The Indo-Pacific Stingray Genus Brevitrygon (Myliobatiformes: Dasyatidae): Clarification of Historical Names and Description of a New Species, B. manjajiae sp. nov., from the Western Indian Ocean †
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Genus Brevitrygon Last, Naylor & Manjaji-Matsumoto, 2016
3.2. Species Treatments
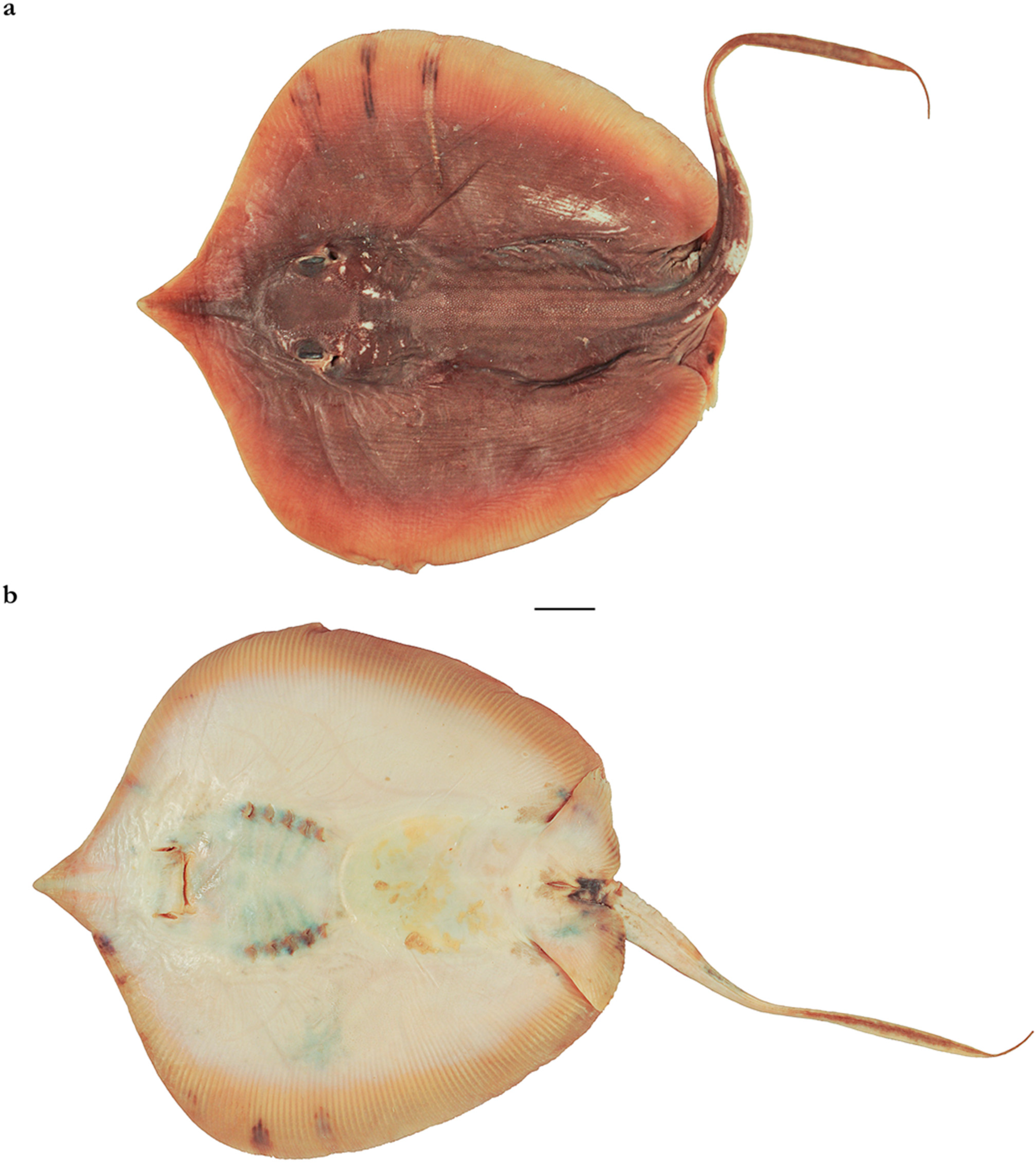
3.2.1. Brevitrygon imbricata (Bloch & Schneider, 1801)
3.2.2. Brevitrygon walga (Müller & Henle, 1841)
“In the original species description, Müller and Henle (1841) mentioned a total of ten syntypes distributed in a number of museums across Europe. However, they did not provide any catalogue number, but some of which were later listed by Eschmeyer (On-Line, ver. 15 February 2002) (i.e., one BMNH; four MNHN, in addition to MNHN 2337 and MNHN 2431, and three RMNH). Some of these putative types were examined by P. Last (pers. comm. December 2000; June 2001). In addition, Last encountered several possible types in the museums where the syntypes were deposited and examined these as well. The number of specimens, including types and possible types of H. walga, examined by Last are seven from BMNH, six MNHN, and six RMNH. In the following, putative and possible types are discussed firstly, followed by non-types”.
“For the BMNH specimens, only one is supposedly the syntype. Thus, minus the syntypes of three species, i.e., H. heterurus, H. dadong and H. nuda, the number is shortlisted to four. Of the four, one is of unknown locality, while each of the other three came from different localities, which is Muscat (Oman), Penang (Malaysia) and Madras (India). Based on the type locality given in the species description, the possible syntype is most likely the Madras specimen. This specimen BMNH 89.2.1.4196, is a female 222 mm DW, with denticle band well developed”.
“For the MNHN specimens, two are confirmed as syntypes (MNHN 2337 and MNHN 2431) in agreement with Eschmeyer. MNHN 2337 is a mature male, 198 mm DW from the Red Sea, and MNHN 2431 is a female (maturity stage undetermined), 170 mm DW from Delta Grange (Ganges, India). The other four specimens appear to conform to the characteristics of the description. However, their locality differs from that of the type locality, with one of them unknown and the rest from ‘Gulf of Thailand.’ The specimen with locality unknown is most likely the syntype referred to in the original description, i.e., ‘the skin of the youngest one quite smooth.’ This specimen, MNHN 2438, 155 mm DW, mature male, has a patch of denticle band above the fontanelle and a narrow band developing at the tail base to sting origin, but otherwise the dorsal surface is smooth. A smaller specimen, ca. 97.5 mm DW (sex not determined), BMNH 1904.5.25 is completely void of denticles on its dorsal surface. All other specimens including those from BMNH and RMNH have a well-developed denticle band, and none as smooth. Therefore, this specimen (MNHN 2438) is designated as one of the syntypes. Other unique characteristics of this specimen are the presence of a well-developed stinging spine, the tail not tapering to a point (although it appears as damaged during its lifetime), disc plain yellowish brown, and tail darkish in color. As for the three specimens from Gulf of Thailand, these remain unconfirmed syntypes of H. walga. It is worth mentioning the denticle bands in all three mature males, size between 144–150 mm DW, are well developed as opposed to the designated syntype (MNHN 2438).
“For the RMNH specimens, three specimens are listed as the syntypes. Based on type locality, two Batavia/ Singapore specimens are excluded as the possible types. One other specimen is anonymously labelled as Trygon chindrakee Cuvier, a name in synonymy thus not available (Eschmeyer). This specimen and the remaining two are all of unknown locality, however, it is likely that all three specimens are the syntypes of H. walga”.
3.2.3. Brevitrygon heterura (Bleeker, 1852)
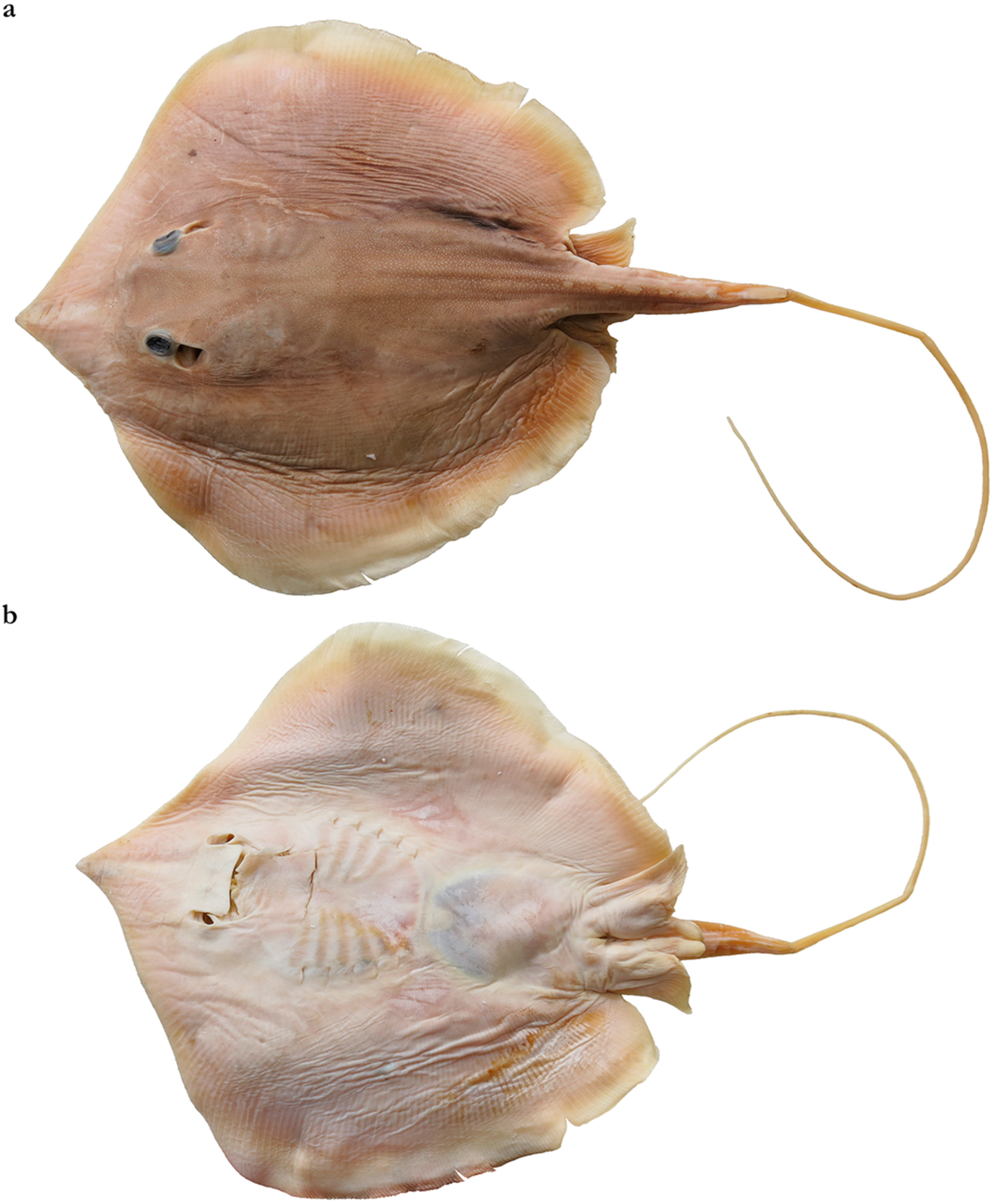
3.2.4. Brevitrygon javaensis (Last & White, 2013)
3.2.5. Brevitrygon manjajiae sp. nov.
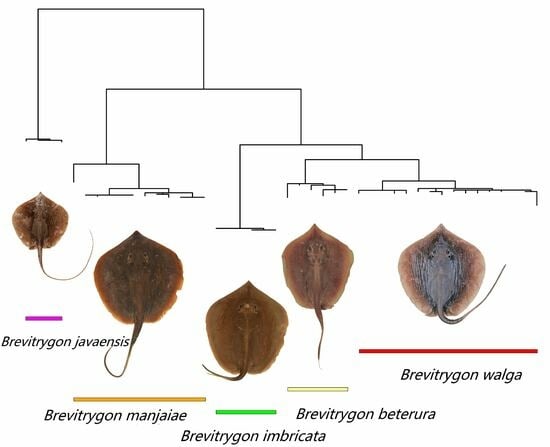
4. Discussion
4.1. Interspecific Comparisons
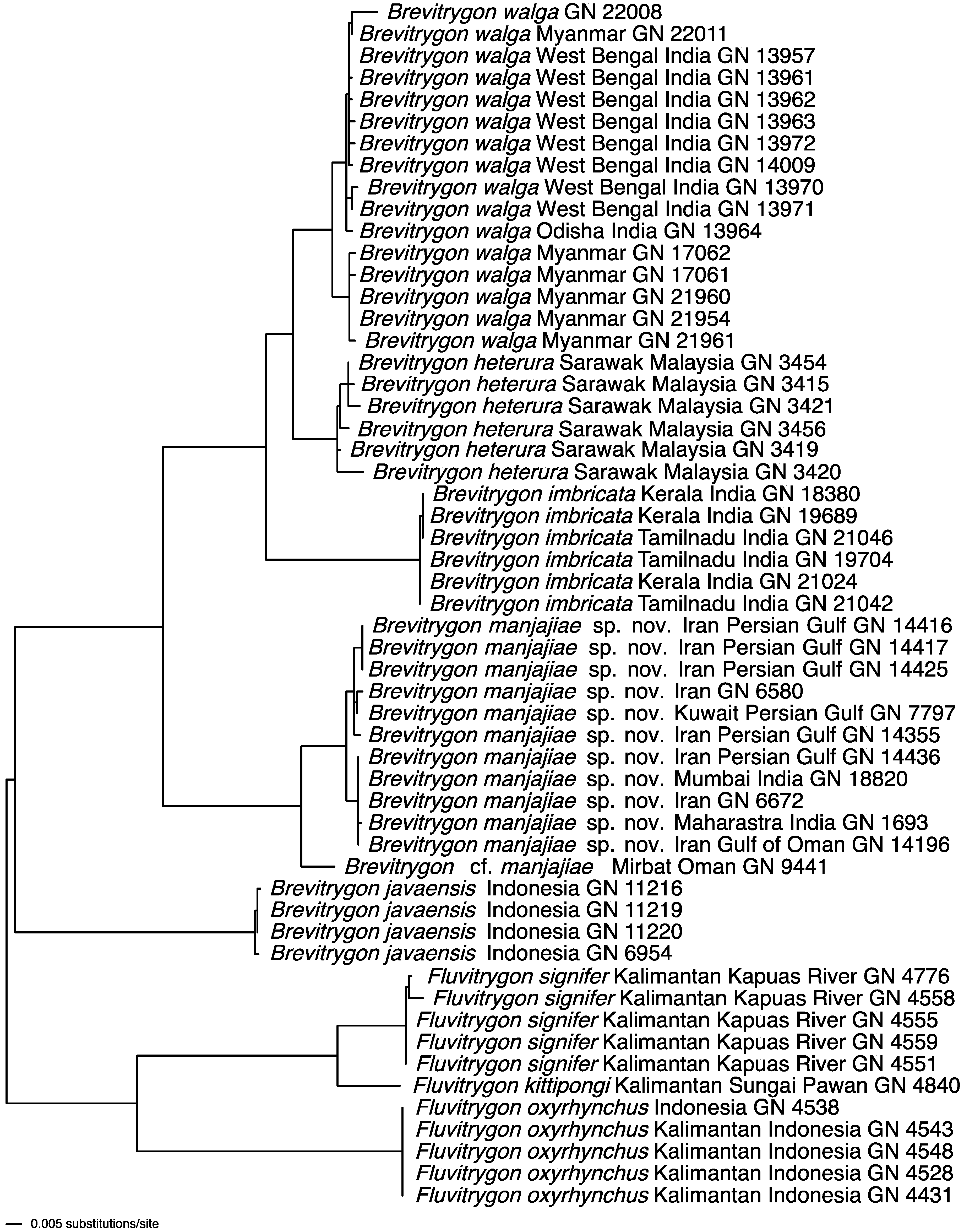
4.2. Phylogenetic Relationships
4.3. Key to Species of the Genus Brevitrygon (Morphometrics Based on Adolecents and Adults)
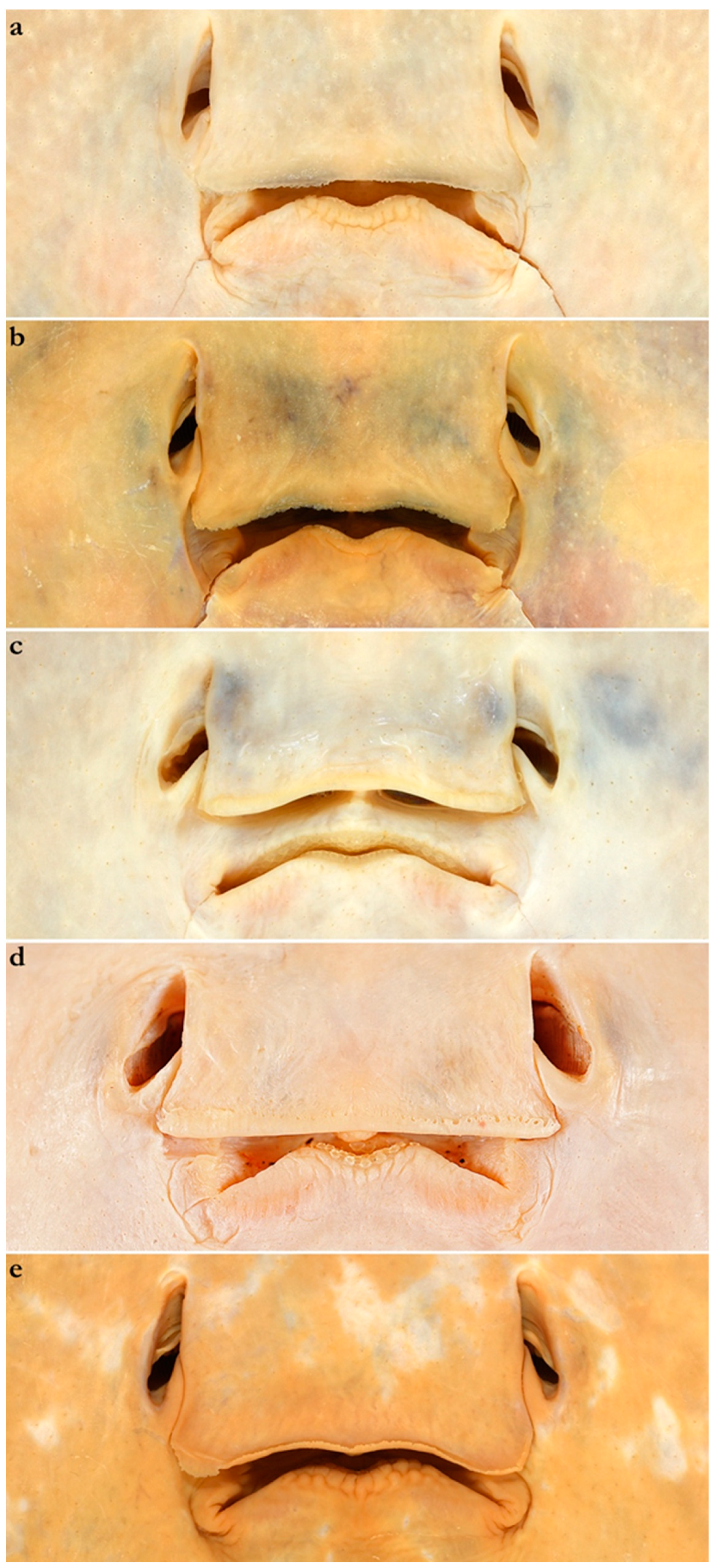
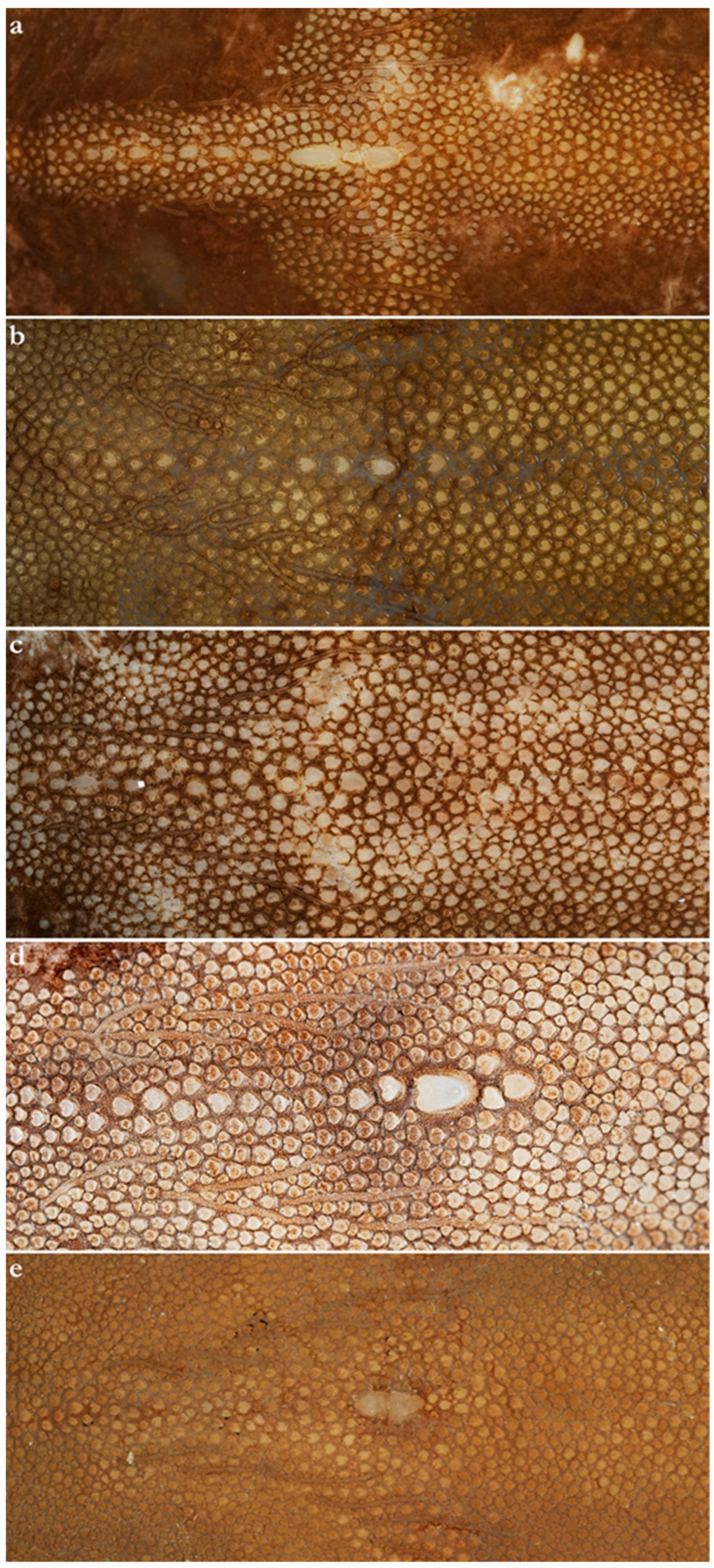
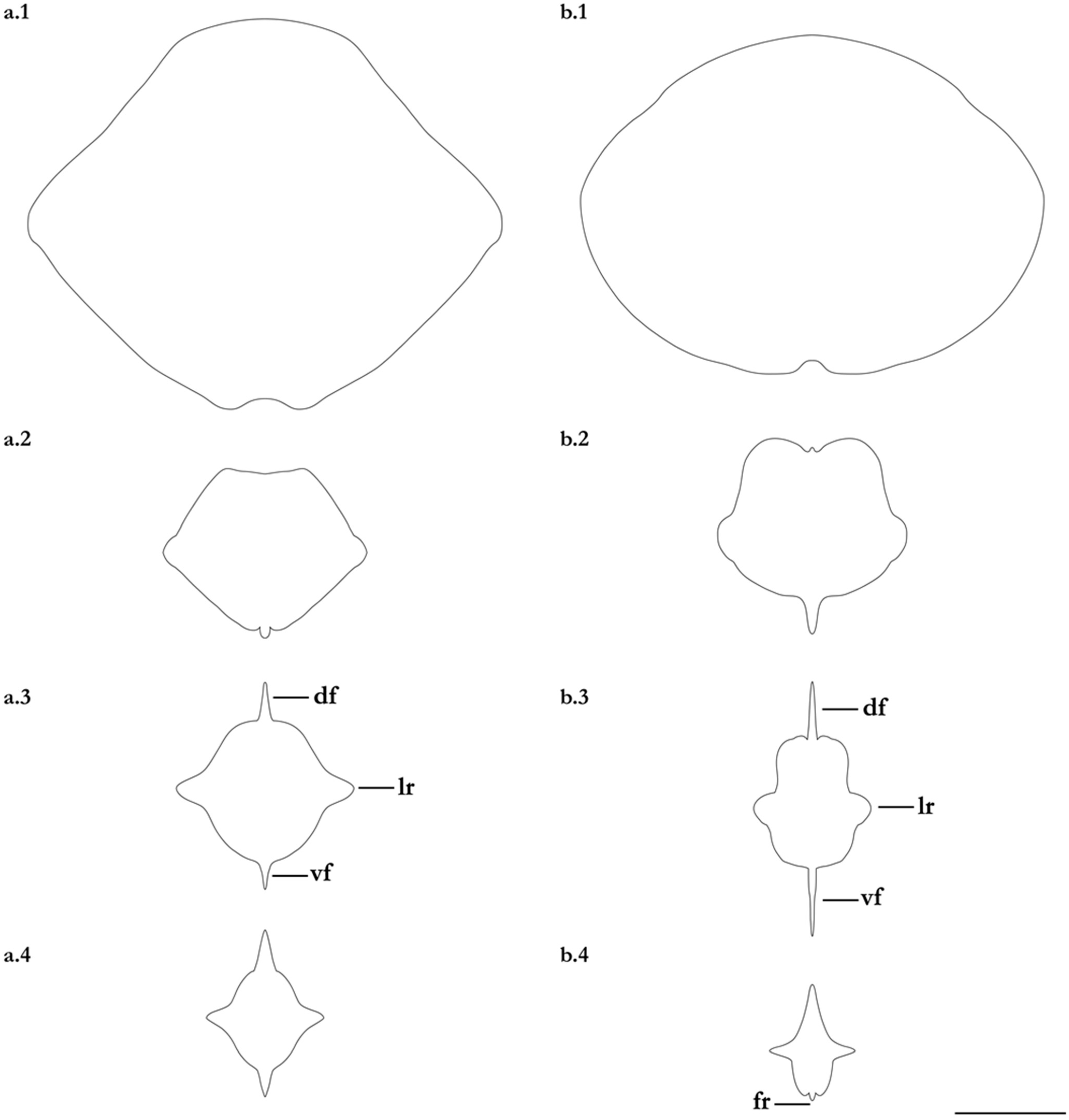
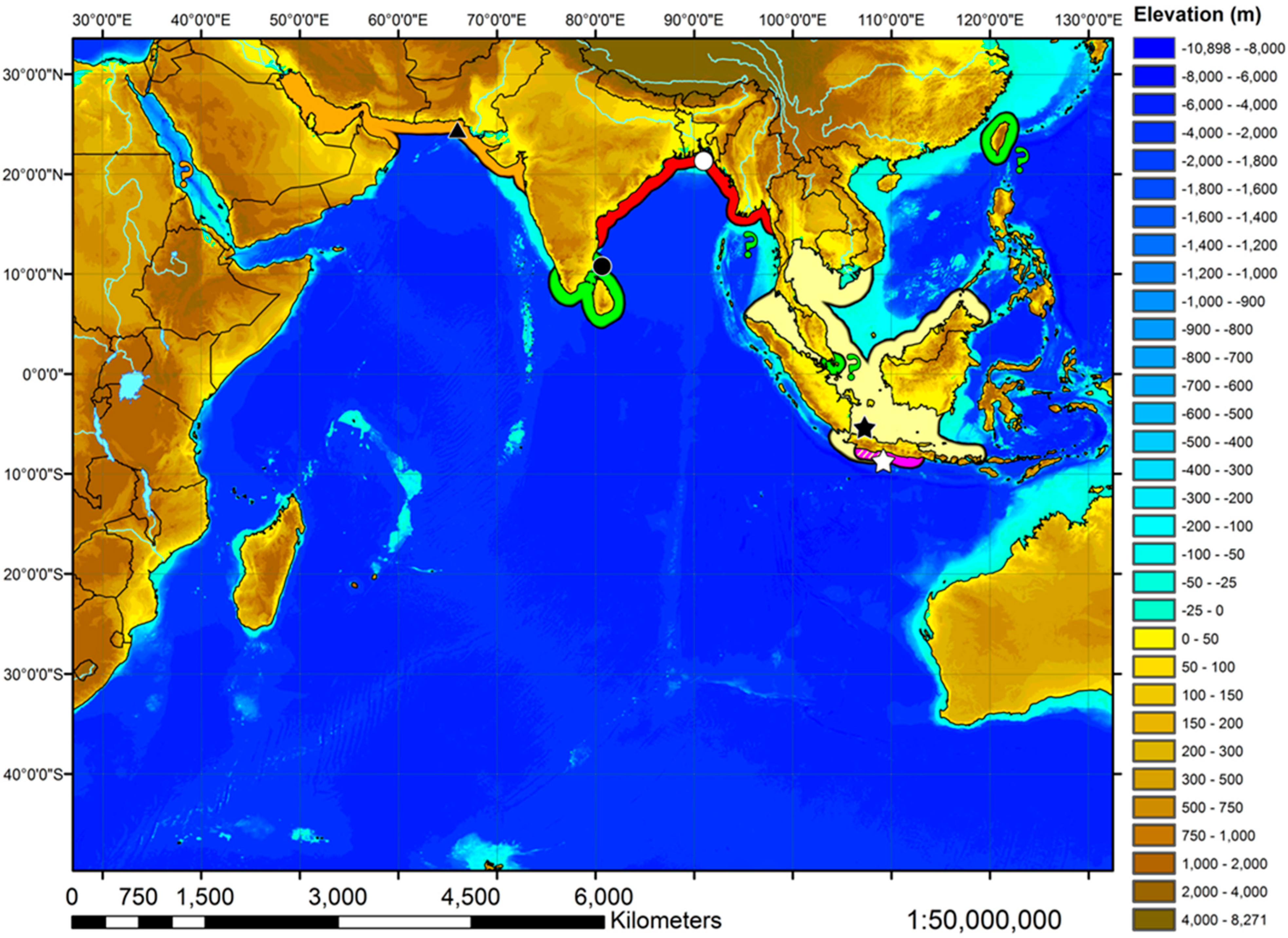
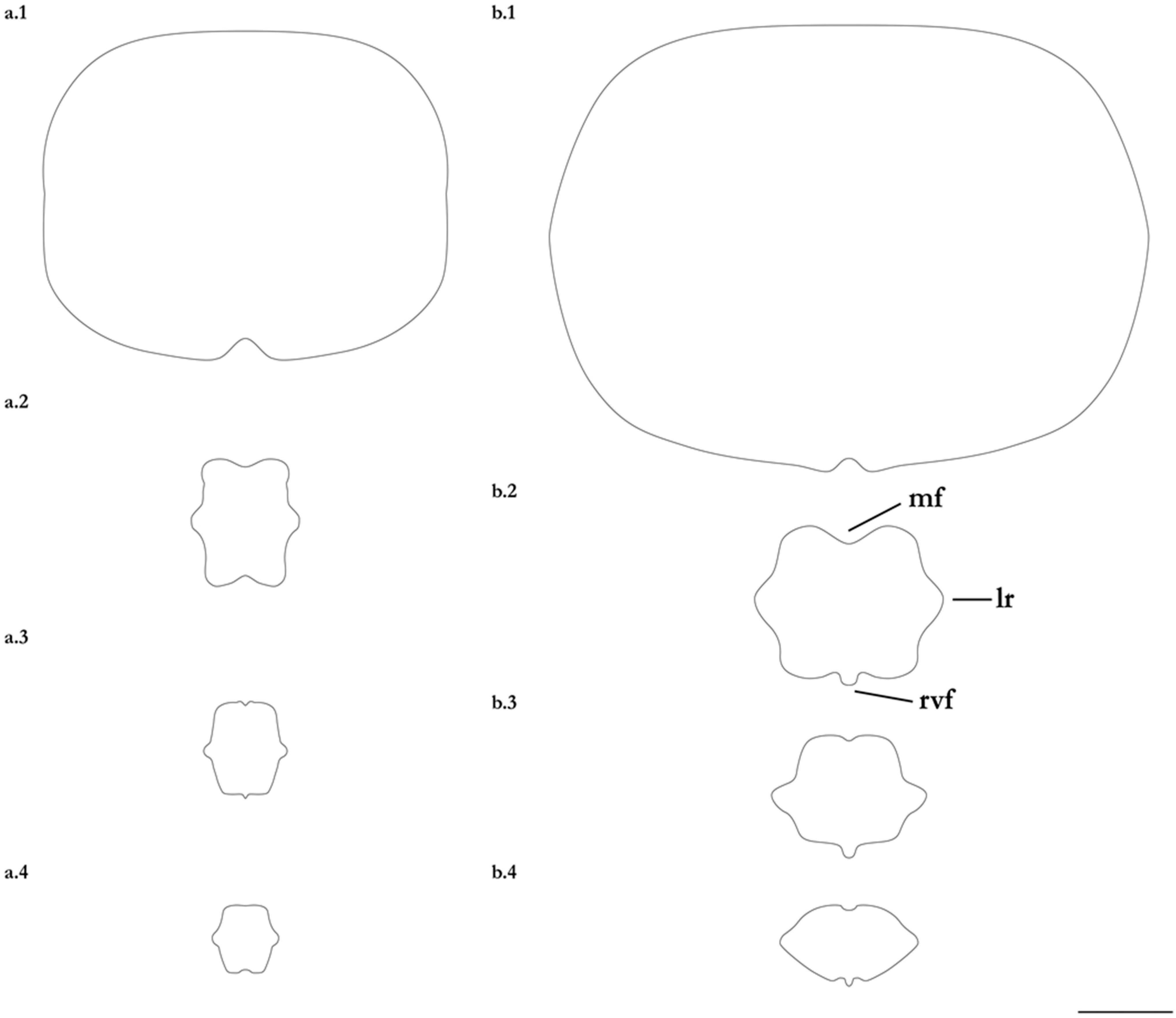
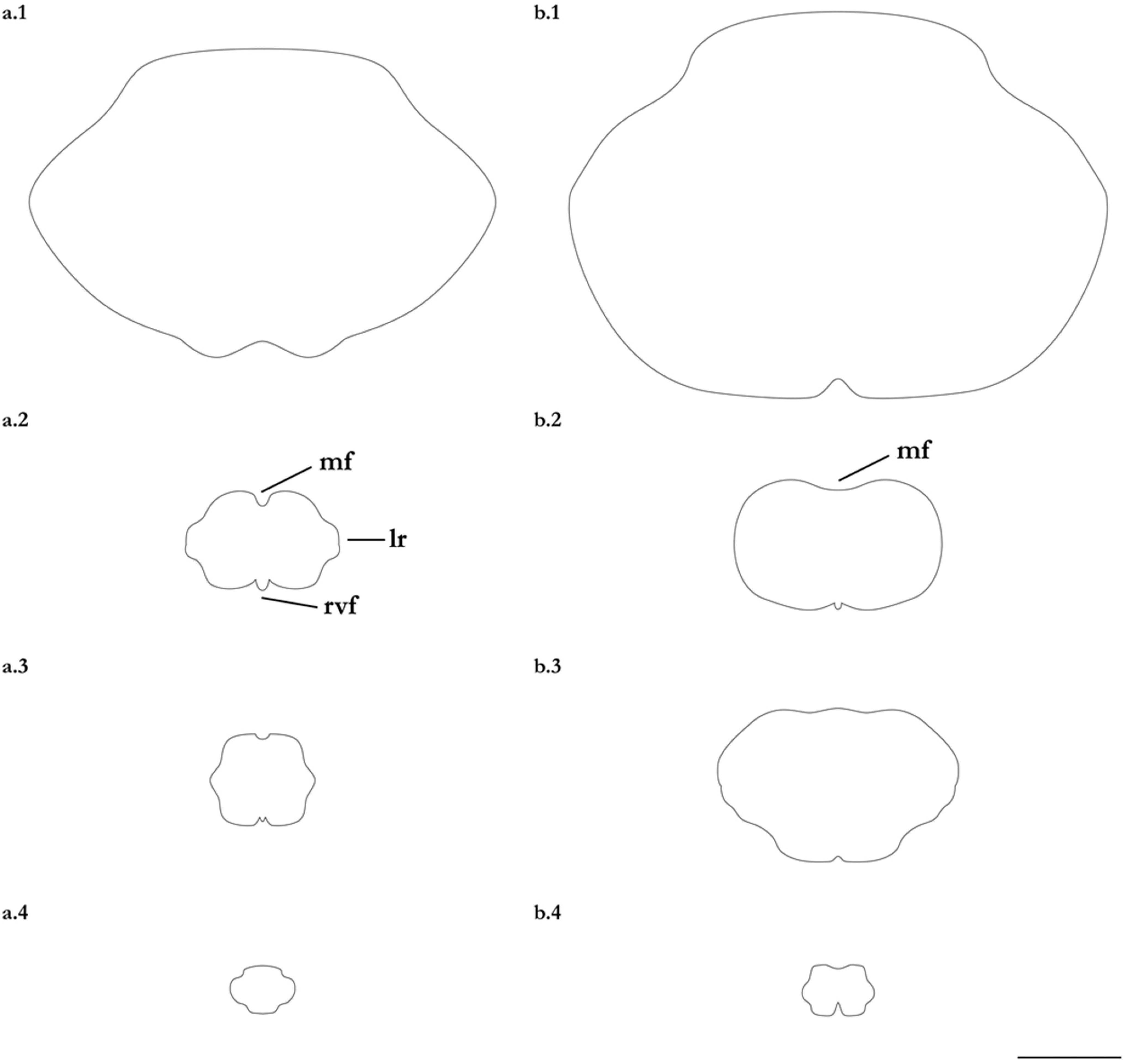
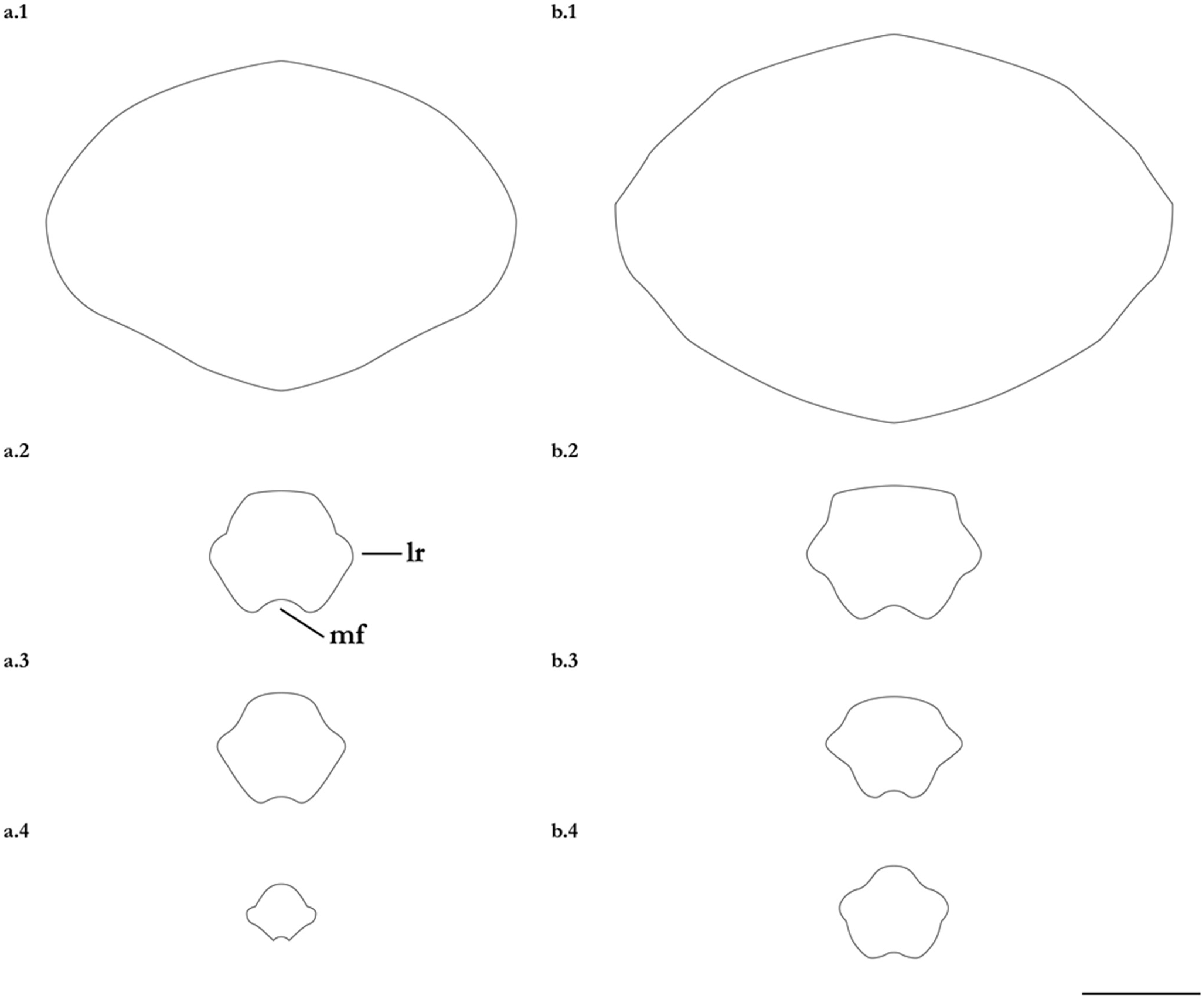
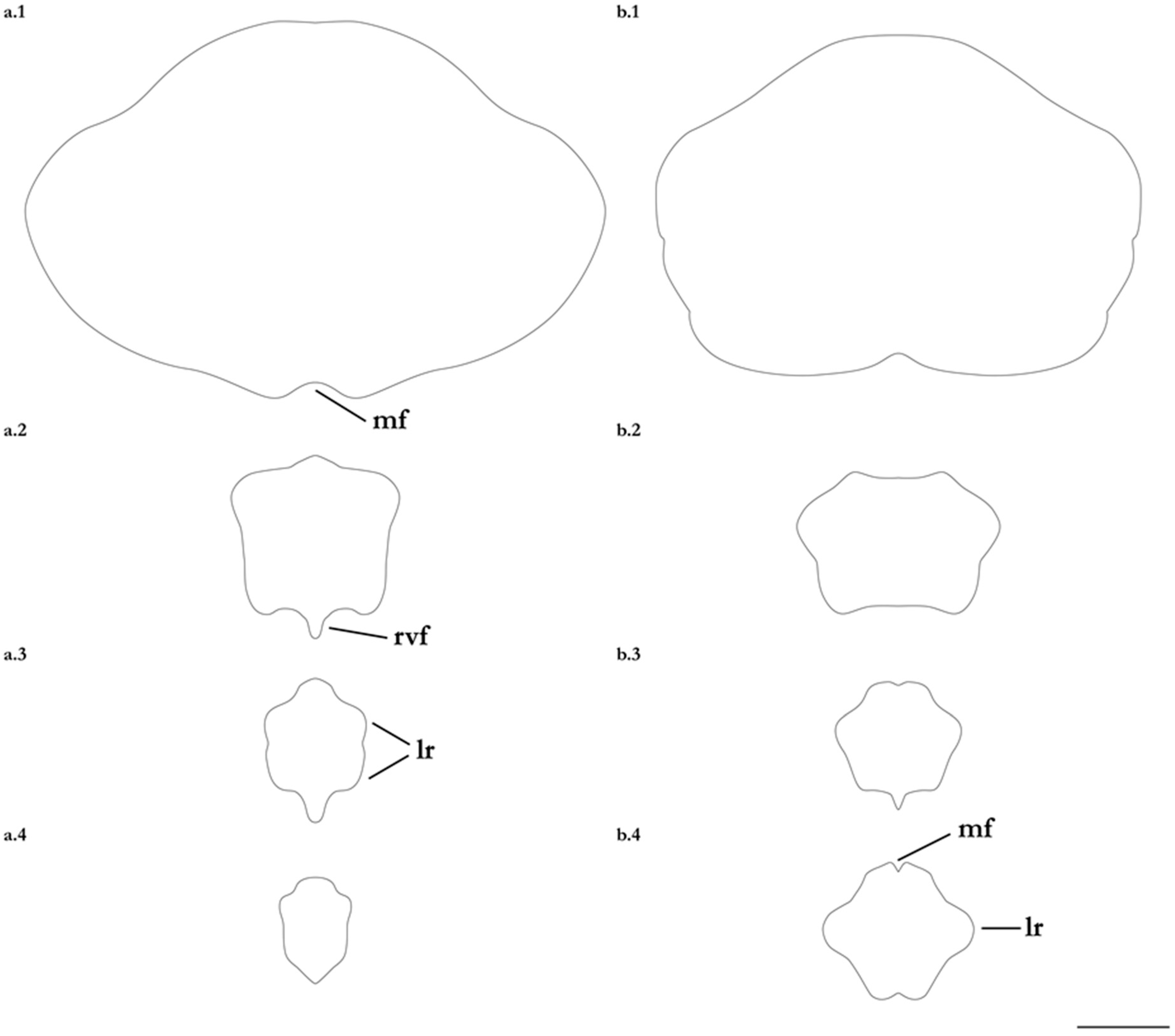
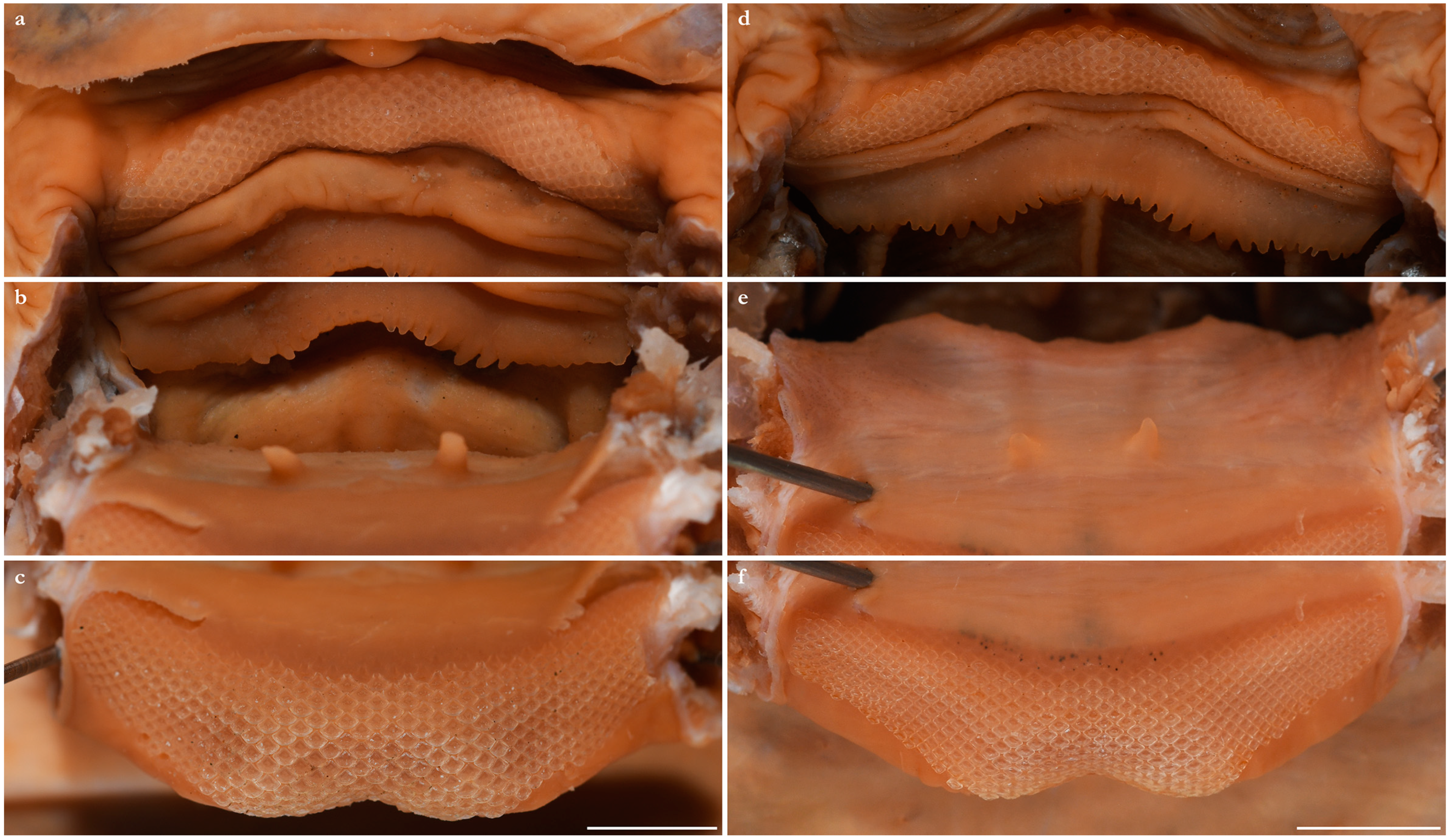
- 1. Tail relatively short, robust beyond sting in both sexes (length when undamaged 0.8–1.2 times DW); post-sting tail with obvious dorsal and ventral cutaneous folds (Figure 3a); secondary denticle band poorly developed in adults, expanded laterally to form a cruciate pattern over scapulocoracoid, very narrow over branchial region and abdomen (narrower than eye diameter), margins not converging posteriorly on disc (see Figure 4a); Bay of Bengal and possibly Western North Pacific ………….………………Brevitrygon imbricata
- - Tail more elongate, semi-rigid to filamentous, often sexually dimorphic (length when undamaged 1.3–2.2 times DW in males); post-sting tail with cutaneous folds absent or as rudimentary ridges (Figure 3b); secondary denticle band well developed in adults, not configured as above (Figure 4b–e) ……………………………………………………….……2
- 2. Tail relatively long, slender (length when undamaged 2.1–2.2 times DW); dorsal post-sting tail appearing speckled due to rows of melanophore clusters each bearing minute denticles (Figure 3f); enlarged denticles and thorns absent from tail (see Figure 5d); southern coast of Java, Indonesia …………………………………………Brevitrygon javaensis
- - Tail slender in males, more robust in females and often bulbous near its tip (length when undamaged 0.6–1.8 times DW); post-sting tail without melanophore clusters; enlarged denticles and/or thorns usually present on midline of pre-sting tail (Figure 5a–c,e) …………3
- 3. Tail beyond sting usually pale (Figure 3d); posterior part of tail in females noticeably bulbous, usually thicker just before tip than at its midlength (Figure 3e); secondary denticle band strongly constricted over branchial region (Figure 4c); relatively large eyes, orbit diameter typically 6–8% DW; large claspers in adult male, postcloacal length 18–23% DW; Indo-Malay Archipelago ……………………………………………………Brevitrygon heterura
- - Tail beyond sting usually dark dorsally and ventrally, fleshy lateral keels white and strongly contrasted with surfaces above and below (Figure 3b,h); posterior part of tail in females not greatly expanded distally, similar in thickness or becoming thinner distally (Figure 3c,i); secondary denticle band not or weakly constricted over branchial region (Figure 4b,e); eyes relatively small, orbit diameter typically 5–6% DW; smaller claspers in adult male, post cloacal length 15–19% DW ……………………………………………..4
- 4. Tail relatively short, length when undamaged 1.3–1.5 times DW in males, 1.0–1.3 times DW in females; preorbital snout short, length usually 28–30% DW; Persian/Arabian Gulf, Arabian Sea, possibly Red Sea ……………..………………...…Brevitrygon manjajiae sp. nov.
- - Tail longer, length when undamaged 1.6–1.8 times DW in males, 1.2–1.6 times DW in females; preorbital snout longer, length 31–34% DW; Bay of Bengal..……Brevitrygon walga
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Last, P.R.; Naylor, G.J.P.; Manjaji-Matsumoto, B.M. A revised classification of the family Dasyatidae (Chondrichthyes: Myliobatiformes) based on new morphological and molecular insights. Zootaxa 2016, 4139, 345. [Google Scholar] [CrossRef]
- Last, P.R.; Compagno, L.J.V. Dasyatidae. In FAO Species Identification Guide for Fishery Purposes. The Living Marine Resources of the Western Central Pacific. Volume 3 Batoid Fishes, Chimaeras and Bony Fishes Part 1 (Elopidae to Linophrynidae); Carpenter, K.E., Niem, V.H., Eds.; FAO: Rome, Italy, 1999; pp. 1479–1510. [Google Scholar]
- Manjaji, B.M. Taxonomy and phylogenetic systematics of the Indo-Pacific Whip-Tailed Stingray genus Himantura Müller & Henle 1837 (Chondrichthyes: Myliobatiformes: Dasyatidae). Ph.D. Thesis, University of Tasmania, Hobart, Australia, 2004. [Google Scholar]
- Weigmann, S. Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. J. Fish Biol. 2016, 88, 837–1037. [Google Scholar] [CrossRef]
- Lim, K.C.; Chong, V.C.; Lim, P.-E.; Yurimoto, T.; Loh, K.H. Feeding ecology of three sympatric species of stingrays on a tropical mudflat. J. Mar. Biol. Ass. 2019, 99, 999–1007. [Google Scholar] [CrossRef]
- Last, P.R.; Manjaji-Matsumoto, B.M.; Naylor, G.J.P.; White, W.T. Stingrays, Family Dasyatidae. In Rays of the World; Last, P.R., White, W.T., de Carvalho, M.R., Séret, B., Stehmann, M.F.W., Naylor, G.J.P., Eds.; CSIRO Publishing: Melbourne, Australia, 2016; pp. 522–618. [Google Scholar]
- Last, P.R.; de Carvalho, M.R.; Corrigan, S.; Naylor, G.J.P.; Séret, B.; Yang, L. The Rays of the World Project—An explanation of Nomenclatural Decisions. In Rays of the World: Supplementary Information; CSIRO Special Publication: Melbourne, Australia, 2016; pp. 1–10. [Google Scholar]
- Simpfendorfer, C.; Moore, A.; Elhassan, I.; Owfi, F.; Akhilesh, K.V. Brevitrygon walga. In The IUCN Red List of Threatened Species: E.T104176764A111015783; IUCN Global Species Programme Red List Unit: Cambridge, UK, 2017. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. Eschmeyer’s Catalog of Fishes: Genera, Species, References. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 23 June 2023).
- Compagno, L.J.V.; Heemstra, P.C. Himantura draco, a new species of stingray (Myliobatiformes: Dasyatidae) from South Africa, with a key to the Dasyatidae and the first record of Dasyatis kuhlii (Müller & Henle, 1841) from southern Africa. J. L. B. Smith Inst. Ichthyol. Spec. Publ. 1984, 33, 1–17. [Google Scholar]
- Last, P.R.; Stevens, J.D. Sharks and Rays of Australia; CSIRO Publishing: Hobart, Tasmania, 1994. [Google Scholar]
- Last, P.R.; Manjaji-Matsumoto, M.; Kailola, P.J. Himantura hortlei n. sp., a new species of whipray (Myliobatiformes: Dasyatidae) from Irian Jaya, Indonesia. Zootaxa 2006, 1239, 19–34. [Google Scholar] [CrossRef]
- Compagno, L.J.V.; Roberts, T.R. Freshwater stingrays (Dasyatidae) of Southeast Asia and New Guinea, with description of a new species of Himantura and reports of unidentified species. Environ. Biol. Fish. 1982, 7, 321–339. [Google Scholar] [CrossRef]
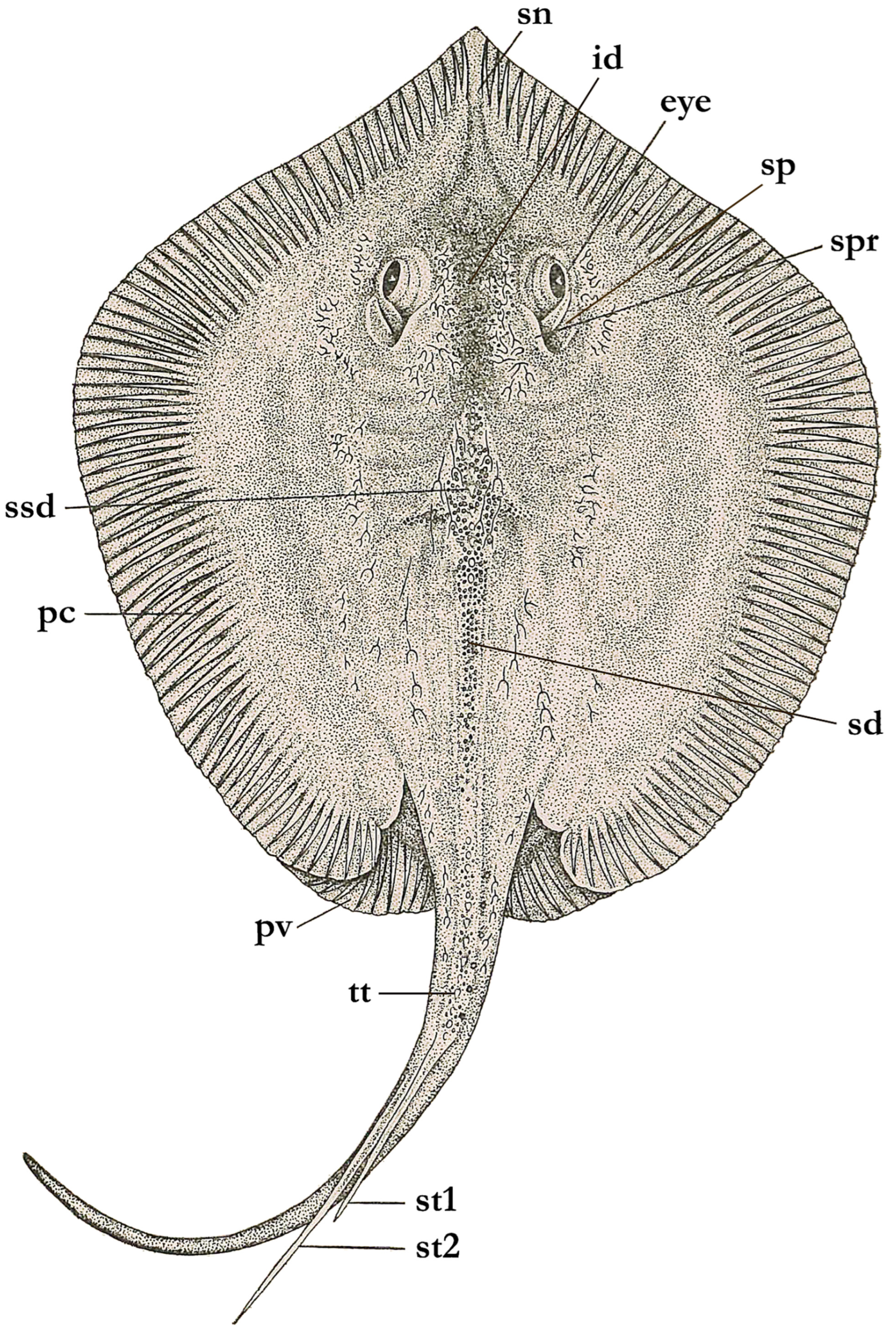
- Compagno, L.J.V. Technical Terms and Measurements. In FAO Species Identification Guide for Fishery Purposes. The Living Marine Resources of the Western Central Pacific. Volume 3 Batoid Fishes, Chimaeras and Bony Fishes Part 1 (Elopidae to Linophrynidae); Carpenter, K.E., Niem, V.H., Eds.; FAO: Rome, Italy, 1999; p. 1398. [Google Scholar]
- Naylor, G.J.P.; Ryburn, J.A.; Fedrigo, O.; López, J. Phylogenetic Relationships among the Major Lineages of Modern Elasmobranchs. In Reprodutive Biology and Phylogeny of Chondrichthyes-Sharks, Batoids and Chimaeras; Hamlett, W.C., Ed.; Science Publishers, Inc.: Enfield, UK, 2005; pp. 1–25. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N. Eschmeyer’s Catalog of Fishes: Guide to Fish Collections. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/collections.asp (accessed on 25 June 2023).
- Last, P.R.; White, W.T. Two new stingrays (Chondrichthyes: Dasyatidae) from the eastern Indonesian Archipelago. Zootaxa 2013, 3722, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Manjaji-Matsumoto, B.M.; de Carvalho, M.R.; Santos, H.R.S.; Gomes, U.L.; Last, P.R. Family Dasyatidae. In Coastal Fishes of the Western Indian Ocean; Heemstra, P.C., Heemstra, E., Ebert, D.A., Holleman, W., Randall, J.E., Eds.; South African Institute for Aquatic Biodiversity: Makhanda, South Africa, 2022; Volume 1, pp. 598–616. [Google Scholar]
- Last, P.R.; White, W.T.; Caira, J.N.; Dharmadi; Fahmi; Jensen, K.; Lim, A.P.K.; Manjaji-Matsumoto, B.M.; Naylor, G.J.P.; Pogonoski, J.J.; et al. Sharks and Rays of Borneo; CSIRO Publishing: Collingwood, ON, Canada, 2010. [Google Scholar]
- Weigmann, S. Contribution to the taxonomy and distribution of eight ray species (Chondrichthyes, Batoidea) from coastal waters of Thailand. Proc. Soc. Nat. Sci. Hamb. 2011, 46, 249–312. [Google Scholar]
- Bloch, M.E.; Schneider, J.G. ME Blochii... Systema Ichthyologiae Iconibus CX Illustratum/Post Obitum Auctoris opus Inchoatum Absoluit, Correxit, Interpolavit Jo. Gottlob Schneider, Saxo; Sumtibus Auctoris Impressum et Bibliopolio Sanderiano Commissum: Berlin, Germany, 1801. [Google Scholar]
- Bleeker, P. Tweede bijdrage tot de kennis der ichthyologische fauna van het eiland Bintang. Natuurk. Tijdschr. Ned. Indië 1856, 10, 345–356. [Google Scholar]
- Duméril, A.H.A. Histoire Naturelle des Poissons, ou, Ichthyologie Générale. In Tome Premier. I. Elasmobranches. Plagiostomes et Holocéphales ou Chimères; Librairie encyclopédique de Roret: Paris, France, 1865. [Google Scholar]
- Günther, A. Catalogue of the Fishes in the British Museum; British Museum (Natural History): London, UK, 1870; Volume 8. [Google Scholar]
- Boeseman, M. Atlas Ichthyologique des Indes Orientales Néêrlandaises, par M. P. Bleeker. In Reproduction for the First Time of Plates Originally Prepared for Unpublished Tomes XI–XIV; Smithsonian Institution Press: Washington, DC, USA, 1983. [Google Scholar]
- Garman, S. The Plagiostomia—Sharks, Skates, and Rays; Memoirs of the Museum of Comparative Zoology at Harvard College; Museum of Comparative Zoology at Harvard College: Cambridge, MA, USA, 1913; Volume 36. [Google Scholar]
- Fowler, H.W. Fishes of the Red Sea and Southern Arabia. In I. Branchiostomida to Polynemida; Weizmann Science Press: Jerusalem, Israel, 1956. [Google Scholar]
- Chandy, M. Memoirs on Indian Animal Type. I. Dasyatis (The Stingray); Maxwell Co. Private Ltd.: Lucknow, India, 1957. [Google Scholar]
- Dor, M. CLOFRES: Checklist of the Fishes of the Red Sea; The Israel Academy of Sciences and Humanities: Jerusalem, Israel, 1984. [Google Scholar]
- Mohsin, A.K.M.; Ambak, M.A. Marine Fishes and Fisheries of Malaysia and Neighbouring Countries; Universiti Pertanian Malaysia Press: Serdang, Malaysia, 1996. [Google Scholar]
- Fricke, R. Fishes of the Mascarene Islands (Réunion, Mauritius, Rodriguez): An Annotated Checklist with Descriptions of New Species; Theses Zoologicae; Koeltz Scientific Books: Königstein, Germany, 1999; ISBN 978-3-87429-411-9. [Google Scholar]
- Rainboth, W.J. FAO Species Identification Field Guide for Fishery Purposes. In Fishes of the Cambodian Mekong; FAO: Rome, Italy, 1996. [Google Scholar]
- Fernando, D.; Bown, R.M.K.; Tanna, A.; Gobiraj, R.; Ralicki, H.; Jockusch, E.L.; Ebert, D.A.; Jensen, K.; Caira, J.N. New insights into the identities of the elasmobranch fauna of Sri Lanka. Zootaxa 2019, 4585, 201–238. [Google Scholar] [CrossRef] [PubMed]
- Haroon, Y.; Kibria, G. Shark Fisheries (Taxonomy, Biology, Ecology) of Bangladesh and Pollution Impacts; Self-publication. 2021. [Google Scholar]
- Karrer, C.; Whitehead, P.J.P.; Paepke, H.-J. Bloch & Schneider’s Systema Ichthyologiae, 1801: History and Autorship [sic] of Fish Names. Mitt. Mus. Nat. Berl. Zool. Reihe 1994, 70, 99–111. [Google Scholar] [CrossRef]
- Müller, J.; Henle, J. Systematische Beschreibung der Plagiostomen; Veit und Comp: Berlin, Germany, 1841. [Google Scholar]
- Bleeker, P. Bijdrage tot de kennis der Plagiostomen van den Indischen Archipel. Verh. Het Bataviaasch Genoot. Kunsten Wet. 1852, 24, 1–92. [Google Scholar]
- Séret, B.; McEachran, J.D. Catalogue critique des types de Poissons du Muséum national d’Histoire naturelle. (Suite) Poissons Batoïdes (Chondrichthyes, Elasmobranchii, Batoidea). Bull. Mus. Natl. Hist. Nat. 1987, 8, 3–50. [Google Scholar] [CrossRef]
- Krishnan, S.; Mishra, S.S. On a Collection of Fish from Kakinada-Gopalpur Sector of the East Coast of India. Rec. Zool. Surv. India 1993, 93, 201–240. [Google Scholar] [CrossRef]
- Scharpf, C. The ETYFish Project. Family Dasyatidae Jordan & Gilbert 1879 (Stingrays). Available online: https://etyfish.org/dasyatidae (accessed on 22 June 2023).
- Golani, D.; Fricke, R. Checklist of the Red Sea Fishes with delineation of the Gulf of Suez, Gulf of Aqaba, endemism and Lessepsian migrants. Zootaxa 2018, 4509, 1–215. [Google Scholar] [CrossRef] [PubMed]
- Jawad, L.A.; Ziyadi, M.S.F.; Näslund, J.; Pohl, T.; Al-Mukhtar, M.A. Checklist of the fishes of the newly discovered coral reef in Iraq, north-west Arabian Gulf, with 10 new records to the Arabian Gulf. Aqua Int. J. Ichthyol. 2018, 24, 89–138. [Google Scholar]
- Golzarianpour, K.; Malek, M.; Golestaninasab, M.; Sarafrazi, A.; Kochmann, J.; Klimpel, S. Insights into the Urogymnid whiprays (Chondrichthyes: Batoidea) in the Persian Gulf and the Gulf of Oman, with an amendment of their diagnostic characteristics and dispersal range. Zootaxa 2020, 4819, 316–334. [Google Scholar] [CrossRef] [PubMed]
- Ralph, G.M.; Stump, E.; Linardich, C.; Bullock, R.W.; Carpenter, K.E.; Allen, D.J.; Hilton-Taylor, C.; Al Mheiri, R.; Alshamsi, O. UAE National Red List of Marine Species: Reef-Building Corals, Cartilaginous Fishes and Select Bony Fishes; Ministry of Climate Change and Environment: Dubai, United Arab Emirates, 2021; p. 50. [Google Scholar]
- Athira, P.P.; Anju, M.V.; Anooja, V.V.; Archana, K.; Neelima, S.; Rosamma, P. A histone H2A-derived antimicrobial peptide, Hipposin from mangrove whip ray, Himantura walga: Molecular and functional characterisation. 3 Biotech 2020, 10, 467. [Google Scholar] [CrossRef]
- Gupta, T.; Warde, K.; Rao, C.; Manoharakrishnan, M. Composition and biology of elasmobranchs in the shore seine catches of Malvan, Maharashtra. J. Mar. Biol. Assoc. India 2022, 64, 80–83. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chaudhuri, A.; Kundu, N.; Mitra, S.; Homechaudhuri, S. Comprehensive Analysis of Fish Assemblages in Relation to Seasonal Environmental Variables in an Estuarine River of Indian Sundarbans. Estuaries Coasts 2013, 36, 192–202. [Google Scholar] [CrossRef]
- Zainal Abidin, D.H.; Lavoué, S.; Mohd Abu Hassan Alshari, N.F.; Nor, S.A.M.; Rahim, A.M.; Mohammed Akib, N.A. Ichthyofauna of Sungai Merbok Mangrove Forest Reserve, northwest Peninsular Malaysia, and its adjacent marine waters. CheckList 2021, 17, 601–631. [Google Scholar] [CrossRef]
- McIvor, A.J. Assessing Sharks and Rays in Shallow Coastal Habitats Using Baited Underwater Video and Aerial Surveys in the Red Sea. Master’s Thesis, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia, 2020. [Google Scholar]
- Al-Faisal, A.; Mutlak, F. Survey of the Marine Fishes in Iraq. Bull. Iraq Nat. Hist. Mus. 2018, 15, 163–177. [Google Scholar] [CrossRef][Green Version]
- Moazzam, M.; Osmany, H.B. Species composition, commercial landings, distribution and conservation of stingrays (Class Pisces: Family Dasyatidae) from Pakistan. Int. J. Biol. Biotech. 2021, 18, 339–376. [Google Scholar]
- Tint, K.M.M.; Thwin, S.; Swe, T.; Tun, S.T.; Htun, T. Preliminary investigation on the occurrence of some estuarine ichthyofauna around Mein Ma Hla Island, Bogale Township, Pyapon district, Ayeyawady Region, Myanmar. Int. J. Fish. Aquat. Stud. 2020, 8, 241–248. [Google Scholar]
- Martens, E.V. Die Preussische Expedition nach Ost-Asien. Zoologischer Thiel. Erster Band. Allgemeines und Wirbelthiere; Verlag der Königlichen Geheimen Ober-Hofbuchdruckerei (R. v. Decker): Berlin, Germany, 1876. [Google Scholar]
- Fowler, H.W. A synopsis of the fishes of China. Part I (concluded). The sharks, rays and related fishes. Hong Kong Nat. 1930, 1, 177–189. [Google Scholar]
- Giltay, L. Résultats scientifiques du voyage aux Indes Orientales Néerlandaises de LL. AA. RR. le Prince et la Princesse Léopold de Belgique Poissons. Mém. Mus. R. Hist. Nat. Belg. 1933, 5, 1–129. [Google Scholar]
- Fowler, H.W. A list of the fishes known from Malaya. Fish. Bull. 1938, 1, 1–268. [Google Scholar]
- White, W.T.; Last, P.R.; Stevens, J.D.; Yearsley, G.K.; Fahmi; Dharmadi. Economically Important Sharks and Rays of Indonesia; ACIAR Publishing: Canberra, Australia, 2006. [Google Scholar]
- Ward, R.D.; Holmes, B.H.; White, W.T.; Last, P.R. DNA barcoding Australasian chondrichthyans: Results and potential uses in conservation. Mar. Freshwater Res. 2008, 59, 57–71. [Google Scholar] [CrossRef]
- White, W.T. Dharmadi Species and size compositions and reproductive biology of rays (Chondrichthyes, Batoidea) caught in target and non-target fisheries in eastern Indonesia. J. Fish Biol. 2007, 70, 1809–1837. [Google Scholar] [CrossRef]
- Sherman, C.S.; Bin Ali, A.; Bineesh, K.K.; Derrick, D.; Dharmadi; Fahmi; Fernando, D.; Haque, A.B.; Maung, A.; Seyha, L.; et al. Brevitrygon javaensis. In The IUCN Red List of Threatened Species; E.T104180270A104180287; IUCN Global Species Programme Red List Unit: Cambridge, UK, 2021. [Google Scholar]
- FAO. The World’s Mangroves 1980–2005; FAO: Rome, Italy, 2007; p. 77. [Google Scholar]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The Loss of Species: Mangrove Extinction Risk and Geographic Areas of Global Concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef]
- Goren, M.; Dor, M. An Updated Checklist of the Fishes of the Red Sea. CLOFRES II; The Israel Academy of Sciences and Humanities: Jerusalem, Israel, 1994. [Google Scholar]
- Moore, A.B.M.; McCarthy, I.D.; Carvalho, G.R.; Peirce, R. Species, sex, size and male maturity composition of previously unreported elasmobranch landings in Kuwait, Qatar and Abu Dhabi Emirate. J. Fish Biol. 2012, 80, 1619–1642. [Google Scholar] [CrossRef] [PubMed]



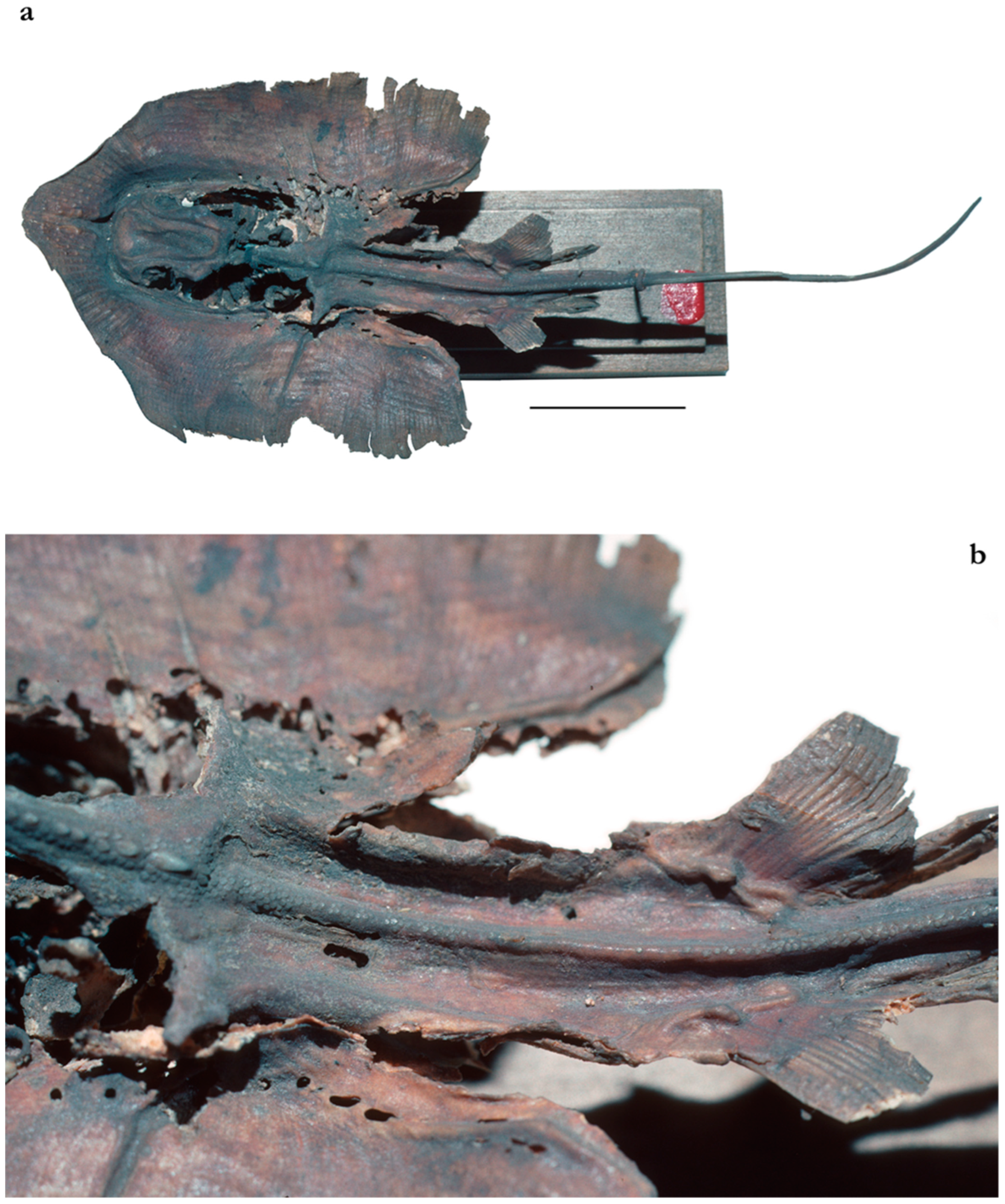








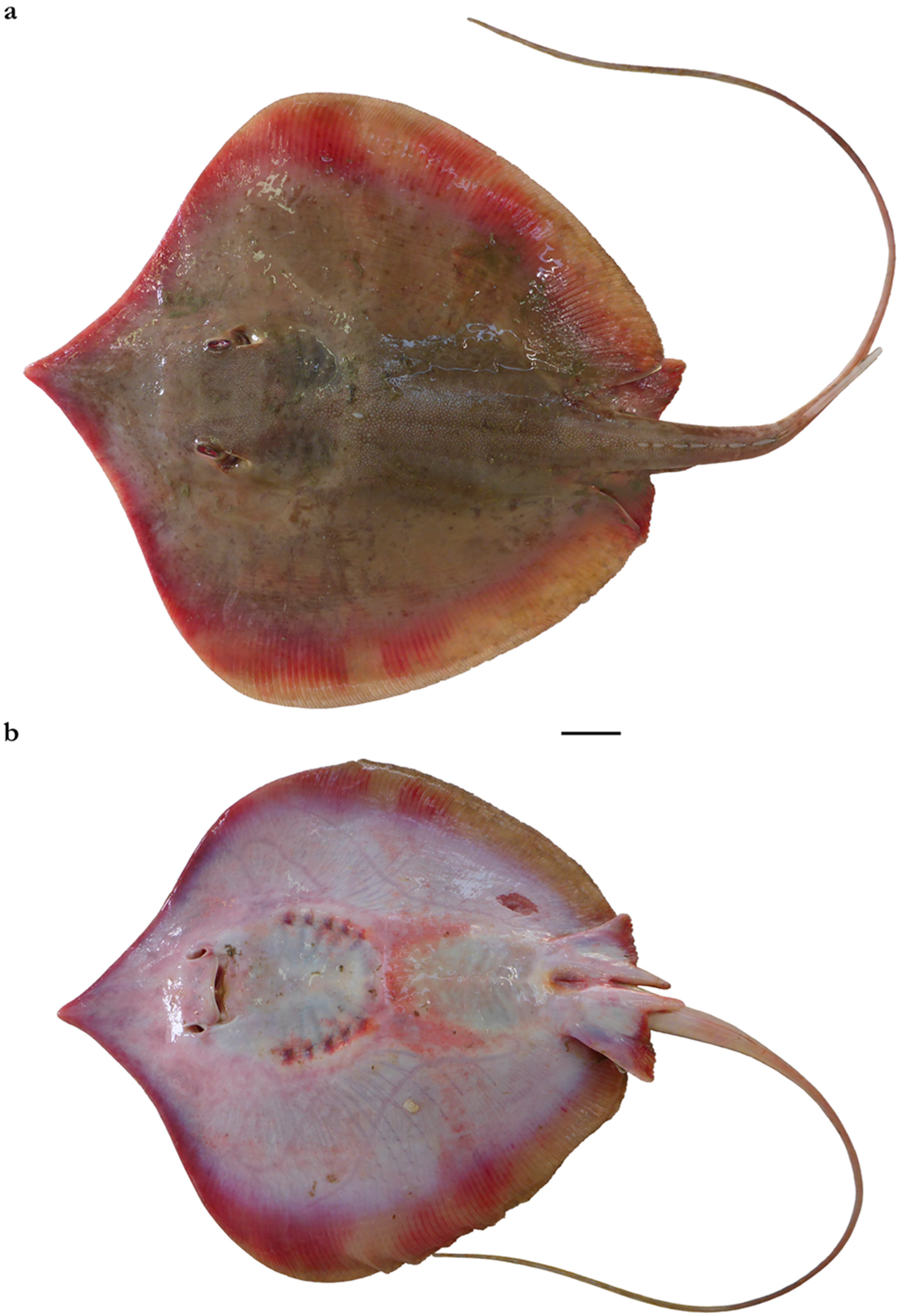
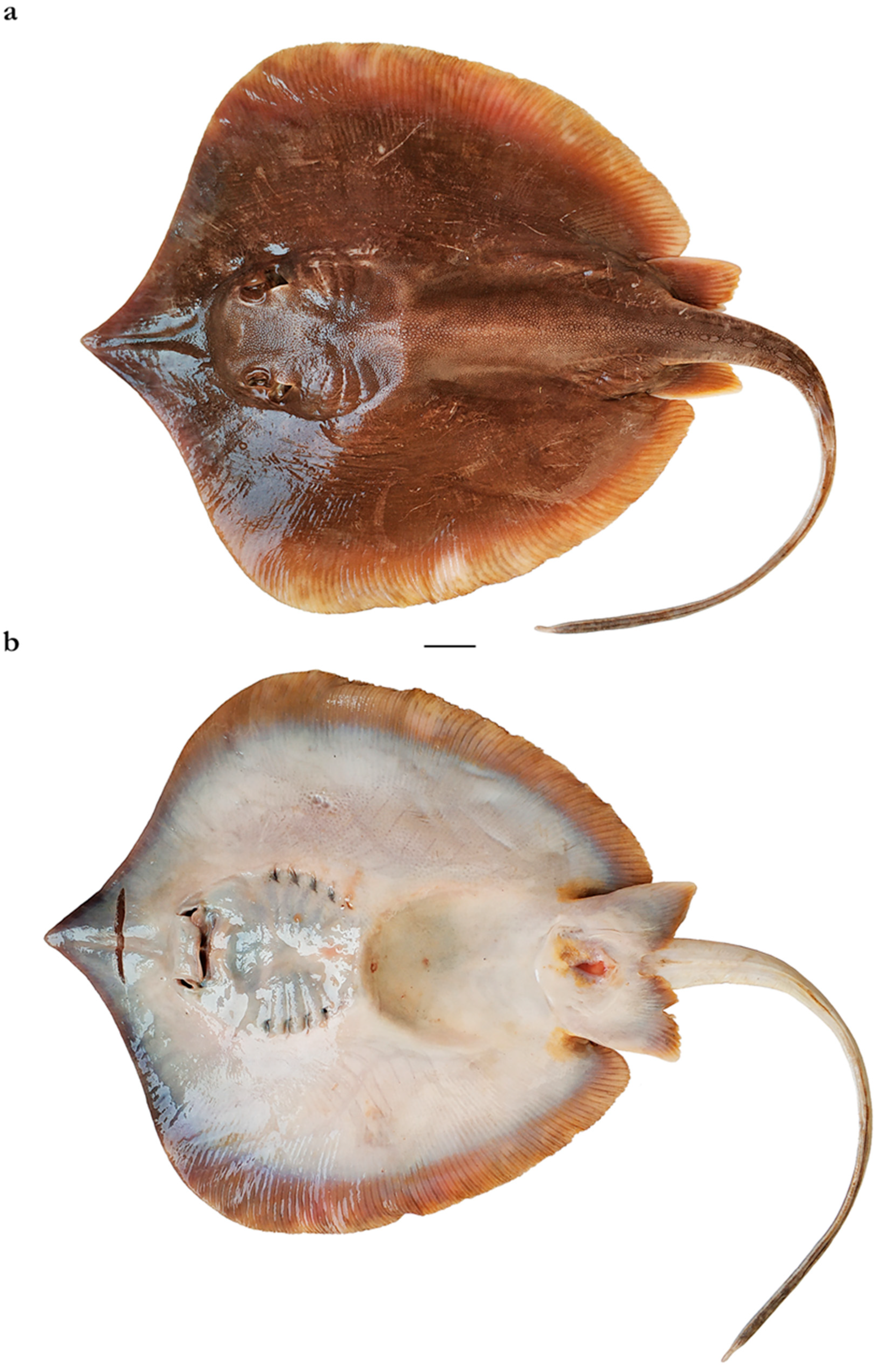
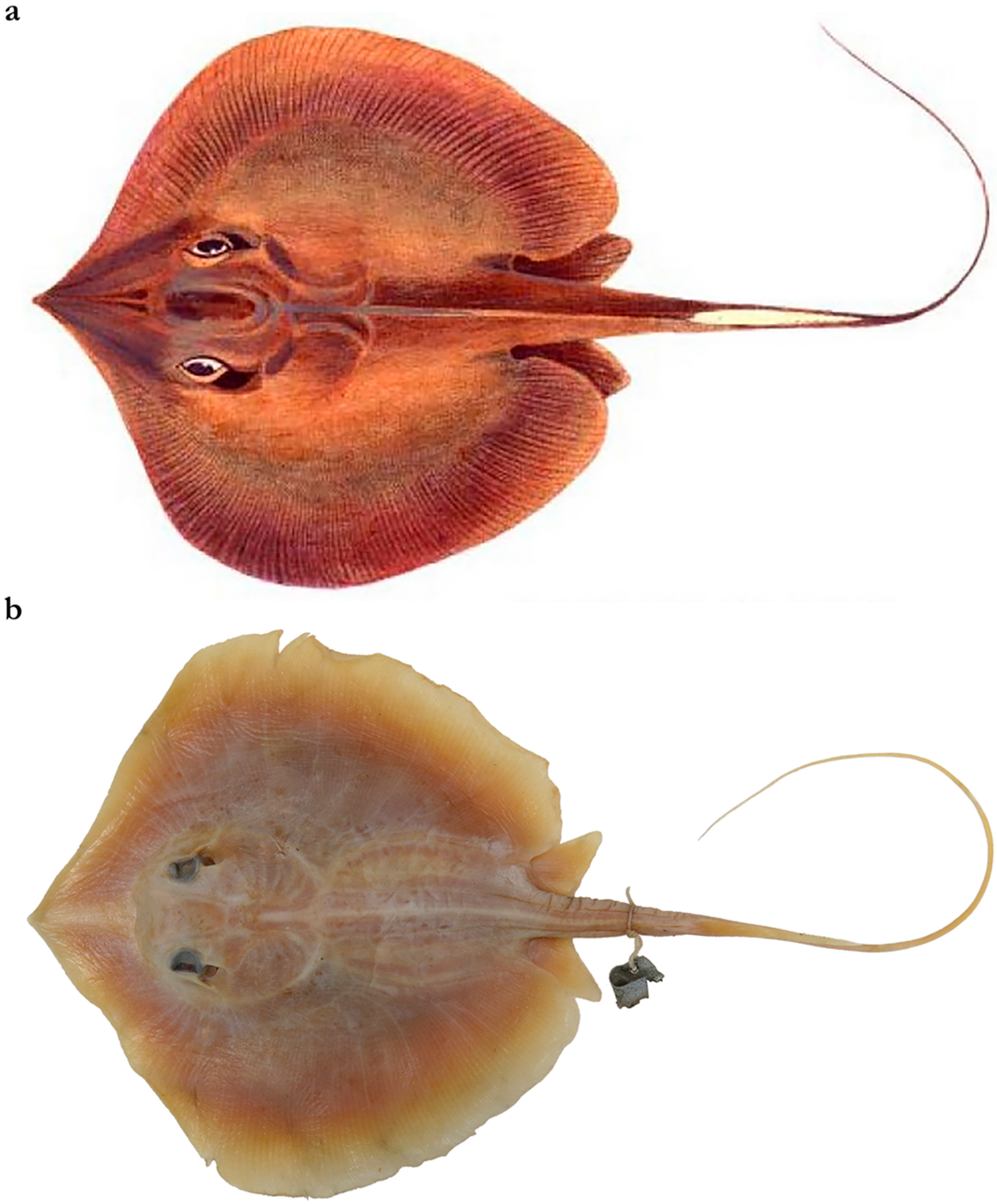
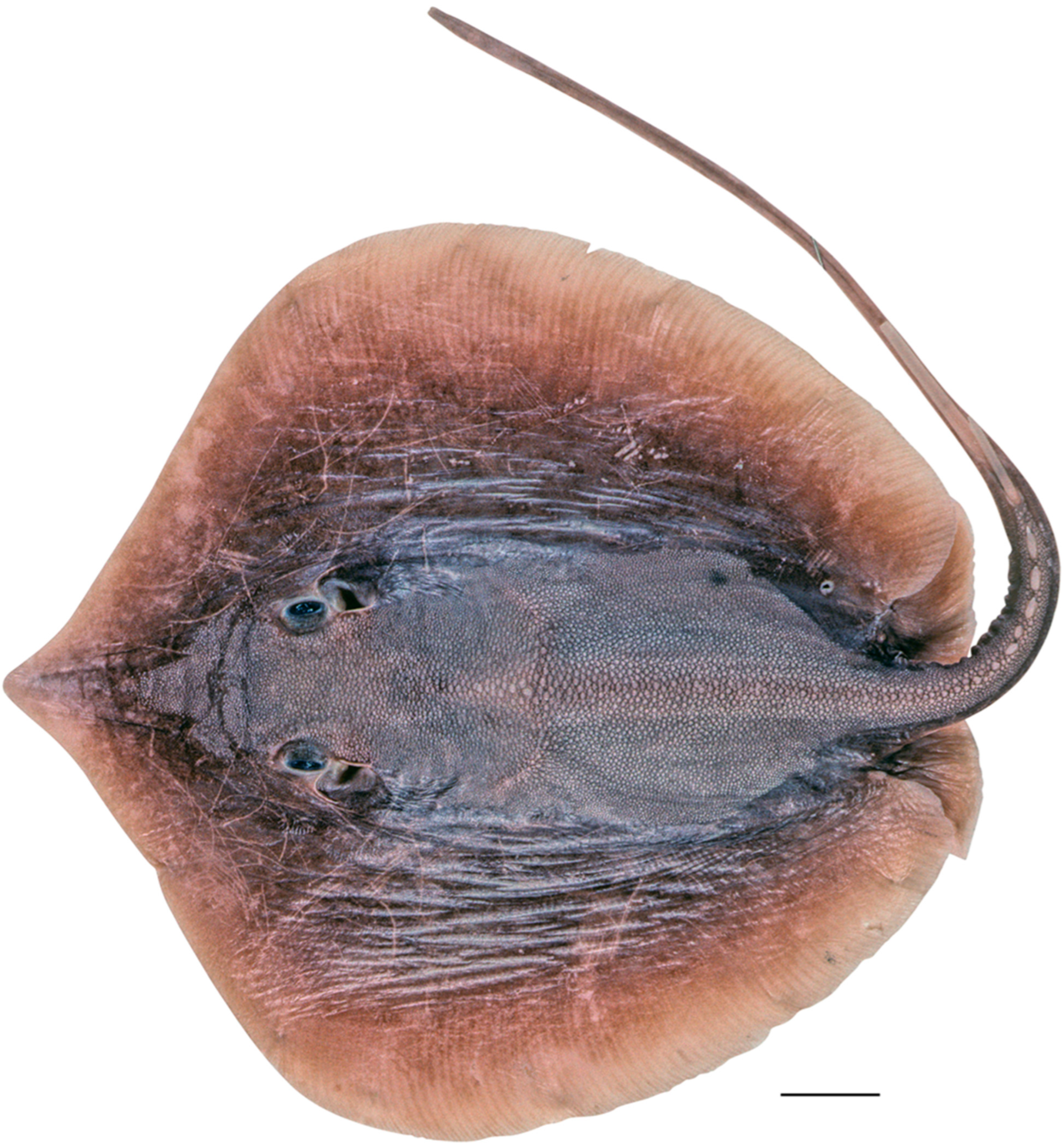










| Pondicherry, India | Southern India and Sri Lanka | Taiwan | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MNHN 2438 | All Data (n = 11) | Males (n = 5) | Females (n = 6) | Males (n = 3) | |||||
| Min | Max | Min | Max | Min | Max | Min | Max | ||
| Disc, width (mm) | 159 | 138 | 205 | 171 | 205 | 138 | 204 | 162 | 182 |
| Total length | 197.6 | 169.8 | 207.9 | 187.2 | 198.5 | 169.8 | 207.9 | 191.9 | 202.0 |
| Disc, length (direct) | 106.9 | 106.1 | 113.3 | 107.1 | 113.1 | 106.1 | 113.3 | 105.7 | 109.4 |
| Disc, thickness | 10.6 | 7.9 | 13.3 | 10.5 | 12.8 | 7.9 | 13.3 | 12.3 | 12.9 |
| Snout to origin of cloaca | 91.2 | 89.0 | 94.7 | 89.0 | 94.7 | 91.2 | 93.4 | 88.8 | 91.9 |
| Cloaca origin to tail tip | 106.4 | 76.6 | 116.5 | 94.4 | 105.4 | 76.6 | 116.5 | 100.9 | 110.7 |
| Snout to pectoral insertion | 98.6 | 95.0 | 103.8 | 95.7 | 103.8 | 95.0 | 103.2 | 95.4 | 98.9 |
| Snout to maximum width | 48.2 | 42.9 | 52.3 | 42.9 | 49.2 | 46.0 | 52.3 | 42.7 | 52.6 |
| End of orbit to pectoral insertion | 62.6 | 58.1 | 66.1 | 59.1 | 65.7 | 58.1 | 66.1 | 61.6 | 63.6 |
| Snout, preorbital (direct) | 30.5 | 29.0 | 31.9 | 29.7 | 31.1 | 29.0 | 31.9 | 28.1 | 31.8 |
| Snout, preorbital (horizontal) | 29.8 | 26.1 | 30.7 | 27.6 | 30.7 | 26.1 | 30.0 | 25.0 | 28.4 |
| Orbit diameter | 6.7 | 5.8 | 7.5 | 6.1 | 6.9 | 5.8 | 7.5 | 7.0 | 7.6 |
| Eye diameter | 4.7 | 3.8 | 5.0 | 3.8 | 5.0 | 4.0 | 4.5 | 4.2 | 4.4 |
| Spiracle length | 6.2 | 5.7 | 6.7 | 5.9 | 6.5 | 5.7 | 6.7 | 5.5 | 6.7 |
| Orbit and spiracle length | 10.0 | 9.7 | 11.1 | 10.4 | 11.1 | 9.7 | 10.8 | 10.1 | 11.3 |
| Interorbital width | 11.6 | 10.0 | 12.1 | 11.0 | 12.1 | 10.0 | 11.5 | 11.1 | 11.3 |
| Intereye width | 17.3 | 14.6 | 17.1 | 15.7 | 17.1 | 14.6 | 16.6 | 15.8 | 17.5 |
| Distance between spiracles | 15.5 | 15.5 | 16.6 | 15.5 | 16.6 | 15.6 | 16.4 | 16.8 | 17.9 |
| Head length (direct) | 57.0 | 55.2 | 58.6 | 56.5 | 58.6 | 55.2 | 57.8 | 55.7 | 59.1 |
| Snout, prenasal (direct) | 24.2 | 24.0 | 26.9 | 24.0 | 26.0 | 24.0 | 26.9 | 22.4 | 26.3 |
| Nostril length | 3.9 | 3.5 | 4.3 | 3.7 | 4.3 | 3.5 | 4.3 | 3.7 | 4.6 |
| Nasal curtain, length | 5.4 | 4.9 | 6.6 | 5.7 | 6.6 | 4.9 | 6.2 | 6.3 | 7.0 |
| Nasal curtain, width | 11.4 | 10.2 | 12.5 | 11.6 | 12.5 | 10.2 | 11.7 | 12.2 | 12.4 |
| Distance between nostrils | 11.8 | 10.1 | 12.1 | 10.7 | 12.1 | 10.1 | 10.9 | 12.3 | 13.1 |
| Snout, preoral (direct) | 30.6 | 29.8 | 32.4 | 29.8 | 31.7 | 30.0 | 32.4 | 27.3 | 32.0 |
| Mouth width | 9.8 | 9.3 | 10.9 | 9.6 | 10.9 | 9.3 | 10.5 | 8.4 | 9.4 |
| Width, 1st gill slit | 2.3 | 2.8 | 3.7 | 2.8 | 3.2 | 2.9 | 3.7 | 3.2 | 3.7 |
| Width, 3rd gill slit | 2.5 | 3.0 | 4.0 | 3.0 | 4.0 | 3.2 | 4.0 | 3.9 | 4.2 |
| Width, 5th gill slit | 1.7 | 2.3 | 2.7 | 2.3 | 2.7 | 2.4 | 2.7 | 2.5 | 2.6 |
| Distance between 1st gill slits | 23.2 | 22.0 | 24.4 | 23.1 | 24.4 | 22.0 | 24.3 | 23.9 | 24.7 |
| Distance between 5th gill slits | 14.9 | 13.5 | 15.3 | 13.6 | 15.3 | 13.5 | 15.3 | 14.6 | 15.4 |
| Length pelvic fin | 21.1 | 18.1 | 22.6 | 18.3 | 20.9 | 18.1 | 22.6 | 20.4 | 21.2 |
| Width across pelvic fin base | 14.7 | 15.5 | 20.3 | 15.5 | 18.2 | 17.1 | 20.3 | 16.4 | 17.1 |
| Greatest width across pelvic fins | 33.6 | 26.6 | 42.9 | 26.6 | 38.2 | 35.6 | 42.9 | 34.9 | 38.0 |
| Tail width, axil of pelvics | 8.1 | 7.3 | 9.8 | 8.2 | 9.8 | 7.3 | 9.1 | 7.8 | 9.7 |
| Tail height, axil of pelvics | 5.4 | 5.0 | 5.7 | 5.0 | 5.6 | 5.1 | 5.7 | 5.9 | 6.1 |
| Tail width, base of sting | 5.2 | 4.1 | 5.2 | 4.2 | 5.2 | 4.1 | 4.9 | 3.7 | 5.5 |
| Tail height, base of sting | 3.9 | 3.2 | 4.2 | 3.2 | 3.7 | 3.2 | 4.2 | 3.4 | 4.2 |
| Cloaca length | 5.4 | 4.8 | 8.9 | 4.8 | 8.9 | 5.9 | 7.0 | 5.5 | 6.6 |
| Clasper, postcloaca length | - | 18.4 | 20.0 | 18.4 | 20.0 | 0.0 | 0.0 | 17.6 | 19.5 |
| Clasper, length from pelvic axil | - | 7.9 | 10.1 | 7.9 | 10.1 | 0.0 | 0.0 | 8.6 | 9.8 |
| Pect. insertion to sting origin | 26.3 | 25.4 | 29.5 | 25.4 | 28.7 | 25.9 | 29.5 | 22.9 | 26.0 |
| Cloaca origin to sting | 28.8 | 29.2 | 36.1 | 29.6 | 36.1 | 29.2 | 35.7 | 29.1 | 32.4 |
| Caudal sting 1 length | - | 17.4 | 31.9 | 17.4 | 25.8 | 21.0 | 31.9 | 21.2 | 22.0 |
| Caudal sting 2 length | - | 22.9 | 34.5 | 24.6 | 30.4 | 22.9 | 34.5 | 31.7 | 32.7 |
| MNHN 2431 | BMNH 1889.2.1.4196 | Other Material (n = 6) | ||
|---|---|---|---|---|
| Lectotype | Syntype T. walga? | Bay of Bengal | ||
| Female | Female | Min | Max | |
| Disc, width (mm) | 170 | 223 | 141 | 233 |
| Total length | 231.2 | 215.8 | 211.4 | 270.8 |
| Disc, length (direct) | 111.5 | 106.0 | 100.6 | 111.7 |
| Disc, thickness | 8.9 | 11.2 | 9.4 | 13.3 |
| Snout to origin of cloaca | 94.2 | 90.5 | 89.5 | 99.7 |
| Cloaca origin to tail tip | 137.1 | 125.3 | 118.1 | 181.3 |
| Snout to pectoral insertion | 101.0 | 97.2 | 95.1 | 103.1 |
| Snout to maximum width | 52.0 | 45.9 | 44.2 | 48.8 |
| End of orbit to pectoral insertion | 62.1 | 61.1 | 56.3 | 108.4 |
| Snout, preorbital (direct) | 33.7 | 31.1 | 31.0 | 33.5 |
| Snout, preorbital (horizontal) | 33.2 | 27.3 | 30.3 | 32.9 |
| Orbit diameter | 6.1 | 5.3 | 5.3 | 5.9 |
| Eye diameter | 4.2 | 3.5 | 3.5 | 3.9 |
| Spiracle length | 7.2 | 6.4 | 5.5 | 6.5 |
| Orbit and spiracle length | 10.9 | 9.8 | 9.2 | 10.4 |
| Interorbital width | 11.4 | 12.6 | 11.4 | 14.0 |
| Intereye width | 16.5 | 15.5 | 15.6 | 16.4 |
| Distance between spiracles | 16.6 | 16.9 | 15.8 | 17.7 |
| Head length (direct) | 61.0 | 57.2 | 57.1 | 59.2 |
| Snout, prenasal (direct) | 30.2 | 25.8 | 26.0 | 28.6 |
| Nostril length | 3.9 | 3.7 | 4.1 | 5.1 |
| Nasal curtain, length | 6.6 | 5.3 | 5.8 | 7.5 |
| Nasal curtain, width | - | 11.1 | 11.7 | 13.1 |
| Distance between nostrils | - | 11.1 | 11.4 | 12.3 |
| Snout, preoral (direct) | - | 31.4 | 31.9 | 34.5 |
| Mouth width | - | 9.4 | 9.3 | 11.0 |
| Width, 1st gill slit | 2.5 | 3.3 | 2.5 | 3.2 |
| Width, 3rd gill slit | 2.4 | 3.5 | 2.4 | 3.3 |
| Width, 5th gill slit | 1.3 | 2.5 | 2.0 | 2.6 |
| Distance between 1st gill slits | 25.4 | 24.9 | 22.4 | 24.4 |
| Distance between 5th gill slits | 14.5 | 15.6 | 13.0 | 15.9 |
| Length pelvic fin | 21.7 | 20.3 | 17.9 | 21.9 |
| Width across pelvic fin base | 17.6 | 17.0 | 14.4 | 21.5 |
| Greatest width across pelvic fins | 39.1 | 13.2 | 32.3 | 40.8 |
| Tail width, axil of pelvics | 10.0 | 8.6 | 6.1 | 10.4 |
| Tail height, axil of pelvics | 7.0 | 5.9 | 5.6 | 7.2 |
| Tail width, base of sting | 5.3 | 4.1 | 4.2 | 6.1 |
| Tail height, base of sting | 3.9 | 3.2 | 3.4 | 4.0 |
| Cloaca length | 5.1 | 6.0 | 4.8 | 7.7 |
| Clasper, postcloaca length | - | - | 12.1 | 16.8 |
| Clasper, length from pelvic axil | - | - | 4.3 | 8.0 |
| Pect. insertion to sting origin | 31.9 | 34.6 | 25.2 | 35.2 |
| Cloaca origin to sting | 36.1 | 37.6 | 32.9 | 39.1 |
| Caudal sting 1 length | - | - | 21.0 | 28.0 |
| Caudal sting 2 length | - | 8.2 | 16.0 | 37.9 |
| Holotype | Non-Types | |||||||
|---|---|---|---|---|---|---|---|---|
| BMNH 1867.11.28.158 | Indonesia (n = 4) | Malaysia (n = 21) | Thailand (n = 16) | Vietnam (n = 1) | ||||
| Female, Java, Indonesia | Min | Max | Min | Max | Min | Max | ||
| Disc, width (mm) | 199 | 163 | 244 | 101 | 210 | 161 | 210 | 176 |
| Total length | 213.6 | 195.6 | 237.6 | 184.9 | 255.1 | 156.3 | 231.6 | - |
| Disc, length (direct) | 110.1 | 105.5 | 112.7 | 103.3 | 109.8 | 103.4 | 110.6 | 107.4 |
| Disc, thickness | 10.2 | 11.0 | 13.6 | 10.6 | 13.4 | 10.6 | 13.5 | 12.3 |
| Snout to origin of cloaca | 91.5 | 88.7 | 97.9 | 85.0 | 92.9 | 85.6 | 95.6 | 91.4 |
| Cloaca origin to tail tip | 122.1 | 100.4 | 142.3 | 93.1 | 168.3 | 64.3 | 142.7 | - |
| Snout to pectoral insertion | 98.9 | 95.2 | 104.7 | 92.7 | 100.8 | 93.5 | 102.1 | 97.4 |
| Snout to maximum width | 50.3 | 47.1 | 52.0 | 39.8 | 53.4 | 43.5 | 49.3 | 46.5 |
| End of orbit to pectoral insertion | 63.2 | 61.7 | 67.2 | 56.9 | 65.7 | 59.5 | 65.0 | 62.9 |
| Snout, preorbital (direct) | 31.7 | 29.6 | 32.4 | 28.3 | 32.1 | 27.6 | 31.1 | 30.0 |
| Snout, preorbital (horizontal) | 30.6 | 28.6 | 31.5 | 27.0 | 30.7 | 25.6 | 29.5 | 27.0 |
| Orbit diameter | 6.6 | 5.3 | 6.7 | 5.8 | 7.7 | 5.8 | 7.9 | 6.5 |
| Eye diameter | 4.4 | 3.3 | 4.8 | 3.7 | 5.2 | 3.8 | 4.6 | 4.1 |
| Spiracle length | 5.5 | 5.2 | 7.0 | 5.2 | 6.9 | 4.9 | 6.2 | 5.7 |
| Orbit and spiracle length | 10.1 | 9.7 | 10.6 | 9.4 | 11.8 | 9.2 | 11.0 | 10.7 |
| Interorbital width | 12.4 | 10.5 | 13.4 | 10.3 | 13.0 | 10.0 | 12.3 | 12.5 |
| Intereye width | 15.3 | 15.8 | 16.9 | 16.1 | 18.6 | 15.7 | 17.2 | 16.9 |
| Distance between spiracles | 16.7 | 16.6 | 18.0 | 15.4 | 18.0 | 15.5 | 17.4 | 18.8 |
| Head length (direct) | 57.6 | 55.9 | 59.3 | 52.3 | 59.3 | 54.6 | 58.8 | 58.2 |
| Snout, prenasal (direct) | 26.4 | 23.9 | 27.2 | 23.5 | 26.7 | 22.7 | 26.6 | 22.7 |
| Nostril length | 4.8 | 4.2 | 5.3 | 3.6 | 5.2 | 3.8 | 5.2 | 3.6 |
| Nasal curtain, length | 5.5 | 5.6 | 7.6 | 5.1 | 7.1 | 6.3 | 7.8 | 6.9 |
| Nasal curtain, width | 11.6 | 11.7 | 13.8 | 10.3 | 17.9 | 11.0 | 14.5 | 13.4 |
| Distance between nostrils | 11.7 | 11.1 | 13.2 | 10.4 | 12.9 | 10.8 | 13.7 | 12.6 |
| Snout, preoral (direct) | 33.0 | 29.5 | 33.4 | 28.5 | 33.1 | 28.2 | 32.8 | 29.7 |
| Mouth width | 9.8 | 9.3 | 10.4 | 7.9 | 9.9 | 8.3 | 10.2 | 10.6 |
| Width, 1st gill slit | 3.1 | 2.7 | 3.7 | 2.6 | 3.4 | 2.6 | 3.7 | 3.0 |
| Width, 3rd gill slit | 3.5 | 2.7 | 4.1 | 2.9 | 3.8 | 3.1 | 3.9 | 3.5 |
| Width, 5th gill slit | 1.9 | 2.0 | 2.6 | 1.4 | 2.5 | 1.9 | 2.5 | 2.0 |
| Distance between 1st gill slits | 23.7 | 22.7 | 25.1 | 21.0 | 25.1 | 22.3 | 24.4 | 25.2 |
| Distance between 5th gill slits | 15.3 | 14.9 | 15.7 | 14.1 | 16.5 | 14.6 | 16.6 | 16.4 |
| Length pelvic fin | 26.8 | 21.2 | 25.1 | 20.0 | 24.1 | 18.6 | 24.5 | 23.7 |
| Width across pelvic fin base | 17.1 | 16.1 | 16.7 | 12.9 | 18.7 | 13.8 | 17.8 | 15.2 |
| Greatest width across pelvic fins | - | 35.6 | 40.4 | 32.1 | 45.8 | 33.5 | 42.5 | 40.3 |
| Tail width, axil of pelvics | 7.8 | 8.4 | 10.0 | 8.1 | 11.2 | 7.4 | 11.2 | 9.7 |
| Tail height, axil of pelvics | 6.6 | 5.4 | 5.9 | 5.0 | 6.9 | 5.3 | 6.6 | 6.0 |
| Tail width, base of sting | 5.0 | 5.7 | 6.2 | 3.7 | 6.4 | 4.2 | 5.9 | - |
| Tail height, base of sting | 4.1 | 4.0 | 4.1 | 2.9 | 4.0 | 3.3 | 4.2 | - |
| Cloaca length | 5.7 | 5.0 | 8.3 | 4.0 | 7.3 | 4.5 | 7.2 | 5.9 |
| Clasper, postcloaca length | - | 18.7 | 20.4 | 12.0 | 22.6 | 20.6 | 22.8 | 19.2 |
| Clasper, length from pelvic axil | - | 9.2 | 13.3 | 7.5 | 20.3 | 9.3 | 17.1 | 13.6 |
| Pect. insertion to sting origin | 28.7 | 29.1 | 29.6 | 23.7 | 32.1 | 20.2 | 27.0 | - |
| Cloaca origin to sting | 34.0 | 32.5 | 33.6 | 29.4 | 37.1 | 26.8 | 31.3 | - |
| Caudal sting 1 length | - | - | - | - | - | - | - | - |
| Caudal sting 2 length | - | - | - | - | - | - | - | - |
| Malaysia (n = 11) | Malaysia (n = 10) | Thailand (n = 7) | Thailand (n = 10) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult Males | Females | Adult Males | Females | |||||||||
| Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | |
| Disc, width (mm) | 107 | 200 | 173 | 136 | 210 | 184 | 170 | 207 | 183 | 161 | 199 | 180 |
| Total length | 227.9 | 255.1 | 239.0 | 185.5 | 215.6 | 202.6 | 215.3 | 231.6 | 224.1 | 156.3 | 205.3 | 182.7 |
| Disc, length (direct) | 103.7 | 108.0 | 105.3 | 104.3 | 109.8 | 107.0 | 103.4 | 107.6 | 105.0 | 105.3 | 110.6 | 108.3 |
| Disc, thickness | 11.2 | 13.4 | 12.6 | 10.6 | 13.2 | 12.1 | 11.8 | 13.5 | 12.7 | 10.9 | 13.1 | 12.4 |
| Snout to origin of cloaca | 86.8 | 92.3 | 88.8 | 87.8 | 92.9 | 90.2 | 85.6 | 93.2 | 88.8 | 88.9 | 95.6 | 92.2 |
| Cloaca origin to tail tip | 139.5 | 168.3 | 150.6 | 93.1 | 127.8 | 112.7 | 122.4 | 142.7 | 134.2 | 64.3 | 116.1 | 90.4 |
| Snout to pectoral insertion | 92.9 | 98.3 | 94.8 | 93.4 | 100.8 | 97.0 | 93.5 | 98.2 | 95.4 | 95.0 | 102.1 | 98.8 |
| Snout to maximum width | 45.4 | 53.4 | 48.7 | 43.0 | 50.6 | 46.5 | 43.5 | 49.1 | 46.3 | 46.5 | 49.3 | 48.0 |
| End of orbit to pectoral insertion | 57.0 | 62.0 | 59.4 | 57.3 | 65.7 | 61.6 | 59.5 | 61.4 | 60.7 | 60.0 | 65.0 | 62.8 |
| Snout, preorbital (direct) | 29.5 | 32.0 | 30.8 | 28.3 | 32.1 | 30.5 | 27.6 | 30.1 | 29.0 | 28.8 | 31.1 | 29.7 |
| Snout, preorbital (horizontal) | 28.2 | 30.6 | 29.4 | 27.0 | 30.7 | 29.4 | 26.9 | 29.5 | 28.1 | 25.6 | 29.0 | 27.8 |
| Orbit diameter | 5.9 | 7.7 | 6.4 | 5.8 | 6.6 | 6.1 | 6.2 | 7.3 | 6.7 | 6.3 | 7.9 | 7.1 |
| Eye diameter | 3.9 | 5.2 | 4.2 | 3.8 | 4.5 | 4.2 | 4.1 | 4.5 | 4.1 | 3.8 | 4.6 | 4.3 |
| Spiracle length | 5.5 | 6.9 | 6.3 | 5.2 | 5.8 | 5.5 | 4.9 | 6.1 | 5.6 | 5.5 | 6.2 | 5.9 |
| Orbit and spiracle length | 10.0 | 11.8 | 10.5 | 9.4 | 10.2 | 9.8 | 10.0 | 11.0 | 10.5 | 9.2 | 10.9 | 10.3 |
| Interorbital width | 11.9 | 13.0 | 12.5 | 10.9 | 11.6 | 11.3 | 10.3 | 12.3 | 11.1 | 10.0 | 11.6 | 10.9 |
| Intereye width | 16.3 | 18.6 | 17.5 | 16.1 | 17.5 | 16.6 | 16.2 | 17.2 | 16.7 | 15.7 | 16.8 | 16.4 |
| Distance between spiracles | 16.7 | 18.0 | 17.1 | 15.4 | 16.8 | 16.1 | 16.7 | 17.4 | 17.0 | 15.5 | 16.4 | 16.0 |
| Head length (direct) | 56.4 | 59.3 | 57.6 | 52.3 | 57.6 | 56.0 | 54.6 | 58.8 | 56.6 | 55.1 | 57.5 | 56.3 |
| Snout, prenasal (direct) | 23.5 | 26.6 | 25.0 | 24.2 | 26.6 | 25.4 | 22.7 | 25.0 | 23.5 | 23.4 | 26.6 | 24.9 |
| Nostril length | 3.6 | 5.2 | 4.3 | 4.2 | 4.9 | 4.5 | 3.8 | 4.9 | 4.5 | 4.4 | 5.1 | 4.7 |
| Nasal curtain, length | 6.4 | 7.1 | 6.8 | 5.4 | 6.9 | 6.2 | 6.7 | 7.8 | 7.4 | 6.3 | 7.1 | 6.8 |
| Nasal curtain, width | 11.2 | 14.1 | 12.7 | 10.9 | 11.7 | 11.3 | 12.9 | 14.5 | 13.2 | 11.0 | 12.6 | 11.7 |
| Distance between nostrils | 11.3 | 12.9 | 12.0 | 10.5 | 11.1 | 10.8 | 12.2 | 13.7 | 12.6 | 10.8 | 11.4 | 11.0 |
| Snout, preoral (direct) | 29.4 | 32.4 | 31.1 | 29.1 | 33.0 | 31.5 | 28.2 | 31.6 | 29.7 | 29.8 | 32.8 | 31.0 |
| Mouth width | 8.8 | 9.9 | 9.5 | 8.3 | 8.8 | 8.5 | 9.0 | 10.2 | 9.7 | 8.3 | 10.2 | 9.0 |
| Width, 1st gill slit | 2.7 | 3.3 | 2.9 | 2.6 | 3.4 | 3.0 | 2.7 | 3.7 | 3.0 | 2.6 | 3.2 | 2.9 |
| Width, 3rd gill slit | 2.9 | 3.7 | 3.4 | 2.9 | 3.6 | 3.2 | 3.1 | 3.9 | 3.4 | 3.1 | 3.7 | 3.3 |
| Width, 5th gill slit | 1.7 | 2.3 | 1.9 | 1.6 | 2.5 | 2.1 | 1.9 | 2.2 | 2.1 | 1.9 | 2.5 | 2.1 |
| Distance between 1st gill slits | 22.3 | 25.1 | 23.2 | 21.3 | 22.4 | 21.9 | 23.0 | 24.4 | 23.8 | 22.3 | 24.4 | 23.3 |
| Distance between 5th gill slits | 15.1 | 16.5 | 15.7 | 14.1 | 15.3 | 14.8 | 14.6 | 16.5 | 15.6 | 14.6 | 16.6 | 15.4 |
| Length pelvic fin | 20.7 | 23.4 | 22.3 | 20.0 | 23.3 | 21.2 | 22.0 | 24.5 | 23.3 | 18.6 | 24.0 | 22.4 |
| Width across pelvic fin base | 12.9 | 15.2 | 13.8 | 13.7 | 18.7 | 16.6 | 13.8 | 15.5 | 14.8 | 15.4 | 17.8 | 16.9 |
| Greatest width across pelvic fins | - | - | - | - | - | - | 40.4 | 42.5 | 41.6 | 34.3 | 42.1 | 37.8 |
| Tail width, axil of pelvics | 8.1 | 10.4 | 9.6 | 10.0 | 11.2 | 10.7 | 7.4 | 9.1 | 8.8 | 9.2 | 11.2 | 10.0 |
| Tail height, axil of pelvics | 5.4 | 6.5 | 5.8 | 5.2 | 6.5 | 5.9 | 5.3 | 6.1 | 5.6 | 5.4 | 6.6 | 5.9 |
| Tail width, base of sting | 3.7 | 6.4 | 5.2 | 4.1 | 5.4 | 4.8 | 4.2 | 5.6 | 5.3 | 5.1 | 5.9 | 5.5 |
| Tail height, base of sting | 3.0 | 4.0 | 3.5 | 2.9 | 3.6 | 3.3 | 3.3 | 3.9 | 3.8 | 3.5 | 4.2 | 3.8 |
| Cloaca length | 4.0 | 5.6 | 4.9 | 4.5 | 7.3 | 5.8 | 4.5 | 5.3 | 4.9 | 4.9 | 7.2 | 5.9 |
| Clasper, postcloaca length | 13.0 | 22.6 | 20.2 | - | - | - | 20.6 | 22.8 | 21.3 | - | - | - |
| Clasper, length from pelvic axil | 7.5 | 20.3 | 15.1 | - | - | - | 9.3 | 17.1 | 15.7 | - | - | - |
| Pect. insertion to sting origin | 25.1 | 32.1 | 28.1 | 24.8 | 30.9 | 27.8 | 20.2 | 26.7 | 23.4 | 20.7 | 27.0 | 23.7 |
| Cloaca origin to sting | 29.4 | 37.1 | 32.2 | 29.7 | 35.6 | 33.4 | 26.8 | 31.3 | 28.7 | 28.8 | 31.3 | 30.1 |
| Caudal sting 1 length | - | - | - | - | - | - | - | - | - | - | - | - |
| Caudal sting 2 length | - | - | - | - | - | - | - | - | - | - | - | - |
| Min | Max | |
|---|---|---|
| Disc, width (mm) | 164 | 234 |
| Total length | 296.3 | 309.2 |
| Disc, length (direct) | 99.8 | 105.5 |
| Disc, thickness | 11.0 | 13.5 |
| Snout to origin of cloaca | 83.8 | 89.5 |
| Cloaca origin to tail tip | 209.8 | 220.7 |
| Snout to pectoral insertion | 90.9 | 96.6 |
| Snout to maximum width | 46.8 | 49.0 |
| End of orbit to pectoral insertion | 57.2 | 61.5 |
| Snout, preorbital (direct) | 28.3 | 30.2 |
| Snout, preorbital (horizontal) | 26.2 | 29.1 |
| Orbit diameter | 5.1 | 6.0 |
| Eye diameter | 3.2 | 3.8 |
| Spiracle length | 5.6 | 6.6 |
| Orbit and spiracle length | 9.3 | 9.9 |
| Interorbital width | 11.4 | 12.9 |
| Intereye width | 15.1 | 16.7 |
| Distance between spiracles | 17.0 | 18.4 |
| Head length (direct) | 52.1 | 55.5 |
| Snout, prenasal (direct) | 24.1 | 26.0 |
| Nostril length | 3.3 | 3.9 |
| Nasal curtain, length | 10.0 | 11.9 |
| Nasal curtain, width | 4.8 | 5.7 |
| Distance between nostrils | 9.0 | 10.3 |
| Snout, preoral (direct) | 28.6 | 30.7 |
| Mouth width | 7.5 | 9.1 |
| Width, 1st gill slit | 2.8 | 3.4 |
| Width, 3rd gill slit | 2.8 | 3.3 |
| Width, 5th gill slit | 2.0 | 2.8 |
| Distance between 1st gill slits | 20.5 | 22.3 |
| Distance between 5th gill slits | 13.2 | 14.3 |
| Length pelvic fin | 17.3 | 19.7 |
| Width across pelvic fin base | 15.1 | 17.9 |
| Greatest width across pelvic fins | 33.7 | 42.2 |
| Tail width, axil of pelvics | 8.3 | 10.0 |
| Tail height, axil of pelvics | 5.2 | 6.7 |
| Tail width, base of sting | 3.8 | 4.6 |
| Tail height, base of sting | 2.9 | 3.2 |
| Cloaca length | 5.5 | 7.3 |
| Clasper, postcloaca length | 18.4 | 18.9 |
| Clasper, length from pelvic axil | 8.1 | 8.7 |
| Pect. insertion to sting origin | 33.9 | 39.1 |
| Cloaca origin to sting | 38.1 | 42.1 |
| Caudal sting 1 length | - | - |
| Caudal sting 2 length | - | - |
| Pakistan | Kuwait | ||||
|---|---|---|---|---|---|
| Holotype | Paratypes | Non-Types | |||
| USNM 222555 | Min | Max | BPBM 33199 | MCZ 59269 (1 of 5) | |
| Disc, width (mm) | 231 | 174 | 226 | 187 | 167 |
| Total length | 224.2 | 220.2 | 239.0 | 191.8 | 238.7 |
| Disc, length (direct) | 103.2 | 101.3 | 102.9 | 109.1 | 107.5 |
| Disc, thickness | 12.6 | 10.6 | 13.2 | 10.9 | 10.1 |
| Snout to origin of cloaca | 87.6 | 84.5 | 87.2 | 92.3 | 93.2 |
| Cloaca origin to tail tip | 136.6 | 134.1 | 152.2 | 98.6 | 145.4 |
| Snout to pectoral insertion | 94.0 | 88.7 | 93.7 | 100.2 | 100.1 |
| Snout to maximum width | 47.0 | 42.9 | 47.4 | 46.2 | 50.7 |
| End of orbit to pectoral insertion | 60.5 | 56.3 | 59.6 | 62.6 | 60.1 |
| Snout, preorbital (direct) | 28.5 | 28.0 | 30.0 | 31.1 | 34.2 |
| Snout, preorbital (horizontal) | 27.5 | 27.2 | 28.4 | 29.2 | 33.8 |
| Orbit diameter | 6.2 | 5.3 | 6.3 | 6.5 | 6.8 |
| Eye diameter | 4.4 | 3.8 | 4.5 | 4.4 | 4.4 |
| Spiracle length | 5.3 | 5.0 | 6.7 | 5.8 | 6.4 |
| Orbit and spiracle length | 10.0 | 9.1 | 10.5 | 11.4 | 11.6 |
| Interorbital width | 13.0 | 12.0 | 13.8 | 12.5 | - |
| Intereye width | 16.9 | 15.1 | 16.8 | 16.6 | - |
| Distance between spiracles | 16.4 | 16.2 | 17.7 | 16.6 | - |
| Head length (direct) | 55.8 | 53.9 | 56.1 | 57.0 | 59.7 |
| Snout, prenasal (direct) | 22.5 | 21.0 | 24.6 | 26.3 | 28.0 |
| Nostril length | 4.6 | 3.6 | 4.6 | 4.7 | 5.0 |
| Nasal curtain, length | 7.3 | 5.6 | 6.7 | 7.1 | 7.0 |
| Nasal curtain, width | 12.5 | 10.8 | 13.3 | 11.6 | 11.6 |
| Distance between nostrils | 11.7 | 11.1 | 12.4 | 10.6 | 11.5 |
| Snout, preoral (direct) | 28.2 | 26.5 | 30.0 | 32.1 | 34.2 |
| Mouth width | 10.0 | 8.8 | 10.4 | 9.8 | - |
| Width, 1st gill slit | 3.1 | 2.7 | 3.6 | 3.7 | 3.3 |
| Width, 3rd gill slit | 3.4 | 3.3 | 3.9 | 3.5 | 3.1 |
| Width, 5th gill slit | 2.6 | 2.1 | 2.8 | 2.6 | 2.5 |
| Distance between 1st gill slits | 24.1 | 21.5 | 23.1 | 21.9 | 22.1 |
| Distance between 5th gill slits | 16.0 | 13.6 | 15.6 | 14.9 | 15.0 |
| Length pelvic fin | 19.3 | 17.1 | 19.3 | 20.2 | 18.1 |
| Width across pelvic fin base | 16.3 | 15.9 | 18.3 | 18.0 | 14.9 |
| Greatest width across pelvic fins | 36.5 | 29.8 | 38.7 | 35.1 | 36.6 |
| Tail width, axil of pelvics | 8.3 | 8.1 | 10.4 | 9.4 | 9.8 |
| Tail height, axil of pelvics | 5.6 | 5.4 | 6.1 | 6.4 | 5.8 |
| Tail width, base of sting | 4.0 | 4.2 | 4.6 | 4.3 | 4.8 |
| Tail height, base of sting | 3.2 | 3.4 | 3.7 | 3.2 | 3.8 |
| Cloaca length | 5.7 | 4.6 | 7.0 | 7.2 | 5.9 |
| Clasper, postcloaca length | 18.5 | 11.7 | 17.1 | - | 17.9 |
| Clasper, length from pelvic axil | 7.2 | 5.5 | 7.4 | - | 8.2 |
| Pect. insertion to sting origin | 30.7 | 28.7 | 35.9 | 31.2 | 30.1 |
| Cloaca origin to sting | 35.8 | 31.5 | 40.2 | 37.4 | 36.0 |
| Caudal sting 1 length | 17.4 | 6.9 | 21.0 | 18.2 | 21.6 |
| Caudal sting 2 length | 24.6 | 23.0 | 23.5 | - | 29.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Last, P.R.; Weigmann, S.; Naylor, G.J.P. The Indo-Pacific Stingray Genus Brevitrygon (Myliobatiformes: Dasyatidae): Clarification of Historical Names and Description of a New Species, B. manjajiae sp. nov., from the Western Indian Ocean. Diversity 2023, 15, 1213. https://doi.org/10.3390/d15121213
Last PR, Weigmann S, Naylor GJP. The Indo-Pacific Stingray Genus Brevitrygon (Myliobatiformes: Dasyatidae): Clarification of Historical Names and Description of a New Species, B. manjajiae sp. nov., from the Western Indian Ocean. Diversity. 2023; 15(12):1213. https://doi.org/10.3390/d15121213
Chicago/Turabian StyleLast, Peter R., Simon Weigmann, and Gavin J. P. Naylor. 2023. "The Indo-Pacific Stingray Genus Brevitrygon (Myliobatiformes: Dasyatidae): Clarification of Historical Names and Description of a New Species, B. manjajiae sp. nov., from the Western Indian Ocean" Diversity 15, no. 12: 1213. https://doi.org/10.3390/d15121213
APA StyleLast, P. R., Weigmann, S., & Naylor, G. J. P. (2023). The Indo-Pacific Stingray Genus Brevitrygon (Myliobatiformes: Dasyatidae): Clarification of Historical Names and Description of a New Species, B. manjajiae sp. nov., from the Western Indian Ocean. Diversity, 15(12), 1213. https://doi.org/10.3390/d15121213