Abstract
Based on the analysis of the botanical composition of the organic-mineral soil layer and peat, dendrochronological and radiocarbon datings, we performed the reconstruction of the development of six pine wooded sphagnum bogs located in the boreal zone of Russia. Most of the bogs under study followed the endogenesis patterns with the vegetation cover gradually changing, peat layer growing, substrate trophicity declining and shrub-sphagnous vegetation forming under modern conditions. Emerging pyrogenic layers and charcoals in the peat indicate that the study sites were constantly affected by fires, which periodically interrupted the endogenous development of the bogs, especially during the warmest Holocene periods.
1. Introduction
Peatlands or mires, along with forest ecosystems, shape the Northern Hemisphere. They play a key role in the conservation of biodiversity and are crucial for supporting the hydrological regime of the surrounding areas. Mires accumulate carbon and influence the content of greenhouse gases in the atmosphere [1,2]. The course of the mire formation process depends on various endogenous and exogenous factors: climate change on a global and regional scale, geomorphological position, hydrological regime, local ecological conditions, various anthropogenic factors (land reclamation, deforestation, fires, etc.) and others. These factors determine the characteristics of each mire complex, structure and composition of mire vegetation [3,4,5]. Under the conditions of the changing climate of the current century, the study of the age of bogs and the identification of factors that determine the bog-forming process during the Holocene are of great importance for solving environmental problems and optimizing nature management [6]. Wildfires are one of the most important factors influencing ecosystems’ structures and functions at the global level, especially in the regions exposed to high fire risks [7,8,9,10,11,12,13]. In these settings, wildfires may play an integral role in the long-term ecosystem function, stability and succession [14], being the most common type of disturbance in boreal forests and forested peatlands [15]. Fires in wetlands can occur with surprising frequency [16] in the form of smoldering fires in the deep organic soils that accumulate in these ecosystems. Recent studies are focusing on the wildfire impact on wetland ecosystems, including water quality, nitrogen and carbon pools, species composition, vegetation type and structure [17,18,19,20,21,22,23,24].
Historically, fires caused by lightning were normal for swamps in many parts of the world. Modern mire fires often result from human activities [16,25]. In the boreal zone, fires play an important role in the functioning of natural mire ecosystems [6,26,27,28]. At mires, fires initiate succession changes in the vegetation cover and increase the soil temperature and the nutrients’ availability [11,29,30,31]. Globally, peatlands contain nearly a third of the terrestrial carbon pool while occupying only 2–3% of the Earth’s land surface [32]. The amount of carbon stored in peat exceeds the vegetation carbon pool and is comparable to the current atmospheric carbon pool. Fires occur in many peat-rich biomes and can damage these carbon stocks [33,34]. Peat fires are dominated by smoldering combustion, which can persist in wet conditions. In undisturbed boreal peatlands, most of the peat carbon stock is typically protected from smoldering, and such resistance has led to a peat-carbon accumulation over long timescales. The reduction of the water table in bogs due to past or present climate fluctuations, as well as human activities, increases the frequency and extent of peat fires. The combustion of deep peat affects the soil carbon that has not been part of the active carbon cycle, and thus will dictate the importance of peat fire emissions to the carbon cycle and feedbacks to the climate [28].
In Russia, in terms of size and peat stocks, boreal-raised bogs are the prevalent type of mires. Among all mires, wooded bogs are most vulnerable to fires. Kucherov and Kutenkov [35] classified these mires as ledum-sphagnum pine forests. Pyavchenko classified such communities as mire forests, growing on peat. Wooded bogs or mire forests are common throughout the entire range of bogs and are characterized by homogenous plant cover, pronounced Pinus story (P. sylvestris, P. sibirica), hummock relief and peat deposit exceeding 30 cm. Pine wooded bogs are presented by numerous pine-dwarf shrub-sphagnous communities, which are the transition from forest to open bog. Such bogs often alternate with sandy ridges occupied by sphagnum pine forests rising 2–4 m above the surface of the peat deposit [35,36,37,38,39].
Due to the occurrence of tree layer species, wooded bogs are often affected by fires. For example, Gromtsev [40] reported Karelian wooded bogs to burn at least once or twice every 100 years. However, due to the high moisture content, most peat soils in natural peatlands are protected from burning, which contribute to the accumulation of peat over centuries or millennia in northern areas [28,41]. The occurrence of peat deposit that holds information about past events on the wooded bogs makes them suitable models for studying climatic and environmental change (of a particular site/bog as well as adjacent areas) throughout their development history. Thus, while the effects of fire on forests and soils in well-drained landscapes have been studied in some detail, the influence of the pyrogenic factor on the formation of bog ecosystems has been studied to a lesser extent. Knowledge of the role of fire in wetland development is essential for modelling vegetation change under changing climate and anthropogenic influences.
The aim of our research was to reconstruct the Holocene development of six boreal wooded bogs located in Northeast Europe and Central Siberia based on the study of peat deposit stratigraphy, the botanical composition of peat, radiocarbon age and dendrochronology and to study the role of the pyrogenic factor in the vegetation successions at these sites.
2. Materials and Methods
2.1. Site Description
The studies were carried out in two regions within the boreal zone of Russia (Figure 1). Three study sites were in the middle taiga subzone of the Komi Republic (KOM) and three were in the boreal zone of the Krasnoyarsk Region (KRA). Both regions have a temperate continental cold climate.
Figure 1.
Location of the study sites. KO_S0—Site 0 (Maksimovsky monitoring site, Komi Republic), KO_S1—Site I (Koygorodsky National Park, Komi Republic), KO_S2—Site II (Pechoro-Ilychsky Reserve, Komi Republic), KRA_S3–Site III (Zotino, Krasnoyarsk Region), KRA_S4—Site IV (Zotino, Krasnoyarsk Region), KRA_S5—Site V (Zotino, Krasnoyarsk Region).
The KOM study sites are located in the northeast of the Russian Plain. The vegetation is dominated by spruce (Picea obovata Ledeb.) and pine forests (Pinus sylvestris L.). Depressions are occupied by raised bogs and aapa mires. The mean annual air temperature is from +0.2 to +1.3 °C. The amount of annual precipitation is from 670 to 790 mm [42].
The KRA study sites are located in the central part of the Krasnoyarsk Region, in the east of Western Siberia in the Middle Siberian Plateau. The vegetation is dominated by coniferous forests (Larix sibirica Ledeb. and Pinus sylvestris, P. sibirica Du Tour). The climate is typically continental with mean annual air temperatures from −10 °C to −3 °C. Annual precipitation is 500–600 mm. The territory is covered with snow for lengthy periods during the year, and has continuous permafrost (Schulze et al., 2002).
The climatic conditions are similar in both regions. The main ecological parameter that distinguishes European Russia from Siberia is the length of the growing season (230 d above 0 °C NE Moscow to 170 d above 0 °C in the east of Western Siberia) and to a lesser extent, precipitation (580 mm NE Moscow to 530 mm in Central Siberia) [43].
The studied wooded bogs are typical for the boreal zone and surrounded by coniferous pine (Pinus sylvestris) or spruce (Picea obovata) forests with birch (Betula pubescens Ehrh.). Plant communities are similar in all study sites and represented mainly by class Oxycocco-Sphagnetea Br.-Bl. et Tüxen ex Westhoff et al. 1946 O. Sphagnetalia medii Kästner et Flössner 1933 and one of the two alliances Sphagnion medii Kästner et Flössner 1933 or Oxycocco microcarpi-Empetrion hermaphroditi Nordhagen ex Du Rietz 1954 [44]. Tree stands are dominated by Pinus sylvestris L. The total canopy density is 0.1–0.4. Tree height is 2–16 m. Single burned tree trunks occur at all study plots. The herb-dwarf shrub layer is formed by Ledum palustre L., Chamaedaphne calyculata (L.) Moench., Rubus chamaemorus L., Eriophorum vaginatum L. and Vaccinium uliginosum L. Species with low abundance are Carex globularis L., Vaccinium oxycoccos L., Melampyrum pratense L. and Vaccinium myrtillus L. Moss dominants are Sphagnum angustifolium (Warnst.) C.E.O.Jensen, S. divinum Flatberg and K. Hasse, S. fuscum (Schimp.) H.Klinggr., S. girgensohnii Russow, Polytrichum commune Hedw. and Pleurozium schreberi (Willd. ex Brid.) Mitt.
Fibric histosols develop in the studied areas, with the exception of area 4 where histic podzols were detected. The soils are characterized by high acidity, high content of carbon and nitrogen in organic horizons and a wide C/N ratio. More detailed information on the physical and chemical characteristics of peat is presented in Dymov et al. [45] and Gorodnitskaya et al. [46]. Site 0 is located near the Maksimovsky monitoring site, Syktyvdinsky district (Komi Republic; 61°41′48″ N, 50°39′17″ E). A soil pit (No. 3–20) was made in the central part of the swampy shrub pine forest (Figure 2A). The depth is 90 cm. The clay parent material contains about 10% of residuals of herbs, sphagnum mosses and burned particles of pine and birch bark. The degree of peat decomposition (R) is 20–50%, and about 5% in the upper horizon.

Figure 2.
The vegetation and the structure of the soil profile. (A)—Site 0 (Maksimovsky monitoring site, Komi Republic), (B)—Site I (Koygorodsky National Park, Komi Republic), (C)—Site II (Pechoro-Ilychsky Reserve, Komi Republic), (D) –Site III (Zotino, Krasnoyarsk Region), (E)—Site IV (Zotino, Krasnoyarsk Region), (F)—Site V (Zotino, Krasnoyarsk Region).
Site I is located near the Koygorodsky National Park (Komi Republic; 59°58′58″ N, 50°09′24″ E). A soil pit (No. 10–19) was made at the wooded edge of the mire (Figure 2B). The peat depth is 115 cm. The upper 70 cm of the peat deposit is formed by forest peats from wood-herb and moss groups. From depths below 70 cm to the mineral bottom, there is an organic-mineral residue with a high degree of plant decomposition and ash content. The share of sand in the mineral component is 20–30%.
Site II is located on the territory of the Pechoro-Ilychsky Reserve (Komi Republic; 61°57′27″ N, 57°55′59″ E), about 5 hundred kilometers northeast of site I. A soil pit (p. 28–19) was made in the central part of the pine wooded dwarf shrub-sphagnum bog (Figure 2C). The peat depth is 220 cm. The parent material is loam. Peat deposits are formed by swamp and forest-swamp subtypes, wood-herbaceous, herbaceous, herbaceous-moss and moss groups.
Site III is located near the Zotino monitoring station (Krasnoyarsk region, 60°52′22″ N, 89°24′16″ E). A soil pit (No. 15-2019) was made at the oligotrophic edge of the water-logged aapa mire in the relief depression (Figure 2D). The depth is 200 cm, and the depth of the peat deposit is 430 cm. We could not make a full-depth section due to strong water-logging of the lower horizons. The upper part of the deposit is from transitional and upper peats of herb-moss and moss groups.
Site IV is located near the Zotino monitoring station (Krasnoyarsk region, 60°44′54″ N; 89°00′18″ E). A soil pit (No. 18–19) was made in the central part of the pine wooded dwarf shrub-sphagnum bog (Figure 2E). The pit depth is 0.65 m. The parent material is loam. Peat deposits are presented by moss and woody-moss peats. A pyrogenic horizon (Tpyr) occurs at a depth of 55 cm at the base of the deposit built of the poorly decomposed (R-5–15%) peat of the moss group.
Site V is located near the Zotino monitoring station (Krasnoyarsk region, 60°48′47″ N; E 89°19′47″ E). A soil pit (No.20-2019) was made in the central part of the pine wooded dwarf shrub-sphagnum bog (Figure 2F). The peat depth is 240 cm. The peat deposits are formed by swamp and forest-swamp subtypes, wood-herbaceous, herbaceous, herbaceous-moss and moss groups.
2.2. Vegetation Study and Peat Sampling
Field studies were conducted in 2019–2020. At the study plots, we described plant communities, collected wood samples from burned trees for dendrochronological studies, made soil pits and collected peat samples with the subsequent analysis of peat botanical composition, determined the degree of peat decomposition and performed radiocarbon dating tests. Plant communities were described using the standard geobotanical methods [47]. For the tree layer, we described its composition. The undergrowth, herb-dwarf shrub, and moss-lichen layers were characterized by the relative abundance of species and the total projective cover of each layer. Plant taxonomy was given according to World Flora Online [48].
2.3. Plant Macrofossil Analysis
A plant macrofossil analysis was conducted to detect and to reconstruct changes in vegetation assemblages. The analysis was performed at 10 cm resolution and in a visually homogeneous deposit at every 20 cm. During sampling and pre-analysis, we carefully noted any visual evidence for the occurrence of charred material. The botanical composition of peat and the peat decomposition degree were analyzed in the Laboratory of Phytocenology and Forestry Science at the Sukachev Forest Institute (Krasnoyarsk, Russia) by L.V. Karpenko using GOST 28245-89. Samples were rinsed with water using a 140 Mkm mesh size sieve, and the residues were analyzed for proportions of the main peat components. A stereomicroscope was used to estimate percentages for the total sample volume and a light microscope (“Leitz Wetzlar” a ×20, ×40 power) for further species identification using identification guides [49,50] and a reference collection of the V.N. Sukachev Institute of Forest SB RAS. Diagrams of peat were created using software ”Korpi” [51].
2.4. Chronology
The radiocarbon dating of peat samples was carried out on the equipment provided by Shared Research Facilities of the Tomsk Scientific Center SB AS (Tomsk, Russia) by the liquid scintillation method using a spectrometer-radiometer Quantulus 1220 (Wallac, Finland). Calibration of the radiocarbon age to calendar age was performed using the CALIB REV–7.10 programs [52]. Calibration of 14C ages and the construction of the age–depth model, which was a basic linear regression between neighboring levels, were carried out in Clam 2.2 [53] for R Workspace (4.1.2; R Development Core Team) using IntCal13 [54]. Dates were calibrated assuming a Gaussian distribution. Ages were reported as calibrated years before present (yr BP; present = 1950 AD/Common Era (CE)). Negative radiocarbon ages were calibrated using post-bomb analyses for the Northern Hemisphere.
The Holocene scheme (division of the Holocene) was given according to Walker et al. [55]. Global climatic characteristics in the Holocene were given according to N.A. Khotinskiy [56], and regional paleoclimatic characteristics for the European North by Golubeva et al. [57]; Andreicheva et al. [58]; for Siberia, by Khotinskii [59].
Tree cuts were sampled according to Madany et al. [60]. On each site, tree cores were taken from living trees, and tree cuts were collected from living pine trunks and dead wood. Preparation of the wood samples (cuts and cores) for the dating of fires was carried out according to Fritts [61] and Grissino-Mayer [62]. The calendar year of each ring and fire scar was determined by means of the TSAPW in the software environment with cross-dating [63].
3. Results
3.1. Age Models and Chronologies
For each pit, the chronology was based on 2–7 radiocarbon measurements taken from bulk peat sediments. The use of this panel of material for 14C dating has been realized in many studies in boreal regions [64,65,66]. We performed 22 radiocarbon dating tests (Table 1). Three dates were excluded from the analysis because the resulting age was questionable (sampling error, analysis error). A detailed description of the core sediment facies can be found in Dymov et al. [67].

Table 1.
Radiocarbon dating of peat.
Recent fires were dated by the results of the dendrochronological analysis (Table 2). Then, the upper charcoal horizons were signed with the same date. The dates of these fires were also used for the calibration of 14C age.

Table 2.
Dendrochronological dating of peat (last fires).
3.2. Macrofossil Analysis
An analysis of peat botanical composition at site 0 allowed us to distinguish three stages in the development of this bog (Figure 3).
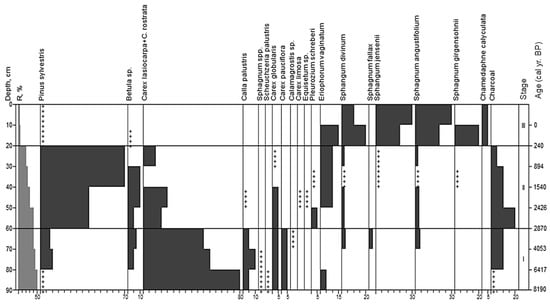
Figure 3.
Macrofossil diagram for site 0. Stages correspond to paleo-communities: (I)—Carex lasiocarpa + C. rostrata; (II)—Pinus sylvestris+Betula sp.-herbs; (III)—Ericales-Eriophorum vaginatum-Sphagnum divinum + S. angustifolium; +—abundance less than 5%.
Stage I; 90–60 cm; R 35–50%; sedge peat; age from 8190 to 2870 yr BP.
The early assemblages dated to ca. 8200 yr BP. The overall composition of peat was dominated by strongly decomposed herbaceous plant residues (65–100%), with the highest value in the lower horizon. The share of sedge residues (Carex rostrata Stokes, C. lasiocarpa Ehrh., C. globularis, C. pauciflora Lightf.) reached 60–90%. The proportion of Eriophorum vaginatum, Equisetum sp. and Calla palustris L. was about 10–15%; Calamagrostis sp. and Scheuchzeria palustris L. were found with single occurrences. The share of wood (Pinus sylvestris and Betula pubescens) was 15%, and that of sphagnum mosses was 10%. Peat samples contained burned particles of bark and wood, the share of which was about 10%.
Stage II; 60–20 cm; wood peat; R 20–40%; age from 2870 to 240 yr BP.
The share of wood was 45–70%. In the wood residues, the share of pine bark and wood reached 70%, and about 10% of the samples were birch bark. In addition, 10–20% of peat was formed by burned wood. Compared to Stage I, the share of herbs was low and reached 20%. The share of sedge residues (Carex rostrata, C. lasiocarpa, C. globularis, C. limosa L.) was 10–25%. Eriophorum vaginatum remained, which disappeared after ca. 6400 yr BP (80 cm) and appeared again around 2400 yr BP (50 cm). The share of Eriophorum vaginatum was 10%. Equisetum sp. and Calla palustris emerged with single occurrences. The share of mosses in the total peat composition was from 1 to 10%: Sphagnum angustifolium, S. divinum, S. jensenii H.Lindb., Pleurozium schreberi.
Stage III; 0–20 cm; upper peat; low decomposed (R 5%); sphagnous; age from 240 yr BP to the present.
At ca. 240 yr BP (20 cm), the plant assemblage changed and was dominated by spore plants. Sphagnum mosses’ remains were 60–80%. The share of Sphagnum angustifolium was 20–30%, S. divinum—10% and S. jensenii–30%. The share of herbaceous plants (Eriophorum vaginatum) was 20–25%, wood residues comprised 5% and were formed by pine, birch and Chamaedaphne calyculata.
Based on the analysis of peat botanical composition at site 1, we distinguished four stages in the development of this bog (Figure 4).
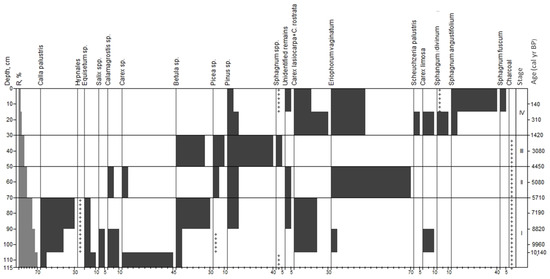
Figure 4.
Macrofossil diagram for site 1. Stages correspond to paleo-communities: (I)—organic-mineral residue; (II)—Pinus sylvestris-Eriophorum vaginatum; (III)—Pinus sylvestris + Betula sp.-Sphagnum spp.; (IV)—Pinus sylvestris-Eriophorum vaginatum + Carex spp.-Sphagnum angustifolium; +—abundance less than 5%.
Stage I; 115–70 cm; organic-mineral residue; age from 10,140 to 5710 yr BP. Herbaceous plants were dominant: Carex lasiocarpa, C. rostrata and C. limosa. Their total share was 20–45%. The proportion of Equisetum sp. was 10%, Calla palustris was 5–20% and Calamagrostis sp. was 10%. In the wood residues, we found bark of Betula (30%), Pinus (10%), Salix (5%) and single particles of Picea (less than 1%). The peat contained rare residues of mosses. There were small quantities of charcoal throughout the layer.
Stage II; 70–50 cm; eriophorum (cotton grass) peat; age from 5710 to 4450 yr BP. In the overall composition of peat, the remains of herbaceous plants (80%) were significantly more prevalent than woody plants (20%). The main peat-former was Eriophorum vaginatum with a share of 70%. The share of remains of Carex rostrata and C. lasiocarpa was 5%, and Calamagrostis sp was less than 1%. Among wood residues, spruce bark prevailed: Pinus sylvestris—10% and Picea sp.—5%. Residues of spore plants were rare and presented by hypnum mosses. There were charcoal particles in the peat.
Stage III; 50–30 cm; wood transitional peat; age from 4450 to 1420 yr BP. The share of wood residues was 75%: bark of Pinus sylvestris—40%, Betula sp.—25% and Picea sp.—10%. Herbaceous residues were presented by Carex rostrata and Eriophorum vaginatum with a total share of 20%. The share of spore plants was low (5%, Sphagnum sp.). There were small amounts of charcoal particles in the peat.
Stage IV; 30–0 cm; transitional peat; age from 1420 to yr BP to the present. Peat botanical composition was dominated by herbaceous (60%) and spore (30%) plant residues. The share of wood residues was 5–10%. Mesotrophic sedges Carex lasiocarpa, C. rostrata, C. limosa (40%) and Eriophorum vaginatum (30%) dominated in herbaceous residues. The share of Scheuchzeria palustris was about 5%. Spore plants were presented by sphagnum mosses, mainly Sphagnum angustifolium (40%), S. divinum (10%) and S. fuscum (5%). In addition, at a 10–15 cm depth, there was a pyrogenic horizon with a thickness of up to 5 cm.
Based on the analysis of peat botanical composition at site 2, we distinguished four stages in the development of this site (Figure 5).
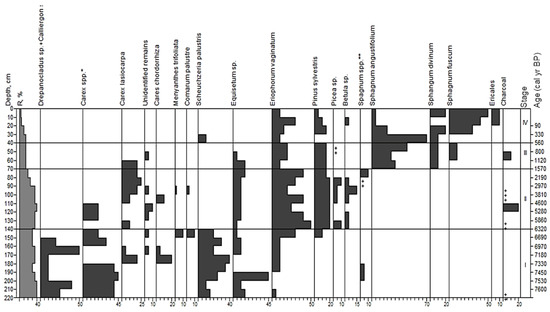
Figure 5.
Macrofossil diagram for site 2. Stages correspond to paleo-communities: (I)—Equisetum fluviatile+Scheuchzeria palustris+Carex spp.-Drepanocladus+Calliergon; (II)—Pinus sylvestris-Carex spp.+Eriophorum vaginatum; (III)—Pinus sylvestris-Eriophorum vaginatum-Sphagnum angustifolium; (IV)—Pinus sylvestris-Eriophorum vaginatum-Sphagnum fuscum. *—Carex lasiocarpa+C. cespitosa+C. limosa/paupercula; **—Sphagnum fuscum + S. divinum + S. angustifolium; +—abundance less than 5%.
Stage I; 220–140 cm; herbaceous lowland peat; age from 7600 to 6320 yr BP. In the overall composition of peat, the remains of herbaceous plants (50–90%) were prevalent. The share of mosses could reach 50% in some samples. Among herbaceous residues, hydrophilous sedges were common (Carex cespitosa L., C. lasiocarpa, C. limosa/C. paupercula Michx.—45%), as well as Scheuchzeria palustris (40%). Other plant species found in the peat were Eriophorum sp. (about 10%), Equisetum fluviatile L. (about 10–15%), Menyanthes trifoliata L. (10%) and Comarum palustre (10%). Among spore plants, the main dominants were Drepanocladus/Warnstorfia, Calliergon (average 25%) and Sphagnum teres (Schimp.) Ångström (5%). The peat samples contained sporadic amounts of charcoal.
Stage II; 140–70 cm; wood-herbaceous transitional peat; age from 6320 to 1570 yr BP. The remains of herbaceous plants (60%) dominated the overall composition of peat. The share of wood residues was 30%. Spore plants were 5% (45% in several samples). Wood residues contained Pinus sylvestris (20%), Betula sp. and Picea sp (5–10%). Among the herbaceous residues, Eriophorum vaginatum dominated (25–50%). The share of sedges was about 25%: Carex lasiocarpa, Carex cespitosa, Carex chordorrhiza L.f. and C. pauciflora. The share of Equisetum fluviatile was about 5%. There were small quantities of charcoal and burned bark in the peat.
Stage III; 70–40 cm; pine-sphagnous upper (bog) peat; age from 1570 to 800 yr BP. The remains of spore plants (>50%) dominated the overall composition of peat. Herbaceous and wood residues were present in roughly equal shares (20%). Among the wood, the remains of Pinus sylvestris dominated. Herbaceous remains contained Eriophorum vaginatum (20%). Carex lasiocarpa and Equisetum sp. were also present in the peat, but disappeared by the end of this stage (50–40 cm). In this stage, sphagnum mosses also appeared. They were presented by Sphagnum angustifolium (20–70%), S. fuscum (10%) and S. divinum (100%).
Stage IV; 30–0 cm; pine-sphagnous upper peat; age from 560 to the present yr BP.
The remains of spore plants (90%) dominated the overall composition of peat. Herbaceous and wood residues were present in roughly equal shares (up to 15–25%). Among the wood, the remains of Pinus sylvestris dominated (15%). We found small amounts of Betula sp. and mire shrubs (10%), and single occurrences of Picea sp. and Pinus sibirica (<1%). Herbaceous remains (30%) contained only Eriophorum vaginatum (10–25), and at a depth of 30–40 cm, small amounts of Scheuchzeria palustris (<5%). Among spore plants, Sphagnum angustifolium dominated (70%) at 70–40 cm, and then this species was replaced by oligotrophic Sphagnum fuscum (10–50%). Furthermore, there were small quantities of S. divinum (5–10%) in the peat.
We distinguished three age stages in the deposit at site 3 (Figure 6).
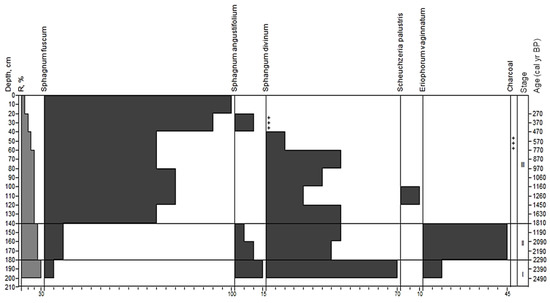
Figure 6.
Macrofossil diagram for site 3. Stages correspond to paleo-communities: (I)—herbs-Sphagnum divinum+S. angustifolium; (II)—Eriophorum vaginatum-Sphagnum divinum; (III)—Ericales-Sphagnum fuscum+ Sphagnum divinum; +—abundance less than 5%.
Stage I.; ?–180 cm; transitional sphagnous peat; age from before 2500 to 2290 yr BP. Residuals of sphagnum mosses with 90% share were prevalent in the overall peat botanical composition. Sphagnum divinum dominated; the share of S. angustifolium was 15%, and S. fuscum was 5%. The share of herbaceous Eriophorum vaginatum was 10%.
Stage II; 180–140 cm; transitional eriophorum-sphagnous peat; age from 2290 to 800 yr BP. In the total peat composition, there were approximately equal amounts of spore and herbaceous plant residues (55% and 45%). The main peat-formers were Eriophorum vaginatum (45%) and Sphagnum divinum (40%). We also noted remnants of Sphagnum angustifolium and S. fuscum.
Stage III; 140–0 cm; upper fuscum peat; age from 800 yr BP to the present. The peat was mostly formed by remnants of sphagnum mosses (90–100%), with a clear prevalence of Sphagnum fuscum (55–100%). In some samples, the share of S. divinum was essential (up to 40%). The upper horizons (60–10 cm) contained small amounts of S. angustifolium. In addition to mosses, the peat revealed only the remnants of Scheuchzeria palustris (10%). There were small quantities of charcoal at the depth of 40–60 cm.
The deposit at site 4 had three age development stages (Figure 7).
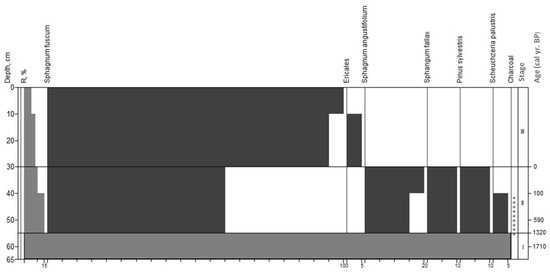
Figure 7.
Macrofossil diagram for site 4. Stages correspond to paleo-communities: (I)—pyrogenic horizon; (II)—Pinus sylvestris-Scheuchzeria palustris-Sphagnum angustifolium; (III)—Ericales-Sphagnum fuscum; +—abundance less than 5%.
Stage I; 65–55 cm; pyrogenic horizon; age 1710 to 1320 yr BP. The horizon (Pyr) was dark-grey colored and had a high level of homogeneity. Plant remains in the samples have lost their anatomical structure.
Stage II; 55–40 cm; sphagnous peat, age from 1320 to 0 yr BP. The peat was mostly formed by remnants of spore plants with a prevalence of Sphagnum fuscum (about 60%). The share of S. angustifolium was 15% and S. fallax H.Klinggr. was 10%. Wood remnants contained the bark (including burned bark) of Pinus sylvestris (10%). The herbaceous peat fraction was formed by the remnants of Scheuchzeria palustris (5%).
Stage III; depth 30–0 cm; sphagnous (fuscum) peat; 0 yr BP to the present. In total, 95–100% of the peat consisted of slightly decomposed Sphagnum fuscum. Roots and stems of ericoid shrubs (Chamaedaphne calyculata, Ledum palustre, Andromeda polifolia L. and Vaccinium uliginosum) formed about 5% of the peat.
Based on the analysis of peat botanical composition at site 5, we distinguished four stages in the development of this site (Figure 8).
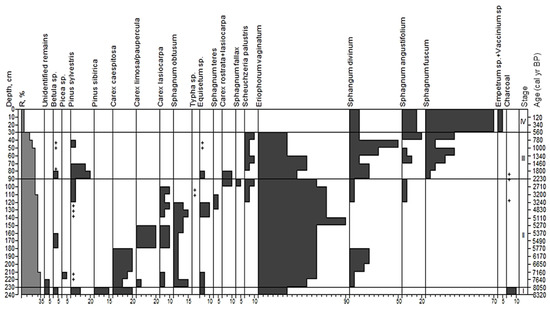
Figure 8.
Macrofossil diagram for site 5. Stages correspond to paleo-communities: (I)—Pinus+Betula-herbaceous-Sphagnum; (II)—herbaceous+Eriophorum vaginatum-Sphagnum spp.; (III)—Pinus sylvestris-Eriophorum vaginatum-Sphagnum spp.; (IV)—Ericales-Sphagnum fuscum; +—abundance less than 5%.
Stage I; 240–230 cm; pyrogenic horizon; type—transitional wooded-eriophorum peat; age from 8320 to 8050 yr BP. In the overall composition of peat, the remains of herbaceous plants were prevalent (55%). The share of wood remains was 30%. Wood peat contained the bark of Pinus sylvestris (10%), P. sibirica (20%) and Betula sp. (5%). The herbaceous fraction was dominated by Eriophorum vaginatum (30%) and Carex cespitosa (20%). The share of Equisetum fluviatile was about 5%. Other remnants (5%) have lost their anatomical structure. The samples contained burned remnants of bark and the wood of pine, birch and spruce. The share of charcoals in the sample was 10%.
Stage II; 230–90 cm; transitional eriophorum peat; age from 8050 to 2230 yr BP. In the overall composition of peat, the remnants of herbaceous plants (70–90%) were prevalent, the share of wood was 5%, and spore plants, 30%. The wood peat fraction contained the bark of Betula, Pinus and Picea. Eriophorum vaginatum was the main peat-former with the share ranging from 50 to 90%. The herbaceous fraction also contained the remains of mesotrophic sedges: Carex cespitosa, Carex lasiocarpa, C. rostrata and C. limosa, with their shares ranging from 10 to 30%. The share of Equisetum fluviatile was 5–10%, and Scheuchzeria palustris, 5%. We found a single occurrence of Typha sp. (<1%). Spore plants were presented by sphagnum mosses. In the lower horizons, hydrophilous mosses were prevalent: Sphagnum obtusum and S. teres. In the upper part of the deposit (higher than 110 cm), mesophilous Sphagnum angustifolium and S. fallax occurred. Remnants of S. divinum occurred throughout the entire peat deposit with the share of up to 15%.
Stage III; 90–30 cm; eriophorum-sphagnous bog peat (R 10–25%); age from 2230 to 560 yr BP. Herbaceous and spore remnants were prevalent in the peat samples (30–60%). The share of wood remnants was up to 25%. Wood residuals were mainly the bark of pine (Pinus sylvestris, up to 20%), and single remnants of birch Betula pubescens. Within the herbaceous fraction, Eriophorum vaginatum prevailed (30–50%); the share of Scheuchzeria palustris was about 10%, and Equisetum fluviatile emerged with single occurrences. Spore plants were oligomesotrophic and oligotrophic sphagnum mosses: Sphagnum angustifolium, S divinum and S. fuscum, the share of each varied from 10 to 30%. In general, the peat demonstrated species impoverishment, and mesotrophic plants (Picea, Carex lasiocarpa, C. cespitosa, Sphagnum obtusum, S. teres) were substituted by oligotrophic ones (such as Sphagnum fuscum). Charcoals occurred at depths of 110–120, 80–90 cm.
Stage IV; 30–0 cm; low decomposed sphagnous bog peat (R 5–10%); age from 560 to the present yr BP. In total, 95% of the peat consisted of sphagnum mosses (Sphagnum fuscum—70%, S. angustifolium—15% and S. divinum—10%). The other part was the roots and stems of mire shrubs (Ledum palustre, Andromeda polifolia and Chamaedaphne calyculata).
4. Discussion
4.1. Post-Glacial Pine Wooded Bogs’ Initiation and Development
In general, the main period of the birth and development of the mires in the circum-arctic regions in the Northern Hemisphere occurred/formed between 12,000 and 8000 yr BP [1,6,68,69,70,71,72,73,74] The formation of mires is linked to global climate warming at the turn of the Late Pleistocene and Holocene [56,59,75,76,77,78,79,80]. At the beginning of the Mid Holocene, there was a change in climatic conditions towards greater humidity with simultaneous warming, which created favorable conditions for intensive water-logging and peat accumulation.
All the studied bogs have dryland swamping/paludification type, but the initial stages of formation took place under different geomorphologic and climatic conditions, which influenced the further course of their development. The data showed three periods in the frequency of peat initiation: one during the Early Holocene, the second at the boundary of the Early and Late Holocene and the third in the Late Holocene.
At all sites, except site 4, the initial stage of bog development occurred during the period after 10,140–8000 yr BP (Figure 9 and Figure 10). During the Mid Holocene, an active mire formation took place in many regions of European Russia and Western Siberia [68]. For instance, about 40% of Karelian bogs formed during this period [81]. During the period 8800–7600 yr BP, the climate was warm and wet, which contributed to the accumulation of the main peat amounts in the bogs [59]. One of the study bogs (Figure 8, site 4) showed a later origin, around 2000 yr BP. In this case, the age of the basal peat horizons indicates the time when the bog system started to recover after a fire, which destroyed the initial plant community [6].
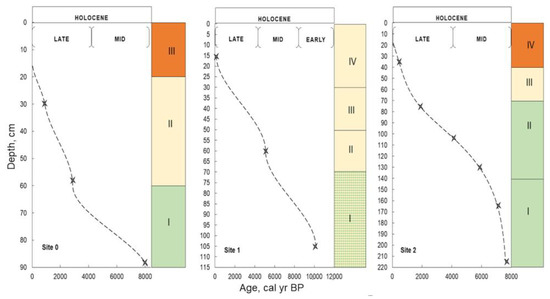
Figure 9.
Peat accumulation rate reconstructed from radiocarbon dates within different stages of the Holocene (KOM). The color scale shows the stages of bogs’ genesis: ombrotrophic (orange), transitional (yellow), minerotrophic (green). I-IV correspond to the paleo-communities from Figure 3, Figure 4 and Figure 5. Stars represent peat sampling depths.
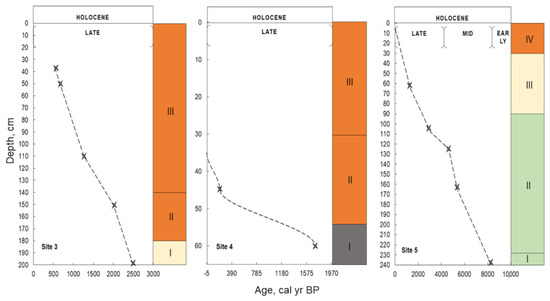
Figure 10.
Peat accumulation rate reconstructed from radiocarbon dates within different stages of the Holocene (KRA). The color scale shows the stages of bogs’ genesis: ombrotrophic (orange), transitional (yellow), minerotrophic (green), pyrogenic horizon (gray). I-IV correspond to the paleo-communities from Figure 6, Figure 7 and Figure 8. Stars represent peat sampling depths.
An analysis of the peat botanical composition revealed that the development of the bogs under study has taken different ways. Some bogs originated from the post-glacial water bodies, while others resulted from forest community water-logging. At sites 1, 2 and probably 3, the formation of bogs began with the overgrowth of residual post-glacial water bodies with hydrophilic plants, as indicated by the presence of sedges, horsetails, Scheuchzeria palustris, Calla palustris, Menyanthes trifoliata and hypnum mosses in the botanical composition of the peat (Figure 4, Figure 5 and Figure 6). This stage of mire formation after the shallowing of post-glacial water bodies is consistent with other records from Europe, Russia and Northern America [71,73,82,83,84]. Most of the Russian Plain’s raised bogs were formed in this way [38].
Species’ composition of paleo-communities at sites 1 and 2, despite the presence of small amounts of macrocarbons in most basal peat samples, indicates that, at the initial stages of mire formation, fires did not affect the vegetation cover of these sites due to their being water-logged and an absence of trees. This is additionally evidenced by the presence of hydrophilic species (e.g., Equisetum, Scheuchzeria, Drepanocladus, etc.) and the absence of forest species and burned waste. However, low-intensity fires may have occurred in some periods. For instance, at site 2 the charcoals were possibly brought in from adjacent areas, which burned regularly.
At sites 0 and 5, the formation of peat deposit began with the forest transformation following swamping, which was directly linked to the influence of the pyrogenic factor (Figure 3 and Figure 8). The warming of the Early Holocene led to an increase in fires, and then the warm and humid climate of the Mid Holocene intensified bog formation [56].
The initial stage of bog formation at site 0 dates from the end of the Early Holocene (about 8200 yr BP; Figure 8). The occurrence of mesophilic forest species (Pinus sylvestris, Carex globularis, etc.) in the bottom peat indicates the presence of a forest community at the early stages. This paleo-forest was transformed by pyrogenic effects, as evidenced by the presence of burned remains of pine and birch in the peat and the underlying horizon.
Peat accumulation at site 5 started also at the end of the Early Holocene (about 8300 yr BP). The share of woody remnants in the bottom peat is up to 30%, which confirms the presence of a well-developed tree layer at the initial stages of the bog development (Figure 8, stage I). At the beginning of the Mid Holocene, there was a large fire or several fires at the site. As a result, the vegetation cover was damaged, as evidenced by the charcoal occurrence in the peat (10%), as well as the burned remnants of bark and the wood of pine, birch and spruce. This fire probably accelerated the processes of peat accumulation, which resulted in the formation of water-logged wood-grass and/or wood-large-grass post-pyrogenic paleo-communities. In general, studies of post-fire paleo-successions [85,86,87] indicate a temporary interruption in peat accumulation after fires and reforestation. However, in some cases, post-fire mineralization and humification of peat can lead to a significant increase in the mineral content of the peat, which then leads to an increase in community productivity. For example, an increase in the rate of peat accumulation after a fire, due to an increase in the productivity of post-pyrogenic communities, was observed in one of the bogs of the Tomsk Region [88]. The presence of autochthonous charcoal in the bottom layers supports the idea of some authors that in some cases water-logging is caused by forest fires [89,90].
4.2. The Bogs’ Development and Fire Activity
At the beginning of the Mid Holocene period, vegetation at site 0 (Figure 3 and Figure 9) was dominated by mire-patched communities with a predominance of mesotrophic sedges (Carex rostrata and C. lasiocarpa) and an open tree layer. Such communities existed for about 5000 years during the entire warm and humid Mid Holocene and most of the colder Late Holocene. The following stage was about 3000 yr BP. Strengthened natural drainage of the top layer of peat due to the growth of peat deposition with the subsequent warming of the Mid Late Holocene (Medieval Warm Period) climate led to the afforestation of the mire [59,91,92]. Wetland grass vegetation was replaced by woodland mire vegetation. The share of mire species Carex rostrata, C. lasiocarpa, Eriophorum vaginatum, Sphagnum angustifolium and S. divinum decreased. The share of forest species (Carex globularis, Sphagnum girgensohnii and Pleurozium schreberi) increased. The tree layer also emerged. As a result, a meso-oligotrophic pine wooded grass-sphagnous mire gradually formed. Most peat samples contain clearly recognizable burned bark and wood fragments of pine and birch. In some samples, their proportion is up to 20%. So, at stages I and II, fires were some of the most important determinants of vegetation development and succession at this site. Intermittent fires delayed oligotrophication, releasing mineral elements from burnt organic matter and increasing the mineralization of peat and the nutrient content of associated bog water [93,94,95], and, together with a warmer climate, promoted reforestation. At site 1 (Figure 4 and Figure 8), a swampy birch forest with a polydominant herbaceous eutrophic cover formed in the Early Holocene (about 9000 yr BP). In modern vegetation, such plant communities are widespread in southern taiga, where they establish wet habitats and are confined to relief depressions and floodplains [96]. This forest-mire stage lasted over 3000 years during the Early and Middle Holocene. Throughout this stage, the area was regularly exposed to wildfires and small charcoals and burned tree bark remains were consistently found in the peat. The transition to the next stage (Figure 4, II) was around 6000 yr BP at the end of the Atlantic period. Forest-mire vegetation was substituted by bog plant communities. The share of woody plants reduced rapidly, and mesotrophic communities with a predominance of Eriophorum vaginatum became prevalent at the site. In addition to the change in the hydrological regime towards a lower mire water level and less flowage, fires might be the reason for these vegetation changes and the subsequent long-term existence of eriophorum communities. We know that the frequency of fires increased as a result of the late Atlantic warming in the south of the European northeast, when mean annual temperatures were 2–3 °C higher than today [57]. Eriophorum vaginatum is one of the first species to invade extensively burnt-out areas of bogs, including water-logged sites. Several recent studies confirmed the increase in Eriophorum occurrence in the post-pyrogenic plant communities [97,98]. The additional ash elements supplied by the fires, against the background of the Mid Holocene warming (about 4500 yr BP), contributed to the re-activation of forest formation and the establishment of transitional tree and sedge communities (Figure 4, III). Over time, the amount of nutrients in the upper peat layers decreased, and more oligotrophic vegetation gradually formed. This process is consistent with the generally accepted pattern of bog development in the taiga zone [68,83,99] from eutrophic to oligotrophic mires. At stage IV, a pine-herb community was substituted by a sedge-eriophorum-sphagnous plant community with an open pine tree layer. Complex vegetation occurred during this period (Figure 4).
At site 2 (Figure 4 and Figure 8), at the end of the Mid Holocene, pine and birch wooded meso-eutrophic herbaceous plant communities became dominant. A high decomposition degree of plant peat-formers and visible and detectable pyrogenic layers indicate a significant influence of fires on the bog history. At this site, we found the highest fire frequency in the period 6300–3000 yr BP, which corresponds to the warmest Holocene periods, when the mean annual temperature increased by 2–4 °C compared to the previous time [100]. As the peat layer accumulated, the plant species’ composition became impoverished, and the sphagnum cover formed. At this stage, the most intense pyrogenic activity was confined to the Medieval Warm Period of the Late Holocene. The share of burned bark remnants reached 10% in the peat at a depth of 50–60 cm (about 1100–800 yr BP). A further increase in the thickness of the peat deposit, combined with a cooler and more arid (dry) climate, had led to the transition of the bog to an oligotrophic (subsequent) development phase (around 800 yr BP and Little Ice Age Period).
Site 5, throughout the Mid and Late Holocene (8045–2234 yr BP), was dominated by mesotrophic paleo-communities with a predominance of eriophorum, eriophorum-sedge and eriophorum-sphagnum, which formed the main part of the peat deposit (Figure 6, stage II and Figure 10). The presence of charcoal and the remains of burnt bark, which occur in small numbers throughout the deposit, provide evidence that the bog has been periodically (regularly) exposed to a pyrogenic factor. At the same time, the high water content of the bog, as indicated by the abundance of hydrophilous plants (e.x. Equisetum sp., Sphagnum obtusum, Carex spp., Scheuchzeria palustris), prevented the peat layer from burning out completely. The high degree of conservation of the peat-forming plant remnants also indirectly confirmed the absence of intensive fires in site 5. According to our data, the strongest fires date from the beginning of the Late Holocene (about 4000 yr BP). In the end of the Late Holocene (about 2200 yr BP), the mire switched to an oligotrophic stage of development. The participation of mesotrophic species (Equisetum sp., Carex rostrata, C. lasiocarpa, Betula sp.) gradually decreased and formed dwarf shrubs-sphagnum communities, based on plants typical of raised (oligitrophic) bogs such as Vaccinium spp., Empetrum sp. and Sphanum fuscum et al. (Figure 6, stages III–IV).
In general, in the study areas confined to enclosed (drainless) small depressions, the impact of fires on the mire vegetation has been regular throughout their history. Due to the regular fires, substrate trophicity, which usually tends to decrease during mire development [83], remained more or less constant. This fact contributed to the maintenance of a clear tree layer of pine, which is well adapted to post-pyrogenic demutations [101].
The development of the bog at site 3 also passed through several stages (Figure 5 and Figure 9), the initial ones of which we were unfortunately unable to sample. From the depth of 200 cm, which corresponds to the Late Holocene (2500 yr BP), the bog had switched to atmospheric nutrition; by this time, oligotrophic sphagnum-fossil paleo-communities had already formed with the predominance of Sphagnum divinum and S. angustifolium, which formed the main part of the peat deposit (180–0 cm). Then, about 1500 yr BP, dwarf shrubs and Sphagnum fuscum became dominants and the formation of a ridge-hollow complex began. The high content of charcoals’ macroparticles in the lower peat layers and absence of visible pyrogenic layers and burned remnants in the upper part of the deposit indicate that fires may have affected the vegetation development only at the early stages. Then, the site became part of a major flooding system, and its further development was influenced by it. Fires, even during the warmest periods (e.g., warming of the Medieval Warm Period), did not directly affect its vegetation cover or peat deposit.
4.3. The Impact of Pyrogenic Factor on the Bog Plant Communities during the Recent (Oligotrophic) Stage of Their Development
The studied bogs assumed their modern appearance in the period from 2200 to 300 yr BP. This period corresponds to the Late Holocene [1,68,69,71,72,74,79,102]. During this time, a slight cooling and an increase in precipitation intensified bog formation and peat accumulation in all mire biomes. Growing bogs began to merge into huge swampy areas covering hundreds of square kilometers [75]. At the present stage of development, the studied bog communities can be divided into those that are regularly affected by pyrogenic factors (sites 0, 2, 4 and 5) and those where the impact of fires on the vegetation is minimal (sites 1 and 3).
So, at site 0, about 300 yr BP until the present, water-logging processes intensified and led to further change in the dominant vegetation cover (Figure 3, stage III). The share of trees and sedges decreased, the share of mosses gradually increased and then a continuous sphagnum cover was formed and heather shrubs appeared. At this stage, fires were an important factor affecting vegetation. Furthermore, an increase in pyrogenic inclusions in the peat at a depth of 16 cm corresponds to this suggestion. At site 0, we also observed living trees with traces of at least two fires. According to Gorbach et al. [103], these fires were dated to the years 1918 and 1874.
At site 2, the growth of peat deposit in view of climate cooling and aridization led to the transition of the bog to an oligotrophic development phase (around 800 yr BP). Mesotrophic pine-herb-sphagnous paleo-communities (Figure 5, stage III) were substituted by pine-dwarf shrub-sphagnous ones. The main dominants of the peat deposit were oligotrophic species Pinus sylvestris, Eriophorum vaginatum, Sphagnum fuscum, S. angustifolium and mire shrubs. By the end of the Holocene (about 400 yr BP), the mire in site 2 had acquired its modern characteristics. The main part of the vegetation was species-poor polydominant pine-dwarf shrub-sphagnum communities. At this study site, during the oligotrophic stage of mire development, the frequency and intensity of fires were low due to a humid climate and temperatures that were 1–3 °C lower than today’s [57]. The low fire impact on site 2 is also confirmed by the absence of visible pyrogenic particles in the upper peat and litter. At the same time, the living trees of Pinus sylvestris around 200 years old show signs of at least two fires.
At site 5, the mire switched to the oligotrophic stage (Figure 8, III–IV) in the Mid of the Late Holocene (about 2200 yr BP). The succession was gradual, with no dramatic changes in moisture levels. Mesotrophic plant species dropped out from the plant communities (Carex lasiocarpa, C. cespitosa, Sphagnum obtusum Warnst., S. teres, etc.) and were substituted by oligotrophic Scheuchzeria palustris, Sphagnum fuscum and S. angustifolium. Bog dwarf shrubs (Ledum palustre, Andromeda polifolia, Chamaedaphne calyculata, etc.) established the plant communities at the final stage of the mire development. As a result, an oligotrophic dwarf shrub-sphagnum community formed, the structure and species composition of which were similar to the modern vegetation cover of this study site. In the second half of the Late Holocene (about the last 2500 years), the impact of fires on the mire was regular. According to Karpenko and Prokushkin [104], the inter-fire interval in the bogs in the study area between 2915 ± 150 and 1430 ± 120 did not exceed 300 years. Recently, there has been a reduction in this interval. Fire scars on live pines indicate that there were at least two fires on the bog in the last 200 years, dating back to 1940 and 1986. It is likely that these fires were not intense, with only the moss and dwarf shrub-herb cover being affected. In addition, there was a highland fire in the surrounding area in 2018, but it did not affect the bog, i.e., even small, wooded bogs burn only in the driest periods and prevent fires from spreading.
Wildfires are a natural factor for these bogs throughout their development. The highest frequency of fires corresponded to the warmest Holocene periods: one after the Last Glacial Maximum (LGM) and two during the Mid to Late Holocene [105]. During these periods, an increase in fire activity was noted in most of the bogs studied. We, like many authors [28,71,73,84,106,107], attribute the increase in fire frequency over the last few hundred years to an increase in anthropogenic influence on natural ecosystems, as well as to a general global increase in temperature [108].
At site 4, vegetation development is closely related to fire activity. Moreover, the pyrogenic factor was crucial in its genesis. The forest and bog phases alternated throughout the bog history. During periods of high fire activity, the vegetation burned out and succession began anew. At the base of the peat deposit, a 10 cm pyrogenic layer is well defined; its age is dated as 1710 yr BP and occurred in the warmest period of the Late Holocene (Figure 7, stage I). It is likely that in this area a major fire or series of fires completely destroyed the previous vegetation and burned out the layer of organic matter down to the mineral horizon. Then, a secondary shallow water body occurred, overgrowing with hydrophilous grasses (Scheuchzeria palustris), sphagnum mosses (Sphagnum fallax, Figure 7, stage II) and then with more xerophitic species (Sphagnum angustifolium, S. fuscum, and Pinus sylvestris). Around 600 yr BP, an oligotrophic shrub-sphagnum community with an open tree layer of Pinus sylvestris emerged (Figure 7, stage III). The dynamics of the modern vegetation cover is also linked to fires. There were at least two fires during the last 100 years: in 1959 and 1938. We suggest this site is not a ”constant“ mire, because the mire stage is temporary as the forest one. Changes in vegetation occur dramatically, under the influence of an external factor, and the interval between successions may differ. According to the literature [80,83,97], such ecosystems are widespread. There is a periodic pattern of water-logging: during wet periods, shallow depressions are covered by mire sedge vegetation, and when the surface water table drops, the process of mire formation slows down or is stopped, and organics often burn out [92].
At site 1, about 1300 yr BP, we found a disappearance of birch and spruce residues in the peat, and an increase in residues of Carex lasiocarpa, C. rostrata, Scheuchzeria palustris, Sphagnum angustifolium and Sphagnum fuscum (Figure 3). This fact indicates an increase in water-logging processes and the formation of mosaic/complex plant cover, where mesotrophic sedge-sphagnum communities established microrelief depressions, and dwarf shrub-sphagnous communities with Sphagnum fuscum occupied hummocks. Overall, for site 1, there has been a decline in the influence of fire on bog development over the last 4000 years. This trend has continued into the modern time. At the same time, local fire events still occur. For example, according to dendrochronological data, there was a low-intensity top fire in the bog about 140 yr BP. We also found pyrogenic inclusions in the peat at a depth of 10 cm. Nevertheless, the fire had little effect on the vegetation and did not interrupt the succession. An oligotrophic pine wooded dwarf-shrub sphagnous mire was gradually formed at the site.
The modern vegetation of site 3 differs from the other studied sites by the absence of a distinct tree layer and the dominance of Sphagnum fuscum in the moss cover. A dwarf shrub-sphagnum plant community formed about 1500 yr BP. At about the same time, the microrelief became fragmented and a water-logged ridge-hollow complex formed. During this period of mire development, which began in the Mid Holocene and continues to the present day, fires had no direct effect on the vegetation cover due to its high water content. The small amounts of macrocharcoals in the peat were probably brought from the forest areas surrounding the bog, which according to Dymov et al. [67] have been regularly exposed to fire.
Overall, the analysis of the composition of the peat deposits at sites 1 and 3 indicates relatively constant environmental conditions during the development of bog ecosystems, and the absence of strong (irreversible) pyrogenic effects on the plant communities. At the same time, we know that the forests adjacent to these bogs have regularly burned and continue burning [67,109,110]. It is likely that the reduced historical pyrogenic impact on sites 1 and 3 compared to sites 0, 2 and 5 is due to their topographic positions. Both sites (1 and 3) are located on the periphery of larger bog systems, which led to their merging and increased watering during subsequent stages of the bog genesis, preventing fires and the formation of a closed pine tree layer. In other words, those areas that become part of larger mire systems (as a result of their merger with other mires) cease to be significantly affected by external factors, e.g., fires. Furthermore, only global changes in the water–thermal balance can affect the reversibility of this process.
5. Conclusions
Based on macrofossil studies of the organic-mineral soil layer and peat, dendrological and radiocarbon dating, we performed the reconstruction of the probable development of six pine wooded sphagnum bogs in the Komi Republic and Krasnoyarsk Region of Russia. All bogs, except site 4, followed the patterns of endogenesis, and the vegetation change was mainly continual and gradual, tending to increase the peat layer, decrease substrate trophicity and form shrub-sphagnous vegetation. However, the pyrogenic layers and charcoals in the peat indicate that the bogs were constantly affected by fires, which periodically interrupted the endogenous development of the study mires, influencing the vegetation cover and causing its shifts. This impact was expressed in the destruction of vegetation and peat deposits, leading to pyrogenic eutrophication and the formation of post-pyrogenic plant communities. The highest frequency of fires corresponded to the warmest Holocene periods. During these periods, an increase in fire activity was noted in most of the bogs studied. However, for some large and water-logged bogs, the modern impact of the pyrogenic factor has decreased compared to the earliest stages of their development. At present, they prevent the spread of fires by performing a protective function, being natural water-logged barriers.
Thus, modern pine wooded shrub-sphagnum bogs with similar species composition and vegetation structure have a different genesis in terms of fire impact. At the same time, geographically remote areas located in similar geomorphologic conditions have many similarities in their development patterns. In the development of pine wooded shrub-sphagnous bogs that occupy small, enclosed and drainless depressions, fires are important and often interrupt the endogenous course of the bog development.
Author Contributions
Conceptualization, N.G. and Y.A.D.; Methodology, N.G., M.M. and I.N.K.; Software, M.M.; Validation, N.G., Y.A.D. and M.M.; Formal Analysis, N.G., M.M. and A.D.; Investigation, N.G.; Resources, A.D.; Data Curation, A.D.; Writing—Original Draft Preparation, N.G. and Y.A.D.; Writing—Review and Editing, N.G., Y.A.D. and M.M.; Visualization, N.G. and M.M.; Supervision, A.D.; Project Administration, A.D.; Funding Acquisition, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Foundation for Basic Research (RFBR) under Grant No. 19-29-05111 mk and budgetary theme of IB FRC Komi SC UB RAS–122040100031-8, 122040600026-9.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data were obtained from herbaria, field surveys and the literature and are available from the first author on reasonable request.
Acknowledgments
We are grateful to Cand. Sci (Biol.) A.S. Prokushkin for his help in the work arrangement, and Cand. Sci (Agric.) E.V. Zhangurov and Cand. Sci (Biol.) V.V Startsev for assistance in the fieldwork. We also thank Cand. Sci (Biol.) L.V. Karpenko for the macrofossil analysis of peat.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, Z.; Loisel, J.; Brosseau, D.P.; Beilman, D.W.; Hunt, S.J. Global peatland dynamics since the last glacial maximum. Geophys. Res. Lett. 2010, 37, L13402. [Google Scholar] [CrossRef]
- Joosten, H. Peatlands, Climate Change Mitigation and Biodiversity Conservation; Nordic Council of Minister: Copenhagen, Denmark, 2015. [Google Scholar]
- Cubizolle, H.; Fassion, F.; Argant, J.; Latour-Argant, C.; Galet, P.; Oberlin, C. Mire initiation, climatic change and agricultural expansion over the course of the Late-Holocene in the Massif Central mountain range (France): Causal links and implications for mire conservation. Quat. Int. 2012, 251, 77–96. [Google Scholar] [CrossRef]
- Lavoie, M.; Pellerin, S.; Larocque, M. Examining the role of allogenous and autogenous factors in the longterm dynamics of a temperate headwater peatland (southern Quebec, Canada). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 386, 336–348. [Google Scholar] [CrossRef]
- Kur’ina, I.V.; Veretennikova, E.E. Impact of climate change of the Holocene on the development of the ridge-hollow swamp complex of Western Siberia. Proc. RAS Geogr. 2015, 2, 74–87. [Google Scholar] [CrossRef]
- Dyakonov, K.N.; Novenko, E.Y.; Mazei, N.G.; Kusilman, M.V. The age of peatlands and peatland formation stages in Polesie landscapes of the East European plain. Dokl. Earth Sci. 2020, 492, 464–470. [Google Scholar] [CrossRef]
- Wein, R.W.; MacLean, D.A. The Role of Fire in Northern Circumpolar Ecosystems; Chichester Wiley: Hoboken, NJ, USA, 1983; pp. 1–18. [Google Scholar]
- Bond, W.J.; Keeley, J.E. Fire as a global ‘herbivore’: The ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 2005, 20, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Bowman, D.M.J.S.; Balch, J.K.; Artaxo, P.; Bond, W.J.; Carlson, J.M.; Cochrane, M.A.; D’Antonio, C.M.; DeFries, R.S.; Doyle, J.C.; Harrison, S.P.; et al. Fire in the Earth system. Science 2009, 324, 481–484. [Google Scholar] [CrossRef]
- Driscoll, D.A.; Lindenmayer, D.B.; Bennett, A.F.; Bode, M.; Bradstock, R.A.; Cary, G.J.; Clarke, M.F.; Dexter, N.; Fensham, R.; Friend, G.; et al. Fire management for biodiversity conservation: Key research questions and our capacity to answer them. Biol. Conserv. 2010, 143, 1928–1939. [Google Scholar] [CrossRef]
- Kettridge, N.; Thompson, D.K.; Waddington, J.M. Impact of wildfire on the thermal behavior of northern peatlands: Observations and model simulations. J. Geophys. Res. 2012, 117, G02014. [Google Scholar] [CrossRef]
- Thompson, D.K. Wildfire Impacts on Peatland Ecohydrology. Ph.D. Thesis, School of Geography and Earth Sciences, McMaster University, Hamilton, ON, Canada, 2012. [Google Scholar]
- Pressler, Y.; Moore, J.C.; Cotrufo, M.F. Belowground community responses to fire: Meta-analysis reveals contrasting responses of soil microorganisms and mesofauna. Oikos 2019, 128, 309–327. [Google Scholar] [CrossRef]
- Doerr, S.H.; Santín, C. Global trends in wildfire and its impacts: Perceptions versus realities in a changing world. Phil. Trans. R. Soc. B 2016, 371, 20150345. [Google Scholar] [CrossRef] [PubMed]
- Kharuk, V.I.; Ponomarev, E.I.; Ivanova, G.A.; Dvinskaya, M.L.; Coogan, S.C.; Flannigan, M.D. Wildfires in the Siberian taiga. Ambio 2021, 50, 1953–1974. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.C.; Kobziar, L.N. Smoldering Combustion and Ground Fires: Ecological Effects and Multi-Scale Significance. Fire Ecol. 2013, 9, 124–132. [Google Scholar] [CrossRef]
- Parish, F.; Sirin, A.; Charman, D.; Joosten, H.; Minayeva, T.; Silvius, M.; Stringer, L. (Eds.) Assessment on Peatlands, Biodiversity and Climate Change: Main Report; Global Environment Centre: Osaka, Japan, 2008; 179p. [Google Scholar]
- Minayeva, T.; Sirin, A.A.; Stracher, G.B. The peat fires of Russia. In Coal and Peat Fires: A Global Perspective; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 2, pp. 376–394. [Google Scholar]
- Bixby, R.J.; Cooper, S.D.; Gresswell, R.E.; Brown, L.E.; Dahm, C.N.; Dwire, K.A. Fire effects on aquatic ecosystems: An assessment of the current state of the science. Freshw. Sci. 2015, 34, 1340–1350. [Google Scholar] [CrossRef]
- Joosten, H.; Sirin, A.; Couwenberg, J.; Laine, J.; Smith, P. The role of peatlands in climate regulation. In Peatland Restoration and Ecosystem Services: Science, Policy and Practice; Bonn, A., Allott, T., Evans, M., Joosten, H., Stoneman, R., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 63–76. [Google Scholar]
- Martin, D.A. At the nexus of fire, water and society. Philos. Trans. R. Soc. B 2016, 371, 20150172. [Google Scholar] [CrossRef]
- Harper, A.R.; Doerr, S.H.; Santin, C.; Froyd, C.A.; Sinnadurai, P. Prescribed fire and its impacts on ecosystem services in the UK. Sci. Total Environ. 2018, 624, 691–703. [Google Scholar] [CrossRef]
- Kiely, L.; Spracklen, D.V.; Wiedinmyer, C.; Conibear, L.A.; Reddington, C.L.; Arnold, S.R.; Knote, C.; Khan, M.F.; Latif, M.T.; Syaufina, L.; et al. Air quality and health impacts of vegetation and peat fires in Equatorial Asia during 2004–2015. Environ. Res. Lett. 2020, 15, 094054. [Google Scholar] [CrossRef]
- Flanagan, N.E.; Wang, H.; Winton, S.; Richardson, C.J. Low-severity fire as a mechanism of organic matter protection in global peatlands: Thermal alteration slows decomposition. Glob. Change Biol. 2020, 36, 3930–3946. [Google Scholar] [CrossRef] [PubMed]
- Joosten, H.; Clarke, D. Wise Use of Mires and Peatlands; International Mire Conservation Group and International Peat Society: Saarijärvi, Finland, 2002; 304p. [Google Scholar]
- Minaeva, T.Y.; Sirin, A.A. Peat fires—causes and ways of prevention. Sci. Ind. 2002, 9, 3–8. [Google Scholar]
- Sirin, A.; Minaeva, T.; Vozbrannaya, A.; Bartalev, S. How to avoid peat fires? Sci. Russ. 2011, 2, 13–21. [Google Scholar]
- Turetsky, M.R.; Benscoter, B.; Page, S.; Rein, G.; Van Der Werf, G.R.; Watts, A. Global vulnerability of peatlands to fire and carbon loss. Nat. Geosci. 2015, 8, 11–14. [Google Scholar] [CrossRef]
- Mętrak, M.; Malawska, M.; Kamiński, J.; Wiłkomirski, B. Chemical changes of peat soils and plant succesion on the deeply burnt mires. Pol. J. Environ. Stud. 2006, 15, 57–66. [Google Scholar]
- Sulwiński, M.; Mętrak, M.; Suska-Malawska, M. Long-term fire effects of the drained open fen on organic soils. Arch. Environ. Prot. 2017, 43, 11–19. [Google Scholar] [CrossRef]
- Van Beest, C.; Petrone, R.; Nwaishi, F.; Waddington, J.M.; Macrae, M. Increased peatland nutrient availability following the Fort Mcmurray Horse River wildfire. Diversity 2019, 11, 142. [Google Scholar] [CrossRef]
- Gorham, E. Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecol. Appl. 1991, 1, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Poulter, B.; Christensen, N.L.; Halpin, P.N. Carbon emissions from a temperate peat fire and its relevance to interannual variability of trace atmospheric greenhouse gases. J. Geophys. Res. 2006, 111, D06301. [Google Scholar] [CrossRef]
- Sirin, A.A.; Makarov, D.A.; Maslov, A.A.; Gul’be, Y.I.; Gummert, I. Depth of peat burning and carbon loss during an underground forest fire. Contemp. Probl. Ecol. 2020, 13, 769–779. [Google Scholar] [CrossRef]
- Kucherov, I.B.; Kutenkov, S.A. Dwarf shrub sphagnum-feathermoss and sphagnum pine forests of northern and middle taiga of Europaean Russia. Trans. Karelian Res. Cent. Russ. Acad. Sci. 2012, 1, 16–32. [Google Scholar]
- Yurkovskaya, T.K. Geography and Cartography of the Vegetation of the Mires of European Russia and Adjacent Territories. Proc. Komarov Bot. Institute 1992, 4, 1–256. [Google Scholar]
- Yurkovskaya, T.K. Regularities of distribution of mires in Russia. Bot. J. 2006, 91, 1777–1786. [Google Scholar]
- Kuznetsov, O.L. Vegetation dynamics of raised bogs. News Samara Sci. Cent. Russ. Acad. Sci. 2012, 14, 1288–1291. [Google Scholar]
- Kutenkov, S.A.; Kuznetsov, O.L. Diversity and dynamics of forested mires and paludified forests on the European North of Russia. In Diversity and Dynamics of Forest Ecosystems in Russia; Publishing house KMK: Moscow, Russia, 2013; Volume 2, pp. 152–204. [Google Scholar]
- Gromtsev, A.N. Fire regime in spontaneous forests of the North-Western taiga landscapes. Ecology 1993, 3, 22–26. [Google Scholar]
- Feurdean, A.; Diaconu, A.-C.; Pfeiffer, M.; Gałka, M.; Hutchinson, S.M.; Butiseaca, G.; Gorina, N.; Tonkov, S.; Niamir, A.; Tantau, I.; et al. Holocene wildfire regimes in forested peatlands in western Siberia: Interaction between peatland moisture conditions and the composition of plant functional types. Clim. Past 2021, 18, 1255–1274. [Google Scholar] [CrossRef]
- Taskaev, A.I. (Ed.) Atlas of the Komi Republic on Climate and Hydrology; DiK, Drofa: Moscow, Russia, 1997; p. 116. [Google Scholar]
- Schulze, E.D.; Vygodskaya, N.N.; Tchebakova, N.M.; Czimczik, C.I.; Kozlov, D.N.; Lloyd, J.; Mollicone, D.; Parfenova, E.; Sidorov, K.N.; Varlagin, A.V.; et al. The Eurosiberian Transect: An introduction to the experimental region. Chem. Phys. Meteorol. 2002, 54, 421–428. [Google Scholar] [CrossRef]
- Jiroušek, M.; Peterka, T.; Chytrý, M.; Jiménez-Alfaro, B.; Kuznetsov, O.L.; Pérez-Haase, A.; Aunina, L.; Biurrun, I.; Dítě, D.; Goncharova, N.; et al. Classification of European bog vegetation of the Oxycocco-Sphagnetea class. Appl. Veget. Sci. 2022, 25, e12646. [Google Scholar] [CrossRef]
- Dymov, A.A.; Gorbach, N.M.; Goncharova, N.N.; Karpenko, L.V.; Gabov, D.N.; Kutyavin, I.N.; Startsev, V.V.; Mazur, A.S.; Grodnitskaya, I.D. Holocene and recent fires influence on soil organic matter, microbiological and physico-chemical properties of peats in the European North-East of Russia. Catena 2022, 217, 106449. [Google Scholar] [CrossRef]
- Gorodnitskaya, I.D.; Karpenko, L.V.; Pashkeeva, O.E.; Goncharova, N.N.; Startsev, V.V.; Baturina, O.A.; Dymov, A.A. Impact of forest fires on the microbiological properties of oligotrophic peat soils and gleyed peat podzols of bogs in the Northern part of the Sym-Dubches Interfluve, Krasnoyarsk Region. Eurasian Soil Sci. 2022, 55, 460–473. [Google Scholar] [CrossRef]
- Ipatov, V.S.; Mirin, D.M. Description of Phythocoenosis. Metodical Recommendations; St. Petersburg State University Press: St. Petersburg, Russia, 2008; 71p. [Google Scholar]
- WFO (2022): World Flora Online. Available online: http://www.worldfloraonline.org (accessed on 22 November 2022).
- Dombrovskaya, A.V.; Koronieva, M.M.; Tyuremnov, S.N. Atlas of Plant Macroremains Occurring in Peat; State Energy Publishing: Moscow, Russia, 1959; pp. 1–89. [Google Scholar]
- Katz, N.Y.; Katz, S.V.; Skobeeva, E.I. Atlas of Plant Remains in Peat; Nedra: Moscow, Russia, 1977; 376p. [Google Scholar]
- Kutenkov, S.A. Korpi software for plotting stratigraphic diagrams of peat composition. Trans. Karelian Res. Cent. Russ. Acad. Sci. 2013, 6, 171–176. [Google Scholar]
- Chichagova, O.A. Radiocarbon Dating of Soil Humus; Nauka: Moscow, Russia, 1985; 157p. [Google Scholar]
- Blaauw, M. Methods and code for ’classical’ age-modelling of radiocarbon sequences. Quat. Geochron. 2010, 5, 512–518. [Google Scholar] [CrossRef]
- Reimer, P.J.; Bard, E.; Bayliss, A.; Beck, J.W.; Blackwell, P.G.; Ramsey, C.B.; Buck, C.E.; Cheng, H.; Edwards, R.L.; Friedrich, M. IntCal13 and Marine13 Radiocarbon Age Calibration Curves 0-50,000 years cal BP. Radiocarbon 2013, 55, 1869–1887. [Google Scholar] [CrossRef]
- Walker, M.; Head, M.J.; Lowe, J.; Berkelhammer, M.; BjÖrck, S.; Cheng, H.; Cwynar, L.C.; Fisher, D.; Gkinis, V.; Long, A.; et al. Subdividing the Holocene Series/Epoch: Formalization of stages/ages and subseries/subepochs, and designation of GSSPs and auxiliary stratotypes. J. Quat. Sci. 2019, 34, 173–186. [Google Scholar] [CrossRef]
- Khotinskiy, N.A. Controversial problems of reconstruction and correlation of Holocene paleoclimates. In Paleoclimates of Later Glaciation and Holocene; Nauka: Moscow, Russia, 1989; pp. 12–17. [Google Scholar]
- Golubeva, Y. Climate and vegetation of the post-glacial period on the territory of the Komi Republic. Lithosphere 2008, 2, 124–132. [Google Scholar]
- Andreicheva, L.N.; Bratushak, Y.V.; Marchenko-Vagapova, T.I. Development of the Natural Environment and Climate in the Pleistocene and Holocene in the North of the European Russia; Geoprint: Syktyvkar, Russia, 2006; 23p. [Google Scholar]
- Khotinskiy, N.A. The Holocene of the Northern Eurasia; Nauka: Moscow, Russia, 1977; 200p. [Google Scholar]
- Madany, M.N.; Swetnam, T.W.; West, N.E. Comparison of two approaches for determining fire dates from tree scars. For. Sci. 1982, 28, 856–861. [Google Scholar] [CrossRef]
- Fritts, H.C. Dendroclimatology and dendroecology. Quat. Res. 1971, 1, 419–449. [Google Scholar] [CrossRef]
- Grissino-Mayer, H.A. Manual and tutorial for the proper use of an increment borer. Tree-Ring Res. 2003, 59, 63–79. [Google Scholar]
- Rinn, F.T. Reference Manual. Computer Program for Tree-Ring Analysis and Presentation; Frank Rinn: Helenberg, Germany, 1996; Version 3.5; 264p. [Google Scholar]
- Ali, M.I.; Feng, F.; Liu, X.; Min, W.K.; Shabir, M. On some new operations in soft set theory. Comput. Math. Appl. 2009, 57, 1547–1553. [Google Scholar] [CrossRef]
- Van Bellen, S.; Garneau, M.; Ali, A.A.; Bergeron, Y. Did fires drive Holocene carbon sequestration in boreal ombrotrophic peatlands of eastern Canada? Quat. Res. 2012, 78, 50–59. [Google Scholar] [CrossRef]
- Ouarmim, S.; Asselin, H.; Hely, C.; Bergeron, Y.; Ali, A.A. Long-term dynamics of fire refuges in boreal mixedwood forests. J. Quat. Sci. 2014, 29, 123–129. [Google Scholar] [CrossRef]
- Dymov, A.A.; Grodnitskaya, I.D.; Yakovleva, E.V.; Dubrovskiy, Y.A.; Kutyavin, I.N.; Startsev, V.V.; Milanovsky, E.Y.; Prokushkin, A.S. Albic Podzols of Boreal Pine Forests of Russia: Soil Organic Matter, Physicochemical and Microbiological Properties across Pyrogenic History. Forests 2022, 13, 1831. [Google Scholar] [CrossRef]
- Liss, O.L.; Abramova, L.I.; Avetov, N.A.; Berezina, N.A.; Inisheva, L.I.; Kurnishkova, T.V.; Sluka, Z.A.; Tolpysheva, T.Y.; Shvedchikova, N.K. The Mire Systems of Western Siberia and Their Environmental Significance; Kuvaev, V.B., Ed.; Grif Publications: Tula, Russia, 2001; 584p. [Google Scholar]
- Kremenetski, K.V.; Velichko, A.A.; Borisova, O.K.; MacDonald, G.M.; Smith, L.C.; Frey, K.E.; Orlova, L.A. Peatlands of the Western Siberian lowlands: Current knowledge on zonation, carbon content and Late Quaternary history. Quat. Sci. Rev. 2003, 23, 703–723. [Google Scholar] [CrossRef]
- Smith, L.C.; Macdonald, G.M.; Velichko, A.A.; Beilman, D.W.; Borisova, O.K.; Frey, K.E.; Kremenetski, K.V.; Sheng, Y. Siberian peatlands a net carbon sink and global methane source since the early Holocene. Science 2004, 303, 353–356. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, G.M.; Beilman, D.W.; Kremenetski, K.V.; Sheng, Y.; Smith, L.C.; Velichko, A.A. Rapid Early Development of Circumarctic Peatlands and Atmospheric CH4 and CO2 Variations. Science 2006, 314, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Korhola, A.; Ruppel, M.; Seppä, H.; Väliranta, M.; Virtanen, T.; Weckström, J. The Importance of northern Peatland Expansion to the Late-Holocene Rise of Atmospheric Methane. Quat. Sci. Rev. 2010, 29, 611–617. [Google Scholar] [CrossRef]
- Ruppel, M.; Väliranta, M.; Virtanen, T.; Korhola, A. Postglacial spatiotemporal peatland initiation and lateral expansion dynamics in North America and northern Europe. Holocene 2013, 23, 1596–1606. [Google Scholar] [CrossRef]
- Kuryina, I.V.; Veretennikova, E.E.; Golovatskaya, E.A.; Blyakharchuk, T.A.; Smirnov, S.V. Dynamics of the level of watering of swamps in the southern taiga subzone of Western Siberia in the middle and late Holocene. Bull. Tomsk State Univ. Biol. 2018, 42, 218–241. [Google Scholar]
- Zemtsov, V.A.; Inisheva, L.I. Mires of Western Siberia—Their Role in the Biosphere, 2nd ed.; TGU: Tomsk, Russia, 2000. [Google Scholar]
- Elina, G.A.; Lukashov, A.D.; Yurkovskaya, T.K. Late Glacial and Holocene Palaeovegetation and Palaeogeography of Eastern Fennoscandia; Finnish Environment Institute: Helsinki, Finland, 2010; Volume 4, 300p. [Google Scholar]
- Borisova, O.K. Landscape and climate change in Holocene. News RAS Geogr. 2014, 2, 5–20. [Google Scholar]
- Andreicheva, L.N.; Marchenko-Vagapova, T.I.; Buravskaya, M.N.; Golubeva, Y.V. The Natural Environment of the Neopleistocene and Holocene in the European Northeast of Russia; GEOS: Moscow, Russia, 2015; p. 224. [Google Scholar]
- Dendievel, A.-M.; Jouffroy-Bapicot, I.; Argant, J.; Scholtès, A.; Tourman, A.; Beaulieu, J.-L.; Cubizolle, H. From natural to cultural mires during the last 15 ka years: An integrated approach comparing 14C14C ages on basal peat layers with geomorphological, palaeoecological and archaeological data (Eastern Massif Central, France). Quat. Sci. Rev. 2020, 233, 106219. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Mazei, N.G.; Kupriyanov, D.A.; Kusilman, M.V.; Olchev, A.V. Peatland initiation in Central European Russia during the Holocene: Effect of climate conditions and fires. Holocene 2021, 31, 545–555. [Google Scholar] [CrossRef]
- Kuznetsov, O.L. Bog ecosystems of the Karelian part of the green belt of Fennoscandia. Tr. Kar. Sci. Cent. RAS 2014, 6, 77–88. [Google Scholar]
- Pyavchenko, N.I. On the age of peatlands and changes in vegetation in the south of Western Siberia in the Holocene. Bull. Quat. Study Comm. 1983, 52, 164–170. [Google Scholar]
- Pyavchenko, N.I. Peat Bogs, Their Natural and Economic Importance; Nauka: Moscow, Russia, 1985; 152p. [Google Scholar]
- Jones, M.C.; Yu, Z. Rapid deglacial and early Holocene expansion of peatlands in Alaska. Proc. Natl. Acad. Sci. USA 2010, 107, 7347–7352. [Google Scholar] [CrossRef] [PubMed]
- Antipin, V.K.; Elina, G.A.; Tokarev, P.N.; Brazovskaya, T.I. Mire ecosystems of ≪Vodlozersky≫ National Nature Park: Past, present and future. Bot. J. 1996, 81, 21–37. [Google Scholar]
- Elina, G.A.; Arslanov, K.A.; Klimanov, V.A.; Usova, L.I. Vegetation and climatochronology of Holocene in Lovozero plain of Kola Peninsula (according to spore-pollen diagrams of pulsa mire). Bot. J. 1995, 80, 1–16. [Google Scholar]
- Elina, G.A.; Arslanov, K.A.; Klimanov, V.A. Stages of development of Holocene vegetation in southern and eastern Karelia. Bot. J. 1996, 81, 1–17. [Google Scholar]
- Efremova, T.T.; Efremov, S.P.; Kosykh, N.P.; Mironicheva-Tokareva, N.P.; Titlyanova, A.A. Biological productivity and soils of southern Vasyugany bogs. Sib. Ecol. J. 1994, 1, 253–269. [Google Scholar]
- Pitkänen, A.; Huttunen, P.; Jungner, H.; Meriläinen, J.; Tolonen, K. Holocene fire history of middle boreal pine forest sites in eastern Finland. Ann. Bot. Fennici. 2003, 40, 15–33. [Google Scholar]
- Franzén, L.G.; Malmgren, B.A. Microscopic charcoal and tar (CHAT) particles in peat: A 6500-year record of palaeo-fires in southern Sweden. Mires Peat 2012, 10, 1–25. [Google Scholar]
- Kozlovskaya, L.S.; Medvedeva, V.M.; Pyavchenko, N.I. Dynamics of Organic Matter during Peat Formation; Nauka: Leningrad, Russia, 1978; 173p. [Google Scholar]
- Glebov, F.Z. The Relationships between Forest and Mire in the Taiga Zone; Science Siberian Department: Novosibirsk, Russia, 1988; 184p. [Google Scholar]
- Vasiliev, S.V. Forests and Wetlands of West Siberia; NTL: Tomsk, Russia, 2007; 276p, ISBN 978-5-89503-334-0. [Google Scholar]
- Akhmetieva, N.P.; Belova, S.E.; Jamalov, R.G.; Kulichevskaya, I.S.; Lapina, E.E.; Mikhailova, A.V. Natural restoration of mires after fires. Water Res. 2014, 41, 343–354. [Google Scholar]
- Badmazhapova, I.A.; Gyninova, A.B.; Gonchikov, B.N. The chemical property change of the drained peat soils under the fire factor influence. Bull. KrasGAU 2014, 5, 50–55. [Google Scholar]
- Vasilevich, V.I. Swampy birch forests of the North-West of European Russia. Bot. J. 1997, 82, 19–29. [Google Scholar]
- Grishutkin, O.G. Influence of the fires of 2010 on the swamp ecosystems of the Mordovian State Nature Reserve. Proc. Smidovich Mordovian State Nat. Reserve 2012, 10, 261–265. [Google Scholar]
- Napreenko-Dorokhova, T.V.; Napreenko, M.G. Development of the natural complex of Tselau (according to the structure of the peat deposit). Bull. Balt. Fed. Univ. I Kant. Nat. Med. Sci. 2015, 1, 50–64. [Google Scholar]
- Pyavchenko, N.I. Forest Swamp Science; Publishing House of the Academy of Sciences of the USSR: Moscow, Russia, 1963; 192p. [Google Scholar]
- Marchenko-Vagapova, T.I.; Golubeva, Y.V. Warming or cooling? The question is still open! Bull. Inst. Geol. Komi SC UB RAS 2010, 189, 45–46. [Google Scholar]
- Furyaev, V.V.; Furyaev, E.A. Pyroecological properties of Scotch pine in Central Siberia. Conifers Boreal Zone 2008, 25, 103–109. [Google Scholar]
- Peteet, D.; Andreev, A.; Bardeen, W.; Mistretta, F. Long-term Arctic peatland dynamics, vegetation and climate history of the Pur-Taz region, western Siberia. Boreas 1998, 27, 115–126. [Google Scholar] [CrossRef]
- Gorbach, N.M.; Kutyavin, I.N.; Startsev, V.V.; Dymov, A.A. Dynamics of fires in the northeast of the European part of Russia in the Holocene. Theor. Appl. Ecol. 2021, 3, 104–110. [Google Scholar] [CrossRef]
- Karpenko, L.V.; Prokushkin, A.S. Genesis and history of the post-glacial evolution of forest bog in the valley of the Dubches river. Sib. For. J. 2018, 5, 33–44. [Google Scholar]
- Kupriyanov, D.A.; Novenko, E.Y. Reconstruction of the dynamics of forest fires in Central Meshchera in the Holocene (according to paleoanthracological analysis data). Sib. Ecol. J. 2019, 26, 253–263. [Google Scholar]
- Marlon, J.R.; Bartlein, P.J.; Walsh, M.K.; Harrison, S.P.; Brown, K.J.; Edwards, M.E.; Higuera, P.E.; Power, M.J.; Anderson, R.S.; Briles, C.; et al. Wildfire responses to abrupt climate change in North America. Proc. Natl. Acad. Sci. USA 2009, 106, 2519–2524. [Google Scholar] [CrossRef]
- Barhoumi, C.; Peyron, O.; Joannin, S.; Subetto, D.; Kryshen, A.; Drobyshev, I.; Girardin, M.P.; Brossier, B.; Paradis, L.; Pastor, T.; et al. Gradually increasing forest fire activity during the Holocene in the northern Ural region (Komi Republic, Russia). Holocene 2019, 29, 1906–1920. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; pp. 3–32. [Google Scholar] [CrossRef]
- Karpenko, L.V.; Prokushkin, A.S. Reconstruction of fires in virgin forests at Sym-Dubches interfluve in Holocene. Sib. For. J. 2019, 5, 61–69. [Google Scholar]
- Ryzhkova, N.I.; Kutyavin, I.N.; Pinto, G.; Kryshen, A.M.; Aleinikov, A.A.; Vozmitel, F.K.; Drobyshev, I.V. 2020 Dynamics of fire activity in the pine forests of the Pechora-Ilychsky reserve according to the data of dendrochronological studies. Proc. Pechora-Ilychsky Reserve 2020, 19, 101–106. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).