Abstract
The mutualistic interspecific relationships of trophobiosis between trophobiont planthoppers (Hemiptera, Fulgoromorpha) providing food to the host called xenobiont, are reviewed. The degree of interspecific relationships between these symbionts varies from occasional or short time duration (a few hours to a few days) to longer ones, with trophobionts left free to escape (optobiotic type) by the xenobiont, or maintained enclosed in nests or ant shelters (cryptobiotic type). Of 267 collected cases, 126 are new illustrated observations. Occasional trophobiosis is documented in 13 families of planthoppers and appears to be quite general in Fulgoromorpha, although it is reported for the first time for Dictyopharidae, Eurybrachidae, and Nogodinidae. Xenobionts associated with planthoppers are reported from ants and other Hymenoptera, Lepidoptera, and Blattodea, but also from Mollusca and even small gekkonid vertebrates. Tettigometridae appear to be exclusively tended by ants, while Fulgoridae significantly more often by cockroaches (40%) than by ants (27%). Long-time trophobiosis occurs always with ants, cryptobiotic ones reported in Cixiidae, Delphacidae, Tettigometridae, Meenoplidae, Flatidae and Hypochthonellidae, while optobiotic ones remain restricted to tettigometrids. A particular focus on Tettigometridae attended by ants is provided with new etho-ecological observations of 92 currently described tettigometrids species, 32 different species (35%) are now known to be able to be ant-attended. In Bulgaria, where fourteen species occur, trophobiosis occurs with at least five species of them (36%). In tettigometrids, subsociality, sessility, and underground life appear to be key factors allowing more complex relationships with ants. However, the planthopper size and thus the amount of food (drops of honeydew) is probably also an important factor. This might explain many new observations in large-sized and often isolated fulgorids with cockroaches. Tapping of trophobiont forewings by cockroaches, moths, or of the bark subtrate by geckos has been observed, but antennal palpation behaviours by ants are the most commonly observed with tettigometrids, although not with larger planthoppers. In tettigometrids, specific tegumentary glands secretions (allomones) of the abdomen pleurites might also mediate their long-term mutualistic associations, even possibly completing honeydew kairomones actions mediating planthopper trophobiosis in general.
1. Introduction
Trophobiosis is defined as a disjunctive symbiotic association based on an interspecific relationship between two symbiotic organisms: one member, the host or trophobiont, providing food to the second. This particular type of interspecific interaction ranges from irregular and facultative relationships to mutualistic long-term associations, some being permanent, where both symbiotic organisms gain benefit. Trophobiosis is best known between ants (Insecta, Hymenoptera, Formicidae) and hemipterans (Insecta, Hemiptera) [1,2]. It also occurs between some other taxa, such as in well-known cases of ants and Lycaenidae (Insecta, Lepidoptera) [3]. Among Hemiptera, aphids and coccids (Sternorrhyncha) are commonly known to enter in mutualistic associations with ants, but this type of association occurs more rarely also with Auchenorrhyncha species of both suborders, Cicadomorpha and Fulgoromorpha [4,5,6].
With Hemiptera, ants might exploit the relationship on a short term (occasional trophobiotic interactions) or on a longer term (trophobiosis evolving into mutualistic interactions) by few to several or many individuals. In exchange, ants obtain drops of honeydew excreted by the host, a sugar-rich source, resulting from feeding from phloem vessels by these phytophagous hoppers. The secreted liquid metabolic waste remains, however, rich in nutrients (various sugars, amino acids) for the attending host [7]. Its chemical composition depends on several biological factors of the hopper (instar, symbiotic bacteria present in the gut) but also of the host plant and the pressure of its infestation (review in [7]). When non-attended, the feeding planthopper regularly produces a drop of honeydew at the end of the anal tube and, by a kicking movement of the abdomen and legs, projects the drop in the near environment of the insect. Honeydew drops act both as a volatile and contact pheromone due to the natural weathering (fermentation, oxidation, etc.) of the sugars and amino acids contained in this excretion [7] and might attract predators and parasitoids. However, it is also an important source of nutrition for attending hosts and particularly for ants which might enter into a mutualistic interaction with the trophobiont providing it. In exchange, protection from predators and parasitoids, transport when environmental conditions are unfavourable, waste removal, and protection of eggs and nymphs are provided by the ants [5]. Costs and benefits of these associations to both hemipterans and ants was recently reviewed in [2].
Most observations and investigations on trophobiosis between ants and Auchenorrhyncha have been made within members of the Cicadomorpha group (mainly Membracidae from the tropics and subtropics), but less so with Fulgoromorpha where it has been reported sporadically in a few planthopper families only: Caliscelidae, Cixiidae, Delphacidae, Issidae, Flatidae, Fulgoridae, Meenoplidae, and Hypochthonellidae (reviewed in [4,6,8]). However, among Fulgoromorpha most mutualistic ant-attended records concern the family Tettigometridae for which both some morphological and behavioural characters (sedentary and gregarious habits) were considered as facilitating ant attendance [4].
In the frame of a wider project about interspecific relationships of the planthoppers with other associated species, we provide here the review of the different aspects of trophobiosis from 13 Fulgoromorpha families (267 cases) with a special focus on tettigometrids, which include 40.8% of the observations.
2. Materials and Methods
Our bibliographic survey on trophobiosis was supplemented both by our personal photographic documents and by those collected for several years by data mining on the Internet. This approach has also been successfully reinforced by the involvement of citizen science—in particular, thanks to the project Knowledge of the Biodiversity of Fulgoridae in Cambodia [9]. This heuristic strategy makes it possible to rely on persistent and verifiable information provided by photographic data by objectively documenting a trophobiotic relationship and by confirming, as precisely as possible, the taxonomic determination of the trophobiont-xenobiont pair.
Additionally, ant-associated tettigometrids were particularly studied in surveys of the Auchenorrhyncha fauna, mainly in Bulgaria, but also in some other Southern European countries—Albania, North Macedonia, Greece, Malta, Italy, and France between 2001 and 2019. Observations of ant visits or co-habitation were recorded and photographed and voucher specimens of trophobionts, ants and host plants were sampled and identified. The tettigometrids were collected by direct sampling or by using an entomological net. In most cases, both tettigometrids and ants were stored in ethyl acetate and transported to the laboratory, while plants were dried using a standard method and stored in an herbarium. During field observations, the life stages, the location on the host plant or another type of microhabitat, the size of aggregations, the presence of direct contact and stimuli, and various type of ant care—antennating, egg protection, transfer of nymphs and pushing of adults—were also documented.
In all symbiotic pairs when the trophobiont could be identified (n = 108), its body length was estimated by the average of the minimum and maximum lengths provided with its original description or taxonomic revisions, or directly from specimens in collection. For larger fulgorid species, it was counted from the top of the cephalic capsule in dorsal view to the end of the tegmina. When the trophobiont was only identified to genus, an approximation to the average length of the known species in the genus and in the distribution area where it was reported was used (Appendix A).
3. Results
Two hundred and sixty-seven trophobiont-xenobiont pairs that have been collected from the published literature and internet resources (Table 1), 126 of which (47.2%) are newly reported, with most being illustrated here. Two observations remain doubtful as being true trophobiosis relationships and were not included in our calculations (Table 1). One hundred and seven different planthoppers were identified to species.

Table 1.
Numbers of trophobiosis observations (distinct trophobiont-xenobiont pairs) reported by planthoppers families, for identified planthopper taxa (at least to genus level), and by xenobiont taxa. For Tettigometridae, figures are also reported by subfamilies. In brackets: 2 probably non-true trophobiosis cases.
Ant-attended planthoppers are the most frequent cases with 204 observations (76.8%) and have been reported for all planthopper families except for Ricaniidae. However, other groups are also reported, particularly cockroaches (35 cases, 13.1%), but also gekkonids (16 cases, 6%), moths (8 cases, 3%) and gastropods (5 cases, 1.9%).
If tettigometrids appear to be exclusively tended by ants, fulgorids are more often tended by cockroaches (31 cases, 40%) than by ants (19 cases, 27%), while the other observations also concern moths (8 cases, 11.4%), gekkonids (8 cases, 11.4%) and Gasteropods (5 cases, 7%). Trophobiosis involving cockroaches are mainly reported for fulgorids (80%) but are also known from Cixiidae (8.6%) and Eurybrachidae, Flatidae, Issidae, and Ricaniidae (2.8% each). For 178 symbiont pairs in which the trophobiont was identified at least to the genus level (147 involving ants and 31 cockroaches), a statistical two-sample Student t-test on the average size of the trophobionts shows significantly (p = 0.0054, threshold 5%) that cockroaches xenobionts are more likely to enter in trophobiose with larger sized trophobionts than ants (Figure 1).
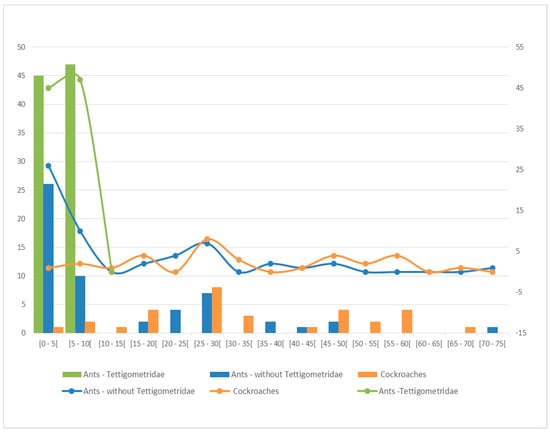
Figure 1.
Number of trophobiosis observations according to average trophobiont size (in mm) being tended by ants for Tettigometridae alone (green), by ants for other planthoppers without tettigometrids (blue) or by cockroaches for all planthoppers (orange).
All data were extracted either from literature references or from original photos collected on the web or newly provided here, and carefully checked to confirm trophobiont identification to species or genus. Thirteen planthoppers families of twenty-one currently recognized [10] are concerned: Caliscelidae (Figure 2), Cixiidae (Figure 3 and Figure 4), Delphacidae (Figure 5), Dictyopharidae and Eurybrachidae (Figure 6), reported for the time, Flatidae (Figure 7), Fulgoridae (Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15, Figure 16, Figure 17, Figure 18, Figure 19, Figure 20, Figure 21 and Figure 22), Nogodinidae (Figure 23) also reported for the first time, Tettigometridae: Nototettigometrinae (Figure 24, Figure 25, Figure 26 and Figure 27), Tettigometridae: Tettigometrinae (Figure 28, Figure 29, Figure 30, Figure 31, Figure 32, Figure 33, Figure 34 and Figure 35), and a few indetermined planthoppers (Figure 36). For the most important families, 109 cases (40.8%) involve 35 different tettigometrid species and 72 cases (26.9%) involve 36 different fulgorid species; delphacids remain poorly documented for 11% of the observations, followed by cixiids (7%) and issids (5%).

Figure 2.
Caliscelidae: (a,b) Caliscelis wallengreni (Stål, 1863) tended by Formicidae: Formicinae: Lasius sp., Hungary: Tiszagyenda vill., 47°23′57.8″ N 20°30′05.8″ E, 25.07.2021, obs. M. de Haas.

Figure 3.
Cixiidae: (a) Bothriocera sp. tended by Formicidae: Formicinae: Camponotus sexguttatus (Fabricius, 1793), USA: Florida, Pinelands, Everglades National Park, Homestead, 07.12.2015, obs. J. Gallagher; (b) Hyalesthes sp. tended by Formicidae: Formicinae: Camponotus piceus (Leach, 1825), France: Provence-Alpes-Côte d’Azur, Solliès-Toucas, 43°11′38.6″ N 6°00′33.2″ E, 29.07.2021, obs. B. Noguès; (c,d) Reptalus sp. tended by Formicidae: Myrmicinae: Aphaenogaster subterranea (Latreille, 1798), Bulgaria: Eastern Rhodopes Mt., Oreshino vill., 41°28′19.3″ N 26°05′30.0″ E, 243 m, 20.04.2012, nymphs in ant nest under stone, obs. I. Gjonov, A. Lapeva-Gjonova; (e) Singabenna praestans (Walker, 1857) tended by Blattodea indet., Malaysia: Sarawak, Borneo, Bako National Park, 1°42′44″ N 110°26′46″ E, 07.01.2021, obs. C. Lee.

Figure 4.
Cixiidae: (a–c) Cixiidae indet. tended by Blattodea indet., Malaysia: Sarawak, Borneo, Bako National Park, 1°43′ N 110°28′ E, 9.07.2016, adult on tree trunk, obs. X. Vermeersch; (d) Cixiidae indet. tended by Blattodea indet., Malaysia: Sarawak, Borneo, Selangor, 3°20′ N 101°30′ E, 05.2016, adult on tree trunk, obs. H.P. Guek; (e) Cixiinae indet. tended by Formicidae: Myrmicinae: Pheidole sp., Madagascar, Namoroka, 16°24′25″ S 45°18′47″ E, 139 m, 24.10.2016, nymphs on tree roots in a cave, obs. G. Kunz.

Figure 5.
Delphacidae: (a) Dicranotropis beckeri Fieber, 1866 tended by Formicidae: Formicinae: Camponotus aethiops (Latreille, 1798), Bulgaria: Vlahina Mt.: Boboshevo, 42°09′50″ N 22°58′47″ E, 730 m, 02.07.2001, 24.06.2010, adult on Poacea gen. sp. (Poaceae), obs. I. Gjonov, A. Lapeva-Gjonova; (b) Tropidocephala sp. tended by Formicidae: Formicinae: Oecophylla smaragdina (Fabricius, 1775), Thailand, Bangkok, 13°42′54.9″ N 100°31′10.8″ E, 07.01.2021, adult on Imperata cylindrica (L.) P.Beauv. (Poaceae), obs. P. Asawadecharit.

Figure 6.
Eurybrachidae (a) Eurybrachys sp. tended by Formicidae indet., India: Amar Sagar, Jaisalmer Rajastan, 18.12.2020, adult male on Crotalaria sp. (Fabaceae), obs. S. Sunder Meena; (b) Eurybrachys sp. tended by Formicidae indet., India: Amar Sagar, Jaisalmer Rajastan, 18.12.2020, adult female on Heliotropium sp. (Boraginaceae), obs. S. Sunder Meena; (c) Gelastopsis insignis Kirkaldy, 1906 tended by Blattodea indet., Australia: Queesnland, Brisbane, 27°28′04″ S 153°01′41″ E, adult on Acacia sp. (Fabaceae), obs. P. Chew.

Figure 7.
Flatidae: (a) Petrusa epilepsis (Kirkaldy, 1767) tended by Formicidae indet. Puerto Rico: San Juan, Paseo del Morro, 18°28′12.6″ N 66°07′28.5″ W, 11.12.2018, nymphs and adults, obs. C. O’Neill; (b) Siphanta sp. tended by Formicidae indet., Australia: Queensland, Cairns, 16°49′10.7″ S 145°37′59.4″ E, nymph, obs. C. Page; (c) Flatoidinae sp. tended by Blattodea indet., Vietnam: Kon Chu Rang National Park, 14°28′28″ N 108°32′27″ E, 19.07.2018, adult on tree trunk, obs. J. Constant.

Figure 8.
Fulgoridae: (a) Alaruasa aerata (Distant, 1887) tended by Lepidoptera: Erebidae: Euclystis sp., Costa Rica: Wildlife Refuge Monteverde, 10°19′ N 84°49′ W, 25.08.2019, adult on tree trunk, obs. J. Carlos; (b) Belbina recurva Lallemand, 1950 tended by Blattodea indet., Madagascar: Andasibe National Park, 14°28′28″ N 108°32′27″ E, 13.02.2015, adult on tree trunk, obs. P. Bertner; (c) Dichoptera sp. tended by Reptilia: Squamata: Gekkonidae indet., Thailand: Phanom Bencha National Park, 8°14′31″ N 98°54′55″ E, 13.12.2019, adult on tree trunk, obs. C. Hübner; (d) Enchophora sanguinea Distant, 1887 tended by Gastropoda indet., Costa Rica: La Selva Biological Station, 10°25′40.6″ N 84°00′22.1″ W, 03.02.2016, adult, obs. G. Kunz.

Figure 9.
Fulgoridae: (a) Enchophora sp. tended by Blattodea: Blattidae: Eurycotis sp., Costa Rica: Puerto Viejo de Sarapiquí, La Selva Biological Station, 10°25′42.54″ N 84°0′26.68″ W, 70 m, 03.02.2014, adult on tree trunk, obs. G. Kunz; (b–d) Enchophora sp. tended by Blattodea: Ectobiidae: Macrophyllodromia maximiliani (Saussure, 1873), Costa Rica: Puerto Viejo de Sarapiquí, La Selva Biological Station, 10°25′54″ N 84°0′38.60″ W, 65 m, 09.02.2018, adult on tree trunk, obs. G. Kunz; (e) Kalidasa lanata (Drury, 1773) tended by Formicidae indet., India: Karnataka, 12°58′12″ N 77°30′ E, 07.2017, adult on tree trunk, obs. A. C. Deodhar.

Figure 10.
Fulgoridae: (a) Kalidasa nigromaculata (Gray, 1832) tended by Lepidoptera: Nolidae: Eligma narcissus (Cramer, 1775), Laos: Vientiane, 17°58′ N 102°36′ E, 10.08.2017, adult on tree trunk, obs. K. Keomani; (b) Lycorma delicatula (White, 1845) tended by Coleoptera indet., USA: Pennsylvania, Berks County, Berks, Oley, 10.10.2017, obs. E. Smyers; (c) L. delicatula tended by Diptera indet., USA: Pennsylvania, Berks County, Berks, Oley, 25.09.2017, on tree trunk, obs. E. Smyers.

Figure 11.
Fulgoridae: (a) Lycorma delicatula (White, 1845) tended by Diptera indet., USA: Pennsylvania, Berks County, Berks, Oley, 25.09.2017, on tree trunk, obs. E. Smyers; (b) L. delicatula tended by Diptera indet., USA: Pennsylvania, Berks County, Berks, Oley, 21.05.2017, nymph on tree leave, obs. E. Smyers; (c) L. delicatula tended by Formicidae indet., USA: Pennsylvania, Berks County, Berks, Oley, 25.09.2017, adults on tree trunk, obs. E. Smyers; (d) L. delicatula tended by Formicidae indet., USA: Pennsylvania, Berks County, Boyertown, 20.06.2017, nymphs on Vitis sp. (Vitaceae), obs. E. Smyers.

Figure 12.
Fulgoridae: (a) Lycorma delicatula (White, 1845) tended by Gastropoda: Limacidae indet., USA: Pennsylvania, Berks County, Berks, Oley, 08.09.2017, adults on tree trunk, obs. by E. Smyers; (b) Lystra lanata (Linnaeus, 1758) tended by Formicidae indet., Peru: Puritania river, 4°17′55.0″ S 73°16′52.7″ W, 24.01.2020, adult on tree trunk, obs. “ampatenaude”; (c) Penthicodes atomaria (Weber, 1801) tended by Blattodea: Blattidae: Periplaneta australasiae (Fabricius, 1775), Cambodia: Cardamom Mts., 04.08.2013, adult on tree trunk, obs. A. Anker; (d) Penthicodes atomaria tended by Blattodea indet., Thailand: Khao Sok National Park, 25.12.2017, adult on tree trunk obs. C. Hübner.

Figure 13.
Fulgoridae: (a) Penthicodes atomaria (Weber, 1801) tended by Blattodea indet., Thailand: Khao Sok National Park, 12.02.2018, adult on tree trunk, obs. C. Hübner; (b) P. atomaria tended by Reptilia: Squamata: Gekkonidae indet., Thailand: Khao Sok National Park, 8°56′12″ N 98°31′49″ E, 24.02.2020, adult on tree trunk, obs. C. Hübner; (c) P. pulchellus (Guérin-Méneville, 1838) tended by Blattodea indet., Cambodia: Koh Kong Province, Tatai, 11°35′13″ N 103°05′50″ E, 13.11.2017, adult on tree trunk, obs. G. Chartier; (d) P. pulchellus tended by Blattodea indet., Cambodia: Ratanakiri, Yeak Laom Lake, 13°44′ N 107°01′ E, 10.10.2017, adult on tree trunk, obs. J. Constant.
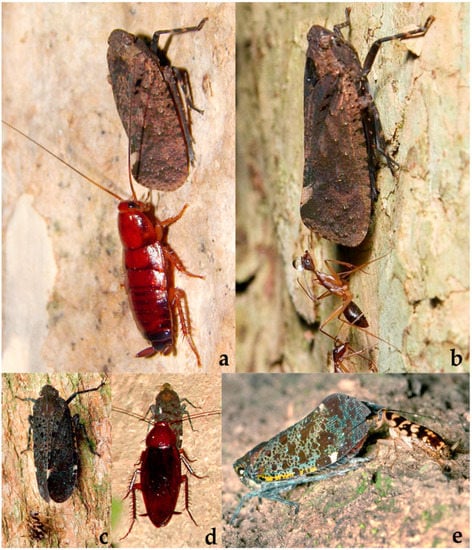
Figure 14.
Fulgoridae: (a) Penthicodes pulchellus (Guérin-Méneville, 1838) tended by Blattodea indet., Vietnam: Phuoc Binh National Park, 12°00′ N 108°47′ E, 30.07.2014, adult on tree trunk, obs. J. Constant; (b) P. pulchellus tended by Formicidae indet., Thailand: Chiang Mai, Doi Suthep Pui, 18°49′ N 98°55′ E, 10.02.2015, adult on tree trunk, obs. W. Bunkhwamdi; (c) P. quadrimaculatus Lallemand, 1963 tended by Blattodea indet., Malaysia: Lambir Hills National Park, 4°11′38.7″ N 114°01′58.3″ E, 19.03.2008, adult on tree trunk, obs. C. Lee; (d) P. variegatus Guérin-Méneville, 1829 tended by Blattodea indet., Vietnam: Nui Chua National park, 11°42′ N 109°09′ E, 8.08.2014, adult on tree trunk, obs. J. Constant; (e) P. variegatus tended by Blattodea indet., Thailand: Khao Sok National Park, 24.05.2019, obs. C. Hübner.

Figure 15.
Fulgoridae: (a) Phrictus quinquepartitus Distant, 1883 tended by Blattodea indet., Costa Rica: Guanacaste, San Jorge, 10°44′2.23″ N 85°18′2.03″ W, 617 m, 01.03.2018, adult on tree trunk, obs. G. Kunz; (b) P. quinquepartitus tended by Formicidae: Formicinae: Camponotus sp., Costa Rica: La Selva Biological Station, 10°25′42.9″ N 84°00′16.0″ W, 04.02.2016, adult, obs. G. Kunz; (c) P. quinquepartitus tended by Gastropoda: Spiraxidae: Euglandina sp., Costa Rica: Puerto Viejo de Sarapiquí, La Selva Biological Station, 10°25′49.97″ N 84° 0′18.36″ W, 53 m, 02.02.2016, adult on tree trunk, obs. G. Kunz; (d) Polydictya thompsoni Constant and Pham, 2019 tended by Lepidoptera: Nolidae: Eligma narcissus (Cramer, 1775), Thailand: Ratchaburi province, Suan Phueng district, 30.10.2020, adults on tree trunk, obs. K. Jiaranaisakul; (e) Polydictya sp. tended by Formicidae: Formicinae: Dinomyrmex gigas (Latreille, 1802), Singapore, 1°17′ N 103°50′ E, 19.10.2016, adult on tree trunk, obs. C. Nijhuis.

Figure 16.
Fulgoridae: (a) Polydictya sp. tended by Formicidae indet., Cambodia: Yeak Laom Lake, 13°44′ N 107°01′ E, 10.10.2017, adult on tree trunk, obs. J. Constant; (b) Polydictya sp. tended by Reptilia: Squamata: Gekkonidae indet., Cambodia: Yeak Laom Lake, 13°44′ N 107°01′ E, 10.10.2017, adult on tree trunk, obs. J. Constant; (c) Pyrops candelaria (Linné, 1758) tended by Reptilia: Squamata: Gekkonidae indet., Laos: Luang Prabang, Royal Palace Garden, 19°53′30″ N 102°08′08″ E, 05.02.2013, adult on tree trunk, obs. H. Rawson; (d) Pyrops candelaria tended by Reptilia: Squamata: Gekkonidae: Hemidactylus platyurus (Schneider, 1797), Thailand, Chiang Mai University Campus, 2006, obs. M. Kemal; (e) Pyrops coelestinus (Stål, 1863) tended by Blattodea indet., Vietnam: Cat Tien National Park, 11°26′ N 107°26′ E, 10.12.2016, adult on tree trunk, obs. D. Gochfeld.

Figure 17.
Fulgoridae: (a) Pyrops connectens (Atkinson, 1885) tended by Blattodea indet., Thailand: Khao Sok National Park, 8°56′12″ N 98°31′49″ E, 23.07.2018, adult on tree trunk, obs. C. Hübner; (b) Pyrops cultellatus yoshiakii Nagai and Porion, 2002 tended by Formicidae indet., Malaysia, 22.12.2015, adult on tree trunk, obs. H.P. Guek; (c) P. cultellatus yoshiakii tended by Formicidae: Formicinae: Dinomyrmex gigas (Latreille, 1802), Malaysia: Gunung Ganing National Park, 1°41′40.1″ N 109°50′40.2″ E, 27.08.2018, adult on tree trunk, obs. C. Lee; (d) P. intricatus (Walker, 1857) tended by Blattodea indet., Malaysia: Gunung Mulu National Park, 4°02′29.8″ N 114°48′56.8″ E, 01.07.2006, adult on tree trunk, obs. C. Lee; (e) P. intricatus tended by Blattodea indet., Malaysia: Gunung Mulu National Park, 4°02′23.6″ N 114°48′58.1″ E, 15.10.2009, adult on tree trunk, obs. C. Lee.

Figure 18.
Fulgoridae: (a) Pyrops sidereus (Distant, 1905) tended by Reptilia: Squamata: Gekkonidae indet., Malaysia: Borneo, Sabah, Abai, Kinabatangan River, 5°41′16″ N 118°23′01″ E, 13.05.2015, adult on tree trunk, obs. R.K. Asuncion; (b) P. spinolae (Westwood, 1842) tended by Blattodea indet., Vietnam: Kon Plong Protected Forest, 14°39′43″ N 108°15′45″ E, 20.08.2019, adult on tree trunk, obs. J. Constant; (c) P. spinolae tended by Blattodea indet., Thailand: Suan Phueng District Ratchaburi, 13°32′54.9″ N 99°12′24.2″ E, 10.08.2021, adult on tree trunk, obs. K. Jiaranaisakul; (d) P. whiteheadi (Distant, 1889) tended by Blattodea indet., Malaysia: Danum Valley Conservation Area, 4°57′48.1″ N 117°48′19.5″ E, 15.12.2008, adult on tree trunk, obs. C. Lee; (e) P. whiteheadi tended by Reptilia: Squamata: Gekkonidae: Gehyra mutilata (Wiegmann, 1834), Malaysia: Sabah, 5°44′03.0″ N 117°48′44.8″ E, 09.2018, adults on tree trunk, obs. C. Lee.

Figure 19.
Fulgoridae: (a) Pyrops whiteheadi (Distant, 1889) tended by Reptilia: Squamata: Gekkonidae indet., Malaysia: Borneo, Sabah, Sandakan, 05°50′ N 118°07′ E, 13.05.2015, adult on tree trunk, obs. R.K. Asuncion; (b) Scamandra polychroma Gerstaecker, 1895 tended by Formicidae: Formicinae: Dinomyrmex gigas (Latreille, 1802), Malaysia: Borneo, Sarawak, Mulu National Park, 4°7′55″ N 114°55′8″ E, 1.05.2006, adult on tree trunk, obs. D. Mezger; (c) S. polychroma tended by Formicidae: Formicinae: Camponotus sp., Malaysia: Borneo, Sarawak, Mulu National Park, 4°7′55″ N 114°55′8″ E, 28.06.2014, adult on tree trunk, obs. A. Kang; (d,e) S. polychroma tended by Formicidae indet., Malaysia: Borneo, Sarawak, Mulu National Park, 4°7′55″ N 114°55′8″ E, 20.09.2015, adult on tree trunk, obs. P. Soltys.
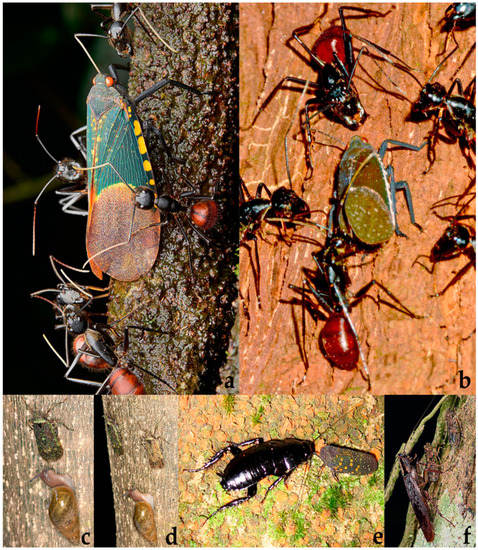
Figure 20.
Fulgoridae: (a) Scamandra polychroma Gerstaecker, 1895 tended by Formicidae indet., Malaysia: Gunung Mulu National Park, 4°08′13.8″ N 114°53′30.5″ E, 25.11.2012, adult female on tree trunk, obs. C. Lee; (b) S. polychroma tended by Formicidae: Formicinae: Dinomyrmex gigas (Latreille, 1802), Malaysia: Borneo, Sarawak, Mulu National Park, 4°7′55″ N 114°55′8″ E, 8.07.2015, adult male on tree trunk, obs. A. Kang; (c,d) Villala canoi Goemans, 2005 tended by Gastropoda: Spiraxidae: Euglandina sp., Costa Rica: Puerto Viejo de Sarapiquí, La Selva Biological Station, 10°25′ N 84°0′ W, 02.02.2012, adult on tree trunk, obs. E. Holzer; (e) Poiocerinae indet. tended by Blattodea indet., Costa Rica: Bosque Eterno de Los Niños, 10°20′08.8″ N 84°44′00.6″ W, 14.05.2011, adult on tree trunk, obs. J. G. Phillips; (f) Fulgoridae indet. tended by Blattodea indet., Ecuador: Orellana, 0°28′00.9″ S 76°50′42.7″ W, 27.02.2018, adults on tree trunk, obs. C. Lee.

Figure 21.
Issidae: (a) Hysteropterum sp. tended by Formicidae: Formicinae: Camponotus aethiops (Latreille, 1798), Croatia: Sibenik-Knin County, 4.5 km SE Primosten, Macchia, 43°34′29″ N 15°56′19″ E, 22.09.2015, 55 m, obs. G. Kunz; (b) Hysteropterum sp. tended by Blattodea: Ectobiidae indet., Croatia: Sibenik-Knin County, 4.5 km SE Primosten, Macchia, 43°34′29″ N 15°56′19″ E, 22.09.2015, 55 m, obs. G. Kunz; (c) Sarimini indet. tended by Formicidae: Formicinae: Camponotus sp., Cambodia: Keo Seima Wildlife Sanctuary, 12°11′39″ N 107°01′01″ E, 19.11.2018, adult on low vegetation, obs. J. Constant.

Figure 22.
Issidae: (a) Thioniini indet. tended by Formicidae: Formicinae: Camponotus sp., Costa Rica: La Leona Eco Lodge, 8°26′54.17″ N; 83°29′13.54″ W, 16.02.2018, adult on tree trunk, obs. G. Kunz; (b) Issidae indet. tended by Formicidae: Formicinae: Dinomyrmex gigas (Latreille, 1802), Brunei: Kg Menengah, 4°26′53.3″ N 115°12′45.5″ E, 11.08.2021, adult, obs. H. Ahmad Sah; (c) Issidae indet. tended by Formicidae indet., Malaysia: Kuala Lumpur, Perdana Botanical Garden, 3°08′20.4″ N 101°41′12.7″ E, 27.10.2019, adult, obs. T. Maul.

Figure 23.
Nogodinidae: (a) Biolleyana fenestra (Gerstaecker, 1895) tended by Formicidae: Formicinae: Camponotus sp., Costa Rica: Rio Agujitas River, 8°41′27.9″ N 83°40′42.7″ W, 02.04.2018, adult on tree trunk, obs. T. Stice; (b) Paravarcia sp. tended by Blattodea indet., Malaysia: Penang Island, 5°25′28″ N 100°16′08.1″ E, 20.01.2021, adult on unidentified tree trunk, obs. A. Kang; (c,d) Paravarcia sp. tended by Formicidae indet., Malaysia: Penang Island, 5°25′28″ N 100°16′08.1″ E, 30.11.2020, adult on unidentified tree trunk, obs. A. Kang.

Figure 24.
Tettigometridae: (a) Egropa sp. tended by Formicidae: Formicinae: Anoplolepis gracilipes (Smith, F., 1857) Malaysia: Selangor, Ampang Jaya, Tamarind Springs Jalan, 3°10′14.7″ N 101°46′14.3″ E, 19.01.2020, adults, obs. T. Maul; (b) Egropa sp. tended by Formicidae: Formicinae: Camponotus sp., Malaysia: Selangor, Ampang Jaya, Tamarind Springs Jalan, 3°10′14.7″ N 101°46′14.3″ E, 26.12.2021, adults and nymphs, obs. T. Maul; (c) Egropa sp. tended by Formicidae: Formicinae: Oecophylla smaragdina (Fabricius, 1775), Cambodia, Phnom Penh, RUPP Campus, 11°34′03.5″ N 104°53′30.2″ E, 13.05.2015, on Ficus sp., obs. J. Constant and V. Sougnez.

Figure 25.
Tettigometridae: (a) Nototettigometra (Nototettigometra) patruelis (Stål, 1855), Formicidae: Formicinae: Camponotus maculatus (Fabricius, 1782), South Africa: St Lucia, St Lucia Park, 28°22′18.8″ S 32°23′28.0″ E, 01.04.2018, nymphs and adults on Searsia chirindensis (Anacardiaceae), obs. “magdastlucia”; (b) N. (Nototettigometra) patruelis tended by Formicidae: Myrmicinae: Pheidole megacephala (Fabricius, 1793), South Africa: St Lucia, St Lucia Park, 28°22′34.3″ S 32°25′05.2″ E, 16.01.2018, nymphs and adults, obs. “magdastlucia”; (c) N. (Nototettigometra) patruelis tended by Formicidae indet., South Africa: St Lucia, iSimangaliso Wetland Park World Heritage Site, 28°22′34.3″ S 32°25′05.2″ E, 05.10.2019, nymphs and adults on Senecio polyanthemoides Sch.Bip. (Asteraceae), obs. “magdastlucia”; (d) N. (Nototettigometra) patruelis tended by Formicidae: Myrmicinae: Crematogaster sp., South Africa: Pietermaritzburg, Kingston Park, 29°33′53.7″ S 30°21′53.2″ E, 15.09.2019, nymphs and adults, obs. A. Manson.

Figure 26.
Tettigometridae: (a,b) Nototettigometra sp. near N. (N.) patruelis tended by Formicidae undet., Kenya, Masai Mara, on Senna didymobotrya (Fresen.) Irwin and Barneby (Fabaceae), 24.10.2013, obs. T. Bourgoin; (c) Nototettigometra sp. tended by Formicidae: Formicinae: Camponotus sp., South Africa: Timbavati Nature Reserve, 24°22′34.9″ S 31°09′40.4″ E, 21.05.2014, eggs and adults on Euphorbia sp. (Euphorbiaceae), obs. W. Uys; (d) Nototettigometra sp. tended by Formicidae: Formicinae undet., South Africa: South Cape, 33°58′00.3″ S 22°05′26.5″ E, 21.04.2019, adults, obs. S. Adam.

Figure 27.
Tettigometridae: (a) Nototettigometra sp. tended by Formicidae indet., Malawi: Liwonda National Park, 14°50′58.2″ S 35°17′39.2″ E, 10.11.2017, adult, obs. A. Joaquin; (b) Nototettigometra sp. tended by Formicidae indet., Botswana: Gaborone, 24°33′24.2″ S 25°57′48.7″ E, 24.04.2020, nymphs on Cassia abbreviata Oliv. (Fabaceae), obs. C. Sydes; (c, d) new genus near Nototettigometra tended by Formicidae undet., Kenya (Masai Mara, Narok), on Grewia sp. (Malvaceae), observed 22.10.2013, obs. T. Bourgoin.

Figure 28.
Tettigometridae: (a) Tettigometra (Tettigometra) atra Hagenbach, 1825 tended by Formicidae: Formicinae: Lasius platythorax Seifert, 1991, Bulgaria: Danibe plane, Rusenski Lom, 43°35′43.0″ N 26°06′25.0″ E, 24.03.2011, adults in soil ant nests under stones, obs. I. Gjonov, A. Lapeva-Gjonova; (b) T. (Tettigometra) brachycephala Fieber, 1865 tended by Formicidae: Dolichoderinae: Tapinoma sp., Croatia: Mravince, 43°32′24.8″ N 16°30′14.1″ E, 08.03.2021, obs. A. Gjeldum; (c) T. (Tettigometra) fusca Fieber, 1865 tended by Formicidae: Dolichoderinae: Tapinoma sp., Ukraine: Mykolayiv, 46°58′30.1″ N 31°59′40.5″ E, 17.03.2019, adults, obs. Roman (efarilis).

Figure 29.
Tettigometridae: (a) Tettigometra (Tettigometra) fusca Fieber, 1865 tended by Formicidae indet., Ukraine: Mykolayiv, 46°58′30.1″ N 31°59′40.5″ E, 17.03.2019, adults, obs. Roman (efarilis); (b,c) T. (Tettigometra) impressifrons Mulsant and Rey, 1855 tended by Formicidae: Dolichoderinae: Tapinoma sp., Italy: Calabria, Capo Colonna, 39°0′46″ N 17°10′49″ E, 27.04.2008, nymphs and adults on Cakile maritima Scopoli (Brassicaceae), obs. I. Gjonov, A. Lapeva-Gjonova.

Figure 30.
Tettigometridae: (a) Tettigometra (Tettigometra) laeta Herrich-Schäffer, 1834 tended by Formicidae: Dolichoderinae: Tapinoma erraticum (Latreille, 1798), Bulgaria: Eastern Rhodopes, Zhelezino vill., 41°26′60″ N 25°56′51″ E, 30.03.2012, adults in soil ant nests under stones, obs. I. Gjonov, A. Lapeva-Gjonova; (b) T. (Tettigometra) laeta tended by Formicidae: Myrmicinae: Tetramorium chefketi Forel, 1911, Albania: Strëmbec, 40°09′24.8″ N 20°28′26.0″ E, 17.06.2019, nymphs and adults on Asteraceae, obs. G. Kunz; (c) T. (Tettigometra) picta Fieber, 1865 tended by Formicidae: Dolichoderinae: Tapinoma nigerrimum (s.l.), Malta: Il-Kalanka bay, 35°49′29.0″ N 14°33′23.3″ E, 02.06.2019; nymphs and adults on Lavatera arborea L. (Malvaceae), obs. I. Gjonov, A. Lapeva-Gjonova.

Figure 31.
Tettigometridae: (a) Tettigometra (Tettigometra) sulphurea Mulsant and Rey, 1855 tended by Formicidae: Myrmicinae: Tetramorium moravicum Kratochvíl, 1941, Bulgaria: Vlahina Mt., 42°09′15.0″ N 23°02′06.0″ E, 29.06.2001, nymphs and adults on Onopordon acanthium L. (Asteraceae), obs. I. Gjonov, A. Lapeva-Gjonova; (b) T. (Tettigometra) sulphurea tended by Formicidae: Formicinae: Camponotus aethiops (Latreille, 1798), North Macedonia: Zhelino vill., 41°59′07.0″ N 21°05′41.0″ E, 16.06.2011, nymphs and adults on Onopordon acanthium (Asteraceae), obs. I. Gjonov, A. Lapeva-Gjonova; (c) T. (Tettigometra) sulphurea tended by Formicidae: Formicinae: Camponotus sp., Croatia: Split: Solin, 43°32′47.4″ N 16°28′44.9″ E, 11.05.2019, adults on Onopordon acanthium (Asteraceae), obs. A. Gjeldum.

Figure 32.
Tettigometridae: (a) Tettigometra (Tettigometra) sulphurea Mulsant and Rey, 1855 tended by Formicidae: Formicinae: Camponotus sp., Greece: Lesbos, Voreio Aigaio, 39°11′21.9″ N 26°27′47.8″ E, 12.02.2021, adults on Onopordon acanthium L. (Asteraceae), obs. S. Zafeiriou; (b) T. (Tettigometra) sulphurea tended by Formicidae: Formicinae: Lasius paralienus Seifert, 1992, Serbia: Frushka gora, Neradin vill., nymphs and adults on Onopordon acanthium (Asteraceae), obs. D. Savic; (c) T. (Eurychila) bifoveolata Signoret, 1866 tended by Formicidae: Dolichoderinae: Tapinoma sp., France: Pyrénées Orientales, Banyuls, 02.2022, adults in ant nest, obs. H. Bouyon.

Figure 33.
Tettigometridae: (a) Tettigometra (Mitricephalus) griseola Fieber, 1864 tended by Formicidae: Dolichoderinae: Tapinoma sp., Italy: Calabria, Capo Colonna, 39°0′46″ N 17°10′49″ E, 27.04.2008, nymphs and adults on Cakile maritima Scopoli (Brassicaceae), obs. I. Gjonov, A. Lapeva-Gjonova; (b,c) T. (Mitricephalus) leucophaea (Preyssler, 1792) tended by Formicidae: Formicinae: Formica cinerea Mayr, 1853, Bulgaria: Northern Black sea coast, Kamchiya river mouth, 43°01′56.0″ N 27°53′17.9″ E, 24.06.2004, nymphs and adults on Ammophila arenaria (L.) (Poaceae), obs. I. Gjonov, A. Lapeva-Gjonova.

Figure 34.
Tettigometridae: (a) Tettigometra (Mitricephalus) macrocephala Fieber, 1864 tended by Formicidae: Formicinae: Camponotus aethiops (Latreille, 1798), Bulgaria: Pirin Mt.: Stara Kresna vill., 41°45′48.0″ N 23°10′09.0″ E, 23.07.2001, nymphs and adults on Eryngium campestre L. (Apiaceae), obs. I. Gjonov, A. Lapeva-Gjonova; (b,c) T. (Mitricephalus) macrocephala tended by Formicidae: Formicinae: Camponotus sp., France, Lot, Cabrerets, 44°31′35″ N 1°39′17″ E, 10.08.2018, adults and nymphs on Eryngium sp. (Apiaceae), obs. J. Constant; (d) T. (Mitricephalus) macrocephala tended by Formicidae: Formicinae: Formica spp., Bulgaria: Stara planina Mt., Saranci vill. 42°43′24.0″ N 23°46′58.0″ E, 25.07.2012, nymphs and adults on Eryngium campestre (Apiaceae), obs. I. Gjonov, A. Lapeva-Gjonova.

Figure 35.
Tettigometridae (a) Tettigometra (Mitricephalus) macrocephala Fieber, 1864 tended by Formicidae: Formicinae: Formica sp., Bulgaria: Stara planina Mt., Saranci vill. 42°43′24.0″ N 23°46′58.0″ E, 25.07.2012, nymphs and adults on Eryngium campestre L. (Apiaceae), obs. I. Gjonov, A. Lapeva-Gjonova; (b) T. (Metroplaca) longicornis Signoret, 1864 tended by Formicidae: Myrmicinae: Crematogaster sordidula (Nylander, 1849), Bulgaria: Primorsko, Maslen Nos Cape, 42°18′31.7″ N 27°47′22.5″ E, 04.06.2021, obs. I. Gjonov, A. Lapeva-Gjonova; (c) T. (Metroplaca) longicornis tended by Formicidae: Myrmicinae: Crematogaster sordidula, France: Alpes de Haute-Provence, Sainte-Croix-du-Verdon, 43°46′21.0″ N 6°05′34.0″ E, 04.06.2003, nymphs and adults on Eryngium campestre (Apiaceae), obs. I. Gjonov; (d) T. (Tettigometra) sp. tended by Formicidae: Formicinae: Lasius sp., Germany: Eichstätt, Bayern, 48°53′10.0″ N 11°12′58.6″ E, 29.03.2021, adult, obs. T. Leilich.

Figure 36.
Unidentified Fulgoromorpha: (a) Probably Cixiidae tended by Reptilia: Squamata: Gekkonidae: Hemidactylus craspedotus Mocquard, 1890, Malaysia: Sabah, Sepilok, 5°50′21.8″ N 117°56′57.2″ E, 20.09.2018, adult on tree trunk, obs. C. Lee; (b) Issidae tended by Formicidae indet., Australia: Queensland, Roma, 26°34′29.7″ S 148°48′30.0″ E, adult, obs. “tjeales”; (c) Ricaniidae or Flatidae nymph tended by Reptilia: Squamata: Gekkonidae: Phelsuma guttata Kaudern, 1922, Madagascar: Marojeji National Park, 14°26′14.9″ S 49°46′32.3″ E, 21.10.2016, adult, obs. C. Lee.
4. Discussion
4.1. Trophobiosis: Terminology
In most specific symbioses, the two partners of the interspecific relationships are just called symbionts, a generic term preventing the ability to differentiate them precisely. In trophobiosis, the first member, which provides some food to the second, is called the trophobiont [11]. The name is derived from the Ancient Greek τροφή, trophē, meaning “nourishment” and—βίωσις -biosis. In order to better identify the second symbiont in this particular symbiotic behaviour from symbionts active in others kind of behaviours occurring in other types of interspecific relationships, we propose to call the symbiont benefiting from the food a xenobiont. The name is derived from the Ancient Greek noun ξένος, xénos, meaning “foreigner, or guest” and—βίωσις-biosis. Accordingly, we use the term trophobiosis in the following definition: a symbiotic association between a pair of organisms or symbionts, where one member, the xenobiont, modifies its usual behaviour, waits, and collects after or without stimulation of it, food provided by the second member, the trophobiont. With this definition we exclude cases of species collecting occasionally and randomly drops of honeydew kicked around by the planthopper, or circumstantial cases where high amounts of honeydew produced by great numbers of planthopper individuals in the same place attracts unusual opportunistic species not developing particular trophobiosis behaviours.
Two main types of trophobiosis, mostly ant attendance, have been reported in the Fulgoromorpha literature [4]. The first type is an occasional one and established for a short time (from some hours to a few days), generally concerning one or few individuals; the second reports for mutualistic long-time attending periods on several weeks to months and involve several individuals (one adult and/or young instars to several/many adults and nymphs). Long-time trophobioses are divided into cryptobiotic and optobiotic ones according to whether the trophobiont is maintained confined or free to escape [4]. This division approaches Donisthorpe’s one [12] who used the terms “extranidal” to denote ant guests that occur and interact with ants outside the ant nest, and “intranidal” for those living in an ant colony, and actively seek out ants. However, Donisthorpe’s division is too restrictive, applying especially for ant-attending cases and active “guests”.
In general, trophobionts seem little affected in their usual behaviours, but they are especially more active in their food intake being solicited by the xenobiont. In reverse, xenobionts display more specific behaviours when initiating a trophobiosis relationship: (1) waiting for drops of honeydew, (2) collection of them on the surrrounding substrate, (3) interception of honeydew stream when they are emitted after kicking, or (4) before, directly at the anal extremity of the trophobiont, (5) guidance of larvae and adults to ant nests with (6) possibly phoresy or transportation of individuals (including eggs) by ants called trophophoresy [13], and (7) active protection against predators or parasites. Opportunistic behaviour by ants collecting honeydew already actively collected by other xenobionts or kleptotrophobiosis has also been reported [14]. Table 2 summarizes the different behaviours observed.

Table 2.
Different types of trophobiosis behaviours observed with Fulgoromorpha (Hemiptera).
4.2. Planthopper Xenobionts
Until the end of the 19th and beginning of the 20th century, trophobiosis was only occasionally reported for planthoppers, mostly for Tettigometridae. In the last forty years, it appeared to be much more common than expected. Since the review of Bourgoin [4] several new examples have been documented [8,9,10,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39] and reported in FLOW [10]. In addition, the development of digital macrophotography and internet tools allowing sharing and dissemination of photos and videos more easily (iNaturalist, Facebook groups, Flickr, etc.), have provided an important new source of very valuable natural history data [9]. They are, however, scattered on the internet, difficult to find, and the species are often not identified. Appendix A summarizes all available information for attended planthoppers and trophobiosis found on the internet together with the papers previously published.
Most published planthopper xenobionts belong to ants, which appear to be engaged in opportunistic or occasional behaviours. From all available information (Appendix A), and particularly for Tettigometridae, which remain the best documented, attending ants belong to one of the five Formicidae subfamilies: Dolichoderinae Forel, 1878, Formicinae Latreille, 1809, Myrmicinae Lepeletier de Saint-Fargeau, 1835, Ponerinae Lepeletier de Saint-Fargeau, 1835, and Dorylinae Leach, 1815, and almost twenty different genera are concerned. However, this pattern appears to be not specific for tettigometrids or planthoppers, but is general for sap sucking Hemiptera [40].
However, several unexpected planthopper attendances by other xenobionts taxa have also recently been reported with other insect groups. A well-known case in Australia is the occasional consumption of poisoned honey for man when domestic honey bees (Hymenoptera, Apidae) collect honeydew produced by the passion-vine ricaniid planthopper, Scolypopa australis (Walker, 1851), when feeding on the poisonous shrub tutu (Coriaria arborea Lindsay) (Cucurbitales, Coriariaceae D.C.) [41,42,43]. Recently, high concentrations of the spotted lanterfly, Lycorma delicatula (White, 1845) (Fulgoridae), newly invasive in the USA, have shown various insects such as hornets, yellow jackets and paper wasps (Hymenoptera, Vespidae), Diptera and gastropods (slugs) to be attracted by the vast amount of honeydew production (Figure 10b,c, Figure 11 and Figure 12a). If most of these cases should be reported as opportunistic collection of honeydew drops on the substrate around the fulgorids (not true trophobiosis), and in some cases with short-time occasional attendance. They may also attract more regularly domestic honey bees, which produce a “spotted lanternfly dark honey” of a complex distinct flavor, now commercialized in Philadelphia Pennsylvania USA [44]. Similar observations were made in Madagascar and Vietnam with high concentrations of flatids feeding on the same plant (personal observations by the authors). More unusual and also only recently reported, are Blattodea [9,14,23,33], and various families of Lepidoptera—Tortricidae, Nolidae, Nymphalidae and Erebidae [14,45]. Several new cases involving Blattodea and Lepidoptera are reported and illustrated in this paper (Figure 3, Figure 4, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 12, Figure 13, Figure 14, Figure 15, Figure 16, Figure 17, Figure 18, Figure 20, Figure 21 and Figure 23).
More spectacular are trophobiosis cases of medium-sized Flatidae planthoppers tended by small Gekkonidae in Madagascar [24,34], although geckos are well known predators mainly feeding on insects, spiders, snails, and small vertebrates [34]. In Thailand Kemal and Koçak [31] reported 20–30 large-sized adult fulgorids Pyrops candelaria (Linnaeus, 1758) attended by 4–5 geckos Hemidactylus platyurus (Schneider, 1797) on tree trunks (Figure 16d) near the bottom of the tree in the morning, but in the upper parts in the noon and afternoon, probably due to the daily heat. While trophobiotic behaviour could not be directly observed in this case, a similar gecko-attendance observation with trophobiosis was however reported later by Constant [33] in Borneo.
Another rather unexpected case of a short-time trophobiosis is the one of the molluscan land snail Euglandina aurantiaca Angas (now Pittieria aurantiaca (Angas, 1879)) (Spiraxidae), reported by Naskrecki and Nishida [14], intercepting droplets of honeydew stream kicked away by three large-sized fulgorids Enchoptera sanguinea Distant, 1887, Enchophora stillifera (Stål, 1862) and Phrictus quinquepartitus Distant, 1883, in Costa Rica. The speed of the ejected droplets of honeydews by the hopper varies with species from 0.796 ms−1 to 1.695 ms−1 (±3–6 km/h) [14]. New cases with molluscs are reported here (Figure 8d, Figure 15c and Figure 20c,d). One very spectacular and up-to-now unique case was also recently documented in Borneo (Figure 19d,e) of a large-sized species of Fulgoridae, Scamandra polychroma Gerstaecker, 1895, totally surrounded by a wall of undetermined small ants. According to P. Soltys, who took this fantastic picture, there was no contact at all between the hopper and the ants; the association disappeared the day after, while probable, direct trophobiosis behaviour was not observed (Soltyspers.com).
4.3. Xenobiont Behaviour
Excluding the dubious record from Lichtenstein [46] reporting of mutilated and even lost wings in ant-attended Tettigometra, the old literature reports mostly tettigometrid species explicitly found in ant nests under stones with the ants but without mentioning any associated host plant or elaborated xenobiont behaviours. Food plant information appears in recent reports only because they have a crucial role in trophobisis, allowing the hoppers to feed and excrete honeydew.
While most are well-known predators, xenobionts are able to approach the planthopper, adult or nymph, without it escaping. They manage to collect or even intercept the honeydew stream produced by the planthopper after a kicking movement, or they can collect the drop of honeydew that the trophobiont produces directly at the anal tube opening, before any planthopper kicking. Elaborated specific behaviours generally appear during long-time trophobiotic relationships with ants. These behaviours provide protection from predators and parasitoids, transport when environmental conditions are unfavourable (trophophoresy [13], waste removal, and protection of eggs and nymphs [5]).
In Africa, Weaving [47] reported Nototettigometra (Nototettigometra) patruelis (Stål, 1855) sometimes traveling down the stem of its host plant and entering tunnels constructed by the ants around the roots. Similar behaviour for Tettigometra (Tettigometra) sulphurea Mulsant and Rey, 1855 was reported by Bourgoin [48], although the specimens never went deeply underground, staying a few centimeters beneath the surface. We report here new observations about trophophoresy and active guiding of tettigometrids by ants (Appendix A). Trophophoresy is well known in Hemiptera: Coccoidea [49], but it remains exceptionally reported with planthoppers, although it was also observed once with nymphs of the meenoplid Phaconeura sp. [21].
Antennal palpations by ants, most often on the side of the trophobiont abdomen and on the forewings, are commonly reported with tettigometrids. However, in those cases they are not necessarily followed by the production of a drop of honeydew [48]. In Cameroon, the delphacid Peregrinus maidis (Ashmead, 1890) has been reported having trophobiotic associations with 16 different ant species, several of them having enclosed the trophobiont into natural shelters or built shelters with vegetal fibres around them [16,18,19,20].
In Blattodea, Naskrecki and Nishida [14] reported physical contacts with various species of Fulgoridae that occur also quite often, either with the cockroach palpating the planthopper’s wings with maxillary palps, or even by resting its front legs on the wings. However, they noted that this contact did not appear to elicit any specific response from the planthopper. In Lepidoptera, the same authors also reported the moth Elaeognatha argyritis Hampson, 1905 (Nolidae) tapping the wings of Enchophora sanguinea Distant, 1887 with its antennae, which resulted in an immediate production of a honeydew drop. With Gastropoda, they reported Pittieria aurantiaca extending the anterior portion of their foot, forming a wide hood above the planthopper’s anal tube, allowing the snail to intercept and catch the stream of honeydew droplets, but there was never a physical contact between the symbionts [14]. They also observed several times Camponotus sp. ants climbing the head of the snail and licking honeydew from its body while the snail was attending Enchophora sanguinea and Phrictus quinquepartitus Distant, 1833. They described this extreme case as kleptotrophobiosis. Finally, and although this observation bears repeating, it should be mentioned the particular behaviour of a gecko tapping the bark of the substrate with its head, as a stimulus [24]—perhaps vibrational—inducing the production of honeydew by the planthopper. The different elaborated behaviours adopted by the xenobionts are summarized in Table 3.

Table 3.
Interaction diversity of xenobionts behaviours according to their taxonomic group and related to the size of the trophobiotic planthopper.
4.4. Planthopper Trophobionts
Trophobiosis is now reported from 13 planthopper families (Appendix A), and all cases reported in planthoppers should be classified as facultative trophobiosis. A few exceptions might be considered as permanent (but not obligatory) mutualistic behaviour: the monospecific family Hypochthonellidae [50], the hypogeic delphacid Notuchus kaori Hoch and Asche, 2006 [25] in New Caledonia, and perhaps the meenoplid taxon Phaconeura sp. in Australia [21], the last two found with Paratrechina sp. (Formicidae). These species exhibit morphological adaptations to hypogeic habitats (interstitial or deeper in caves habitats) that are difficult to separate from possible myrmecophilous morphological adaptations, but that might at least favor attendance by ants.
Underground attendance for long period in ant nests (cryptobiotic) is documented in members of Cixiidae, Delphacidae, Hypochthonellidae and Tettigometridae [4,6,48] and also recently reported in Flatidae: Budginmaya eulae Fletcher, 2009 [51] and for four undetermined flatid species [8]. Cryptobiotic attendance under aerial shelters built by ants is reported in Delphacidae only [4,5,16,18,19,20,26]. Optobiotic ant attendance is reported here for the first time in the families Dictyopharidae, Nogodinidae and Eurybrachidae and several new cases are reported for Caliscelidae, Ricaniidae and Issidae, taxa for which such behaviour remained exceptional (Appendix A).
In most optobiotic cases, trophobionts are adult planthoppers only, but in cryptobiotic situations probably also nymphs (Delphacidae, Tettigometridae, Flatidae). However, in a few observations only nymphs seem to act as trophobionts. Cixiidae nymphs of Reptalus sp. in Bulgaria for instance were regularly found in the soil nests of Aphaenogaster subterranea (Latreille, 1798) (Figure 3c,d) but adult individuals of Reptalus inhabiting and feeding on the above-ground parts of the plants were never observed attended by ants. Lörinczi [29] reported a similar observation with Reptalus panzeri (Löw, 1883) in Hungary, and it is probably also the case for the New Guinea flatid nymphs found in nests of the ant Pseudolasius Emery, 1887 [8]. In Madagascar, nymphs of Cixiidae—probably Oliarus sp.—were observed feeding on roots and attended by Pheidole sp. ants (Figure 4e). They were collected in a subterranean environment of the caves in the Tsingy of Namoroka Strict Nature Reserve, a limestone karst region in northwest Madagascar, but adults were not observed.
4.5. Ant Attendance in Tettigometridae
- -
- Tettigometrid behaviour as trophobiont.
Tettigometridae form a small group of planthoppers, for which major life-traits still remain very poorly known [52], but for which several cases of long-time attendance by ants were reported since the 19th and at the beginning of the 20th century. According to Silvestri [53], the first observations of ants with tettigometrids seems to be from Savi who mentioned the occurrence of a “petite cicadelle brunâtre” [small brownish leafhopper] in an ant nest, probably Tettigometra (Tettigometra) impressifrons Mulsant and Rey, 1855 and the xenobiont Tapinoma nigerrimum (Nylander, 1856). However, the earliest direct observation of trophobiosis behaviour recorded was published in France by Rouget [54] for Tettigometra laeta Herrich-Schäffer, 1835 with ants Tapinoma erraticum (Latreille, 1798). Successively new observations were quickly published [46,53,55,56,57,58,59,60,61,62,63,64,65,66,67]. These early publications were found in single published notes, concentrated in a short period some hundred years ago, and provided records for three Tettigometra species only (T. (Eurychila) bifoveolata Signoret, 1866, T. (Mitricephalus) macrocephala Fieber, 1865 and T. (Tettigometra) virescens Panzer, 1799). A careful study of this old literature allowed the retention of only about 25 original observations [4,48]. It is only recently that new observations were added, showing new interest in these behaviours, probably boosted by the development of macrophotography.
To date, 106 observations of tettigometrids attended by ants have now been published or attested by macrophotographic images published on the web. They concern 18 tettigometrid species ant-attended, representing some 20% of the family species. All major suprageneric tettigometrid taxa are concerned except the Phalixinae Ghauri, 1964, Plesiometrini Bourgoin, 2018 and the Cyranometrini Bourgoin, 1987 [68]. We provide and document here several new data on ant attendance in Tettigometridae (Appendix A, Figure 24, Figure 25, Figure 26, Figure 27, Figure 28, Figure 29, Figure 30, Figure 31, Figure 32, Figure 33 and Figure 34), including more specific field observations on twelve European species: Tettigometra (Tettigometra) atra Hagenbach, 1825 in Malta and Bulgaria (Figure 28a), T. (Tettigometra) brachycephala Fieber, 1865 in Croatia (Figure 28b), T. (Tettigometra) fusca Fieber, 1865 in Ukraine (Figure 28c and Figure 29a), T. (Tettigometra) impressifrons Mulsant and Rey, 1855 in Italy (Figure 29b,c), T. (Tettigometra) laeta Herrich-Schäffer, 1834 in Bulgaria (Figure 30a) and Albania (Figure 30b), T. (Tettigometra) picta Fieber, 1865 in Malta (Figure 30c), T. (Tettigometra) sulphurea Mulsant and Rey, 1855 in Bulgaria (Figure 31a), Greece (Figure 31b), including Lesbos Island (Figure 32a), Croatia (Figure 31c) and Serbia (Figure 32b), T. (Eurychila) bifoveolata Signoret, 1866 in France (Figure 32c), T. (Mitricephalus) griseola Fieber, 1864 in Italy (Figure 33a), T. (Mitricephalus) leucophaea (Preyssler, 1792) in Bulgaria (Figure 33b,c), T. (Mitricephalus) macrocephala Fieber, 1864 in Bulgaria (Figure 34a,d and Figure 35a) and France (Figure 34b,c), T. (Metroplaca) longicornis Signoret, 1865 in Bulgaria (Figure 35b) and France (Figure 35c).
Trophobiosis is reported for the first time for T. griseola and T. picta. The Mediterranean species Tettigometra picta (nymphs and adults) was found to be tended by Tapinoma nigerrimum (s.l.) on all studied host plants (about 20) of Lavatera arborea L. (Malvaceae). Besides aggregation of individuals on host plants, transportation by ants was also observed.
Of all the Tettigometra species we observed with ants, three were found in early spring—T. atra, T. laeta and T. (M.) longicornis; the earliest one was T. atra in March (Figure 28a). All of them were observed in ant nests, located under stones, although T. (M.) longicornis (Figure 35c) was also found during summer on the host plant Eryngium campestre L. (Apiales, Apiaceae). The presence of adults during the spring months suggests that they have most likely overwintered in the ant nests.
Ant attendance was observed on different parts of plants, sometimes depending on their growth. For instance, in the beginning of April when the plant leaves are in an early stage of development and they form a rosette close to the ground, the adults of T. (T.) sulphurea were amongst the leaves or on the ground, under the leaves. Other Tettigometra species mentioned in this study were observed over an extended period of time—from April to September.
- -
- Xenobiont preferences in tettigometrids.
The current study increases the known range of ant species partnering in trophobiotic interactions with four further Tettigometra species. Comparing the study results with literature data, some relationships between Tettigometra species and ant species are documented for the first time: T. atra in nests of Tapinoma simrothi Krausse, 1911 and Lasius platythorax Seifert, 1991, T. macrocephala in trophobiotic interactions with Formica cinerea Mayr, 1853 and Camponotus aethiops (Latreille, 1798), T. longicornis in soil nests of Crematogaster sordidula (Nylander, 1849), T. sulphurea tended by Tetramorium chefketi Forel, 1911, Camponotus piceus (Leach, 1825), Formica pratensis Retzius, 1783, Lasius niger (Linnaeus, 1758) and L. paralienus Seifert, 1992.
However, trophobiotic relationships appear non-specific, and one tettigometrid species can be tended by different ant species. In our newly reported observations, six ant genera were involved: Tapinoma (Dolichoderinae), Formica, Lasius, and Camponotus (Formicinae), Tetramorium and Crematogaster (Myrmicinae). Interestingly, some plurispecific situations were found: T. laeta and T. atra (Figure 30a, Appendix A) together tended by Tapinoma erraticum in an ant nest under a stone, and T. impressifrons (Figure 29b,c) and T. griseola (Figure 33a) located together on the host plant Cakile maritima Scop. (Brassicales, Brassicaceae), both also tended by Tapinoma erraticum. In reverse, literature and new observations show that Reptalus (Cixiidae) seems closely associated only with Aphaenogaster subterranea.
- -
- Aggregations and ethological notes in tettigometrids.
The formation of groups of individuals is one of the most characteristic features of the ant attendance in tettigometrids [4]. The lower ones in number were usually those that were found under stones (usually not more than five individuals).
In T. (T.) macrocephala, nymphs and adults form small groups (Figure 34a–c and Figure 35b) (up to 15 specimens) on the host plant Eryngium campestre around the nodes, leaf veins, and branches of the plants while entrances to the ant nest are situated at the base of the plant. Often, nymphs and adults from the hidden entrances in the ant nest go up on the plant in the evening. In case of unfavourable conditions, the planthoppers were herded back into the underground area of the ant nest. During autumn, several adults are often found on the plant base, close to entrances of ant nests.
Most frequent cases observed were with T. (T.) sulphurea (Figure 31 and Figure 32a,b), always on Onopordum acanthium L. (Asterales, Asteraceae), visited by five different ant species. Females lay their eggs in large groups close to each other at the base of plants. Eggs are pale yellow with an elongated oval form (Figure 31b). In their interaction with Tetramorium chefketi, we observed the building of a shelter around the base of plants, where the female planthopper inserts the eggs and stays almost all the time. In other observations, T. (T.) sulphurea females are most often found near the entrances of the ant nests on the basal parts of the host plant or on the ground surface. Young nymphs may live in dense large groups close to the base of plants. Older nymphs and adults may climb higher on the plant, but always stay near the base of the leaves. In addition, some nymphs were hiding in holes of unknown origin. For T. (M.) longicornis in France, small groups of about 10 individuals of nymphs and adults were found on the host plant Eryngium campestre. In Bulgaria, several adults were also found in a soil nest of Crematogaster sordidula in the early spring.
Unlike all other tettigometrid observations, only single adults and nymphs standing on the leaves of the plant Ammophila arenaria (L.) (Poales, Poaceae) were always observed for T. leucophaea (Figure 33b,c). In only two species (T. atra and T. laeta), direct trophobiotic relationships was not observed, probably due to their specific habitat (nests under stones), but when being disturbed, they used to go alone or were transported by ants inside the nest. In all other cases, ant-antennating, transport of individuals, guiding of adults, and direct collection of honeydew from tettigometrids anal openings were observed.
4.6. Cockroach-Attendance in Fulgoridae
We report trophobiosis involving cockroaches in six planthopper families. This symbiotic relation appears as a new major life-trait pattern of Hemiptera planthoppers behaviour, unknown until now. According to the collected observations, when medium and large planthoppers species are involved, cockroach xenobionts enter more often than ants in trophobiose. Interestingly, 80% of the cases involve the Fulgoridae. At this stage, we cannot say whether this observation corresponds to a particular behaviour of the Fulgoridae, due to the fact that they are generally larger in size than the other families of planthoppers, and therefore more attractive in terms of the quantity of food excreted, or whether this is a simple observation bias (species more easily observable, more collected, etc.). However, it should be noted that these large planthoppers are often observed on the same trees over months, showing slight aggregating behaviour that favours the deposit of kicked honeydew drops on the surrounding substrate and that subsequently might attract attending cockroaches.
5. Conclusions
The new observations collected and reported here put into focus the unexpected importance of trophobiosis as a more general and important life trait of fulgoromorph planthoppers in the interspecific relationships they involve. Trophobiosis not only concerns specifically ant-attended tettigometrids, but also cockroach attended fulgorids, and it appears as a potential behavioural trait probably general for most planthoppers. These observations raise several interesting questions that still need more investigations before starting to answer them.
- -
- Trophobiosis is an interspecific interaction between a trophobiont and a xenobiont. In tettigometrids and probably most planthoppers (except perhaps for Hypochthonella caeca and Notuchus kaori for which we have too little data), these interactions are non-permanent. Several factors need to be considered for such a behaviour taking place. Feeding and honeydew production is a regular physiological activity for the planthopper. However, if this criterion is necessary, it is not sufficient as this is general for all phytophagous Hemiptera but not all of them develop trophobiotic relationships. A priori, the interaction starter is given by the xenobiont that stops its other activities, stays around, avoids making the trophobiont escape, and does not predate it. The interaction could be maintained all the time the trophobiont regularly excretes honeydew and even longer in cryptobiotic conditions. Honeydew, which is rich in sugars and amino acids, constitutes a nutritional resource for xenobionts and acts as a volatile and a contact kairomone, stimulating some particular behaviours of predators and parasitoids: searching, preys/hosts locations and attacks [7]. As these behaviours are induced, might trophobiosis be therefore initiated by the honeydew production, alerting the xenobionts around?
- -
- With ants, such interactions seem facilitated by sessility and subsocial traits of the tettigometrid trophobionts that guarantee higher benefits for the xenobiont: easier and abundant source of feeding at a smaller cost [4]. However, all recent new observations of larger fulgorid species show that if sessility and sub-social behaviours are facilitating factors (several of the large fulgorids are often observed in small groups of individuals on the same tree), how also is the amount of food produced an important criterion?
- -
- Absence of trophobiosis interactions reported with Achilidae, which are often found grouped (few adults and nymphs at different instars) under tree bark, but which are though to feed from fungal hyphae [43], also raise the issue of honeydew quality as an additional criterion. Do Achilids produce honeydew which is less nutrient-rich than other sap-sucking planthopper taxa and perhaps without semiochemical kairomone stimulating xenobionts to start any trophobiotic behaviour? Our current knowledge about achilid natural history does not allow any better hypothesis.
- -
- Another aspect of planthopper-ant interactions is that the starter (or their maintenance) could also, at least in the case of the tettigometrids, be an active action of the trophobiont itself. Indeed, the old observations, confirmed by our recent ones, show that tettigometrids are often found in ant nests, under stones, probably overwintering for some species, but without food plants mentioned around, neither roots. In these cases, the regular production of honeydew should be clearly reduced or even stopped for a while. How then are interactions with ants maintained, without the ants starting to predate the hoppers? Bourgoin [4] has mentioned antennal palpations by ants that might encourage the honeydew drop production (as observed with non-ant xenobionts), are also located in the body parts that are rich in pore tegumentary glands [69]. The hypothesis that they might use allomones to chemically manage ant behaviour to their benefit cannot be discarded. It is probable that trophobiosis and mutualistic interactions with ants in tettigometrids are ruled by both allomones and kairomones and are much more widespread than thought until now, and occur with most of the species of the family.
- -
- Trophobiosis, which seems very common although facultative in tettigometrids, also raises other interesting questions which are difficult to answer at this time: how far is the behaviour general in the family? Why have tettigometrids not yet been observed attended by cockroaches? Are they kept away by ants? How important are ants in maintaining tettigometrid populations? Indeed, there is a demonstrated tendency in some species such as Nototettigometra (N.) patruelis, Tettigometra (M.) fusca, T. impressopunctata Dufour, 1846 or T. (M.) leucophaea of drastic and sudden downsizing of their populations, up to totally disappearing from places where they were previously very commonly known [47,70,71]. There is suspicion of cryptic species in the family [52]: would precise observations of such sister or closely related species, allow a smart comparative analysis to better understand how trophobiosis takes place and is maintained over time?
- -
- In large-sized fulgorids, xenobionts belong to other insect orders than ants, such as cockroaches and moths, and also to mollusca and even small gekkonid vertebrates. Obviously, if ant attendance seems relatively common with tettigometrids, xenobiontic Blattodea appear as the most common cases observed after ant attendances, and the main one for the Fulgoridae. What behaviours are occurring at night with these nocturnal xenobionts? How tiny is the frontier between trophophosis and predation for all predator xenobionts?
- -
- Ants, currently dated from the Early Cretaceous from around 110 Mya [72,73] to 140 Mya [2] represent a much younger insect lineage than cockroaches, which dates back to the Permian [74], more than 100 My older. If plants (including fungi) represent the main framework for the interspecific interactions of planthoppers, which are obligate phytophagous insects, the role of these mutualistic behaviours in food webs remains to be further analyzed. For instance, Compton and Robertson [75] have shown a net beneficial effect of the association of the tettigometrid N. (N.) patruelis and its associated ants by protecting fig seeds and pollinators despite the phytophagous action of the planthopper on fig trees. During the evolution of fulgoromorphs, to what extent have these interactions, and in particular a new step in the mode of trophobiosis from cockroach xenobionts to ant xenobionts, also supported diversification of planthoppers, as previously suggested by Grimaldi and Engel [76] and more generally for Hemiptera and their associations with ants?
To answer all these questions, it is clear that beside the few primary biodiversity data related about which is present, where and when (taxonomical data and their associated biological data), we just know almost nothing about life traits in planthoppers! Many more observations are yet needed before we could start investigating all these questions, and how, in the evolutionary framework of the phylogeny, they could help us to better understand how important trophobiosis has been for the evolution of planthoppers.
Author Contributions
Conceptualization, T.B.; Data curation, T.B., I.G., A.L.-G., J.C. and G.K.; Formal analysis, T.B. and S.R.; Funding acquisition, I.G., A.L.-G. and J.C.; Investigation, T.B., I.G., A.L.-G., J.C. and G.K.; Methodology, T.B., J.C. and G.K.; Project administration, T.B.; Resources, I.G. and A.L.-G.; Supervision, T.B.; Validation, T.B., I.G., A.L.-G., J.C., G.K. and M.R.W.; Visualization, T.B., I.G. and S.R.; Writing—original draft, T.B. and I.G.; Writing—review and editing, T.B., I.G., A.L.-G., S.R., J.C., G.K. and M.R.W. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by National Science Fund, Ministry of Education and Science of the Republic of Bulgaria, Grant KP-06-M31/4 (IG). The research is also part of “A step further in the Entomodiversity of Vietnam” and “A step further in the Entomodiversity of Cambodia”, both supported through grants issued by the capacity building Program of the Belgian Global Taxonomy Initiative National Focal Point (CEBioS program) with financial support from the Belgian Directorate-General for Development Cooperation (JC).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data presented in this study will be openly available in FLOW (Fulgoromorpha Lists on The Web): a world knowledge base dedicated to Fulgoromorpha at https://hemiptera-databases.org/flow/.
Acknowledgments
For the numerous photographic documents, they shared with us, already available on the internet or still private, and for their kind authorization to use them to illustrate this paper the authors are greatly indebted to: Aadita Chintamani Deodhar (India), Ahmet Omer Koçak (Turkey), Alan Manson (South Africa), Albert Kang (Malaysia), Anthony James Eales (Australia), Anton Gjeldum (Croatia), Arthur Anker (Brasil), Carol Page (Australia), Chien C. Lee (Malaysia), Christian Hübner (Germany), Christian Nijhuis (Singapore), Christine Sydes (Scotland), Claire O’Neill (USA)—Earthwise Aware (earthwiseaware.org), Dirk Mezger (Germany), Doug Gochfeld (USA), Erica Smyers (USA), Erwin Holzer (Austria), Gerard Chartier (Cambodia), Hock Ping Guek (Malaysia), Howard Rawson (Australia), Jean Carlo Rodríguez (Costa Rica), John G. Phillips (USA), Kai Joaquin (USA), Kawin Jiaranaisakul (Thailand), Keo Keomani (Laos), Magda Botha (South Africa), Marco de Haas (The Netherlands), Muhabbet Kemal (Turkey), Paul Bertner (Canada), Peter Chew (Australia), Peter Soltys (Australia), Pongpan Asawadecharit (Thailand), Roman Stepoviy (Ukraine), Ronald K. Asuncion (Malaysia), Sally Adam (South Africa), Savvas Zafiriou (Greece), Tomas Maul (Malaysia), Tracie Stice (Costa Rica), Vincent Sougnez (France), Hervé Bouyon (France), Wynand Uys (South Africa), Xavier Vermeersch (Belgium). For her help in some tettigometrid identifications, we also thanks Fariba Mozaffarian (Iran).
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
| Fulgoromorpha Trophobiont Species | Medium Size Lenght (mm) | Xenobiont Species | Original Reference/New Data with Location and Associate Biological Information | |
| Caliscelidae | ||||
| Asarcopus palmarum Horvath, 1921 | 3 | Formicidae: Formicinae: Camponotus sp. | [77] | |
| Figure 2a,b | Caliscelis wallengreni (Stål, 1863) | 4 | Formicidae: Formicinae: Lasius sp. | New data: Hungary: Tiszagyenda vill., 47°23′57.8″ N 20°30′05.8″ E, 25.07.2021, observed by Marco de Haas |
| Cixiidae | ||||
| Figure 3a | Bothriocera sp. | 4.55 | Formicidae: Formicinae: Camponotus sexguttatus (Fabricius, 1793) | New data: USA: Florida, Pinelands, Everglades National Park, Homestead, 07.12.2015, observed by Judy Gallagher |
| Fipsianus picturatus (Distant, 1917) | 4.7 | Formicidae: Dolichoderinae: Technomyrmex albipes (Smith, 1861) | [28] | |
| Fipsianus picturatus (Distant, 1917) | 4.7 | Formicidae: Formicinae: Camponotus thomasseti Forel, 1912 | [28] | |
| Fipsianus picturatus (Distant, 1917) | 4.7 | Formicidae: Formicinae: Anoplolepis gracilipes (F. Smith, 1857) | [28] | |
| Figure 3b | Hyalesthes sp. | Formicidae: Formicinae: Camponotus piceus (Leach, 1825) | New data: France: Var, Solliès-Toucas, 43°11′38.6″ N 6°00′33.2″ E, 29.07.2021, observed by Bernard Noguès https://www.inaturalist.org/observations/89103332 | |
| Oecleus borealis Van Duzee, 1912 | 6 | Formicidae: Formicinae: Nylanderia arenivaga (Wheeler, 1905) | [78] | |
| Oliarus vicarius Walker, 1851 | Formicidae: Myrmicinae: Solenopsis invicta Buren, 1972 | [79,80] | ||
| Oliarus vicarius Walker, 1851 | Formicidae: Formicinae: Nylanderia vividula (Nylander, 1846) | [79,80] | ||
| Oliarus vicarius Walker, 1851 | Formicidae: Myrmicinae: Aphaenogaster carolinensis Wheeler, 1915 | [79,80] | ||
| Pentastiridius formicarius (Mitjaev, 1967) | 6.2 | Formicidae: Formicinae: Camponotus turkestanicus Emery, 1887 | [81] | |
| Pentastiridius haloxyli (Mitjaev, 1971) | 4.7 | Formicidae: Formicinae: Camponotus turkestanicus Emery, 1887 | [32] | |
| Pentastiridius subterraneus Emeljanov, 1995 | 5.7 | Formicidae: Formicinae: Camponotus turkestanicus Emery, 1887 | [32] | |
| Reptalus panzeri (Löw, 1883) | 7.5 | Formicidae: Myrmicinae: Aphaenogaster subterranea (Latreille, 1798) | [29] | |
| Reptalus sp. | 5.65 | Formicidae: Myrmicinae: Aphaenogaster subterranea (Latreille, 1798) | New data: Bulgaria: Vitosha Mt., Bosnek vill., 42°30′11″ N 23°10′01″ E, 1000 m., 25.03.2012, nymphs in ant nest under stone, observed by I. Gjonov, A. Lapeva-Gjonova | |
| Figure 3c,d | Reptalus sp. | 5.65 | Formicidae: Myrmicinae: Aphaenogaster subterranea (Latreille, 1798) | New data: Bulgaria: Eastern Rhodopes Mt., Oreshino vill., 41°28′19.3″ N 26°05′30.0″ E, 243 m, 20.04.2012, nymphs in ant nest under stone, observed by I. Gjonov, A. Lapeva-Gjonova |
| Figure 3e | Singabenna praestans (Walker, 1857) | 7.36 | Blattodea indet. | New data: Malaysia: Malaysia: Sarawak, Borneo, Bako National Park, 1°42′44″ N 110°26′46″ E, 07.01.2021, observed by Chien Lee https://www.inaturalist.org/observations/67788138 |
| Figure 4a–c | Cixiidae indet. | Blattodea indet. | New data: Malaysia: Sarawak, Borneo, Bako National Park, 1°43′ N 110°28′ E, 9.07.2016, adult on tree trunk, observed by X. Vermeersch | |
| Figure 4d | Cixiidae indet. | Blattodea indet. | New data: Malaysia: Sarawak, Borneo, Selangor, 3°20′ N 101°30′ E, 05.2016, adult on tree trunk, observed by H.P. Guek | |
| Figure 4e | Cixiinae indet. | Formicidae: Myrmicinae: Pheidole sp. | New data: Madagascar, Namoroka, 16°24′25″ S 45°18′47″ E, 139 m, 24.10.2016, nymphs on tree roots in a cave, observed by G. Kunz | |
| Koroana arthuria Myers, 1924 | Formicidae: Myrmicinae: Monomorium sp. | [82] | ||
| Mnemosyne cubana Stål, 1866 | Formicidae: Ponerinae: Odontomachus haematodus Linné, 1758 | [83]: together with another smaller cixiid, a woodlouse, a cockroach and a scale insect Mixorthezia sp. (Coccoidea: Ortheziidae) | ||
| Delphacidae | ||||
| Figure 5a | Dicranotropis beckeri Fieber, 1866 | 3.125 | Formicidae: Formicinae: Camponotus aethiops (Latreille, 1798) | New data: Bulgaria: Vlahina Mt.: Boboshevo, 42°09′50″ N 22°58′47″ E, 730 m, 02.07.2001, 24.06.2010, adult on Poaceae gen. sp. (Poaceae), observed by I. Gjonov, A. Lapeva-Gjonova |
| Notuchus spp. | 3.85 | Formicidae: Formicinae: Paratrechina sp. | [25] | |
| Peregrinus maidis (Ashmead, 1890) | 3.95 | Formicidae: Formicinae: Camponotus acvapimensis Mayr, 1862 | [18,19,20] | |
| Peregrinus maidis (Ashmead, 1890) | 3.95 | Formicidae: Formicinae: Camponotus brutus Forel, 1886 | [18,19,20] | |
| Peregrinus maidis (Ashmead, 1890) | 3.95 | Formicidae: Formicinae: Camponotus chrysurus Gerstäcker, 1871 | [18,19,20] | |
| Peregrinus maidis (Ashmead, 1890) | 3.95 | Formicidae: Formicinae: Camponotus compressus (Fabricius, 1787) | [18,19,20] | |
| Peregrinus maidis (Ashmead, 1890) | 3.95 | Formicidae: Myrmicinae: Crematogaster castanea Smith, 1858 | [18,19,20] | |
| Peregrinus maidis (Ashmead, 1890) | 3.95 | Formicidae: Myrmicinae: Crematogaster clariventris Mayr, 1895 | [18,19,20] | |
| Peregrinus maidis (Ashmead, 1890) | 3.95 | Formicidae: Myrmicinae: Crematogaster striatula Emery, 1892 | [18,19,20] | |
| Peregrinus maidis (Ashmead, 1890) | 3.95 | Formicidae: Myrmicinae: Myrmicaria opaciventris Emery, 1893 | [18,19,20] | |
| Peregrinus maidis (Ashmead, 1890) | 3.95 | Formicidae: Formicinae: Paratrechina longicornis (Latreille, 1802) | [18,19,20] | |
| Peregrinus maidis (Ashmead, 1890) | 3.95 | Formicidae: Myrmicinae: Pheidole megacephala (Fabicius, 1793) | [18,19,20] | |
| Peregrinus maidis (Ashmead, 1890) | 3.95 | Formicidae: Dolichoderinae: Tapinoma sp. | [18,19,20] | |
| Figure 5b | Tropidocephala sp. | 3.9 | Formicidae: Formicinae: Oecophylla smaragdina (Fabricius, 1775) | New data: Thailand, Bangkok, 13°42′54.9″ N 100°31′10.8″ E, 07.01.2021, adult on Imperata cylindrica (L.) P. Beauv. (Poaceae), observed by P. Asawadecharit |
| Delphacidae indet. | Formicidae: Myrmicinae: Cataulacus sp. | [26] | ||
| Delphacidae indet. | Formicidae: Myrmicinae: Lophomyrmex bedoti Emery, 1893 | [26] | ||
| Delphacidae indet. | Formicidae: Myrmicinae: Crematogaster modiglianii Emery, 1900 | [26] | ||
| Delphacidae indet. | Formicidae: Dolichoderinae: Dolichoderus maschwitzi Dill, 2002 | [26] | ||
| Delphacidae indet. | Formicidae: Dolichoderinae: Dolichoderus indrapurensis Forel, 1912 | [26] | ||
| Delphacidae indet. | Formicidae: Dolichoderinae: Dolichoderus thoracicus (Smith, 1860) | [26] | ||
| Delphacidae indet. | Formicidae: Dolichoderinae: Technomyrmex cf. albipes (Smith, 1861) | [26] | ||
| Delphacidae indet. | Formicidae: Dolichoderinae: Technomyrmex sp. | [26] | ||
| Delphacidae indet. | Formicidae: Formicinae: Colobopsis cf. saundersi (Emery, 1889) | [26] | ||
| Delphacidae indet. | Formicidae: Formicinae: Camponotus (Tanaemyrmex) cf. arrogans (Smith, 1858) | [26] | ||
| Delphacidae indet. | Formicidae: Formicinae: Camponotus (Myrmotarsus) cf. irritabilis (Smith, 1857) | [26] | ||
| Delphacidae indet. | Formicidae: Formicinae: Paratrechina sp.1 | [26] | ||
| Delphacidae indet. | Formicidae: Formicinae: Paratrechina sp.4 | [26] | ||
| Delphacidae indet. | Formicidae: Formicinae: Polyrhachis s. str. bihamata-group | [26] | ||
| Delphacidae indet. | Formicidae: Myrmicinae: Carebara cf. pygmaeus (Emery, 1887) | [26] | ||
| Dictyopharidae | ||||
| Parorgerius platypus (Fieber, 1866) | 6.3 | Formicidae: Formicinae: Camponotus aethiops (Latreille, 1798) | New data: Bulgaria: Vlahina Mt.: Boboshevo, 42°09′50″ N 22°58′47″ E, 730 m, 02.07.2001, 24.06.2010, adult on Eryngium campestre L. (Apiaceae), observ. by I. Gjonov, A. Lapeva-Gjonova | |
| Eurybrachidae | ||||
| Figure 6a,b | Eurybrachys sp. | Formicidae indet. | New data: India: Rajastan, Jaisalmer, Amar Sagar, 26°55′04″ N 70°48′15″ E, 18.12.2020, adult and nymphs on Crotalaria sp. (Fabaceae) and Heliotropium sp. (Boraginaceae), observed by Dr Shyam Sunder Meena | |
| Figure 6c | Gelastopsis insignis Kirkaldy, 1906 | 8.75 | Blattodea indet. | New data: Australia: Queensland, Brisbane, 27°28′04″ S 153°01′41″ E, adult on Acacia sp. (Fabaceae), observed by P. Chew |
| Flatidae | ||||
| Budginmaya eulae Fletcher, 2009 | 4.5 | Formicidae: Formicinae: Camponotus terebrans (Lowne, 1865) | [51] | |
| Bythopsyrna circulata (Guérin-Méneville, 1844) | Formicidae: Formicinae: Dinomyrmex gigas (Latreille, 1802) | [84] | ||
| Figure 7a | Petrusa epilepsis (Kirkaldy, 1767) | Formicidae indet. | New data: Puerto Rico: San Juan, Paseo del Morro, 18°28′12.6″ N 66°07′28.5″ W, 11.12.2018, nymphs and adults, observed by C. O’Neill https://www.inaturalist.org/observations/18980798 | |
| Figure 7b | Siphanta sp. | Formicidae indet. | New data: Australia: Queensland, Cairns, 16°49′10.7″ S 145°37′59.4″ E, 06.01.2016, nymph, observed by C. Page. https://www.inaturalist.org/observations/26160583 | |
| Figure 7c | Flatoidinae sp. | 14.5 | Blattodea indet. | New data: Vietnam: Kon Chu Rang National Park, 14°28′28″ N 108°32′27″ E, 19.07.2018, adult on tree trunk, observed by J. Constant |
| Flatidae indet. (sp1) | Formicidae: Formicinae: Pseudolasius karawajewi Donisthorpe, 1942 | [8] | ||
| Flatidae indet. (sp1) | Formicidae: Formicinae: Pseudolasius karawajewi Donisthorpe, 1942 | [8] | ||
| Flatidae indet. (sp2) | Formicidae: Formicinae: Pseudolasius karawajewi Donisthorpe, 1942 | [8] | ||
| Flatidae indet. (sp3) | Formicidae: Formicinae: Pseudolasius karawajewi Donisthorpe, 1942 | [8] | ||
| Flatidae indet. (sp4) | Formicidae: Formicinae: Pseudolasius breviceps Emery, 1887 | [8] | ||
| Fulgoridae | ||||
| Figure 8a | Alaruasa aerata (Distant, 1887) | 22.2 | Lepidoptera: Erebidae: Euclystis sp. | New data: Costa Rica: Wildlife Refuge Monteverde, 10°19′ N 84°49′ W, 25.08.2019, adult on tree trunk, observed by J. Carlos |
| Figure 8b | Belbina recurva Lallemand, 1950 | 25 | Blattodea indet. | New data: Madagascar: Andasibe National Park, 14°28′28″ N 108°32′27″ E, 13.02.2015, adult on tree trunk, observed by P. Bertner |
| Copidocephala guttata (White, 1846) | 33.5 | Blattodea: Ectobiidae: Macrophyllodromia maximiliani (Saussure, 1873) | [14,23] | |
| Figure 8c | Dichoptera sp. | 22.5 | Reptilia: Squamata: Gekkonidae indet. | New data: Thailand: Phanom Bencha National Park, 8°14′31″ N 98°54′55″ E, 13.12.2019, adult on tree trunk, observed by C. Hübner |
| Enchophora sanguinea Distant, 1887 | 26.75 | Blattodea: Blattidae: Eurycotis sp. | [14] | |
| Enchophora sanguinea Distant, 1887 | 26.75 | Blattodea: Ectobiidae: Macrophyllodromia sp. | [14,23] | |
| Enchophora sanguinea Distant, 1887 | 26.75 | Lepidoptera: Erebidae: Euclystis proba Schaus, 1911) | [14] | |
| Enchophora sanguinea Distant, 1887 | 26.75 | Lepidoptera: Noctuidae indet. | [14] | |
| Enchophora sanguinea Distant, 1887 | 26.75 | Lepidoptera: Nolidae: Elaeognatha argyritis Hampson, 1905 | [14] | |
| Enchophora sanguinea Distant, 1887 | 26.75 | Lepidoptera: Tortricidae indet. | [14] | |
| Enchophora sanguinea Distant, 1887 | 26.75 | Lepidoptera: Tigridia acesta (Linnaeus, 1758) | [14] | |
| Enchophora sanguinea Distant, 1887 | 26.75 | Gastropoda: Spiraxidae: Pittieria aurantiaca (Angas, 1879) | [14] | |
| Figure 8d | Enchophora sanguinea Distant, 1887 | 26.75 | Gastropoda indet. | New data: Costa Rica: La Selva Biological Station, 10°25′40.6″ N 84°00′22.1″ W, 03.02.2016, adult, observed by G. Kunz https://www.inaturalist.org/observations/46069686 |
| Figure 9a | Enchophora sp. | 28.5 | Blattodea: Blattidae: Eurycotis sp.? | New data: Costa Rica: Puerto Viejo de Sarapiquí, La Selva Biological Station, 10°25′42.54″ N 84° 0′26.68″ W, 70 m, 03.02.2014, adult on tree trunk, observed by G. Kunz https://www.inaturalist.org/observations/45712391 |
| Figure 9b–d | Enchophora sp. | 28.5 | Blattodea: Ectobiidae: Macrophyllodromia maximiliani (Saussure, 1873) | New data: Costa Rica: Puerto Viejo de Sarapiquí, La Selva Biological Station, 10°25′54″ N 84° 0′38.60″ W, 65m, 09.02.2018, adult on tree trunk, observed by G. Kunz |
| Figure 9e | Kalidasa lanata (Drury, 1773) | 25 | Formicidae indet. | New data: India: Karnataka, 12°58′12″ N 77°30′ E, 07.2017, adult on tree trunk, observed by A. C. Deodhar |
| Figure 10a | Kalidasa nigromaculata (Gray, 1832) | 24.5 | Lepidoptera: Nolidae: Eligma narcissus (Cramer, 1775) | New data: Laos: Vientiane, 17°58′ N 102°36′ E, 10.08.2017, adult on tree trunk, observed by Keo Keomani https://www.facebook.com/groups/TheEntomologyGroup/permalink/10155157846588393/ |
| Figure 10b | Lycorma delicatula (White, 1845) | 23.65 | Coleoptera indet. | New data: USA: Pennsylvania, Berks County, Berks, Oley, 40°23′15″ N 75°47′23″ W, 10.10.2017, observed by Erica Smyers |
| Figure 10c and Figure 11a,b | Lycorma delicatula (White, 1845) | 23.65 | Diptera indet. | New data: USA: Pennsylvania, Berks County, Berks, Oley, 40°23′15″ N 75°47′23″ W (c,d) 25.09.2017, (e) 21.05.2017, on Vitis sp. (Vitaceae) and other plant species, observed by Erica Smyers |
| Figure 11c,d | Lycorma delicatula (White, 1845) | 23.65 | Formicidae indet. | New data: USA: Pennsylvania, Berks County, Berks, Oley, 40°23′15″ N 75°47′23″ W, (f) 25.09.2017, Boyertown, 40°23′15″ N 75°47′23″ W, 20.06.2017, on Vitis sp. (Vitaceae) and other plant species, observed by Erica Smyers |
| Figure 12a | Lycorma delicatula (White, 1845) | 23.65 | Gastropoda: Limacidae indet. | New data: USA: Pennsylvania, Berks County, Berks, Oley, 08.09.2017, observed by Erica Smyers |
| Figure 12b | Lystra lanata (Linnaeus, 1758) | 24.2 | Formicidae indet. | New data: Peru: Puritania river, 4°17′55.0″ S 73°16′52.7″ W, 24.01.2020, adult on tree trunk, observed by “ampatenaude” https://www.inaturalist.org/observations/38372280 |
| Figure 12c | Penthicodes atomaria (Weber, 1801) | 28 | Blattodea: Blattidae: Periplaneta australasiae (Fabricius, 1775) | New data: Cambodia: Cardamom Mts., 12°00′ N 103°15′ E, 04.08.2013, adult on tree trunk, observed by Arthur Anker |
| Figure 12d and Figure 13a | Penthicodes atomaria (Weber, 1801) | 28 | Blattodea indet. | New data: Constant et al. 2016; Thailand: Khao Sok National Park, 8°56′12″ N 98°31′49″ E, 25.12.2017 and 12.02.2018, observed by C. Hübner |
| Penthicodes atomaria (Weber, 1801) | 28 | Formicidae: Myrmicinae: Crematogaster sp. | [38] | |
| Figure 13b | Penthicodes atomaria (Weber, 1801) | 28 | Reptilia: Squamata: Gekkonidae indet. | New data: Thailand: Khao Sok National Park, 8°56′12″ N 98°31′49″ E, 24.02.2020, adult on tree trunk, observed by C. Hübner |
| Figure 13c | Penthicodes pulchella (Guérin-Méneville, 1838) | 19.75 | Blattodea indet. | New data: Cambodia: Koh Kong Province, Tatai, 11°35′13″ N 103°05′50″ E, 13.11.2017, adult on tree trunk, observed by G. Chartier |
| Figure 13d | Penthicodes pulchella (Guérin-Méneville, 1838) | 19.75 | Blattodea indet. | New data: Cambodia: Ratanakiri, Yeak Laom Lake, 13°44′ N 107°01′ E, 10.10.2017, adult on tree trunk, observed by J. Constant |
| Figure 14a | Penthicodes pulchella (Guérin-Méneville, 1838) | 19.75 | Blattodea indet. | New data: Vietnam: Phuoc Binh National Park, 12°00′ N 108°47′ E, 30.07.2014, adult on tree trunk, observed by J. Constant |
| Figure 14b | Penthicodes pulchella (Guérin-Méneville, 1838) | 19.75 | Formicidae indet. | New data: Thailand: Chiang Mai, Doi Suthep Pui, 18°49′ N 98°55′ E, 10.02.2015, adult on tree trunk, observed by W. Bunkhwamdi |
| Figure 14c | Penthicodes quadrimaculata Lallemand, 1963 | 25 | Blattodea indet. | New data: Malaysia: Lambir Hills National Park, 4°11′38.7″ N 114°01′58.3″ E, 19.03.2008, adult on tree trunk, observed by C. Lee. https://www.inaturalist.org/observations/54558240 |
| Figure 14d | Penthicodes variegata Guérin-Méneville, 1829 | 31.15 | Blattodea indet. | New data: Vietnam: Nui Chua National Park, 11°42′ N 109°09′ E, 08.08.2014, adult on tree trunk, observed by J. Constant |
| Figure 14e | Penthicodes variegata Guérin-Méneville, 1829 | 31.15 | Blattodea indet. | New data: Thailand: Khao Sok National Park, 8°56′12″ N 98°31′49″ E, 24.05.2019, observed by C. Hübner |
| Figure 15a | Phrictus quinquepartitus Distant, 1883 | 45.5 | Blattodea indet. | New data: Costa Rica: Guanacaste, San Jorge, 10°44′2.23″ N 85°18′2.03″ W, 617 m, 01.03.2018, adult on tree trunk, observed by G. Kunz |
| Figure 15b | Phrictus quinquepartitus Distant, 1883 | 45.5 | Formicidae: Formicinae: Camponotus sp. | New data: Costa Rica: La Selva Biological Station, 10°25′42.9″ N 84°00′16.0″ W, 04.02.2016, adult, observed by G. Kunz https://www.inaturalist.org/observations/46068546 |
| Phrictus quinquepartitus Distant, 1883 | 45.5 | Formicidae: Formicinae: Camponotus sp. | [14] | |
| Figure 15c | Phrictus quinquepartitus Distant, 1883 | 45.5 | Gastropoda: Spiraxidae: Euglandina sp. | New data: Costa Rica: Puerto Viejo de Sarapiquí, La Selva Biological Station, 10°25′49.97″ N 84° 0′18.36″ W, 53 m, 02.02.2016, adult on tree trunk, observed by G. Kunz https://www.inaturalist.org/observations/45716031 |
| Polydictya thompsoni Constant and Pham, 2019 | 26.9 | Formicidae: Formicinae: Camponotus sp. | [39] | |
| Figure 15d | Polydictya thompsoni Constant and Pham, 2019 | 26.9 | Lepidoptera: Nolidae: Eligma narcissus (Cramer, 1775) | New data: Thailand: Ratchaburi province, Suan Phueng district, 13°32′54.9″ N 99°12′24.2″ E, 30.10.2020, adults on tree trunk, observed by K. Jiaranaisakul |
| Figure 15e | Polydictya sp. | Formicidae: Formicinae: Dinomyrmex gigas (Latreille, 1802) | New data: Singapore: 1°17′ N 103°50′ E, 19.10.2016, adult on tree trunk, observed by C. Nijhuis | |
| Figure 16a | Polydictya sp. | 23.15 | Formicidae indet. | New data: Cambodia: Yeak Laom Lake, 13°44′ N 107°01′ E, 10.10.2017, adult on tree trunk, observed by J. Constant |
| Figure 16b | Polydictya sp. | 23.15 | Reptilia: Squamata: Gekkonidae indet. | New data: Cambodia: Yeak Laom Lake, 13°44′ N 107°01′ E, 10.10.2017, adult on tree trunk, observed by J. Constant |
| Pyrops candelaria (Linné, 1758) | 71 | Formicidae: Formicinae: Camponotus sp. | [38] | |
| Figure 16c | Pyrops candelaria (Linné, 1758) | 71 | Reptilia: Squamata: Gekkonidae indet. | New data: Laos: Luang Prabang, Royal Palace Garden, 19°53′30″ N 102°08′08″ E, 05.02.2013, adult on tree trunk, observed by H. Rawson |
| Figure 16d | Pyrops candelaria (Linné, 1758) | 71 | Reptilia: Squamata: Gekkonidae: Hemidactylus platyurus (Schneider, 1797) | [31]: Thailand, Chiang Mai University Campus, 2006, photo by M. Kemal |
| Figure 16e | Pyrops coelestinus (Stål, 1863) | 67 | Blattodea indet. | New data: Vietnam: Cat Tien National Park, 11°26′ N 107°26′ E, 10.12.2016, adult on tree trunk, observed by D. Gochfeld |
| Figure 17a | Pyrops connectens (Atkinson, 1885) | 58 | Blattodea indet. | New data: Thailand: Khao Sok National Park, 8°56′12″ N 98°31′49″ E, 23.07.2018, adult on tree trunk, observ. by C. Hübner |
| Pyrops cultellatus cultellatus (Walker, 1857) | 43.95 | Formicidae indet. | [37] | |
| Figure 17b | Pyrops cultellatus yoshiakii Nagai and Porion, 2002 | 36.5 | Formicidae indet. | New data: Malaysia: Selangor, Sungai Tua, 3°19′54.3″ N 101°43′22.1″ E, 22.12.2015, adult on tree trunk, observed by H.P. Guek |
| Figure 17c | Pyrops cultellatus yoshiakii Nagai and Porion, 2002 | 36.5 | Formicidae: Formicinae: Dinomyrmex gigas (Latreille, 1802) | New data: Malaysia: Gunung Ganing National Park, 1°41′40.1″ N 109°50′40.2″ E, 27.08.2018, adult on tree trunk, observed by C. Lee https://www.inaturalist.org/observations/54558235 |
| Figure 17d | Pyrops intricatus (Walker, 1857) | 57.5 | Blattodea indet. | New data: Malaysia: Gunung Mulu National Park, 4°02′29.8″ N 114°48′56.8″ E, 01.07.2006, adult on tree trunk, observed by C. Lee https://www.inaturalist.org/observations/57803615 |
| Figure 17e | Pyrops intricatus (Walker, 1857) | 57.5 | Blattodea indet. | New data: Malaysia: Gunung Mulu National Park, 4°02′23.6″ N 114°48′58.1″ E, 15.10.2009, adult on tree trunk, observed by C. Lee https://www.inaturalist.org/observations/54558243 |
| Pyrops intricatus (Walker, 1857) | 57.5 | Blattodea indet. | Bosuang et al., 2017 | |
| Figure 18a | Pyrops sidereus (Distant, 1905) | 60 | Reptilia: Squamata: Gekkonidae indet. | New data: Malaysia: Borneo, Sabah, Abai, Kinabatangan River, 5°41′16″ N 118°23′01″ E, 13.05.2015, adult on tree trunk, observed by R.K. Asuncion |
| Figure 18b | Pyrops spinolae (Westwood, 1842) | 52 | Blattodea indet. | New data: Vietnam: Kon Plong Protected Forest, 14°39′43″ N 108°15′45″ E, 20.08.2019, adult on tree trunk, observed by J. Constant |
| Figure 18c | Pyrops spinolae (Westwood, 1842) | 52 | Blattodea indet. | New data: Thailand: Suan Phueng District Ratchaburi, 13°32′54.9″ N 99°12′24.2″ E, 10.08.2021, adult on tree trunk, observed by Kawin Jiaranaisakul https://www.inaturalist.org/observations/90767059 |
| Pyrops whiteheadi (Distant, 1889) | 45 | Blattodea: Blattidae: Dorylaea sp. | [33] | |
| Pyrops whiteheadi (Distant, 1889) | 45 | Blattodea: Ectobiidae: Pseudophyllodromiinae indet. | [23] | |
| Figure 18d | Pyrops whiteheadi (Distant, 1889) | 45 | Blattodea indet. | New data: Malaysia: Danum Valley Conservation Area, 4°57′48.1″ N 117°48′19.5″ E, 15.12.2008, adult on tree trunk, observed by C. Lee https://www.inaturalist.org/observations/54558242 |
| Figure 18e | Pyrops whiteheadi (Distant, 1889) | 45 | Reptilia: Squamata: Gekkonidae: Gehyra mutilata (Wiegmann, 1834) | [33]; New data: Malaysia: Sabah, 5°44′03.0″ N 117°48′44.8″ E, 09.2018, adults on tree trunk, observed by Chien Lee https://www.inaturalist.org/observations/79122196 |
| Figure 19a | Pyrops whiteheadi (Distant, 1889) | 45 | Reptilia: Squamata: Gekkonidae indet. | New data: Malaysia: Borneo, Sabah, Sandakan, 05°50′ N 118°07′ E, 13.05.2015, adult on tree trunk, observed by R.K. Asuncion |
| Saiva gemmata (Westwood, 1848) | 40 | Blattodea indet. | [9] | |
| Figure 19b | Scamandra polychroma Gerstaecker, 1895 | 26.5 | Formicidae: Formicinae: Dinomyrmex gigas (Latreille, 1802) | New data: Malaysia: Borneo, Sarawak, Mulu National Park, 4°7′55″ N 114°55′8″ E, 01.05.2006, adult on tree trunk, observed by D. Mezger |
| Figure 19c | Scamandra polychroma Gerstaecker, 1895 | 26.5 | Formicidae: Formicinae: Camponotus sp. | New data: Malaysia: Borneo, Sarawak, Mulu National Park, 4°7′55″ N 114°55′8″ E, 28.06.2014, adult on tree trunk, observed by A. Kang |
| Figure 19d,e | Scamandra polychroma Gerstaecker, 1895 | 26.5 | Formicidae indet. | New data: Malaysia: Borneo, Sarawak, Mulu National Park, 4°7′55″ N 114°55′8″ E, 20.09.2015, adult on tree trunk, observed by P. Soltys |
| Figure 20a | Scamandra polychroma Gerstaecker, 1895 | 26.5 | Formicidae indet. | New data: Malaysia: Gunung Mulu National Park, 4°08′13.8″ N 114°53′30.5″ E, 25.11.2012, adult on tree trunk, observed by C. Lee |
| Figure 20b | Scamandra sp. | 24.5 | Formicidae: Formicinae: Dinomyrmex gigas (Latreille, 1802) | New data: Malaysia: Borneo, Sarawak, Mulu National Park, 4°7′55″ N 114°55′8″ E, 08.07.2015, adult on tree trunk, observed by A. Kang |
| Figure 20c,d | Villala canoi Goemans, 2005 | 28.5 | Gastropoda: Spiraxidae: Euglandina sp. | New data: Costa Rica: Puerto Viejo de Sarapiquí, La Selva Biological Station, 10°25′ N 84°0′ W, 02.02.2012, adult on tree trunk, observed by E. Holzer |
| Figure 20e | Poiocerinae indet. | Blattodea indet. | New data: Costa Rica: Bosque Eterno de Los Niños, 10°20′08.8″ N 84°44′00.6″ W, 14.05.2011, adult on tree trunk, observed by John G. Phillips https://www.inaturalist.org/observations/15191736 | |
| Figure 20f | Fulgoridae indet. | Blattodea indet. | New data: Equador: Orellana, 0°28′00.9″ S 76°50′42.7″ W, 27.02.2018, adults on tree trunk, observed by C. Lee https://www.inaturalist.org/observations/54469653 | |
| Hypochthonellidae | ||||
| Hypochthonella caeca China and Fennah, 1952 | Formicidae: Dorylinae: Dorylus fulvus (Westwood, 1839), and other spp. | [50] | ||
| Issidae | ||||
| Agalmatium bilobum (Fieber, 1877) | 4.5 | Formicidae: Formicinae: Camponotus ionius Emery, 1920 | [35] | |
| Agalmatium flavescens (Olivier, 1791) | Formicidae: Formicinae: Camponotus aethiops (Latreille, 1798) | [36] | ||
| Argepara lyra (Berg 1883) | 4.5 | Formicidae: Formicinae: Camponotus sp. | [27] | |
| Asarcopus palmarum Horvath, 1921 | 3 | Formicidae: Formicinae: Camponotus sp. | [77] | |
| Issus sp. | Formicidae: Formicinae: Camponotus vagus (Scopoli, 1763) | [59] | ||
| Figure 21a | Hysteropterum sp. | 3.75 | Formicidae: Formicinae: Camponotus aethiops (Latreille, 1798) | New data: Croatia: Sibenik-Knin County, 4.5 km SE Primosten, Macchia, 43°34′29″ N 15°56′19″ E, 22.09.2015, 55 m Excursion with Johanna Gunczy and Roel van Klink, observed by G. Kunz |
| Figure 21b | Hysteropterum sp. | 3.75 | Blattodea: Ectobiidae indet. | New data: Croatia: Sibenik-Knin County, 4.5 km SE Primosten, Macchia, 43°34′29″ N 15°56′19″ E, 22.09.2015, 55 m Excursion with Johanna Gunczy and Roel van Klink, observed by G. Kunz |
| Mycterodus colossicus (Dlabola, 1987) | Formicidae: Formicinae: Camponotus ionius Emery, 1920 | [35] | ||
| Picumna sp. | 5.85 | Formicidae indet. | [85] | |
| Figure 21c | Sarimini indet. | 8.85 | Formicidae: Formicinae: Camponotus sp. | New data: Cambodia: Keo Seima Wildlife Sanctuary, 12°11′39″ N 107°01′01″ E, 19.11.2018, adult on low vegetation, observed by J. Constant |
| Figure 22a | Thioniini indet. | 8.25 | Formicidae: Formicinae: Camponotus sp. | New data: Costa Rica: La Leona Eco Lodge, 8°26′54.17″ N 83°29′13.54″ W, 16.02.2018, adult on tree trunk, observed by G. Kunz. https://www.inaturalist.org/observations/20131049 |
| Figure 22b | Issidae indet. | Formicidae: Formicinae: Dinomyrmex gigas (Latreille, 1802) | New data: Brunei: Kg Menengah, 4°26′53.3″ N 115°12′45.5″ E, 11.08.2021, adult, observed by H. Ahmad Sah https://www.inaturalist.org/observations/97481701 | |
| Figure 22c | Issidae indet. | Formicidae indet. | New data: Malaysia: Kuala Lumpur, Perdana Botanical Garden, 3°08′20.4″ N 101°41′12.7″ E, 27.10.2019, adult, observed by T. Maul. https://www.inaturalist.org/observations/34958087 | |
| Meenoplidae | ||||
| Phaconeura sp. | Formicidae: Formicinae: Paratrechina sp. | [21] | ||
| Meenoplidae indet. | Formicidae: Formicinae: Anoplolepis steingroeveri (Forel, 1894) | [30] | ||
| Nogodinidae | ||||
| Figure 23a | Biolleyana fenestra (Gerstaecker, 1895) | Formicidae: Formicinae: Camponotus sp. | New data: Costa Rica: Rio Agujitas River, 8°41′27.9″ N 83°40′42.7″ W, 02.04.2018, adult on tree trunk, observed by T. Stice. https://www.inaturalist.org/observations/37784649 | |
| Figure 23b | Paravarcia sp. | 17.75 | Blattodea indet. | New data: Malaysia: Penang Island, 5°25′28″ N 100°16′08.1″ E, 20.01.2021, adult on unidentified tree trunk, observ. by A. Kang |
| Figure 23c,d | Paravarcia sp. | 17.75 | Formicidae indet. | New data: Malaysia: Penang Island, 5°25′28″ N 100°16′08.1″ E, 30.11.2020, adult on unidentified tree trunk, observ. by A. Kang |
| Ricaniidae | ||||
| Pocharica sp. or Pochazoides sp. | Reptilia: Squamata: Gekkonidae: Geckolepis sp. (cf. G. maculata) | [34] | ||
| Scolypopa australis (Walker, 1851) | Hymenoptera: Apidae | [41,42,43] | ||
| Tettigometridae | ||||
| Tettigometridae Egropinae | ||||
| Egropa malayensis (Distant, 1908) | Formicidae undet. | New data: Vietnam, Kien Luong, Hon Chong, 10°09′48″ N 104°37′57″ E, 31.05.2008, on Inga sp. (Fabaceae), observed by T. Bourgoin | ||
| Figure 24a | Egropa sp. | 4.5 | Formicidae: Formicinae: Anoplolepis gracilipes (Smith, F., 1857) | New data: Malaysia: Selangor, Ampang Jaya, Tamarind Springs Jalan, 3°10′14.7″ N 101°46′14.3″ E, 19.01.2020, adults, observed by T. Maul. https://www.inaturalist.org/observations/37743145 |
| Figure 24b | Egropa sp. | 4.5 | Formicidae: Formicinae: Camponotus sp. | New data: Malaysia: Selangor, Ampang Jaya, Tamarind Springs Jalan, 3°10′14.7″ N 101°46′14.3″ E, 26.12.2021, adults, observed by T. Maul. https://www.inaturalist.org/observations/103740021 |
| Figure 24c | Egropa sp. | 4.5 | Formicidae: Formicinae: Oecophylla smaragdina (Fabricius, 1775) | New data: Cambodia, Phnom Penh, RUPP Campus, 11°34′03.5″ N 104°53′30.2″ E, 13.05.2015, on Ficus sp. (Moraceae), observed by J. Constant and V. Sougnez |
| Tettigometridae Nototettigometrinae | ||||
| Euphyonarthex phyllostoma Schmidt, 1912 | 8.5 | Formicidae: Dolichoderinae: Tapinoma melanocephalum (Fabricius, 1793) | [15] | |
| Euphyonarthex phyllostoma Schmidt, 1912 | 8.5 | Formicidae: Dolichoderinae: Tapinoma sp. | [15] | |
| Euphyonarthex phyllostoma Schmidt, 1912 | 8.5 | Formicidae: Dolichoderinae: Technomyrmex sp. | [15] | |
| Euphyonarthex phyllostoma Schmidt, 1912 | 8.5 | Formicidae: Myrmicinae: Crematogaster striatula Emery, 1892 | [15] | |
| Euphyonarthex phyllostoma Schmidt, 1912 | 8.5 | Formicidae: Myrmicinae: Crematogaster (Acrocoelia) sp. | [15] | |
| Euphyonarthex phyllostoma Schmidt, 1912 | 8.5 | Formicidae: Myrmicinae: Monomorium sp. | [15] | |
| Euphyonarthex phyllostoma Schmidt, 1912 | 8.5 | Formicidae: Myrmicinae: Myrmicaria opaciventris Emery, 1893 | [15] | |
| Euphyonarthex phyllostoma Schmidt, 1912 | 8.5 | Formicidae: Myrmicinae: Pheidole megacephala (Fabicius, 1793) | [15] | |
| Euphyonarthex phyllostoma Schmidt, 1912 | 8.5 | Formicidae: Myrmicinae: Pheidole sp. | [15] | |
| Euphyonarthex phyllostoma Schmidt, 1912 | 8.5 | Formicidae: Formicinae: Camponotus acvapimensis Mayr, 1862 | [15] | |
| Euphyonarthex phyllostoma Schmidt, 1912 | 8.5 | Formicidae: Formicinae: Camponotus brutus Forel, 1886 | [15] | |
| Euphyonarthex phyllostoma Schmidt, 1912 | 8.5 | Formicidae: Formicinae: Camponotus chrysurus Gerstäcker, 1871 | [15] | |
| Euphyonarthex phyllostoma Schmidt, 1912 | 8.5 | Formicidae: Formicinae: Camponotus sp. | [15] | |
| Euphyonarthex phyllostoma Schmidt, 1912 | 8.5 | Formicidae: Formicinae: Paratrechina sp. | [15] | |
| Nototettigometra (Nototettigometra) patruelis (Stål, 1855) | 5 | Formicidae: Myrmicinae: Crematogaster castanea Smith, F., 1858 | [47] | |
| Nototettigometra (Nototettigometra) patruelis (Stål, 1855) | 5 | Formicidae: Myrmicinae: Pheidole megacephala (Fabicius, 1793) | [47] | |
| Figure 25a | Nototettigometra (Nototettigometra) patruelis (Stål, 1855) | 5 | Formicidae: Formicinae: Camponotus maculatus (Fabricius, 1782) | New data: South Africa: St Lucia, St Lucia Park, 28°22′18.8″ S 32°23′28.0″ E, 01.04.2018, nymphs and adults on Searsia chirindensis (Baker f.) Moffett (Anacardiaceae), observ. by “magdastlucia” https://www.inaturalist.org/observations/10553418 (bug) https://www.inaturalist.org/observations/10553414 (plant) https://www.inaturalist.org/observations/10553415 (ant) |
| Figure 25b | Nototettigometra (Nototettigometra) patruelis (Stål, 1855) | 5 | Formicidae: Myrmicinae: Pheidole megacephala (Fabicius, 1793) | New data: South Africa: St Lucia, St Lucia Park, 28°22′34.3″ S 32°25′05.2″ E, 16.01.2018, nymphs and adults, observed by “magdastlucia”. https://www.inaturalist.org/observations/9530115 (bug). https://www.inaturalist.org/observations/9530116 (ant) |
| Nototettigometra (Nototettigometra) patruelis (Stål, 1855) | 5 | Formicidae: Formicinae: Anoplolepis custodiens (Smith, F., 1858) | [75] | |
| Nototettigometra (Nototettigometra) patruelis (Stål, 1855) | 5 | Formicidae: Formicinae: Camponotus acvapimensis Mayr, 1862 | [86] | |
| Nototettigometra (Nototettigometra) patruelis (Stål, 1855) | 5 | Formicidae: Formicinae: Camponotus sp. | [47] | |
| Nototettigometra (Nototettigometra) patruelis (Stål, 1855) | 5 | Formicidae: Formicinae: Polyrhachis schistacea (Gerstäcker, 1859) | [75] | |
| Nototettigometra (Nototettigometra) patruelis (Stål, 1855) | 5 | Formicidae indet. | New data: South Africa: Mtunzini, 28°57′12.6″ S 31°46′06.2″ E, 14.04.2019, nymphs and adults, observed by “magdastlucia”. https://www.inaturalist.org/observations/23220905 | |
| Figure 25c | Nototettigometra (Nototettigometra) patruelis (Stål, 1855) | 5 | Formicidae indet. | New data: South Africa: St Lucia, Simangaliso Wetland Park World Heritage Site, 28°22′34.3″ S 32°25′05.2″ E, 05.10.2019, nymphs and adults on Senecio polyanthemoides Sch.Bip. (Asteraceae), observed by “magdastlucia”. https://www.inaturalist.org/observations/33903815 (bug) https://www.inaturalist.org/observations/33903837 (plant) https://www.inaturalist.org/observations/33903799 (ant) |
| Figure 25d | Nototettigometra (Nototettigometra) patruelis (Stål, 1855) | 5 | Formicidae indet. | New data: South Africa: Pietermaritzburg, Kingston Park, 29°33′53.7″ S 30°21′53.2″ E, 15.09.2019, nymphs and adults, observed by A. Manson. https://www.inaturalist.org/observations/38824625 |
| Nototettigometra (Nototettigometra) rubrospersa (Fennah, 1958) | Formicidae: Formicinae: Camponotus sp. (brutus group) | [4,15] | ||
| Nototettigometra (Nototettigometra) undata (Walker, 1851) | Formicidae: Formicinae: Camponotus acvapimensis Mayr, 1862 | [15,16] | ||
| Nototettigometra (Nototettigometra) undata (Walker, 1851) | Formicidae: Formicinae: Camponotus brutus Forel, 1886 | [15,16] | ||
| Nototettigometra (Prodhilda) proteacola (Bourgoin and Pajor, 2000) | 5.75 | Formicidae: Myrmicinae: Crematogaster sp. | [22] | |
| Nototettigometra (Prodhilda) proteacola (Bourgoin and Pajor, 2000) | 5.75 | Formicidae: Myrmicinae: Myrmicaria sp. | [22] | |
| Nototettigometra (Prodhilda) proteacola (Bourgoin and Pajor, 2000) | 5.75 | Formicidae: Myrmicinae: Pheidole megacephala (Fabicius, 1793) | [22] | |
| Nototettigometra (Prodhilda) proteacola (Bourgoin and Pajor, 2000) | 5.75 | Formicidae: Formicinae: Anoplolepis custodiens (Smith, F., 1858) | [22] | |
| Figure 26a,b | Nototettigometra sp. apud N. (N.) patruelis | Formicidae undet. | New data: Kenya, Narok, Masai Mara, 01°29′ S 35°08′ E, 24.10.2013, on Senna didymobotrya (Fresen.) H.S. Irwin and Barneby (Fabaceae), observed by T. Bourgoin | |
| Figure 26c | Nototettigometra sp. | 4.95 | Formicidae: Formicinae: Camponotus sp. | New data: South Africa: Timbavati Nature Reserve, 24°22′34.9″ S 31°09′40.4″ E, 21.05.2014, eggs and adults on Euphorbia sp. (Euphorbiaceae), observed by W. Uys. https://www.inaturalist.org/observations/10993430 |
| Figure 26d | Nototettigometra sp. | 4.95 | Formicidae: Formicinae undet. | New data: South Africa: South Cape, 33°58′00.3″ S 22°05′26.5″ E, 21.04.2019, adults, observed by S. Adam. https://www.inaturalist.org/observations/22878347 |
| Figure 27a | Nototettigometra sp. | 4.95 | Formicidae indet. | New data: Malawi: Liwonda National Park, 14°50′58.2″ S 35°17′39.2″ E, 10.11.2017, adult, observed by A. Joaquin. https://www.inaturalist.org/observations/9065660 |
| Nototettigometra sp. | 4.95 | Formicidae indet. | New data: South Africa: St Lucia, iSimangaliso Wetland Park, 28°22′34.3″ S 32°25′05.2″ E, 17.09.2016, 16.01.2018 and 17.08.2018, nymphs and adults, observed by “magdastlucia”. https://www.inaturalist.org/observations/1120533 (bug) https://www.inaturalist.org/observations/9530116 (ant) https://www.inaturalist.org/observations/31306876 | |
| Figure 27b | Nototettigometra sp. | 4.95 | Formicidae indet. | New data: Botswana: Gaborone, 24°33′24.2″ S 25°57′48.7″ E, 24.04.2020, nymphs on Cassia abbreviata Oliv. (Fabaceae), observed by C. Sydes. https://www.inaturalist.org/observations/44759407 |
| Figure 27c,d | New genus apud Nototettigometra | Formicidae undet. | New data: Kenya, Narok, Masai Mara, 01°29′ S 35°08′ E, 22.10.2013, on Grewia sp. (Malvaceae), observed by T. Bourgoin | |
| Tettigometridae Tettigometrinae | ||||
| Tettigometra (Tettigometra) atra Hagenbach, 1825 | 3.39 | Formicidae indet. | In ant nests: [11,55,87,88] | |
| Tettigometra (Tettigometra) atra Hagenbach, 1825 | 3.39 | Formicidae: Dolichoderinae: Tapinoma erraticum (Latreille, 1798) | [65,66] | |
| Tettigometra (Tettigometra) atra Hagenbach, 1825 | 3.39 | Formicidae: Dolichoderinae: Tapinoma erraticum (Latreille, 1798) | New data: Bulgaria: Eastern Rhodopes, Zhelezino vill., 41°27′00.0″ N 25°56′51.0″ E, 30.03.2012, adults in soil ant nests under stones, observed by I. Gjonov, A. Lapeva-Gjonova | |
| Tettigometra (Tettigometra) atra Hagenbach, 1825 | 3.39 | Formicidae: Dolichoderinae: Tapinoma simrothi Krausse, 1911 | New data: Malta: Malta isl., Paradise Bay, 35°58′54.0″ N 14°19′58.0″ E, 19.11.2017, adults in soil ant nest under stones, observed by I. Gjonov, A. Lapeva-Gjonova | |
| Tettigometra (Tettigometra) atra Hagenbach, 1825 | 3.39 | Formicidae: Myrmicinae: Tetramorium caespitum (Linnaeus, 1758) | [89] | |
| Tettigometra (Tettigometra) atra Hagenbach, 1825 (as T. piceola Burmeister, 1835) | 3.39 | Formicidae: Formicinae: Lasius niger (Linnaeus, 1758) | [60] | |
| Tettigometra (Tettigometra) atra Hagenbach, 1825 | 3.39 | Formicidae: Formicinae: Lasius platythorax Seifert, 1991 | New data: Bulgaria: Vitosha Mt., Zheleznitsa vill., 42°32′07.0″ N 23°20′05.0″ E, 03.03.2002, observed by I. Gjonov, A. Lapeva-Gjonova | |
| Figure 28a | Tettigometra (Tettigometra) atra Hagenbach, 1825 | 3.39 | Formicidae: Formicinae: Lasius platythorax Seifert, 1991 | New data: Bulgaria: Danibe plane, Rusenski Lom, 43°35′43.0″ N 26°06′25.0″ E, 24.03.2011, adults in soil ant nests under stones, observed by I. Gjonov, A. Lapeva-Gjonova |
| Tettigometra (Tettigometra) barbata Mitjaev, 1971 | Formicidae: Formicinae: Camponotus turkestanicus Emery, 1887 | [90] | ||
| Figure 28b | Tettigometra (Tettigometra) brachycephala Fieber, 1865 | Formicidae: Dolichoderinae: Tapinoma sp. | New data: Croatia: Mravince, 43°32′24.8″ N 16°30′14.1″ E, 08.03.2021, observed by Anton Gjeldum. https://www.inaturalist.org/observations/70816240 | |
| Tettigometra (Tettigometra) fusca Fieber, 1865 | 6.745 | Formicidae: Dolichoderinae: Tapinoma nigerrimum (Nylander, 1856) | [11] | |
| Tettigometra (Tettigometra) fusca Fieber, 1865 | 6.745 | Formicidae: Formicinae: Formica pratensis Retzius, 1783 | [71,91] | |
| Figure 28c and Figure 29a | Tettigometra (Tettigometra) fusca Fieber, 1865 | 6.745 | Formicidae: Formicinae: Lasius sp. | New data: Ukraine: Mykolayiv, 46°58′30.1″ N 31°59′40.5″ E, 17.03.2019, adults, observed by Roman (efarilis) https://www.inaturalist.org/observations/21321863, https://www.inaturalist.org/observations/21321852 |
| Tettigometra (Tettigometra) impressifrons Mulsant and Rey, 1855 | 3.7 | Formicidae: Dolichoderinae: Tapinoma erraticum (Latreille, 1798) | [48] | |
| Figure 29b,c | Tettigometra (Tettigometra) impressifrons Mulsant and Rey, 1855 | 3.7 | Formicidae: Dolichoderinae: Tapinoma sp. | New data: Italy: Calabria, Capo Colonna, 39°0′46″ N 17°10′49″ E, 27.04.2008, nymphs and adults on Cakile maritima Scopoli (Brassicaceae), observed by I. Gjonov, A. Lapeva-Gjonova |
| Tettigometra (Tettigometra) impressifrons Mulsant and Rey, 1855 | 3.7 | Formicidae: Dolichoderinae: Tapinoma nigerrimum (Nylander, 1856) (as: T. erratico-nigerrimum Nyl.) | [53,92] | |
| Tettigometra (Tettigometra) impressifrons Mulsant and Rey, 1855 | 3.7 | Formicidae: Dolichoderinae: Tapinoma simrothi Emery, 1925 | [93,94] | |
| Tettigometra (Tettigometra) impressifrons Mulsant and Rey, 1855 | 3.7 | Formicidae: Myrmicinae: Myrmica sp. | [95] | |
| Tettigometra (Tettigometra) impressifrons Mulsant and Rey, 1855 | 3.7 | Formicidae: Formicinae: Formica rufibarbis Fabricius, 1793 | [87] | |
| Tettigometra (Tettigometra) laeta Herrich-Schäffer, 1834 | 4.52 | Formicidae: Dolichoderinae: Tapinoma erraticum (Latreille, 1798) (as Formica erraticum) | Probably 3 different observations: [56,57,60] Recited by: [11,54,55,88] (for ‘Rouget, 1966’ sic), [63,64] (under T. atra), [92] | |
| Figure 30a | Tettigometra (Tettigometra) laeta Herrich-Schäffer, 1834 | 4.52 | Formicidae: Dolichoderinae: Tapinoma erraticum (Latreille, 1798) | New data: Bulgaria: Eastern Rhodopes, Zhelezino vill., 41°26′60″ N 25°56′51″ E, 30.03.2012, adults in soil ant nests under stones, observed by I. Gjonov, A. Lapeva-Gjonova |
| Tettigometra (Tettigometra) laeta Herrich-Schäffer, 1834 | 4.52 | Formicidae: Myrmicinae: Tetramorium caespitum (Linnaeus, 1758) | [96] | |
| Figure 30b | Tettigometra (Tettigometra) laeta Herrich-Schäffer, 1834 | 4.52 | Formicidae: Myrmicinae: Tetramorium chefketi Forel, 1911 | New data: Albania: Strëmbec, 40°09′24.8″ N 20°28′26.0″ E, 17.06.2019, nymphs and adults on Asteraceae, observed by G. Kunz |
| Tettigometra (Tettigometra) laeta Herrich-Schäffer, 1834 | 4.52 | Formicidae: Formicinae: Formica cunicularia Latreille, 1798 | [96] | |
| Tettigometra (Tettigometra) laeta Herrich-Schäffer, 1834 | 4.52 | Formicidae: Formicinae: Lasius psammophilus Seifert, 1992 | [96] | |
| Tettigometra (Tettigometra) nasicornis Mityaev, 1971 | Formicidae: Formicinae: Camponotus turkestanicus Emery, 1887 | [90] | ||
| Figure 30c | Tettigometra (Tettigometra) picta Fieber, 1865 | 3.86 | Formicidae: Dolichoderinae: Tapinoma nigerrimum (s.l.) | New data: Malta: Il-Kalanka bay, 35°49′29.0″ N 14°33′23.3″ E, 02.06.2019; nymphs and adults on Lavatera arborea L. (Malvaceae), observed by I. Gjonov, A. Lapeva-Gjonova |
| Tettigometra (Tettigometra) sulphurea Mulsant and Rey, 1855 | 5.98 | Formicidae: Dolichoderinae: Tapinoma erraticum (Latreille, 1798) | [48] | |
| Tettigometra (Tettigometra) sulphurea Mulsant and Rey, 1855 | 5.98 | Formicidae: Myrmicinae: Tetramorium caespitum (Linnaeus, 1758) | [48] | |
| Tettigometra (Tettigometra) sulphurea Mulsant and Rey, 1855 | 5.98 | Formicidae: Myrmicinae: Tetramorium meridionale Emery, 1870 | [48] | |
| Figure 31a | Tettigometra (Tettigometra) sulphurea Mulsant and Rey, 1855 | 5.98 | Formicidae: Myrmicinae: Tetramorium moravicum Kratochvíl, 1941 | New data: Bulgaria: Vlahina Mt., 42°09′15.0″ N 23°02′06.0″ E, 29.06.2001, nymphs and adults on Onopordon acanthium L. (Asteraceae), observed by I. Gjonov, A. Lapeva-Gjonova |
| Tettigometra (Tettigometra) sulphurea Mulsant and Rey, 1855 | 5.98 | Formicidae: Formicinae: Camponotus aethiops (Latreille, 1798) | [48] | |
| Figure 31b | Tettigometra (Tettigometra) sulphurea Mulsant and Rey, 1855 | 5.98 | Formicidae: Formicinae: Camponotus aethiops (Latreille, 1798) | New data: Greece: Serres, Strimoniko, 41°02′12.0″ N 23°17′07.0″ E, 22.04.2011; North Macedonia: Zhelino vill., 41°59′07.0″ N 21°05′41.0″ E, 16.06.2011, nymphs and adults on Onopordon acanthium L. (Asteraceae), observed by I. Gjonov, A. Lapeva-Gjonova |
| Tettigometra (Tettigometra) sulphurea Mulsant and Rey, 1855 | 5.98 | Formicidae: Formicinae: Camponotus piceus (Leach, 1825) | New data: Bulgaria: Northern Black Sea coast, Cape Kaliakra, 43°22′03.0″ N 28°27′57.0″ E, 30 m, 23.06.2008, nymphs and adults on Onopordon acanthium L. (Asteraceae), observed by I. Gjonov, A. Lapeva-Gjonova | |
| Figure 31c | Tettigometra (Tettigometra) sulphurea Mulsant and Rey, 1855 | 5.98 | Formicidae: Dolichoderinae: Tapinoma sp. | New data: Croatia: Split: Solin, 43°32′47.4″ N 16°28′44.9″ E, 11.05.2019, adults on Onopordon acanthium L. (Asteraceae), observed by A. Gjeldum https://www.inaturalist.org/observations/24932089 |
| Figure 32a | Tettigometra (Tettigometra) sulphurea Mulsant and Rey, 1855 | 5.98 | Formicidae: Formicinae: Camponotus sp. | New data: Greece: Lesbos, Voreio Aigaio, 39°11′21.9″ N 26°27′47.8″ E, 12.02.2021, adults on Onopordon acanthium L. (Asteraceae), observed by Savvas Zafeiriou https://www.inaturalist.org/photos/130244418 |
| Tettigometra (Tettigometra) sulphurea Mulsant and Rey, 1855 | 5.98 | Formicidae: Formicinae: Formica pratensis Retzius, 1783 | New data: Bulgaria: Vlahina Mt., 42°09′15.0″ N 23°02′06.0″ E, 29.06.2001; 42°09′24.0″ N 22°59′49.0″ E, 09.07.2006, nymphs and adults on Onopordon acanthium L. (Asteraceae), observed by I. Gjonov, A. Lapeva-Gjonova | |
| Tettigometra (Tettigometra) sulphurea Mulsant and Rey, 1855 | 5.98 | Formicidae: Formicinae: Lasius niger (Linnaeus, 1758) | New data: Bulgaria: Vlahina Mt., 42°09′15.0″ N 23°02′06.0″ E, 29.06.2001, nymphs and adults on Onopordon acanthium L. (Asteraceae), observed by I. Gjonov, A. Lapeva-Gjonova | |
| Figure 32b | Tettigometra (Tettigometra) sulphurea Mulsant and Rey, 1855 | 5.98 | Formicidae: Formicinae: Lasius paralienus Seifert, 1992 | New data: Serbia: Frushka gora, Neradin vill., 45°07′ N 19°54′ E, nymphs and adults on Onopordon acanthium L. (Asteraceae), observed by D. Savic |
| Tettigometra (Tettigometra) sulphurea Mulsant and Rey, 1855 | 5.98 | Formicidae: Formicinae: Lasius psammophilus Seifert, 1992 | New data: Bulgaria: Vlahina Mt., 42°09′15.0″ N 23°02′06.0″ E, 29.06.2001, nymphs and adults on Onopordon acanthium L. (Asteraceae), observed by I. Gjonov, A. Lapeva-Gjonova | |
| Tettigometra (Tettigometra) virescens (Panzer, 1799) | 4 | Formicidae: Myrmicinae: Myrmica sp. | Probably same recited observation: [58,59,62,63,64,88,92,97] | |
| Tettigometra (Tettigometra) virescens (Panzer, 1799) | 4 | Formicidae: Formicinae: Camponotus vagus (Scopoli, 1763) (as: Formica pubescens) | Probably same recited observation: [58,59,62,63,64,88,92,97] | |
| Figure 32c | Tettigometra (Eurychila) bifoveolata Signoret, 1866 | 4.68 | Formicidae: Dolichoderinae: Tapinoma sp. | New data: France: Pyrénées Orientales, Banyuls, 42°29′ N, 03°08′ E, 02.2022, adults in ant nest, observed by H. Bouyon |
| Tettigometra (Eurychila) bifoveolata Signoret, 1866 | 5 | Formicidae indet. | In ant nests: [11,55,87] (as T. atra), [92] | |
| Tettigometra (Eurychila) decorata Signoret, 1866 | 5 | Formicidae: Dolichoderinae: Tapinoma nigerrimum (Nylander, 1856) (as: T. erratico-nigerrimum Nyl.) | Probably same recited observation: [11,62,63,64,88] | |
| Figure 33a | Tettigometra (Mitricephalus) griseola Fieber, 1864 | 4.325 | Formicidae: Dolichoderinae: Tapinoma sp. | New data: Italy: Calabria, Capo Colonna, 39°00′46.0″ N 17°10′49.0″ E, 27.04.2008, observed by I. Gjonov, A. Lapeva-Gjonova; Sicily: Punta Delle Formiche, 36°39′41.0″ N 15°03′22.0″ E, 06.05.2008, nymphs and adults on Cakile maritima Scopoli (Brassicaceae), observed by I. Gjonov, A. Lapeva-Gjonova |
| Tettigometra (Mitricephalus) leucophaea (Preyssler, 1792) (as: T. obliqua Panzer, 1799) | Formicidae: Formicinae: Formica cinerea Mayr, 1853 | Same observation: [67,92] | ||
| Tettigometra (Mitricephalus) leucophaea (Preyssler, 1792) (as: T. obliqua Panzer, 1799) | Formicidae: Formicinae: Formica lugubris Zetterstedt, 1838 (as F. congerens) | [57] | ||
| Tettigometra (Mitricephalus) leucophaea (Preyssler, 1792) (as: T. obliqua Panzer, 1799) | Formicidae: Formicinae: Formica pratensis Retzius, 1783 | Same observation: [66,87] | ||
| Tettigometra (Mitricephalus) leucophaea (Preyssler, 1792) (as: T. obliqua Panzer, 1799) | Formicidae: Formicinae: Lasius niger (Linnaeus, 1758) | Same observation: [67,92] | ||
| Figure 33b,c | Tettigometra (Mitricephalus) leucophaea (Preyssler, 1792) | Formicidae: Formicinae: Formica cinerea Mayr, 1853 | New data: Bulgaria: Northern Black sea coast, Kamchiya river mouth, 43°01′56.0″ N 27°53′17.9″ E, 24.06.2004, nymphs and adults on Ammophila arenaria (L.) (Poaceae), observed by I. Gjonov, A. Lapeva-Gjonova | |
| Tettigometra (Mitricephalus) leucophaea (Preyssler, 1792) | Formicidae: Formicinae: Lasius niger (Linnaeus, 1758) | New data: Bulgaria: Western Pre-Balkan, Belogradchik, 43°37′20″ N 22°41′11″ E, 20.09.2012, adults around plant roots, observed by I. Gjonov, A. Lapeva-Gjonova | ||
| Tettigometra (Mitricephalus) macrocephala Fieber, 1864 | 4.715 | Formicidae: Formicinae: Camponotus aethiops (Latreille, 1798) | New data: Bulgaria: Vlahina Mt., Boboshevo, 42°09′50.0″ N 22°58′47.0″ E, 02.07.2001, nymphs and adults on Eryngium campestre L. (Apiaceae), observed by I. Gjonov, A. Lapeva-Gjonova | |
| Figure 34a | Tettigometra (Mitricephalus) macrocephala Fieber, 1864 | 4.715 | Formicidae: Formicinae: Camponotus aethiops (Latreille, 1798) | New data: Bulgaria: Pirin Mt.: Stara Kresna vill., 41°45′48.0″ N 23°10′09.0″ E, 23.07.2001, nymphs and adults on Eryngium campestre L. (Apiaceae), observed by I. Gjonov, A. Lapeva-Gjonova |
| Figure 34b,c | Tettigometra (Mitricephalus) macrocephala Fieber, 1864 | 4.715 | Formicidae: Formicinae: Camponotus sp. | New data: France, Lot, Cabrerets, 44°31′35″ N 1°39′17″ E, 10.08.2018, adults and nymphs on Eryngium sp. (Apiaceae), observed by J. Constant |
| Figure 34d and Figure 35a | Tettigometra (Mitricephalus) macrocephala Fieber, 1864 | 4.715 | Formicidae: Formicinae: Formica spp. | New data: Bulgaria: Stara planina Mt., Saranci vill. 42°43′24.0″ N 23°46′58.0″ E, 25.07.2012, nymphs and adults on Eryngium campestre L. (Apiaceae), observed by I. Gjonov, A. Lapeva-Gjonova |
| Tettigometra (Mitricephalus) macrocephala Fieber, 1864 | 4.715 | Formicidae: Formicinae: Formica sp. | [63,64] | |
| Tettigometra (Metroplaca) baranii Signoret, 1866 | 3.35 | Formicidae: Myrmicinae: Crematogaster sordidula (Nylander, 1849) | Same observation: [61,88] | |
| Tettigometra (Metroplaca) longicornis Signoret, 1865 | 3.3 | Formicidae: Dolichoderinae: Tapinoma erraticum (Latreille, 1798) | Probably same recited observation: [11,57,63,64,87] | |
| Figure 35b | Tettigometra (Metroplaca) longicornis Signoret, 1865 | 3.3 | Formicidae: Myrmicinae: Crematogaster sordidula (Nylander, 1849) | New data: Bulgaria: Primorsko, Maslen Nos Cape, 42°18′31.7″ N 27°47′22.5″ E, 04.06.2021, observ. I. Gjonov, A. Lapeva-Gjonova |
| Tettigometra (Metroplaca) longicornis Signoret, 1865 | 3.3 | Formicidae: Myrmicinae: Crematogaster sordidula (Nylander, 1849) | New data: Bulgaria: Eastern Rhodopes: Zhelezino vill., 41°27′00.0″ N 25°56′51.0″ E, 30.03.2012, observed by I. Gjonov, A. Lapeva-Gjonova | |
| Figure 35c | Tettigometra (Metroplaca) longicornis Signoret, 1865 | 3.3 | Formicidae: Myrmicinae: Crematogaster sordidula (Nylander, 1849) | New data: France: Alpes de Haute-Provence, Sainte-Croix-du-Verdon, 43°46′21.0″ N 6°05′34.0″ E, 04.06.2003, nymphs and adults on Eryngium campestre L. (Apiaceae), observ. by I. Gjonov |
| Tettigometra (Stirometra) costulata Fieber, 1865 | 3.89 | Formicidae: Myrmicinae: Myrmica sp. | Probably same recited observation: [46,63,64,87,88,95] | |
| Tettigometra (Stirometra) costulata Fieber, 1865 (as T. parviceps and T. costata) | 3.89 | Formicidae: Dolichoderinae: Tapinoma nigerrimum (Nylander, 1856) | [11] | |
| Tettigometra (Stirometra) costulata Fieber, 1865 (as T. parviceps and T. costata) | 3.89 | Formicidae: Dolichoderinae: Tapinoma nigerrimum (Nylander, 1856) (as: T. erratico-nigerrimum Nyl.) | Same observation: [53,92] | |
| Figure 35d | Tettigometra (Tettigometra) sp. | Formicidae: Formicinae: Lasius sp. | New data: Germany: Eichstätt, Bayern, 48°53′10.0″ N 11°12′58.6″ E, 29.03.2021, adult, observed by Tim Leilich https://www.inaturalist.org/observations/72365528 | |
| Fulgoroidea indet. | ||||
| Figure 36a | Probably Cixiidae (?) | Reptilia: Squamata: Gekkonidae: Hemidactylus craspedotus Mocquard, 1890 | New data: Malaysia: Sabah, Sepilok, 5°50′21.8″ N 117°56′57.2″ E, 20.09.2018, adult on tree trunk, observed by C. Lee https://www.inaturalist.org/observations/52416629 | |
| Figure 36b | Issidae (?) | Formicidae indet. | New data: Australia: Queensland, Roma, 26°34′29.7″ S 148°48′30.0″ E, 28.04.2016, adult, observed by “tjeales” https://www.inaturalist.org/observations/26032540 | |
| Figure 36c | Ricaniidae or Flatidae (?) | Reptilia: Squamata: Gekkonidae: Phelsuma guttata Kaudern, 1922 | New data: Madagascar: Marojeji National Park, 14°26′14.9″ S 49°46′32.3″ E, 21.10.2016, adult, observed by C. Lee https://www.inaturalist.org/observations/49399703 |
References
- Way, M.J. Mutualism between ants and honeydew producing Homoptera. Annu. Rev. Entomol. 1963, 8, 307–344. [Google Scholar] [CrossRef]
- Nelson, A.S.; Mooney, K.A. The evolution and ecology of interactions between ants and honeydew-producing hemipteran insects. Annu. Rev. Ecol. Evol. Syst. 2022, 53, 379–402. [Google Scholar] [CrossRef]
- Pierce, N.E.; Braby, M.F.; Heath, A.; Lohman, D.J.; Mathew, J.; Rand, D.B.; Travassos, M.A. The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annu. Rev. Entomol. 2002, 47, 733–771. [Google Scholar] [CrossRef]
- Bourgoin, T. Habitat and ant-attendance in Hemiptera: A phylogenetic test with emphasis on trophobiosis in Fulgoromorpha. In The Origin of Biodiversity in Insects: Phylogenetic Tests of Evolutionary Scenarios; Grandcolas, P., Ed.; Mémoire du Muséum national d’Histoire Natuelle: Paris, France, 1997; pp. 109–124. [Google Scholar]
- Delabie, J.H.C. Trophobiosis between Formicidae and Hemiptera (Stenorrhyncha and Auchenorrhyncha): An overview. Neotrop. Entomol. 2001, 30, 501–516. [Google Scholar] [CrossRef]
- Bourgoin, T.; Dehalleux, A.; Constant, J. Macropatterns of Planthopper interspecific relationships (Hemiptera, Fulgoromorpha). In Proceedings of the 15th International Auchenorrhyncha Congress and 10th International Workshop on Leafhoppers and Planthoppers of Economic Importance, IAC 2017, Mendes, Brazil, 9–15 July 2017; pp. 22–23. [Google Scholar]
- Leroy, P.; Capella, Q.; Haubruge, E. L’impact du miellat de puceron au niveau des relations tritrophiques entre les plantes-hôtes, les insectes ravageurs et leurs ennemis naturels. Biotechnol. Agron. Soc. Environ. 2009, 13, 325–334. [Google Scholar]
- Klimes, P.; Borovanska, M.; Plowman, N.S.; Leponce, M. How common is trophobiosis with hoppers (Hemiptera: Auchenorrhyncha) inside ant nests (Hymenoptera: Formicidae)? Novel interactions from New Guinea and a worldwide overview. Myrmecol. News 2018, 26, 31–45. [Google Scholar]
- Constant, J.; Phauk, S.; Bourgoin, T. Updating lanternflies biodiversity knowledge in Cambodia (Hemiptera: Fulgoromorpha: Fulgoridae) by optimizing field work surveys with citizen science involvement through Facebook networking and data access in FLOW website. Belg. J. Entomol. 2016, 37, 1–16. [Google Scholar]
- Bourgoin, T. FLOW (Fulgoromorpha Lists on The Web): A World Knowledge Base Dedicated to Fulgoromorpha. Version 8. 2003. Available online: https://hemiptera-databases.org/flow/ (accessed on 27 December 2022).
- Wasmann, S.J. Kritisches Verzeichniss der Myrmekophilen und Termitophilen Arthropdode; F. L. Dames: Berlin, Germany, 1894; 231p. [Google Scholar]
- Donisthorpe, H. The ants (Formicidae), and some myrmecophiles, of Sicily. Entomol. Rec. 1927, 12, 6–9. [Google Scholar]
- La Polla, J.S.; Cover, S.P.; Mueller, U.G. Natural history of the mealybug-tending ant Acropyga epedana, with descriptions of the male and queen castes. Trans. Am. Entomol. Soc. 2002, 128, 367–376. [Google Scholar]
- Naskrecki, P.; Nishida, K. Novel trophobiotic interactions in lantern bugs (Insecta: Auchenorrhyncha: Fulgoridae). J. Nat. Hist. 2007, 41, 2397–2402. [Google Scholar] [CrossRef]
- Dejean, A.; Bourgoin, T. Relationships between ants (Hymenoptera: Formicidae) and Euphyonarthex phyllostoma (Hemiptera: Tettigometridae). Sociobiology 1998, 32, 91–100. [Google Scholar]
- Dejean, A.; Bourgoin, T.; Gibernau, M. Ant species that protect figs against other ants: Result of territoriality induced by a mutualistic homopteran. Ecoscience 1997, 4, 446–453. [Google Scholar] [CrossRef]
- Dejean, A.; Bourgoin, T.; Orivel, J. Ant defense of Euphyonarthex phyllostoma (Homoptera: Tettigometridae) during trophobiotic associations. Biotropica 2000, 32, 112–119. [Google Scholar] [CrossRef]
- Dejean, A.; Ngneugueu, P.R.; Bourgoin, T. Trophobiosis between ants and Peregrinus maidis (Hemiptera, Fulgoromorpha, Delphacidae). Sociobiology 1996, 28, 111–120. [Google Scholar]
- Dejean, A.; Ngneugueu, P.R.; Durand, J.L.; Bourgoin, T. The influence of ants (Hymenoptera: Formicidae), particularly tramp species, on the proliferation of a maize pest. Sociobiology 1997, 30, 85–93. [Google Scholar]
- Dejean, A.; Orivel, J.; Durand, J.L.; Ngneugueu, P.R.; Bourgoin, T.; Gibernau, M. Interference between ant species distribution in different habitats and the density of a maize pest. Sociobiology 2000, 35, 175–189. [Google Scholar]
- Humphreys, W.F. Phaconeura (Homoptera: Meenoplidae) attended by ants of the genus Paratrechina (Hymenoptera: Formicidae) in caves. Aust. Entomol. 1998, 25, 23–27. [Google Scholar]
- Bourgoin, T.; Pajor, I. A new Hilda species (Hemiptera, Fulgoromorpha, Tettigometridae) on Protea sp. (Proteaceae) from Kwazulu-natal, South Africa. Dt. Entomol. Z. 2000, 47, 51–56. [Google Scholar] [CrossRef]
- Roth, L.; Naskrecki, P. Trophobiosis between a blattellid cockroach (Macrophyllodromia spp.) and fulgorids (Enchophora and Copidocephala spp.) in Costa Rica. J. Orthoptera Res. 2001, 10, 189–194. [Google Scholar] [CrossRef]
- Fölling, M.; Knogge, C.; Böhme, W. Geckos are milking honeydew-producing planthoppers in Madagascar. J. Nat. Hist. 2001, 35, 279–284. [Google Scholar] [CrossRef]
- Hoch, H.; Asche, M.; Burwell, C.; Monteith, G.M.; Wessel, A. Morphological alteration in response to endogeic habitat and ant association in two new planthopper species from New Caledonia (Hemiptera: Auchenorrhyncha: Fulgoromorpha: Delphacidae). J. Nat. Hist. 2006, 40, 1867–1886. [Google Scholar] [CrossRef]
- Mezger, D.; Bluthgen, N. Trophobioses on Borneo Climbing Bamboo—Diversity and ecology of ant-hemipteran associations on Dinochloa trichogona (Poaceae). Asian Myrmecol. 2007, 1, 59–68. [Google Scholar]
- Gnezdilov, V.M.; O’Brien, L.B. New taxa and combinations in Neotropical Issidae (Hemiptera: Fulgoroidea). Insecta Mundi 2008, 31, 1–26. [Google Scholar]
- Holzinger, W.E. A novel trophobiosis between ants (Hymenoptera: Formicidae) and a palm-feeding planthopper (Hemiptera: Cixiidae). Afr. Entomol. 2009, 17, 115–118. [Google Scholar] [CrossRef]
- Lőrinczi, G. A novel association between Aphaenogaster subterranea (Hymenoptera: Formicidae) and the nymphs of Reptalus panzeri (Hemiptera: Cixiidae). Eur. J. Entomol. 2012, 109, 509–515. [Google Scholar] [CrossRef]
- Picker, M.D.; Ross-Gillespie, V.; Vlieghe, K.; Moll, E. Ants and the enigmatic Namibian fairy circles—Cause and effect? Ecol. Entomol. 2012, 37, 33–42. [Google Scholar] [CrossRef]
- Kemal, M.; Koçak, A.Ö. A report on the mutualism between Hemidactylus (Gekkonidae) and Laternaria (Fulgoridae) in Thailand. Cesa News 2012, 82, 15–18. [Google Scholar]
- Emeljanov, A.F. Planthoppers of the family Cixiidae of Russia and adjacent territories. Keys Fauna Russ. 2015, 177, 1–252. [Google Scholar]
- Constant, J. Review of the effusus group of the Lanternfly genus Pyrops Spinola, 1839, with one new species and notes on trophobiosis (Hemiptera: Fulgoromorpha: Fulgoridae). Eur. J. Taxon. 2015, 128, 1–23. [Google Scholar] [CrossRef]
- Jono, T. Feeding behavior on tree sap and planthopper-derived honeydew by a fish-scale Gecko (Geckolepis sp.) in a dry forest of Madagascar. Curr. Herpetol. 2015, 34, 85–88. [Google Scholar] [CrossRef]
- Gnezdilov, V.M. Planthoppers of the family Issidae (Hemiptera, Fulgoroidea) of Western Palaearctic. Ph.D. Thesis, Institute of Zoology, Russian Academy of Sciences, Saint-Peterburg, Russia, 2016; 506p. [Google Scholar]
- Gjonov, I.V.; Lapeva-Gjonova, A. Agalmatium flavescens (Hemiptera, Issidae) and Camponotus aethiops (Hymenoptera, Formicidae)—An unknown trophobiotic association. ZooNotes 2017, 109, 1–3. [Google Scholar]
- Bosuang, S.; Audibert, C.; Porion, T. A Guide to Lanterflies of Borneo; Natural History Publications (Borneo) Sdn. Bhd. (edts): Kota Kinabalu, Malaysia, 2017; 119p. [Google Scholar]
- Constant, J.; Bartlett, C.R. New records and species in five planthopper families from Keo Seima Wildlife Sanctuary, Cambodia with checklist of Cambodian planthoppers (Hemiptera: Fulgoromorpha). Belg. J. Entomol. 2019, 83, 1–27. [Google Scholar]
- Constant, J.; Pham, H.T. Polydictya lanternflies of the Indochinese region: Six new species and identification key (Hemiptera: Fulgoromorpha: Fulgoridae). Belg. J. Entomol. 2019, 86, 1–42. [Google Scholar]
- Hölldobler, B.; Wilson, E. The Ants; Belknap (Harvard University Press): Cambridge, MA, USA, 1990; Volume xiv, 732p. [Google Scholar]
- Palmer-Jones, T.; Sutherland, M.D.; Paterson, C.R.; Harris, W.F.; Filmer, D.W. A recent outbreak of honey poisoning. N. Z. J. Sci. Technol. 1947, A29, 107–143. [Google Scholar]
- Larsen, L.; Joyce, N.I.; Sansom, C.E.; Cooney, J.M.; Jensen, D.J.; Perry, N.B. Sweet Poisons: Honeys Contaminated with Glycosides of the Neurotoxin Tutin. Journal of Natural Products 2015, 78, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, L.B. The wonderful world of Fulgoromorpha. Denisia 2002, 4, 83–102. [Google Scholar]
- Cunningham, C. Finally, Something Spotted Lanternflies Are Good for: Honey. 2020. Available online: https://www.phillymag.com/foobooz/2020/11/23/spotted-lanternfly-honey-pennsylvania/ (accessed on 27 December 2022).
- Jiaranaisakul, K.; Constant, J. The lanternflies (Hemiptera: Fulgoromorpha, Fulgoridae) of Khao Krachom Mountain, Thailand. Far. East. Entomol. 2021, 435, 7–19. [Google Scholar] [CrossRef]
- Lichtenstein, J. Communication du 1 avril 1870. Petites Nouv. Entomol. 1870, 2, 74. [Google Scholar]
- Weaving, A.J.S. Observations on Hilda patruelis Stal. (Homoptera: Tettigometridae) and its infestation of the groundnut crop in Rhodesia. J. Entomol. Soc. South Afr. 1980, 43, 151–167. [Google Scholar]
- Bourgoin, T. Une association méconnue: Les Tettigometridae (Hemiptera, Fulgoromorpha) et les fourmis (Hymenoptera, Formicidae). Entomol. Gall. 1985, I, 233–234. [Google Scholar]
- Wilson, E.O. The Insect Societies; The Belknap Press of Harvard University Press: Cambridge, MA, USA, 1971; 548p. [Google Scholar]
- China, W.E.; Fennah, R. A remarkable new genus and species of Fulgoroidea (Homoptera) representing a new family. Ann. Mag. Nat. Hist. 1952, 12, 189–199. [Google Scholar] [CrossRef]
- Fletcher, M.J.; Moir, M.L. Budginmaya eulae gen. et sp. nov., a myrmecophilous planthopper (Hemiptera: Fulgoromorpha: Flatidae) from Western Australia. Aust. J. Entomol. 2009, 48, 36–39. [Google Scholar] [CrossRef]
- Mozaffarian, F.; Bourgoin, T. Altitudinal spectrum of tettigometridae in Iran: Relaxing trophic specialization for a wider etho-ecological adaptation or cryptic speciation? In Proceedings of the 16th International Auchenorrhyncha Congres & 12th International Workshop on Leafhoppers and Planthoppers of Economic Importance, Cuc-Phong, Cuc-Phong, Vietnam, 1–7 July 2019; pp. 101–103. [Google Scholar]
- Silvestri, F. Contribuzioni alla conoscenza dei Mirmecofili. 1. Osservazioni su alcuni mirmecofili dei dintorni di Portici. Annu. Mus. Zool. Della Univ. Napoli 1903, 1, 1–5. [Google Scholar]
- Rouget, A. Note on Tettigometra laeta with Tapinoma erraticum. Ann. Société Entomol. Fr. 1867, 5, LXXXIII–LXXXIV. [Google Scholar]
- Puton, A. Note on the Hemiptera living in ants-nests. Petites Nouv. Entomol. 1869, 12, 45. [Google Scholar]
- Bellevoye, M.A. Notes sur les Hémiptères. Petites Nouv. Entomol. 1870, 1, 62. [Google Scholar]
- Rouget, A. Note on Tettigometra obliqua with Formica pratensis. Ann. Société Entomol. Fr. 1870, 10, LXXVI–LXXVII. [Google Scholar]
- Delpino, F. On the fondness of ants for certain Homoptera. Entomol. Mon. Mag. 1872, 12, 10–12. [Google Scholar]
- Delpino, F. Altre osservazioni sui rapporti tra Cicadelle e Formiche. Bull. Della Soc. Entomol. Ital. 1875, 46, 61–65. [Google Scholar]
- Reiber, F.; Puton, A. Catalogue des Heéméiptères-Hétéroptères (Cicadines et Psyllides) de l’Alsace et de la Lorraine; Bulletin de la Société d’Histoire naturelle de Colmar: Failly, France, 1880; pp. 51–80. [Google Scholar]
- Schneider, O. San Remo und Siene Thierwelt im Winter; Sitzungsberichte und Abhandlungen der Naturwissenschaftlichen Gesellschaft Isis: Dresden, Germany, 1893; pp. 3–62. [Google Scholar]
- Forel, A. Eine myrmekologische Ferienreise nach Tunesien und Ostalgerien nebst einer Beobachtung des Herrn Gleadow in Indien über Aenictus. Humboldt 1890, 9, 296–306. [Google Scholar]
- Lesne, P. Les relations des Fourmis avec les Hémiptères homoptères de la famille des Fulgorides; domestication des Tettigometra. Comptes Rendus Séances Mémoires Société Biol. 1905, 57, 1005–1007. [Google Scholar]
- Lesne, P. Les relations des Fourmis avec les Hémiptères homoptères de la famille des Fulgorides; domestication des Tettigometra. Bull. Société Entomol. Fr. 1905, 11, 161–164. [Google Scholar] [CrossRef]
- Roubal, P.C.J. Notizen zur Biologie von Tettigometra atra Hagenb. Wien. Entomol. Ztg. 1905, 24, 167–168. [Google Scholar]
- Roubal, P.C.J. Prodromus myrmecophilů ýeských. (Studie zoogeografická s ethologickymi poznámkami.). Vestník Královské ceské spolecnosti náuk. Trída mathematicko-prírodovedecká. Sitzungsberichte der Königl. Böhmischen Gesellschaft der Wissenschaften. Math. Nat. Cl. 1905, 15, 1–44. [Google Scholar]
- Torka, V. Tettigometra obliqua Panz. Z. Wiss. Insektenbiologie 1905, 1, 451–455. [Google Scholar]
- Mozaffarian, F.; Bourgoin, T.; Wilson, M.R. Nomenclatural changes in the higher classification of the family Tettigometridae (Hemiptera: Fulgoroidea) with description of a new tribe and new species and a review of the Iranian tettigometrid fauna. Zootaxa 2018, 4392, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Bourgoin, T. Les glandes tégumentaires chez les Tettigometridae (Hemiptera Fulgoromorpha). Ann. Société Entomol. Fr. 1986, 22, 139–144. [Google Scholar]
- Holzinger, W.E.; Kammerlander, I.; Nickel, H. The Auchenorrhyncha of Central Europe. 2003,Vol. 1: Fulgoromorpha, Cicadomorpha excl. Cicadellidae; Brill Academic Publishers: Leiden, The Netherlands, 2003; 673 p. [Google Scholar]
- Malenovský, I.; Lauterer, P. Additions to the fauna of planthoppers and leafhoppers (Hemiptera: Auchenorrhyncha) of the Czech Republic. Acta Musei Moraviae Scientiae Biologicae (Brno) 2010, 95, 49–122. [Google Scholar]
- Wilson, E.O.; Hölldobler, B. The rise of the ants: A phylogenetic and ecological explanation. Proc. Natl. Acad. Sci. USA 2005, 102, 7411–7414. [Google Scholar] [CrossRef]
- Borowiec, M.L.; Rabeling, C.; Brady, S.G.; Fisher, B.L.; Schultz, T.R.; Ward, P.S. Compositional heterogeneity and outgroup choice influence the internal phylogeny of the ants. Mol. Phylogenetics Evol. 2019, 134, 111–121. [Google Scholar] [CrossRef]
- Legendre, F.; Nel, A.; Svenson, G.J.; Robillard, T.; Pellens, R.; Grandcolas, P. Phylogeny of Dictyoptera: Dating the Origin of Cockroaches, Praying Mantises and Termites with Molecular Data and Controlled Fossil Evidence. PLoS ONE 2015, 10, e0130127. [Google Scholar] [CrossRef] [PubMed]
- Compton, S.G.; Robertson, H.G. Complex interactions between mutualisms: Ants tending Homopterans protect fig seeds and pollinators. Ecology 1988, 69, 1302–1305. [Google Scholar] [CrossRef]
- Grimaldi, D.A.; Engel, M.S. Evolution of the Insects; Cambridge University Press: Cambridge, UK, 2005; 770p. [Google Scholar]
- O’Brien, L.B. Taxonomic Changes in North American Issidae (Homoptera: Fulgoroidea). Ann. Entomol. Soc. Am. 1988, 81, 865–869. [Google Scholar] [CrossRef]
- Thompson, C.R. Association of Paratrechina arenivaga (Hymenoptera, Formicidae) with nymphs of Oecleus borealis (Homoptera, Cixiidae). J. N. Y. Entomol. Soc. 1984, 92, 35–41. [Google Scholar]
- Sheppard, C.; Martin, P.B.; Mead, F.W. A planthopper (Homoptera: Cixiidae) associated with red imported fire ant (Hymenoptera: Formicidae) mounds. J. Ga. Entomol. Soc. 1979, 14, 140–144. [Google Scholar]
- Thompson, C.R.; Nickerson, J.C.; Mead, F.W. Nymphal habitat of Oliarus vicarius (Homoptera: Cixiidae), and possible association with Aphaenogaster and Paratrechina (Hymenoptera: Formicidae). Psyche 1979, 86, 321–325. [Google Scholar] [CrossRef]
- Mitjaev, I.D. New and little known species of planthoppers and leafhoppers (Homoptera, Auchenorrhyncha) from the Eastern Kazakhstan. Entomologicheskoe Obozrenie 1967, 46, 712–723, (In Russian with English Summary). [Google Scholar]
- Myers, J.G. The New Zealand planthoppers of the family Cixiidae (Homoptera). Transactions of the Peninsula Horticultural Society. Bull. State Board Agric. 1924, 55, 315–326. [Google Scholar]
- Myers, J.G. Observations on the biology of two remarkable planthoppers (Homoptera) from Cuba. Psyche 1929, 36, 283–293. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Linsenmair, K.E. Trophobiosis in a tropical rainforest on Borneo: Giant ants Camponotus gigas (Hymenoptera: Formicidae) herd wax cicadas Bythopsyrna circulata (Auchenorrhyncha: Flatidae). Asian Myrmecol. 2007, 1, 105–119. [Google Scholar]
- Dietrich, C.; MacKamey, S.H. Three new idiocerine leafhoppers (Homoptera: Cicadellidae) from Guyana with notes on ant-mutualism and subsociality. Proc. Entomol. Soc. Wash. 1990, 92, 214–223. [Google Scholar]
- Jerath, M.L. An Encyrlid parasite of Hilda undata Walk. (Homoptera: Tettigometridae) in Ibadan. Nigerian Entomol. Mag. 1968, 1, 43. [Google Scholar]
- André, E. Description des fourmis d’Europe pour servir à létude des insectes myrmécophiles. Rev. Mag. Zool. 1874, 3, 152–235. [Google Scholar]
- Janet, C. Etudes sur les Fourmis, les Guêpes et les Abeilles, Note 14: Rapports des Animaux Myrmécophiles avec les Fourmis; Ducourtieux: Limoges, France, 1897; pp. 1–99. [Google Scholar]
- Ivanov, S.P. Beiträge zur Kenntnis des Geschlechtsapparats der Homoptera Fulgoroidea. Rev. Russe d’Entomol. 1928, 22, 53–67. [Google Scholar]
- Mitajaev, I.D. Cicadina of Kazakhstan (Homoptera, Cicadinea). Key for identification. Publishing House ‘Science’ of Kasakh SSR: Alma-Ata, Kazakhstan, 1971; 212 p. [Google Scholar]
- Gösswald, K. 1934. Ueber Ameisengäste und-schmarotzer des mittleren Maingebiets. Entomologische Zeitschrift 1934, 48, 165–167. [Google Scholar]
- Forel, A. Le Monde Social des Fourmis du Globe Comparé à celui de l’homme; Kunding: Genève, Switzerland, 1922; Volume 3, pp. 121–127. [Google Scholar]
- Menozzi, C. Nuovi contributi alla conoscenza della fauna delle Isole italiane dell’Egeo. VI. Hymenoptera—Formicidae. Boll. Lab. Zool. Gen. E Agrar. Della Fac. Agrar. Portici 1936, 29, 262–311. [Google Scholar]
- Menozzi, C. Nuovi contributi alla conoscenza della fauna delle Isole italiane dell’Egeo. 15. mirmecofili di rodi e Scarpento. Boll. Lab. Zool. Gen. E Agrar. Della Fac. Agrar. Portici 1936, 32, 3–10. [Google Scholar]
- Lichtenstein, J. Quelques feuillets de mon journal. Mitt. Scheiz. Ges. 1880, 5, 197–303. [Google Scholar]
- Lehouk, V.; Bonte, D.B.; Dekoninck, W.; Maelfait, J.-P.E. Trophobiotic relationships between ants (Hymenoptera: Formicidae) and Tettigometridae (Hemiptera: Fulgoromorpha) in the grey dunes of Belgium. Eur. J. Entomol. 2004, 101, 547–553. [Google Scholar] [CrossRef]
- Delpino, F. Sui Rapporti delle Formiche colle Tettigometre e sulla genealogia degli Afidi e dei Coccidi. Bull. Della Soc. Entomol. Ital. 1872, 4, 343–351. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).