Abstract
Space utilization by animals is vital for species ecology but also a valuable predictor of habitat conditions and environment capacity for a given species. We investigated the ranging behavior of the white-tailed eagle, an apex predator experiencing a significant population increase and saturation. Comparing five adult floaters and two breeding males tracked with GPS loggers in Poland for 1–5 years, we observed substantial differences in space utilization. Breeding males occupied approximately 63 to 122 km2 (using 90% kernel density), while floaters ranged over roughly 6000 to 60,000 km2. Breeding males expanded their home ranges during successful breeding, with one male frequently flying 29 km to a foraging site when raising chicks but hardly doing so in other seasons. Both breeding males revisited nests more frequently in April and May (up to seven times daily, typically two to four), exhibiting distinct seasonal daily movement patterns. Floaters had slightly higher daily movement rates with a weak seasonal pattern. We conclude that breeding males’ ranging behavior depended on proximity to optimal foraging sites, while adult floaters engaged in prolonged wandering.
1. Introduction
Animals vary in their spatial utilization requirements. Typically, larger species require larger areas [1,2,3]. Nevertheless, dietary preferences also exert an influence on spatial necessities [1,3,4]. A multitude of factors influence spatial utilization within a species, encompassing factors such as sex, landscape characteristics, and climatic variables [5]. This underscores the significant role played by both internal and external factors in shaping animal space utilization. Consequently, space utilization not only holds ecological importance, but also serves as a metric describing environmental quality and the availability of resources within a given habitat. Thus, comprehensive studies on animal movement conducted within broad biogeographical and ecological contexts are indispensable for comprehending the relationship between space utilization and habitat conditions.
Mobility represents a fundamental characteristic of animals. Despite their capability to cover considerable distances, animals tend to confine their movements to specific areas where they fulfill essential activities like feeding, mating, sheltering, and caring for offspring. This area is commonly referred to as the “home range” [6,7]. The home range holds a central position in species ecology and is frequently investigated as a parameter of ranging behavior. It is also anticipated to serve as an indicator of an individual’s territorial quality. For central-place foragers and territorial species, smaller home ranges are indicative of higher quality, particularly concerning foraging opportunities [8,9]. Hence, comparative studies on home range size within a given species are regularly undertaken to assess optimal or suboptimal conditions across different populations. This information is also instrumental for estimating the suitability of various habitats for a particular species [10]. In addition to home range, other ranging parameters such as step length and daily movement distance are valuable indicators of resource distribution and landscape quality. These parameters assume that movement incurs costs and is subject to optimization. Therefore, animals tend to minimize movement when resources are abundant. This is especially true for central-place foragers [11] that are tethered to specific areas, like nest sites, to which they repeatedly return with resources. Such animals are expected to optimize the radius of their repeated daily movements. This behavior is characteristic of birds during the breeding season and territorial birds before and after breeding. However, not all individuals within a population are breeders; some individuals, although already mature enough, are showing delayed breeding, due to the low availability of mates or territories, or due to gaining benefits from such delay [12]. In birds, such individuals are called “floaters” as they have no mate, no fixed territory, and therefore display nomadic movements [13]. Do these individuals optimize their movements, even though they do not bear the costs of reproduction? Possibly not, rather they may range over large areas in order to find suitable and free territory and a mate. How mobile are they compared to breeders? Do they exhibit similar patterns of spatial utilization?
We conducted an investigation addressing the aforementioned issues within the white-tailed eagle, an apex predator characterized by a slow life history. During the breeding season it feeds on fish, waterfowl and other relatively large prey [12,13,14], which are hunted mainly in aquatic and wetland habitat. Therefore, the distribution of favorable habitats moderate the suitability of habitats (especially for breeders) and, in turn, the distribution of nest sites [14]. White-tailed eagles typically commence breeding in their fifth calendar year [15] and attain full adult plumage at that time [16]. However, owing to a substantial increase in their population numbers in recent decades [17,18,19], many adult individuals may still not establish territories, as the age of first reproduction is a density-dependent trait in avian predators [20]. This has led to an increase in the proportion of floaters in the population. Floaters seeking for their own territory and territorial adults have to compete for the resources and defend the space they need to breed from competitors. This is especially visible in the increase of territorial behavior in May [21], when eaglets experience their most significant growth and exhibit heightened food demands [22].
Given the expanding populations of breeders and the rising number of floaters in this apex predator species, a crucial question arises: what are the spatial requirements of white-tailed eagles, and do these requirements vary over time and space? Earlier data, primarily based on visual observations, suggested that white-tailed eagles utilize approximately 20–30 km2, 19–115 km2, or 60–120 km2 in Germany, depending on the specific study (as reviewed by Krone and co-authors 2013) [23]. In Russia, home ranges were estimated to be 60–80 km2 or 28–30 km2, while in Norway, only 6–9 km2 (as reviewed by Mizera [24]). Current and objective movement data for adult eagles remain exceedingly scarce within this species. This scarcity is primarily due to the difficulty of trapping adults, while tagging juvenile eagles rarely results in tags surviving until they reach the age of first breeding. A limited number of adult individuals were tracked in Estonia, Latvia, and Russia [25,26,27], but as of now, their space utilization results have not been published. The sole published data on the movements of breeding adult white-tailed eagles stem from Germany, where eight breeding adults were GPS-tracked between 2003 and 2009 [28]. All of these eagles were baited with food and captured as adults in the wild. Their home ranges were estimated using the 95% kernel density estimate (kde) method, ranging from 2.4 to 83.7 km2 (with an average of 13.7 ± 28.9 km2) and the 95% minimum convex polygon (mcp) method, ranging from 6.0 to 392.5 km2 (with an average of 91.2 ± 130.9 km2). These adults ventured up to 38 km from their nests, although the registered locations were on average approximately 3.3 ± 1.6 km from their nests. The greater the distance to potential hunting sites, mostly waterbodies, the more extensive the distances the eagles had to cover. Some feeding sites were located 15–40 km away from the intact territories around the nests [28]. Unfortunately, data on the ranging behavior of adult floaters are conspicuously absent in the existing literature. For instance, a single individual reported from Germany ranged over an area of a thousand square kilometers [28], which is considerably more extensive than in breeding adults, but still less than in dispersing juveniles [29].
2. Materials and Methods
2.1. GPS Telemetry Dataset
All the white-tailed eagles included in our study were equipped with GPS loggers primarily in the North-Eastern part of Poland, particularly in the Podlasie region. However, they frequently roamed into neighboring countries, primarily Lithuania, Belarus, and Russia. Notably, one individual (HALB11) was also tracked in central Poland. These eagles were obtained from two distinct sources (as detailed in Table 1): First, four eagles in full adult plumage were captured from the wild when they were weakened or injured, rehabilitated, and subsequently released with GPS loggers attached. The second source consisted of three individuals equipped with loggers while still in their nests, and tracked for a sufficient duration to reach adulthood [30,31]. In the case of the latter group, we exclusively utilized data collected during their sixth and seventh calendar years (cy). The sex of the juveniles was determined using molecular methods in the laboratory of the University of Łódź, while the sex of the adult eagles was identified based on their weight and biometric measurements. Our study focused on data from years when there were at least three full months of individual movement records available. This timeframe was deemed adequate for estimating home range size in resident and territorial birds of prey, as even as little as 15 days of data have been shown to be sufficient for reliable home range assessments in species like Bonelli’s eagles and Golden eagles [32]. In the case of non-territorial individuals, three months of data are also informative in terms of assessing the scale of their displacements.

Table 1.
White-tailed eagle GPS telemetry dataset characteristics.
The primary objective of our study was to investigate the ranging behavior of adult white-tailed eagles. In this long-living species, we classified individuals as adults based on their full adult plumage, in accordance with the description by Forsman [18]. Consequently, the rehabilitated adults included in this study were at least in their fifth year, and were considered potential breeders or individuals with the potential to breed in the near future. Remarkably, two out of the four rehabilitated eagles turned out to be territorial individuals, returning immediately to territories, where they stayed and bred during the whole tracking period. The other two rehabilitated adults were not confirmed to be breeding, similar to individuals tracked from fledging. In most of the individuals and different seasons in which they were tracked, we noted high mobility during the breeding period (February–June). The (i) absence of a central-place foraging pattern typical for feeding adults (especially males) and (ii) the lack of very limited movement, restricted to one forest location, typical of incubating females, was considered an indication of floating behavior. In one case, we noted a central-place foraging pattern in spring and attempted to confirm breeding directly in the field.
All the eagles were fitted with GPS loggers manufactured by the Ecotone company (different models were used: Saker, Kite, and Griffon), weighing between 35 g and 50 g. These devices were mounted as backpacks using Teflon ribbons sewn at the sternum using the “Y” method, a method recommended for Bald eagles by Buehler et al. [33]. The GPS fixes were acquired at intervals ranging from 0.5 to 6 h, depending on the battery level of the individual being tracked. During spring and summer, shorter intervals of typically between 0.5 and 2 h were employed, while in late autumn, when solar charging potential was reduced, longer intervals between 2 and 6 h were utilized. An exception was the HALB11, which was equipped with a modern logger transmitting data at 6 h intervals through GSM and at 15 min intervals through GPRS. The first dataset was used in a manner consistent with other units for estimating home range, whereas the second dataset (one with higher frequency data) was particularly useful for calculating nest re-visitations.
The dataset was provided with information on the breeding success of the breeders. Each season, we monitored nests of the breeding males BIEL05 and HALB11 at least twice. First, we did so to confirm their breeding in the given year (nests were visited from the ground in the break of winter and spring). Second, we did so to check the breeding success and count the number of fledglings (nests were visited in June).
2.2. Data Analyses
All data analyses were performed using R 4.1.1 software, and the preparation of maps was conducted in QGIS 3.22. Home ranges were estimated using two well-established methods: mcp and kde. Both of these estimators are commonly recognized and have been utilized in the limited studies on adult white-tailed eagle home ranges [28,34]. Employing these same estimators allowed us to make meaningful comparisons of home range sizes with existing data. The calculations for both mcp and kde home ranges were carried out using the ‘adehabitatHR’ package [35] for the 50th, 75th, 90th, and 95th percentiles. The kde home range was estimated using a reference bandwidth, a bivariate normal kernel, an extent of 1.5, and different grids for each individual, but with each grid of the same size (250).
The daily distances covered by the individuals were computed using the ‘moveHMM’ package [36]. Step lengths between consecutive relocations were calculated and summarized on a daily basis. Generalized additive models, implemented in the ‘mgcv’ package [37], were utilized to model the daily mobility of breeding eagles and floaters over time. These models explained the daily distance traveled with respect to the day of the year, using a gamma distribution, and smoothed the data with regression splines.
To investigate the factors compelling breeding eagles to increase their mobility during the breeding season, we examined the number of potential prey deliveries to the nests. This analysis focused exclusively on data from white-tailed eagle males that successfully bred (e.g., 3 seasons of BIEL05, 1 season of HALB11). GPS tracking locations were limited to the period from February to August, starting from the beginning of incubation until the time when the juveniles left the nest vicinity. The count of nest revisits was conducted within a radius of 200 m from the nest using the ‘recurse’ package [38]. This radius encompassed the nest and its immediate vicinity, which is the area where male eagles might transfer prey to the female or fledglings.
3. Results
3.1. GPS Telemetry Dataset
The dataset of rehabilitated individuals included three individuals recovered after poisoning and one after electrocution (HALB11). Among them, BIEL01 and BIEL05 were successfully tracked for 2 and 5 years, respectively. BIEL01 ceased transmitting with no indications of mortality, while BIEL05 managed to shed its tag after a full 5 years of tracking. HALB11 has been successfully transmitting data for over 2 years to date. Regrettably, HALB08 was tracked for only half a year, after which it was found weakened once again, rehabilitated, released, and ultimately discovered deceased a few weeks later. The data from the shorter tracking period following the second rehabilitation were not included in this dataset.
Among the eight juveniles equipped with loggers in 2017–2018, as documented in previous studies [30,31], only three still transmitted in their 6th calendar year. HALB04 ceased transmitting data at the beginning of 2023 but did not establish a territory during the entire tracking period. Similarly, HALB02 is still transmitting data, and it did not exhibit signs of territorial behavior, manifested by attachment to relatively limited area. Only HALB05 displayed indications of its first breeding attempt in its sixth calendar year, with very limited movement during the incubation period and central-place foraging during the transition from spring to summer. However, the potential nesting site was located in Belarus, near the border with Lithuania, making it impossible for us or local ornithologists to inspect (although we did attempt to do so). HALB05 may have attempted to breed, but the fact that it left the potential territory in the early summer suggests that the breeding attempt was unsuccessful, or rather this individual might have built the first nest there but did not settle. Such a situation was observed on a tagged eagle that built its first nest in early spring of the eighth year, and left this site in May [26]. Therefore, finally we classified this individual as a floater.
3.2. Home Range
White-tailed eagles ranged over large areas, but often overlapped significantly between following seasons (Figure 1). The annual home range size of white-tailed eagles varied greatly depending on the estimator used (Table 2). Core home ranges (kde50%, mcp50%) might be as small as 5–30 km2 in breeding individuals, but also dozens of thousands of square kilometers in floaters. The mean 50% kde annual home range of floaters reached 5892 km2 (±5059), while 95% kde reached 21,209 km2 (±18,744). Breeding males used a much smaller area. The mean annual home range of two breeding males reached 278 km2 (±232), when estimated with 95% kde, but only 102 km2 (±59) when estimated with 90% kde. Mean core home ranges (50% kde) reached 17.7 km2 (±9.3). Both males differed in their mean annual home range almost two-fold in the case of the 95% kde home range, which reached 336 and 162 km2, respectively in BIEL05 and HALB11, and in case of 90% kde, which reached 122 and 63 km2, respectively. The difference in core home range (50% kde) was less significant and showed the opposite trend. The first male used less space than the second one (15 vs. 23 km2).
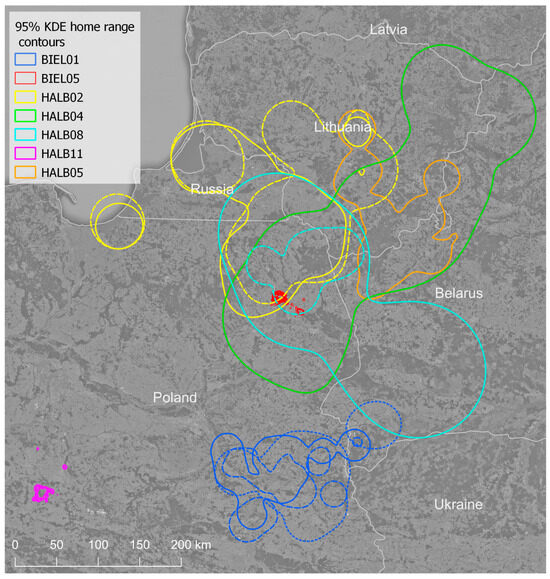
Figure 1.
Annual home ranges of adult white-tailed eagles (breeders and floaters) calculated with 95% kernel density estimation in course of this study. Colors represent different individuals, while different line styles relate to different years, if more than a single season was studied. BIEL05 and HALB11 are breeding individuals.

Table 2.
Home range estimates of the GPS-tracked white-tailed eagles from Poland, calculated with two methods (KDE—kernel density estimation, MCP—minimum convex polygon) and different percentile values.
The home ranges of white-tailed eagles clearly exhibited differences between breeders and floaters, as depicted in Figure S1 (kde 95%, W = 0, p < 0.0001). Within the group of floaters, there was no significant difference in home range size between males and females (kde 95%, W = 14, p = 0.26). In the group of breeders, annual home range size was significantly larger in years of successful than unsuccessful breeding (W = 1, p = 0.032). However, it is important to note that this result was primarily driven by one of the two tested males, while the other showed similar home range sizes, regardless of nesting success.
The home ranges of breeding males extended to encompass external foraging sites, as illustrated in Figure 2. HALB11, during one of the studied years, utilized a waterbody located 45 km from its nest (Figure 2A). In the case of BIEL05, during years of successful breeding (Table S1), it frequently employed a waterbody located 29 km from its nest for foraging (Figure 2B and Figure S2). In other years, its primary foraging site consisted of a large artificial channel situated on the periphery of a vast wetland, specifically within the Biebrza National Park.
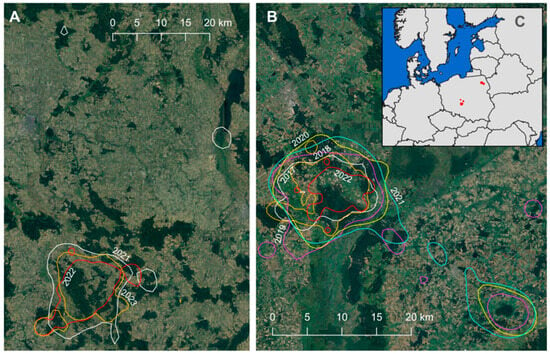
Figure 2.
Annual home range estimations (kde 95%) of two white-tailed eagle males: HALB11 (A) and BIEL05 (B) tracked with GPS loggers and their general location (C).
3.3. Daily Movements
White-tailed eagles exhibited distinct movement patterns between breeders and floaters. On average, breeding males covered approximately 16.3 km per day (±20.9), which was less than the daily distance covered by floaters, averaging 19.2 km per day (±29.2). However, the average distance values may not be highly informative because daily distances covered by breeding males displayed considerable variation throughout the year (Figure 3, Table S2). Male eagles tended to move the most between mid-April and mid-June, while their movements were less pronounced during the winter months (Figure 3A). The model explained almost 30% of the daily distance variance in breeding males, with day of the year showing a much greater effect than the random effect of the individual (Table S1). There were substantial differences in the daily distances covered between different years, with a sevenfold variation.
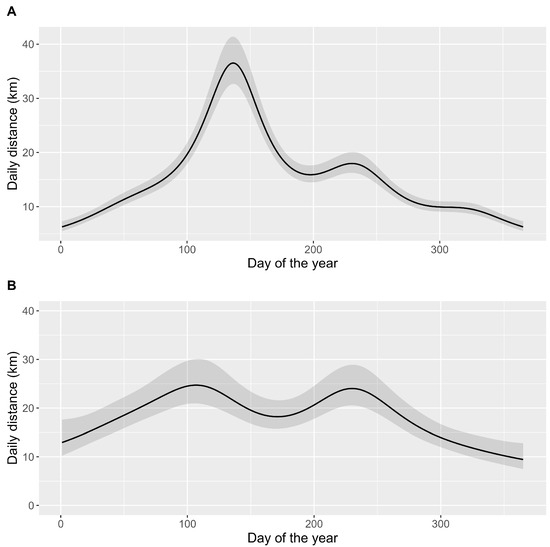
Figure 3.
Comparison of adult white-tailed eagle daily distance covered by breeders (A) and floaters (B) tracked with GPS loggers. Black line shows the trend calculated with generalized additive models with 95% confidence interval (dark grey).
In contrast, the daily distance covered by floaters showed a smaller, twofold difference throughout the year (Figure 3B), and the model explained only 4.4% of the variance in daily distances (Table S2). The random effect of the individual was relatively high, but lower than the effect of the day of the year. The peak mobility of floaters was observed around mid-April and mid-August.
3.4. Prey Delivery Movements
The mobility of white-tailed eagles was indeed influenced by the food requirements of their chicks. Males that successfully raised their offspring not only covered longer daily distances during the spring, but also made a higher number of nest revisits, which can be interpreted as prey deliveries. In a successful breeding season, which extended from incubation in February to the late chick stage in August, white-tailed eagle males revisited their nest sites from one to seven times per day. One of the males (BIEL05) displayed a distinct peak in April and May, during which he made an average of 4.3 and 4.1 nest revisits per day, respectively. The overall average number of nest revisits throughout the entire breeding season was 2.85, with a higher number of revisits in the early breeding phase (3.2 in March) compared to the late phase (1.8 in July and August) (Figure 4A).
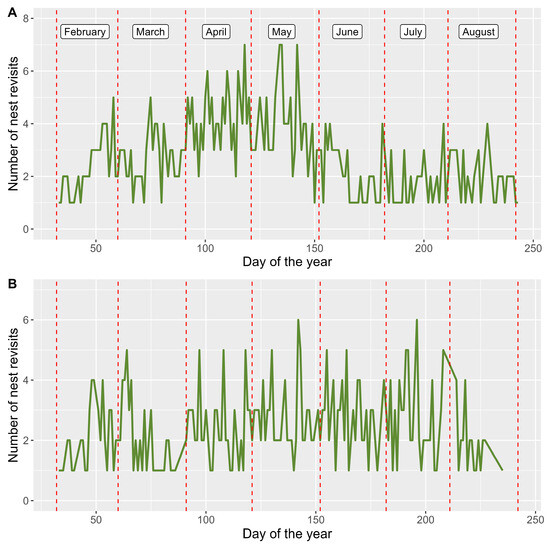
Figure 4.
Number of daily nest revisits during successful breeding seasons, as a proxy of number of prey deliveries by two white-tailed eagle males, BIEL05 (A) and HALB11 (B), followed with GPS loggers.
The other male (HALB11) revisited the nest site less frequently, averaging 1.99 times per day during a successful breeding season. The number of nest revisits reached its peaks in April, May, and June, with 2.4, 2.6, and 2.5 revisits per day, respectively. However, the number of revisits dropped significantly in August (0.9), when he ceased visiting the nest for approximately half of the month.
4. Discussion
4.1. Ranging Behavior of Breeders
Breeders and floaters exhibited marked differences in their ranging behavior, encompassing not only the extent of their spatial coverage but also their annual mobility patterns. Breeding individuals, as expected, displayed a strong attachment to their nest during the breeding period, although this attachment might decrease post-breeding. Historically, breeding white-tailed eagles were believed to occupy more extensive territories, covering areas of up to 600 square kilometers [24]. Moreover, even in Europe, they exhibited seasonal movements during severe winters [23], a behavior still observed in this species in Siberia and Kamchatka [25,39].
Our current study demonstrates that breeding males in Poland maintained relatively stable home ranges, ranging from approximately 62 (90% kde) to 119 km2 (95% kde) during unsuccessful breeding attempts. Nevertheless, when caring for their offspring and undertaking longer travels, the size of the home range may expand to nearly 700 km2. This latter value is primarily a consequence of the density of observations along their frequent flight paths to a limited foraging area, which covers only about 5 km2 but is located roughly 29 km from the nest. While the utilization distribution method employing the 95th percentile of data (excluding 5% of outliers) is commonly used for home range estimation, for highly mobile species like the white-tailed eagle, we recommend considering the 90th percentile. In our case, the difference between the 90th and 95th percentiles resulted in a doubling of the estimated utilization area, albeit with only a 5% increase in the likelihood of its use by the focal individual. Therefore, we consider the 90th KDE to be a more reliable measure of white-tailed eagle space utilization requirements in this context. Nonetheless, the mean home range during the breeding season in Poland ranged from 63 to 122 km2 (see Table 2), which is significantly larger than the home ranges of German eagles, which spanned from 2.4 to 83.7 km2 [28]. Several factors could account for the smaller home ranges observed in Germany compared to Poland. First, both studies were conducted at similar latitudes, but the climate conditions in northeastern Poland are more continental. More importantly, the landscapes in the two regions differed. The German study was primarily conducted in the lake-dominated landscape of Mecklenburg–Western Pomerania, and partly in Brandenburg, which features numerous water bodies. In contrast, one of the breeding males studied in Poland (BIEL05) inhabited the periphery of the natural river valley (Biebrza Valley), lacking direct access to the main river, which was already a foraging site for other territorial birds. Therefore, the primary territory offered relatively modest foraging opportunities, with the optimal foraging site located approximately 29 km away. The other male (HABL11) occupied a mosaic of forests and farmland, with two fish pond complexes situated 5 and 7 km apart serving as his main foraging areas. In comparison, the German eagles were found an average of 3.3 km from their nests [28], and their distance to optimal foraging sites was shorter, resulting in smaller home ranges. The dietary analysis also suggested that white-tailed eagles in lower-quality landscapes compensated for less optimal prey by traveling greater distances to capture larger prey [16], thereby influencing their ranging behavior. Secondly, methodological considerations could contribute to the significant disparity in home range sizes between German and Polish eagles. In our study, we monitored two individuals over three and five breeding seasons, collecting over 2300 GPS fixes from the first individual and over 23,000 GPS fixes from the second. In contrast, earlier German studies employed lower-frequency data collection, resulting in a smaller number of GPS fixes, ranging from 81 to 571 for most individuals and just over 4500 points for a single individual [28]. The sample size used for home range estimation, to some extent, influences the results [40], and the smoothing parameter employed in the kernel density function significantly impacts the final estimate [41].
4.2. Seasonal Difference in the Ranging Behavior of Breeders
One of the studied males displayed a tendency to cover extensive distances only during the years when he successfully raised chicks. In instances where breeding failed before hatching, this male seldom visited remote foraging sites (see Figure S2). The other male did not exhibit a similar response, and continued to use the same waterbodies on a daily basis, regardless of the breeding outcome. However, it is important to note that the waterbodies in this case were located five to six times closer. Consequently, the comparison of annual home ranges in years when males bred successfully and unsuccessfully revealed a significant difference. Nevertheless, it should be acknowledged that this result was influenced by the behavior of one of these males. Krone and Treu [28] did not observe differences in home ranges related to sex and breeding status, but it is worth noting that their sample size suffered from a low number of cases, with only one male and one female achieving breeding success in a single season.
The ranging behavior of breeding males exhibited distinct annual dynamics. Daily distances covered were at their lowest from October to February when they were not engaged in breeding activities. However, from January during the pre-breeding period, daily distances gradually increased. The primary peak of activity was clearly associated with the heightened demands for food provisioning to growing chicks. Daily distances sharply increased from the beginning of April when chicks hatched, peaking around the 10th of May, when chicks were approximately halfway to fledging. Daily distances then returned to an average level around the 20th of June when the chicks were fully grown but still in close proximity to the nest [42]. During the summer and early autumn, adults continued to be more mobile than during the winter months since they continued to provide supplementary food to their offspring until they dispersed, which usually occurred in September and October [29,30,31,43].
The increase in mobility was also reflected in the number of nest revisits, which we consider as a proxy for prey deliveries. Both males increased the frequency of nest revisits during successful breeding seasons, and in one of them, the daily distance covered and the number of nest revisits closely overlapped. White-tailed eagles have been reported to deliver prey to the nest once every 5–13 h [42], which translates to 2–4 prey deliveries per day, generally aligning with our data from the HALB11 male. However, the other male might have made up to 7 deliveries at maximum and continued revisiting the nest daily throughout the entire season.
4.3. Ranging Behavior of Floaters
Floaters were expected to differ from breeders in terms of their space use. In another apex predator species, the Eagle Owl Bubo bubo, floaters used smaller home ranges than breeders, but exhibited higher activity throughout the whole night, while breeders exhibited the most activity at its beginning and ending [44]. On the contrary, in our study, floaters showed to range further, compared to breeders. Annually, they covered areas spanning thousands or even tens of thousands of square kilometers. Despite being adults, they did not differ substantially from dispersing juveniles from the Czech Republic, Austria, and Hungary, who roamed over an area of 13, 376 km2 (median 95% mcp) [29]. Ranging data suggested that immature eagles roamed relatively vast territories, but subadults in their fourth calendar year began seeking breeding territories, often in proximity to their natal sites, and some even initiated breeding attempts [17]. It is important to note that these findings were based on ringing recoveries from the period between 1977 and 2006, and the white-tailed eagle population is currently much more saturated, with young adults still in search of territories. For example, one adult male tracked in Latvia did not breed for the first time until its ninth calendar year [26].
In our study, there was no significant difference in the areas covered by males and females. There might be no globally consistent trend in white-tailed eagles’ ranging behavior between the sexes. During the post-fledging ranging behavior of juveniles, no differences were noted in Germany [45]. However, males roamed over larger areas in a study conducted in the Czech Republic, Austria, and Hungary [29]. Conversely, females were the ones dispersing further in Norway [46].
On average, floaters moved slightly greater distances than breeding males, but their daily movement rates were less predictable throughout the season (the model explaining daily distance with the date of the year explained only a marginal part of the variance, as shown in Table S1). The contrast between spring and summer movements, compared to autumn and winter movements, was much less pronounced than in the case of breeders. Nevertheless, there were two minor peaks of greater movement in mid-April and mid-August, which might be associated with exploratory behavior or shifts in foraging sites. The average daily distance reached 19 km, although it is worth noting that this value is not absolute, as the daily distance calculated from telemetry data is significantly influenced by the sampling interval in order to account for the tortuosity of movement paths [47].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15121208/s1, Figure S1: Comparison of 95% kernel density estimated home ranges of white-tailed eagle breeders and floaters and successfully and unsuccessfully breeding males; Figure S2: Movements of white-tailed eagle male, Table S1: Productivity (number of chicks fledged) of white-tailed eagle breeding males during the tracking period. Zeros mean unsuccessful breeding Table S2: Summary of generalized additive models on daily distance covered by white-tailed eagle breeders and floaters across the year.
Author Contributions
Conceptualization, P.M. and D.A.; methodology, P.M.; software, P.M.; validation, P.M. and D.A.; formal analysis, P.M.; resources, D.A. and P.M.; data curation, P.M. and D.A.; writing—original draft preparation, P.M.; writing—review and editing, P.M. and D.A.; visualization, D.A.; project administration, D.A.; funding acquisition, D.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by the Forest Fund under the grant “Studies of behavior and dispersal of juvenile white-tailed eagles in Wigry National Park with the use of GPS/GSM trackers”, stage 1 (grant number EZ.0290.1.39.2017) and 2 (EZ.0290.1.36.2018).
Institutional Review Board Statement
Permission to tag wild eagles was given by the Regional Directorate for Environmental Protection in Białystok and the Ministry of Environment (in the case of individuals tagged in Wigry National Park).
Data Availability Statement
GPS telemetry data of floaters are stored in Movebank under ID 384868221. GPS telemetry data of breeders are hidden as they show current nest sites of a species under strict protection and zone protection of nest sites.
Acknowledgments
The authors would like to thank Dorota Zawadzka, Grzegorz Zawadzki, Krzysztof Henel, Tomasz Janiszewski, Stefan Lewandowski and Jarosław Obierzalski for their help in the field. They also thank Wigry National Park for their help and permission in carrying out part of this research on juvenile eagles in their park. Lastly, we also thank the two anonymous reviewers for their comments that helped us to improve this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Milton, K.; May, M.L. Body weight, diet and home range area in primates. Nature 1976, 259, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, D.; Cairns, S.C.; Oliverio, M.; Boitani, L. Body mass as a predictive variable of home-range size among Italian mammals and birds. J. Zool. 2006, 269, 317–330. [Google Scholar] [CrossRef]
- Ofstad, E.G.; Herfindal, I.; Solberg, E.J.; Sæther, B.-E. Home ranges, habitat, and body mass: Simple correlates of home range size in ungulates. Proc. Roy. Soc. B 2016, 283, 20161234. [Google Scholar] [CrossRef] [PubMed]
- Peery, M.Z. Factors Affecting Interspecies Variation in Home-range Size of Raptors. Auk 2000, 117, 511–517. [Google Scholar] [CrossRef]
- Mirski, P.; Cenian, Z.; Dagys, M.; Daróczi, S.; Dementavičius, D.; Maciorowski, G.; Menderski, S.; Nowak, D.; Pongrácz, Á.; Prommer, M.; et al. Sex-, landscape- and climate-dependent patterns of home-range size—A macroscale study on an avian generalist predator. IBIS 2021, 163, 641–657. [Google Scholar] [CrossRef]
- Burt, W.H. Territoriality and home range concepts as applied to mammals. J. Mammal. 1943, 24, 346–352. [Google Scholar] [CrossRef]
- Seaman, D.E.; Powell, R.A. Identifying Patterns and Intensity of Home Range Use. Bears Biol. Manag. 1990, 8, 243–249. [Google Scholar] [CrossRef]
- Bjørneraas, K.; Herfindal, I.; Solberg, E.J.; Sæther, B.E.; van Moorter, B.; Rolandsen, C.M. Habitat quality influences population distribution, individual space use, and functional responses in habitat selection by a large herbivore. Oecologia 2012, 168, 231–243. [Google Scholar] [CrossRef]
- Patenaude-Monette, M.; Bélisle, M.; Giroux, J.-F. Balancing Energy Budget in a Central-Place Forager: Which Habitat to Select in a Heterogeneous Environment? PLoS ONE 2014, 9, e102162. [Google Scholar] [CrossRef]
- Šálek, M.; Drahníková, L.; Tkadlec, E. Carnivores along the natural–urban habitat gradient. Mammal. Rev. 2015, 45, 1–14. [Google Scholar] [CrossRef]
- Rosenberg, D.K.; McKelvey, K.S. Estimation of Habitat Selection for Central-Place Foraging Animals. J. Wildl. Manag. 1999, 63, 1028–1038. [Google Scholar] [CrossRef]
- Zack, S.; Stutchbury, B.J. Delayed breeding in avian social systems: The role of territory quality and “floater’ tactics. Behaviour 1992, 123, 194–219. [Google Scholar] [CrossRef]
- Smith, S.M. The “Underworld” in a Territorial Sparrow: Adaptive Strategy for Floaters. Am. Nat. 1978, 112, 571–582. [Google Scholar] [CrossRef]
- Dementavičius, D.; Rumbutis, S.; Virbickas, T.; Vaitkuviene, D.; Dagys, M.; Treinys, R. Spatial and temporal variations in the White-tailed Eagle Haliaeetus albicilla breeding diet revealed by prey remains. Bird Study 2020, 67, 206–216. [Google Scholar] [CrossRef]
- Zawadzka, D.; Zawadzki, G. Changes in Avian Top-Predator Diet in the 21st Century in Northeast (NE) Poland. Diversity 2023, 15, 1144. [Google Scholar] [CrossRef]
- Mirski, P.; Komar, E. The White-Tailed Eagle, the Apex Predator, Adjusts Diet towards Larger Prey in Suboptimal Territories. Diversity 2023, 15, 747. [Google Scholar] [CrossRef]
- Struwe-Juhl, B.; Grünkorn, T. Results of colour-ringing White-tailed Sea Eagles Haliaeetus albicilla in Schleswig-Holstein: Site fidelity, movements, dispersal, age of first breeding, age structure and breeding of siblings. Vogelwelt 2007, 128, 117–129. [Google Scholar]
- Forsman, D. The Raptors of Europe and the Middle East: A Handbook of Field Identification; T. & A.D. Poyser: London, UK, 1997. [Google Scholar]
- Bělka, T.; David, H. The White-tailed Eagle Haliaeetus albicilla in the Czech Republic. Denisia 2009, 27, 65–77. [Google Scholar]
- Herrmann, C.; Krone, O.; Stjernberg, T.; Helander, B. Population development of Baltic bird species: White-tailed Sea Eagle Haliaeetus albicilla. In HELCOM Indicator Fact Sheets; HELCOM: Helsinki, Finland, 2009. [Google Scholar]
- Treinys, R.; Dementavičius, D.; Rumbutis, S.; Švažas, S.; Butkauskas, D.; Sruog, A.; Dagys, M. Settlement, habitat preference, reproduction, and genetic diversity in recovering the white-tailed eagle Haliaeetus albicilla population. J. Ornithol. 2016, 157, 311–323. [Google Scholar] [CrossRef]
- Katzenberger, J.; Gottschalk, E.; Balkenhol, N.; Waltert, M. Density-dependent age of first reproduction as a key factor for population dynamics: Stable breeding populations mask strong floater declines in a long-lived raptor. Anim. Conserv. 2021, 24, 862–875. [Google Scholar] [CrossRef]
- Krone, O.; Nadjafzadeh, M.; Berger, A. White-tailed Sea Eagles Haliaeetus albicilla defend small home ranges in north-east Germany throughout the year. J. Ornithol. 2013, 154, 827–835. [Google Scholar] [CrossRef]
- Mizera, T.; Bielik. Monografie Przyrodnicze, Zeszyt 4; Lubuski Klub Przyrodników: Świebodzin, Poland, 1999; p. 195. ISBN 83–87846–04-X. [Google Scholar]
- Ptchelintsev, V.G. Movements of the mature White-tailed Eagle specimens. In Proceedings of the Collection of Abstracts and Short Notes of the SEAEAGLE 2017 Conference 2017, Roosta, Estonia, 5–7 October 2017; Eagle Club: Lääne County, Estonia; p. 89. [Google Scholar]
- Kuze, J. Temporary nests built by the tagged newly territorial adult White-tailed Eagle. In Proceedings of the Collection of Abstracts and Short Notes of the SEAEAGLE 2017 Conference, Roosta, Estonia, 5–7 October 2017; Eagle Club: Lääne County, Estonia; pp. 60–61. [Google Scholar]
- Birdmap. Available online: https://birdmap.5dvision.ee (accessed on 30 October 2023).
- Krone, O.; Treu, G. Movement patterns of white-tailed sea eagles near wind turbines. J. Wildl. Manag. 2018, 82, 1367–1375. [Google Scholar] [CrossRef]
- Rymešová, D.; Raab, R.; Machálková, V.; Horal, D.; Dorňáková, D.; Rozsypalová, L.; Spakovszky, P.; Literák, I. First-year dispersal in white-tailed eagles Haliaeetus albicilla. Eur. J. Wildl. Res. 2021, 67, 44. [Google Scholar] [CrossRef]
- Mirski, P.; Anderwald, D.; Lewandowski, S.; Pieczyński, P.; Zawadzka, D. Przemieszczenia juwenalnych bielików z Wigierskiego Parku Narodowego po opuszczeniu gniazd. Stud. I Mater. Cent. Edukac. Przyr.-Leśnej 2017, 53, 56–66. [Google Scholar]
- Mirski, P.; Anderwald, D.; Pieczyński, P.; Zawadzka, D. Znaczenie miejsca gniazdowego i przebieg usamodzielniania się młodych bielików z Wigierskiego Parku Narodowego. Stud. I Mater. Cent. Edukac. Przyr.-Leśnej 2018, 54, 134–144. [Google Scholar]
- Morollón, S.; Urios, V.; López-López, P. Fifteen days are enough to estimate home-range size in some long-lived resident eagles. J. Ornithol. 2022, 163, 849–854. [Google Scholar] [CrossRef]
- Buehler, D.A.; Fraser, D.A.; Fuller, M.R.; McAllister, L.S.; Seegar, J.K.D. Captive and field-tested radio transmitter attachments for bald eagles. J. Field Ornithol. 1995, 66, 173–180. [Google Scholar]
- Krone, O.; Treu, G.; Grünkorn, T. Satellite Tracking of White-Tailed Sea Eagles in Mecklenburg-Western Pomerania and Brandenburg. In Birds of Prey and Wind Farms; Hötker, H., Krone, O., Nehls, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 207–225. [Google Scholar]
- Calenge, C. The package adehabitat for the R software: Tool for the analysis of space and habitat use by animals. Ecol. Model. 2006, 197, 1035. [Google Scholar] [CrossRef]
- Michelot, T.; Langrock, R.; Patterson, T.A. moveHMM: An R package for the statistical modelling of animal movement data using hidden Markov models. Meth. Ecol. Evol. 2016, 7, 1308–1315. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An introduction with R, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Bracis, C.; Bildstein, K.; Mueller, T. Revisitation analysis uncovers spatio-temporal patterns in animal movement data. Ecography 2018, 41, 1801–1811. [Google Scholar] [CrossRef]
- Ueta, M.; Sato, F.; Lobkov, E.G.G.; Mita, N. Migration route of White-tailed Sea Eagles Haliaeetus albicilla in northeastern Asia. IBIS 1998, 140, 684–686. [Google Scholar] [CrossRef]
- Girard, I.; Ouellet, J.-P.; Courtois, R.; Dussault, C.; Breton, L. Effects of Sampling Effort Based on GPS Telemetry on Home-Range Size Estimations. J. Wildl. Manag. 2002, 66, 1290–1300. [Google Scholar] [CrossRef]
- Worton, B.J. Using Monte Carlo Simulation to Evaluate Kernel-Based Home Range Estimators. J. Wildl. Manag. 1995, 59, 794–800. [Google Scholar] [CrossRef]
- Hardey, J.; Crick, H.; Wernham, C.; Riley, H.; Etheridge, B.; Thompson, D. Raptors: A Field Guide to Survey and Monitoring, 3rd ed.; The Stationery Office: Edinburgh, UK, 2013. [Google Scholar]
- Anderwald, D.; Lubińska, K. Proces usamodzielniania się śledzonych telemetrycznie bielików Haliaeetus albicilla z Parku Narodowego “Bory Tucholskie” w okresie post-pisklęcym. For. Res. Pap. 2021, 82, 131–142. [Google Scholar] [CrossRef]
- Penteriani, V.; del Mar Delgado, M.; Campioni, L. Quantifying space use of breeders and floaters of a long-lived species using individual movement data. Sci. Nat. 2015, 102, 21. [Google Scholar] [CrossRef]
- Engler, M.; Krone, O. Movement patterns of the White-tailed Sea Eagle Haliaeetus albicilla: Post-fledging behaviour, natal dispersal onset and the role of the natal environment. IBIS 2021, 164, 188–201. [Google Scholar] [CrossRef]
- Bevanger, K.; Berntsen, F.; Clausen, S.; Dahl, E.L.; Flagstad, Ø.; Follestad, A.; Halley, D.; Hanssen, F.; Hoel, P.L.; Johnsen, L.; et al. Pre- and post-construction studies of conflicts between birds and wind turbines in coastal Norway (BirdWind). Progress Report 2009. In NINA Report; Norsk Institutt for Naturforskning: Trondheim, Norway, 2009; Volume 505. [Google Scholar]
- Schlaich, A.; Bouten, W.; Bretagnolle, V.; Heldbjerg, H.; Klaassen, R.; Sørensen, I.; Villers, A.; Both, C. A circannual perspective on daily and total flight distances in a long-distance migratory raptor, the Montagu’s harrier, Circus pygargus. Biol. Lett. 2017, 13, 20170073. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).