What Do Butterflies Tell Us about an Intermediate Disturbance in a Dry Tropical Forest Context?
Abstract
1. Introduction
2. Materials and Methods
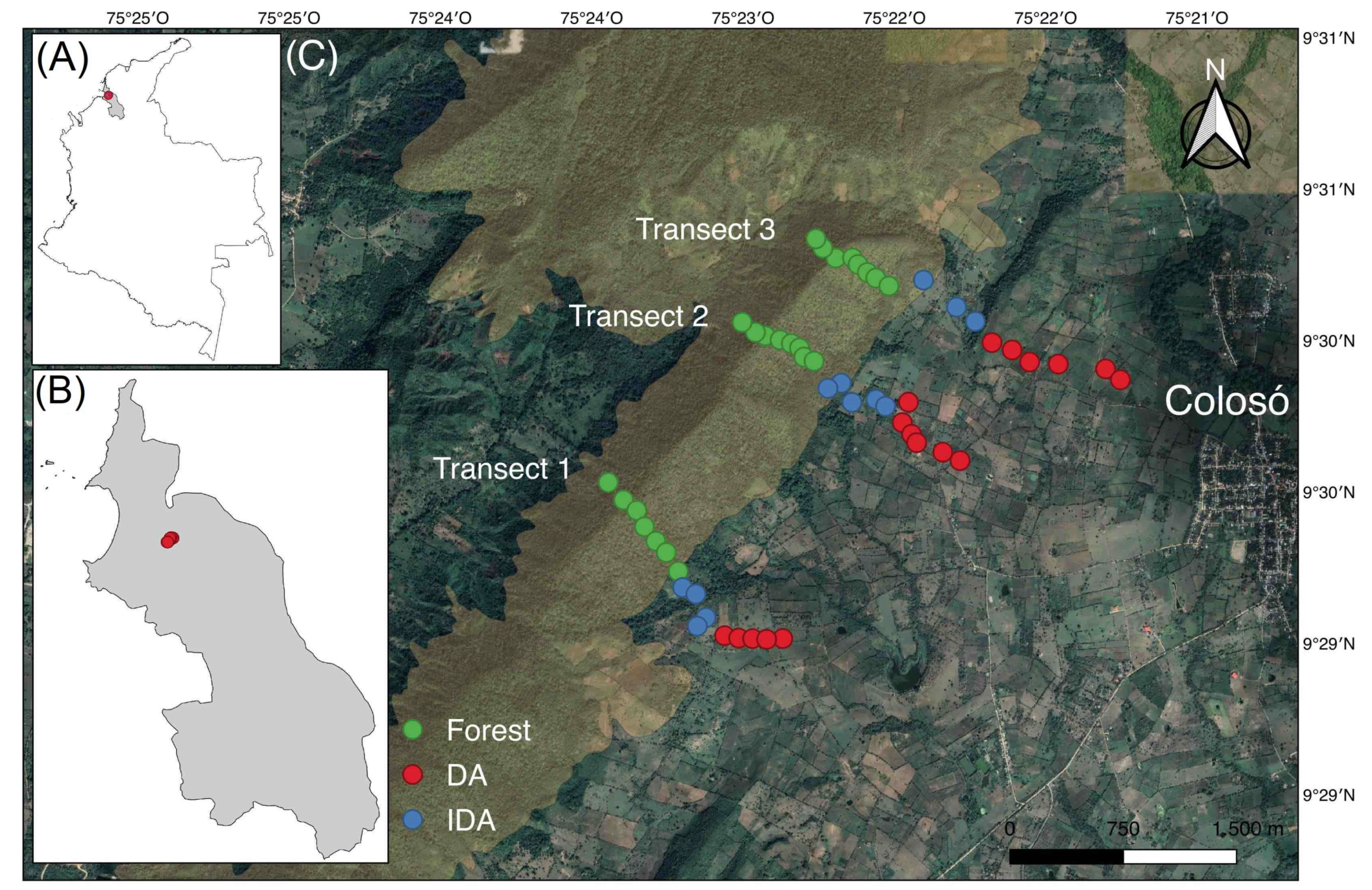
2.1. Study Area
2.2. Biological Material
2.3. Taxonomical Identification
2.4. Community Structure Analyses
3. Results
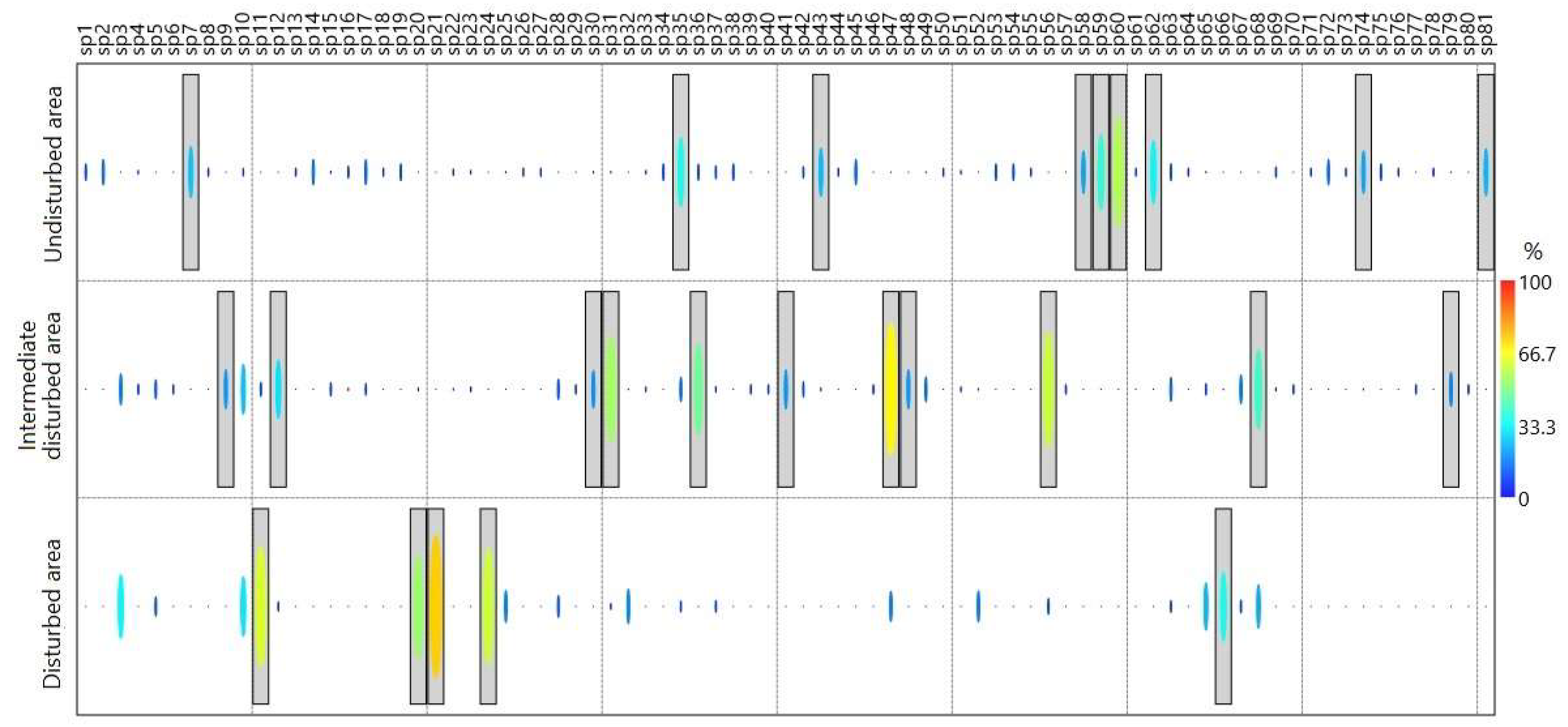
3.1. Taxonomic Composition
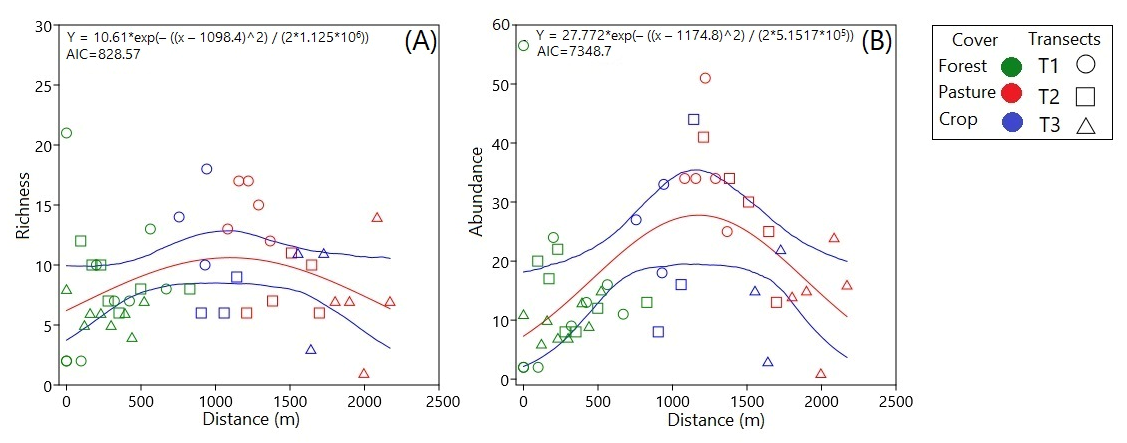
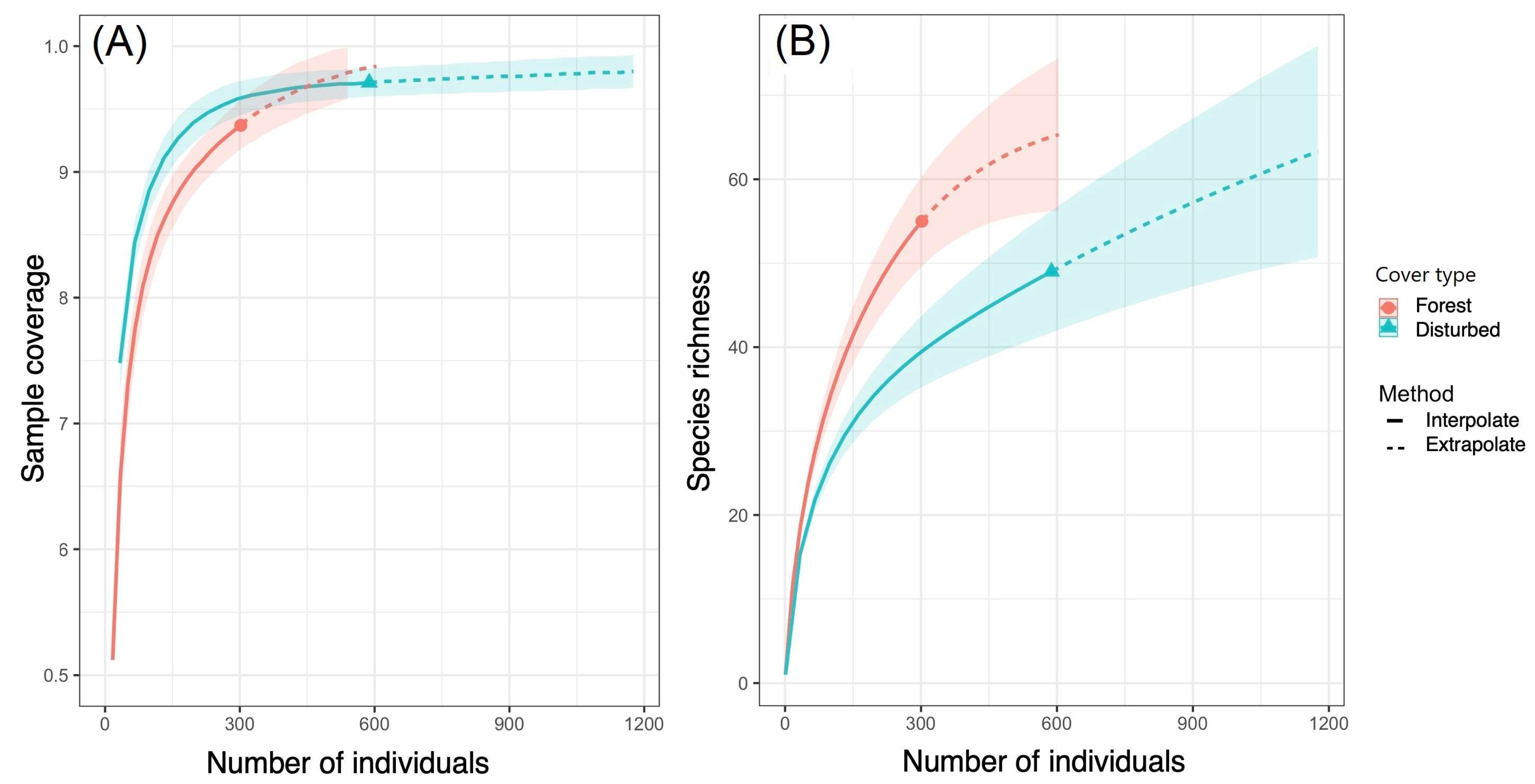
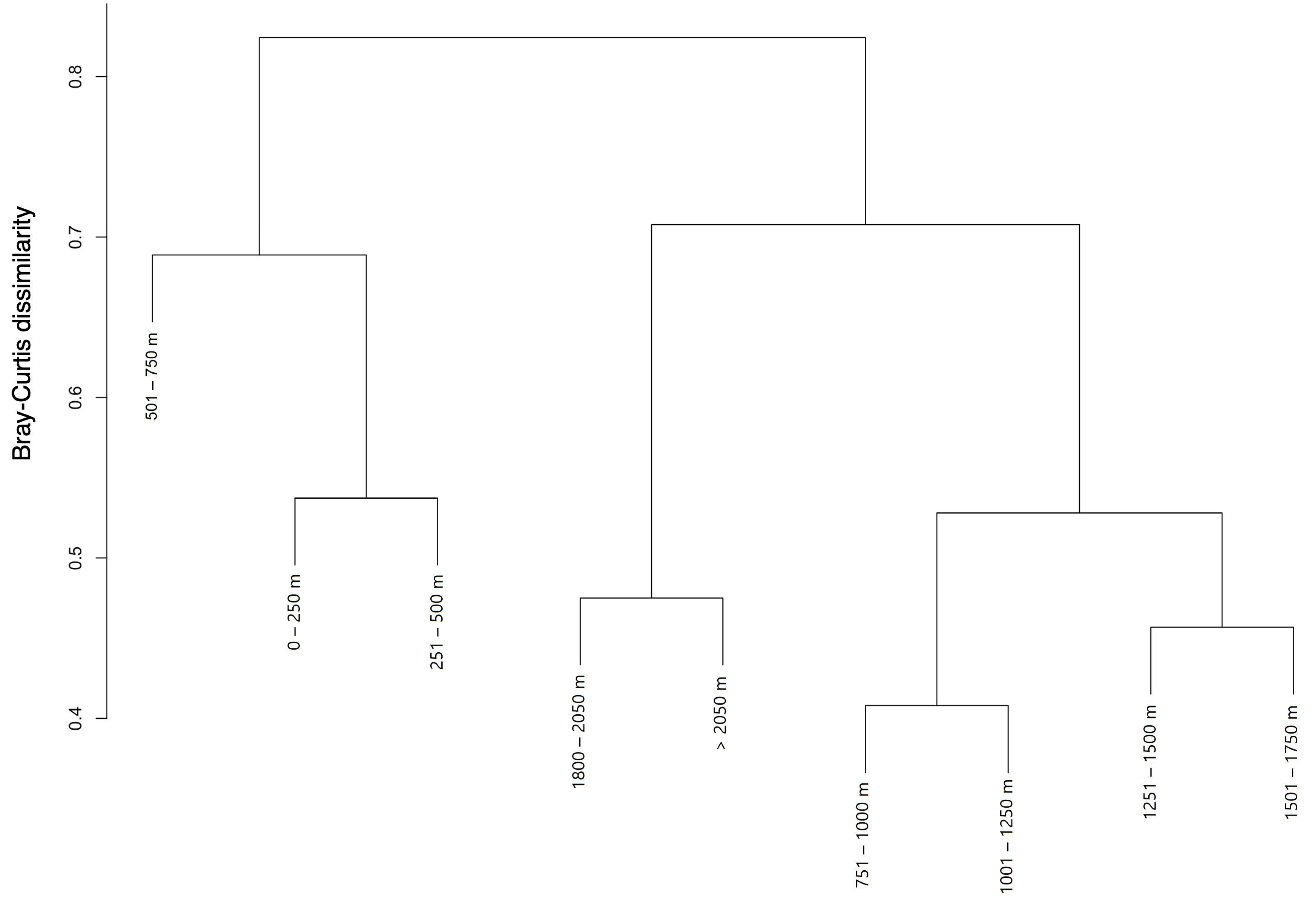
3.2. Ecological Structure of Communities
4. Discussion
4.1. Taxonomic Composition
4.2. Community Structure Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, P.; Pickett, S. Natural disturbance and patch dynamics: An introduction. In The Ecology of Natural Disturbance and Patch Dynamics; Pickett, S.T.A., White, P.S., Eds.; Academic Press: San Diego, CA, USA, 1985; pp. 3–13. [Google Scholar] [CrossRef]
- Séguin, A.; Gravel, D.; Archambault, P. Effect of disturbance regime on alpha and beta diversity of rock pools. Diversity 2014, 6, 1–17. [Google Scholar] [CrossRef]
- Socolar, J.B.; Gilroy, J.J.; Kunin, W.E.; Edwards, D.P. How should beta-diversity inform biodiversity conservation? Trends Ecol. Evol. 2016, 31, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.A.; Chase, J.M.; Crandall, R.M.; Jiménez, I. Disturbance alters beta-diversity but not the relative importance of community assembly mechanisms. J. Ecol. 2015, 103, 1291–1299. [Google Scholar] [CrossRef]
- Barlow, J.; Gardner, T.A.; Araujo, I.S.; Ávila-Pires, T.C.; Bonaldo, A.B.; Costa, J.E.; Esposito, M.C.; Ferreira, L.V.; Hawes, J.; Hernandez, M.I.M.; et al. Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc. Natl. Acad. Sci. USA 2007, 104, 18555–18560. [Google Scholar] [CrossRef]
- Dornelas, M. Disturbance and change in biodiversity. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2010, 365, 3719–3727. [Google Scholar] [CrossRef] [PubMed]
- Bendix, J.; Wiley, J.J.; Commons, M.G. Intermediate disturbance and patterns of species richness. Phys. Geogr. 2017, 38, 393–403. [Google Scholar] [CrossRef]
- Santillan, E.; Seshan, H.; Constancias, F.; Drautz-Moses, D.I.; Wuertz, S. Frequency of disturbance alters diversity, function, and underlying assembly mechanisms of complex bacterial communities. Biofilms Microbiomes 2019, 5, 8. [Google Scholar] [CrossRef]
- Svensson, J.R.; Lindegarth, M.; Jonsson, P.R.; Pavia, H. Disturbance-diversity models: What do they really predict and how are they tested? Proc. R. Soc. B Biol. Sci. 2012, 279, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Bongers, F.; Poorter, L.; Hawthorne, W.D.; Sheil, D. The intermediate disturbance hypothesis applies to tropical forests, but disturbance contributes little to tree diversity. Ecol. Lett. 2009, 12, 798–805. [Google Scholar] [CrossRef]
- Catford, J.A.; Daehler, C.C.; Murphy, H.T.; Sheppard, A.W.; Hardesty, B.D.; Westcott, D.A.; Rejmánek, M.; Bellingham, P.J.; Pergl, J.; Horvitz, C.C.; et al. The intermediate disturbance hypothesis and plant invasions: Implications for species richness and management. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 231–241. [Google Scholar] [CrossRef]
- Webb, C. Exploring the phylogenetic structure of ecological communities: An example for rain forest trees. Am. Nat. 2000, 156, 145–155. [Google Scholar] [CrossRef]
- Feng, G.; Svenning, J.-C.; Mi, X.; Jia, Q.; Rao, M.; Ren, H.; Bebber, D.P.; Ma, K. Anthropogenic disturbance shapes phylogenetic and functional tree community structure in a subtropical forest. For. Ecol. Manag. 2014, 313, 188–198. [Google Scholar] [CrossRef]
- Santo-Silva, E.E.; Santos, B.A.; Arroyo-Rodríguez, V.; Melo, F.P.L.; Faria, D.; Cazetta, E.; Mariano-Neto, E.; Hernández-Ruedas, M.A.; Tabarelli, M. Phylogenetic dimension of tree communities reveals high conservation value of disturbed tropical rain forests. Divers. Distrib. 2018, 24, 776–790. [Google Scholar] [CrossRef]
- Checa, M.F.; Donoso, D.; Levy, E.; Mena, S.; Rodriguez, J.; Willmott, K. Assembly mechanisms of neotropical butterfly communities along an environmental gradient. bioRxiv 2019. [Google Scholar] [CrossRef]
- Pellissier, L.; Alvarez, N.; Espíndola, A.; Pottier, J.; Dubuis, A.; Pradervand, J.-N.; Guisan, A. Phylogenetic alpha and beta diversities of butterfly communities correlate with climate in the western Swiss Alps. Ecography 2013, 36, 541–550. [Google Scholar] [CrossRef]
- Addo-Fordjour, P.; Osei, B.; Kpontsu, E. Butterfly community assemblages in relation to human disturbance in a tropical upland forest in Ghana, and implications for conservation. J. Insect Biodivers. 2015, 3, 1–18. [Google Scholar] [CrossRef][Green Version]
- Ghazoul, J. Impact of logging on the richness and diversity of forest butterflies in a tropical dry forest in Thailand. Biodivers. Conserv. 2002, 11, 521–541. [Google Scholar] [CrossRef]
- León-Cortés, J.L.; Caballero, U.; Miss-Barrera, I.D.; Girón-Intzin, M. Preserving butterfly diversity in an ever expanding urban landscape? A case study in the highlands of Chiapas, MÈxico. J. Insect Conserv. 2019, 23, 405–418. [Google Scholar] [CrossRef]
- Raguso, R.A.; Llorente-Bousquets, J. The Butterflies (Lepidoptera) of the Tuxtlas Mts., Veracruz, México, Revisited: Species-Richness and Habitat Disturbance. J. Res. Lepid. 1990, 29, 266622. [Google Scholar] [CrossRef]
- Spitzer, K.; Jaroš, J.; Havelka, J.; Lepš, J. Effect of small-scale disturbance on butterfly communities of an Indochinese montane rainforest. Biol. Conserv. 1997, 80, 9–15. [Google Scholar] [CrossRef]
- Mercado-Gómez, J.; Prieto-Torres, D.A.; Gonzalez, M.; Morales-Puentes, M.; Escalante, T.; Rojas, O. Climatic affinities of neotropical species of Capparaceae: An approach from ecological niche modelling and numerical ecology. Bot. J. Linn. Soc. 2020, 193, 263–275. [Google Scholar] [CrossRef]
- Janzen, D.H. Tropical dry forest: The most endangered major tropical ecosystem. In Biodiversity; Wilson, E.O., Ed.; National Academy Press: Washington, DC, USA, 1988; pp. 130–137. [Google Scholar]
- Janzen, D.H. Management of Habitat Fragments in a Tropical Dry Forest: Growth. Ann. Mo. Bot. Gard. 1988, 75, 105–116. [Google Scholar] [CrossRef]
- Vellend, M.; Verheyen, K.; Flinn, K.; Jacquemyn, H.; Kolb, A.; Van calster, H.; Peterken, H.; Graae, B.; Bellemare, J.; Honnay, O.; et al. Homogenization of forest plant communities and weakening of species–environment relationships via agricultural land use. J. Ecol. 2007, 95, 565–573. [Google Scholar] [CrossRef]
- Uehara-Prado, M.; Brown, K.S., Jr.; Freitas, A.V.L. Species richness, composition and abundance of fruit-feeding butterflies in the Brazilian Atlantic Forest: Comparison between a fragmented and a continuous landscape. Glob. Ecol. Biogeogr. 2007, 16, 43–54. [Google Scholar] [CrossRef]
- Linares-Palomino, R.; Oliveira-Filho, A.T.; Pennington, R.T. Neotropical seasonally dry forests: Diversity, endemism, and biogeography of woody plants. In Seasonally Dry Tropical Forest. ECOLOGY and Conservation; Dirzo, R., Young, H., Mooney, H., Ceballos, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–21. [Google Scholar] [CrossRef]
- Banda, K.; Delgado-Salinas, A.; Dexter, K.G.; Linares-Palomino, R.; Oliveira-Filho, A.; Prado, D.; Pullan, M.; Quintana, C.; Riina, R.; Rodríguez, G.M.; et al. Plant diversity patterns in neotropical dry forests and their conservation implications. Science 2016, 353, 1383–1387. [Google Scholar] [CrossRef]
- Pizano, C.; Garcia, H. El Bosque Seco Tropical en Colombia; Instituto de Investigacion de Recursos Biologicos Alexander von Humboldt (IAvH): Bogota, Colombia, 2014. [Google Scholar]
- García, H.; Corzo, G.; Isaac, P.; Etter, A. Distribución y estado actual de los remanentes del bioma de bosque seco tropical en Colombia: Insumos para su conservación. In El Bosque Seco Tropical en Colombia; Pizano, C., García, H., Eds.; Instituto de Investigacion de Recursos Biológicos, Alexander von Humboldt: Bogota, Colombia, 2014; Volume 90, pp. 228–251. [Google Scholar]
- Olascuaga, D.; Sánchez-Montaño, R.; Mercado-Gómez, J. Análisis de la vegetación sucesional en un fragmento de bosque seco tropical en Toluviejo-Sucre (Colombia). Colomb. For. 2016, 19, 23–40. [Google Scholar] [CrossRef]
- Mercado-Gómez, J.; Solano, C.; Hoffman, W. Recursos florales usados por dos especies de Bombus en un fragmento de bosque subandino (Pamplonita-Colombia). Recia 2017, 9, 31–37. [Google Scholar]
- Sampedro, M.A.; Gómez, F.H.; Ballut, D.G. Estado de la vegetación en localidades abandonadas por “desplazamiento”, en los montes de María Sucre, Colombia. Recia 2014, 6, 184–193. [Google Scholar] [CrossRef]
- Aguilera, M. La Mojana: Riqueza Natural y Potencial Económico; Serie de documentos de trabajo sobre economía regional Nº 48; Banco de la República: Cartagena, Colombia, 2005; p. 122. [Google Scholar]
- Herazo-Vitola, F.; Mendoza-Cifuentes, H.; Mercado-Gómez, J. Estructura y composición florística del bosque seco tropical en los Montes de María (Sucre–Colombia). Cienc. Desarro. 2017, 8, 79–90. [Google Scholar] [CrossRef]
- Elias, M.; Gompert, Z.; Jiggins, C.; Willmott, K. Mutualistic Interactions Drive Ecological Niche Convergence in a Diverse Butterfly Community. PLoS Biol. 2008, 6, 300–308. [Google Scholar] [CrossRef]
- Rydon, A. Notes on the use of butterfly traps in East Africa. J. Lepid. Soc. 1964, 18, 51–58. [Google Scholar]
- Villanueva-Espinoza, R.; Condo, F. Sinopsis de la familia Acanthaceae en el Perú. Rev. For. Del Perú 2019, 34, 21–40. [Google Scholar] [CrossRef]
- Martins, L.P.; Araujo Junior, E.d.C.; Martins, A.R.P.; Colins, M.S.; Almeida, G.C.F.; Azevedo, G.G. Butterflies of Amazon and Cerrado remnants of Maranhão, Northeast Brazil. Biota Neotrop. 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Francini, R.B.; Duarte, M.; Mielke, O.H.H.; Caldas, A.; Freitas, A.V.L. Butterflies (Lepidoptera, Papilionoidea and Hesperioidea) of the “Baixada Santista” region, coastal São Paulo, southeastern Brazil. Rev. Bras. Entomol. 2011, 55, 55–68. [Google Scholar] [CrossRef]
- García-Martínez, S.; Mercado-Gómez, J. Diversidad de briófitos en fragmentos de bosque seco tropical, Montes de María, Sucre, Colombia. Rev. Mex. Biodivers. 2017, 88, 824–831. [Google Scholar] [CrossRef]
- Lucci Freitas, A.V.; Agra Iserhard, C.; Pereira Santos, J.; Oliveira CarreiraI, J.Y.; Bandini Ribeiro, D.; Alves Melo, D.H.; Batista Rosa, A.H.; Marini-filho, O.J.; Mattos Accacio, G.; Uehara-prado, M. Studies with butterfly bait traps: An overview. Rev. Colomb. Entomol. 2014, 40, 203–212. [Google Scholar]
- Borror, D.; Delong, D.; Triplehorn, C.; Johnson, N. An Introduction of the Study of Insects; Harcourt Brace Jovanovich College: Philadelphia, PA, USA, 1982. [Google Scholar]
- Triplehorn, C.; Johnson, N. Borror and DeLong’s Lntroduction to the Study of Insects, 7th ed.; Thomson Brooks Cole Cengage Learning: Boston, MA, USA, 2005. [Google Scholar]
- Warren, A.D.; Davis, K.J.; Stangeland, E.M.; Pelham, J.P.; Willmott, K.R.; Grishin, N.V. Butterflies of America. Available online: http://www.butterfliesofamerica.com/ (accessed on 14 May 2023).
- Le Crom, J.F.; Constantino, L.M.; Salazar, J.A. Mariposas de Colombia Tomo 1 Papilionoidae; CARLEC Ltda: Bogota, Colombia, 2002. [Google Scholar]
- Le Crom, J.F.; Constantino, L.M.; Salazar, J.A. Mariposas de Colombia Tomo 2: Pieridae; CARLEC Ltda: Bogota, Colombia, 2004. [Google Scholar]
- Lamas, G. Atlas of Neotropical Lepidoptera. In CheckList: Part 4A Hesperioidea-Papilionoidea; Heppner, J.B., Ed.; Scientific Publishers: New Delhi, India.
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–57. [Google Scholar] [CrossRef]
- Hsieh, T.; Ma, K.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Colwell, R.; Chao, A.; Gotelli, N.; Shang-Yi, L.; Chang Xuan, M.; Robin, L.C.; Jhon, T.L. Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J. Plant Ecol. 2012, 5, 3–21. [Google Scholar] [CrossRef]
- Iriarte-Cárdenas, S.; García-Martínez, S.; Mercado-Gómez, J.D. Beta diversity analyses reveal distinct bryophyte community assemblages on different substrates in Neotropical seasonally dry forest fragments of the Colombian Caribbean. J. Bryol. 2022, 43, 355–367. [Google Scholar] [CrossRef]
- Gonzalez-Hidalgo, J. Las divisorias de aguas como elementos del paisaje. Geographycalia 1990, 27, 47–62. [Google Scholar] [CrossRef][Green Version]
- Akaike, H. Akaike, H. A new look at the statistical model identification. In Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1974; pp. 215–222. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, C.D.L. Betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-2. 2018. Available online: https://CRAN.R-project.org/package=vegan (accessed on 14 May 2023).
- Baselga, A. Partitioning abundance-based multiple-site dissimilarity into components: Balanced variation in abundance and abundance gradients. Methods Ecol. Evol. 2017, 8, 799–808. [Google Scholar] [CrossRef]
- Marcon, E.; Hérault, B. Entropart: An R Package to Measure and Partition Diversity. J. Stat. Softw. 2015, 67, 26. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Moreno, C.; Castillo-Campos, G.; Verdú, J. Taxonomic diversity as complementary information to assess plant species diversity in secondary vegetation and primary tropical deciduous forest. J. Veg. Sci. 2009, 20, 935–943. [Google Scholar] [CrossRef]
- Warwick, R.; Clarke, R. New ’biodiversity’ measures reveal a decrease in taxonomic distinctness with increasing stress. Marine Ecol. Prog. Ser. 1995, 129, 301–305. [Google Scholar] [CrossRef]
- Tucker, C.M.; Cadotte, M.W.; Carvalho, S.B.; Davies, T.J.; Ferrier, S.; Fritz, S.A.; Grenyer, R.; Helmus, M.R.; Jin, L.S.; Mooers, A.O.; et al. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biol. Rev. 2017, 92, 698–715. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.; Warwick, R. A taxonomic distinctness index and its statistical properties. J. Appl. Ecol. 1998, 35, 523–531. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Henao-Bañol, E.R.; Gantiva-Q, C.H. Mariposas (Lepidoptera: Hesperioidea-Papilionoidae) del bosque seco tropical (BST) en Colombia. Conociendo la diversidad en un ecosistema amenazado. Boletín Científico Cent. De Mus. Mus. De Hist. Nat. 2020, 24, 150–196. [Google Scholar] [CrossRef]
- Millan, C.; Chacon, P.; Giraldo, A. Estudio de la comunidad de Lepidopteros diurnos en zonas naturales y sistemas productivos del municipio de Caloto (Cauca, Colombia). Bol. Cient. Mus. Hist. Nat. 2009, 13, 185–195. [Google Scholar]
- Gaviria-Ortiz, F.; Henao-Bañol, E. Diversidad de mariposas diurnas (Hesperioidea-Papilionoidea) del parque natural regional el vinculo (Buga-Valle del Cauca). Boletín Científico Cent. De Mus. Mus. De Hist. Nat. 2011, 15, 115–133. [Google Scholar]
- Henao-Bañol, E.R.; Andrade-C, M. Registro del género Megaleas (Lepidoptera: Hesperiidae: Hesperiinae) para Colombia con descripción de una nueva especie. Rev. Acad. Colomb. De Cienc. Exactas 2013, 37, 29–35. [Google Scholar]
- Orozco, S.; Muriel, S.; Palacio, J. Diversidad de Lepidopteros diurnos en una área de Bosque Seco Tropical del Occidente antioqueño. Actual Biol. 2009, 90, 31–41. [Google Scholar]
- Peña, J.; Reinoso, G. Mariposas diurnas de tres fragmentos de bosque seco tropical del alto valle del Magdalena. Tolima-Colombia. Rev. De La Asoc. Colomb. De Cienc. Biológicas 2016, 1, 57–66. [Google Scholar]
- Prince-Chacon, S.; Vargas-Zapata, M.; Salazar-E, J.; Martinez-Hernandez, N. Mariposas Papilionoidea y Hesperioidea (Insecta:Lepidoptera) en dos fragmentos de Bosque Seco Tropical en Corrales de San Luis, Atlantico, Colombia. Bol. De La Soc. Entomol. Aragonesa 2011, 48, 243–252. [Google Scholar]
- Casas-Pinilla, L.; Mahecha, J.; Dumar, R.; Rios-Malaver, I. Diversidad de mariposas en un paisaje de bosque seco tropical, en la Mesa de los Santos, Santander, Colombia (Lepidoptera: Papilionoidea). SHILAP Rev. Lepid 2017, 45, 83–108. [Google Scholar]
- Vargas-Zapata, M.; Boom-Urueta, C.; Seña-Ramos, L.; Echeverry-Iglesias, A.; Martinez-Hernandez, N. Composicion vegetal, preferencias alimenticias y abundancia de Biblidinae (Lepidoptera:Nymphalidae) en un fragmento de bosque seco tropical en el departamento del Atlantico, Colombia. Acta Biol. Colomb. 2015, 20, 79–92. [Google Scholar] [CrossRef]
- Carlos, P.; Dahners, H.W. Eumaeini (Lepidoptera: Lycaenidae) del cerro San Antonio: Dinámica de la riqueza y comportamiento de Hilltopping. Rev. Colomb. Entomol. 2006, 32, 179–190. [Google Scholar]
- Montero, F.; Moreno, M.; Gutiérrez, L. Mariposas (Lepidoptera: Hesperioidea y papilionoidea) asociadas a fragmentos de bosque seco tropical en el departamento del Atlántico, Colombia. Boletín Científico Cent. Mus. Mus. Hist. Nat. 2009, 13, 157–173. [Google Scholar]
- Vargas Zapata, M.A.; Martinez Hernandez, N.J.; Gutierrez Moreno, L.C.; Prince Chacon, S.; Herrera Colon, V.; Torres Periñan, L.F. Riqueza y Abundancia de Hesperioidea y Papilionoidea(Lepidoptera) en la Reserva Natural las Delicias, Santa Marta Magdalena, Colombia. Acta Biol. Colomb. 2011, 16, 43–59. [Google Scholar]
- Diaz, J.M. Bosque Seco Tropical en Colombia; Banco de Occidente: Cali, Colombia, 2006. [Google Scholar]
- Connell, J. Diversity in tropical rain forests and coral reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Beck, J.; Schulze, C.H. Diversity of fruit-feeding butterflies (Nymphalidae) along a gradient of tropical rainforest succession in Borneo with some remarks on the problem of “pseudoreplicates”. Lepid. Sci. 2000, 51, 89–98. [Google Scholar] [CrossRef]
- Vanschoenwinkel, B.; Buschke, F.; Brendonck, L. Disturbance regime alters the impact of dispersal on alpha and beta diversity in a natural metacommunity. Ecology 2013, 94, 2547–2557. [Google Scholar] [CrossRef] [PubMed]
- Cadotte, M.W.; Tucker, C.M. Should environmental filtering be abandoned? Trends Ecol. Evol. 2017, 32, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Heino, J.; Melo, A.S.; Bini, L.M. Reconceptualising the beta diversity-environmental heterogeneity relationship in running water systems. Freshw. Biol. 2015, 60, 223–235. [Google Scholar] [CrossRef]
- Pavoine, S.; Baguette, M.; Stevens, V.M.; Leibold, M.A.; Turlure, C.; Bonsall, M.B. Life history traits, but not phylogeny, drive compositional patterns in a butterfly metacommunity. Ecology 2014, 95, 3304–3313. [Google Scholar] [CrossRef]
- Pérez Hernández, C. Distintividad taxonómica: Evaluación de la diversidad en la estructura taxonómica en los ensambles. In La Biodiversidad en un Mundo Cambiante: Fundamentos Teóricos y Metodológicos Para su Estudio; Moreno, C., Ed.; Universidad Autónoma del Estado de Hidalgo: Pachuca, Mexico; Libermex: Mexico City, Mexico, 2019; pp. 285–306. [Google Scholar]
- Angulo, D.F.; Ruiz-Sanchez, E.; Sosa, V. Niche conservatism in the Mesoamerican seasonal tropical dry forest orchid Barkeria (Orchidaceae). Evol. Ecol. 2012, 26, 991–1010. [Google Scholar] [CrossRef]
- Swenson, N.G.; Enquist, B. Opposing assembly mechanisms in a Neotropical dry forest: Implications for phylogenetic and functional community ecology. Ecology 2009, 90, 2161–2170. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Wiens, J.J.; Graham, C.H. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Syst. 2005, 36, 519–539. [Google Scholar] [CrossRef]
- Webb, C.; Ackerly, D.; McPeek, M.; Donoghue, M. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Robinson, W.H. Insects and mite pests in the human environment. In Urban Entomology; Chapman & Hall: London, UK, 1996; p. 430. [Google Scholar]
- Ramirez -Restrepo, L.; Chacon, P.; Constantino, L. Diversidad de Mariposas Diurnas (Lepidoptera: Papilionoidea y Hesperoidea) en Santiago de Cali, Valle del Cauca, Colombia. Rev. Colomb. De Entomol. 2007, 33, 54–63. [Google Scholar] [CrossRef]
- Brown JR, K. Borboletas da Serra do Japi: Diversidade, habitats, recursos alimentares e variação temporal. In Istoria Natural da Serra do Japi: Ecologia e Preservação de Uma Área Florestal no Sudeste do Brasil; Morellato, L., Ed.; Unicamp: Campinas, Brazil, 1992; pp. 142–187. [Google Scholar]
- Fagua, G. Comunidad de mariposas y artropodofauna asociada con el suelo de tres tipos de vegetación de la Serranía de Taraira (Vaupés, Colombia). Una prueba del uso de mariposas como bioindicadores. Rev. Colomb. De Entomología. 1996, 22, 143–151. [Google Scholar] [CrossRef]
- Master, L.L. Assesing treats and setting priorices for conservation. Conserv. Biol. 1991, 5, 559–563. [Google Scholar] [CrossRef]
| Adelpha fessonia ernestoi 1 | Euptoieta hegesia 3 | Mesosemia carissima 1 |
| Adelpha iphicleola 1 | Eurema agave 3 | Mestra hersilia 3 |
| Agraulis vanillae 3 | Eurema arbela 2 | Microtia elva 3 |
| Anartia amathea 3 | Eurema daira 2 | Morpho helenor 1 |
| Anartia jatrophae 3 | Eurema elathea 3 | Myscelia leucocyana 1 |
| Anteos maerula 3 | Fountainea halice 2 | Neographium anaxilaus 1 |
| Archaeoprepona demophon 1 | Glutophrissa drusilla 1 | Nica flavilla 1 |
| Archaeoprepona demophoon 1 | Hamadryas februa 2 | Parides anchises serapis 2 |
| Aricoris erostratus 3 | Hamadryas feronia 2 | Parides eurimedes mycale 1 |
| Ascia monuste 2 | Heliconius erato 2 | Parides iphidamas 2 |
| Battus polydamas 2 | Heliconius ethilla 1 | Phoebis agarithe 3 |
| Biblis hyperia 3 | Heraclides thoasnealces 3 | Phoebis argante 2 |
| Caligo brasiliensis morpheus 1 | Hermeuptychia hermes 1 | Phoebis sennae 3 |
| Callicore pitheas 1 | Historis odius 3 | Prepona laertes 2 |
| Chlosyne lacinia 2 | Hypna clytemnestra 2 | Pseudolycaena marsyas 3 |
| Chlosyne poecile 2 | Itaballia demophile 2 | Pyrisitia dina 1 |
| Cissia themis 2 | Itaballia pandosia 1 | Pyrisitia leuce 1 |
| Colobura dirce 1 | Janatella leucodesma 1 | Pyrrhogyra neaerea 1 |
| Consul fabius 1 | Juditha sp. 3 | Siderone galanthis 2 |
| Danaus eresimus 3 | Junonia sp1 3 | Siproeta stelenes 1 |
| Danaus gilippus 2 | Junonia sp2 3 | Smyrna blomfildia 1 |
| Detritivora hermodora 2 | Leptotes cassius 3 | Strymon sp. 3 |
| Doxocopa pavon theodora 2 | Libytheana carinenta 1 | Taygetis laches 1 |
| Dryadula phaetusa 3 | Lycorea halia 2 | Temenis laothoe 2 |
| Dryas iulia 2 | Marpesia chiron 3 | Thereus cithonius 3 |
| Ectima erycinoides 1 | Melanis electron 1 | Zaretis ellos 1 |
| Eunica tatila 1 | Memphis arginussa 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercado-Gómez, Y.L.; Mercado-Gómez, J.D.; Giraldo-Sánchez, C.E. What Do Butterflies Tell Us about an Intermediate Disturbance in a Dry Tropical Forest Context? Diversity 2023, 15, 927. https://doi.org/10.3390/d15080927
Mercado-Gómez YL, Mercado-Gómez JD, Giraldo-Sánchez CE. What Do Butterflies Tell Us about an Intermediate Disturbance in a Dry Tropical Forest Context? Diversity. 2023; 15(8):927. https://doi.org/10.3390/d15080927
Chicago/Turabian StyleMercado-Gómez, Yarlenis L., Jorge D. Mercado-Gómez, and Carlos E. Giraldo-Sánchez. 2023. "What Do Butterflies Tell Us about an Intermediate Disturbance in a Dry Tropical Forest Context?" Diversity 15, no. 8: 927. https://doi.org/10.3390/d15080927
APA StyleMercado-Gómez, Y. L., Mercado-Gómez, J. D., & Giraldo-Sánchez, C. E. (2023). What Do Butterflies Tell Us about an Intermediate Disturbance in a Dry Tropical Forest Context? Diversity, 15(8), 927. https://doi.org/10.3390/d15080927






