Tham Chiang Dao: A Hotspot of Subterranean Biodiversity in Northern Thailand
Abstract
1. Introduction
2. Materials and Methods
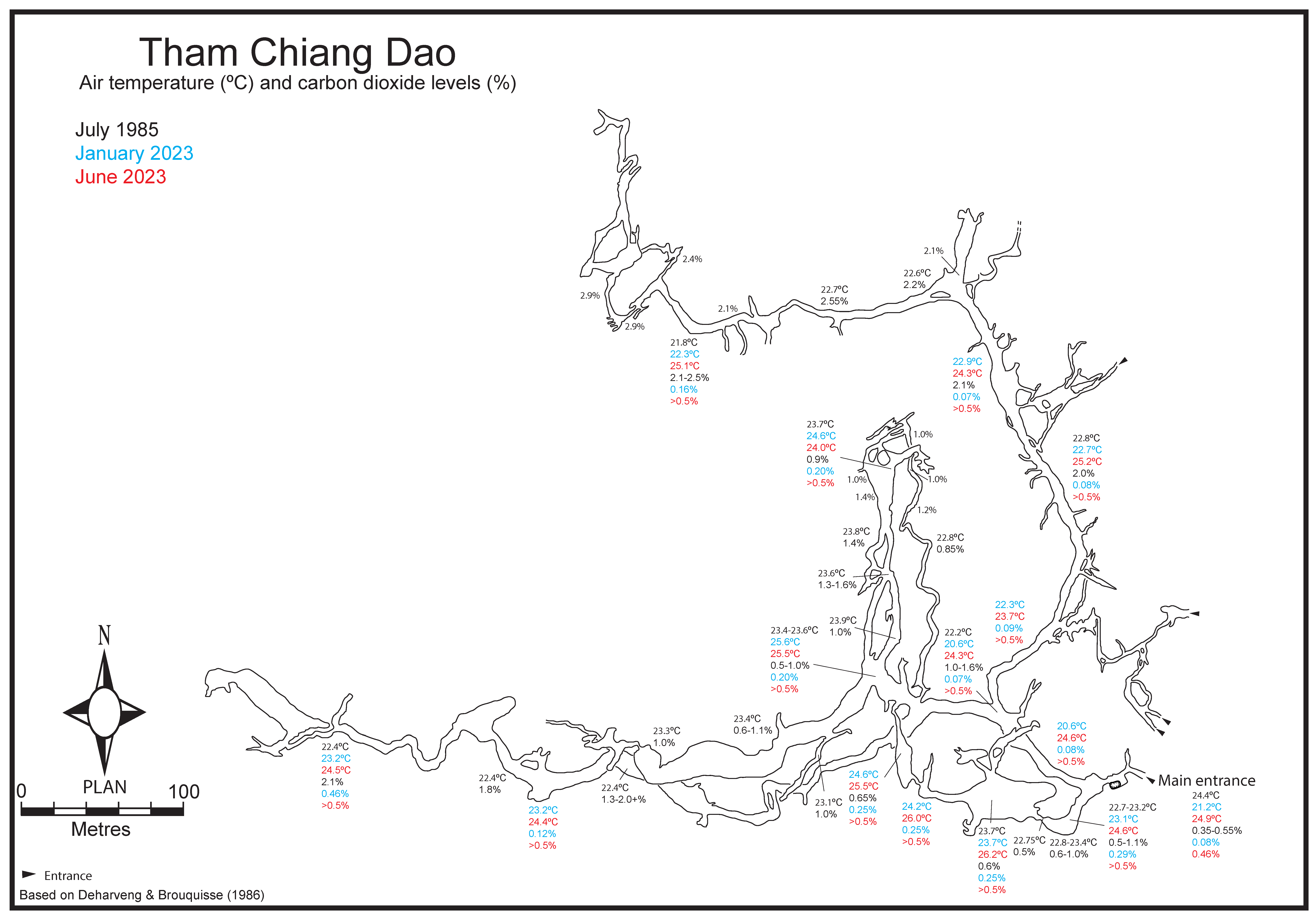
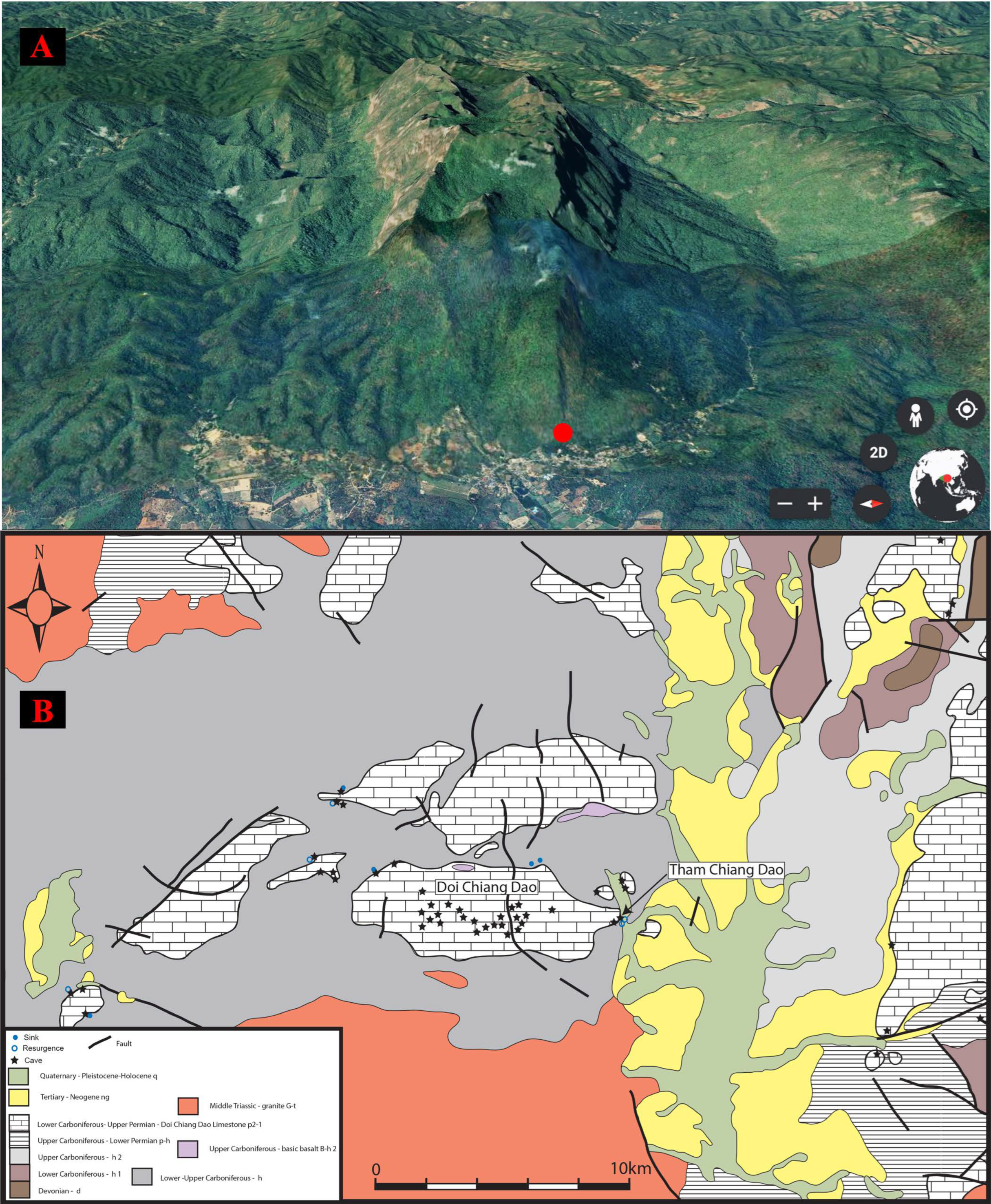
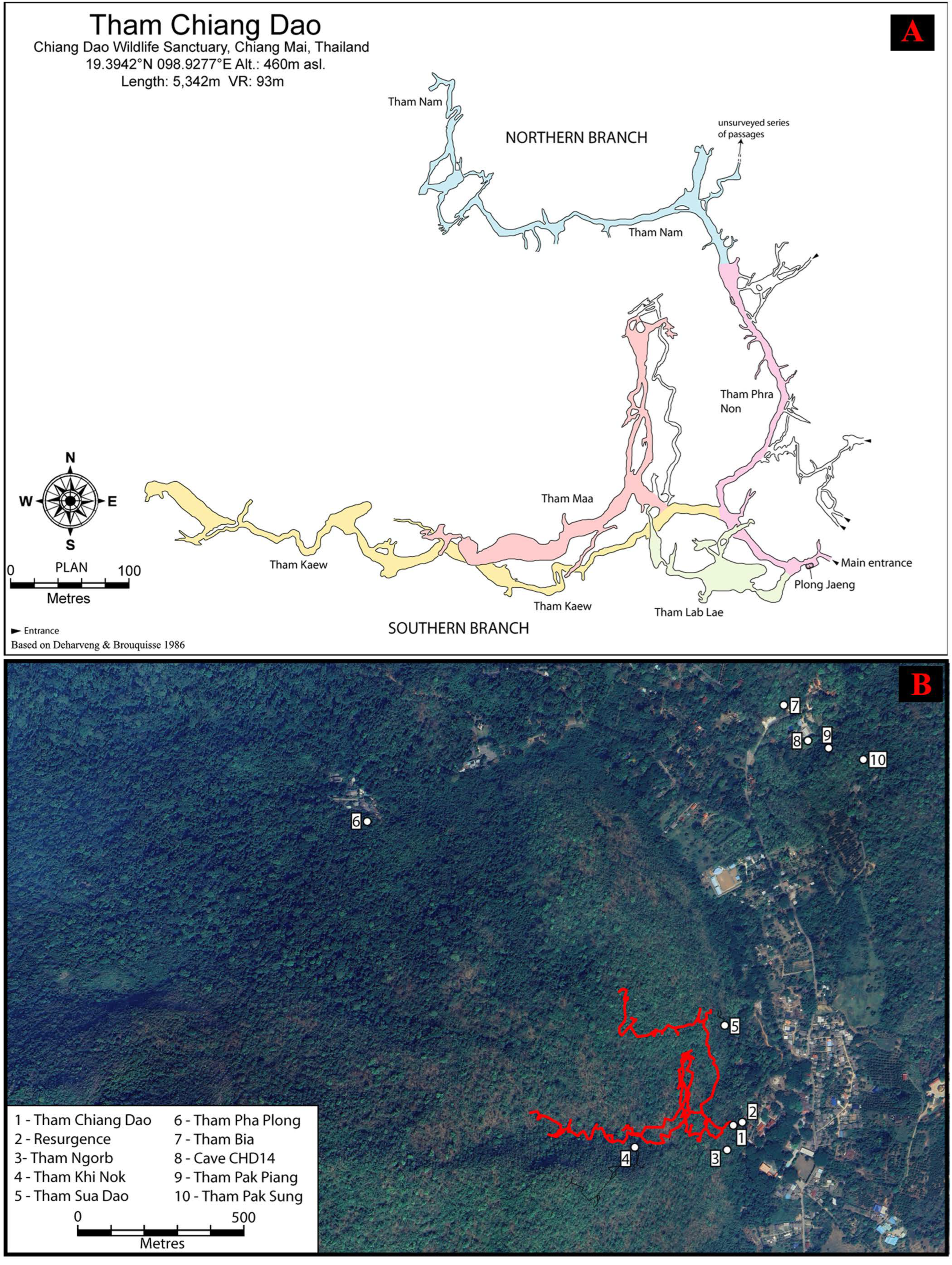
2.1. A Historical Overview of Tham Chiang Dao
2.2. A Brief History of Cave Fauna Investigation
| Date | Researchers | Institution | Biological Survey | Notes | Reference |
|---|---|---|---|---|---|
| January 1913 | T. H. Lyle | British consul, Nan | Bats | [16] | |
| 25 June 1914 | N. Gyldenstolpe | Swedish Zoological Expeditions to Siam | Biological survey | No bats seen in the cave, but there were large deposits of guano | [17] |
| March–June 1937 | Harvard Asiatic Primate Expedition, USA | Bats | [18] | ||
| 19 January 1958 | T. Umesao and K. Yoshikawa | Osaka City University, Japan | General cave fauna collecting | [19] | |
| 18 July 1958 | B. Degerbøl Hansen | Zoological Museum, University of Copenhagen, Denmark | General cave fauna collecting | [20] | |
| 1967 | F. Stone and R. Montgomery | Cornell University, USA | General cave fauna collecting | [21] | |
| 11 and 19 December 1969 | B. A. Harrison and K. Mongkolpanya | SEATO Laboratory, Bangkok | Mosquitos | [22] | |
| 1968–1971 | C. Boutin | Faculté des Sciences de Phnom Penh | Diptera | [23] | |
| 27 December 1972 | F. Stone | Bishop Museum, Honolulu, USA | Invertebrates | [24] | |
| May 1974 | J. Sedlacek | Bishop Museum, Honolulu, USA | General cave fauna collecting | [25] | |
| 15 February 1975 | P. Strinati | Switzerland | General cave fauna collecting | [26] | |
| December 1980–January 1981 | L. Deharveng and A. Gouze | Université Paul Sabatier, Toulouse, France | General cave fauna collecting | Cave exploration and survey | [11] |
| 1980–1987 | M. Kottelat | Laboratoire d’Ichthyologie, Delémont, Switzerland | Fish | [27] | |
| July 1981 | F. Stone | Bishop Museum, Honolulu, USA | Invertebrates | [28] | |
| 14 and 16 August 1981 | F. Stone | Bishop Museum, Honolulu, USA | Invertebrates | [29] | |
| 24 December 1983 | R. Hemperly | Thailand Karst Hydrologic Project, USA | Bats | Cave exploration and survey | [30] |
| 10 June 1984 and November 1984 | P. Beron and S. Andreev | National Museum of Natural History, Bulgaria | General cave fauna collecting | [31] | |
| July 1985 | L. Deharveng, P. Leclerc, A. Bedos, J.-P. Besson et al. | Association Pyrénéenne de Spéléologie, France | General cave fauna collecting | Cave exploration and survey | [32] |
| 5 and 31 July 1986 | F. Stone | Bishop Museum, Honolulu, USA | General cave fauna collecting | [24,28] | |
| 10 January 1989 | J. Trautner and K. Geigenmüller | Staatliches Museum für Naturkunde, Stuttgart, Germany | General cave fauna collecting | [33] | |
| 6 March 1989 | M. Anderson and H. Read | Natural History Museum of Denmark | Spiders | [34] | |
| 2007–2010 | S. Watiroyram | Nakhon Phanom University, Nakhon Phanom | Copepods | [35] | |
| 2010 | L. Chintapitasakul and colleagues | National Institute of Animal Health, Bangkok | Bat viruses | [36] | |
| 24, 25 and 28 June 2014 | P. Jaeger, S. Li, E. Shaw and E. Grall | Senckenberg Museum, Frankfurt am Main, Germany | Spiders | [37] | |
| 25 October 2015 | Animal Systematics Research Unit | Chulalongkorn University, Bangkok | Molluscs | [38] | |
| 10 March 2019 | S. Jantarit | Prince of Songkla University, Hat Yai | Collembola | [39] | |
| 8–11 January 2023 | S. Jantarit, R. Promdam, P. Pitaktunsakul, N. Boonkanpai, B. Noipracha, Y. Tokiri, C. Siripornpibul, W. Jaitrong, T. Jeenthong, K. Thongsri | DMR/Kanchanaburi Rajabhat University | General cave fauna collecting | First field visit | [14] |
| 9–11 June 2023 | S. Jantarit, R. Promdam, P. Pitaktunsakul, N. Boonkanpai, B. Noipracha, Y. Tokiri, C. Siripornpibul, W. Jaitrong, T. Jeenthong, K. Thongsri | DMR/Kanchanaburi Rajabhat University | General cave fauna collecting | Second field visit | [14] |
2.3. Cave System
2.4. Checklist and Sampling of Cave Fauna
3. Results and Discussion
3.1. Diversity of Cave Fauna in Tham Chiang Dao
| Phylum | Class | Order | Family | No. | Species | Reference(s) | Status |
|---|---|---|---|---|---|---|---|
| Mollusca | Gastropoda | Architaenioglossa | Pupinidae | 1 | Pupina artata Benson, 1856 | [38] | TP |
| Stylommatophora | Achatinidae | 2 | Allopeas gracile (Hutton, 1834) | [14] | TP | ||
| Annelida | Clitellata | Haplotaxida | Haplotaxidae | 3 | Heterochaetella glandularis (Yamaguchi, 1953) | [47,48] | SB |
| Arthropoda | Arachnida | Opiliones | Assamiidae | 4 | Bandona palpalis Roewer, 1927 | [14,29] | TP |
| 5 | Neopygoplus siamensis Suzuki, 1985 | [20] | TP | ||||
| Pseudoscorpiones | Chernetidae | 6 | Megachernes trautneri Schawaller, 1994 * | [33] | TP, TL | ||
| Palpigradi | Eukoeneniidae | 7 | Eukoenenia thais Condé, 1988 * | [49] | TB, TL | ||
| Araneae | Clubionidae | 8 | Systaria lannops Jäger, 2018 | [37] | TB | ||
| Psilodercidae | 9 | Althepus tibiatus Deeleman-Reinhold, 1985 * | [24] | TB, TL | |||
| Ochyroceratidae | 10 | Theotima minutissima (Petrunkevitch, 1929) | [24] | TP | |||
| Sparassidae | 11 | Heteropoda venatoria Linnaeus, 1767 | [14] | TP | |||
| 12 | Sinopoda ruam Grall & Jäger, 2020 * | [34] | TB, TL | ||||
| Nesticidae | 13 | Nesticella beccus Grall & Jäger, 2016 | [34] | TP | |||
| 14 | Nesticella mogera (Yaginuma, 1972) | [43] | TP | ||||
| Theridiidae | 15 | Nesticodes rufipes (Lucas, 1846) | [43] | TP | |||
| Gnaphosidae | 16 | Micythus anopsis Deeleman-Reinhold, 2001 * | [50] | TB, TL | |||
| Liocranidae | 17 | Jacaena schwendingeri (Deeleman-Reinhold, 2001) | Unpublished record. Specimen in SMF | TX | |||
| Chilopoda | Scolopendromorpha | Scolopendridae | 18 | Scolopendra dehaani Brandt, 1840 | [51] | TP | |
| Diplopoda | Polydesmida | Paradoxosomatidae | 19 | Tylopus perarmatus Hoffman, 1973 | [14,52] | TX | |
| Haplodesmidae | 20 | Eutrichodesmus gremialis (Hoffman, 1982) * | [14,26] | TB, TL | |||
| Maxilliopoda | Cyclopoida | Cyclopidae | 21 | Tropocyclops prasinus (Fischer, 1860) | [43] | SP | |
| Harpacticoida | Canthocamptidae | 22 | Elaphoidella namnaoensis Brancelj, Watiroyram & Sanoamuang, 2010 | [53] | SB | ||
| 23 | Epactophanes richardi Mrázek, 1893 | [53] | SP | ||||
| Malacostraca | Bathynellacea | Parabathynellidae | 24 | Siambathynella janineana Camacho & Leclerc, 2022 * | [54] | SB | |
| Isopoda | Oniscidae | 25 | Exalloniscus beroni Taiti & Ferrara, 1988 * | [14,31] | TB, TL | ||
| Decapoda | Palaemonidae | 26 | Macrobrachium yui Holthuis, 1950 | [14] | SP | ||
| Collembola | Entomobryomorpha | Isotomidae | 27 | Folsomides parvulus Stach, 1922 | [14,43] | TB | |
| 28 | Folsomina onychiurina Denis, 1931 | [43] | TP | ||||
| Paronellidae | 29 | Salina pulchella Goto, 1955 | [19,55] | TX | |||
| 30 | Troglopedetes fredstonei Deharveng 1988 * | [14,56] | TB, TL | ||||
| 31 | Troglopedetes leclerci Deharveng, 1990 * | [28] | TB, TL | ||||
| Entomobryoidae | 32 | Pseudosinella chiangdaoensis Deharveng, 1990 * | [14,28,55] | TB, TL | |||
| 33 | Coecobrya guanophila Deharveng, 1990 * | [28] | TB, TL | ||||
| 34 | Coecobrya similis Deharveng, 1990 | [14,28,55] | TB | ||||
| Poduromorpha | Hypogastruridae | 35 | Acherontiella colotlipana Palacios-Vargas & Thibaud, 1985 | [14,57] | TB | ||
| Symphypleona | Arrhopalitidae | 36 | Arrhopalites anulifer Nayrolles, 1990 | [58] | TP | ||
| 37 | Arrhopalites chiangdaoensis Nayrolles, 1990 * | [15,58] | TB, TL | ||||
| Insecta | Coleoptera | Carabidae | 38 | Itamus castaneus Schmidt-Goebel, 1846 | [14] | TP | |
| Staphylinidae | 39 | Bironium troglophilum Löbl, 1990 | [25] | TB | |||
| Lepidoptera | Tineidae | 40 | Crypsithyris spelaea Meyrick, 1908 | [43] | TP | ||
| 41 | Tinea antricola Meyrick, 1924 | [14,43] | TB | ||||
| 42 | Wegneria cerodelta (Meyrick, 1911) | [43] | TP | ||||
| Pscoptera | Liposcelididae | 43 | Liposcelis bostrychophilus Badonnel, 1931 | [14,43] | TP | ||
| 44 | Liposcelis entomophilus Enderlein, 1907 | [43] | TP | ||||
| Psyllipsocidae | 45 | Psocathropos lachlani Ribaga, 1899 | [43] | TP | |||
| Diptera | Culicidae | 46 | Culex harrisoni Sirivanakorn, 1977 * | [22] | TB, TL | ||
| Hymenoptera | Formicidae | 47 | Carebara diversa (Jerdon, 1851) | [14] | TX | ||
| 48 | Anoplolepis gracilipes Smith, 1857 | [14] | TX | ||||
| Chordata | Actinopterygii | Cypriniformes | Cyprinidae | 49 | Neolissochilus stracheyi (Day, 1871) | [14] | TX |
| Reptilia | Squamata | Colubridae | 50 | Elaphe taeniura (Cope 1861) | [14] | TP | |
| Mammalia | Chiroptera | Soricidae | 51 | Suncus murinus (Linnaeus, 1766) | [59] | TX | |
| Hipposideridae | 52 | Aselliseus stoliczkanus Dobson, 1871 | [14,30] | TX | |||
| 53 | Hipposideros armiger (Hodgson, 1835) | [14,16,59] | TX | ||||
| 54 | Hipposideros diadema (Geoffroy, 1813) | [60] | TX | ||||
| 55 | Hipposideros lylei Thomas, 1913 | [14,16,59] | TX, TL | ||||
| Pteropodidae | 56 | Eonycteris spelaea (Dobson, 1871) | [60] | TX | |||
| 57 | Macroglossus sobrinus Andersen, 1911 | [60] | TX | ||||
| 58 | Rousettus leschenaulti (Desmarest, 1820) | [60] | TX | ||||
| Rhinolophidae | 59 | Rhinolophus pusillus lakkhanae Yoshiyuki, 1990 | [14,59] | TX | |||
| Vespertilionidae | 60 | Ia io Thomas 1902 | [18] | TX | |||
| 61 | Pipistrellus paterculus (Thomas, 1915) | [59] | TX |
3.2. The Subterranean Fauna of Tham Chiang Dao
3.2.1. Terrestrial Fauna
- (1)
- Gastropoda
- (2)
- Acari
- (3)
- Araneae
- (4)
- Opiliones
- (5)
- Palpigradi
- (6)
- Pseudoscorpion
- (7)
- Schizomida
- (8)
- Diplopoda
- (9)
- Isopoda
- (10)
- Collembola
- (11)
- Diplura
- (12)
- Blattodea
- (13)
- Orthoptera
- (14)
- Hymenoptera
- (15)
- Coleoptera
- (16)
- Diptera
- (17)
- Lepidoptera
3.2.2. Aquatic Fauna
- (1)
- Nematoda
- (2)
- Annelida
- (3)
- Harpacticoida
- (4)
- Bathynellacea
3.2.3. Other Fauna
| # | TB/SB | Species | Taxonomic Classification | Notes | Reference(s) |
|---|---|---|---|---|---|
| 1 | SB | Heterochaetella glandularis (Yamaguchi, 1953) | Clitellata: Haplotaxida: Haplotaxidae | (TM) | [47] |
| 2 | SB? | Undetermined sp. | Clitellata: Enchytraeida: Enchytraeidae | [47] | |
| 3 | TB | Acmella sp. | Gastropoda: Caenogastropoda: Assimineidae | TM? | [14] |
| 4 | TB | Undetermined sp. | Arachnida: Acari: Leeuwenhoekiidae (?) | TM | [14,43] |
| 5 | TB | Systaria lannops Jäger, 2018 | Arachnida: Araneae: Clubionidae | [37] | |
| 6 | TB | Micythus anopsis Deeleman-Reinhold, 2001 | Arachnida: Araneae: Gnaphosidae | * TM | [50] |
| 7 | TB | Spermophora sp. | Arachnida: Araneae: Pholcidae | TM | [43] |
| 8 | TB | Althepus tibiatus Deeleman-Reinhold, 1985 | Arachnida: Araneae: Psilodercidae | TL | [24,75] |
| 9 | TB | Sinopoda ruam Grall & Jäger, 2020 | Arachnida: Araneae: Sparassidae | * | [34] |
| 10 | TB | Paratakaoia sp. | Arachnida: Opiliones: Epedanidae | TM | [43] |
| 11 | TB | Eukoenenia thais Condé, 1988 | Arachnida: Palpigradi: Eukoeneniidae | * TM | [41,69] |
| 12 | TB | Eukoenenia sp. (E. cf. lyrifer Condé, 1992) | Arachnida: Palpigradi: Eukoeneniidae | [69] | |
| 13 | TB | Tyrannochthonius sp. | Arachnida: Pseudoscorpiones: Chthoniidae | (TM) | [14,43] |
| 14 | TB? | Undetermined sp. | Arachnida: Schizomida: Hubbardiidae | G | [43] |
| 15 | TB | Eutrichodesmus gremialis Hoffman, 1982 | Diplopoda: Polydesmida: Haplodesmidae | * | [26,76] |
| 16 | TB | Undetermined sp. | Diplopoda: Polydesmida: Opisotretidae | [14] | |
| 17 | SB | Elaphoidella namnaoensis Brancelj, Watiroyram & Sanoamuang, 2010 | Maxillopoda: Harpacticoida: Canthocamptidae | [47,53] | |
| 18 | SB | Siambathynella janineana Camacho & Leclerc, 2022 | Malacostraca: Bathynellacea: Parabathynellidae | * | [47,54] |
| 19 | TB | Cubaris sp. | Malacostraca: Isopoda: Armadillidae | (TM) G | [14,43] |
| 20 | TB | Exalloniscus beroni Taiti & Ferrara, 1988 | Malacostraca: Isopoda: Oniscidae | * (TM) | [31] |
| 21 | TB? | Undetermined sp. | Malacostraca: Isopoda: Philosciidae | (TM) | [43] |
| 22 | TB | Coecobrya guanophila Deharveng, 1990 | Collembola: Entomobryomorpha: Entomobryidae | * G | [28] |
| 23 | TB | Pseudosinella chiangdaoensis Deharveng, 1990 | Collembola: Entomobryomorpha: Entomobryidae | * (TM) | [28] |
| 24 | TB | Troglopedetes fredstonei Deharveng 1988 | Collembola: Entomobryomorpha: Paronellidae | * TM | [56] |
| 25 | TB | Troglopedetes leclerci Deharveng, 1990 | Collembola: Entomobryomorpha: Paronellidae | * G | [28] |
| 26 | TB | Acherontiella colotlipana Palacios-Vargas & Thibaud, 1985 | Collembola: Poduromorpha: Hypogastruridae | G | [57] |
| 27 | TB | Arrhopalites chiangdaoensis Nayrolles, 1990 | Collembola: Symphypleona: Arrhopalitidae | * | [58] |
| 28 | TB? | Undetermined sp. | Insecta: Diplura: Japygidae | (TM) | [43] |
| 29 | TB | Helmablatta sp. | Insecta: Blattodea: Nocticolidae | TM | [14] |
| 30 | TB | Spelaeoblatta sp. | Insecta: Blattodea: Nocticolidae | TM | [14] |
| 31 | TB | Myrmecophilus sp. | Insecta: Orthoptera: Myrmecophilidae | TM | [14] |
| 32 | TB? | Brachyponera sp. | Insecta: Hymenoptera: Formicidae | [14] | |
| 33 | TB | Bironium troglophilum Löbl, 1990 | Insecta: Coleoptera: Scaphidiidae | [25] | |
| 34 | TB? | Undetermined sp. | Insecta: Coleoptera: Staphylinidae: Oxytelinae | (TM) | [14,43] |
| 35 | TB | Tinea antricola Meyrick, 1924 | Insecta: Lepidoptera: Tineidae | G | [43] |
| 36 | TB? | Culex harrisoni Sirivanakorn, 1977 | Insecta: Diptera: Culicidae | TL | [22] |
| 37 | TB? | Chetoneura sp. | Insecta: Diptera: Keroplatidae | [14,23] |
4. Cave Management and Conservation
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baum, F.; Hahn, L. Geological Map of Northern Thailand 1:250,000-Sheet 2 Chiang Rai; German Geological Mission in Thailand/Department of Mineral Resources, Federal Institute of Geosciences and Natural Resources: Hannover, Germany, 1976. [Google Scholar]
- Baum, F.; Hahn, L. Geological Map of Northern Thailand 1:250,000-Sheet 3 Phayao; German Geological Mission in Thailand/Department of Mineral Resources, Federal Institute of Geosciences and Natural Resources: Hannover, Germany, 1977. [Google Scholar]
- Baum, F.; Hahn, L. Geological Map of Northern Thailand 1:250,000-Sheet 4 Chiang Dao; German Geological Mission in Thailand/Department of Mineral Resources, Federal Institute of Geosciences and Natural Resources: Hannover, Germany, 1979. [Google Scholar]
- Miyahigashi, A.; Ueno, K.; Charoentitirat, T. Late Permian (Lopingian) foraminifers from the Doi Chiang Dao limestone in the Inthanon zone of Northern Thailand. Acta Geosci. Sin. 2009, 30, 40–43. [Google Scholar]
- Ellis, M. (Shepton Mallet Caving Club, Somerset, UK). Thailand Cave Database. 2023; Unpublished database. [Google Scholar]
- Ridd, M.F.; Barber, A.J.; Crow, M.J. (Eds.) The Geology of Thailand; Geological Society of London: London, UK, 2011; pp. 1–312. [Google Scholar]
- Ueno, K.; Charoentitirat, T.; Sera, Y.; Miyahigashi, A.; Suwanprasert, J.; Sordsud, A.; Boonlue, H.; Pananto, S. The Doi Chiang Dao Limestone: Paleo-Tethyan mid-oceanic carbonates in the Inthanon Zone of North Thailand. In Proceedings of the International Symposia on Geoscience Resources and Environments of Asian Terranes (GREAT 2008) 4th IGCP 516 and 5th APSEG, Bangkok, Thailand, 24–26 November 2008; pp. 42–48. [Google Scholar]
- Putiyanan, S.; Maxwell, J.F. Survey and herbarium specimens of medicinal vascular flora of Doi Chiang Dao. CMU. J. Nat. Sci. 2007, 6, 159. [Google Scholar]
- Chiang Dao Wildlife Sanctuary. Significant Wild Animal. 2023. Available online: https://www.chiangdao-biosphere.com/ (accessed on 5 August 2023).
- Windecker, R.C.; Mangkrontong, N.; Siriwitayakorn, S.; Chiraprapoosak, S. Map of Chiang Dao Cave. Tripod-mounted compass and tape survey 1972–73. Nat. Hist. Bull. Siam. Soc. 1975, 26, 1–10. [Google Scholar]
- Deharveng, L.; Gouze, A. Expédition en Thailande-Rapport Speleologique; Privately Circulated Report; Université Paul Sabatier: Toulouse, France, 1983; pp. 1–14. [Google Scholar]
- Deharveng, L.; Brouquisse, F. Le massif du Doï Chiang Dao. Les karsts de l’est de Chiang Mai. Expédition Thaï-Maros 85, Rapport Spéléologique et Scientifique; Association Pyrénéenne de Spéléologie: Toulouse, France, 1986; pp. 23–30. [Google Scholar]
- Sidisunthorn, P.; Gardner, S.; Smart, D. Caves of Northern Thailand; River Books: Bangkok, Thailand, 2006; pp. 1–392. [Google Scholar]
- Department of Mineral Resources, Ministry of Natural Resources and Environment, Thailand. Biodiversity Assessment of Tham Chiang Dao System, Chiang Mai Province; Kanchanaburi Rajabhat University and Parties: Kanchanaburi, Thailand, 2023; pp. 1–152. [Google Scholar]
- McGilvary, D. A Half Century among the Siamese and the Lao, an Autobiography; Fleming H. Revell Company: New York, NY, USA, 1912; pp. 1–435. [Google Scholar]
- Thomas, O. Some new Ferae from Asia and Africa, Annals and Magazine of Natural History Series 9 Vol. 12 pp. 88–89, reprinted as a new species of bat from Siam. Nat. Hist. Bull. Siam. Soc. 1913, 1, 49–50. [Google Scholar]
- Gyldenstolpe, N. Zoological results of the Swedish Zoological Expeditions to Siam 1911–1912 & 1914–1915. V. Mammals II. Kungl. Svenska Vetenskapsakad. Handl. 1916, 57, 3–59. [Google Scholar]
- Allen, G.M.; Coolidge, H.J., Jr. Mammal and bird collections of the Asiatic Primate Expedition. Mammals. Bull. Mus. Comp. Zool. 1940, 87, 131–166. [Google Scholar]
- Yosii, R. On some Collembola from Thailand. Nat. Life SE Asia 1961, 1, 171–201. [Google Scholar]
- Suzuki, S. A synopsis of the Opiliones of Thailand (Arachnida) I. Cyphophthalmi and Laniatores. Steenstrupia 1985, 11, 69–110. [Google Scholar]
- Stone, F.D. Caving in South-east Asia. New York State Grotto Dripstone 1967, 5, 3–5. [Google Scholar]
- Sirivanakarn, S. Redescription of four Oriental species of Culex (Culiciomyia) and the description of a new species from Thailand (Dipteria: Culicidae). Mosq. Syst. 1977, 9, 93–111. [Google Scholar]
- Boutin, C. Observations biospelologiques en Asie du Sud-Est. Ann. Fac. Sc. Phnom Penh 1971, 4, 168–185. [Google Scholar]
- Deeleman-Reinhold, C.L. New Althepus Species from Sarawak, Sumatra and Thailand (Arachnida: Araneae: Ochyroceratidae). Sarawak Mus. J. SMJ 1985, 34, 115–123. [Google Scholar]
- Löbl, I. Review of the Scaphidiidae (Coleoptera) of Thailand. Rev. Suisse Zool. 1990, 97, 505–621. [Google Scholar] [CrossRef]
- Hoffman, R.L. A new genus and species of Doratodesmid millipede from Thailand. Arch. Sci. Genève 1982, 35, 87–93. [Google Scholar]
- Kottelat, M. Two species of cavefishes from Northern Thailand in the genera Nemacheilus and Homaloptera (Osteichthyes: Homalopteridae). Rec. Aust. Mus. 1988, 40, 225–230. [Google Scholar] [CrossRef]
- Deharveng, L. Fauna of Thai Caves II: New Entomobryoides Collembola from Chiang Dao Cave, Thailand. Occas. Pap. Bernice P. Bishop Mus. 1990, 30, 279–287. [Google Scholar]
- Suzuki, S.; Stone, F.D. The fauna of Thai caves I: Three phalangides from Thailand (Arachnida). Occas. Pap. Bernice P. Bishop Mus. 1986, 26, 123–127. [Google Scholar]
- Blood, B.R.; McFarlane, D.A. Notes on some bats from northern Thailand with comments on the subgeneric status of Myotis altarium. Z. Säugetierkd. 1988, 53, 276–280. [Google Scholar]
- Taiti, S.; Ferrara, F. Revision of the genus Exalloniscus Stebbing, 1911 (Crustacea: Isopoda: Oniscidea). Zool. J. Linn. Soc. 1988, 94, 339–377. [Google Scholar] [CrossRef]
- Association Pyrénéenne de Spéléologie. Expédition Thai-Maros 85: Rapport Spéléologique et Scientifique; Association Pyrénéenne de Spéléologie: Toulouse, France, 1986; pp. 1–215. [Google Scholar]
- Schawaller, W. Pseudoskorpione aus Thailand (Arachnidae: Pseudoscorpiones). Rev. Suisse Zool. 1994, 101, 725–759. [Google Scholar] [CrossRef]
- Grall, E.; Jäger, P. Forty-seven new species of Sinopoda from Asia with a considerable extension of the distribution range to the South and description of a new species group (Sparassidae: Heteropodinae). Zootaxa 2020, 4797, 1–101. [Google Scholar] [CrossRef] [PubMed]
- Watiroyram, S. Species diversity and distribution of freshwater Copepoda in caves in Northern Thailand. Ph.D. Thesis, Khon Kaen University, Khon Kaen, Thailand, 2012; pp. 1–186. [Google Scholar]
- Chintapitasakul, L.; Choengern, N.; Bumrungsri, S.; Chalamat, M.; Molee, L.; Parchariyanon, S.; Ratanamungklanon, S.; Kongsuwan, K. RT-PCR survey of emerging Paramyxoviruses in cave-dwelling bats. Thai J. Vet. Med. 2012, 42, 29–36. [Google Scholar] [CrossRef]
- Jäger, P. On the genus Systaria (Araneae: Clubionidae) in Southeast Asia: New species from caves and forests. Zootaxa 2018, 4504, 524–544. [Google Scholar] [CrossRef] [PubMed]
- Jirapatrasilp, P.; Sutcharit, C.; Panha, S. Annotated checklist of the operculated land snails from Thailand (Mollusca, Gastropoda, Caenogastropoda): The family Pupinidae, with descriptions of several new species and subspecies, and notes on classification of Pupina Vignard, 1829 and Pupinella Gray, 1850 from mainland Southeast Asia. ZooKeys 2022, 1119, 1–115. [Google Scholar]
- Jantarit, S. Cavernicolus Springtails (Hexapoda: Collembola) in Northern and Western Part of Thailand; Thailand Research Fund: Bangkok, Thailand, 2021; pp. 1–151. [Google Scholar]
- Soisook, P.; Jantarit, S.; Rodcharoen, E.; Sa-ardrit, P.; Samoh, A.; Promdam, R.; Mitpuangchon, N.; Pimsai, A.; Wattanasen, S.; Watcharakul, S.; et al. Management of Biodiversity and Indigenous Knowledge of the Cave Ecosystem in Satun UNESCO Global Geopark for Sustainable Tourism; National Science and Technology Development Agency: Khlong Luang, Thailand, 2020; pp. 1–380. [Google Scholar]
- Department of Mineral Resources, Ministry of Natural Resources and Environment, Thailand. Biodiversity Assessment of Tham Khao Chang Hai, Na Muen Si, Na Yong, Trang Province; Kanchanaburi Rajabhat University and Parties: Kanchanaburi, Thailand, 2022; pp. 1–180. [Google Scholar]
- Jantarit, S.; Thongtip, U.; Boonrotpong, S.; Soisook, P.; Sa-ardrit, P.; Promdam, R.; Mitpuangchon, N.; Pimsai, A. An Interdisciplinary Approach to Evaluate Geological Structures, Physical Factors and Biodiversity of Cave Ecosystem: A Case Study in Tourist Caves of Satun, Phatthalung and Trang Province; Biodiversity-Based Economy Development Office (Public Organization) (BEDO): Bangkok, Thailand, 2020; pp. 1–200. [Google Scholar]
- Deharveng, L.; Bedos, A. The cave fauna of Southeast Asia. Origin, evolution and ecology. In Ecosystems of the World Vol. 30: Subterranean Ecosystems; Wilkens, H., Culver, D.C., Humphreys, W.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 603–632. [Google Scholar]
- Jantarit, S.; Ellis, M. The Cave Fauna of Thailand; Prince of Songkla University Press: Songkhla, Thailand, 2023; pp. 1–396. [Google Scholar]
- Deharveng, L.; Rahmadi, C.; Suhardjono, Y.R.; Bedos, A. The Towakkalak System, a hotspot of subterranean biodiversity in Sulawesi, Indonesia. Diversity 2021, 13, 392. [Google Scholar] [CrossRef]
- Moseley, M.; Lim, T.W.; Lim, T.T. Fauna reported from Batu Caves, Selangor, Malaysia: Annotated checklist and bibliography. Cave Karst Sci. 2012, 39, 77–92. [Google Scholar]
- Gibert, J. Le système karstique du Doi Chiang Dao (Thailande). Peuplements aquatiques souterrains, repartition, relations entre le milieu karstique et le sous-ecoulement de l’exutoire. In Expédition Thaï-Maros 86, Rapport Spéléologique et Scientifique; Association Pyrénéenne de Spéléologie: Toulouse, France, 1987; pp. 117–128. [Google Scholar]
- Giani, N.; Bouguenec, V. La Faune Aquatique de Thailande Généralités et Catalogue. Expeditions de l’APS en Asie du Sud-Est: Travaux Scientifiques–1; Association Pyrénéenne de Spéléologie: Toulouse, France, 1988; pp. 29–38. [Google Scholar]
- Condé, B. Nouveaux Palpigrades de Trieste, de Slovenié, de Malte, du Paraguay, de Thaïlande et de Bornéo. Rev. Suisse Zool. 1988, 95, 723–750. [Google Scholar] [CrossRef]
- Deeleman-Reinhold, C.L. Forest spiders of South East Asia: With a revision of the sac and ground spiders (Araneae: Clubionidae, Corinnidae, Liocranidae, Gnaphosidae, Prodidomidae and Trochanterrudae); Brill Academic Publishers: Leiden, The Netherlands, 2001; pp. 1–591. [Google Scholar]
- Siriwut, W.; Edgecombe, G.D.; Sutcharit, C.; Tongkerd, P.; Panha, S. A taxonomic review of the centipede genus Scolopendra Linnaeus, 1758 (Scolopendromorpha, Scolopendridae) in mainland Southeast Asia, with description of a new species from Laos. ZooKeys 2016, 590, 1–124. [Google Scholar]
- Likhitrakarn, N.; Golovatch, S.I.; Prateepasen, R.; Panha, S. Review of the genus Tylopus Jeekel, 1968, with descriptions of five new species from Thailand (Diplopoda, Polydesmida, Paradoxosomatidae). ZooKeys 2010, 72, 23–68. [Google Scholar]
- Watiroyram, S.; Brancelj, A.; Sanoamuang, L. Two new stygobiotic species of Elaphoidella (Crustacea: Copepoda: Harpacticoida) with comments on geographical distribution and ecology of harpacticoids from caves in Thailand. Zootaxa 2015, 3919, 81–99. [Google Scholar] [CrossRef]
- Camacho, A.I.; Leclerc, P. A new species of the genus Siambathynella Camacho, Watiroyram & Brancelj, 2011 (Crustacea, Bathynellacea, Parabathynellidae) from a Thai cave. Subterr. Biol. 2022, 44, 139–152. [Google Scholar]
- Jantarit, S.; Bedos, A.; Deharveng, L. An annotated checklist of the Collambolan fauna of Thailand. Zootaxa 2016, 4169, 301–360. [Google Scholar] [CrossRef] [PubMed]
- Deharveng, L. A new troglomorphic Collembola from Thailand: Troglopedetes fredstonei, n. sp. (Collembola: Paronellidae). Occas. Pap. Bernice P Bishop Mus. 1988, 28, 95–98. [Google Scholar]
- Thibaud, J. Révision du genre Acherontiella Absolon, 1913 (Insecta, Collembola). Bull. Mus. Natl. 1990, 12, 401–414. [Google Scholar] [CrossRef]
- Nayrolles, P. Fauna of Thai caves III: Two new cavernicolous species of Arrhopalites from Thailand (Insecta: Collembola). Occas. Pap. Bernice P Bishop Mus. 1990, 30, 288–293. [Google Scholar]
- Roguin, L. Expéditions Thai-Maros 85 et Thai 87-Mammiferes. Expeditions de l’APS en Asie du Sud-est: Travaux Scientifiques–1; Association Pyrénéenne de Spéléologie: Toulouse, France, 1988; pp. 47–52. [Google Scholar]
- Yenbutra, S.; Felton, H. Bat species and their distribution in Thailand according to the collections in TISTR and SMF. In Contributions to the Knowledge of the Bats of Thailand; Felton, H., Ed.; Courier Forschungsinstitut Senckenberg: Frankfurt, Germany, 1986; Volume 87, pp. 9–45. [Google Scholar]
- Culver, D.C.; Sket, B. Hotspots of subterranean biodiversity in caves and wells. J. Caves Karst Stud. 2000, 62, 11–17. [Google Scholar]
- Camacho, A.I.; Puch, C. Ojo Guareña: A hotspot of subterranean biodiversity in Spain. Diversity 2021, 13, 199. [Google Scholar] [CrossRef]
- Brad, T.; Iepure, S.; Sarbu, S.M. The chemoautotrophically based Movile cave groundwater ecosystem, a hotspot of subterranean biodiversity. Diversity 2021, 13, 128. [Google Scholar] [CrossRef]
- Polak, S.; Pipan, T. The subterranean fauna of Križna Jama, Slovenia. Diversity 2021, 13, 210. [Google Scholar] [CrossRef]
- Hutchins, B.T.; Gibson, J.R.; Diaz, P.H.; Schwartz, B.F. Stygobiont diversity in the San Marcos artesian well and Edwards aquifer groundwater ecosystem, Texas, USA. Diversity 2021, 13, 234. [Google Scholar] [CrossRef]
- Zagmajster, M.; Polak, S.; Fišer, C. Postojna-Planina cave system in Slovenia, a hotspot of subterranean biodiversity and a cradle of Speleobiology. Diversity 2021, 13, 271. [Google Scholar] [CrossRef]
- Faille, A.; Deharveng, L. The Coume Ouarnède system, a hotspot of subterranean biodiversity in Pyrenees (France). Diversity 2021, 13, 419. [Google Scholar] [CrossRef]
- Jäger, P. The second true troglobiont Heteropoda species from a limestone cave system in Palawan, Philippines (Araneae: Sparassidae: Heteropodinae). Arachnology 2018, 17, 427–431. [Google Scholar] [CrossRef]
- Condé, B. Palpigrades cavernicoles et endogés de Thaïlande et de Célèbes (1ere Note). Rev. Suisse Zool. 1992, 99, 665–672. [Google Scholar] [CrossRef]
- Harvey, M.S.; Ratnaweera, P.B.; Udagama, P.; Wijesinghe, M.T. A new species of the pseudoscorpion genus Megachernes (Pseudoscorpiones: Chernetidae) associated with a threatened Sri Lankan rainforest rodent, with a review of host associations of Megachernes. J. Nat. Hist. 2012, 46, 2519–2535. [Google Scholar] [CrossRef]
- Jantarit, S. Biodiversity of Collembola in Subterranean Habitat of Thailand; Thailand Research Fund: Bangkok, Thailand, 2022; pp. 1–136. [Google Scholar]
- Zhang, F.; Deharveng, L.; Chen, J. New species and rediagnosis of Coecobrya (Collembola: Entomobryidae), with a key to the species of the genus. J. Nat. Hist. 2009, 43, 2597–2615. [Google Scholar] [CrossRef]
- Vidlička, Ľ.; Vršanský, P.; Kúdelová, T.; Kúdela, M.; Deharveng, L.; Hain, M. New genus and species of cavernicolous cockroach (Blattaria, Nocticolidae) from Vietnam. Zootaxa 2017, 4232, 361–375. [Google Scholar] [CrossRef]
- Ševčík, J. Pseudochetoneura gen. nov., a peculiar new genus from Ecuador, with notes on Chetoneura (Diptera: Keroplatidae). Acta Entomol. Mus. Natl. Pragae. 2012, 52, 281–288. [Google Scholar]
- Deeleman-Reinhold, C.L. The Ochyroceratidae of the Indo-Pacific Region (Araneae). Raffles Bull. Zool. Suppl. 1995, 2, 1–103. [Google Scholar]
- Golovatch, S.I.; Geoffroy, J.-J.; Mauriès, J.-P.; VandenSpiegel, D. Review of the millipede genus Eutrichodesmus Silvestri, 1910 (Diplopoda, Polydesmida, Halpodesmidae), with descriptions of new species. ZooKeys 2009, 12, 1–46. [Google Scholar] [CrossRef][Green Version]
- Castello, M. Species diversity of Bryophytes and ferns of lampenflora in Grotta Gigante (NE Italy). Acta Carsologica 2014, 43, 185–193. [Google Scholar] [CrossRef]
- Mulec, J.; Kosi, G. Lampenflora algae and methods of growth control. J. Cave Karst Stud. 2009, 71, 109–115. [Google Scholar]
- Kurniawan, I.D.; Rahmadi, C.; Ardi, T.E.; Nasrullah, R.; Willyanto, M.I.; Setiabudi, A. The impact of lampenflora on cave-dwelling arthropods in Gunungsewu karst, Java, Indonesia. Biosaintifika 2018, 10, 275–283. [Google Scholar] [CrossRef][Green Version]
- O’Dowd, D.J. Crazy Ant Attack. Wingspan 1999, 9, 7. [Google Scholar]
- Health Protection Agency; Chilcott, R.P. Compendium of Chemical Hazards: Kerosene (Fuel Oil). Available online: https://silo.tips/download/health-protection-agency (accessed on 12 August 2023).
- Lam, N.L.; Smith, K.R.; Gauthier, A.; Bates, M.N. Kerosene: A review of household uses and their hazards in low- and middle-income countries. J. Toxicol. Environ. Health B Crit. Rev. 2012, 15, 396–432. [Google Scholar] [CrossRef]
- Tedsen, E. Black Carbon Emissions from Kerosene Lamps; Ecologic Institute: Berlin, Germany, 2013; pp. 1–45. [Google Scholar]
- Deharveng, L.; Bedos, A. Gaz carbonique. In Expédition Thaï-Maros 85 Rapport Spéléologique et Scientifique; Association Pyrénéenne de Spéléologie: Toulouse, France, 1986; pp. 144–152. [Google Scholar]
- Howarth, F.G.; Stone, F.D. Elevated carbon dioxide levels in Bayliss Cave, Australia: Implications for the evolution of obligate cave species. Pac. Sci. 1990, 44, 207–218. [Google Scholar]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deharveng, L.; Ellis, M.; Bedos, A.; Jantarit, S. Tham Chiang Dao: A Hotspot of Subterranean Biodiversity in Northern Thailand. Diversity 2023, 15, 1076. https://doi.org/10.3390/d15101076
Deharveng L, Ellis M, Bedos A, Jantarit S. Tham Chiang Dao: A Hotspot of Subterranean Biodiversity in Northern Thailand. Diversity. 2023; 15(10):1076. https://doi.org/10.3390/d15101076
Chicago/Turabian StyleDeharveng, Louis, Martin Ellis, Anne Bedos, and Sopark Jantarit. 2023. "Tham Chiang Dao: A Hotspot of Subterranean Biodiversity in Northern Thailand" Diversity 15, no. 10: 1076. https://doi.org/10.3390/d15101076
APA StyleDeharveng, L., Ellis, M., Bedos, A., & Jantarit, S. (2023). Tham Chiang Dao: A Hotspot of Subterranean Biodiversity in Northern Thailand. Diversity, 15(10), 1076. https://doi.org/10.3390/d15101076