Abstract
The continuing impact of local and global stressors on coral reefs worldwide is prompting the exploration of novel approaches aimed at mitigating and improving the bleak future projections for corals. Ex situ aquaculture has the potential to provide a standardized supply of source corals to support active intervention-based research endeavours as well as complementary reef restoration initiatives. To effectively achieve this supply of coral, however, ex situ aquaculture systems need to be able to efficiently maintain reproducing colonies, and have the capacity to support each coral life stage. We monitored the monthly reproduction of the brooding coral, Pocillopora acuta, for one year in two distinct aquaculture systems: a natural seawater-supplied flow-through system (FTS) and an artificial seawater-supplied recirculating aquaculture system (RAS). The coral colonies in both aquaculture systems showed relatively consistent reproduction over time (on average > 70% of all colonies reproducing each month) and maintained natural diel reproduction patterns even after 1 year of ex situ culture. Coral colony reproductive output and timing (i.e., lunar day of release) differed between aquaculture systems in some, but not all, seasons. Planulae released from colonies settled and grew to reproductive size after ~16 months of culture, resulting in the ex situ production of an F2 generation of a brooding coral within two distinct aquaculture systems. This demonstrated that the use of ex situ aquaculture could be directly applied to research, restoration, and conservation aimed at mitigating coral decline in a future marred by climate change and local impacts.
1. Introduction
Bleak projections for the fate of coral reefs in the near future (e.g., ≥ 70% loss of corals globally by 2050 [1]) due to climate change and local stressors [2,3] have prompted urgent investigation into a diverse range of novel protection and active intervention approaches. Some of these include the identification and protection of coral in climate change resistant/resilient reef environments [4,5], investigation into the role of the coral holobiont composition and epigenetic mechanisms for enhanced coral thermal tolerance [6,7], development of cryogenic techniques to safeguard coral biodiversity into the future [8,9], and the recent prioritization of coral restoration as a needed tool in combating the current coral crisis [10,11]. Complementary to these approaches, there is also a renewed and growing interest in exploring the potential role that aquaculture can play in coral conservation [12,13], research-based inquiry (e.g., testing efficacy of probiotic treatments [14] and thermal ‘hardening’ [15]), and reef recovery [16,17].
Aquaculture-based cultivation of corals can use a wide range of methods, each with their own benefits and drawbacks. For example, in situ aquaculture (i.e., coral nurseries established in the marine environment) is preferable in some circumstances due to the typically lower cost and maintenance requirements, but ex situ aquaculture (i.e., tank systems in land-based aquaria) is usually more suitable for experiments because biotic/abiotic conditions can be carefully controlled [18,19]. To date, most ex situ coral aquaculture has been conducted in natural seawater-supplied flow-through systems that necessitate a close proximity to the ocean. Yet, recent advances in recirculating aquaculture system tanks (e.g., highly customizable modular tanks that do not need to be close to the ocean [20,21]), the identification of improved culture techniques (e.g., grazer co-culture [22], active feeding [23], and the inclusion of ‘live rock’ as a biofilter [24]), and a better understanding of optimal culture conditions (see [25,26] for review) highlight the potential for expanding the use of recirculating aquaculture systems. Interestingly, a direct comparison of coral performance in flow-through and recirculating aquaculture systems could reveal both opportunities and pinpoint areas in need of improvement to facilitate site-, species-, and project-specific aquaculture requirements.
A major challenge to ex situ coral aquaculture—in both flow-through and recirculating aquaculture systems—remains: the relatively limited ability to maintain reproducing colonies across long-term time scales. This challenge likely persists because coral reproduction relies on a suite of natural cues (e.g., temperature, moonlight, wind fields [27,28,29]), which can be challenging to simulate outside of the marine environment. This is especially relevant to broadcast spawning corals that only reproduce once a year. Yet, through customization of ex situ culture conditions, successful reproduction has been achieved for several broadcast spawning coral species in recirculating aquaculture systems (e.g., [20,30]). Complementary to this achievement, offspring produced in a recirculating system have been grown to reproductive age, resulting in the production of an F2 generation (i.e., completing the entire coral life cycle) [31]. In contrast to broadcast spawning species, long-term ex situ reproduction in brooding corals (i.e., species that release competent planulae rather than eggs/sperm, typically on a monthly basis) remains relatively understudied (but see [32] for an example of long-term reproduction, and [33] for a preliminary report of successful multigenerational culture ex situ). Often studies involving ex situ coral reproduction in brooding corals are conducted in flow-through aquaculture systems and rely on the frequent and repeated collection of colonies from natural reefs (e.g., [34]), or alternatively are relatively short-term experiments that are undertaken over a small number of reproductive cycles (e.g., [35,36,37]). The paucity of studies reporting consistent reproduction beyond a few reproductive cycles (i.e., >5 months) coupled with a declining trend in reproduction over time (e.g., [36]) suggests a reproductive bottleneck in ex situ coral aquaculture for brooding corals.
Here, we explored the feasibility of maintaining long-term ex situ reproduction using the brooding coral, Pocillopora acuta. We compared the reproductive output and timing of colonies held in a natural seawater-supplied flow-through system (FTS) and an artificial seawater-supplied recirculating aquaculture system (RAS) over the course of 1 year. We also assessed the diel timing of reproduction after ~1 year of ex situ culture (i.e., to check if it matches that of natural populations), and investigated the ability of an F1 generation to produce an F2 generation (i.e., to test the ability to complete the entire life cycle). Consistent long-term ex situ reproduction using the same colonies could reduce the impact of research on reefs (i.e., mitigating the need for repeated colony collection), open up opportunities for multigenerational conditioning studies, and provide a consistent broodstock for experimental inquiry and restoration initiatives.
2. Materials and Methods
2.1. Study Species and Colony Collection
The brooding reef-building coral P. acuta (often mistaken as P. damicornis [38,39]) was selected as the study species for this long-term ex situ culture experiment because it is well-studied and is commonly used in both reproduction and thermal tolerance experiments (e.g., [40,41]). The colonies used in this study were originally sourced from two reefs in Nanwan Bay (Inlet reef [21.954°E, 120.753°N] and Outlet reef [21.932°E, 120.744°N]) in southern Taiwan. Nanwan Bay has high daily thermal variability due to internal tide-induced upwelling [42], and Outlet reef has been exposed to chronic elevated temperatures due to warm water outflow from an adjacent nuclear power plant [43]. The coral colonies were not explicitly collected for the purpose of this study, but rather had been previously collected for other experiments (colony collection dates ranged from September 2020–February 2021) and subsequently remained within mesocosm tanks at the research facilities of the National Museum of Marine Biology and Aquarium after the experiment’s completion. None of the colonies underwent any treatment exposure in previous experiments; the colonies were only used for larval collection purposes. Of the 27 colonies of P. acuta colonies used in this study, 5 were from Inlet reef (colony diameter, mean ± SD: 9.9 ± 2.0 cm) and 22 were from Outlet reef (colony diameter, mean ± SD: 15.4 ± 2.1 cm); all colonies were collected from depths of 3–5 m. Twelve colonies were placed haphazardly in three FTS tanks (4 colonies/tank), and another 12 colonies were placed haphazardly in three RAS tanks (4 colonies/tank). The reproductive output and timing of P. acuta colonies (n = 12/aquaculture system) were monitored from lunar February 2021–lunar January 2022 (Gregorian calendar months: ~March 2021–February 2022). Three colonies in the RAS were removed and replaced with new colonies in lunar May due to the mortality of three of the original colonies; therefore, a total of 15 colonies were used in the RAS, but only 12 colonies were in the tanks at any given time.
2.2. Ex Situ Aquaculture Systems
For the two distinct aquaculture systems used in this study (FTS and RAS), there were three independent replicate tanks representing each aquaculture system (tank length x width x height, FTS: 300 × 200 × 100 cm; RAS: 125 × 60 × 70 cm) (Figure 1a,c). Each tank was equipped with two flow motors (GP03, Maxspect, China) set to alternate every 6 h from high (mean ± SD, FTS: 23.24 ± 0.77 cm s−1; RAS: 17.46 ± 2.81 cm s−1) to low flow (FTS: 19.64 ± 0.54 cm s−1; RAS: 14.38 ± 1.54 cm s−1) to mimic tidal flow and facilitate water circulation. The tanks were lit with LED lights (FTS: HLG-185H-36B, Mean Well, Taiwan; RAS: HLG-480H_C2100B, Mean Well, Taiwan) generating a light level of ~250 µmol quanta m−2 s−1 across a 12 h:12 h light:dark cycle (lights on from 6:00–18:00). The base of each tank was covered with ‘live sand’, and co-cultured sea urchins (Hemicentrotus pulcherrimus), snails (Trochus sp.) and sea cucumbers (Holothuria atra) were used to control algae and detritus accumulation. ‘Live rocks’ were also placed within the tanks to act as a biofilter. Natural seawater in the FTS was sourced offshore of the National Museum of Marine Biology & Aquarium and was sand filtered before entering the experimental tanks (flow rate: 3 mL s−1). Artificial seawater in the RAS was made by combining Red Sea salt (Red Sea, TX, USA) with reverse osmosis (RO) water. Salinity was maintained at ~35% with a Mato-200 osmoregulator (AutoAqua, Taiwan), which compensated for evaporated water loss with RO water automatically. Each RAS tank had an independent life support system (positioned below the culture tank) that was comprised of three sections: the first section had a 200 µm filter bag (Power Filter, Tetra, Blacksburg, VA, USA); the second section had a protein skimmer (CO-2, JNS, Aquaria, Taiwan), and the third section had live rocks, a heater (350 W, Ista, Taiwan), a primary pump (6000 L/H, Mr. Aqua, Taichung City, Taiwan) and a titration system (CS072A-1, Johnlen, Taiwan) to maintain calcium, magnesium, and alkalinity levels. At the beginning of each month, a commercial nitrifying bacteria (NBL A-5, Pandora, Taiwan) was added to the third section of the life support tank to mitigate the accumulation of organic waste.

Figure 1.
Aquaculture systems in which Pocillopora acuta colonies were held from lunar February 2021–lunar January 2022 (Gregorian calendar months: ~March 2021–February 2022): (a) Flow-through aquaculture system set up (FTS; n = 3 independent tanks) and (b) FTS feeding tank; (c) recirculating aquaculture system set up (RAS; n = 3 independent tanks) and (d) RAS feeding tank. (e) Planulae were collected daily using a meshed bottle in which the coral colonies were held from ~17:00–09:00. (f) F1 corals that had been previously reared in an outdoor tank for ~8 months were moved into the FTS and RAS and cultured for ~8 months (n = 15 F1 corals/aquaculture system); scale bar shows 2 cm.
Abiotic environmental parameters in each tank were monitored regularly to ensure the stability of conditions in the aquaculture systems. Light intensity was measured with a light portable meter (LI-250A, LI-COR, Lincoln, NE, USA) connected to a light sensor (LI-193SA, LI-COR, Lincoln, NE, USA), flow rate was measured with a propeller current meter (GR20, Kenek, Tokyo, Japan) connected to a detector (GR3T-2–20N, Kenek, Tokyo, Japan) and water temperature in each tank was recorded every 10 min using a HOBO pendant logger (HOBO pendant, Onset Computer Corporation, Bourne, MA, USA). The temperature in FTS tanks fluctuated in sync with natural conditions over the seasons, but to mitigate the potential heat stress in summer, a chiller (CL650, Resun, Shenzhen, China) limited the maximum temperature to 28 °C ± 1 °C. The RAS tanks were located in a separate room and an air conditioner, also set to 28 °C, was used to control the maximum water temperature. Water chemistry samples were collected from each tank on a monthly basis to quantify multiple parameters including: calcium, magnesium, ammonia, nitrite, nitrate, phosphate, alkalinity, pH, and salinity (Salifert Profi Test, Holland; WaterLink SpinTouchFX, La Motte, Chestertown, MD, USA; Cond3110, WTW, Weilheim in Oberbayern, Germany).
2.3. Artemia Preparation and Coral Feeding
Corals were fed Artemia naupii twice a week (concentration (mean ± SD): 32.2 ± 2.75 individual A. naupii mL−1), with each feeding session lasting 4 h in duration (10:00–14:00). Prior to feeding, the corals were moved from their culture tanks into a meshed PVC frame positioned within independent system-specific feeding tanks (Figure 1b,d); two bubble stones were placed at the corners of each of the meshed frames to promote water circulation. The coral colonies were suspended in the tanks with fishing line, which allowed them to be moved quickly and easily between the culture tanks and their system-specific feeding tanks. During feeding, the lights were turned off in the RAS culture room, and a non-airtight lid was placed over the FTS feeding tank to induce darkness. The RAS feeding tank was equipped with a life support tank similar to the RAS culture tanks, but with the addition of a bioreactor (BioReact 150, Reef Octopus, Manila, Philippines) and a macroalgae reactor (MBR127, Skimz, Singapore) containing Chaetomorpha linum to help eliminate organic waste more efficiently [44]. Commercial nitrifying bacteria (NBL A-5, Pandora, Taiwan) were also added into the life support tank monthly.
Food was prepared by mixing a ratio of 5 g A. naupii cysts (Golden West Artemia, Supreme plus, South Ogden, UT, USA) to 1 L of seawater (natural seawater was used for making FTS corals’ food, and synthetic seawater was used for making RAS corals’ food). This mixture was then incubated in an aerated container for 48 h. Two hours prior to feeding, 4.5 mL of 100 ppm Pack Boost 10 Enrichment Diets (Omega, Chuan Kuan Enterprise, Taiwan) were added to the food container. Prior to the collection of A. naupii, the bottom of the container was exposed to light for 5 min to allow for the separation of living A. naupii from unhatched cysts or shells floating on the surface. Live A. naupii were then collected through a 200-µm mesh net and rinsed with the seawater from the system-specific feeding tanks for cleaning and poured into the meshed frames within the feeding tanks.
2.4. Reproductive Output and Timing
The coral colonies were individually placed in a meshed (100-µm plankton mesh) 6 L plastic container (Figure 1e) from 17:00–09:00 each day to quantify reproductive output and timing within each aquaculture system. In the morning, the containers were removed and planulae that had been released within the containers overnight were enumerated. The onset of reproduction was determined as the first lunar day on which the colonies began releasing planulae; on a given day, only colonies that produced ≥ 5 planulae were defined as reproducing colonies. The mean lunar day of reproduction was determined for each colony by calculating the weighted (by colony-specific reproductive output) mean lunar day of planulae release. Reproductive output was not assessed on weekends when corals had stopped reproducing (i.e., during the interim time between reproductive cycles), but if any coral colony was reproducing or anticipated to be reproducing on weekends, we checked for planulae release. Colonies were hung in the tanks in close proximity to one another in an effort to increase the probability of sexual reproduction [40].
2.5. Diel Timing of Reproduction
The diel timing of planulae release was assessed on a subset of colonies (n = 9/aquaculture system) after ~1 year of ex situ culture (assessed after 10 months [FTS] or 11 months [RAS] within the aquaculture systems; diel timing was not assessed during the same month for both systems due to logistical/personnel limitations). The number of planulae released was counted hourly over the course of a 48 h period using the same collection containers as described in Section 2.4. Planulae were considered to be produced at night if released between 19:00–06:00, and were considered to be produced during the day if released between 07:00–18:00.
2.6. Preliminary Assessment of Growth and Reproduction in an F1 Generation
Quantifying the settlement, growth, and ultimately reproduction of an F1 generation of corals was not the primary focus of this study, yet interestingly we were able to conduct a preliminary assessment that highlighted the feasibility of completing the entire life cycle of a brooding coral ex situ using both an FTS and RAS approach. Planulae released from our experimental colonies upon their initial collection (i.e., ~3–4 months prior to the start of our ex situ culture experiment) were pooled and settled using similar techniques/containers as described in [37]. In brief, 40 planulae each were placed into meshed plastic containers (n = 12 containers, volume 540 cm3) with multiple small ceramic tiles (2.5 × 2.5 cm) covering the bottom of each container; tiles had been held for 1 month in a tank rich in crustose coralline algae to help facilitate coral settlement. The settlement containers were then placed evenly in 60 L flow-through tanks (n = 3) for 5 days, after which, the tiles that had settled recruits were removed and moved to a semi-enclosed outdoor common garden tank and were grown at ambient temperature and light for ~8 months. These F1 generation corals were not fed during this time, but had access to food suspended within natural seawater supplied to the tank. In lunar June (Gregorian month: May), a subset (n = 30) of these F1 corals were placed evenly in the FTS and RAS tanks (n = 5/tank); the diameter of each F1 coral was measured before being placed into the tanks, and photographs were taken approximately monthly (Figure 1f). After 8 months of culture within our aquaculture systems (i.e., in lunar February at ~16 months of age) the size (total linear extension) and reproductive output of these F1 corals were assessed. Each of the F1 corals were placed in a small meshed (100-µm plankton mesh) 300 mL plastic container from 17:00–09:00, and the number of planulae produced was counted each day at 09:00 for 1 lunar month.
2.7. Statistical Analyses
The temperature in the aquaculture systems was compared using a linear mixed-effects model, with aquaculture system, season, and their interaction as fixed effects, and lunar month as a random effect to mitigate for temporal autocorrelation. Water chemistry parameters were evaluated by using a linear mixed-effects model (separately for each water chemistry parameter), with system as a fixed effect and lunar month as a random effect due to repeated measurements; tank was also initially included as a random effect (it generally did not have a significant effect as assessed by the rand function in lme4), but was dropped to improve the model fit. A Tobit regression model, with system as a fixed effect, was used in cases when zero-inflation was observed in the water chemistry data.
Reproductive output was assessed using a zero-inflated negative binomial mixed-effects model with aquaculture system, season, and colony size as fixed effects, and colony and tank as random effects; an autocorrelation structure (AR1) was incorporated due to repeated measures over time on the same colonies. Interactions between system*season and system*colony size were also included in the model. Tank temperature was not included in the reproductive output model due to model convergence issues, but was assessed independently using the same zero-inflated negative binomial mixed-effects model approach, with daily mean temperature and aquaculture system (and their interaction) as fixed effects, and colony and tank as random effects. Reproductive timing was assessed by examining the mean lunar day (MLD) of planulae release (weighted by colony-specific reproductive output each month) using a linear mixed-effects model with system and season (and their interaction) as fixed effects, and tank and colony as random effects. The relationship between temperature and reproductive timing was assessed using linear regression, independently for each aquaculture system.
Diel reproductive timing was assessed by comparing the percentage of the hourly reproductive output over a 48 h period using a linear mixed-effects model with system and diel period (i.e., day or night), and their interaction, as fixed effects and tank and colony as random effects. The initial size (diameter) of the F1 corals was compared between systems using a student t-test. Two separate linear mixed-effects models (with aquaculture system as a fixed effect and tank as a random effect) were used to compare reproductive output and total linear extension of F1 corals after ~8 months of culture within the FTS or the RAS.
All analyses were conducted in R [45] using the packages lme4 [46], lmerTest [47], emmeans [48], car [49], AER [50], glmmTMB [51], and DHARMa [52]. Model assumptions were assessed by a visual inspection of the model residuals and calculation of variance inflation factors, except for the zero-inflated negative binomial mixed-effects model assumptions, which were assessed using the DHARMa package. All data and R scripts are publicly available at (https://github.com/CJ-McRae/Lam-et-al_Diveristy-submission, uploaded on 31 October 2022).
3. Results
3.1. Aquaculture System Temperature and Water Chemistry
Temperature differed between the aquaculture systems in each season (Figure S1), with the RAS being warmer in all seasons except for summer (mean ± SD for FTS and RAS, respectively: spring [27.4 ± 1.4 °C, 28.0 ± 0.2 °C], summer [28.4 ± 0.2 °C, 27.8 ± 0.2 °C], fall [27.4 ± 1.3 °C, 28.0 ± 0.4 °C], and winter [24.6 ± 0.9 °C, 27.8 ± 0.4 °C]) (linear mixed-effect model, post hoc contrasts, all t ≥ 3.54, all p < 0.001). There was no significant seasonal change in the temperature in the RAS, but in the FTS, winter was significantly cooler than the other seasons (post hoc contrasts, all t ≥ 4.65, all p ≤ 0.009). Water chemistry parameters also differed between aquaculture systems, whereby the RAS tanks had higher calcium, magnesium, alkalinity, pH, and salinity than the FTS tanks (linear mixed effects models, all t ≥ 3.09, all p ≤ 0.003), but no differences were found for ammonia or nitrate (Table S1, Figure S2). Nitrite and phosphate concentrations were not statistically compared because all concentrations in the RAS were zero.
3.2. Coral Colony Reproductive Output
The coral colonies in both aquaculture systems showed relatively consistent reproduction over the course of the 12-month study, with an average (± SD) of 79.9 ± 20.2% (FTS) and 70.8 ± 26.5% (RAS) colonies reproducing each month. Reproductive output did not differ between aquaculture systems in the spring and winter, but differences were found between the aquaculture systems in summer and fall (Figure 2). In the summer, colonies in the RAS produced more planulae (mean ± SD; 1417 ± 2546) than colonies in the FTS (389 ± 430) (zero-inflated negative binomial mixed-effects model, post hoc contrast, t = 3.39, p < 0.001). The opposite trend was found in fall, with colonies in the FTS producing more planulae (630 ± 1162) than colonies in the RAS (196 ± 319) (post hoc contrast, t = 3.92, p < 0.001). Neither colony size, nor the interaction between colony size*aquaculture system had a significant effect on reproductive output. Within systems, the number of planulae produced remained similar across the seasons for colonies in the FTS, whereas colonies in the RAS produced more planulae in summer than in other seasons (post hoc contrasts, all t ≥ 2.67, all p < 0.04). Temperature, which was analyzed in a separate zero-inflated negative binomial mixed-effects model due to over-parameterization when included in the main reproductive output model, did not have a significant effect on reproductive output in either aquaculture system (Figure S3). The monthly reproductive output for individual colonies is reported in Figure S4.
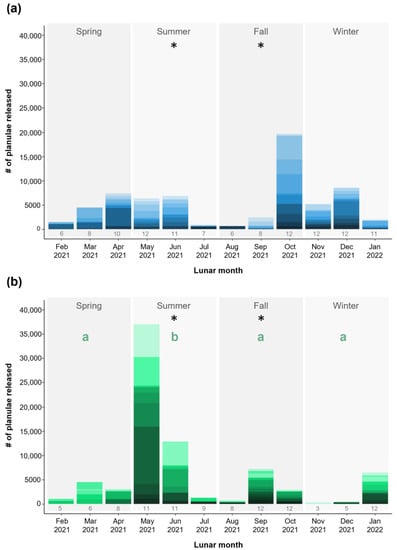
Figure 2.
Reproductive output of Pocillopora acuta colonies cultured within a flow-through aquaculture system (FTS; n = 3 independent tanks) and a recirculating aquaculture system (RAS; n = 3 independent tanks) from lunar February 2021–lunar January 2022 (Gregorian calendar months: ~March 2021–February 2022). Total monthly reproductive output for colonies within the (a) FTS (blue-toned bars) and (b) RAS (green-toned bars); specific colours in each bar represent individual colony reproductive output each month and the number below the bar indicates the number of colonies reproducing that month. Letters indicate within system seasonal differences, and asterisks indicate seasonal differences in reproductive output between aquaculture systems.
3.3. Coral Colony Reproductive Timing
The reproductive timing of coral colonies differed between aquaculture systems in summer and winter, but not in spring and fall (Figure 3). In summer, the mean lunar day (MLD ± SD; values were weighted by reproductive output) of planulae release in the FTS occurred later in the lunar cycle (18.0 ± 6.7) than colonies in the RAS (14.2 ± 9.1) (linear mixed-effects model, post hoc contrast, t = 2.26, p = 0.03). The opposite trend, however, occurred in winter, with colonies in the RAS releasing planulae later in the lunar cycle (21.9 ± 11.3) than colonies in the FTS (11.0 ± 4.8) (t = 5.90, p < 0.001). Seasonal variation in reproductive timing also occurred within aquaculture systems. In the FTS, the MLD of planulae release occurred later in the lunar cycle in the summer compared to the spring and winter (both t ≥ 2.94, both p ≤ 0.02); the MLD in fall did not differ significantly from any other season. In the RAS, the MLD of planulae release in the spring was earlier than all other seasons (all t ≥ 2.67, all p < 0.001), and the MLD of planulae release in winter was later than all other seasons (all t ≥ 2.86, all p ≤ 0.04); no difference in the MLD was found between the summer and fall. Overall, monthly reproductive timing for individual colonies showed relatively high variability across time in each aquaculture system. The colony-specific reproductive timing is reported in Figure S5. Temperature did not influence reproductive timing in the RAS colonies, but increased temperature was associated with reproduction later in the lunar cycle in the FTS colonies (linear regression, t = 4.02, p < 0.001, adjusted R2 = 0.12; Figure S6).
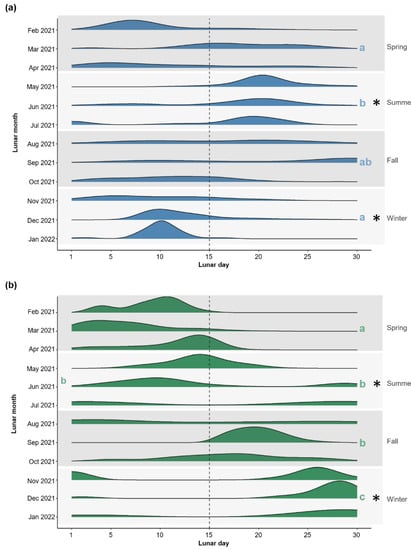
Figure 3.
Reproductive timing of Pocillopora acuta colonies cultured within (a) a flow-through aquaculture system (FTS [blue]; n = 3 independent tanks) and (b) a recirculating aquaculture system (RAS [green]; n = 3 independent tanks) from lunar February 2021–lunar January 2022 (Gregorian calendar months: ~March 2021–February 2022). Ridge lines represent reproduction time weighted by colony-specific reproductive output; horizontal dashed line shows lunar day 15 (i.e., the full moon) and letters show seasonal differences independently for each aquaculture system. Asterisks indicate significant seasonal differences in reproductive timing between aquaculture systems.
The number of reproductive days (i.e., the number of days/lunar month in which a colony released ≥ 5 planulae) did not differ between aquaculture systems in any season (linear mixed-effects model, post hoc contrasts, all t ≤ 1.76, all p > 0.05); overall, the number of reproductive days (mean ± SD) for colonies per month was 8.0 ± 3.1 (FTS) and 6.6 ± 3.1 (RAS) (Figure S7). Within the FTS, the number of reproductive days did not differ across seasons, but the RAS colonies had more reproductive days in summer compared to spring and winter (both t ≥ 2.64, both p ≤ 0.04); there was no difference in the number of reproductive days between fall and summer.
Over the course of a 48 h period (after ~1 year of ex situ culture), the colonies in both aquaculture systems demonstrated clear diel trends in reproductive timing, with a higher percentage of larval release occurring during the night (FTS: 80.4%, RAS: 84.5%) compared to the day (FTS: 19.6%, RAS: 15.5%, linear mixed-effects model post hoc contrasts, both t ≥ 18.44, both p < 0.001) (Figure 4). There was no significant difference in the percentage of planulae released between aquaculture systems in the day or the night, respectively.
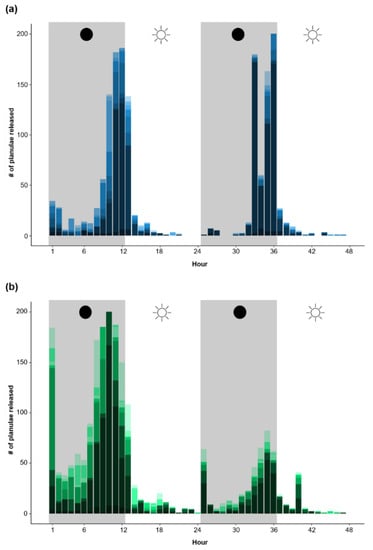
Figure 4.
Diel reproductive timing of Pocillopora acuta colonies over the course of a 48 h period after being cultured for ~1 year within (a) a flow-through aquaculture system (FTS [blue]; n = 3 independent tanks) and (b) a recirculating aquaculture system (RAS [green]; n = 3 independent tanks). Grey shading indicates the night (i.e., when colonies were in the dark), and the specific colours within the bars represent total reproductive output for each individual colony per hour.
3.4. Growth and Reproduction of F1 Generation Corals
There was no difference in initial size (mean diameter ± SD) of F1 corals when introduced into the aquaculture systems (FTS: 1.72 ± 0.37 cm, RAS: 1.79 ± 0.42 cm; two sample t-test p > 0.05), but after 8 months of culture, F1 corals in the FTS were significantly larger than those in the RAS (total linear extension mean ± SD, FTS: 12.5 ± 1.25 cm, RAS: 8.36 ± 1.48 cm; linear mixed-effects model t = 5.38, p = 0.006). The reproductive capacity of F1 corals was assessed in lunar February and revealed that 93% (FTS) and 73% (RAS) of F1 corals released more than five planulae during the reproductive cycle; RAS F1 coral reproduction range: 0–79 planulae; and FTS F1 reproduction range: 0–360 planulae (Figure 5a). The reproductive output of F1 corals did not differ between aquaculture systems (linear mixed-effects model with square root transformation, t = 1.44, p = 0.22), and only F1 corals in the RAS showed a significant relationship between size and reproductive output (linear regression, t = 2.54, p = 0.02, R2 = 0.33; Figure 5b).
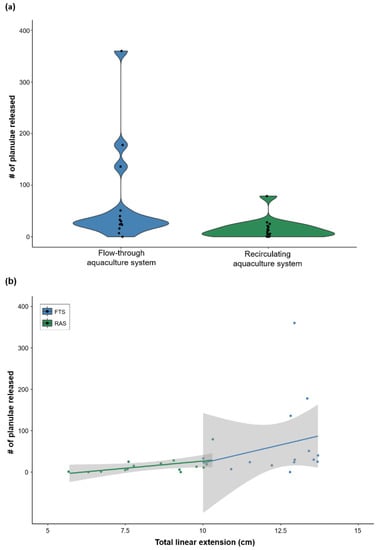
Figure 5.
Reproductive output of F1 corals cultured within (a) a flow-through aquaculture system (FTS [blue]; n = 3 tanks) and a recirculating aquaculture system (RAS [green]; n = 3 tanks); n = 15 F1 corals/system. (b) Reproductive output relative to F1 coral size (total linear extension); lines show smoothed mean and grey shading indicates 95% confidence intervals. Reproduction was enumerated in lunar February after ~16 months of ex situ culture; first 8 months of culture in a semi-enclosed outdoor tank exposed to ambient light and temperature, and then last 8 months of culture in either the FTS or RAS tanks.
4. Discussion
We cultured Pocillopora acuta colonies in two distinct aquaculture systems (a flow-through system (FTS) and a recirculating aquaculture system (RAS)) and were able to achieve consistent monthly reproduction for over 1 year, with the offspring grown in our systems being capable of producing an F2 generation. This is, to the best of our knowledge, the first direct comparison of long-term/multi-generational culture of a brooding coral in two distinct aquaculture systems. Although the temporal trends in reproductive output and timing differed in some seasons between aquaculture systems, we did not find definitive evidence to suggest that one system was markedly superior to the other. Despite the coral recruits growing more in the FTS compared to the RAS, the reproductive output of F1 generation corals was similar. Collectively, these findings suggest that consistently reproducing colonies of P. acuta can be effectively cultured long-term using both a FTS and RAS approach.
4.1. Potential Reasons for Successful Long-Term Ex Situ Reproduction
The amount of planulae produced by colonies in both systems showed some variation across time, but relatively consistent monthly reproduction persisted over the course of 1 year. A possible explanation for this consistency may be related to the provision of a steady food supply to the colonies. Heterotrophy is known to enhance coral growth and can be beneficial to corals under heat stress [53,54], but the direct influence of feeding on reproduction is less well understood. Yet, using the brooding coral Stylophora pistillata, Bellworthy et al. [55] showed that after 5 months of culture (in an FTS), colonies that were fed Artemia produced three times more planulae than unfed colonies; this higher reproductive output was associated with higher protein concentrations in the fed colonies. In contrast, feeding Rotifers (Brachionus sp.) reduced the reproductive output in the spawning coral Pocillopora verrucosa [56]. This inconsistency highlights the need for more research examining feeding effects on reproduction with a particular focus placed on multi-species responses to different food types (e.g., akin to approaches used in feeding studies measuring coral growth responses [57]). In our study, the reproductive output of colonies in both the FTS and RAS was similar (or sometimes greater) to that of colonies freshly collected from local natural populations [34], suggesting that our aquaculture conditions, including feeding, may have had a positive effect on reproductive output. The consumption of a steady supply of enriched Artemia in our aquaculture systems likely meant that energy needs were effectively met, reducing the need for periodic trade-offs between reproduction and colony growth/maintenance (e.g., [58,59]).
Consistent reproduction over time was also likely possible because colonies were not held in isolation from one another. In many reproduction-based studies, it is common to maintain brooding colonies within independent tanks, as this allows for straightforward colony-specific planulae collection and enumeration (e.g., [37]). While this approach is often needed to meet experimental design requirements, it could potentially lead to a reduction of reproduction (or perhaps even cessation in some populations) over long-term time scales due to the inhibition of sperm transfer among colonies. P. acuta has a mixed reproductive mode with the ability to produce both asexually and sexually derived planulae [60,61], with a relatively unique capacity for simultaneous sperm release and larval planulation [40,62]. Since our planulae collection containers negated the need for ‘single-housed’ colonies, the opportunity for multiple/combined modes of reproduction remained possible. Grouping colonies together in tanks may therefore provide an advantage to coral colonies held long-term in ex situ aquaculture systems. A direct assessment of sperm production and evaluation of offspring genetics was outside of the scope of our study, but future research focused on this topic could help elucidate the temporal dynamics of reproductive strategies as well as the genetic diversity potential within ex situ aquaculture.
Finally, active efforts that were made to reduce potential stress-related impacts on the colonies may have also contributed to the long-term reproduction in corals within both the FTS and RAS. For example, the corals were fed in independent system-specific tanks, rather than within the culture tanks, to mitigate algal growth and exposure to high nutrient levels that could adversely affect coral health [23,63]. Further, we actively monitored the condition of each coral colony daily to check for signs of stress (e.g., colour change, tissue loss) which, if found, were addressed in a timely manner to help facilitate a good outcome for the coral. For example, in some reproductive cycles, planulae release coincided with reproduction in guard crabs (Trapezia spp.), after which some colonies showed signs of tissue sloughing. The presence of these crabs within the FTS and RAS colonies is not typically cause for concern, as they live in symbiosis quite commonly on natural reefs [64], but an abundance of gametes released by the crabs at night coupled with relatively low flow within the collection containers may have resulted in the acute degradation of the water quality. If the coral colony condition did not improve after several days, then that section of the coral was removed using bone cutters to prevent possible spread to healthy parts of the colony. These partial fragmentations were relatively rare throughout our study (only ~4 undertaken over the course of 1 year) but they may have helped to reduce the deterioration of individual colonies. We took an adaptive management approach [65] in our decision to conduct these partial fragmentations, but future work would benefit from comprehensively testing the efficacy of this approach.
4.2. Comparing Aquaculture Systems: Reproductive Output
Seasonal variation in colony reproductive output was subtly observed in our study, and trends differed between aquaculture systems. The most prominent change in reproductive output was observed in the RAS colonies in lunar May, where the total monthly reproductive output surpassed 35,000 planulae (sourced from n = 11 reproducing colonies). This reproductive output was higher than any other month in either aquaculture system. A potential reason for this high reproductive output may have been due to the replacement of three colonies that had died with three freshly collected colonies; the three original colonies experienced tissue degradation and subsequent death in lunar April. Interestingly, it was not the new colonies that contributed most to the high reproductive output, but rather it was six other colonies that had already been in the RAS that released the most planulae (see Figure S4 for colony specific reproductive output). A possible explanation for this high reproduction may have been that the three dying colonies invested their remaining energy into reproduction via sperm release (i.e., in line with the ‘abandoning the ship hypothesis’, whereby organisms in a stressed state invest highly in sexual reproduction [66,67]). This sperm may have then been used for fertilization and subsequent brooding of planulae by healthy colonies. The relatively high number of reproductive days in lunar May (~12 days; see Figure S7) coupled with a flexible window for brooding times (i.e., ranging from a few days to several weeks [68]) may explain the high reproductive output in the RAS in lunar May.
Periods of relatively lower reproduction, in summer in the FTS and in fall in the RAS, may be a reflection of episodic system-specific challenges. The lower reproduction in the FTS in the summer may be a result of water quality perturbations due to seasonal tropical storms and typhoons. Monthly monitoring of water quality showed relatively higher nitrate and phosphate levels in summer in the FTS tanks, which may be attributed to the effect of substantial run-off caused by heavy rain. Studies that assess the effects of nutrients on coral reproduction are typically focused on spawning coral species (e.g., see [69] for review) and show a negative effect of nutrients (dissolved nitrogen in particular) on coral fertilization success. In brooding corals, high nutrient levels have also been shown to reduce the number of planulae produced [70]. The potential for local perturbations in summer highlights the ‘Achilles heel’ of using a FTS for coral culture, whereby the quality of the culture conditions is intrinsically linked to the quality of an unpredictable seawater source. Similarly, the lower reproduction in the RAS colonies in fall compared to the FTS colonies was potentially a result of relatively higher nutrient concentrations in the RAS feeing tank at that time, which could have impacted coral reproduction.
4.3. Comparing Aquaculture Systems: Reproductive Timing
The reproductive timing varied between systems and showed system-specific seasonal differences. At the onset of our study, the mean lunar day of reproduction for colonies in both systems was similar (i.e., before the full moon), but as time passed, differing timing trends emerged. In brief, the FTS colony reproductive timing shifted to later in the lunar cycle in summer, was highly dispersed across the lunar cycle in fall, and then returned to early in the lunar cycle in winter, whereas the reproductive timing of colonies in the RAS showed a relatively steady gradual shift to later in the lunar cycle (i.e., after the full moon) across the study months. Changes in temperature can induce a shift in reproductive timing, with evidence of warmer temperatures being associated with reproduction earlier in the lunar cycle shown in both temperature manipulation lab experiments [35,37] and seasonally in field-sourced populations [34]. It is therefore surprising that colonies in the FTS reproduced later in the lunar cycle in summer (and earlier in the lunar cycle in winter), and that constant exposure to 28 °C in the RAS resulted in a shift in reproduction to later, rather than earlier in the lunar cycle. The reason for this mismatch from the expected responses remains unclear, but could be related to abundant energy availability due to food provision, whereby a longer brooding time may have provided benefits to the planulae (e.g., larger size and high lipid content can improve offspring dispersal range and recruitment success [71,72]). In accordance with bet-hedging theory, corals are known to produce a range of planulae phenotypes throughout a single reproductive cycle [73], so perhaps delaying reproduction when resources are abundant is another strategy to maximize colony fitness. Alternatively, acclimation to aquaculture tanks coupled with the absence of moonlight and tidal cues may have offset natural reproductive timing patterns [28], or perhaps brooding corals have more plasticity in reproductive timing than originally expected; for example, Linden et al. [74] showed that the reproductive timing in Stylophora pistillata colonies was not necessarily closely linked to the lunar cycle or environmental conditions, but rather followed a circatrigintan pattern. Despite uncertainty regarding the specific mechanism/driver of the reproductive timing patterns in our study, the different timing patterns among colonies in the FTS and RAS presents a unique benefit: the almost daily production of planulae. Collectively, the use of these two distinct aquaculture systems demonstrates the practicality of using ex situ culture to provide a consistent broodstock supply.
Diel reproductive timing was assessed after ~1 year of ex situ culture and showed that colonies in both the FTS and RAS retained natural timing, with the majority of planulae being released in the ‘dark’ hours [75]. This pattern likely remained undisrupted by ex situ culture conditions, as light/dark periods in tanks were set to approximately mimic local conditions. By releasing the majority of their planulae under dark conditions, the colonies are likely maximizing the survival odds of their offspring as the risk of predation may be lower than during the daylight hours [76].
4.4. Multi-Generational Coral Culture
Our preliminary assessment of F1 coral culture as a means of completing the coral life-cycle in two distinct ex situ aquaculture systems clearly passed the ‘proof of concept’ check with the successful production of an F2 generation in both the FTS and RAS. We do, however, recognize that our sample size was relatively small and the partial culture of F1 corals in a common garden prior to introduction into our tank systems introduces some unintended variation, which should be improved upon in the future. Yet, reaching reproductive size at ~16 months (or potentially earlier as we only assessed F1 reproduction at the end of the experiment) is promising and opens up opportunities for multiple life-stage culture using both FTS and RAS approaches to ex situ culture.
4.5. Potential Application of Ex Situ Culture to Support Climate Change Research
Many active interventions aimed at improving coral performance under climate change require long-term experiments and/or culture within ex situ aquaculture systems. For example, multi-generational treatment exposure (e.g., temperature and/or pCO2 conditioning) is needed to effectively test the capacity for, and duration of, epigenetic responses [77]. Therefore, the ability to maintain reproducing colonies and offspring beyond an F2 generation in ex situ aquaculture systems could broaden our understanding of epigenetics in marine organisms, which to date has been predominately focused on shorter-lived organisms (see [78] for review). Further, selective breeding experiments aimed at producing more thermally resistant corals can also benefit from ex situ culture; e.g., Humanes et al. found increased survival outcomes with an 11-month (as opposed to a 5-month) ex situ culture period before outplanting to natural reefs [79]. Planulae produced and grown within ex situ aquaculture systems could serve as a stepping-stone to support similar selective breeding experiments by determining species-specific ‘optimal’ outplanting size. Additionally, ex situ aquaculture systems have recently been pointed out as useful testing grounds to tackle a major bottleneck in probiotic applications in corals, i.e., determining an effective delivery method for beneficial microorganisms to corals on natural reefs [80]. The specific ex situ culture of reproducing colonies—as well as each subsequent life-stage—would allow for controlled experimentation of life-stage-specific testing of probiotic delivery methods. Lastly, many studies do not explicitly require colonies or planulae collected directly from natural reef environments, so corals from ex situ aquaculture systems could be used to support these experiments (e.g., eco-toxicity experiments, cryogenic preservation experiments) to reduce the impact of research collections on reefs that are already being severely impacted by climate change and local stressors.
5. Conclusions
The successful long-term culture of reproducing colonies and the ex situ production of multiple generations opens up diverse opportunities to support research and restoration projects. Here, we have demonstrated the potential for long-term culture of P. acuta using both FTS and RAS approaches. Exploring the feasibility of our described techniques with other species, as well as further ‘fine-tuning’ of ex situ food provision (e.g., testing other food sources and feeding concentrations), and optimization of recruitment culture conditions to maximize growth rates and fecundity would help further improve coral aquaculture. It is important, however, to be clear that ex situ culture should not be seen as a solution to mitigate the impacts of climate change or local stressors, but rather it should be viewed as a complementary tool to support larger more comprehensive plans (e.g., [11,81]) aimed at improving the future outlook for corals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15020218/s1, Figure S1: Mean daily temperature within aquaculture system tanks; Figure S2: Water chemistry within aquaculture system tanks; Figure S3: Total monthly reproductive output of Pocillopora acuta colonies relative to aquaculture system-specific mean monthly temperature; Figure S4: Total monthly reproductive output of individual Pocillopora acuta colonies cultured within a flow-through aquaculture system or a recirculating aquaculture system; Figure S5: Reproductive timing of individual Pocillopora acuta colonies cultured within a flow-through aquaculture system or a recirculating aquaculture system; Figure S6: Mean lunar day of planulae release of Pocillopora acuta colonies relative to aquaculture system-specific mean monthly temperature; Figure S7: Mean number of reproductive days of Pocillopora acuta colonies cultured within a flow-through aquaculture system or a recirculating aquaculture system; Table S1: Water chemistry comparison analyses between aquaculture system tanks.
Author Contributions
Conceptualization, K.-W.L., T.-Y.F. and C.J.M.; methodology, K.-W.L., T.-Y.F., C.J.M. and X.-C.Z.; formal analysis, C.J.M. and K.-W.L.; investigation, K.-W.L., X.-C.Z., Z.-M.Y., Y.-T.Q., M.-Q.J., T.-H.C. and G.K.C.; resources, T.-Y.F.; data curation, C.J.M., K.-W.L. and X.-C.Z.; writing—original draft preparation, K.-W.L., C.J.M. and T.-Y.F.; writing—review and editing, all authors; visualization, C.J.M. and K.-W.L.; supervision, T.-Y.F.; project administration, K.-W.L., X.-C.Z., Z.-M.Y., Y.-T.Q., M.-Q.J., T.-H.C. and G.K.C.; funding acquisition, T.-Y.F. and C.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology (Taiwan), grant number MOST 110-2611-M-291-002.
Data Availability Statement
Data and R scripts used in this study are publicly available on GitHub (https://github.com/CJ-McRae/Lam-et-al_Diveristy-submission, uploaded on 31 October 2022).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript; or in the decision to publish the results.
References
- Hoegh-Guldberg, O.; Kennedy, E.V.; Beyer, H.L.; McClennen, C.; Possingham, H.P. Securing a long-term future for coral reefs. Trends Ecol. Evol. 2018, 33, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Barnes, M.L.; Bellwood, D.R.; Cinner, J.E.; Cumming, G.S.; Jackson, J.B.C.; Kleypas, J.; van de Leemput, I.A.; Lough, J.M.; Morrison, T.H.; et al. Coral reefs in the Anthropocene. Nature 2017, 546, 82–90. [Google Scholar] [CrossRef]
- Donovan, M.K.; Burkepile, D.E.; Kratochwill, C.; Shlesinger, T.; Sully, S.; Oliver, T.A.; Hodgson, G.; Freiwald, J.; van Woesik, R. Local conditions magnify coral loss after marine heatwaves. Science 2021, 372, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Abelson, A.; Nelson, P.A.; Edgar, G.J.; Shashar, N.; Reed, D.C.; Belmaker, J.; Krause, G.; Beck, M.W.; Brokovich, E.; France, R.; et al. Expanding marine protected areas to include degraded coral reefs. Conserv. Biol. 2016, 30, 1182–1191. [Google Scholar] [CrossRef]
- Camp, E.F. Contingency planning for coral reefs in the Anthropocene; The Potential of Reef Safe Havens. Emerg. Top. Life Sci. 2022, 6, 107–124. [Google Scholar] [CrossRef]
- Voolstra, C.R.; Suggett, D.J.; Peixoto, R.S.; Parkinson, J.E.; Quigley, K.M.; Silveira, C.B.; Sweet, M.; Muller, E.M.; Barshis, D.J.; Bourne, D.G.; et al. Extending the natural adaptive capacity of coral holobionts. Nat. Rev. Earth Environ. 2021, 2, 747–762. [Google Scholar] [CrossRef]
- Putnam, H.M. Avenues of reef-building coral acclimatization in response to rapid environmental change. J. Exp. Biol 2021, 224, jeb239319. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, M.; van Oppen, M.J.H.; Carter, V.; Henley, M.; Abrego, D.; Puill-Stephan, E.; Negri, A.; Heyward, A.; MacFarlane, D.; Spindler, R. First frozen repository for the Great Barrier Reef coral created. Cryobiology 2012, 65, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Toh, E.-C.; Liu, K.-L.; Tsai, S.; Lin, C. Cryopreservation and cryobanking of cells from 100 coral species. Cells 2022, 11, 2668. [Google Scholar] [CrossRef] [PubMed]
- Shaver, E.C.; McLeod, E.; Hein, M.Y.; Palumbi, S.R.; Quigley, K.; Vardi, T.; Mumby, P.J.; Smith, D.; Montoya-Maya, P.; Muller, E.M.; et al. A roadmap to integrating resilience into the practice of coral reef restoration. Glob. Change Biol. 2022, 28, 4751–4764. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, N.; Corcoran, E.; Felis, T.; Ferse, S.; de Goeij, J.; Grottoli, A.; Harding, S.; Kleypas, J.; Mayfield, A.; Miller, M.; et al. Rebuilding Coral Reefs: A Decadal Grand Challenge; International Coral Reef Society and Future Earth Coasts: Bremen, Germany, 2021. [Google Scholar] [CrossRef]
- Froehlich, H.E.; Gentry, R.R.; Halpern, B.S. Conservation aquaculture: Shifting the narrative and paradigm of aquaculture’s role in resource management. Biol. Conserv. 2017, 215, 162–168. [Google Scholar] [CrossRef]
- Zoccola, D.; Ounais, N.; Barthelemy, D.; Calcagno, R.; Gaill, F.; Henard, S.; Hoegh-Guldberg, O.; Janse, M.; Jaubert, J.; Putnam, H.; et al. The World Coral Conservatory (WCC): A Noah’s ark for corals to support survival of reef ecosystems. PLoS Biol. 2020, 18, e3000823. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, C.; Høj, L.; Bourne, D.G. Probiotics for coral aquaculture: Challenges and considerations. Curr. Opin. Biotech. 2022, 73, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Henley, E.M.; Bouwmeester, J.; Jury, C.P.; Toonen, R.J.; Quinn, M.; Lager, C.V.A.; Hagedorn, M. Growth and survival among Hawaiian corals outplanted from tanks to an ocean nursery are driven by individual genotype and species differences rather than preconditioning to thermal stress. PeerJ 2022, 10, e13112. [Google Scholar] [CrossRef]
- Gibbs, M.T. Technology requirements, and social impacts of technology for at-scale coral reef restoration. Technol. Soc. 2021, 66, 101622. [Google Scholar] [CrossRef]
- Osinga, R.; Schutter, M.; Wijgerde, T.; Rinkevich, B.; Shafir, S.; Shpigel, M.; Luna, G.M.; Danovaro, R.; Bongiorni, L.; Deutsch, A.; et al. The CORALZOO Project: A synopsis of four years of public aquarium science. J. Mar. Biol. Assoc. 2012, 92, 753–768. [Google Scholar] [CrossRef]
- Barton, J.A.; Willis, B.L.; Hutson, K.S. Coral Propagation: A review of techniques for ornamental trade and reef restoration. Rev. Aquac. 2017, 9, 238–256. [Google Scholar] [CrossRef]
- Merck, D.E.; Petrik, C.G.; Manfroy, A.A.; Muller, E.M. Optimizing seawater temperature conditions to increase the productivity of ex situ coral nurseries. PeerJ 2022, 10, e13017. [Google Scholar] [CrossRef]
- Craggs, J.; Guest, J.R.; Davis, M.; Simmons, J.; Dashti, E.; Sweet, M. Inducing broadcast coral spawning ex situ: Closed system mesocosm design and husbandry protocol. Ecol. Evol. 2017, 7, 11066–11078. [Google Scholar] [CrossRef]
- Rocha, R.J.M.; Bontas, B.; Cartaxana, P.; Leal, M.C.; Ferreira, J.M.; Rosa, R.; Serôdio, J.; Calado, R. Development of a standardized modular system for experimental coral culture. J. World Aquac. Soc. 2015, 46, 235–251. [Google Scholar] [CrossRef]
- Craggs, J.; Guest, J.; Bulling, M.; Sweet, M. Ex situ co culturing of the sea urchin, Mespilia globulus and the coral Acropora millepora enhances early post-settlement survivorship. Sci. Rep. 2019, 9, 12984. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Mayfield, A.B.; Fan, T.-Y. Effects of feeding on the physiological performance of the stony coral Pocillopora acuta. Sci. Rep. 2020, 10, 19988. [Google Scholar] [CrossRef] [PubMed]
- Yuen, Y.S.; Yamazaki, S.S.; Nakamura, T.; Tokuda, G.; Yamasaki, H. Effects of live rock on the reef-building coral Acropora digitifera cultured with high levels of nitrogenous compounds. Aquac. Eng. 2009, 41, 35–43. [Google Scholar] [CrossRef]
- Leal, M.C.; Ferrier-Pagès, C.; Petersen, D.; Osinga, R. Coral aquaculture: Applying scientific knowledge to ex situ production. Rev. Aquac. 2016, 8, 136–153. [Google Scholar] [CrossRef]
- Osinga, R.; Schutter, M.; Griffioen, B.; Wijffels, R.H.; Verreth, J.A.J.; Shafir, S.; Henard, S.; Taruffi, M.; Gili, C.; Lavorano, S. The biology and economics of coral growth. Mar. Biotechnol. 2011, 13, 658–671. [Google Scholar] [CrossRef]
- Crowder, C.M.; Meyer, E.; Fan, T.-Y.; Weis, V.M. Impacts of temperature and lunar day on gene expression profiles during a monthly reproductive cycle in the brooding coral Pocillopora damicornis. Mol. Ecol. 2017, 26, 3913–3925. [Google Scholar] [CrossRef]
- Lin, C.-H.; Takahashi, S.; Mulla, A.J.; Nozawa, Y. Moonrise timing is key for synchronized spawning in soral Dipsastraea speciosa. Proc. Natl. Acad. Sci. USA 2021, 118, e2101985118. [Google Scholar] [CrossRef]
- van Woesik, R. Calm before the spawn: Global coral spawning patterns are explained by regional wind fields. Proc. R. Soc. B 2010, 277, 715–722. [Google Scholar] [CrossRef]
- O’Neil, K.L.; Serafin, R.M.; Patterson, J.T.; Craggs, J.R.K. Repeated ex situ spawning in two highly disease susceptible corals in the family Meandrinidae. Front. Mar. Sci. 2021, 8, 669976. [Google Scholar] [CrossRef]
- Craggs, J.; Guest, J.; Davis, M.; Sweet, M. Completing the life cycle of a broadcast spawning coral in a closed mesocosm. Invertebr. Reprod. Dev. 2020, 64, 244–247. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Ito, R.Y.; Liu, P.M. Night irradiance and synchronization of lunar release of planula larvae in the reef coral Pocillopora damicornis. Mar. Biol. 1985, 88, 167–174. [Google Scholar] [CrossRef]
- Cantin, N.; Stephenson, S.; Drury, C.; Majerova, E.; Roper, C.; van Oppen, M.J.H. Pre-Conditioning Three Generations of Pocillopora acuta to Explore Mechanisms Underpinning Coral Acclimation to Climate Change Stress; International Coral Reef Society: Bremen, Germany, 2022; p. 1018. [Google Scholar]
- Fan, T.; Hsieh, Y.; Lin, K.; Kuo, F.; Soong, K.; McRae, C.; Edmunds, P.; Fang, L. Plasticity in lunar timing of larval release of two brooding Pocilloporid corals in an internal tide-induced upwelling reef. Mar. Ecol. Prog. Ser. 2017, 569, 117–127. [Google Scholar] [CrossRef]
- Crowder, C.M.; Liang, W.-L.; Weis, V.M.; Fan, T.-Y. Elevated temperature alters the lunar timing of planulation in the brooding coral Pocillopora damicornis. PLoS ONE 2014, 9, e107906. [Google Scholar] [CrossRef] [PubMed]
- Nietzer, S.; Moeller, M.; Kitamura, M.; Schupp, P.J. Coral larvae every day: Leptastrea purpurea, a brooding species that could accelerate coral research. Front. Mar. Sci. 2018, 5, 466. [Google Scholar] [CrossRef]
- McRae, C.J.; Huang, W.-B.; Fan, T.-Y.; Côté, I.M. Effects of thermal conditioning on the performance of Pocillopora acuta adult coral colonies and their offspring. Coral Reefs 2021, 40, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.F.; Ravago-Gotanco, R. Rarity of the “common” coral Pocillopora damicornis in the western Philippine Archipelago. Coral Reefs 2018, 37, 1209–1216. [Google Scholar] [CrossRef]
- Johnston, E.C.; Forsman, Z.H.; Toonen, R.J. A Simple molecular technique for distinguishing species reveals frequent misidentification of Hawaiian corals in the Genus Pocillopora. PeerJ 2018, 6, e4355. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.A.; Moya, A.; Cantin, N.E.; van Oppen, M.J.H.; Torda, G. Observations of simultaneous sperm release and larval planulation suggest reproductive assurance in the coral Pocillopora acuta. Front. Mar. Sci. 2019, 6, 362. [Google Scholar] [CrossRef]
- Putnam, H.M.; Ritson-Williams, R.; Cruz, J.A.; Davidson, J.M.; Gates, R. Environmentally-induced parental or developmental conditioning influences coral offspring ecological performance. Sci. Rep. 2020, 10, 13664. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Lee, H.-J.; Zheng, Q.; Lai, J.-W.; Su, F.-C.; Ho, C.-R. Tide-induced periodic sea surface temperature drops in the coral reef area of Nanwan Bay, southern Taiwan. J. Geophys. Res. Ocean 2020, 125, e2019JC015226. [Google Scholar] [CrossRef]
- Keshavmurthy, S.; Kuo, C.-Y.; Huang, Y.-Y.; Carballo-Bolaños, R.; Meng, P.-J.; Wang, J.-T.; Chen, C.A. Coral reef resilience in Taiwan: Lessons from long-term ecological research on the coral reefs of Kenting National Park (Taiwan). JMSE 2019, 7, 388. [Google Scholar] [CrossRef]
- Aquilino, F.; Paradiso, A.; Trani, R.; Longo, C.; Pierri, C.; Corriero, G.; de Pinto, M.C. Chaetomorpha linum in the bioremediation of aquaculture wastewater: Optimization of nutrient removal efficiency at the laboratory scale. Aquaculture 2020, 523, 735133. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022.
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear mixed-effects models using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.; Christensen, R. LmerTest Package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Length, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, R Package Version 1.7.4-1. 2022.
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Kleiber, C.; Zeileis, A. Applied Econometrics with R; Springer-Verlag: New York, NY, USA, 2008; ISBN 978-0-387-77316-2. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. GlmmTMB Balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models, R package Version 0.4.5. 2022.
- Huffmyer, A.S.; Johnson, C.J.; Epps, A.M.; Lemus, J.D.; Gates, R.D. Feeding and thermal conditioning enhance coral temperature tolerance in juvenile Pocillopora acuta. R. Soc. Open Sci. 2021, 8, 210644. [Google Scholar] [CrossRef]
- Conti-Jerpe, I.E.; Thompson, P.D.; Wong, C.W.M.; Oliveira, N.L.; Duprey, N.N.; Moynihan, M.A.; Baker, D.M. Trophic strategy and bleaching resistance in reef-building corals. Sci. Adv. 2020, 6, eaaz5443. [Google Scholar] [CrossRef] [PubMed]
- Bellworthy, J.; Spangenberg, J.E.; Fine, M. Feeding increases the number of offspring but decreases parental investment of Red Sea coral Stylophora pistillata. Ecol. Evol. 2019, 9, 12245–12258. [Google Scholar] [CrossRef] [PubMed]
- Séré, M.G.; Massé, L.M.; Perissinotto, R.; Schleyer, M.H. Influence of heterotrophic feeding on the sexual reproduction of Pocillopora verrucosa in Aquaria. J. Exp. Mar. Biol. Ecol. 2010, 395, 63–71. [Google Scholar] [CrossRef]
- Conlan, J.A.; Bay, L.K.; Severati, A.; Humphrey, C.; Francis, D.S. Comparing the capacity of five different dietary treatments to optimise growth and nutritional composition in two scleractinian corals. PLoS ONE 2018, 13, e0207956. [Google Scholar] [CrossRef]
- Ward, S. Two patterns of energy allocation for growth, reproduction and lipid storage in the scleractinian coral Pocillopora damicornis. Coral Reefs 1995, 14, 87–90. [Google Scholar] [CrossRef]
- Leuzinger, S.; Willis, B.L.; Anthony, K.R.N. Energy allocation in a reef coral under varying resource availability. Mar. Biol. 2012, 159, 177–186. [Google Scholar] [CrossRef]
- Combosch, D.J.; Vollmer, S.V. Mixed asexual and sexual reproduction in the Indo-Pacific reef coral Pocillopora damicornis. Ecol. Evol. 2013, 3, 3379–3387. [Google Scholar] [CrossRef]
- Yeoh, S.-R.; Dai, C.-F. The production of sexual and asexual larvae within single broods of the scleractinian coral, Pocillopora damicornis. Mar. Biol. 2010, 157, 351–359. [Google Scholar] [CrossRef]
- Schmidt-Roach, S.; Miller, K.J.; Woolsey, E.; Gerlach, G.; Baird, A.H. Broadcast spawning by Pocillopora species on the Great Barrier Reef. PLoS ONE 2012, 7, e50847. [Google Scholar] [CrossRef]
- Chang, T.-C.; Mayfield, A.B.; Fan, T.-Y. Culture systems influence the physiological performance of the soft coral Sarcophyton glaucum. Sci. Rep. 2020, 10, 20200. [Google Scholar] [CrossRef]
- Canizales-Flores, H.M.; Rodríguez-Troncoso, A.P.; Rodríguez-Zaragoza, F.A.; Cupul-Magaña, A.L. A long-term symbiotic relationship: Recruitment and fidelity of the crab Trapezia on its coral host Pocillopora. Diversity 2021, 13, 450. [Google Scholar] [CrossRef]
- Holling, C.S.; Walters, C. Adaptive Environmental Assessment and Management; John Wiley and Sons: Toronto, Canada, 1978. [Google Scholar]
- Gerber, N.; Kokko, H. Abandoning the ship using sex, dispersal or dormancy: Multiple escape routes from challenging conditions. Philos. Trans. R. Soc. B 2018, 373, 20170424. [Google Scholar] [CrossRef]
- Griffiths, J.G.; Bonser, S.P. Is sex advantageous in adverse environments? A test of the abandon-ship hypothesis. Am. Nat. 2013, 182, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Permata, W.; Kinzie, R.; Hidaka, M. Histological studies on the origin of planulae of the coral Pocillopora damicornis. Mar. Ecol. Prog. Ser. 2000, 200, 191–200. [Google Scholar] [CrossRef]
- Nalley, E.M.; Tuttle, L.J.; Conklin, E.E.; Barkman, A.L.; Wulstein, D.M.; Schmidbauer, M.C.; Donahue, M.J. A systematic review and meta-analysis of the direct effects of nutrients on corals. Sci. Total Environ. 2023, 856, 159093. [Google Scholar] [CrossRef]
- Loya, Y.; Lubinevsky, H.; Rosenfeld, M.; Kramarsky-Winter, E. Nutrient enrichment caused by in situ fish farms at Eilat, Red Sea is detrimental to coral reproduction. Mar. Pollut. Bull. 2004, 49, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Isomura, N.; Nishihira, M. Size variation of planulae and its effect on the lifetime of planulae in three Pocilloporid corals. Coral Reefs 2001, 20, 309–315. [Google Scholar] [CrossRef]
- de Putron, S.J.; Lawson, J.M.; White, K.Q.L.; Costa, M.T.; Geronimus, M.V.B.; MacCarthy, A. Variation in larval properties of the Atlantic brooding coral Porites astreoides between different reef sites in Bermuda. Coral Reefs 2017, 36, 383–393. [Google Scholar] [CrossRef]
- Cumbo, V.R.; Fan, T.-Y.; Edmunds, P.J. Physiological development of brooded larvae from two Pocilloporid corals in Taiwan. Mar. Biol. 2012, 159, 2853–2866. [Google Scholar] [CrossRef]
- Linden, B.; Huisman, J.; Rinkevich, B. Circatrigintan instead of lunar periodicity of larval release in a brooding coral species. Sci. Rep. 2018, 8, 5668. [Google Scholar] [CrossRef]
- Fan, T.; Lin, K.; Kuo, F.; Soong, K.; Liu, L.; Fang, L. Diel patterns of larval release by five brooding scleractinian corals. Mar. Ecol. Prog. Ser. 2006, 321, 133–142. [Google Scholar] [CrossRef]
- Goodbody-Gringley, G. Diel Planulation by the brooding coral Favia fragum (Esper, 1797). J. Exp. Mar. Biol. Ecol. 2010, 389, 70–74. [Google Scholar] [CrossRef]
- Torda, G.; Donelson, J.M.; Aranda, M.; Barshis, D.J.; Bay, L.; Berumen, M.L.; Bourne, D.G.; Cantin, N.; Foret, S.; Matz, M.; et al. Rapid adaptive responses to climate change in corals. Nat. Clim. Change 2017, 7, 627–636. [Google Scholar] [CrossRef]
- Byrne, M.; Foo, S.A.; Ross, P.M.; Putnam, H.M. Limitations of cross- and multigenerational plasticity for marine invertebrates faced with global climate change. Glob. Change Biol. 2020, 26, 80–102. [Google Scholar] [CrossRef]
- Humanes, A.; Beauchamp, E.A.; Bythell, J.C.; Carl, M.K.; Craggs, J.R.; Edwards, A.J.; Golbuu, Y.; Lachs, L.; Martinez, H.M.; Palmowski, P.; et al. An experimental framework for selectively breeding corals for assisted evolution. Front. Mar. Sci. 2021, 8, 669995. [Google Scholar] [CrossRef]
- Peixoto, R.S.; Sweet, M.; Villela, H.D.M.; Cardoso, P.; Thomas, T.; Voolstra, C.R.; Høj, L.; Bourne, D.G. Coral probiotics: Premise, promise, prospects. Annu. Rev. Anim. Biosci. 2021, 9, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Kleypas, J.; Allemand, D.; Anthony, K.; Baker, A.C.; Beck, M.W.; Hale, L.Z.; Hilmi, N.; Hoegh-Guldberg, O.; Hughes, T.; Kaufman, L.; et al. Designing a blueprint for coral reef survival. Biol. Conserv. 2021, 257, 109107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).