are consistent with Delfan et al. (2021) [23]. It has been shown that large polychaetes are more effective at oxygenating sediments and cycling nutrients in mangrove ecosystems [62]. Posey (1987) [63] found that the maximum body size decreased as the number of pneumatophores increased, possibly because of dense root structures that inhibit the burrowing and feeding of large-sized species, while the maximum body size was larger in the mud habitat. We found that all regions and habitats were dominated by burrower and crawler trait modalities, but the crawler polychaetes increased slightly in vegetation habitats. Also in other studies, surface crawlers are frequently found in habitats with sandy sediments [64], bare mud, algal mats, and pneumatophores [22,31].
According to our findings, the predatory polychaetes were slightly higher in vegetated habitats and omnivorous polychaetes dominated all habitats and regions. Probably, the large variety and amount of food led to an increase in surface deposit feeders, which led to an increase in predatory species in vegetated habitats [65,66]. The increase in omnivorous polychaetes in mangrove ecosystems is due to the presence of Nereididae (i.e., N. glandicincta), which showed high abundance in all habitats and regions. The success of omnivores against other feeding strategies is due to their broad dietary flexibility, which allows them to adjust to environmental and resource fluctuations [67]. Therefore, they balance their diet as a result of feeding needs, food quality, and the availability of alternative foods [68].
Polychaete assemblages are affected by a variety of environmental variables, such as temperature, salinity, sediment type, and TOC and TON in sediments [69,70]. The results of DistLM showed that the TOC variable in Gwadar, silt/clay, TOC, and TON variables in Sirik, and temperature and silt/clay variables in Nayband contributed the most to the taxonomic structure of polychaetes. Carvalho et al. (2013) [71] stated that environmental processes controlling patterns of diversity could ultimately be taxon- as well as locality-specific. Shillabeer and Tapp (1989) [72] stated that the mangrove environment was much more dynamic than the purely marine environment and therefore, there is every possibility of variation in species occurrence. Our results are consistent with previous studies worldwide and indicate that sediment variables (e.g., sediment grain size, TOC, and TON) are more relevant than water column variables (temperature and salinity) in the distribution pattern of polychaetes [31,70,71,73,74].
In the present study, TOC, silt/clay, and salinity were the main drivers of polychaete traits in three mangrove ecosystems (the DistLM analysis), which can influence the difference in the expression of traits between mangrove ecosystems (Gwader, Sirik, and Nayband) and habitats (vegetated and mudflat). The results of this study are in accordance with Nasi et al. (2018) [12], which suggests that habitat and sediment descriptors, such as particles of sand or clay, and the depth of TOC and TON, are related to polychaete assemblages taxonomically and functionally. The study by Medeiros et al. (2021) [25] revealed that polychaetes’ functional nesting patterns were driven, regardless of seasonality, by salinity gradients throughout the year. Therefore, salinity determined the functional nestedness of the polychaete community. There were a number of functional traits present in the polychaete community that were nested subsets of those observed in higher salinity sites, where a wider range of categories of functional traits was encountered [25].
Along with other environmental variables, such as temperature or food availability, salinity may also affect polychaetes’ physiology, regeneration, and growth [74,75,76,77]. Arrighetti and Penchaszadeh (2010) [78] proposed that sediment characteristics, dissolved oxygen, and salinity were the main factors affecting polychaete communities. Kim et al. (2021) [79] stated that the sediment composition (gravel, sand, and clay) and hydrological variables (dissolved oxygen, depth, and temperature) were important factors in polychaete distribution patterns. Whether the environmental variables included in the analysis change this pattern structure solely or whether incorporating other variables in the analysis changes the observed polychaete pattern is difficult to determine [12,79,80]. It is important to examine how each set of environmental characteristics (such as salinity, particle size, and organic matter) and human impacts affect ecological functions.
In the current study, changes in the taxonomic and functional structure of polychaetes in mangrove ecosystems were observed, which showed that the species and trait composition of polychaetes depends on the structural complexity of the respective habitat. As reported by Van der Linden et al. (2016) [9], polychaetes exhibited higher taxonomic and functional diversity than molluscs, suggesting that in comparison with molluscs, they may be utilizing the resources more efficiently, resulting in changes in the functioning of the systems. An evaluation of the changes and relationships between traits and diverse mangrove habitats can lead to a better understanding of the functioning of these habitats and their effects on the environment.
5. Conclusions
According to the results, polychaetes were distributed differently in the three studied mangrove ecosystems, which may be due to their characteristics and the local environmental conditions of each ecosystem. It was found that the abundance and biodiversity of polychaetes in the mudflat habitats were higher than the vegetated habitats. Vegetated habitats had higher proportions of mobile (crawler) predatory species that are longer lived (3–5 years), with larger body size (80–100 mm), and are upward conveyors, whereas mudflat habitats had higher proportions of mobile (burrower) omnivore species that are moderately lived (1–3 years), with larger body size (>100 mm), and are a biodiffuser. These traits played important roles in determining the functional composition of a polychaete assemblage in mangrove ecosystems. The results showed that the species and trait composition of polychaetes in two habitats of a mangrove ecosystem is distinct, and the increasing abundance of polychaetes may be related to the structural complexity of their respective habitats. The advantage and importance of using taxonomic diversity and BTA of polychaetes and the impact of environmental variables on them generate information on how to respond and change the functioning of mangrove ecosystems, allowing us to make more efficient conclusions about biodiversity conservation and ecosystem functionality in future research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15090998/s1, Table S1: Taxonomic list and mean density (ind.m−2 ± SE) of polychaetes in the mangrove vegetated habitats and mudflats in the Persian Gulf and Gulf of Oman; Table S2: Result of SIMPER analysis determining the major species leading to the polychaete community structure difference between regions (GT—Gwadar, SK—Sirik, and NY—Nyband), habitats (V—vegetated and M—mudflat), and seasons (W—winter and S—summer) in mangrove ecosystems in the Persian Gulf and Gulf of Oman (≥50% cumulative frequency); Table S3: Result of SIMPER analysis determining the major traits leading to the polychaete community structure difference between regions (GT—Gwadar, SK—Sirik, and NY—Nyband), habitats (V—vegetated and M—mudflat), and seasons (W—winter and S—summer) in mangrove ecosystems in the Persian Gulf and Gulf of Oman (≥50% cumulative frequency).
Author Contributions
Conceptualization, M.M., J.S. and M.G.S.; methodology, M.M., M.G.S. and H.R.; software, M.M., M.G.S. and M.V.; validation, J.S., M.G.S. and H.R.; formal analysis, M.M., J.S. and M.G.S.; investigation, M.M. and M.G.S.; data curation, M.M.; writing—original draft preparation, M.M. and M.G.S.; writing—review and editing, J.S., H.R. and M.V.; supervision, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the supplementary data files as well as from the corresponding author on reasonable request.
Acknowledgments
We gratefully acknowledge the financial support provided by the Department of Marine Biology, Faculty of Natural Resources and Marine Sciences, Tarbiat Modares University, Noor, Iran.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, R.S. A review of biodiversity studies of soil dwelling organisms in indian mangroves. Zoos Print J. 2000, 7, 221–227. [Google Scholar] [CrossRef]
- Pawar, P.R. Monitoring of Pollution Using Density, Biomass and Diversity Indices of Macrobenthos from Mangrove Ecosystem of Uran, Navi Mumbai, West Coast of India. J. Bioremediation Biodegrad. 2015, 6, 299. [Google Scholar] [CrossRef]
- Adame, M.F.; Reef, R.; Santini, N.S.; Najera, E.; Turschwell, M.P.; Hayes, M.A.; Masque, P.; Lovelock, C.E. Mangroves in arid regions: Ecology, threats, and opportunities. Estuar. Coast. Shelf Sci. 2021, 248, 106796. [Google Scholar] [CrossRef]
- Davidson, N.C.; Finlayson, C.M.; Davidson, N.C.; Finlayson, C.M. Updating global coastal wetland areas presented in Davidson and Finlayson (2018). Mar. Freshw. Res. 2019, 70, 1195–1200. [Google Scholar] [CrossRef]
- Pan, S.-H.; Ho, C.-W.; Lin, C.-W.; Huang, S.-C.; Lin, H.-J. Differential Response of Macrobenthic Abundance and Community Composition to Mangrove Vegetation. Forests 2021, 12, 1403. [Google Scholar] [CrossRef]
- Coblentz, K.E.; Henkel, J.R.; Sigel, B.J.; Taylor, C.M. Technical Note: The Use of Laser Diffraction Particle Size Analyzers for Inference on Infauna-Sediment Relationships. Estuaries Coasts 2015, 38, 699–702. [Google Scholar] [CrossRef]
- Fauchald, K.; Jumars, P.A. The diet of worms: A study of polychaete feeding guilds. Oceanogr. Mar. Biol. Annu. Rev. 1979, 17, 193–284. [Google Scholar]
- Cutrim, A.; Sousa, L.; Ribeiro, R.; Oliveira, V.; Almeida, Z. Structure of a polychaete community in a mangrove in the northern coast of Brazil. Acta Biol. Colomb. 2018, 23, 286–294. [Google Scholar] [CrossRef]
- Van der Linden, P.; Borja, A.; Rodríquez, J.G.; Muxika, I.; Galparsoro, I.; Patrício, J.; Veríssimo, H.; Marques, J.C. Spatial and temporal response of multiple trait-based indices to natural- and anthropogenic seafloor disturbance (effluents). Ecol. Indic. 2016, 69, 617–628. [Google Scholar] [CrossRef]
- Wouters, J.M.; Gusmao, J.B.; Mattos, G.; Lana, P. Polychaete functional diversity in shallow habitats: Shelter from the storm. J. Sea Res. 2018, 135, 18–30. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Duffy, J.E.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef]
- Nasi, F.; Nordström, M.C.; Bonsdorff, E.; Auriemma, R.; Cibic, T.; Del Negro, P. Functional biodiversity of marine soft-sediment polychaetes from two Mediterranean coastal areas in relation to environmental stress. Mar. Environ. Res. 2018, 137, 121–132. [Google Scholar] [CrossRef]
- Laureto, L.M.O.; Cianciaruso, M.V.; Samia, D.S.M. Functional diversity: An overview of its history and applicability. Nat. Conserv. 2015, 13, 112–116. [Google Scholar] [CrossRef]
- Bremner, J.; Rogers, S.; Frid, C. Assessing functional diversity in marine benthic ecosystems: A comparison of approaches. Mar. Ecol. Prog. Ser. 2003, 254, 11–25. [Google Scholar] [CrossRef]
- Culhane, F.E.; Briers, R.A.; Tett, P.; Fernandes, T.F. Structural and functional indices show similar performance in marine ecosystem quality assessment. Ecol. Indic. 2014, 43, 271–280. [Google Scholar] [CrossRef]
- Degen, R.; Aune, M.; Bluhm, B.A.; Cassidy, C.; Kędra, M.; Kraan, C.; Vandepitte, L.; Włodarska-Kowalczuk, M.; Zhulay, I.; Albano, P.G.; et al. Trait-based approaches in rapidly changing ecosystems: A roadmap to the future polar oceans. Ecol. Indic. 2018, 91, 722–736. [Google Scholar] [CrossRef]
- Winemiller, K.O.; Fitzgerald, D.B.; Bower, L.M.; Pianka, E.R. Functional traits, convergent evolution, and periodic tables of niches. Ecol. Lett. 2015, 18, 737–751. [Google Scholar] [CrossRef]
- Dolédec, S.; Chessel, D.; ter Braak, C.J.F.; Champely, S. Matching species traits to environmental variables: A new three-table ordination method. Environ. Ecol. Stat. 1996, 3, 143–166. [Google Scholar] [CrossRef]
- Usseglio-Polatera, P.; Bournaud, M.; Richoux, P.; Tachet, H. Biological and ecological traits of benthic freshwater macroinvertebrates: Relationships and definition of groups with similar traits. Freshw. Biol. 2000, 43, 175–205. [Google Scholar] [CrossRef]
- Darr, A.; Gogina, M.; Zettler, M.L. Functional changes in benthic communities along a salinity gradient—A western Baltic case study. J. Sea Res. 2014, 85, 315–324. [Google Scholar] [CrossRef]
- Gogina, M.; Darr, A.; Zettler, M.L. Approach to assess consequences of hypoxia disturbance events for benthic ecosystem functioning. J. Mar. Syst. 2014, 129, 203–213. [Google Scholar] [CrossRef]
- Hajializadeh, P.; Safaie, M.; Naderloo, R.; Shojaei, M.G.; Gammal, J.; Villnäs, A.; Norkko, A. Species Composition and Functional Traits of Macrofauna in Different Mangrove Habitats in the Persian Gulf. Front. Mar. Sci. 2020, 7, 575480. [Google Scholar] [CrossRef]
- Delfan, N.; Shojaei, M.G.; Naderloo, R. Patterns of structural and functional diversity of macrofaunal communities in a subtropical mangrove ecosystem. Estuar. Coast. Shelf Sci. 2021, 252, 107288. [Google Scholar] [CrossRef]
- Nozarpour, R.; Shojaei, M.G.; Naderloo, R.; Nasi, F. Crustaceans functional diversity in mangroves and adjacent mudflats of the Persian Gulf and Gulf of Oman. Mar. Environ. Res. 2023, 186, 105919. [Google Scholar] [CrossRef]
- Medeiros, C.R.; Paiva, F.F.; Ligeiro, R.; Molozzi, J.; Melo, A.S. Saline gradient drives functional nestedness of polychaete communities in tropical estuaries. Estuar. Coast. Shelf Sci. 2021, 251, 107185. [Google Scholar] [CrossRef]
- Akbari Noghabi, N.; Shojaei, M.G.; Farahani, M.M.; Weigt, M. Stable Isotopes Reveal the Food Sources of Benthic Macroinvertebrates in the Arid Mangrove Ecosystem of the Persian Gulf. Estuaries Coasts 2022, 45, 2241–2253. [Google Scholar] [CrossRef]
- Vorsatz, L.D.; Pattrick, P.; Porri, F. Quantifying the in situ 3-dimensional structural complexity of mangrove tree root systems: Biotic and abiotic implications at the microhabitat scale. Ecol. Indic. 2021, 121, 107154. [Google Scholar] [CrossRef]
- Bernardino, A.F.; Gomes, L.E.d.O.; Hadlich, H.L.; Andrades, R.; Correa, L.B. Mangrove clearing impacts on macrofaunal assemblages and benthic food webs in a tropical estuary. Mar. Pollut. Bull. 2018, 126, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Checon, H.H.; Corte, G.N.; Silva, C.F.; Schaeffer-Novelli, Y.; Amaral, A.C.Z. Mangrove vegetation decreases density but does not affect species richness and trophic structure of intertidal polychaete assemblages. Hydrobiologia 2017, 795, 169–179. [Google Scholar] [CrossRef]
- Corte, G.; Checon, H.; Shah Esmaeili, Y.; Lefcheck, J.; Amaral, A. Mangrove fragments as key coastal reservoirs of taxonomic and functional biodiversity. Biodivers. Conserv. 2021, 30, 1573–1593. [Google Scholar] [CrossRef]
- Leung, J.Y.S. Habitat Heterogeneity Determining the Macrobenthic Infaunal Community in a Mangrove Swamp in South China: Implication for Plantation and Plant Invasion. J. Coast. Res. 2015, 313, 624–633. [Google Scholar] [CrossRef]
- Leung, J.Y.S.; Tam, N.F.Y. Influence of plantation of an exotic mangrove species, Sonneratia caseolaris (L.) Engl., on macrobenthic infaunal community in Futian Mangrove National Nature Reserve, China. J. Exp. Mar. Biol. Ecol. 2013, 448, 1–9. [Google Scholar] [CrossRef]
- Milani, A.S. Mangrove Forests of the Persian Gulf and the Gulf of Oman. In Threats to Mangrove Forests: Hazards, Vulnerability, and Management; Makowski, C., Finkl, C.W., Eds.; Coastal Research Library; Springer International Publishing: Cham, Switzerland, 2018; pp. 53–75. ISBN 978-3-319-73016-5. [Google Scholar]
- Amiri, S.N.; Sajadi, J.; Sadough Vanini, S.H. Application of Vegetation Indices Derived from IRS Data for Detecting the Avicennia Forest Area Near the South Pars Oil Apparatus. Environ. Sci. 2010, 8, 69–84. [Google Scholar]
- Taherizadeh, M.; Sharifinia, M. Applicability of ecological benthic health evaluation tools to three subtropical estuaries (Azini, Jask and Khalasi) from the Iranian coastal waters. Environ. Earth Sci. 2015, 74, 3485–3499. [Google Scholar] [CrossRef]
- Erfani, M.; Nouri, G.; Danekar, A.; Marvi Mohajer, M.R.; Mahmoudi, B. Vegetative parameters of Mangrove forest on the Govater bay in southeast of Iran. Taxon. Biosyst. 2009, 1, 33–46. [Google Scholar]
- Bonyadi Naeini, A.; Rastegar-Pouyani, N.; Rastegar Pouyani, E.; Glasby, C.; Rahimian, H. Nereididae (Annelida: Phyllodocida) of the Persian Gulf and Gulf of Oman, including description of two new species and 11 new records. Zootaxa 2017, 4244, 91. [Google Scholar] [CrossRef] [PubMed]
- Bonyadi Naeini, A.; Rahimian, H. Intertidal scale worms (Polychaeta, Polynoidae and Sigalionidae) from the northern coasts of the Persian Gulf and Gulf of Oman. ZooKeys 2009, 31, 53–71. [Google Scholar] [CrossRef][Green Version]
- Jones, D.A. A Field Guide to the Sea Shores of Kuwait and the Arabian Gulf; University of Kuwait: Kuwait City, Kuwait, 1986. [Google Scholar]
- Rahimian, H.; Yousefi, S.; Nabavi, S.; Glasby, C. Nereididae (Annelida: Polychaeta) from intertidal habitats in the Gulf of Oman, Iran. Zootaxa 2011, 3013, 48–64. [Google Scholar] [CrossRef]
- Bhowmik, M.; Ghoshal, P.; Salazar-Vallejo, S.; Mandal, S. Sigambra sundarbanensis sp. nov. (Annelida, Pilargidae) from the Indian sector of Sundarbans Estuarine System, with remarks on parapodial glands. Eur. J. Taxon. 2021, 744, 49–66. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 595–624. ISBN 978-0-89118-977-0. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer Method Improved for Making Particle Size Analyses of Soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Chevene, F.; Doléadec, S.; Chessel, D. A fuzzy coding approach for the analysis of long-term ecological data. Freshw. Biol. 1994, 31, 295–309. [Google Scholar] [CrossRef]
- Jumars, P.A.; Dorgan, K.M.; Lindsay, S.M. Diet of worms emended: An update of polychaete feeding guilds. Annu. Rev. Mar. Sci. 2015, 7, 497–520. [Google Scholar] [CrossRef] [PubMed]
- Muscarella, R.; Uriarte, M. Do community-weighted mean functional traits reflect optimal strategies? Proc. Biol. Sci. 2016, 283, 20152434. [Google Scholar] [CrossRef]
- Clarke, K.; Gorley, R.N. Primer v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006; pp. 52–182. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Götzenberger, L.; de Bello, F.; Dias, A.T.C.; Moretti, M.; Berg, M.P.; Carmona, C.P. Trait-Based Ecology Tools in R 2020; CSIC-GV-UV-Centro de Investigaciones sobre Desertificación (CIDE): Moncada, Spain, 2020. [Google Scholar] [CrossRef]
- Palmia, B.; Bartoli, M.; Laini, A.; Bolpagni, R.; Ferrari, C.; Viaroli, P. Effects of Drying and Re-Wetting on Litter Decomposition and Nutrient Recycling: A Manipulative Experiment. Water 2019, 11, 708. [Google Scholar] [CrossRef]
- Martins, A.D.; Barros, F. Ecological Functions of Polychaetes Along Estuarine Gradients. Front. Mar. Sci. 2022, 9, 780318. [Google Scholar] [CrossRef]
- Mooraki, N.; Moghadasi, B.; Manoochehri, H.; Changizi, R. Determining the concentration of Heavy metals and evaluating the contamination degree of Haleh Estuary and Nayband Gulf sediment’s and their effects on foraminifera assemblages. J. Wetl. Ecobiol. 2016, 8, 45–58. [Google Scholar]
- Evin, L.; Talley, T. Influences of Vegetation and Abiotic Environmental Factors on Salt Marsh Invertebrates. In Concepts and Controversies in Tidal Marsh Ecology; Springer: Berlin/Heidelberg, Germany, 2002; pp. 661–707. ISBN 978-0-7923-6019-3. [Google Scholar]
- Ringold, P. Burrowing, root mat density, and the distribution of fiddler crabs in the eastern United States. J. Exp. Mar. Biol. Ecol. 1979, 36, 11–21. [Google Scholar] [CrossRef]
- Kawaida, S.; Nanjo, K.; Ohtsuchi, N.; Kohno, H.; Sano, M. Cellulose digestion abilities determine the food utilization of mangrove estuarine crabs. Estuar. Coast. Shelf Sci. 2019, 222, 43–52. [Google Scholar] [CrossRef]
- Chen, G.-C.; Ye, Y.; Lu, C.-Y. Changes of macro-benthic faunal community with stand age of rehabilitated Kandelia candel mangrove in Jiulongjiang Estuary, China. Ecol. Eng. 2007, 31, 215–224. [Google Scholar] [CrossRef]
- Capehart, A.A.; Hackney, C.T. The potential role of roots and rhizomes in structuring salt-marsh benthic communities. Estuaries 1989, 12, 119–122. [Google Scholar] [CrossRef]
- Netto, S.A.; Lana, P.C. The role of above- and below-ground components of Spartina alterniflora (Loisel) and detritus biomass in structuring macrobenthic associations of Paranaguá Bay (SE, Brazil). Hydrobiologia 1999, 400, 167–177. [Google Scholar] [CrossRef]
- Gimenez, B.C.G.; Lana, P. Functional redundancy in polychaete assemblages from a tropical Large Marine Ecosystem (LME). Zoosymposia 2020, 19, 72–90. [Google Scholar] [CrossRef]
- Gerlach, S.A.; Hahn, A.E.; Schrage, M. Size spectra of benthic biomass and metabolism. Mar. Ecol. Prog. Ser. 1985, 26, 161–173. [Google Scholar] [CrossRef]
- Norkko, A.; Villnäs, A.; Norkko, J.; Valanko, S.; Pilditch, C. Size matters: Implications of the loss of large individuals for ecosystem function. Sci. Rep. 2013, 3, 2646. [Google Scholar] [CrossRef]
- Posey, M.H. Influence of relative mobilities on the composition of benthic communities. Mar. Ecol. Prog. Ser. Oldendorf 1987, 39, 99–104. [Google Scholar] [CrossRef]
- Liu, K.; Lin, H.; He, X.; Huang, Y.; Li, Z.; Lin, J.; Mou, J.; Zhang, S.; Lin, L.; Wang, J.; et al. Functional trait composition and diversity patterns of marine macrobenthos across the Arctic Bering Sea. Ecol. Indic. 2019, 102, 673–685. [Google Scholar] [CrossRef]
- Bluhm, B.A.; Gradinger, R.; Hopcroft, R.R. Editorial—Arctic Ocean Diversity: Synthesis. Mar. Biodivers. 2011, 41, 1–4. [Google Scholar] [CrossRef]
- Iken, K.; Bluhm, B.; Dunton, K. Benthic food-web structure under differing water mass properties in the southern Chukchi Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 71–85. [Google Scholar] [CrossRef]
- Muro-Torres, V.M.; Amezcua, F.; Soto-Jiménez, M.; Balart, E.F.; Serviere-Zaragoza, E.; Green, L.; Rajnohova, J. Primary Sources and Food Web Structure of a Tropical Wetland with High Density of Mangrove Forest. Water 2020, 12, 3105. [Google Scholar] [CrossRef]
- Cole, V.J.; McQuaid, K.A.; McQuaid, C.D. Examination of small- and large-scale influences on the diet of an omnivorous polychaete indicates weak effects of upwelling. J. Exp. Mar. Biol. Ecol. 2012, 436–437, 28–35. [Google Scholar] [CrossRef]
- Maurer, D.; Keck, R.T.; Tinsman, J.C.; Leathem, W.A. Vertical migration and mortality of benthos in dredged material—Part I: Mollusca. Mar. Environ. Res. 1981, 4, 299–319. [Google Scholar] [CrossRef]
- Murugesan, P.; Sarathy, P.P.; Muthuvelu, S.; Mahadevan, G. Diversity and Distribution of Polychaetes in Mangroves of East Coast of India. In Mangrove Ecosystem Ecology and Function; Sharma, S., Ed.; InTech: London, UK, 2018; ISBN 978-1-78984-277-7. [Google Scholar]
- Carvalho, R.; Wei, C.-L.; Rowe, G.; Schulze, A. Complex depth-related patterns in taxonomic and functional diversity of polychaetes in the Gulf of Mexico. Deep Sea Res. Part Oceanogr. Res. Pap. 2013, 80, 66–77. [Google Scholar] [CrossRef]
- Shillabeer, N.; Tapp, J.F. Improvements in the benthic fauna of the Tees estuary after a period of reduced pollution loadings. Mar. Pollut. Bull. 1989, 20, 119–123. [Google Scholar] [CrossRef]
- Maghsoudlou, A.; Momtazi, F.; Hashtroudi, M.S. Ecological Quality Status (EcoQs) of Chabahar sub-tropical bay based on multimetric macrobenthos-indexes approach: Response of bio-indexes to sediment structural/pollutant variables. Reg. Stud. Mar. Sci. 2020, 40, 101524. [Google Scholar] [CrossRef]
- Sobczyk, R.; Czortek, P.; Serigstad, B.; Pabis, K. Modelling of polychaete functional diversity: Large marine ecosystem response to multiple natural factors and human impacts on the West African continental margin. Sci. Total Environ. 2021, 792, 148075. [Google Scholar] [CrossRef]
- Qiu, J.-W.; Qian, P.-Y. Combined effects of salinity, temperature and food on early development of the polychaete Hydroides elegans. Mar. Ecol. Prog. Ser. MEPS 1997, 152, 79–88. [Google Scholar] [CrossRef]
- Freitas, R.; Pires, A.; Velez, C.; Almeida, Â.; Wrona, F.J.; Soares, A.M.V.M.; Figueira, E. The effects of salinity changes on the Polychaete Diopatra neapolitana: Impacts on regenerative capacity and biochemical markers. Aquat. Toxicol. 2015, 163, 167–176. [Google Scholar] [CrossRef]
- Thi Thu, E.V.; Rahman, M.; Phoo, W.W.; Kim, C. Salinity Effects on Growth and Survival of the Polychaete Rockworm Marphysa sanguinea (Montagu, 1813) Juveniles and Adults. J. Aquac. Res. Dev. 2019, 10, 1–7. [Google Scholar]
- Arrighetti, F.; Penchaszadeh, P. Macrobenthos–sediment relationships in a sandy bottom community off Mar del Plata, Argentina. J. Mar. Biol. Assoc. UK 2010, 90, 933–939. [Google Scholar] [CrossRef]
- Kim, S.L.; Lee, H.G.; Yu, O.H. Correlation between rocky reefs and surrounding benthic habitats: Distribution and diversity patterns of polychaetes in the macrobenthic community in the East Sea of South Korea. J. Sea Res. 2021, 174, 102083. [Google Scholar] [CrossRef]
- Martinez-Garcia, E.; Sanchez-Jerez, P.; Aguado-Giménez, F.; Ávila, P.; Guerrero, A.; Sánchez-Lizaso, J.L.; Fernandez-Gonzalez, V.; González, N.; Gairin, J.I.; Carballeira, C.; et al. A meta-analysis approach to the effects of fish farming on soft bottom polychaeta assemblages in temperate regions. Mar. Pollut. Bull. 2013, 69, 165–171. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).