An Updated Checklist of Freshwater Gastropods (Mollusca: Gastropoda) of Bosnia and Herzegovina, with Emphasis on Crenobiotic Species
Abstract
1. Introduction
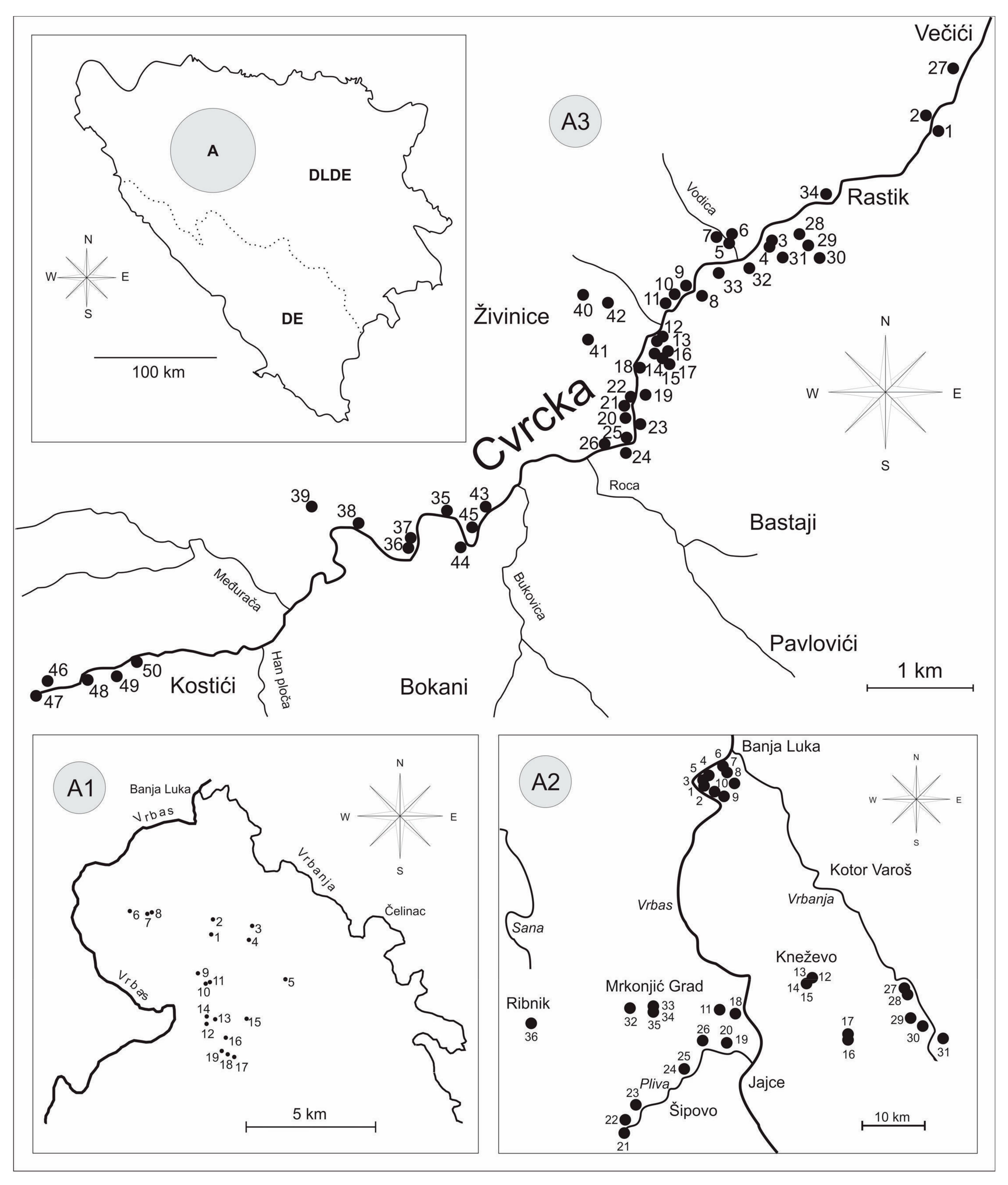
2. Materials and Methods
3. Results and Discussion
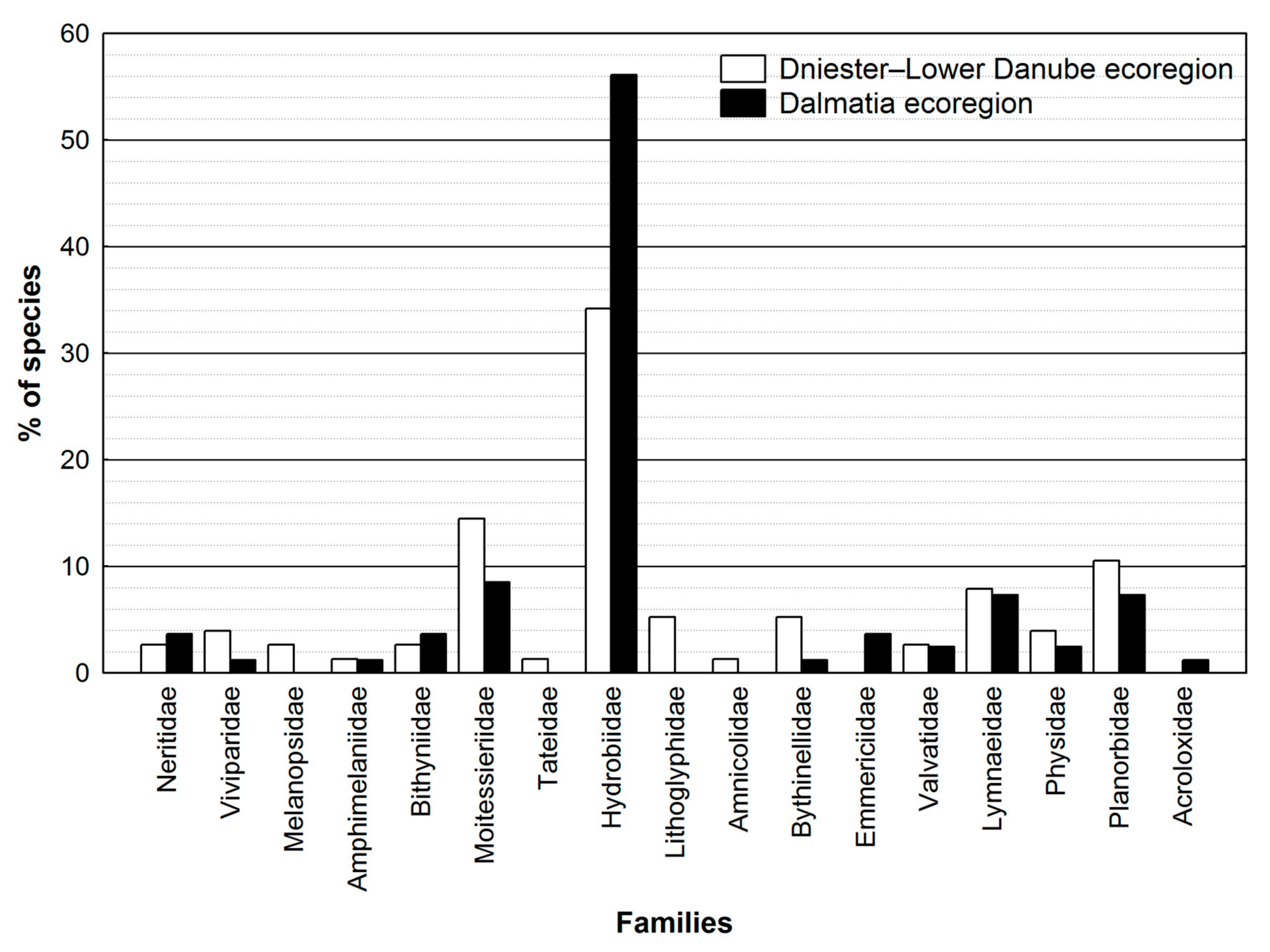
3.1. Diversity of Freshwater Gastropods in Bosnia and Herzegovina
3.2. Endemism Patterns
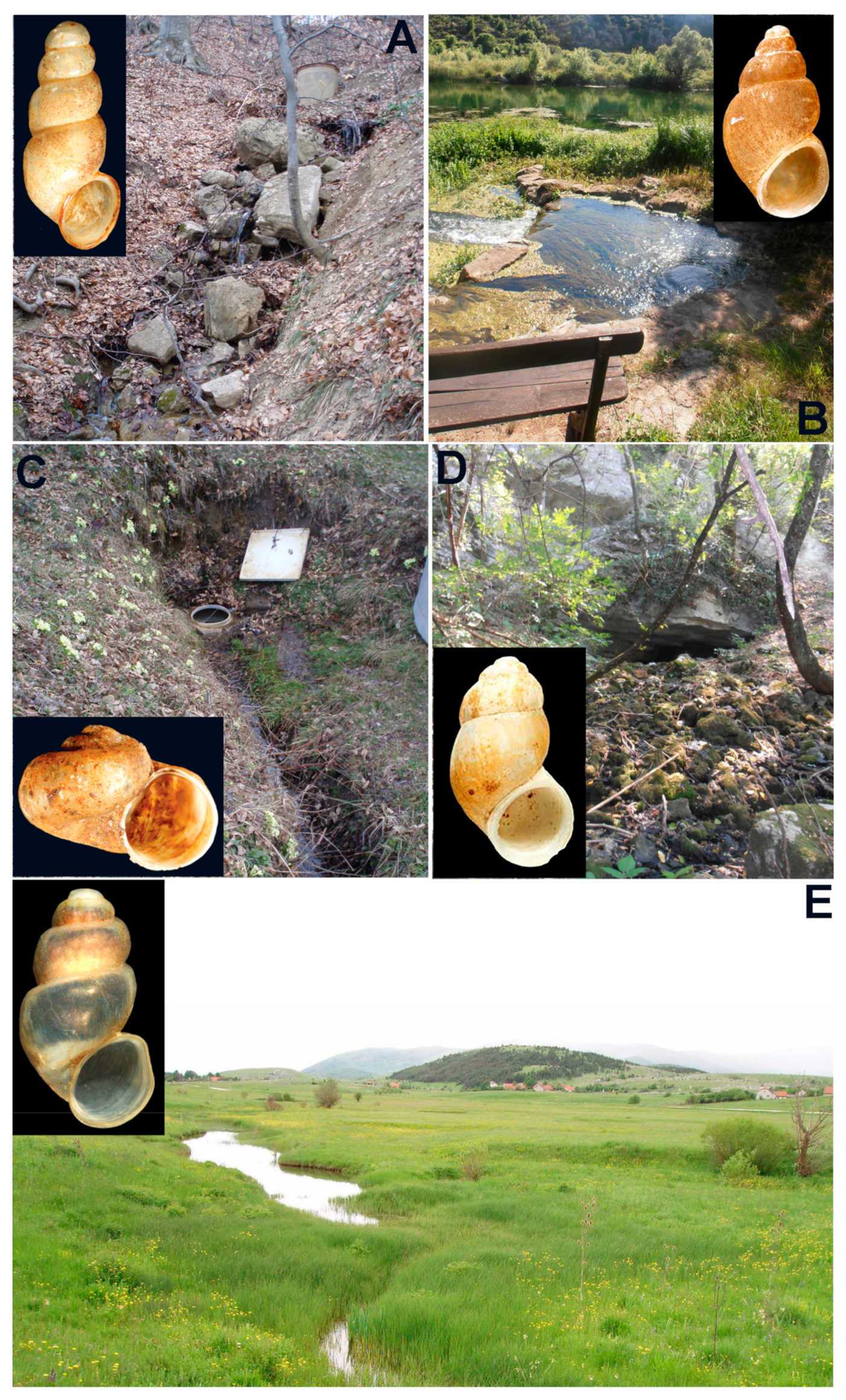
3.3. Diversity of Freshwater Gastropods in Spring Habitats
3.4. IUCN Red List Assessments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Group I permanent: average similarity = 6.44 Group II intermittent: average similarity = 24.25 | Group I and II: average dissimilarity = 92.65 | |||
| species | Av. Abund. | Av. Sim. | Contrib. % | Cum. % |
| Group I | ||||
| Bythinella opaca | 12.22 | 3.52 | 54.75 | 54.75 |
| Belgrandiela bozidarcurcici | 31.65 | 2.08 | 32.35 | 87.1 |
| Galba truncatula | 3.3 | 0.32 | 5.05 | 92.14 |
| Group II | ||||
| Bythinella opaca | 31.8 | 24.25 | 100 | 100 |
| N | Mean | Std. Deviation | Z | Sig. | ||
|---|---|---|---|---|---|---|
| Water_temperature | modified | 25 | 11.28 | 3.37 | −1.850 | 0.064 |
| natural | 59 | 12.34 | 4.23 | |||
| pH_value | modified | 25 | 7.92 | 0.22 | −0.688 | 0.491 |
| natural | 59 | 7.86 | 0.28 | |||
| Conductivity | modified | 25 | 4.76 | 2.83 | −1.135 | 0.256 |
| natural | 59 | 4.73 | 3.58 | |||
| Oxygen_concentration | modified | 25 | 7.18 | 0.90 | −1.837 | 0.066 |
| natural | 59 | 6.91 | 1.12 | |||
| Discharge | modified | 25 | 2.68 | 1.11 | −1.806 | 0.071 |
| natural | 59 | 2.20 | 1.08 | |||
| Group I modified: average similarity = 6.53 Group II natural: average similarity = 7.56 | Group I and II: average dissimilarity = 94.23 | |||
| species | Av. Abund. | Av. Sim. | Contrib. % | Cum. % |
| Group I | ||||
| Bythinella opaca | 16.35 | 3.49 | 53.41 | 53.41 |
| Galba truncatula | 7.6 | 1.35 | 20.62 | 74.03 |
| Belgrandiela bozidarcurcici | 12.9 | 1.09 | 16.66 | 90.69 |
| Group II | ||||
| Bythinella opaca | 11.18 | 4.25 | 56.21 | 56.21 |
| Belgrandiela bozidarcurcici | 40.75 | 2.5 | 33.01 | 89.22 |
| Ancylus recurvus | 0.89 | 0.63 | 8.3 | 97.53 |
References
- Piechocki, A.; Wawrzyniak-Wydrowska, B. Guide to Freshwater and Marine Mollusca of Poland; Bogucki Wydawnictwo Naukowe: Poznan, Poland, 2016; pp. 52–62. ISBN 9788379861095. [Google Scholar]
- Raković, M.; Tomović, J.; Popović, N.; Jovičić, K.; Stanković, J.; Slavevska Stamenković, V.; Paunović, M. Diverzitet slatkovodnih mekušaca (Gastropoda) u malim vodnim telima zapadnog Balkana—Pritisci i ugroženost. In Zbornik Radova 51. Godišnje Konferencije o Aktuelnim Temama Korišćenja i Zaštite Voda (VODA 2022), Vrnjačka Banja, Srbija, 26–28 Oktobar 2022; Đukić, A., Ed.; Srpsko Društvo za Zaštitu Voda: Belgrade, Serbia, 2022; pp. 43–52. [Google Scholar]
- Von Möllendorff, O. Beiträgezur Fauna Bosniens, Inauguraldissertation; Hoffman & Reiber: Görlitz, Germany, 1873; pp. 30–73. [Google Scholar]
- Berberović, L.J. Mollusca Bosne i Hercegovine. In Problemi Inventarizacije Životinjskog Svijeta Bosne i Hercegovine–Stanje i Perspektive; Vuković, T., Ed.; Posebna izdanja 47, Knjiga 8; Akademija Nauka i Umjetnosti Bosne i Hercegovine: Sarajevo, Bosnia and Herzegovina, 1980; pp. 57–60. [Google Scholar]
- Brancsik, K. Nachträge zur Conchylien-Fauna Bosniens. Nachr. Dtsch. Malakozool. Ges. 1888, 20, 161–169. [Google Scholar]
- Brancsik, C. Consignatio systematica specierum in itinere bosnensi anno 1888 per me collectarum, novaque data ad faunam molluscarum Bosniae ac Hercegovinae. Jahresh. Nat. Ver. Trencsiner Com. 1888–1889, 11–12, 68–76. [Google Scholar]
- Brancsik, K. Einige Daten zur Conchylienfauna Bosniens, der Hercegovina u. Dalmatiens. Jahresh. Nat. Ver. Trencsiner Com. 1896–1897, 19–20, 86–90. [Google Scholar]
- Servain, G. Excursions malacologiques en Bosnie aux environs de Sarajewo et aux sources de la Bosna. Ann. Malacol. 1884, 1, 341–380. [Google Scholar]
- Boettger, O. Beitrag zur Kenntniss der Schneckenfauna von Central-Bosnien, sowie des südlichsten Dalmatiens und Westmontenegros. Jahrbücher Dtsch. Malakozool. Ges. 1885, 12, 53–71. [Google Scholar]
- Bourguignat, J.R. Étude Sur Les Noms Génériques des Petites Paludinidées à Opercule Spirescent; Suivie de la Description du Nouveau Genre Horatia; M.V. Tremblay: Paris, France, 1887. [Google Scholar]
- Jaeckel, S.G.; Klemm, W.; Meise, W. Die Land- und Süsswasser-Mollusken der nördlichen Balkanhalbinsel. Abh. Ber. Staatl. Mus. Tierkd. Dresd. 1957, 23, 141–205. [Google Scholar]
- Bole, J. Nove Hidrobide (Gastropoda) iz podzemeljskih voda zahodnega Balkana. Biološki Vestn. 1961, 9, 59–69. [Google Scholar]
- Bole, J. Prispevek k poznavanju anatomije in taksonomije podzemeljskih hidrobiid (Gastropoda, Prosobranchia). Razprave IV Razred SAZU 1970, 13, 85–111. [Google Scholar]
- Bole, J.; Velkovrh, F. Nove vrste podzemeljskih polžev Jugoslavije. Razprave IV Razred SAZU 1987, 28, 69–83. [Google Scholar]
- Boeters, H.D. Die Gattung Microna Clessin, 1890 (Prosobranchia, Hydrobiidae). Arch. Molluskenkd. 1970, 100, 113–145. [Google Scholar]
- Schütt, H. Zur Höhlenschneckenfauna Montenegros. Arch. Molluskenkd. 1959, 88, 185–190. [Google Scholar]
- Schütt, H. Weitere neue Süßwasser-Höhlenschnecken aus Dalmatien. Arch. Molluskenkd. 1961, 90, 139–144. [Google Scholar]
- Schütt, H. Die Plagigeyeria-Arten Dalmatiens. Arch. Molluskenkd. 1961, 90, 131–137. [Google Scholar]
- Schütt, H. Vier bemerkenswerte Höhlenschnecken. Arch. Molluskenkd. 1963, 92, 205–213. [Google Scholar]
- Schütt, H. Verwandtschaftliche Beziehungen höhlenbewohnender Rissoaceen Dalmatiens. Arch. Molluskenkd. 1968, 98, 103–111. [Google Scholar]
- Schütt, H. Neue Formen höhlenbewohnender Hydrobiiden des Balkan und ihre Beziehungen zu Paladilhiopsis Pavlović 1913. Arch. Molluskenkd. 1970, 100, 305–317. [Google Scholar]
- Schütt, H. Die Formen der Gattung Iglica A.J. Wagner. Arch. Molluskenkd. 1975, 106, 1–14. [Google Scholar]
- Radoman, P. Speciation of the genus Emmericia (Gastropoda) in the Adriatic area. Basteria 1967, 31, 27–43. [Google Scholar]
- Radoman, P. New classification of fresh and brakish water Prosobranchia from the Balkans and Asia Minor. Poseb. Izd. Prir. Muz. Beogr. 1973, 32, 1–30. [Google Scholar]
- Radoman, P. Contribution à la connaissance des Gastéropodes des eaux douces de Bosnie et d’Herzégovine. Bull. Mus. Hist. Nat. Marseille 1973, 33, 227–237. [Google Scholar]
- Radoman, P. Ein Beitrag zur Kenntnis der Höhlenfauna Bosniens. Arch. Molluskenkd. 1974, 104, 81–84. [Google Scholar]
- Radoman, P. Specijacija u okviru roda Belgrandiella i njemu srodnih rodova na Balkanskom poluostrvu. Bull. Nat. Hist. Mus. Belgrade B 1975, 30, 29–69. [Google Scholar]
- Radoman, P. Hydrobioidea a Superfamily of Prosobranchia (Gastropoda) I. Systematics; Monographs 547, Department of Sciences, 57; Serbian Academy of Sciences and Arts: Belgrade, Serbia, 1983. [Google Scholar]
- Karaman, B.J. Former Investigations of the Fauna of Snails (Mollusca, Gastropoda) in Bosnia & Herzegovina. Nat. Montenegrina 2006, 5, 55–66. [Google Scholar]
- Bank, R.A.; Neubert, E. Checklist of the Land and Freshwater Gastropoda of Europe. MolluscaBase. 16 July 2017. Available online: https://www.molluscabase.org/aphia.php?p=sourcedetails&id=279050 (accessed on 19 December 2022).
- Boeters, H.D.; Glöer, P.; Pešić, V. Some new freshwater gastropods from Southern Europe (Mollusca: Gastropoda: Truncatelloidea). Folia Malacol. 2013, 21, 225–235. [Google Scholar] [CrossRef][Green Version]
- Glöer, P.; Pešić, V. Two new species of the genus Bythinella Moquin-Tandon, 1856 (Mollusca: Gastropoda: Hydrobiidae) from the Western Balkan Peninsula. Ecol. Montenegrina 2014, 1, 249–255. [Google Scholar] [CrossRef]
- Glöer, P.; Pešić, V. Belgrandiella bozidarcurcici n. sp., a new species from Bosnia and Herzegovina (Gastropoda: Hydrobiidae). Arch. Biol. Sci. 2014, 66, 461–464. [Google Scholar] [CrossRef]
- Glöer, P.; Grego, J. New subterranean freshwater molluscs from Bosnia & Hercegovina (Mollusca: Hydrobiidae). Ecol. Montenegrina 2015, 2, 307–314. [Google Scholar] [CrossRef]
- Grego, J.; Glöer, P. A new Hydrobiid (Mollusca, Gastropoda, Hydrobiidae) genus of Bosnia and Hercegovina. Ecol. Montenegrina 2019, 21, 86–89. [Google Scholar] [CrossRef]
- Grego, J. Revision of the stygobiont gastropod genera Plagigeyeria Tomlin, 1930 and Travunijana Grego & Glöer, 2019 (Mollusca; Gastropoda; Moitessieriidae and Hydrobiidae) in Hercegovina and adjacent regions. Eur. J. Taxon. 2020, 691, 1–56. [Google Scholar] [CrossRef]
- Hofman, S.; Rysiewska, A.; Osikowski, A.; Falniowski, A. A New Species of Kerkia Radoman, 1978 (Caenogastropoda, Hydrobiidae) from Bosnia and Herzegovina. ZooKeys 2020, 973, 17–33. [Google Scholar] [CrossRef]
- Falniowski, A.; Lewarne, B.; Rysiewska, A.; Osikowski, A.; Hofman, S. Crenobiont, stygophile and stygobiont molluscs in the hydrographic area of the Trebišnjica River Basin. ZooKeys 2021, 1047, 61–89. [Google Scholar] [CrossRef] [PubMed]
- Glöer, P.; Mulaomerović, J. Four new hydrobiid species from Bosnia-Herzegovina (Mollusca: Gastropoda: Hydrobiidae). Nachr. Ersten Vorarlb. Malakol. Ges. 2021, 28, 63–66. [Google Scholar]
- Haase, M.; Grego, J.; Erőss, Z.P.; Farkas, R.; Fehér, Z. On the origin and diversification of the stygobiotic freshwater snail genus Hauffenia (Caenogastropoda: Hydrobiidae) with special focus on the northern species and the description of two new species. Eur. J. Taxon. 2021, 775, 143–184. [Google Scholar] [CrossRef]
- Hofman, S.; Grego, J.; Rysiewska, A.; Osikowski, A.; Falniowski, A. Two new species of the Balkan genus Paladilhiopsis Pavlović, 1913 (Caenogastropoda, Moitessieriidae). ZooKeys 2021, 1046, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Delicado, D.; Hauffe, T. Shell features and anatomy of the springsnail genus Radomaniola (Caenogastropoda: Hydrobiidae) show a different pace and mode of evolution over five million years. Zool. J. Linn. Soc. 2022, 196, 393–441. [Google Scholar] [CrossRef]
- Hofman, S.; Grego, J.; Beran, L.; Jaszczyńska, A.; Osikowski, A.; Falniowski, A. Kerkia Radoman, 1978 (Caenogastropoda: Hydrobiidae): Endemism, apparentlymorphostatic evolution and cryptic speciation. Molluscan Res. 2022, 42, 295–319. [Google Scholar] [CrossRef]
- Aescht, E.; Bisenberger, A. The mollusc collection at the Upper Austrian Museum in Linz (Austria): History of curatorial and educational activities concerning molluscs, checklists and profiles of main contributers. Denisia 2019, 42, 595–686. [Google Scholar]
- Beran, L. Aquatic molluscan fauna (Mollusca) of the Korana River (Croatia). Nat. Croat. 2013, 22, 223–234. [Google Scholar]
- Boettger, O. Eine neue Form der Paludinidengattung Emmericia im Mainzer Becken. Nachr. Dtsch. Malakozool. Ges. 1904, 36, 112–116. [Google Scholar]
- Brancsik, K. Sechs Wochen durch Dalmatien, Hercegovina und Bosnien. Jahresh. Nat. Ver. Trencsiner Com. 1904–1905, 27–28, 136–193. [Google Scholar]
- Čičić-Močić, A.; Škrijelj, R.; Đug, S. Gastropods in the Basin of the River Fojnička. Ribarstvo 2008, 66, 119–129. [Google Scholar]
- Clessin, S. Neue Süsswasserschnecken. Nachr. Dtsch. Malakozool. Ges. 1910, 42, 71–73. [Google Scholar]
- Clessin, S. Neue Arten. Nachr. Dtsch. Malakozool. Ges. 1911, 43, 74–77. [Google Scholar]
- Dmitrović, D.; Savić, A.; Pešić, V. Discharge, substrate type and temperature as factors affecting gastropod assemblages in springs in Northwestern Bosnia and Herzegovina. Arch. Biol. Sci. 2016, 68, 613–621. [Google Scholar] [CrossRef]
- Glöer, P.; Mulaomerović, J. Prvi nalaz Ancylus recurvus Martens, 1873 i novipodaci o vrsti Islamia valvataeformis (Möllendorff, 1873) u Bosni i Hercegovini. Naš Krš 2017, 50, 69–74. [Google Scholar]
- Glöer, P.; Pešić, V. The morphological plasticity of Theodoxus fluviatilis (Linnaeus, 1758) (Mollusca: Gastropoda: Neritidae). Ecol. Montenegrina 2015, 2, 88–92. [Google Scholar] [CrossRef]
- Glöer, P. The Freshwater Gastropods of the West-Palaearctis, Volume 1: Fresh- and Brackish Waters Except Spring and Subterranean Snails. Identification Key, Anatomy, Ecology, Distribution; Glöer, P.: Hetlingen, Germany, 2019. [Google Scholar]
- Glöer, P. The Freshwater Gastropods of the West-Palaearctis, Volume 2: Moitessieriidae, Bythinellidae, Stenothyridae. Identification Key, Anatomy, Ecology, Distribution; Glöer, P.: Hetlingen, Germany, 2022. [Google Scholar]
- Glöer, P. The Freshwater Gastropods of the West-Palaearctis, Volume 3: Hydrobiidae. Identification Key, Anatomy, Ecology, Distribution; Glöer, P.: Hetlingen, Germany, 2022. [Google Scholar]
- Glöer, P.; Mulaomerović, J.; Mitrović, B. Some taxonomic notes on Horatia spp. of the Balkans with the designation of the neotype of Horatia knorri Schütt, 1961 (Gastropoda: Hydrobiidae). Folia Malacol. 2022, 30, 109–116. [Google Scholar] [CrossRef]
- Hofman, S.; Osikowski, A.; Rysiewska, A.; Grego, J.; Gloeer, P.; Dmitrović, D.; Falniowski, A. Sarajana Radoman, 1975 (Caenogastropoda: Truncatelloidea): Premature invalidation of a genus. J. Conchol. 2019, 43, 407–418. [Google Scholar]
- Jackiewicz, M. New European locality of Lymnaea (Stagnicola) occulta (Jackiewicz, 1959) (Gastropoda: Basommatophora: Lymnaeidae). Malakol. Abh. 1997, 18, 255–259. [Google Scholar]
- Kukavica, B.; Davidović-Plavšić, B.; Dmitrović, D.; Šukalo, G.; Savić, A.; Pešić, V. Seasonal Dynamics of Oxidative and Antioxidative Parameters in Sadleriana fluminensis (Gastropoda: Hydrobiidae). Malacologia 2021, 64, 57–67. [Google Scholar] [CrossRef]
- Kuščer, L. Prispevek k poznavnju podzemskih gastropodov Dalmacije in Hercegovine. Zagreb. Prirodosl. Istraživanja Kralj. Jugosl. 1933, 18, 59–67. [Google Scholar]
- Von Möllendorff, O. Excursionsberichte aus Bosnien. Nachr. Dtsch. Malakozool. Ges. 1871, 3, 65–70. [Google Scholar]
- Mulaomerović, J. Physa fontinalis (Linnaeus, 1758)–nova vrsta slatkovodnog puža u Bosni i Hercegovini (?). Naš Krš 2016, 49, 71–73. [Google Scholar]
- Mulaomerović, J.; Giöer, P. First record of the species Bithynia zeta Glöer & Pešić, 2007 (Gastropoda: Hydrobiidae) in Bosnia and Herzegovina. Nat. Slo. 2021, 23, 35–36. [Google Scholar]
- Mulaomerović, J.; Glöer, P.; Husanović, M. Prilog rasprostranjenosti izvorskih i vodenih puževa na kraškim poljima. Voda i Mi 2021, 105, 43–46. [Google Scholar]
- Savić, A.; Dmitrović, D.; Glöer, P.; Pešić, V. Assessing environmental response of gastropod species in karst springs: What species response curves say us about niche characteristic and extinction risk? Biodivers. Conserv. 2020, 29, 695–708. [Google Scholar] [CrossRef]
- Trožić-Borovac, S.; Šanjta, A.; Krlić-Avdibegović, S. Diverzitet gastropoda u izvorima na području spomenika prirode Vrela Bosne. Voda i Mi 2013, 81, 23–30. [Google Scholar]
- Wagner, A. Beschreibungen neuer Land- und Süßwasserschnecken aus Südösterreich, Kroatien und Bosnien. Verh. Kais. Königlichen Zool. Bot. Ges. Wien 1912, 62, 246–260. [Google Scholar]
- Wagner, A. Höhlenschnecken aus Süddalmatien und der Hercegovina. Sitz. Kais. Akad. Wiss. Wien Math. Nat. Kl. I. Abt. 1914, 123, 33–48. [Google Scholar]
- Wagner, A. Studien zur Molluskenfauna der Balkanhalbinsel mit besonderer Berücksichtigung Bulgariens und Thraziens, nebst monographischer Bearbeitung einzelner Gruppen. Ann. Zool. Musei Pol. Hist. Nat. 1927, 6, 263–399. [Google Scholar]
- MolluscaBase. Available online: https://www.molluscabase.org (accessed on 10 February 2023).
- Freshwater Ecoregions of the World (FEOW). Available online: https://www.feow.org (accessed on 22 December 2022).
- Pešić, V.; Dmitrović, D.; Savić, A.; Milošević, Đ.; Zawal, A.; Vukašinović-Pešić, V.; Von Fumetti, S. Application of macroinvertebrate multimetrics as a measure of the impact of anthropogenic modification of spring habitats. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 341–352. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-e: Auckland, New Zealand, 2015; Available online: http://updates.primer-e.com/primer7/manuals/Getting_started_with_PRIMER_7.pdf (accessed on 22 December 2022).
- Cuttelod, A.; Seddon, M.; Neubert, E. European Red List of Non-Marine Molluscs; European Commission: Luxembourg, 2011; pp. 48–64. ISBN 978-92-79-20198-1. [Google Scholar]
- Cordeiro, J.; Perez, K.; The IUCN Red List of Threatened Species. Ferrissia californica. Available online: https://www.iucnredlist.org/species/189119/8686887 (accessed on 22 December 2022).
- Van Damme, D.; The IUCN Red List of Threatened Species. Potamopyrgus antipodarum. Available online: https://www.iucnredlist.org/species/155980/738398 (accessed on 22 December 2022).
- Slapnik, R.; Jalzic, B.; The IUCN Red List of Threatened Species. Saxurinator sketi. Available online: https://www.iucnredlist.org/species/156082/4888492 (accessed on 22 December 2022).
- Raković, M.; Paunović, M.; Tomović, J.; Popović, N.; Csányi, B.; Jovanović, M.; Glöer, P.; Pešić, V. Do Molluscs Assemblages reflect River Typology: A Case Study of Montenegro. In The Rivers of Montenegro; Pešić, V., Paunović, M., Kostianoy, A.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 265–285. ISBN 978-3-030-55712-6. [Google Scholar]
- Marković, V.; Gojšina, V.; Novaković, B.; Božanić, M.; Stojanović, K.; Karan-Žnidaršič, T.; Živić, I. The freshwater molluscs of Serbia: Annotated checklist with remarks on distribution and protection status. Zootaxa 2021, 5003, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Pešić, V.; Gadawski, P.; Gligorović, B.; Glöer, P.; Grabowski, M.; Kovács, T.; Murány, D.; Płóciennik, M.; Šundić, D. The Diversity of the zoobenthos communities of the Lake Skadar/Shkodra Basin. In The Skadar/Shkodra Lake Environment; Pešić, V., Karaman, G., Kostianoy, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 255–294. ISBN 978-3-319-99249-5. [Google Scholar]
- Pešić, V.; Dmitrović, D.; Savić, A.; von Fumetti, S. Studies on eucrenal-hypocrenal zonation of springs along the river mainstream: A case study of a karst canyon in Bosnia and Herzegovina. Biologia 2016, 71, 809–817. [Google Scholar] [CrossRef]
- Płóciennik, M.; Dmitrović, D.; Pešić, V.; Gadawski, P. Ecological patterns of Chironomidae assemblages in Dynaric karst springs. Knowl. Manag. Aquat. Ecosyst. 2016, 417, 11:1–11:19. [Google Scholar] [CrossRef]
- Von Fumetti, S.; Dmitrović, D.; Pešić, V. The influence of flooding and river connectivity on macroinvertebrate assemblages in rheocrene springs along a third-order river. Fundam. Appl. Limnol. 2017, 190, 251–263. [Google Scholar] [CrossRef]
- Savić, A.; Dmitrović, D.; Pešić, V. Ephemeroptera, Plecoptera, and Trichoptera assemblages of karst springs in relationto some environmental factors: A case study in central Bosnia and Herzegovina. Turk. J. Zool. 2017, 41, 119–129. [Google Scholar] [CrossRef]
- Pešić, V.; Dmitrović, D.; Savić, A. Riparian Springs-Challenges from a Neglected Habitat. In Small Water Bodies of the Western Balkans; Pešić, V., Milošević, D., Miliša, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 109–127. ISBN 978-3-030-86477-4. [Google Scholar]
- Stanić-Koštroman, S.; Kamberović, J.; Dmitrović, D.; Dedić, A.; Škobić, D.; Lasić, A.; Gligora Udovič, M.; Herceg, N. Ecological Characteristics and Specifics of Spring Habitats in Bosnia and Herzegovina. In Small Water Bodies of the Western Balkans; Pešić, V., Milošević, D., Miliša, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 129–145. ISBN 978-3-030-86477-4. [Google Scholar]
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org (accessed on 24 December 2022).
- Pešić, V.; Glöer, P. A new freshwater snail genus (Hydrobiidae, Gastropoda) from Montenegro, with a discussion on gastropod diversity and endemism in Skadar Lake. ZooKeys 2013, 281, 69–90. [Google Scholar] [CrossRef]
- Žganec, K.; Lajtner, J.; Ćuk, R.; Crnčan, P.; Pušić, I.; Atanacković, A.; Kralj, T.; Valić, D.; Jelić, M.; Maguire, I. Alien macroinvertebrates in Croatian freshwaters. Aquat. Invasions 2020, 15, 593–615. [Google Scholar] [CrossRef]
- Raković, M.; Tomović, J.; Popović, N.; Pešić, V.; Dmitrović, D.; Slavevska Stamenković, V.; Hinić, J.; Stefanovska, N.; Lajtner, J.; Paunović, M. Gastropods in Small Water Bodies of the Western Balkans—Endangerments and Threats. In Small Water Bodies of the Western Balkans; Pešić, V., Milošević, D., Miliša, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 227–249. ISBN 978-3-030-86477-4. [Google Scholar]
- Uredba o strogo zaštićenim i zaštićenim divljim vrstama. Službeni Glas. Repub. Srp. 2020, 65, 2–33.

| TAXA LIST | I | II | A | B | C | D | CS |
|---|---|---|---|---|---|---|---|
| Familia: Neritidae Rafinesque, 1815 | |||||||
| Theodoxus baeticus (Lamarck, 1822) | + | + | |||||
| Theodoxus danubialis (C. Pfeiffer, 1828) | + | + | LC | ||||
| Theodoxus fluviatilis (Linnaeus, 1758) | + | + | + | + | LC | ||
| Theodoxus subterrelictus Schütt, 1963 * | + | + | EN | ||||
| Familia: Viviparidae J. E. Gray, 1847 | |||||||
| Viviparus acerosus Bourguignat, 1862 | + | LC | |||||
| Viviparus contectus (Millet, 1813) | + | + | + | + | LC | ||
| Viviparus viviparus (Linnaeus, 1758) | + | + | LC | ||||
| Familia: Melanopsidae H. Adams and A. Adams, 1854 | |||||||
| Esperiana esperi (Férussac, 1823) | + | + | LC | ||||
| Microcolpia daudebartii acicularis (A. Férussac, 1823) | + | + | LC | ||||
| Familia: Amphimelaniidae P. Fischer and Crosse, 1891 | |||||||
| Holandriana holandrii (C. Pfeiffer, 1828) | + | + | + | LC | |||
| Familia: Bithyniidae Gray, 1857 | |||||||
| Bithynia leachii (Sheppard, 1823) | + | LC | |||||
| Bithynia mostarensis Möllendorff, 1873 * | + | + | DD | ||||
| Bithynia tentaculata (Linnaeus, 1758) | + | + | + | + | + | LC | |
| Bithynia zeta Glöer and Pešić, 2007 | + | + | EN | ||||
| Familia: Moitessieriidae Bourguignat, 1864 | |||||||
| Bosnidilhia vreloana Boeters, Glöer and Pešić, 2013 ** | + | + | + | ||||
| Lanzaia bosnica Bole, 1970 ** | + | + | DD | ||||
| Lanzaia edlaueri Schütt, 1961 * | + | + | DD | ||||
| Lanzaia vjetrenicae Kuščer, 1933 * | + | + | VU | ||||
| Paladilhiopsis absoloni (A. J. Wagner, 1914) * | + | + | LC | ||||
| Paladilhiopsis arion Rysiewska and Osikowski, 2021 ** | + | + | |||||
| Paladilhiopsis blihensis (Glöer and Grego, 2015) ** | + | + | |||||
| Paladilhiopsis bosniaca (Clessin, 1910) ** | + | + | DD | ||||
| Paladilhiopsis brandisi (Clessin, 1911) * | + | + | DD | ||||
| Paladilhiopsis maroskoi (Glöer and Grego, 2015) ** | + | + | |||||
| Paladilhiopsis montenegrina Schütt, 1959 * | + | + | EN | ||||
| Paladilhiopsis solida Kuščer, 1933 * | + | + | DD | ||||
| Bythiospeum dervovici Glöer and Mulaomerović, 2021 ** | + | + | |||||
| Bythiospeum hrustovoense Glöer and Grego, 2015 ** | + | + | |||||
| Bythiospeum petroedei Glöer and Grego, 2015 ** | + | + | |||||
| Bythiospeum plivense Glöer and Grego, 2015 ** | + | + | |||||
| Iglica bagliviaeformis Schütt, 1970 | + | + | EN | ||||
| Iglica bosnica Schütt, 1975 ** | + | + | DD | ||||
| Familia: Tateidae Thiele, 1925 | |||||||
| Potamopyrgus antipodarum (J. E. Gray, 1843) | + | + | LC | ||||
| Familia: Hydrobiidae W. Stimpson, 1865 | |||||||
| Pseudamnicola confinis (Brancsik, 1897) ** | + | + | + | DD | |||
| Pseudamnicola troglobia Bole, 1961 ** | + | + | LC | ||||
| Pseudamnicola virescens (Küster, 1853) | + | DD | |||||
| Montenegrospeum bogici (Pešić and Glöer, 2012) | + | + | |||||
| Narentiana albida Radoman, 1973 | + | +k | NT | ||||
| Narentiana vjetrenicae (Radoman, 1973) ** | + | + | EN | ||||
| Litthabitella chilodia (Westerlund, 1886) | + | + | + | + | LC | ||
| Bracenica plana (Bole, 1961) | + | + | + | NT | |||
| Hauffenia edlaueri (Schütt, 1961) * | + | + | DD | ||||
| Hauffenia steffeki Haase, Grego, Eröss, Farkas and Fehér, 2021 ** | + | + | + | NT | |||
| Belgrandiella bajraktarevici Glöer and Mulaomerović, 2021 ** | + | + | |||||
| Belgrandiella bozidarcurcici Glöer and Pešić, 2014 ** | + | +k | |||||
| Belgrandiella dabriana Radoman, 1975 ** | + | +k | NT | ||||
| Belgrandiella driniana (Radoman, 1975) * | + | +k | |||||
| Belgrandiella erythropoma Schütt, 1959 ** | + | +k | |||||
| Belgrandiella fontinalis (F. J. Schmidt, 1847) | + | +k | LC | ||||
| Belgrandiella koprivnensis Radoman, 1975 * | + | +k | DD | ||||
| Belgrandiella kurtovici Glöer and Mulaomerović, 2021 ** | + | + | |||||
| Belgrandiella substricta (Kuščer, 1932) | + | VU | |||||
| Belgrandiella travnicensis (Radoman, 1975) ** | + | + | +k | ||||
| Graziana glinensis Radoman, 1975 * | + | +k | LC | ||||
| Graziana lacheineri (Küster, 1853) | + | +k | LC | ||||
| Graziana vrbasensis Radoman, 1975 ** | + | +k | DD | ||||
| Sarajana apfelbecki (Brancsik, 1888) ** | + | + | +k | DD | |||
| Plagigeyeria angyaldorkae Grego 2020 ** | + | + | |||||
| Plagigeyeria erossi Grego 2020 ** | + | + | |||||
| Plagigeyeria inflata (A. J. Wagner, 1928) ** | + | + | |||||
| Plagigeyeria jakabi Grego, 2020 ** | + | + | |||||
| Plagigeyeria konjicensis Grego, 2020 ** | + | + | |||||
| Plagigeyeria lewarnei Grego, 2020 ** | + | + | |||||
| Plagigeyeria listicaensis Grego, 2020 ** | + | + | |||||
| Plagigeyeria ljutaensis Grego, 2020 ** | + | + | |||||
| Plagigeyeria mostarensis Kuščer, 1933 * | + | + | DD | ||||
| Plagigeyeria olsavskyi Grego, 2020 ** | + | + | |||||
| Plagigeyeria ozimeci Grego, 2020 ** | + | + | |||||
| Plagigeyeria plagiostoma (A. J. Wagner, 1914) ** | + | + | DD | ||||
| Plagigeyeria pseudocostellina Grego, 2020 ** | + | + | |||||
| Plagigeyeria reischuetzorum Grego, 2020 ** | + | + | |||||
| Plagigeyeria vriosticaensis Grego, 2020 ** | + | + | |||||
| Plagigeyeria zetatridyma Schütt, 1960 | + | + | |||||
| Travunijana edlaueri (Schütt, 1961) * | + | + | DD | ||||
| Travunijana gloeri Grego, 2020 ** | + | + | |||||
| Travunijana nitida (Schütt, 1963) * | + | + | DD | ||||
| Travunijana ovalis (Kuščer, 1933) ** | + | + | |||||
| Travunijana robusta (Schütt, 1959) * | + | + | DD | ||||
| Travunijana tribunicae (Schütt, 1963) * | + | + | CR | ||||
| Travunijana vruljakensis Grego and Glöer, 2019 ** | + | +k | |||||
| Saxurinator brandti Schütt, 1968 * | + | + | VU | ||||
| Saxurinator orthodoxus Schütt, 1960 | CR | ||||||
| Cilgia dalmatica (Schütt, 1961) * | + | + | DD | ||||
| Kerkia briani Rysiewska and Osikowski, 2020 ** | + | + | |||||
| Kerkia minuta Grego and Hofman, 2022 ** | + | + | |||||
| Kerkia tabanensis (Boeters, Glöer and Pešić, 2014) | + | + | |||||
| Horatia klecakiana Bourguignat, 1887 | + | + | LC | ||||
| Iglicopsis butoti Falniowski and Hofman, 2021 ** | + | + | |||||
| Sadleriana fluminensis (Küster, 1853) | + | + | + | + | + | LC | |
| Sadleriana sadleriana (Frauenfeld, 1863) | + | + | LC | ||||
| Sadleriana schmidtii (Menke, 1849) | LC | ||||||
| Radomaniola bosniaca (Radoman, 1973) * | + | +k | DD | ||||
| Radomaniola curta germari (Frauenfeld, 1863) | + | +k | LC | ||||
| Radomaniola curta mostarensis (Radoman, 1973) * | + | + | + | LC | |||
| Radomaniola curta narentana (Radoman, 1973) ** | + | + | + | LC | |||
| Radomaniola curta pivensis (Radoman, 1973) * | + | + | LC | ||||
| Radomaniola nachtigallae Delicado and Hauffe, 2022 ** | + | +k | |||||
| Anagastina hadouphylax (Schütt, 1959) * | + | + | CR | ||||
| Islamia bosniaca Radoman, 1973 ** | + | + | + | VU | |||
| Islamia buturovici Glöer and Mulaomerović, 2021 ** | + | + | |||||
| Islamia dmitroviciana Boeters, Glöer and Pešić, 2013 ** | + | + | + | ||||
| Islamia steffeki Glöer and Grego, 2015 ** | + | + | |||||
| Islamia valvataeformis (Möllendorff, 1873) ** | + | + | + | DD | |||
| Pyrgula annulata (Linnaeus, 1758) | + | + | LC | ||||
| Familia: LithoglyphidaeTryon, 1866 | |||||||
| Dabriana bosniaca Radoman, 1974 ** | + | + | NT | ||||
| Lithoglyphus fuscus (C. Pfeiffer, 1828) | + | + | LC | ||||
| Lithoglyphus naticoides (C. Pfeiffer, 1828) | + | + | LC | ||||
| Lithoglyphus pyramidatus Möllendorff, 1873 ** | + | + | |||||
| Familia: Amnicolidae Tryon, 1863 | |||||||
| Marstoniopsis vrbasi Bole and Velkovrh, 1987 ** | + | + | CR | ||||
| Familia: Bythinellidae Locard, 1893 | |||||||
| Bythinella austriaca austriaca (Frauenfeld, 1857) | + | + | LC | ||||
| Bythinella marici Glöer and Pešić, 2014 ** | + | + | |||||
| Bythinella opaca (M. von Gallenstein, 1848) | + | + | +k | LC | |||
| Bythinella samecana Clessin, 1911 ** | + | +k | DD | ||||
| Familia: Emmericiidae Brusina, 1870 | |||||||
| Emmericia narentana Bourguignat, 1881 | + | + | + | DD | |||
| Emmericia patula (Brumati, 1838) | + | + | + | LC | |||
| Emmericia ventricosa Brusina, 1870 | + | + | + | VU | |||
| Familia: Valvatidae Gray, 1840 | |||||||
| Valvata cristata O. F. Müller, 1774 | + | LC | |||||
| Valvata macrostoma Mörch, 1864 | LC | ||||||
| Valvata montenegrina Glöer and Pešić, 2008 | + | + | EN | ||||
| Valvata piscinalis piscinalis (O. F. Müller, 1774) | + | + | + | + | + | LC | |
| Familia: Lymnaeidae Rafinesque, 1815 | |||||||
| Galba truncatula (O. F. Müller, 1774) | + | + | + | + | + | LC | |
| Ladislavella occulta (Jackiewicz, 1959) | + | ||||||
| Stagnicola palustris (O. F. Müller, 1774) | + | + | LC | ||||
| Radix auricularia (Linnaeus, 1758) | + | + | + | + | LC | ||
| Ampullaceana balthica (Linnaeus, 1758) | + | + | + | + | LC | ||
| Peregriana labiata (Rossmässler, 1835) | + | + | + | + | + | LC | |
| Lymnaea stagnalis (Linnaeus, 1758) | + | + | + | + | LC | ||
| Familia: Physidae Fitzinger, 1833 | |||||||
| Physa fontinalis (Linnaeus, 1758) | + | + | + | + | + | LC | |
| Physella acuta (Draparnaud, 1805) | + | + | + | + | LC | ||
| Aplexa hypnorum (Linnaeus, 1758) | + | + | LC | ||||
| Familia: Planorbidae Rafinesque, 1815 | |||||||
| Planorbis carinatus O. F. Müller, 1774 | LC | ||||||
| Planorbis planorbis (Linnaeus, 1758) | + | + | + | LC | |||
| Anisus spirorbis (Linnaeus, 1758) | + | + | LC | ||||
| Anisus vortex (Linnaeus, 1758) | LC | ||||||
| Bathyomphalus contortus (Linnaeus, 1758) | + | + | LC | ||||
| Gyraulus albus (O. F. Müller, 1774) | + | + | + | + | + | LC | |
| Gyraulus parvus (Say, 1817) | + | LC | |||||
| Planorbarius corneus (Linnaeus, 1758) | + | + | LC | ||||
| Hippeutis complanatus (Linnaeus, 1758) | + | + | LC | ||||
| Segmentina nitida (O. F. Müller, 1774) | LC | ||||||
| Ancylus fluviatilis O. F. Müller, 1774 | + | + | + | + | LC | ||
| Ancylus recurvus E. von Martens, 1873 | + | + | + | + | VU | ||
| Ferrissia californica (Rowell, 1863) | + | + | LC | ||||
| Familia: Acroloxidae Thiele, 1931 | |||||||
| Acroloxus lacustris (Linnaeus, 1758) | + | + | LC |
| IUCN Red List Category | Taxa |
|---|---|
| Critically Endangered (CR) | Travunijana vruljakensis |
| Endangered (EN) | Belgrandiella dabriana |
| Belgrandiella erythropoma | |
| Belgrandiella travnicensis | |
| Graziana vrbasensis | |
| Sarajana apfelbecki | |
| Radomaniola nachtigallae | |
| Vulnerable (VU) | Narentiana albida |
| Belgrandiella bozidarcurcici | |
| Belgrandiella driniana | |
| Belgrandiella koprivnensis | |
| Graziana glinensis | |
| Radomaniola bosniaca | |
| Bithynella samecana |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitrović, D.; Savić, A.; Šukalo, G.; Pešić, V. An Updated Checklist of Freshwater Gastropods (Mollusca: Gastropoda) of Bosnia and Herzegovina, with Emphasis on Crenobiotic Species. Diversity 2023, 15, 357. https://doi.org/10.3390/d15030357
Dmitrović D, Savić A, Šukalo G, Pešić V. An Updated Checklist of Freshwater Gastropods (Mollusca: Gastropoda) of Bosnia and Herzegovina, with Emphasis on Crenobiotic Species. Diversity. 2023; 15(3):357. https://doi.org/10.3390/d15030357
Chicago/Turabian StyleDmitrović, Dejan, Ana Savić, Goran Šukalo, and Vladimir Pešić. 2023. "An Updated Checklist of Freshwater Gastropods (Mollusca: Gastropoda) of Bosnia and Herzegovina, with Emphasis on Crenobiotic Species" Diversity 15, no. 3: 357. https://doi.org/10.3390/d15030357
APA StyleDmitrović, D., Savić, A., Šukalo, G., & Pešić, V. (2023). An Updated Checklist of Freshwater Gastropods (Mollusca: Gastropoda) of Bosnia and Herzegovina, with Emphasis on Crenobiotic Species. Diversity, 15(3), 357. https://doi.org/10.3390/d15030357









