Abstract
Cyanobacteria represent a cosmopolitan group of oxyphototrophic bacteria. Although free-living cyanobacteria thriving in aquatic habitats as well as cyanobacteria in terrestrial symbiotic systems (lichens) have been extensively studied in Finland, the diversity of terrestrial rock-inhabiting cyanobacteria is overlooked. As part of an ongoing effort to study terrestrial epilithic cyanobacteria from Finland, we isolated two Pseudanabaena-like cyanobacterial strains and characterized them using a polyphasic approach. Although the two strains were firmly placed within the Pseudanabaena clade in the 16S rRNA phylogenetic analyses, relationships among species were better resolved when phylogenetic analyses were based on a concatenate alignment of 16S rRNA gene and 16S–23S Internal Transcribed Spacer (ITS) region. In addition, 16S–23S ITS percent dissimilarity proved to be more useful for species discrimination in Pseudanabaena compared to secondary structures of conserved 16S–23S ITS domains (D1–D1′, box B, V2 and V3 helices). Considering morphological, molecular and ecological information, we describe P. epilithica sp. nov. and P. suomiensis sp. nov. under the provisions of the International Code of Nomenclature for Algae, Fungi and Plants. Neither toxins nor antimicrobial metabolites were detected during LC-MS analysis or antimicrobial susceptibility testing, respectively. Lastly, our phylogenetic analyses revealed that many Pseudanabaena strains are misidentified and highlight the need for taxonomic revision in this poorly studied cyanobacterial genus.
1. Introduction
Cyanobacteria are an ancient group of photosynthetic prokaryotes with considerable morphological diversity [1]. Their long and complex evolutionary history is considered to have contributed to the successful colonization of a wide range of habitats from polar to temperate and tropical regions [1].
The taxonomy of cyanobacteria is considered complicated and has undergone several changes throughout the years. Traditionally, cyanobacteria were classified simply based on morphological traits as seen in natural and culturable material [2]. This morphology-based approach led to the classification of morphologically similar but phylogenetically distant taxonomic entities under existing cyanobacterial genera, thus resulting in significant underestimation of cyanobacterial biological diversity [3]. Advances in electron microscopy and molecular techniques strongly supported the need for taxonomic revision and led to a classification system in which genera and species were described based on a combination of morphological, ultrastructural and molecular data deriving from 16S rRNA gene and, later on, the secondary hypothetical structures of the 16S–23S Internal Transcribed Spacer (ITS) region [4,5,6,7]. Furthermore, the 16S–23S ITS percent dissimilarity has been utilized in a great number of studies to support the separation of cryptic species [8,9,10,11,12,13,14,15,16]. Based on the work of Erwin and Thacker [14], strains in the same genus that share between 0 and 3% 16S–23S ITS dissimilarity values belong to the same species, whereas percent 16S–23S ITS dissimilarity values greater than 7% are considered strong evidence of species separation. These findings have been confirmed by several previous studies [8,9,10,11,12,13,15,16]. At the same time, intermediate values of 16S–23S ITS dissimilarity, i.e., values between 3 and 7%, are considered ambiguous. In the latter case, the decision to assign two or more strains to the same or different species should be based on morphological and ecological data; see [16]. The combination of morphology with other lines of evidence, i.e., molecular information and ecology, is known as the ‘polyphasic approach’ and has resulted in the establishment of several new taxa, including Oculatella [17], Pinocchia [18], Stenomitos [19], Nodosilinea [20] and many others. Furthermore, it served as the basis for the development of a modern classification system introduced by Komárek et al. in 2014 [21], in which new taxonomic entities are described based on a combination of morphological, ecological and molecular information and the most recent cyanobacterial classification system proposed by Strunecký et al. [22] that utilizes all available genomic and 16S rRNA information and phenotypic apomorphies to provide a more stable, up to date and “ready to use” taxonomic system.
Pseudanabaena, with P. catenata as its type species, is a cosmopolitan genus of simple cyanobacteria thriving in both mesophilic and extremophilic habitats [2,23,24]. Species belonging to this genus are characterized by thin (usually <2 μm wide), usually sheathless, mostly non-motile trichomes with distinctive constrictions at the cross walls and parietal thylakoid arrangement. Cells are usually cylindrical, sometimes almost barrel-shaped, and longer than they are wide. Apical cells are neither differentiated nor characterized by calyptra or thickened cell walls. Reproduction is carried out by the production of hormogonia without the help of necridic cells. The shape of the apical cell and presence of aerotopes further divide the genus into three subgenera, i.e., Illyonema, Skujanema and Pseudanabaena [2]. Previous studies have recognized that Pseudanabaena most likely represents a polyphyletic genus [2,18]. However, no extensive revision has been carried out yet for this genus. The only genera separated from Pseudanabaena by applying a polyphasic approach workflow are Pinocchia [18], Stenomitos [19] and Thalassoporum [25]. To date, there are more than 40 taxonomically accepted species in Pseudanabaena, but only three of them, i.e., P. foetida, P. yagii and P. cinerea, have been described based on a polyphasic approach [2,23,26,27,28]. Interestingly, these newly established species have been isolated from Japanese waterbodies where they produced 2-methylisoborneol (2-MIB), an odorous metabolite that affects the quality of drinking water [26,27,28]. Apart from P. foetida, P. yagii and P. cinerea, other Pseudanabaena strains thriving in aquatic habitats have been found to produce microcystins [29] as well as bioactive metabolites with anticrustacean [30] and anticancer [31,32] activities. Furthermore, phycoerythrin-rich cells of P. tenuis were able to protect renal cells against mercury-induced poisoning [33]. Recently, several fractions of “Pseudanabaena galeata” CCNP1313 obtained through liquid chromatography exhibited strong inhibitory activity against SARS-CoV-2 papain-like protease, a molecule that is considered a promising drug target due to its essential role in the suppression of host innate immune response and viral replication [34].
In this study, two new free-living Pseudanabaena species isolated from Finnish terrestrial epilithic habitats are studied using a combination of morphological, molecular and ecological data and described under the provisions of the International Code of Nomenclature for Algae, Fungi and Plants [35]. Furthermore, the ability of the two strains to produce toxins as well as antimicrobial metabolites is assessed by liquid chromatography and antimicrobial susceptibility testing, respectively.
2. Materials and Methods
2.1. Microbial Strains, Microbiological Media and Growth Conditions
2.1.1. Origin of Cyanobacterial Strains, Isolation and Maintenance
Environmental samples were collected from wet and dry rocks near Kuhakoski waterfall (KK) and Karkkila forest area (KAR), respectively. Samplings were carried out using sterile scalpels and forceps. A portion of the collected material was fixed with an aquatic solution of formaldehyde at a final concentration of 2.5% and was used for comparison reasons. The remaining part was placed in sterile 50 mL falcon tubes containing 30 mL sterile Z8 liquid medium [36]. Environmental samples were allowed to grow for 1–3 months under daylight (northwest-facing window) at 23 °C. After this period, single filaments of two Pseudanabaena-like morphotypes (KK-SYN4 and KAR-SYN4) were isolated on Z8 agarose-solidified media according to previously described methods [37]. The strains were then transferred to 200 mL Erlenmeyer flasks containing 100 mL of Z8 medium and maintained at 18 °C under constant illumination with white fluorescent light (10–12 μmol of photons s−1 m−2). For the bioactivity assays, strains were grown in 5 L Erlenmeyer flasks containing 2.5 L of Z8 liquid media under constant illumination of 20–25 μmol of photons s−1 m−2 and permanent sterile aeration at 18 °C for 30 days. Cells were harvested by centrifugation at 7000× g for 10 min at room temperature (RT) and were stored at −20 °C until lyophilization.
2.1.2. Bacteria and Fungi
All bacterial and fungal strains used in this study were obtained from HAMBI (University of Helsinki, Department of Microbiology) microbial culture collection (https://www.helsinki.fi/en/infrastructures/biodiversity-collections/infrastructures/microbial-domain-biological-resource-centre-hambi (accessed on 17 May 2019)). These included the non-antibiotic-resistant strains Staphylococcus aureus HAMBI 66, Enterococcus faecium HAMBI 1821, Bacillus cereus HAMBI 1881, Micrococcus luteus HAMBI 2688, Pseudomonas aeruginosa HAMBI 25, Acinetobacter baumannii HAMBI 1760, Enterobacter aerogenes HAMBI 1898, Salmonella enterica HAMBI 2331, Candida albicans HAMBI 484, C. krusei HAMBI 486, C. parapsilosis HAMBI 487, Cryptococcus neoformans HAMBI 488, Mucor sp. HAMBI 831 and Aspergillus flavus HAMBI 829. All microbial strains were grown as described previously [38].
2.2. Morphological Evaluation and Type Material Preparation
2.2.1. Microscopy
The cyanobacterial strains Pseudanabaena sp. KK-SYN4 and Pseudanabaena sp. KAR-SYN4 were studied under a Zeiss Axioskop 2 plus Light Microscope (Jena, Germany). Light photomicrographs were acquired using an Axiocam 305 color digital camera and processed using ZEISS ZEN 3.1 (blue edition) software. We analyzed the shape and size of trichomes and vegetative cells in at least 20 trichomes from each strain in both isolated morphotypes and formaldehyde-fixed samples, summarizing 50 cells. Other features, including the presence/absence of calyptra or thickened cell walls, aerotopes, necridic cells as well as hormogonia, were also recorded and compared to data available for all known Pseudanabaena species [2,23,24,26,27,28,39,40].
2.2.2. Type Material Preparation
Type material was prepared for each strain by filtering (vacuum filtration) an active culture (exponential phase) through a 47 mm Whatman glass microfiber filter (GE Healthcare Life Sciences, Chalfont St Giles, UK) and allowing the filter to air-dry in a sterile petri dish at RT; a total of two filters were prepared for each strain. The filters were then placed in acid-free herbarium envelopes and deposited in the Finnish Museum of Natural History (LUOMUS) under the accession numbers H6124570 and H6124571.
Reference strains have been deposited in HAMBI/UHCC (University of Helsinki, Department of Microbiology) microbial culture collection under the accession numbers UHCC 1008 (strain KK-SYN4) and UHCC 1009 (strain KAR-SYN4).
2.3. Molecular Characterization
2.3.1. DNA Extraction, PCR Amplification and Sequencing
Total genomic DNA from Pseudanabaena sp. UHCC 1008 (KK-SYN4) and Pseudanabaena sp. UHCC 1009 (KAR-SYN4) was extracted using the DNeasy PowerSoil Pro kit (Qiagen, GmbH, Hilden, Germany) according to the manufacturer’s protocol with some modifications to increase DNA yield and remove impurities (see below). All step numbers mentioned below correspond to the step numbers seen in the manufacturer’s protocol. During steps 3 and 4, PowerBead tubes were secured on a flat-bed vortex mixer and were vortexed at full speed for 15 min instead of 10 min. At steps 7 and 10, samples were incubated on ice for 15 min instead of 5 min. Having completed step 18, samples remained at RT for 10 min to ensure that residual ethanol included in Solution C5 was completely removed. Lastly, during step 19, samples were incubated for 10 min at RT before final centrifugation.
Partial 16S and the 16S–23S internal transcribed spacer (ITS) region were amplified using the cyanobacteria-specific primers 16S106F 5′-CGGACGGGTGAGTAACGCGTGA-3′ after Nübel et al. [41] and 23S30R 5′-CTTCGCCTCTGTGTGCCTAGGT-3′ after Taton et al. [42]. The PCR reactions were prepared in 50 μL aliquots containing 1X Q5 reaction buffer (New England Biolabs, Ipswich, MA, USA), 0.2 mM of each deoxynucleotide triphosphates (dNTPs; Thermo Scientific, Waltham, MA, USA), 0.5 μΜ of each of the primers (Sigma-Aldrich, St. Louis, MI, USA), 0.02 U of Q5 High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA, USA) and 70–80 ng of genomic DNA, determined using NanoDrop One/One Microvolume UV-Vis Spectrophotometer (Thermo Scientific). Amplifications were run in a SimpliAmp Thermal Cycler (ThermoFisher Scientific). The profile used included an initial denaturation step at 98 °C for 30 s, followed by 35 cycles of denaturation at 98 °C for 10 s, annealing at 65 °C for 30 s and extension at 72 °C for 2 min. A final extension step was carried out at 72 °C for 2 min.
PCR products were analyzed on a 0.8% (w/v) agarose gel stained with EtBr solution (0.625 mg mL−1; Thermo Scientific, Waltham, MA, USA). PCR products were purified using the NucleoSpin Gel and PCR Clean-up kit (Macherey-Nagel, Hoerdt, France) and sequenced using BigDye Terminator v3.1 Cycle Sequencing Kit on an ABI 3730xl DNA analyzer (Applied Biosystems, Waltham, MA, USA). The primers used for Sanger sequencing included 16S106F and 23S30R; 359F and 781R [41]; 979F and 1092R [43]; P17 [44], ILE373R, which is complementary to P17 [45]; and P5 and P6 sensu Boyer et al. [46] (i.e., P14 and P15 from Wilmotte et al. [44]). All primer sequences are presented in Table S1. Sequencing was carried out at the Sequencing Unit of the Institute for Molecular Medicine Finland (FIMM) Technology Center. The generated sequences were edited with CodonCode Aligner v.9.0.1 (CodonCode Corporation, Centerville, MA, USA) and consensus sequences were obtained. All bases had Phred quality score higher than 20. The sequences were checked for chimeras using DECIPHER v.1 [47] (http://www2.decipher.codes/FindChimeras.html; accessed on 15 June 2022) and deposited in GenBank database under the accession numbers OP594631–OP594632 for 16S rRNA gene and OP595723–OP595724 for 16S–23S ITS region.
2.3.2. Phylogenetic Analysis
The newly obtained 16S rRNA nucleotide sequences were compared to 16S rRNA nucleotide sequences available in the National Center for Biotechnology Information (NCBI) database using the BLAST tool [48]. For 16S rRNA phylogenetic analysis, the dataset was composed of 243 OTUs, including the newly obtained sequences and the outgroup. All selected sequences were at least 1100 bp long. Unfortunately, the only 16S rRNA gene nucleotide sequence of Pseudanabaena rutilus-viridis CCPC 697 available in NCBI was too short (685 bp) to be included in the phylogenetic analyses. Synechococcus elongatus PCC 6301 (NR_074309) was used as the outgroup. A combined analysis of 16S rRNA and 16S–23S ITS region was carried out to gain more insights into the phylogenetic relationships of the newly isolated strains and other Pseudanabaena species. The dataset for this analysis included 38 nucleotide sequences of the Pseudanabaena clade, for which 16S–23S ITS regions were available and contained both tRNA genes and Thalassoporum komareki TAU-MAC 1515. All multiple sequence alignments (16S rRNA and combined phylogenetic analyses) were performed using ClustalX v2.1 [49]. The Akaike Information Criterion [50] as implemented in jModeltest v.2.1.10 [51] was used to select the best-fit models of nucleotide substitution. The GTR + I + G model was chosen for phylogenetic analysis of 16S rRNA. For the combined analysis, GTR + I + G was selected for both 16S rRNA gene and 16S–23S ITS region. Prior to the combined phylogenetic analysis, the aligned 16S–23S ITS dataset was concatenated to the associated 16S rRNA gene using Concatenator v.0.2.1 [52]. Following this step, the alignment was partitioned, and the abovementioned nucleotide substitution model was applied to each partition. Bayesian Inference (BI) analysis was carried out in MrBayes v3.2.6 [53]. Two runs with four chains each (one cold and three heated chains) were run simultaneously for 17 × 106 Markov Chain Monte Carlo (MCMC) generations starting with a random tree for the 16S rRNA analysis, whereas, for the combined analysis, the two runs were run for 10 × 106 MCMC generations. Sampling frequency was every 1000th generation. Furthermore, the average standard deviation between the two MCMC runs was below 0.01, and the potential scale reduction factor (PSRF) value for all estimated parameters was 1.00 in both analyses. The first 25% of trees were discarded as burn-in, and a 50% majority rule consensus tree was calculated including posterior probabilities. ML analyses for both the 16S rRNA gene and combined analyses were performed using the IQ-Tree v1.6.12 [54] with 1000 bootstrap replicates. The phylogenetic trees were visualized using FigTree v1.4.2 software [55] and re-drawn in Inkscape v0.48.4 software [56]. All taxa used in the phylogenetic analyses and their GenBank accession numbers are presented in Table S2 (16S rRNA) and Table S3 (combined analysis). For the 16S rRNA dataset, a similarity matrix (p-distance) was generated in MEGA 7 [57] using the Kimura-2 parameter model [58]. The 16S–23S ITS aligned dataset used for the combined analysis was also used for calculating the percent dissimilarity using the Kimura-2 parameter model as described above.
2.3.3. Study of 16S–23S Internal Transcribed Spacer (ITS) Region
Identification of conserved 16S–23S ITS regions was carried out manually according to Iteman et al. [45], whereas V3 helix was identified based on Bohunická et al. [59]. The online tool tRNAscan-SE 2.0 (http://lowelab.ucsc.edu/tRNAscan-SE/ (accessed on 2 September 2022) [60]) was used to identify tRNAs. Hypothetical secondary structures of D1–D1′, Box B, V2 and V3 were predicted using Mfold web server [61] with the default settings except for the structure-drawing mode, which was set to ″Untangle with loop fix”, choosing secondary structures with minimum free energy (ΔG). The obtained structures were compared with the available homologous structures of Pseudanabaena species that were previously described based on a polyphasic approach, P. catenata, as well as strains that shared >99% 16S rRNA sequence similarity with our strains and contained both tRNAIle and tRNAAla. All figures were re-drawn in Adobe Photoshop v.23.2.0 (Adobe Systems Inc., San Jose, CA, USA). The length (bp) of each region was also recorded. Similarly to 16S rRNA phylogenetic analysis, the 16S–23S ITS sequence of Pseudanabaena rutilus-viridis CCPC 697 could not be used for the determination of secondary structures as it lacks more than half of D1–D1′ helix.
2.4. Biochemical Evaluation
2.4.1. Antimicrobial Susceptibility Tests
Extract preparation and antimicrobial susceptibility assays were carried out as described by Christodoulou et al. [38].
2.4.2. Extraction of Phycobiliproteins
Cultures growing in 100 mL Z8 liquid medium were harvested during the exponential phase of growth by centrifugation at 5000× g for 5 min at room temperature (RT) and stored at −20 °C until lyophilization. Phycobiliproteins were then extracted from freeze-dried biomass as described by Zavřel and co-workers [62]. Absorbance spectra were recorded in the range of 350 to 750 nm using a UV-2401PC UV/Vis spectrophotometer and UVProbe v.2.7 software (Shimadzu Corporation, Kyoto, Japan).
2.4.3. Liquid Chromatography
For bioactive metabolite and toxin analysis, 40 mg of freeze-dried biomass from each strain was transferred in two (series A and B) 2 mL screw-cap tubes (20 mg per tube) together with glass beads (0.5 mm diameter, Scientific Industries Inc, Bohemia, New York, NY, USA). Following this step, 1 mL of 100% LC/MS grade methanol (MeOH; Merck, Darmstadt, Germany) was added to series A, and 1 mL of 0.1% trifluoroacetic acid (TFA; Merck, Darmstadt, Germany) in MeOH was added to series B samples. Cells were disrupted using a FastPrep-24® cell disrupter (MP Biomedicals, Santa Ana, CA, USA) two times for 20 s at a speed of 6.5 m s−1 at RT. Intracellular bioactive compounds were obtained after centrifugation at 10,000× g for 5 min at RT. Supernatants were then transferred in 4 mL amber glass vials. A total of 300 μL of series A and B samples were filtered and used for further analysis. A third set of samples (series C) was obtained after mixing three volumes of 100% MeOH-processed samples (series A) with 1 volume of acetonitrile (ACN; VWR Chemicals, Darmstadt, Germany). Series C samples were then filtered, and all samples were further studied by means of liquid chromatography, as described below.
All analyses were conducted using an Acquity UPLC system (Waters, Manchester, UK) using two different liquid chromatography columns, aiming to identify both hydrophobic and hydrophilic compounds, including cyanotoxins. Series A and C samples (100% MeOH, MeOH-ACN) were run as described in Christodoulou et al. [38]. Series B samples (0.1% TFA in MeOH) were run using an Acquity UPLC BEH amide Column (100 × 2.1 mm, 1.7 µm, 130 Å, Waters, Manchester, UK). The two solvents used in this analysis were 0.2% ammonium formate (Sigma-Aldrich, St. Louis, MI, USA) in MQ water as solvent A and HPLC grade acetonitrile (ACN) 100% (VWR Chemicals, Darmstadt, Germany) as solvent B. The strength of solvent B was decreased from 90% to 60% in 9 min and was held at 60% for 1 min. Injection volume and UPLC running parameters were the same as above.
Mass spectra were recorded with a Waters Synapt G2-Si mass spectrometer (Waters, Manchester, UK), and measurements were performed using negative and positive electrospray ionization (ESI) in resolution mode. Ions were scanned in a range from m/z 50 to 2000, and UV detector range from 210 to 800 nm. Mass spectrometry (MS) analyses were performed with scan times of 0.1 s. Capillary voltage was 2.5 kV, source temperature was 120 °C, sampling cone was 40.0, source offset was 80.0, desolvation temperature was 600 °C, desolvation gas flow was 1000 L h−1, and nebulizer gas flow was 6.5 bar. Leucine-encephalin was used as a lock mass reference compound, and calibration was carried out with sodium formate and Ultramark 1621®. UV data were also collected from 210 to 800 nm. Results were compared against all known bioactive metabolites included in CyanometDB [63].
3. Results
3.1. Strain Morphology and Habitat
Strain UHCC 1008 was collected from a wet rock in a man-made cavity that was not exposed to direct sun light. The collected biofilm was very thin and purplish brown to olive-green in color. Microscopic evaluation of the preserved (i.e., formaldehyde-fixed) material revealed a group of sheathless Pseudanabaena-like cyanobacteria of purplish to purplish brown color mixed with other thin filamentous cyanobacteria as well as Microcoleus-like strains and unicellular green algae (Figure 1a,b). The Pseudanabaena-like trichomes were long, densely entangled and their width ranged from 1.7 to 1.9 μm. Furthermore, the trichomes, had cells that varied in length and were characterized by the presence of refractive granules. Similar refractive granules were also observed at the tip of their apical cells. In addition, trichomes lacked necridic cells (see Section 3.5 for detailed description). The morphological evaluation of the isolated UHCC 1008 morphotype growing on solid and liquid media (see Section 3.5) revealed that the strain retained all characteristics observed in the formaldehyde-fixed material, including the purplish brown coloration, trichome and cell morphology. Furthermore, it revealed that UHCC 1008 was motile. On dried solid media (>3 months), the color of the aged colonies turned from purplish brown to dark brown, but all other morphological features remained the same. Similar findings were observed for aged trichomes (2–3 months old) growing in liquid media. Strain UHCC 1009 was collected from a dry rock exposed to direct sunlight. The sample had the form of a greenish to olive-green to brownish-colored patina growing on the rock surface. The sample was dominated by ensheathed morphotypes, most of which were rich in phycoerythrin (Figure 1c,d). Other thin, sheathless morphotypes, including a Pseudanabaena-like cyanobacterium, were less frequently observed. Further study of the Pseudanabaena-like morphotype revealed that it was characterized by short and straight trichomes that were brown in color. The trichomes were 1.6 to 1.9 μm wide, and cells were always longer than wide. Many trichomes were also characterized by the presence of one or two small refractive granules, mostly at the tip of the apical cells. The microscopic assessment of the isolated morphotype confirmed the morphological features observed in the formaldehyde-fixed sample. In addition, it showed that the isolated UHCC 1009 morphotype had brown-colored cells and exhibited motility. Furthermore, when trichomes of UHCC 1009 grew on solid media they were arranged in parallel, thus forming small fascicles. Apart from changes observed in thallus coloration (greenish to olive-green) in aged cultures (>3 months) growing on solid or liquid media, the trichome width, total length, motility as well as cell morphology remained the same throughout the life cycle of the strain.
3.2. Phylogenetic Analysis of 16S rRNA Gene and Phylogeny Based on Concatenate Alignment
The 16S rRNA nucleotide sequences of UHCC 1008 and UHCC 1009 were 1391 bp and 1376 bp long, respectively.
A total of 243 taxa were used in ML and BI phylogenetic analyses. The ML and BI analyses yielded nearly identical topologies and were mapped on the same phylogenetic tree (Figure 2 and Figure 3). In both analyses, many clades were supported by high bootstrap and posterior probability values. The genus Pseudanabaena formed a highly supported clade in both analyses (100 and 1.00 bootstrap values and posterior probabilities, respectively) and appeared to be closely related to the recently described genus Thalassoporum. Although phylogeny based on 16S rRNA gene resolved the genus Pseudanabaena, relationships between species were not well resolved. As seen in Figure 3, only P. yagii, P. cinerea and P. foetida, as well as strains assigned to P. mucicola, are clearly separated from the remaining Pseudanabaena species.
The newly isolated strains UHCC 1008 and UHCC 1009 were firmly placed within the Pseudanabaena clade. Overall, strains UHCC 1008 and UHCC 1009 shared 99.27% 16S rRNA sequence similarity with each other (Table S4). UHCC 1008 clustered with the planktic strain Ak1199 assigned to P. limnetica, with whom it shared 100% 16S rRNA sequence similarity (Table S4). It also appeared to be highly similar to all strains classified as P. catenata (99.69–99.90%) as well as P. limnetica [lim1, LIM1, Ak1201 and PS1303 (99.79–99.90%)], P. biceps (99.27%), “P. galeata” CCNP1313 (99.69%), and P. minima strains GSE-PSE20-05C and CHAB705 (98.95–99.06%). Furthermore, UHCC 1008 shared 98.85–99.90% 16S rRNA sequence similarity with Pseudanabaena spp. (strains PCC 6903, PCC 7402, Sai012, CZS-35E, GSE-PSE-MK15-19B and GSE-PSE-MK21-19D). Similarly to UHCC 1008, UHCC 1009 was highly similar (99.16–99.58%) to P. catenata, P. limnetica, P. biceps and P. minima strains mentioned above, Pseudanabaena sp. [dqh15, Sai012, CZS_35E, GSE-PSE-MK15-19B and GSE-PSE-MK21-19D (98.64–99.37%)] as well as P. galeata strains SAG 13.83 and CCNP1313 (98.87% and 99.16%, respectively). Interestingly, strains PG, CHAB774, CHAB786 and CHAB792, currently assigned to P. limnetica, clustered together in a separate clade (Figure 1b) and shared 93.83–94.40% 16S rRNA sequence similarity with UHCC 1008 and UHCC 1009 and 93.25–95.51% 16S rRNA sequence similarity with the remaining strains of the Pseudanabaena clade.
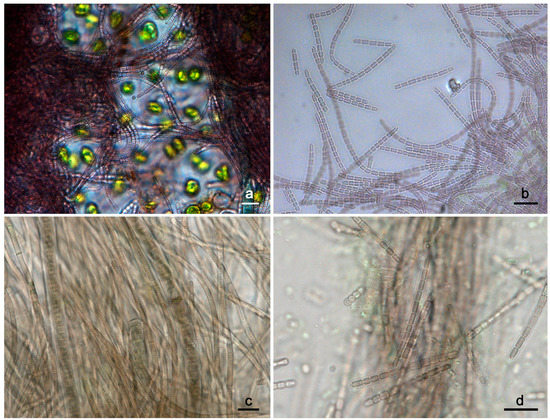
Figure 1.
Light micrographs of the preserved material collected from Kuhakoski (a,b) and Karkkila (c,d) locations. The trichomes of the UHCC 1008 morphotype were long, flexuous, and densely entangled, forming purple-colored clusters among other cyanobacteria and eukaryotic microorganisms (a). Trichomes of UHCC 1008 morphotype (preserved material) bearing refractive granules (b). The preserved material collected from Karkkila area was dominated by phycoerythin-rich, ensheathed morphotypes (c). The less frequently observed trichomes of the Pseudanabaena-like morphotype UHCC 1009 were always short and characterized by the presence of refractive granules at the tip of the apical cell (d). Scale bars correspond to 10 μm.
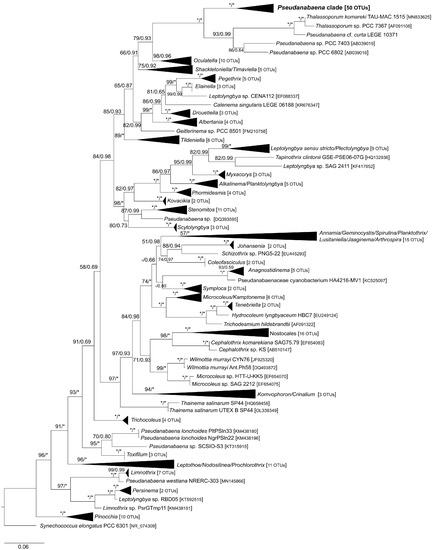
Figure 2.
Phylogenetic relationships inferred from Maximum Likelihood analysis of 16S rRNA gene (243 OTUs) showing the phylogenetic position of Pseudanabaena and closely related genera. Numbers on nodes correspond to bootstrap values (≥50%) and posterior probabilities (≥0.50) obtained from Maximum Likelihood and Bayesian analyses, respectively. Asterisk (*) represents bootstrap values of 100% for Maximum Likelihood and posterior probabilities of 1.0 for Bayesian analysis. Synechococcus elongatus PCC 6301 was the designated outgroup. GenBank accession numbers are given in brackets. The scale corresponds to substitutions/site.
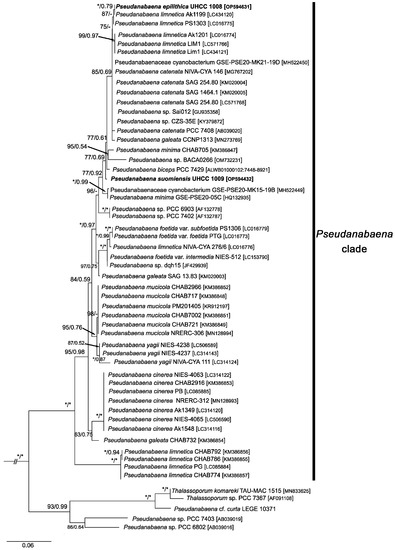
Figure 3.
Uncollapsed clades of Pseudanabaena, Thalassoporum and closely related strains seen in Figure 2. The nucleotide sequences of P. epilithica UHCC 1008T and P. suomiensis UHCC 1009T are shown in bold. Numbers on nodes correspond to bootstrap values (≥50%) and posterior probabilities (≥0.50) obtained from Maximum Likelihood and Bayesian analyses, respectively. Asterisk (*) represents bootstrap values of 100% for Maximum Likelihood and posterior probabilities of 1.0 for Bayesian analysis. Synechococcus elongatus PCC 6301 was the designated outgroup. GenBank accession numbers are given in brackets. The scale corresponds to substitutions/site.
The combined analysis (partial 16S and 16S–23S ITS region) allowed for a better separation of different Pseudanabaena species compared to the 16S rRNA-based phylogeny (Figure 4 for ML and Figure S1 for BI). Based on this analysis, the new strain UHCC 1008 was clustered with Pseudanabaena sp. BACA0266 isolated from a lake in the Azores (Portugal). Furthermore, both UHCC 1008 and BACA0266 were closely related to UHCC 1009. Similarly to 16S rRNA analysis, only strains assigned to P. mucicola, P. cinerea, P. foetida and P. yagii were well separated from the remaining species. Interestingly, strains assigned to P. limnetica formed two separate clusters (lim1, LIM1, Ak1201, Ak1199 and PS1303), while PG, CHAB774, CHAB786 and CHAB792, also assigned to P. limnetica, clustered together in a separate clade.
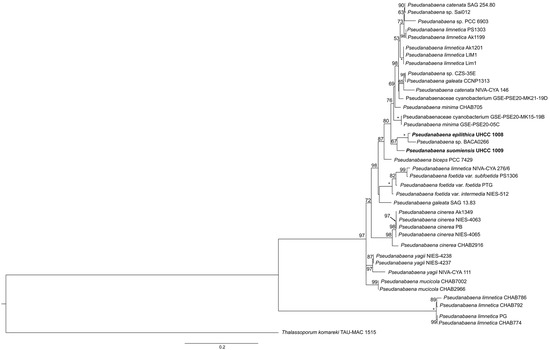
Figure 4.
Phylogenetic relationships of Pseudanabaena strains inferred from Maximum Likelihood analysis based on 16S rRNA concatenated with 16S–23S ITS nucleotide sequences. The new species P. epilithica UHCC 1008T and P. suomiensis UHCC 1009T are shown in bold. Numbers on nodes correspond to bootstrap values (≥50%). Asterisk (*) represents 100% support. T. komareki TAU-MAC 1515 was used as the outgroup. GenBank accession numbers of all nucleotide sequences are given in Table S3. The scale corresponds to substitutions/site.
3.3. Study of 16S–23S ITS
One complete 16S–23S ITS region containing both tRNAs was obtained for each studied strain (484 and 516 nucleotides long for P. epilithica UHCC 1008T and P. suomiensis UHCC 1009T, respectively).
The percent dissimilarity (100 × p-distance) between the new species and other Pseudanabaena strains ranged from 1.41 to 41.02 (Table S5). P. epilithica UHCC 1008T and P. suomiensis UHCC 1009T had 7.70% dissimilarity of 16S–23S ITS. At the same time, P. epilithica UHCC 1008T and Pseudanabaena sp. BACA0266 shared 1.41% dissimilarity values. In addition, the percent dissimilarity between P. epilithica UHCC 1008T and other members of Pseudanabaena clade ranged between 9.37 and 41.01. As seen in Table S5, 16S–23S ITS percent dissimilarity between P. suomiensis UHCC 1009T and other Pseudanabaena strains ranged between 8.20 and 39.49.
3.3.1. D1–D1′ Helix
The D1 stem region was 63 nucleotides (nt) long for all strains assigned to Pseudanabaena (Table S6). The hypothetical secondary structures of D1–D1′ helices presented in Figure 5 (structures 1–27) showed that Pseudanabaena strains can be divided into four groups based on their D1 stem region. The D1–D1′ helix in the first group has a five bp-long basal stem (GACCU−AGGUC) followed by a seven-residue right bulge (CAUCCCA), a ten bp-long stem region, a one-residue left bulge, a four bp-long stem region and a seventeen bp-long terminal hairpin. Strains belonging to the first group included P. epilithica UHCC 1008T, P. suomiensis UHCC 1009T, P. yagii (NIVA-CYA 111, NIES-4237, NIES-4238), strains assigned to P. limnetica (Ak1199, PS1303, Ak1201, Lim1 and LIM1), Pseudanabaena sp. BACA0266, P. catenata NIVA-CYA 146, P. mucicola (CHAB2966, CAB7002), strains classified as P. minima (CHAB705, GSE-PSE20-05C), “P. galeata” CCNP1313, as well as Pseudanabaena sp. CZS_35E, PCC 6903, GSE-PSE-MK15-19B and GSE-PSE-MK21-19D (Figure 5; structures 1,2,4–6,8,9,16–24). The second group included P. catenata SAG 254.80, Pseudanabaena sp. Sai012, P. cinerea (NIES-4063, NIES-4065, Ak1349, PB and CHAB2916) and P. foetida var. foetida PTG (Figure 5; structures 3,7,10,25). The only structural differences observed between the first two groups were located in the subterminal and terminal regions (three bp-long stem region and a nineteen bp-long terminal hairpin). In the third group of strains (P. foetida var. subfoetida PS1306, P. foetida var. intermedia NIES-512, P. galeata SAG 13.83, “P. limnetica” NIVA-CYA 276/6 and P. biceps PCC 7429), the basal stem and middle part of D1–D1′ helix was structurally identical to all other Pseudanabaena strains but differed from them in the subterminal and terminal part (Figure 5; structures 11–15). Here, the one-residue left bulge was followed by a three-nucleotide-long stem, a nine-residue asymmetrical internal loop, a two-residue stem region and a six-residue terminal hairpin. Strains PG, CHAB774, CHAB786 and CHAB792 that formed the fourth group had an identical D1–D1′ helix (Figure 5; structure 26) that was structurally similar to Pseudanabaena group 1 strains. Here, although the basal stem was identical to Pseudanabaena group 1, these four “P. limnetica” strains had a smaller right bulge (five instead of seven nucleotides), longer stem region (11 instead of 10 bp long), a one-residue left bulge, a four bp-long stem region and a sixteen bp-long instead of seventeen bp-long terminal hairpin.
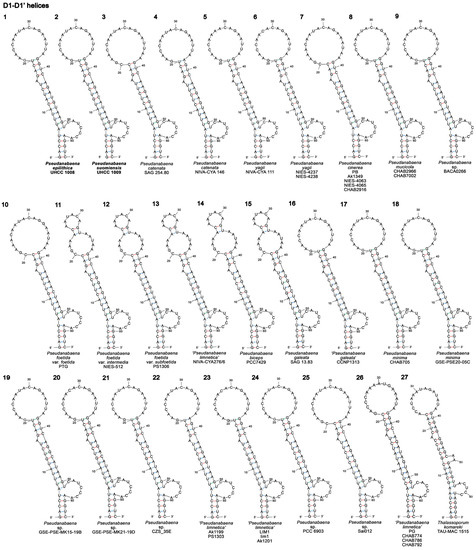
Figure 5.
Hypothetical secondary structures of conserved D1–D1′ helices of 16S–23S ITS region containing both tRNA genes in 26 Pseudanabaena strains (structures 1–26) and the closely related genus Thalassoporum (structure 27). GenBank accession numbers of all studied strains are presented in Table S3.
The D1 stem region of P. epilithica UHCC 1008T (Figure 5; structure 1) was identical to Pseudanabaena sp. BACA0266 (Figure 5; structure 9) and P. minima CHAB705 (Figure 5; structure 17) in terms of primary and secondary structure, whereas the D1–D1′ helix of P. suomiensis UHCC 1009T (Figure 5; structure 2) was identical to “P. galeata” CCNP1313 (Figure 5; structure 16) and Pseudanabaena sp. CZS_35E (Figure 5; structure 21). Lastly, the D1–D1′ helix in T. komareki TAU-MAC 1515 (Figure 5; structure 27) was significantly different from the D1 stem regions of Pseudanabaena strains. Here, the D1 stem region was 67 nt long (63 nt in Pseudanabaena) and comprised a six bp-long basal stem, a five-residue right bulge, a six bp-long stem region, a two-residue right bulge, a five bp-long stem region, a one-residue left bulge, a five bp-long stem region and a fifteen-residue terminal hairpin.
3.3.2. Box B Secondary Structure
Unlike the D1 stem region that appeared to be conserved among different Pseudanabaena species, box B structures were more variable in terms of both primary and secondary structure. The length of the box B region ranged between 45 and 57 nucleotides, with the most common length being 47 nucleotides (Table S6). As seen in Figure 6 (structures 1–29), the box B secondary structures of all Pseudanabaena strains except from PG, CHAB774, CHAB786 and CHAB792, had an identical four bp-long basal stem (AGCA−UGCU) followed by a three-residue asymmetrical internal loop (A−AC), a three bp-long stem region (CUC−GAG or CUU−GAG), a one-residue left bulge and a six bp-long (UCUAGU−ACUAGA) stem region (seven bp-long in P. mucicola strains and Pseudanabaena sp. Sai012; Figure 6, structures 8,25). This highly conserved region was followed by a right bulge or asymmetrical or symmetrical loops, a three to four-residue stem region and a terminal hairpin. The box B hypothetical secondary structure of P. epilithica UHCC 1008T (Figure 6; structure 1) was identical to P. galeata SAG 13.83 (Figure 6; structure 15) in terms of primary and secondary structure. Furthermore, it was structurally identical to P. foetida var. subfoetida PS1306, “P. limnetica” NIVA-CYA 276/6 and Pseudanabaena sp. BACA0266 (Figure 6; structures 9,12,13). The minor differences observed in the box B structures of these strains included the presence of adenine (A) at position 31 in P. epilithica UHCC 1008T instead of cytosine (C), as in P. foetida var. subfoetida PS1306 and P. limnetica NIVA-CYA 276/6, as well as the nucleotide sequence of the terminal hairpin(Figure 6; structures 1,12,13). A single transition mutation from A to G at position 23 separates the box B structures of P. epilithica UHCC 1008T and Pseudanabaena sp. BACA0266(Figure 6; structures 1,9). The primary and secondary box B structure of P. suomiensis UHCC 1009T (Figure 6; structure 2) was identical to P. limnetica strains LIM1, lim1 and Ak1201 (Figure 6; structure 23). In addition, it was structurally identical to P. limnetica Ak1199 and PS1303, as well as P. catenata NIVA−CYA 146, “P. galeata” CCNP1313, and Pseudanabaena sp. CZS_35E(Figure 6; structures 4,16,21,22). The box B secondary structures of the four “P. limnetica” strains (PG, CHAB774, CHAB786 and CHAB792) appeared to be significantly different from the remaining Pseudanabaena strains. As seen in Figure 6 (structures 26–28) and Table S6, the box B helices of these four strains were 52 nt long and were characterized by an identical seventeen bp-long stem region, a four-residue asymmetrical internal loop (six-residue asymmetrical internal loop in CHAB786), a five bp-long stem region (four bp-long in CHAB786) and a four-residue terminal hairpin (GAGA). The box B secondary structure of T. komareki TAU-MAC 1515 seen in Figure 6 (structure 29) was significantly different from Pseudanabaena in terms of both primary and secondary nucleotide sequence. The 53 nt-long box B region of this species comprised a five bp-long basal stem, a two-residue right bulge, a five bp-long stem region, a six-residue symmetrical internal loop, an eleven bp-long stem region and a three-residue terminal hairpin.
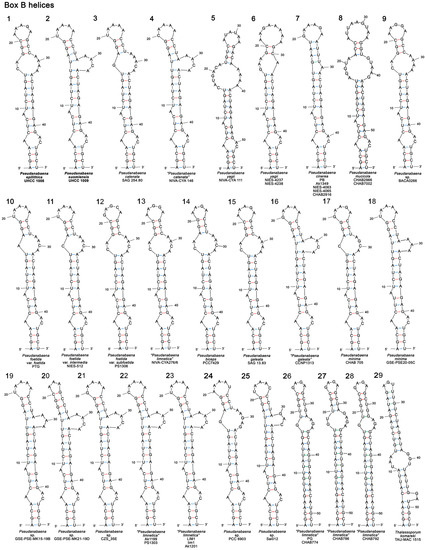
Figure 6.
Hypothetical secondary structures of box B helices of 16S–23S ITS region containing both tRNA genes in Pseudanabaena (structures 1–28) and the closely related genus Thalassoporum (structure 29). GenBank accession numbers of all studied strains can be found in Table S3.
3.3.3. V2 and V3 Helices
The V2 helices of all Pseudanabaena strains presented in Figure 7 (structures 1–31) appeared to be highly variable in length and primary and secondary structure. The V2 helix of the new species P. epilithica UHCC 1008T (Figure 7; structure 1) was structurally identical to Pseudanabaena sp. BACA0266(Figure 7; structure 12). As seen in Figure 7, the V2 helices of UHCC 1008T and BACA0266 were characterized by a three bp-long basal stem, followed by an eight nucleotide-long symmetrical internal loop, a seven bp-long stem region and a six-residue terminal hairpin(structures 1,12). The only differences observed between these two strains were the presence of adenine (A) at positions 14 and 30 of P. epilithica UHCC 1008T instead of guanine (G), as in Pseudanabaena sp. BACA0266(Figure 7; structure 12). The V2 helix of P. suomiensis UHCC 1009T (Figure 7; structure 2) had a unique primary and secondary structure. Briefly, it was characterized by a three bp-long basal stem followed by a six-residue symmetrical internal loop, an eight bp-long stem region, a second six-residue symmetrical internal loop, a three bp-long stem region and a four nucleotide-long terminal hairpin. The V2 secondary structures of PG, CHAB774, CHAB786 and CHAB792 appeared to be unique and highly conserved among these four strains(Figure 7; structures 29–31). The V2 region of T. komareki TAU-MAC 1515 was only four nt long.
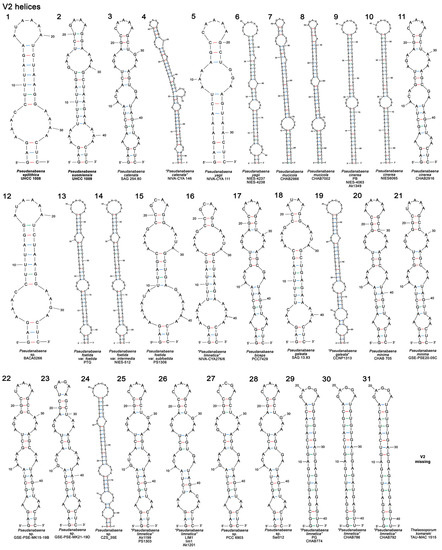
Figure 7.
The highly variable hypothetical secondary structures of V2 helices of 16S–23S ITS region containing both tRNA genes in Pseudanabaena (structures 1–31). The V2 region of Thalassoporum komareki TAU-MAC 1515 was only four nucleotides long. GenBank accession numbers of all studied strains can be found in Table S3.
The V3 region of Pseudanabaena strains ranged from 12 to 14 nucleotides and was not as variable as box B or V2 regions. More than half of the studied strains (n = 23) had an identical V3 structure that was 12 nt long (GUAAUAAGAAGC). These strains (Figure 8; structure 1) included P. epilithica UHCC 1008T, P. suomiensis UHCC 1009T, Pseudanabaena sp. BACA0266, P. limnetica (Ak1199, PS1303, Ak1201, Lim1, LIM1 and NIVA-CYA 276/6), P. catenata (SAG 254.80 and NIVA-CYA 146), P. minima (CHAB705 and GSE-PSE20-05C), P. cinerea (NIES-4063, NIES-4065, Ak1349 and PB), P. galeata (SAG 13.83 and CCNP1313) and Pseudanabaena sp. strains (CZS_35E, GSE-PSE-MK15-19B, GSE-PSE-MK21-19D, PCC 6903). P. foetida var. foetida PTG, P. foetida var. subfoetida PS1306 and P. foetida var. intermedia NIES-512 had a V3 hypothetical secondary structure that was 12 nt long too but differed from the previously mentioned strains by the presence of adenine (A) at position five instead of uracil (U), as in the abovementioned group of strains(Figure 8; structure 2). The V3 helix of the three P. yagii strains (NIVA-CYA 111, NIES-4237 and NIES-4238) was 13 nt long and identical to each other (Figure 8; structure 3). As seen in Figure 8, the V3 helix of CHAB2916 (structure 4), currently assigned to P. cinerea, differed from the remaining P. cinerea strains (Figure 8; structure 1) and P. yagii strains(Figure 8; structure 3). The V3 helices of the studied P. mucicola strains (CHAB2966 and CHAB7002) were identical to each other and 14 nucleotides long (Figure 8; structure 5). Regarding the V3 helices of strains PG, CHAB774, CHAB786 and CHAB792, it is noted that they were 14 nt long too but the nucleotide sequence was considerably different from P. mucicola strains(Figure 8; structures 6,7). The V3 secondary structures of Pseudanabaena biceps PCC 7409 and T. komareki TAU-MAC 1515 could not be determined, whereas the V3 helix for Pseudanabaena sp. Sai012 was missing due to an incomplete nucleotide sequence of 16S–23S ITS region.
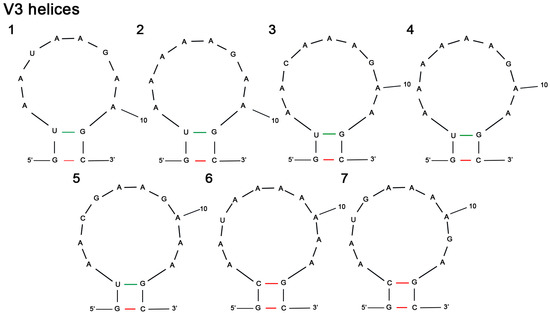
Figure 8.
V3 hypothetical secondary structures of 16S–23S ITS region containing both tRNA genes in Pseudanabaena strains: (1) P. epilithica UHCC 1008T, P. suomiensis UHCC 1009T, Pseudanabaena sp. BACA0266, P. limnetica (Ak1199, PS1303, Ak1201, Lim1, LIM1 and NIVA-CYA 276/6), P. catenata (SAG 254.80 and NIVA-CYA 146), P. minima (CHAB705 and GSE-PSE20-05C), P. cinerea (NIES-4063, NIES-4065, Ak1349 and PB), P. galeata (SAG 13.83 and CCNP1313), Pseudanabaena sp. strains (CZS_35E, GSE-PSE-MK15-19B, GSE-PSE-MK21-19D, PCC 6903); (2) P. foetida var. foetida PTG, P. foetida var. subfoetida PS1306 and P. foetida var. intermedia NIES-512; (3) P. yagii NIVA-CYA 111, NIES-4237 and NIES-4238; (4) P. cinerea CHAB2916; (5) P. mucicola CHAB2966 and CHAB7002; (6) “P. limnetica” PG, CHAB774 and CHAB792; (7) “P. limnetica” CHAB786.
3.4. Biochemical Analysis
3.4.1. Antimicrobial Susceptibility Tests and Bioactive Metabolites Screening
Neither P. epilithica UHCC 1008T nor P. suomiensis UHCC 1009T inhibited the growth of the microbial potential pathogens used in this study. No UV-screening metabolites or cyanobacterial toxins were detected during liquid chromatography analysis.
3.4.2. Phycobiliproteins
The absorption spectra of P. epilithica UHCC 1008T and P. suomiensis UHCC 1009T (Figure 9) showed that both strains contained phycoerythrin and phycocyanin with major peaks at 564 and 620 nm for P. epilithica UHCC 1008T and at 562 and 620 nm for P. suomiensis UHCC 1009T. A shoulder peak at 656 nm, which is close to the absorption maximum of allophycocyanin, was also recorded.
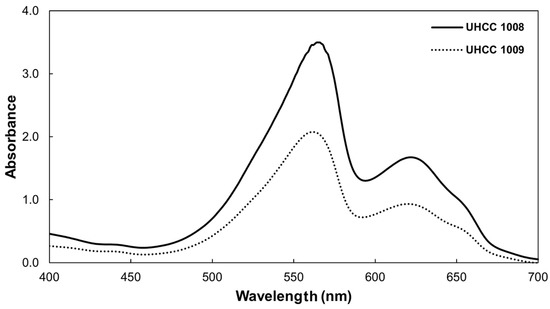
Figure 9.
Absorption spectra of crude extracts of P. epilithica UHCC 1008T (solid line) and P. suomiensis UHCC 1009T (dotted line) with phycoerythrin as the dominant phycobiliprotein in both species.
3.5. Taxonomic Assessments
Description of Pseudanabaena epilithica sp. nov. under the provisions of the International Code of Nomenclature for Algae, Fungi and Plants
Pseudanabaena epilithica Christodoulou et Sivonen sp. nov.
Diagnosis
Ecologically close to P. spelaea and P. skujae. Differs from P. spelaea by having wider and sheathless trichomes and refractive granules at the tip of the apical cell and by exhibiting motility. Differs from P. skujae by having narrower trichomes, rounded instead of elongated and conically pointed apical cells and exhibiting infrequent forward movement as opposed to slow gliding motion observed in P. skujae. Ecologically and phylogenetically (16S rRNA) close to P. suomiensis. Differs from P. suomiensis by having long and flexuous trichomes, isodiametric cells and purplish brown color. In addition, differs from P. suomiensis by length and secondary structure of V2 helix and secondary structure of box B helix of the 16S–23S ITS region.
Description (Figure 10a–h)
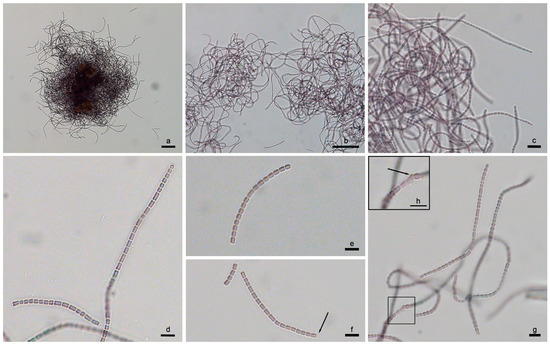
Figure 10.
Light micrographs of P. epilithica UHCC 1008T at different magnifications. (a–c) Densely and irregularly arranged trichomes observed at the exponential phase; note the trichome length and flexibility. (d–f) Young trichomes and hormogonia of P. epilithica UHCC 1008T; note the refractive granule at the apical cell (arrow). (g,h) Trichomes in a relatively old culture showing an involution cell (arrow). Scale bars (a) 50 μm (200×), (b) 50 μm (400×), (c) 10 μm (1000×), (d–h) 5 μm (1000×).
In nature, the species forms very thin purplish brown to olive-green-colored biofilm on wet rocks together with other thin filamentous cyanobacteria and Microcoleus-like strains.
On Z8 agarose-solidified media, colonies are flat and more or less spherical, with a smooth and shiny surface and purple to deep brown color. In liquid cultures, trichomes form densely arranged, purple to deep brown-colored irregular clusters, which are loosely attached to the glass culture vessel. Young trichomes are usually bent. Mature trichomes are cylindrical, long, flexuous, (1.5) 1.7–1.9 (2.1) μm wide, constricted at the translucent cross walls, not attenuated toward the end and motile (infrequent forward movement). Cells are purplish brown in color, cylindrical to barrel-shaped, ± isodiametric to slightly longer than wide or up to 2.5–3 μm long depending on the phase of division, sometimes with one to two granules and/or one to two refractive granules (aerotopes?) on either side of the cross walls. Cell content differentiated into chromatoplasma and centroplasma. Apical cells rounded without calyptra or thickened cell walls, occasionally with characteristic ring-like or cupola-like large refractive granules (aerotopes?). Involution cells are rarely observed. Necridic cells are not observed. Cell division by binary fission in one plane, perpendicular to the longitudinal axis of the trichome. Reproduction via hormogonia.
Etymology: e.pi.li’.thi.ca. N.L. adj. epilithicus -a -um (from Gr. masc. adj. επιλιθικός, epilithic); N.L. fem. adj. epilithica (=growing on lithic surfaces), referring to the habitat of the species.
Holotype here designated: Exsiccate accession number H6124570, deposited in the Finnish Museum of Natural History (LUOMUS), Helsinki, Finland.
Type strain (Reference strain): UHCC 1008T, University of Helsinki Cyanobacteria Collection (HAMBI/UHCC).
Habitat: The environmental sample containing P. epilithica was collected from a wet rock within a man-made/artificial cavity next to a waterfall; the cavity was not exposed to direct sunlight.
Type locality: Kuhakoski waterfall (Nurmijärvi, Southern Finland; 60.432611° N, 24.712667° E), collected by M. Christodoulou in July 2018 from a man-made/artificial cavity by scraping the substrate using sterile scalpels and spatulas.
Reference nucleotide sequences: Partial nucleotide sequence of 16S rRNA gene and complete nucleotide sequence of 16S–23S ITS region deposited to NCBI under the accession numbers OP594631 and OP595723, respectively.
Taxonomic notes: P. epilithica UHCC 1008T is close to P. suomiensis UHCC 1009T in terms of habitat preferences and 16S rRNA phylogeny (Tables S4 and S7). Regarding the latter, the two new species share 99.27% similarity of 16S rRNA, which is above the threshold for species demarcation (98.65%). However, examination of 16S–23S ITS percent dissimilarity values showed that the two strains are more than 7% different, suggesting that they represent two different species. In addition, both box B and V2 hypothetical secondary structures differ greatly between the two species (Figure 6; structures 1,2 and Figure 7; structures 1,2). The morphological differences between P. epilithica UHCC 1008T and P. suomiensis UHCC 1009T further support the findings of 16S–23S ITS studies.
In addition to P. suomiensis UHCC 1009T, the 16S rRNA phylogenetic analyses showed that P. epilithica UHCC 1008T shares more than 98.65% similarity with strains assigned to P. catenata (SAG 254.80, NIVA-CYA146, SAG 1464-1, PCC 7408), P. limnetica (PS1303, Ak1199, Ak1201, LIM1 and Lim1), P. minima (GSE-PSE20-05C, CHAB705), P. biceps (PCC 7429), ‘P. galeata’ (CCNP1313) and strains classified as Pseudanabaena sp. (Sai012, PCC 6903, CZS 35E, PCC 7402, GSE-PSE-MK15-19B, GSE-PSE-MK21-19D). P. epilithica UHCC 1008T can be distinguished from the abovementioned Pseudanabaena strains by their morphology, habitat preferences (i.e., strains isolated from aquatic habitats) and percent dissimilarity of 16S–23S ITS region. Since many of the abovementioned strains have been wrongly assigned to existing species, all available information on their morphology is presented in Table 1. Overall, strains PCC 7408 (=NIVA-CYA146 and SAG 1464-1), CHAB 705, PCC 6903, PCC 7402, CCNP1313 and PCC 7429 have wider trichomes (2.0 to 3.5 μm) compared to P. epilithica UHCC 1008 (1.7–1.9, rarely up to 2.1 μm). In addition, strains CHAB 705, PCC 6903, PCC 7402 and CCNP1313 are characterized by short trichomes (up to 10 cells). Furthermore, strains PS1303, Ak1199, Ak1201, Lim1 (=LIM1) have narrower trichomes (max 1.5 μm wide) compared to the UHCC 1008 taxon. Apart from the obvious morphological and ecological differences, the separation of our taxon from the abovementioned strains is further supported by the 11.16–13.10% dissimilarity values of 16S–23S ITS (Table S6), which are above the 7% threshold for species separation.

Table 1.
Morphological characteristics available for strains sharing >98.65% 16S rRNA sequence similarity with the studied strains.
Lastly, P. epilithica UHCC 1008T shares 1.41% dissimilarity values with the aquatic Pseudanabaena sp. BACA0266 (Azores, Portugal). While this value indicates that these strains might belong to the same species, they share only 98.10% 16S rRNA sequence similarity and originate from different habitats.
Other notes: No morphological differences were observed between the trichomes present in the preserved material (i.e., formaldehyde-fixed environmental sample) and the active culture, despite its repeated sub-culturing in liquid media for 5 years. Furthermore, no changes were observed in trichome coloration by increasing the light intensity from 10 to 25 μmol of photons s−1 m−2.
Description of Pseudanabaena suomiensis sp. nov. under the provisions of the International Code of Nomenclature for Algae, Fungi and Plants
Pseudanabaena suomiensis Christodoulou et Sivonen sp. nov.
Diagnosis
Ecologically close to P. spelaea and P. skujae. Differs from P. spelaea by having trichomes that are wider and shorter in length, cells that are longer than wide and refractive granules at the tip of the apical cell. Additionally differs from P. spelea by the absence of sheath, by exhibiting motility and its brown-colored cells. Differs from P. skujae by having short and narrower trichomes, brown instead of faint bluish color, rounded instead of elongated and conically pointed apical cell and by exhibiting forward movement as opposed to slow gliding motion observed in P. skujae. Ecologically and phylogenetically (16S rRNA) close to P. epilithica UHCC 1008T. Differs from P. epilithica UHCC 1008T by having short and straight trichomes arranged in parallel or subparallel fascicles, cells that are longer than wide and its brown color. In addition, differs from P. epilithica UHCC 1008T by length and secondary structure of V2 helix and secondary structure of box B helix of the 16S–23S ITS region.
Description (Figure 11a–e)
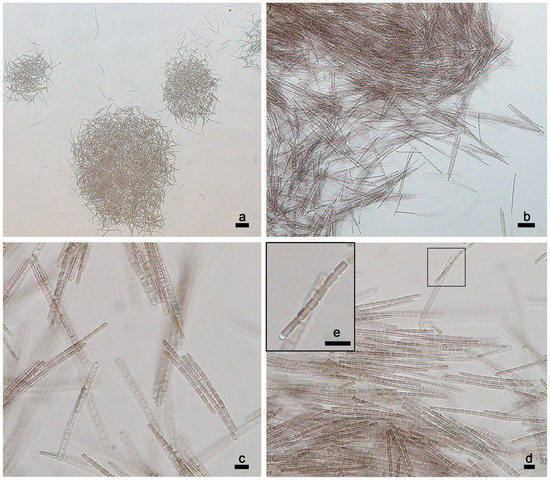
Figure 11.
Light micrographs of P. suomiensis UHCC 1009T at different magnifications. (a–c) Trichomes arranged in parallel or subparallel fascicles during the exponential phase. Note the trichome length. (d,e) Trichomes of P. suomiensis bearing refractive granules at the tip of their apical cells. Scale bars (a) 50 μm (100×), (b) 20 μm (400×), (c–e) 5 μm (1000×).
In nature, thallus appears as a greenish to olive-green to brownish-colored patina spreading on the surface of a dry rock together with other simple trichal cyanobacteria, as well as Phormidum-like strains and eukaryotic microorganisms.
On Z8 agarose-solidified media, trichomes form macroscopic, brownish-colored fascicles, spreading on the solid media surface, sometimes slightly penetrating the solid media. In liquid cultures, young trichomes are usually arranged in parallel or subparallel fascicles, later forming a thin brownish biofilm, which can be attached firmly to the bottom of the Erlenmeyer flask and/or grow at the liquid media/air interface. Trichomes are cylindrical, motile (forward movement; clockwise–anticlockwise rotation rarely observed), usually few-celled or up to 200–300 μm long, mostly straight or slightly bent, (1.5) 1.6–1.9 (2.1) μm wide, constricted at the translucent cross walls and not attenuated toward the end. Cells are brown in color, cylindrical with rounded ends, usually longer than wide, up to 3.5 μm long (depending on phase of division) and usually have one to three granules; sometimes, one refractive granule is observed on either side of the cross walls. Cell content differentiated into chromatoplasma and centroplasma. Apical cells rounded without calyptra or thickened cell walls but with two small (or one large) ring-like or cupola-like refractive granules (aerotopes?). Necridic cells are not observed. Cell division by binary fission in one plane, perpendicular to the longitudinal axis of the trichome. Reproduction via production of motile hormogonia (trembling).
Etymology: su.o.mi.e’.nsis referring to the country of origin (Suomi = the name of the country Finland in Finnish language).
Holotype here designated: Exsiccate accession number H6124571, deposited in the Finnish Museum of Natural History (LUOMUS), Helsinki, Finland.
Type strain (Reference strain): UHCC 1009T, University of Helsinki Cyanobacteria Collection (HAMBI/UHCC).
Habitat: Environmental sample was collected from dry rocks exposed to sunlight.
Type locality: Forest area close to Pitkälänkoski rapid (Karkkila, southern Finland, 60.521889° N, 24.227667° E), collected by M. Christodoulou in July 2019 by scraping the surface of a dry rock using sterile scalpels.
Reference nucleotide sequences: Partial nucleotide sequence of 16S rRNA gene and complete nucleotide sequence of 16S–23S ITS region deposited to NCBI under the accession numbers OP594632 and OP595724, respectively.
Taxonomic notes: Results deriving from the 16S rRNA phylogenetic analyses showed that P. suomiensis UHCC 1009T shared 98.85–99.58% similarity with strains assigned to P. catenata (SAG 254.80, NIVA-CYA146, SAG 1464-1, PCC 7408), P. limnetica (PS1303, Ak1199, Ak1201, LIM1 and Lim1), P. minima (GSE-PSE20-05C, CHAB705), P. biceps (PCC 7429), P. galeata SAG 13.83, ‘P. galeata’ (CCNP1313) and strains classified as Pseudanabaena sp., isolated mostly from aquatic habitats (Sai012, CZS_35E, GSE-PSE-MK15-19B, GSE-PSE-MK21-19D). Compared with the abovementioned strains (Table 1), our taxon has intermediate trichome and cell width and different habitat preferences (epilithic). Furthermore, P. suomiensis UHCC 1009T can be distinguished from the abovementioned Pseudanabaena strains by sharing 8.5–13.55% percent dissimilarity values of 16S–23S ITS.
The unique combination of D1–D1′, box B, V2 and V3 helices of 16S–23S ITS region of P. suomiensis UHCC 1009T as well as percent dissimilarity of 16S–23S ITS region (8.23–39.49%) further separates this species from the remaining Pseudanabaena strains for which the 16S–23S ITS region is available.
Other notes: No morphological differences were observed between the trichomes present in the preserved material (i.e., formaldehyde-fixed environmental sample) and the active culture, despite its repeated sub-culturing in liquid media for 4 years. Furthermore, no changes were observed in trichome coloration by increasing the light intensity from 10 to 25 μmol of photons s−1 m−2.
4. Discussion
Two new phycoerythrin-rich Pseudanabaena species from Finland, i.e., P. epilithica UHCC 1008T and P. suomiensis UHCC 1009T, are described herein based on a combination of morphological, molecular and ecological criteria. Despite the absence of inhibitory activity against microbial pathogens and lack of toxins in both P. epilithica UHCC 1008T and P. suomiensis UHCC 1009T, both strains produce high amounts of C-phycoerythrin (C-PE). R-phycoerythrin, a form of phycoerythrin produced by red algae, is known for its antioxidant, anticancer, anti-inflammatory and anti-neurodegenerative activities (see [69,70] and references therein). Furthermore, it is currently used, inter alia, as a colorant in food and cosmetics as well as in biomedical research [69]. However, the number of studies exploring the biological activities and potential applications of C-phycoerythrin, the form of phycoerythrin found in cyanobacteria, is rather low. This can be attributed to the fact that many cyanobacteria produce water-soluble cyanotoxins [69]. Moreover, many phycoerythrin-rich species have high cultivation and production costs combined with slower growth rates [69]. Further complicating the situation is the fact that the purified C-PE faces several stability problems [see 69]. To date, it has been shown that oral administration of C-PE reduced oxidative stress in C. elegasns [71], as well as HgCl2-induced oxidative stress in mice [72] and CCl4-induced toxicity in rats [73]. Furthermore, oral treatment with C-PE was found to have a protective role against diabetic complications in rats [74]. Since neither of the two new Pseudanabaena strains produces any toxins, they could be potentially used as a source of C-PE for biotechnological purposes.
4.1. Morphology and Ecology
Pseudanabaena is a relatively old cyanobacterial genus with cosmopolitan distribution [2]. The majority of Pseudanabaena species thrive in freshwater environments except for P. apiculato-flexuosa and P. belizensis, which are found in alkaline marshes [23]; P. oblonga, P. thermalis and P. lonchoides, which are reported from thermal springs; and P. spelaea and P. skujae, described from cave environments. The new rock-inhabiting species are distinguished from the ecologically similar P. spelaea and P. skujae by having intermediate cell width and purplish brown (P. epilithica UHCC 1008T) or brown (P. suomiensis UHCC 1009T)-colored cells observed in both preserved (environmental sample) and culturable (unicyanobacterial) specimens. Morphological features including, inter alia, cell coloration, trichome width, presence/absence of thallus, the shape of apical cells, motility as well as habitat preferences separate our taxa from the remaining Pseudanabaena species. The minimum and maximum width of both P. epilithica UHCC 1008T and P. suomiensis UHCC 1009T overlaps with many taxonomically accepted Pseudanabaena species [2]. Based on their morphology and the presence of refractive granules at the tip of their apical cells, P. epilithica UHCC 1008T and P. suomiensis UHCC 1009T are closer to P. galeata. P. epilithica UHCC 1008T differs from P. galeata by having longer trichomes that are densely entangled, forming purple-brown clusters, whereas P. galeata has fine, thin indefinite, expanded and prostrated colonies; hemispherical, bubble-like formations; or erected flaky clusters, usually bright blue-green or emerald-green to olive-green in color. Furthermore, P. epilithica UHCC 1008T is characterized by isodiametric cells that are purplish brown in color as opposed to the pale blue-green to gray-blue coloration of P. galeata cells. The formation of a thin brownish biofilm by P. suomiensis UHCC 1009T separates this species from P. galeata. Furthermore, trichomes of P. suomiensis UHCC 1009T are always straight, brown in color and arranged in parallel or subparallel clusters, as opposed to P. galeata, which has straight or variously curved and entangled trichomes and is pale blue-green to gray-blue in color. Lastly, the two new strains have different habitat preferences compared to P. galeata. The latter is characterized as benthic, epiphytic or endogloeic as opposed to the epilithic habitat of P. epilithica (wet rock in a dim light cavity) and P. suomiensis (dry rocks exposed to sunlight).
4.2. 16S rRNA and Combined Phylogenetic Analyses
Even though Pseudanabaena contains more than 40 taxonomically accepted species, the majority of them have been described between 1915 and 2001 based mainly on morphological features, leading Komárek and Anagnostidis [2] to characterize Pseudanabaena as heterogeneous and highlight the need for molecular evaluation. While the polyphyletic status of this genus has been recognized by many other authors [18,21,22,66], the taxonomy of Pseudanabaena remains problematic despite the plethora of 16S rRNA gene and 16S–23S ITS nucleotide sequences available in GenBank. This can be partially attributed to the lack of sequencing data from the reference strain of the type species, P. catenata [66]. Further complicating the situation is the absence of molecular data from the reference strains of other Pseudanabaena species described based solely on morphological criteria (e.g., P. minima, P. mucicola, etc.). In connection with the abovementioned issues, many publicly available nucleotide sequences derive from incorrectly identified strains. This problem concerns not only Pseudanabaena but the majority of cyanobacterial genera and can potentially lead to confusing results in many phylogenetic analyses [5,75,76,77].
Herein, information deriving from 16S rRNA phylogenetic analyses strongly supports the placement of the two newly isolated Finnish strains under the genus Pseudanabaena. The 97.89−100% 16S rRNA sequence similarity P. epilithica UHCC 1008T and P. suomiensis UHCC 1008T shared with other strains of the Pseudanabaena clade is higher than the 94.5% cut-off value proposed by Yarza et al. [78] for genus delimitation. Furthermore, the new strains shared 99.27% 16S rRNA sequence similarity with each other and 98.85–100% 16S rRNA sequence similarity with other strains of the Pseudanabaena clade assigned to P. catenata (SAG 254.80, NIVA-CYA146, SAG 1464-1, PCC 7408), P. biceps (PCC 7409), P. galeata (SAG 13.83), “P. limnetica” (e.g., Ak1199, Lim1, LIM1) and several other unclassified Pseudanabaena strains. These values are much higher than the recommended threshold of 98.65%, which is today considered clear evidence of speciation [79,80]. Based on these findings, this marker is ambiguous in resolving species relationships within Pseudanabaena and, if the taxonomic assessment was based solely on 16S rRNA gene sequence similarity thresholds, the new strains UHCC 1008 and UHCC 1009 would have been assigned to existing Pseudanabaena species.
4.3. The Use of 16S–23S ITS in Polyphasic Approach Studies
The failure of 16S rRNA gene to provide sufficient resolution at the species level has been observed previously in other genera, such as Oculatella [8], Roholtiella [9], Cyanocohniella [10], Nodularia [81] and Mojavia [11], highlighting the need for additional tools that would lead to unambiguous discriminations among different species. This led Boyer et al. [6] to propose the use of 16S–23S ITS hypothetical secondary structures for this purpose. Since then (2001), several studies have used the differences observed primarily in D1–D1′ and box B regions of 16S–23S ITS as a means to distinguish between studied species [8,12,13,16,38,59,82,83,84]. In addition, the percent dissimilarity of aligned 16S–23S ITS in orthologous ribosomal operons is becoming increasingly popular and has been successfully applied in many studies to support the establishment of new taxa [8,9,10,11,12,13,14,15]. Based on results presented therein, strains within the same genus sharing 0–3% dissimilarity values of 16S–23S ITS region are considered to belong to the same species, whereas 16S–23S ITS percent dissimilarity values above 7% are considered good evidence for species separation. At the same time, combined analysis based on concatenated alignments of 16S rRNA gene and 16S–23S ITS region has been successfully used to investigate the phylogenetic relationships between species (see [8]).
Although almost complete 16S–23S ITS nucleotide sequences exist for the recently described Pseudanabaena species, the different 16S–23S ITS regions were either missing from previous studies (e.g., box B) or improperly presented and, in many cases, not even compared to other publicly available 16S–23S ITS data of Pseudanabaena strains. In the present study, the hypothetical secondary structures of D1–D1′, box B, V2 and V3 helices of 39 strains assigned to Pseudanabaena and the closely related genus Thalassoporum are presented and compared for the first time. Of all 16S–23S ITS domains studied herein, D1–D1′ region was the most conserved, followed by box B and V2 helices. A highly conserved D1 stem region is also seen in other cyanobacterial genera, including Oculatella, Pegethrix, Kaiparpowitsia, Tildeniella, Cyanocohniella and Roholtiella [8,9,10,13]. Interestingly, many Pseudanabaena strains have structurally identical D1–D1′, box B and V2 helices, leading to confusion. Dividing the strains into two groups, i.e., “Pseudanabaena described based on a polyphasic approach” and “Pseudanabaena spp. in need for revision”, led to an interesting observation: species established based on a polyphasic approach workflow can be discriminate from each other based on a unique combination of D1–D1′, box B and V2 helices. Furthermore, differences observed in the D1–D1′, box B or V2 helices of strains belonging to the same species (e.g., P. yagii) can be justified by intraspecies variability of ITS region, which is commonly observed in other cyanobacterial taxa (see [38] and references therein). However, this does not apply to Pseudanabaena strains that require taxonomic revision (see paragraph 4.4). For example, the D1–D1′, box B and V2 helices of Pseudanabaena minima GSE-PSE20-05C are structurally identical to strains Ak1201, Lim1, LIM1, Ak1199 and PS1303 assigned to P. limnetica.
In addition to the study of secondary structures, percent dissimilarity, calculated for the first time for Pseudanabaena, provided useful information on the relationships between different strains. These results are also in line with the ones obtained from the combined analysis. In the case of P. suomiensis UHCC 1009T, separation from the remaining strains is supported by a unique combination of D1–D1′, box B and V2 structures together with the high values (7.70–39.49%) of 16S–23S ITS percent dissimilarity that this species shared with the remaining studied Pseudanabaena strains. The morphological findings and ecology of P. suomiensis UHCC 1009T further corroborate the establishment of this new species. Likewise, P. epilithica UHCC 1008T shared more than 7.00% dissimilarity values with other Pseudanabaena, except Pseudanabaena sp. BACA0266 with whom it shared only 1.41% dissimilarity values, suggesting that these two strains most likely belong to the same species. Moreover, the two strains clustered together in the combined analysis. Although morphological evaluation is not available for BACA0266, information obtained from NCBI shows that this strain has been isolated from a lake in the Azores. Therefore, apart from geographical separation, P. epilithica UHCC 1008T and Pseudanabaena sp. BACA0266 thrive in different habitats. Furthermore, the two strains share only 98.10% 16S rRNA sequence similarity values, which is lower than the recommended threshold of 98.65% for bacterial species demarcation [80]. Based on the abovementioned information, the two strains cannot be regarded as the same species. While many validly described Pseudanabaena used in this study share > 7% dissimilarity values with each other, strains assigned to P. minima, P. limnetica, P. catenata and Pseudanabaena spp. had percent dissimilarity values between 3 and 7%. In such cases, proper morphological evaluation, which is currently missing for many of them as well as data on their ecology, must be taken into consideration to reach safe conclusions (see below).
4.4. A Polyphasic Approach Workflow Is Necessary for Accurate Taxonomic Assignments
Apart from supporting the establishment of the two new species, the polyphasic approach applied herein revealed some interesting points related to the taxonomic assignments of several Pseudanabaena strains. The first point concerns the Pseudanabaena foetida group of strains. In 2016, Niiyama et al. [26] described the new species P. foetida and P. subfoetida (strains PTG and PS1306, respectively). Although differences in 16S–23S ITS secondary structures of the two species were not properly identified, presented and discussed therein, the two species could still be discriminated based on differences in their trichome width (1.0–1.5 μm in P. foetida, 2.1–2.9 μm in P. subfoetida). Later on, these strains, together with strain NIES-512, were subsumed to P. foetida (P. foetida var. foetida PTG, P. foetida var. subfoetida PS1306 and P. foetida var. intermedia NIES-512) by Tuji and Niiyama [27] based on the fact that they all produced the odorous metabolite 2-MIB. Even though the 16S–23S ITS percent dissimilarity values presented in our study (2.69%) confirm that P. foetida var. foetida PTG and P. foetida var. intermedia NIES-512 belong to the same species, the 9.11–10.14% dissimilarity that P. foetida var. subfoetida PS1306 shares with these two strains suggest that PS1306 should be considered a separate species. Furthermore, the 1.55% dissimilarity value that P. foetida var. subfoetida PS1306 shares with “P. limnetica” NIVA-CYA276/6 indicates that these two strains most likely belong to the same species. This is further supported by the almost identical description of the two strains provided by Niiyama et al. [26]. In fact, although these authors acknowledged the morphological similarities of the two strains, they considered “P. limnetica” NIVA-CYA276/6 a different species due to the lack of 2-MIB production that characterizes P. foetida var. subfoetida PS1306.
Second, results obtained from 16S rRNA, combined analyses, and 16S–23S ITS percent dissimilarity values in conjunction with morphological evaluations presented in previous studies [26,64,65,66] show that many strains are assigned to known Pseudanabaena species based solely on preliminary morphological assessments and highlight once again the need for taxonomic revision. For example, strains NIVA-CYA146, SAG 1464-1, PCC7408 and SAG 254.80 are assigned to P. catenata. The work of Rippka et al. [64] shows that NIVA-CYA146, SAG 1464-1 and PCC7408 belong to the same isolate, which is characterized by motile trichomes, 2.0–2.5 μm wide and a lack of polar gas vacuoles [64]. According to the description given in Komárek and Anagnostidis [2], the width of P. catenata trichomes ranges between 1.2 and 2.0 μm, and the presence of gas vacuoles is facultative. These findings indicate that strains NIVA-CYA146, SAG 1464-1 and PCC 7408 most likely represent a different species. However, further studies are required to confirm these findings. A morphological evaluation of SAG 254.80 is also recommended to confirm whether it corresponds to the true P. catenata or not. Moreover, our dataset included four strains that, according to their depositors, belong to P. galeata: SAG 13.83, isolated by L. Geitler from a freshwater environment in Austria (deposited in SAG collection between 1982 and 1983); CCNP1313, isolated from the Baltic Sea [67]; and CHAB732 and CHAB2916, originating from Chinese lakes [66]. Apart from SAG 13.83, for whom there is no description available, closer study of the photographic documentation and brief description provided for the remaining strains suggests that none of them correspond to the description of P. galeata given in Komárek and Anagnostidis [2]. In fact, our phylogenetic analyses showed that CHAB732 and CHAB2916 appear to be related to P. cinerea. Assuming that SAG 13.83 corresponds to P. galeata, the relatively low 16S rRNA gene similarity values between the abovementioned strains and P. galeata SAG 13.83 (97.58–98.43%) as well as information deriving from the combined analysis, 16S–23S ITS secondary structures and high percent dissimilarity values (15.00–16.40%) further support these findings. Another enigmatic group is the freshwater strains PS1303, Ak1199, PTB (=Ak1201), LIM1 and Lim1, which are currently assigned to P. limnetica but do not cluster together in either 16S rRNA or combined analyses as one would expect from strains belonging to the same species. Instead, PS1303 is clustered with Ak1199, while PTB (=Ak1201), Lim1 and LIM1 form a separate cluster. As seen in the work of Niiyama et al. [26] (Tables 1 and 2 therein) and Tuji and Niiyama [65] (Table 1 therein), strains PS1303 and Ak1199 are characterized by trichomes that are 1.1–1.5 μm wide, bright blue-green in color and their apical cell is long cylindrical with rounded ends. The trichomes of PTB (=Ak1201) and Lim1 (=LIM1) are 0.9–1.3 μm wide and pale brownish green in color, with their apical cells being long cylindrical with rounded ends. Apart from morphological differences, the 16S–23S ITS percent dissimilarity between strains of the same clusters is 0.00%, but the percent dissimilarity between the two clusters is lower than the 7.00% threshold for species recognition (5.39–5.61%). Moreover, their folded 16S–23S ITS domains presented herein were structurally identical. Although trichome width overlaps with the minimum and maximum width of P. limnetica (1.2–1.5 μm), the question of whether one of these groups represents P. limnetica or not must be answered after a detailed morphological evaluation of culturable and, if possible, preserved material combined with molecular evaluation (including whole genome sequencing).
The taxonomic placement of strains CHAB774, CHAB786 and CHAB792 isolated from West Lake (Hangzhou, China) as well as strain PG (Serikawa dam, Japan), which are also regarded as P. limnetica, is another point worth discussing. Based solely on the morphological evaluation and the photographic documentation of Yu et al. [66], the three CHAB strains resemble Pseudanabaena. However, they differ from P. limnetica by having bright blue-green-colored trichomes that are 1.3–2.6 μm wide, as opposed to the pale blue-green to olive blue-green-colored trichomes of P. limnetica, which are 1.2–1.5 μm in width. Unfortunately, no description is available for the PG strain. Nevertheless, the 16S rRNA phylogenetic analysis presented herein shows that PG strain, together with the abovementioned CHAB strains, is placed in a clade that is supported by high bootstrap and posterior probability values (100% and 1.0, respectively) and appears to be separated from the remaining Pseudanabaena strains. Furthermore, these four strains share 99.90–100% 16S rRNA gene similarity values with each other and only 93.25–95.51% 16S rRNA gene sequence similarity with all strains of Pseudanabaena clade. These values are close to the generic cut-off points set by Yarza et al. [78] as well as Stackebrandt and Goebel [85] (94.5 and 95%, respectively). Lower 16S rRNA sequence similarity values were also reported in the study of Yu et al. [66] where the three CHAB strains shared 92.6–93.7% 16S rRNA sequence similarity with the remaining strains of Pseudanabaena clade (Table 2 therein). These findings, combined with differences observed in 16S–23S ITS secondary structures, indicate that these four strains require further evaluation, including genomic studies, to reach safe conclusions on their taxonomic position.
5. Conclusions
P. epilithica and P. suomiensis from terrestrial epilithic habitats are described in this study based on a polyphasic approach. Although Pseudanabaena strains have been previously identified from Finnish aquatic environments, P. epilithica and P. suomiensis represent the first Pseudanabaena species established from Finland. Despite the high 16S rRNA sequence similarity values, morphological features, habitat preferences and 16S–23S ITS percent dissimilarity values separate the new species from previously described Pseudanabaena species. As discussed previously, misidentified strains add more confusion to the already complicated taxonomy of cyanobacteria. This work revealed that many Pseudanabaena strains are misidentified, highlighting the need for taxonomic revision in this poorly studied genus using a polyphasic approach.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15080909/s1, Figure S1: Phylogenetic relationships inferred from Bayesian analysis based on a concatenate alignment of 16S rRNA gene and 16S rRNA gene and 16S–23S ITS of Pseudanabaena strains. The new species P. epilithica UHCC 1008T and P. suomiensis UHCC 1009T are shown in bold. Numbers on nodes correspond to posterior probabilities (≥0.50). Asterisk (*) represents support of 1.00. Thalassoporum komareki TAU-MAC 1515 was used as the outgroup. GenBank accession numbers of sequences are given in Table S3. The scale corresponds to substitutions/site. Table S1: cyanobacteria-specific primers used for PCR amplification and/or Sanger sequencing of 16S rRNA gene and 16S–23S ITS region. Table S2: the 243 taxa included in the 16S rRNA phylogenetic analyses and their GenBank accession numbers. Table S3: Nucleotide sequences used in the 16S rRNA and 16S–23S ITS combined analyses. Table S4: comparison of the 16S rRNA sequence similarity between Pseudanabaena and other closely related strains. Similarity = 100 × [1 − (p-distance)]. Table S5: 16S–23S ITS percent dissimilarity matrix (100 * p-distance) for members of the Pseudanabaena clade and Thalassoporum. The p-distance was calculated in MEGA 7 using the Kimura-2 parameter model. Percent dissimilarity values are less than 3% within species and above 7% between species. Intermediate values (3–7%) are considered ambiguous. Table S6: lengths of conserved domains of the 16S–23S Internal Transcribed Space (ITS) of Pseudanabaena species and Thalassoporum. -; missing information due to incomplete 16S–23S ITS nucleotide sequence. Table S7. Morphological comparison of the newly described species P. epilithica and P. suomiensis and all taxonomically accepted species of Pseudanabaena presented in Komárek and Anagnostidis [1], Skuja [2], Niiyama et al. [3], Tuji and Niiyama [4], Kling et al. [5] and Turicchia et al. [6]. Names of all taxonomically accepted Pseudanabaena species have been obtained from AlgaeBase [7]. N/A = not available, −; no.
Author Contributions
Conceptualization, M.C. and K.S.; methodology, M.C. (isolation of cyanobacteria, morphological and molecular evaluation, type material preparation, antimicrobial susceptibility testing, phycobiliprotein extraction) and M.W. (LC-MS analysis); validation, M.C. and M.W.; formal analysis, M.C.; investigation, M.C.; resources, M.C. and K.S.; data curation, M.C.; writing—original draft preparation, M.C.; writing—review and editing, M.C. and K.S.; visualization, M.C.; supervision, K.S.; project administration, K.S.; funding acquisition, M.C. and K.S. All authors have read and agreed to the published version of the manuscript.
Funding
M.C. is supported by a scholarship provided by the Vilho, Yrjö and Kalle Väisälä Foundation of the Finnish Academy of Science and Letters. This work was supported by a NordForsk grant (NordAqua; 82845) to K.S. M.C. would like to thank the senior museum curators and technicians Henry Väre, Pertti Salo and Sanna Laine for their help during holotype deposition at LUOMUS. Open access funding provided by University of Helsinki.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All nucleotide sequences used in this study are available from NCBI. For accession numbers of all nucleotide sequences used in phylogenetic analyses, please refer to Supplementary Materials. Aligned datasets used in phylogenetic analyses can be provided by the corresponding author upon request. Active cultures of both P. epilithica UHCC 1008T and P. suomiensis UHCC 1008T are deposited in the University of Helsinki Cyanobacteria Collection (HAMBI/UHCC) and are publicly available for ordering (https://www.helsinki.fi/en/infrastructures/biodiversity-collections/infrastructures/microbial-domain-biological-resource-centre-hambi#ordering) (accessed on 25 April 2023). The holotypes of both strains are publicly available through the Finnish Museum of Natural History (LUOMUS).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Whitton, B.A.; Potts, M. Introduction to the Cyanobacteria. In Ecology of Cyanobacteria II; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–13. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 2. Teil/2nd Part: Oscillatoriales. In Süsswasserflora von Mitteleuropa 19/2; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Elsevier/Spektrum: Heidelberg, Germany, 2005; p. 759. [Google Scholar]
- Engene, N.; Choi, H.; Esquenazi, E.; Rottacker, E.C.; Ellisman, M.H.; Dorrestein, P.C.; Gerwick, W.H. Underestimated biodiversity as a major explanation for the perceived rich secondary metabolite capacity of the cyanobacterial genus Lyngbya. Environ. Microbiol. 2011, 13, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.; Komárek, J.; Kaštovský, J. System of cyanoprokaryotes (cyanobacteria)—State in 2004. Arch. Hydrobiol. Suppl. Algol. Stud. 2005, 117, 95–115. [Google Scholar] [CrossRef]
- Komárek, J. A polyphasic approach for the taxonomy of cyanobacteria: Principles and applications. Eur. J. Phycol. 2016, 51, 346–353. [Google Scholar] [CrossRef]
- Boyer, S.L.; Flechtner, V.R.; Johansen, J.R. Is the 16S-23S rRNA internal transcribed spacer region a good tool for use in molecular systematics and population genetics? A case study in cyanobacteria. Mol. Biol. Evol. 2001, 18, 1057–1069. [Google Scholar] [CrossRef]
- Johansen, J.R.; Casamatta, D.A. Recognizing cyanobacterial diversity through adoption of a new species paradigm. Arch. Hydrobiol. Suppl. Algol. Stud. 2005, 117, 71–93. [Google Scholar] [CrossRef]
- Osorio-Santos, K.; Pietrasiak, N.; Bohunická, M.; Miscoe, L.H.; Kovácik, L.; Martin, M.P.; Johansen, J.R. Seven new species of Oculatella (Pseudanabaenales, Cyanobacteria): Taxonomically recognizing cryptic diversification. Eur. J. Phycol. 2014, 49, 450–470. [Google Scholar] [CrossRef]
- Bohunická, M.; Pietrasiak, N.; Johansen, J.R.; Gómez, E.B.; Hauer, T.; Gaysina, L.A.; Lukešová, A. Roholtiella, gen. nov. (Nostocales, Cyanobacteria)—A tapering and branching cyanobacteria of the family Nostocaceae. Phytotaxa 2015, 197, 84–103. [Google Scholar] [CrossRef]
- Jung, P.; Sommer, V.; Karsten, U.; Lakatos, M. Salty twins: Salt-tolerance of terrestrial Cyanocohniella strains (Cyanobacteria) and description of C. rudolphia sp. nov. point towards a marine origin of the genus and terrestrial long distance dispersal patterns. Microorganisms 2022, 10, 968. [Google Scholar] [CrossRef]
- Baldarelli, L.M.; Pietrasiak, N.; Osorio-Santos, K.; Johansen, J.R. Mojavia aguilerae and M. dolomitestris—Two new Nostocaceae (Cyanobacteria) species from the Americas. J. Phycol. 2022, 58, 502–516. [Google Scholar] [CrossRef]
- Pietrasiak, N.; Osorio-Santos, K.; Shalygin, S.; Martin, M.P.; Johansen, J.R. When is a lineage a species? A case study in Myxacorys gen. nov. (Synechococcales: Cyanobacteria) with the description of two new species from the Americas. J. Phycol. 2019, 55, 976–996. [Google Scholar] [CrossRef]
- Mai, T.; Johansen, J.R.; Pietrasiak, N.; Bohunická, M.; Martin, M.P. Revision of the Synechococcales (Cyanobacteria) through recognition of four families including Oculatellaceae fam. nov. and Trichocoleaceae fam. nov. and six new genera containing 14 species. Phytotaxa 2018, 365, 1–59. [Google Scholar] [CrossRef]
- Erwin, P.M.; Thacker, R.W. Cryptic diversity of the symbiotic cyanobacterium Synechococcus spongiarum among sponge hosts. Mol. Ecol. 2008, 17, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Mareš, J.; Johansen, J.R.; Hauer, T.; Zima, J.; Ventura, S.; Cuzman, O.; Tiribilli, B.; Kaštovský, J. Taxonomic resolution of the genus Cyanothece (Chroococcales, Cyanobacteria), with a treatment on Gloeothece and three new genera, Crocosphaera, Rippkaea, and Zehria. J. Phycol. 2019, 55, 578–610. [Google Scholar] [CrossRef] [PubMed]
- González-Resendiz, L.; Johansen, J.R.; León-Tejera, H.; Sánchez, L.; Segal-Kischinevzky, C.; Escobar-Sánchez, V.; Morales, M. A bridge too far in naming species: A total evidence approach does not support recognition of four species in Desertifilum (Cyanobacteria). J. Phycol. 2019, 55, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Zammit, G.; Billi, D.; Albertano, P. The subaerophytic cyanobacterium Oculatella subterranea (Oscillatoriales, Cyanophyceae) gen. et sp. nov.: A cytomorphological and molecular description. Eur. J. Phycol. 2012, 47, 341–354. [Google Scholar] [CrossRef]
- Dvořák, P.; Jahodářová, E.; Hašler, P.; Gusev, E.; Poulíčková, A. A new tropical cyanobacterium Pinocchia polymorpha gen et sp nov derived from the genus Pseudanabaena. Fottea 2015, 15, 113–120. [Google Scholar] [CrossRef]
- Miscoe, L.H.; Johansen, J.R.; Kociolek, J.P.; Lowe, R.L.; Vaccarino, M.A.; Pietrasiak, N.; Sherwood, A.R. Novel Cyanobacteria from Caves on Kauai, Hawaii. In The Diatom Flora and Cyanobacteria from Caves on Kauai, Hawaii; Miscoe, L.H., Johansen, J.R., Kociolek, J.P., Lowe, R.L., Vaccarino, M.A., Pietrasiak, N., Sherwood, A.R., Eds.; J. Cramer: Stuttgart, Germany, 2016; pp. 75–152. ISBN 9783443600471. [Google Scholar]
- Perkerson, R.B.; Johansen, J.R.; Kovácik, L.; Brand, J.; Kaštovský, J.; Casamatta, D.A. A unique pseudanabaenalean (cyanobacteria) genus Nodosilinea gen. nov. based on morphological and molecular data. J. Phycol. 2011, 47, 1397–1412. [Google Scholar] [CrossRef]
- Komárek, J.; Kaštovský, J.; Mareš, J.; Johansen, J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Strunecký, O.; Ivanova, A.P.; Mareš, J. An updated classification of cyanobacterial orders and families based on phylogenomic and polyphasic analysis. J. Phycol. 2023, 59, 12–51. [Google Scholar] [CrossRef]
- Turicchia, S.; Ventura, S.; Komárková, J.; Komárek, J. Taxonomic evaluation of cyanobacterial microflora from alkaline marshes of northern Belize. 2. Diversity of oscillatorialean genera. Nov. Hedwig. 2009, 89, 165–200. [Google Scholar] [CrossRef]
- Skuja, H. Some Algae and Protists. In Vegetation of Amchitka Island, Aleutian Islands, Alaska; Shacklette, H.T., Durrell, L.W., Erdman, J.A., Keith, J.R., Klein, W.M., Krog, H., Persson, H., Skuja, H., Weber, W.A., Eds.; United States Government Printing Office: Washington, DC, USA, 1969. [Google Scholar]
- Konstantinou, D.; Voultsiadou, E.; Panteris, E.; Gkelis, S. Revealing new sponge-associated cyanobacterial diversity: Novel genera and species. Mol. Phylogenet. Evol. 2021, 155, 106991. [Google Scholar] [CrossRef]
- Niiyama, Y.; Tuji, A.; Takemoto, K.; Ichise, S. Pseudanabaena foetida sp. nov. and P. subfoetida sp. nov. (cyanophyta/cyanobacteria) producing 2–methylisoborneol from Japan. Fottea 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Tuji, A.; Niiyama, Y. The Identity and phylogeny of Pseudanabaena strain, NIES-512, producing 2-methylisoborneol (2-MIB). Bull. Natl. Mus. Nat. Sci. Ser. B 2016, 42, 83–89. [Google Scholar]
- Tuji, A.; Niiyama, Y. Two new Pseudanabaena (Cyanobacteria, Synechococcales) species from Japan, Pseudanabaena cinerea and Pseudanabaena yagii, which produce 2-methylisoborneol. Phycol. Res. 2018, 66, 291–299. [Google Scholar] [CrossRef]
- Chorus, I.; Walker, M. Cyanobacterial Toxins. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Chorus, I., Walker, M., Eds.; CRC Press, on behalf of the World Health Organization: Geneva, Switzerland, 2021; pp. 15–162. [Google Scholar]
- Olvera-Ramírez, R.; Centeno-Ramos, C.; Martínez-Jerónimo, F. Toxic effects of Pseudanabaena tenuis (cyanobacteria) on the cladocerans Daphnia magna and Ceriodaphnia dubia. Hidrobiologica 2010, 20, 203–212. [Google Scholar]
- Humisto, A.; Herfindal, L.; Jokela, J.; Karkman, A.; Bjørnstad, R.; Choudhury, R.R.; Sivonen, K. Cyanobacteria as a source for novel anti-leukemic compounds. Curr. Pharm. Biotechnol. 2016, 17, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Felczykowska, A.; Pawlik, A.; Mazur-Marzec, H.; Toruńska-Sitarz, A.; Narajczyk, M.; Richert, M.; Wegrzyn, G.; Herman-Antosiewicz, A. Selective inhibition of cancer cells’ proliferation by compounds included in extracts from Baltic Sea cyanobacteria. Toxicon 2015, 108, 1–10. [Google Scholar] [CrossRef]
- Cano-Europa, E.; Ortiz-Butrón, R.; Gallardo-Casas, C.A.; Blas-Valdivia, V.; Pineda-Reynoso, M.; Olvera-Ramírez, R.; Franco-Colin, M. Phycobiliproteins from Pseudanabaena tenuis rich in c-phycoerythrin protect against HgCl2-caused oxidative stress and cellular damage in the kidney. J. Appl. Phycol. 2010, 22, 495–501. [Google Scholar] [CrossRef]
- Cegłowska, M.; Szubert, K.; Grygier, B.; Lenart, M.; Plewka, J.; Milewska, A.; Lis, K.; Szczepański, A.; Chykunova, Y.; Barreto-Duran, E.; et al. Pseudanabaena galeata CCNP1313—Biological activity and peptides production. Toxins 2022, 14, 330. [Google Scholar] [CrossRef]
- Turland, N.; Wiersema, J.; Barrie, F.; Greuter, W.; Hawksworth, D.; Herendeen, P.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017; Turland, N., Wiersema, J., Barrie, F., Greuter, W., Hawksworth, D., Herendeen, P., Knapp, S., Kusber, W.-H., Li, D.-Z., Marhold, K., et al., Eds.; Regnum Vegetabile; Koeltz Botanical Books: Glashütten, Germany, 2018; Volume 159, ISBN 9783946583165. [Google Scholar]
- Kotai, J. Instructions for preparation of modified nutrient solution Z8 for algae. Nor. Inst. Water Res. 1972, 11, 1–5. [Google Scholar]
- Rippka, R.; Waterbury, J.B.; Stanier, R.Y. Isolation and purification of cyanobacteria: Some general principles. In The Prokaryotes; Starr, M.P., Stolp, H., Trüper, H.G., Balows, A., Schlegel, H.G., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 212–220. [Google Scholar]
- Christodoulou, M.; Jokela, J.; Wahlsten, M.; Saari, L.; Economou-Amilli, A.; de Fiore, M.F.; Sivonen, K. Description of Aliinostoc alkaliphilum sp. nov. (Nostocales, Cyanobacteria), a new bioactive metabolite-producing strain from Salina Verde (Pantanal, Brazil) and taxonomic distribution of bioactive metabolites in Nostoc and Nostoc-like genera. Water 2022, 14, 2470. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication, National University of Ireland: Galway, Ireland, 2022. [Google Scholar]
- Kling, H.J.; Dail Laughinghouse, I.; Šmarda, J.; Komárek, J.; Acreman, J.; Bruun, K.; Watson, S.B.; Chen, F. A new red colonial Pseudanabaena (Cyanoprokaryota, Oscillatoriales) from North American large lakes. Fottea 2012, 12, 327–339. [Google Scholar] [CrossRef]
- Nübel, U.; Garcia-Pichel, F.; Muyzer, G. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl. Environ. Microbiol. 1997, 63, 3327–3332. [Google Scholar] [CrossRef] [PubMed]
- Taton, A.; Grubisic, S.; Brambilla, E.; De Wit, R.; Wilmotte, A. Cyanobacterial diversity in natural and artificial microbial mats of lake Fryxell (McMurdo Dry Valleys, Antarctica): A morphological and molecular approach. Appl. Environ. Microbiol. 2003, 69, 5157–5169. [Google Scholar] [CrossRef] [PubMed]
- Hrouzek, P.; Ventura, S.; Lukešová, A.; Mugnai, M.-A.; Turicchia, S.; Komárek, J. Diversity of soil Nostoc strains: Phylogenetic and phenotypic variability. Arch. Hydrobiol. Suppl. Algol. Stud. 2005, 117, 251–264. [Google Scholar] [CrossRef]
- Wilmotte, A.; Van der Auwera, G.; De Wachter, R. Structure of the 16 S ribosomal RNA of the thermophilic cyanobacterium Chlorogloeopsis HTF (‘Mastigocladus laminosus HTF’) strain PCC7518, and phylogenetic analysis. FEBS Lett. 1993, 317, 96–100. [Google Scholar] [CrossRef]
- Iteman, I.; Rippka, R.; De Marsac, N.T.; Herdman, M. Comparison of conserved structural and regulatory domains within divergent 16S rRNA-23S rRNA spacer sequences of cyanobacteria. Microbiology 2000, 146, 1275–1286. [Google Scholar] [CrossRef]
- Boyer, S.L.; Johansen, J.R.; Flechtner, V.R.; Howard, G.L. Phylogeny and genetic variance in terrestrial Microcoleus (cyanophyceae) species based on sequence analysis of the 16s rRNA gene and associated 16S–23S ITS region. J. Phycol. 2002, 38, 1222–1235. [Google Scholar] [CrossRef]
- Wright, E.S.; Yilmaz, L.S.; Noguera, D.R. DECIPHER, a search-based approach to chimera identification for 16S rRNA sequences. Appl. Environ. Microbiol. 2012, 78, 717–725. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Vences, M.; Patmanidis, S.; Kharchev, V.; Renner, S.S. Concatenator, a user-friendly program to concatenate DNA sequences, implementing graphical user interfaces for MAFFT and FastTree. Bioinform. Adv. 2022, 2, vbac050. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating Maximum-Likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 20 April 2022).
- The Inkscape team. The Inkscape Project. Available online: https://inkscape.org (accessed on 10 May 2018).
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Bohunická, M.; Johansen, J.R.; Fučíková, K. Tapinothrix clintonii sp. nov. (Pseudanabaenaceae, Cyanobacteria), a new species at the nexus of five genera. Fottea 2011, 11, 127–140. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Zavřel, T.; Chmelík, D.; Sinetova, M.A.; Červený, J. Spectrophotometric determination of phycobiliprotein content in cyanobacterium Synechocystis. J. Vis. Exp. 2018, 2018, 58076. [Google Scholar] [CrossRef]
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G.; et al. CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria. Water Res. 2021, 196, 117017. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Tuji, A.; Niiyama, Y. The taxonomy of Pseudanabaena (Cyanobacteria) and characters for judging the production of odor compounds. Japanese J. Water Treat. Biol. 2018, 54, 115–120. [Google Scholar] [CrossRef]
- Yu, G.; Zhu, M.; Chen, Y.; Pan, Q.; Chai, W.; Li, R. Polyphasic characterization of four species of Pseudanabaena (Oscillatoriales, Cyanobacteria) from China and insights into polyphyletic divergence within the Pseudanabaena genus. Phytotaxa 2015, 192, 1–12. [Google Scholar] [CrossRef]
- Cegłowska, M.; Toruńska-Sitarz, A.; Stoń-Egiert, J.; Mazur-Marzec, H.; Kosakowska, A. Characteristics of cyanobacterium Pseudanabaena galeata CCNP1313 from the Baltic Sea. Algal Res. 2020, 47, 101861. [Google Scholar] [CrossRef]
- Albrecht, M.; Pröschold, T.; Schumann, R. Identification of cyanobacteria in a eutrophic coastal lagoon on the Southern Baltic Coast. Front. Microbiol. 2017, 8, 923. [Google Scholar] [CrossRef]
- Tan, H.T.; Yusoff, F.M.; Khaw, Y.S.; Noor Mazli, N.A.I.; Nazarudin, M.F.; Shaharuddin, N.A.; Katayama, T.; Ahmad, S.A. A review on a hidden gem: Phycoerythrin from blue-green algae. Mar. Drugs 2023, 21, 28. [Google Scholar] [CrossRef]
- Dagnino-Leone, J.; Figueroa, C.P.; Castañeda, M.L.; Youlton, A.D.; Vallejos-Almirall, A.; Agurto-Muñoz, A.; Pavón Pérez, J.; Agurto-Muñoz, C. Phycobiliproteins: Structural aspects, functional characteristics, and biotechnological perspectives. Comput. Struct. Biotechnol. J. 2022, 20, 1506–1527. [Google Scholar] [CrossRef]
- Patel, S.N.; Sonani, R.R.; Jakharia, K.; Bhastana, B.; Patel, H.M.; Chaubey, M.G.; Singh, N.K.; Madamwar, D. Antioxidant activity and associated structural attributes of Halomicronema phycoerythrin. Int. J. Biol. Macromol. 2018, 111, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Blas-Valdivia, V.; Rojas-Franco, P.; Serrano-Contreras, J.I.; Sfriso, A.A.; Garcia-Hernandez, C.; Franco-Colín, M.; Cano-Europa, E. C-phycoerythrin from Phormidium persicinum prevents acute kidney injury by attenuating oxidative and endoplasmic reticulum stress. Mar. Drugs 2021, 19, 589. [Google Scholar] [CrossRef] [PubMed]
- Soni, B.; Visavadiya, N.P.; Madamwar, D. Ameliorative action of cyanobacterial phycoerythrin on CCl4-induced toxicity in rats. Toxicology 2008, 248, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Soni, B.; Visavadiya, N.P.; Madamwar, D. Attenuation of diabetic complications by C-phycoerythrin in rats: Antioxidant activity of C-phycoerythrin including copper-induced lipoprotein and serum oxidation. Br. J. Nutr. 2009, 102, 102–109. [Google Scholar] [CrossRef]
- Komárek, J. Several problems of the polyphasic approach in the modern cyanobacterial system. Hydrobiologia 2018, 811, 7–17. [Google Scholar] [CrossRef]
- Dvořák, P.; Jahodářová, E.; Casamatta, D.A.; Hašler, P.; Poulíčková, A. Difference without distinction? Gaps in cyanobacterial systematics; when more is just too much. Fottea 2018, 18, 130–136. [Google Scholar] [CrossRef]
- Komárek, J. Quo vadis, taxonomy of cyanobacteria (2019). Fottea 2020, 20, 104–110. [Google Scholar] [CrossRef]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Rosselló-Móra, R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Jonas, E. Taxonomic parameters revisited: Tarnished gold standards. Microbiol. Today 2006, 8, 152–155. [Google Scholar]
- Kim, M.; Oh, H.S.; Park, S.C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef]
- Řeháková, K.; Mareš, J.; Lukešová, A.; Zapomělová, E.; Bernardová, K.; Hrouzek, P. Nodularia (Cyanobacteria, Nostocaceae): A phylogenetically uniform genus with variable phenotypes. Phytotaxa 2014, 172, 235–246. [Google Scholar] [CrossRef]
- Martins, M.D.; Rigonato, J.; Taboga, S.R.; Branco, L.H.Z. Proposal of Ancylothrix gen. nov., a new genus of Phormidiaceae (Cyanobacteria, Oscillatoriales) based on a polyphasic approach. Int. J. Syst. Evol. Microbiol. 2016, 66, 2396–2405. [Google Scholar] [CrossRef]
- Buch, B.; Martins, M.D.; Branco, L.H.Z. A widespread cyanobacterium supported by polyphasic approach: Proposition of Koinonema pervagatum gen. & sp. nov. (Oscillatoriales). J. Phycol. 2017, 53, 1097–1105. [Google Scholar] [CrossRef]
- Martins, M.D.; Branco, L.H.Z. Potamolinea gen. nov. (Oscillatoriales, Cyanobacteria): A phylogenetically and ecologically coherent cyanobacterial genus. Int. J. Syst. Evol. Microbiol. 2016, 66, 3632–3641. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic note: A Place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).