Katagnymene terrestris sp. nov. (Gomontiellaceae, Cyanobacteria) Isolated from the Soil between Rocks in the Republic of Korea
Abstract
1. Introduction
2. Materials and Methods
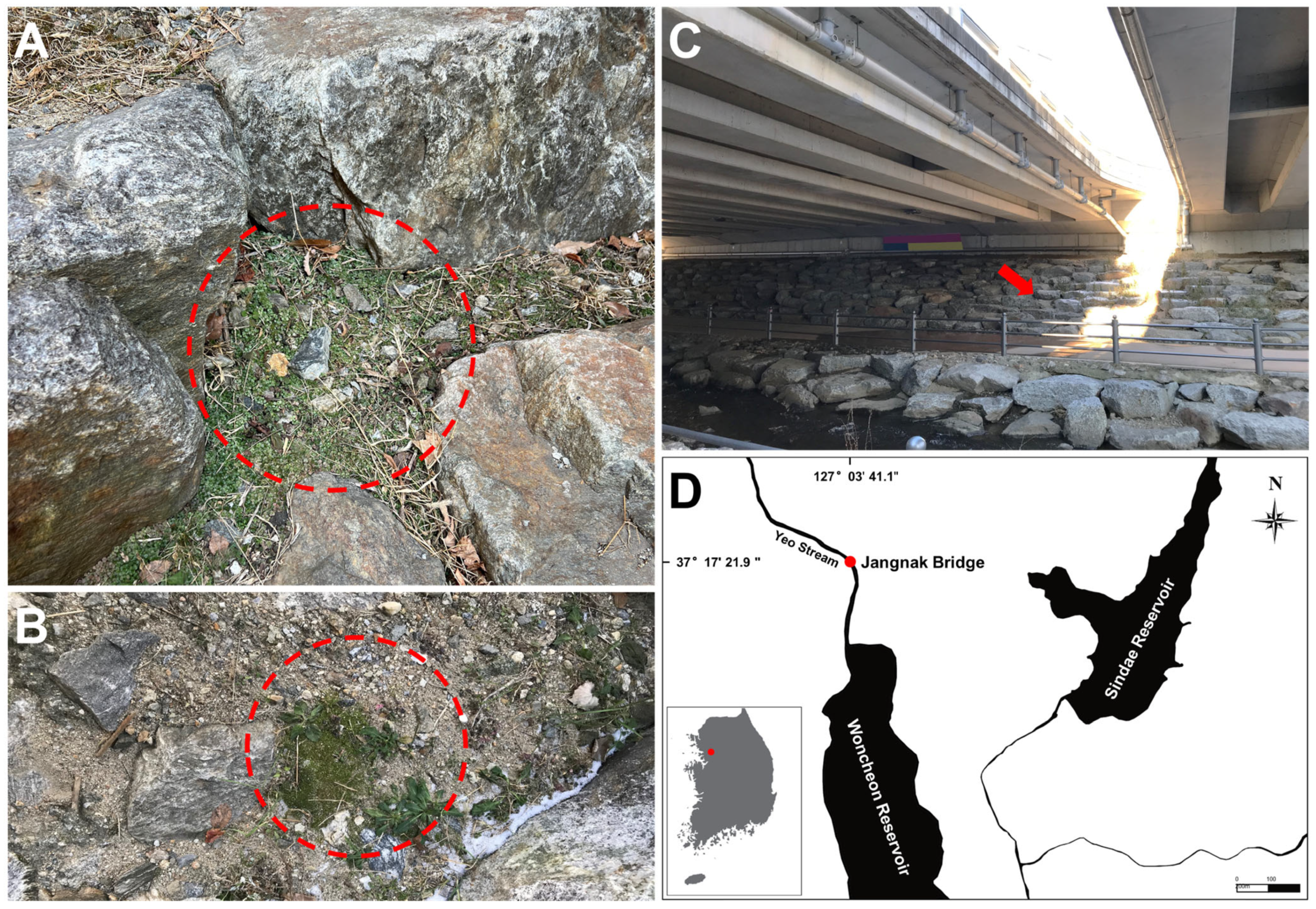
2.1. Sample Collections and Cultures
2.2. Morphological Analysis and Characterization
2.3. DNA Extraction, PCR, and Sequencing
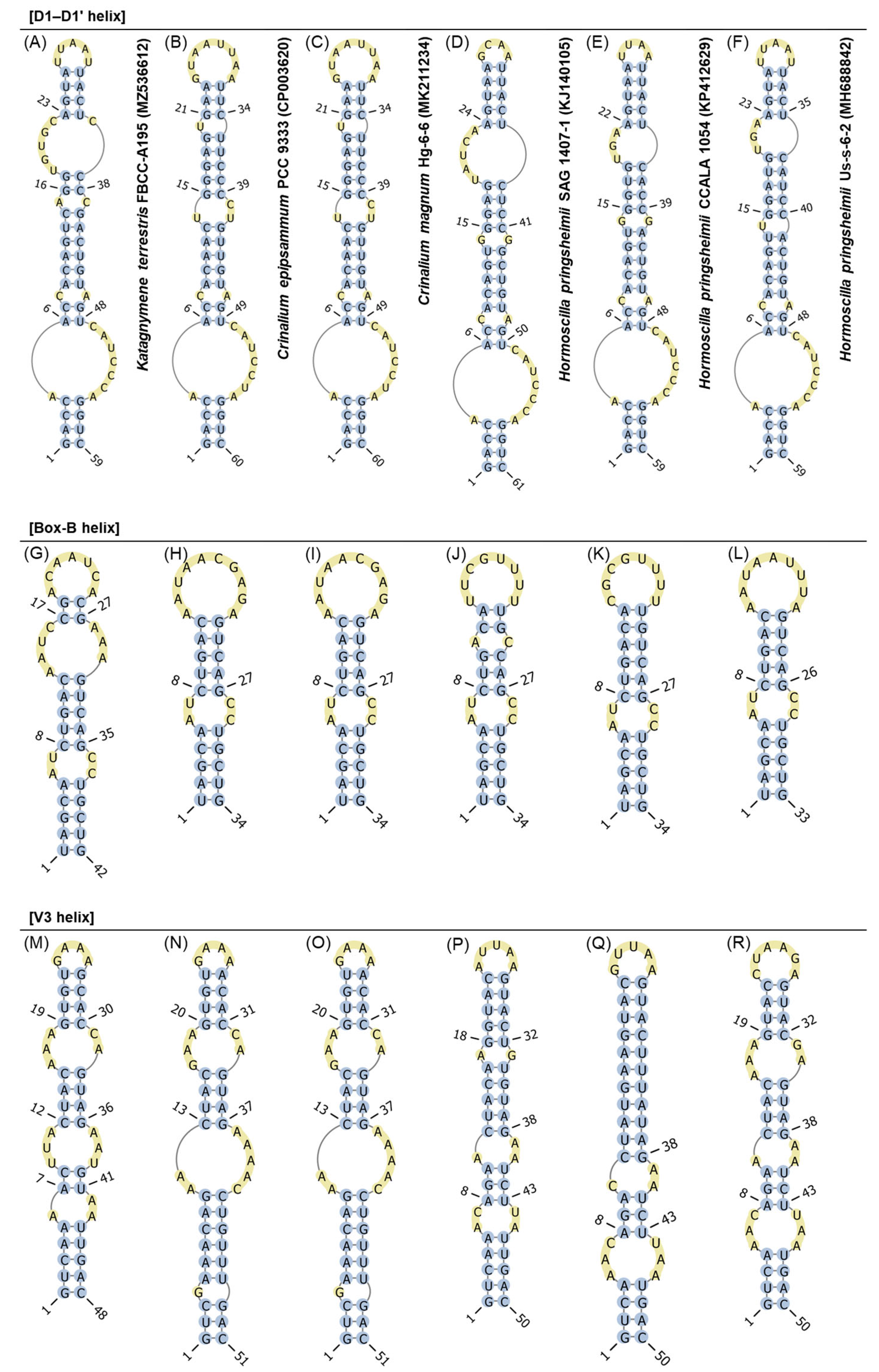
2.4. Alignment, Phylogenetic Analyses, and Secondary Structure
2.5. Alternative Topology Tests
3. Results
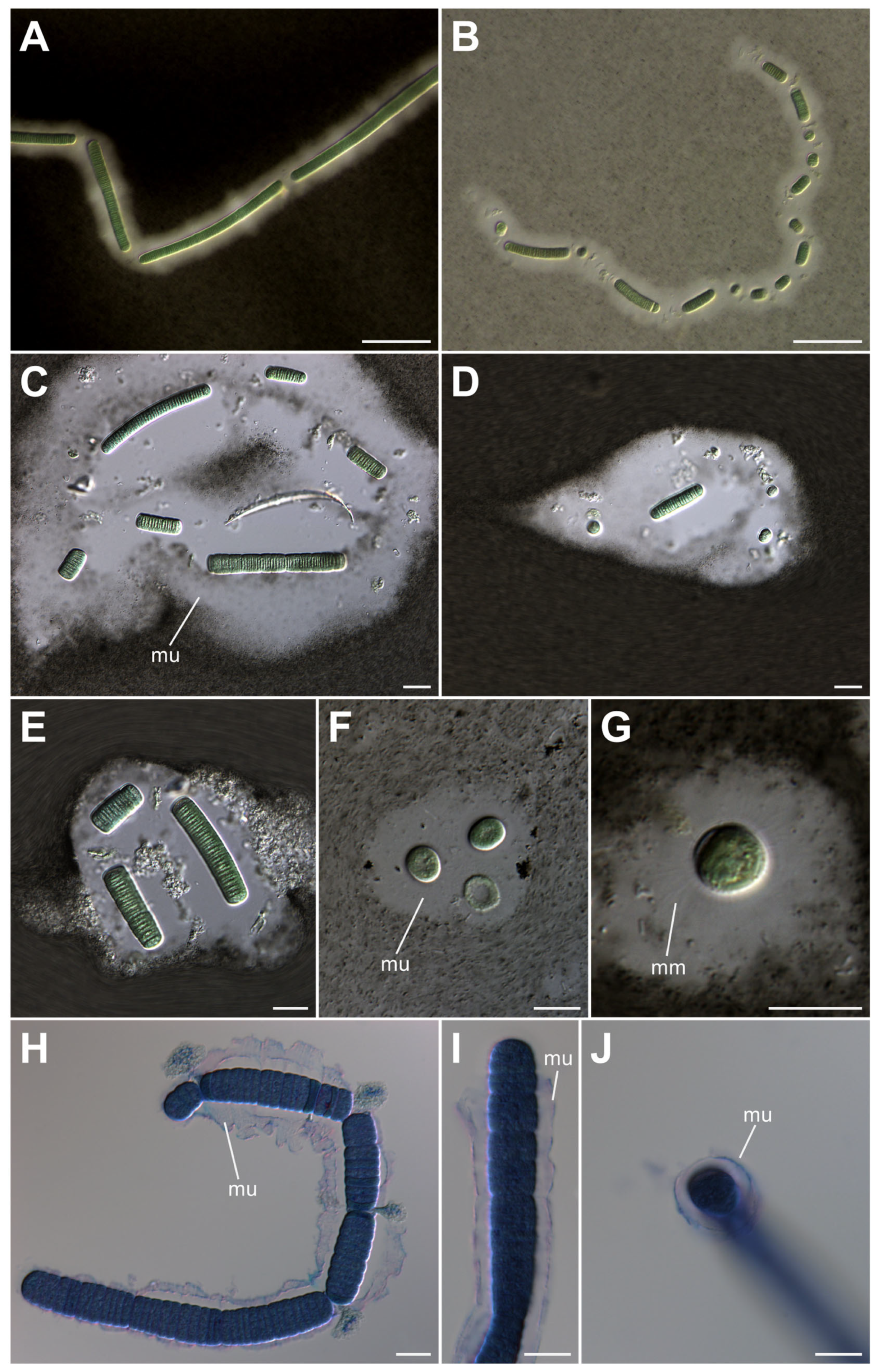
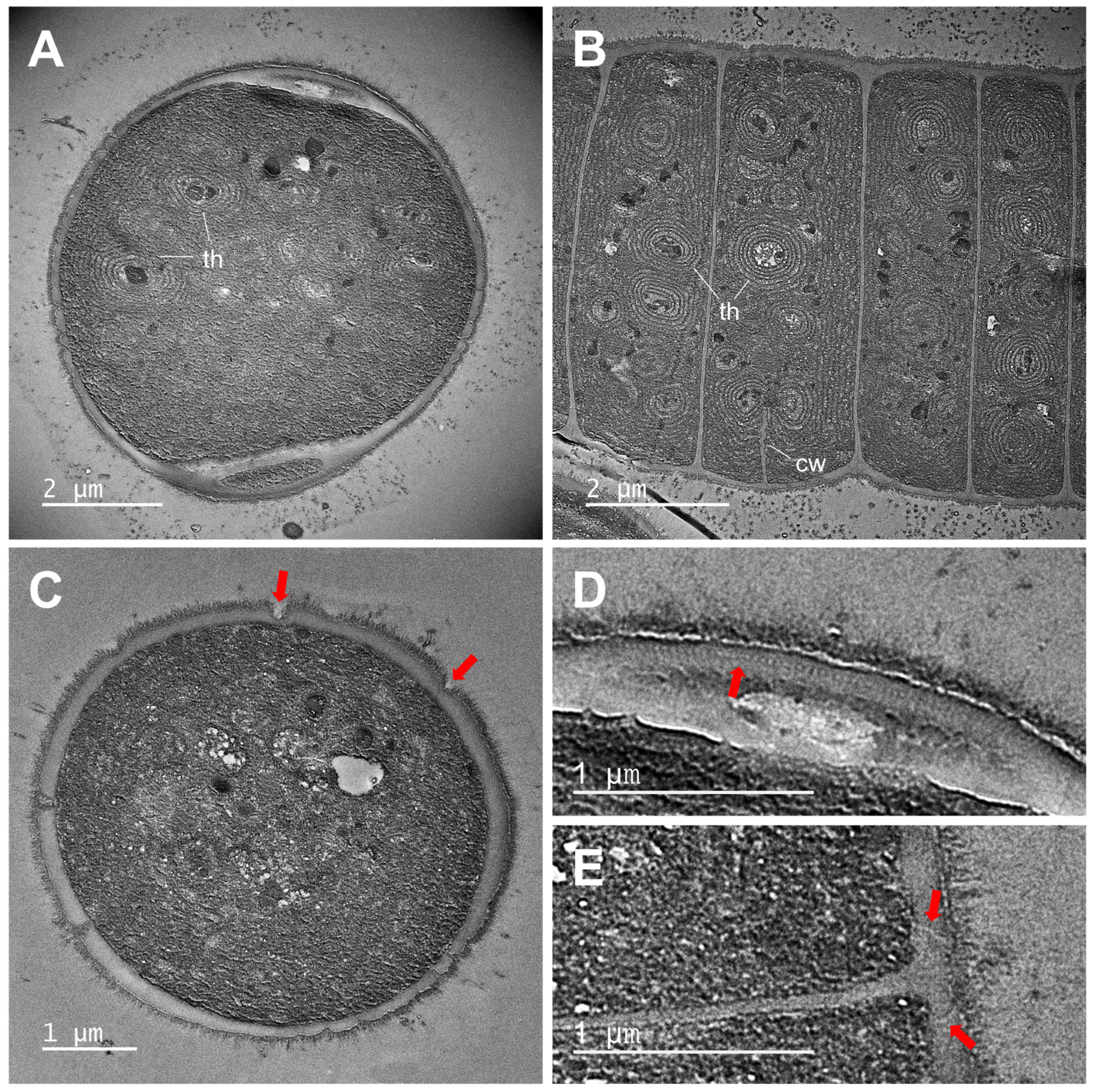
3.1. Morphological Characterization
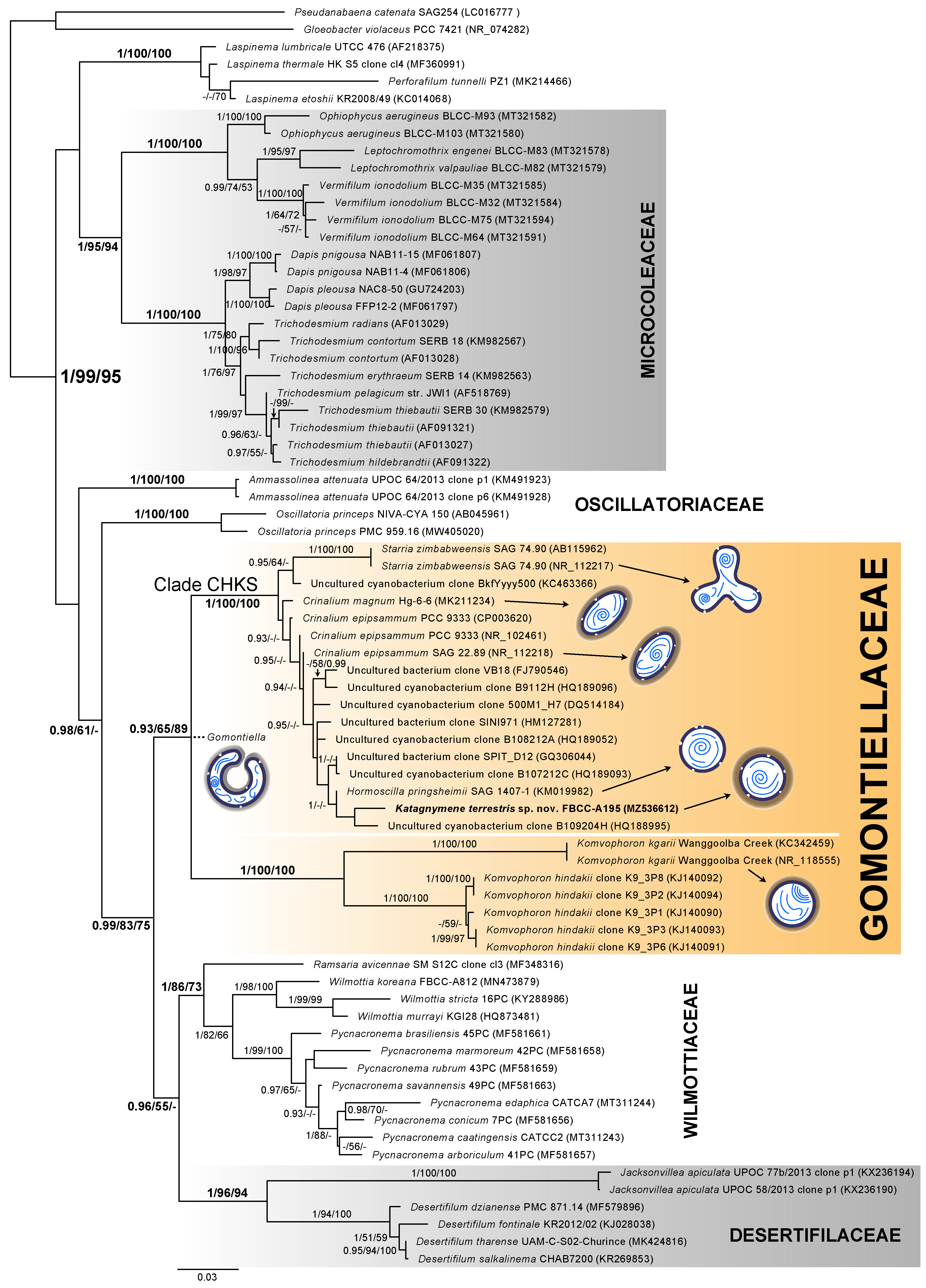
3.2. 16S rRNA Characteristics and Phylogeny
3.3. Secondary Structure of 16S–23S ITS Region
3.4. Taxonomic Treatment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bohunická, M.; Mareš, J.; Hrouzek, P.; Urajová, P.; Lukeš, M.; Šmarda, J.; Komárek, J.; Gaysina, L.A.; Strunecký, O. A combined morphological, ultrastructural, molecular, and biochemical study of the peculiar family Gomontiellaceae (Oscillatoriales) reveals a new cylindrospermopsin-producing clade of cyanobacteria. J. Phycol. 2015, 51, 1040–1054. [Google Scholar] [CrossRef]
- Mikhailyuk, T.; Vinogradova, O.; Holzinger, A.; Glaser, K.; Samolov, E.; Karsten, U. New record of the rare genus Crinalium Crow (Oscillatoriales, Cyanobacteria) from sand dunes of the Baltic Sea, Germany: Epitypification and emendation of Crinalium magnum Fritsch et John based on an integrative approach. Phytotaxa 2019, 400, 165–179. [Google Scholar] [CrossRef]
- Komárek, J.; Kaštovský, J.; Mareš, J.; Johansen, J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota Teil 2: Oscillatoriales. In Süsswasserflora von Mitteleuropa Band 19/2; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Elsevier: Heidelberg, Germany, 2005; pp. 1–759. [Google Scholar]
- Elenkin, A.A. Ob osnovnych principach sistemy Cyanophyceae. Sov. Bot. 1934, 5, 51–83. [Google Scholar]
- Anagnostidis, K.; Komárek, J. Modern approach to the classification system of cyanophytes. 3-Oscillatoriales. Arch. Hydrobiol. 1988, 80, 327–472. [Google Scholar]
- McGregor, G.B.; Sendall, B.C. Komvophoron kgarii sp. nov. (Oscillatoriales), a new epipelic cyanobacterium from subtropical eastern Australia. Phycologia 2013, 52, 472–480. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2023. Available online: www.algaebase.org (accessed on 17 July 2023).
- Strunecký, O.; Ivanova, A.P.; Mareš, J. An updated classification of cyanobacterial orders and families based on phylogenomic and polyphasic analysis. J. Phycol. 2023, 59, 12–51. [Google Scholar] [CrossRef]
- Lemmermann, E. Ergebnisse einer Reise nach dem Pacific. Abh. Naturwiss. Ver. Bremen 1899, 16, 313–398. [Google Scholar]
- Lundgren, P.; Söderbäck, E.; Singer, A.; Carpenter, E.J.; Bergman, B. Katagnymene: Characterization of a novel marine diazotroph. J. Phycol. 2001, 37, 1052–1062. [Google Scholar] [CrossRef]
- Orcutt, K.M.; Rasmussen, U.; Webb, E.A.; Waterbury, J.B.; Gundersen, K.; Bergman, B. Characterization of Trichodesmium spp. by genetic techniques. Appl. Environ. Microbiol. 2002, 68, 2236–2245. [Google Scholar]
- Lundgren, P.; Janson, S.; Jonasson, S.; Singer, A.; Bergman, B. Unveiling of novel radiations within Trichodesmium cluster by hetR gene sequence analysis. Appl. Environ. Microbiol. 2005, 71, 190–196. [Google Scholar] [CrossRef]
- Kiel, G.; Gaylarde, C.C. Bacterial diversity in biofilms on external surfaces of historic buildings in Porto Alegre. World J. Microbiol. Biotechnol. 2006, 22, 293–297. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (Order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Lee, N.-J.; Bang, S.-D.; Kim, T.; Ki, J.-S.; Lee, O.-M. Pseudoaliinostoc sejongens gen. & sp. nov. (Nostocales, Cyanobacteria) from floodplain soil of the Geum River in Korea based on polyphasic approach. Phytotaxa 2021, 479, 55–70. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jeong, M.S.; Kim, D.-Y.; Her, S.; Wie, M.-B. Zinc oxide nanoparticles induce lipoxygenase-mediated apoptosis and necrosis in human neuroblastoma SH-SY5Y cells. Neurochem. Int. 2015, 90, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-J.; Seo, Y.; Ki, J.-S.; Lee, O.-M. Morphology and molecular description of Wilmottia koreana sp. nov. (Oscillatoriales, Cyanobacteria) isolated from the Republic of Korea. Phytotaxa 2020, 447, 237–251. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A toll for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Byun, Y.; Han, K. PseudoViewer3: Generating planar drawings of large-scale RNA structures with pseudoknots. Bioinformatics 2009, 25, 1435–1437. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, H. An approximately unbiased test of phylogenetic tree selection. Syst. Biol. 2002, 51, 492–508. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, H.; Hasegawa, M. CONSEL: For assessing the confidence of phylogenetic tree selection. Bioinformatics 2001, 17, 1246–1247. [Google Scholar] [CrossRef] [PubMed]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017; Regnum Vegetabile 159; Koeltz Botanical Books: Glashütten, Germany, 2018; pp. 1–254. [Google Scholar] [CrossRef]
- Crow, W.B. Crinalium, a new genus of Cyanophyceae, and its bearing on the morphology of the group. Ann. Bot. 1927, 41, 161–166. [Google Scholar] [CrossRef]
- Zimba, P.V.; Shalygin, S.; Huang, I.S.; Momčilović, M.; Abdulla, H. A new boring toxin producer—Perforafilum tunnelli gen. & sp. nov. (Oscillatoriales, Cyanobacteria) isolated from Laguna Madre, Texas, USA. Phycologia 2021, 60, 10–24. [Google Scholar] [CrossRef]


| Species (Acc. No.) | [1] | [2] | [3] | [4] | [5] | [6] | [7] | [8] | [9] | [10] | [11] | [12] | [13] | [14] | [15] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [1] | Katagnymene terrestris FBCC-A195 (MZ536612) | |||||||||||||||
| [2] | Crinalium epipsammum SAG 22.89 (NR_112218) | 2.22 | ||||||||||||||
| [3] | Crinalium magnum Hg-6-6 (MK211234) | 2.19 | 0.97 | |||||||||||||
| [4] | Hormoscilla pringsheimii SAG 1407-1 (KM019982) | 1.71 | 0.90 | 1.09 | ||||||||||||
| [5] | Starria zimbabweënsis SAG 74.90 (NR_112217) | 3.18 | 3.46 | 3.39 | 3.25 | |||||||||||
| [6] | Komvophoron hindakii clone K9_3P8 (KJ140092) | 8.73 | 9.01 | 9.23 | 9.32 | 9.88 | ||||||||||
| [7] | Komvophoron kgarii Wanggoolba Creek (NR_118555) | 12.00 | 11.50 | 11.67 | 12.04 | 13.03 | 8.80 | |||||||||
| [8] | Trichodesmium contortum (AF013028) | 10.25 | 10.24 | 9.83 | 10.24 | 10.80 | 12.58 | 13.63 | ||||||||
| [9] | Trichodesmium erythraeum SERB-14 (KM982563) | 10.75 | 10.52 | 10.11 | 10.53 | 11.08 | 13.20 | 14.39 | 1.95 | |||||||
| [10] | Trichodesmium hildebrandtii (AF091322) | 10.62 | 10.54 | 10.27 | 10.54 | 11.17 | 12.61 | 13.97 | 2.09 | 2.38 | ||||||
| [11] | Trichodesmium pelagicum str. JWI1 (AF518769) | 13.54 | 13.41 | 13.76 | 13.74 | 13.54 | 17.83 | 16.56 | 1.52 | 2.06 | 0.51 | |||||
| [12] | Trichodesmium radians (AF013029) | 10.38 | 10.23 | 9.82 | 10.24 | 10.65 | 12.73 | 13.91 | 0.84 | 1.95 | 2.23 | 2.24 | ||||
| [13] | Trichodesmium thiebautii (AF091321) | 10.49 | 10.41 | 10.13 | 10.41 | 11.03 | 12.53 | 13.81 | 1.95 | 2.30 | 0.56 | 0.53 | 2.09 | |||
| [14] | Dapis pleousa FFP12-2 (MF061797) | 10.53 | 10.43 | 10.59 | 10.83 | 11.31 | 12.43 | 13.63 | 2.55 | 3.19 | 3.19 | 2.74 | 2.78 | 3.11 | ||
| [15] | Dapis pnigousa NAB11-15 (MF061807) | 10.12 | 9.78 | 9.95 | 10.19 | 10.74 | 12.13 | 13.62 | 2.55 | 3.10 | 3.10 | 2.88 | 3.10 | 3.03 | 1.83 | |
| p-distance (%) | ||||||||||||||||
| Genus | Cross Section | Fragmentation | Mucilage | Colony | Thylakoids |
|---|---|---|---|---|---|
| Crinalium | Flattened, Oval to cylindrical (with rounded ends) | Hormogonia | Envelope | Irregular agglomerations | Helically twisted Swirl-like |
| Gomontiella | C-shaped | Hormogonia | - | Solitary | Swirl-like or parietal |
| Hormoscilla | Cylindrical, circular | By necridia | Without | Irregular agglomerations | Swirl-like or parietal |
| Katagnymene | Cylindrical, circular | Mucilaginous separation disk or necridic cell | Envelope | Solitary | Irregularly dispersed Swirl-like structures |
| Starria | Triangular, triradiate | Without necridic cell | - | Irregular clusters | Swirl-like structures |
| Trichodesmium | Cylindrical, circular | Middle of trichomes | Envelope | Parallel or radially arranged | Radial thylakoid with gas vesicle |
| Species | Filaments | Envelopes | Trichome | Cells | Apical Cells | Ecology |
|---|---|---|---|---|---|---|
| Katagnymene terrestris FBCC-A195 | Straight or bent | 50.0–53.0 μm diffluent, thick | W. 4.0–14.6 slightly constrictions at cross walls | L. 1.35–1.54 | Rounded, truncate, convex, O | Soil |
| K. accurata | Screw-like | 2.2–2.5 (–5.5) μm | W. 9.5–11.0 slightly constrictions at cross walls | L. 1.4–2.6 | Convex, rounded, X | Freshwater |
| K. bergii | Straight | 75.0–90.0 μm | W. 20.0 not constrictions | L. 2.5–3.0 | Flattened, O | Ocean |
| K. mucigera | Straight or flexuous | - μm thick | W. 10.0–14.0 constrictions at cross walls | L. 2.5–7.0 | - | Freshwater |
| K. palustris | Slightly curved | - μm thick | W. 28.0 not constrictions | L. 2.8–3.0 | Truncate, X | Freshwater |
| K. pelagica T | Straight or bent | 93.0–100.0 μm very wide | W. 16.0–27.0 not or slightly constrictions | L. 3.0–4.0 | Rounded, O or X | Ocean |
| K. spiralis | Irregularly | 150.0–168.0 μm | W. 20.0–22.0 not constrictions | L. 3.0–4.0 | Rounded - | Ocean |
| K. spirulinoides | Regularly or loosely spirally | 17.0–18.0 | W. 2.5 - | L. - | Conical - | Freshwater |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, N.-J.; Kim, D.-H.; Yang, E.-C.; Lee, O.-M. Katagnymene terrestris sp. nov. (Gomontiellaceae, Cyanobacteria) Isolated from the Soil between Rocks in the Republic of Korea. Diversity 2023, 15, 926. https://doi.org/10.3390/d15080926
Lee N-J, Kim D-H, Yang E-C, Lee O-M. Katagnymene terrestris sp. nov. (Gomontiellaceae, Cyanobacteria) Isolated from the Soil between Rocks in the Republic of Korea. Diversity. 2023; 15(8):926. https://doi.org/10.3390/d15080926
Chicago/Turabian StyleLee, Nam-Ju, Do-Hyun Kim, Eun-Chan Yang, and Ok-Min Lee. 2023. "Katagnymene terrestris sp. nov. (Gomontiellaceae, Cyanobacteria) Isolated from the Soil between Rocks in the Republic of Korea" Diversity 15, no. 8: 926. https://doi.org/10.3390/d15080926
APA StyleLee, N.-J., Kim, D.-H., Yang, E.-C., & Lee, O.-M. (2023). Katagnymene terrestris sp. nov. (Gomontiellaceae, Cyanobacteria) Isolated from the Soil between Rocks in the Republic of Korea. Diversity, 15(8), 926. https://doi.org/10.3390/d15080926






