Population Structure of an African Cycad: Fire May Stimulate the Coning Phenology of Encephalartos lanatus (Zamiaceae) and Also Predispose Its Cones to Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Locality and Study Species
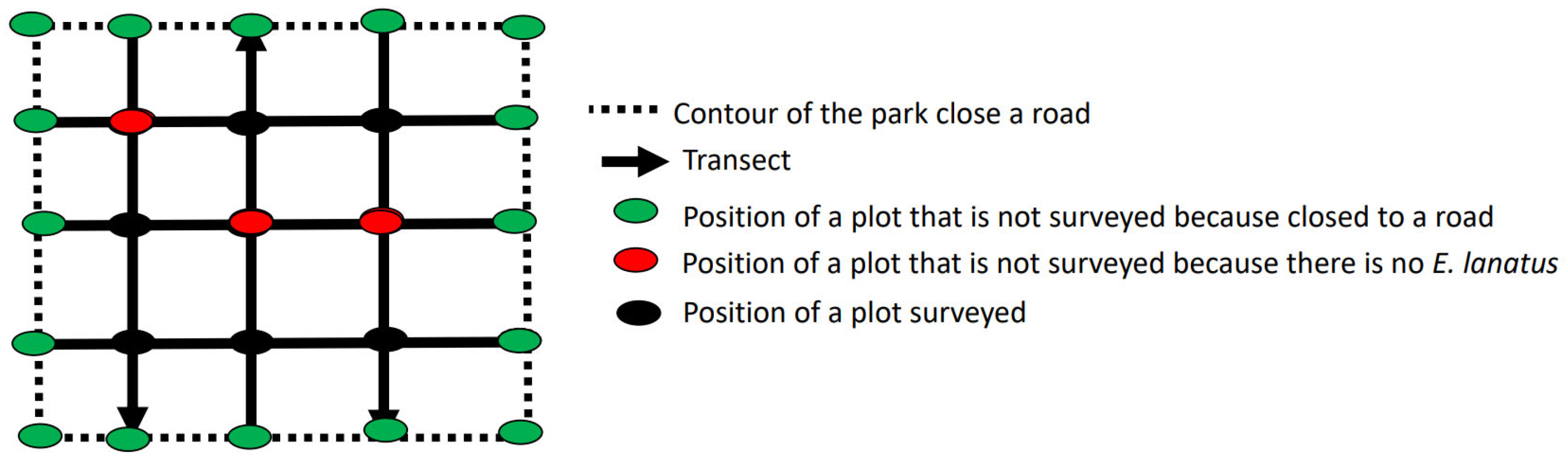
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Population Structure
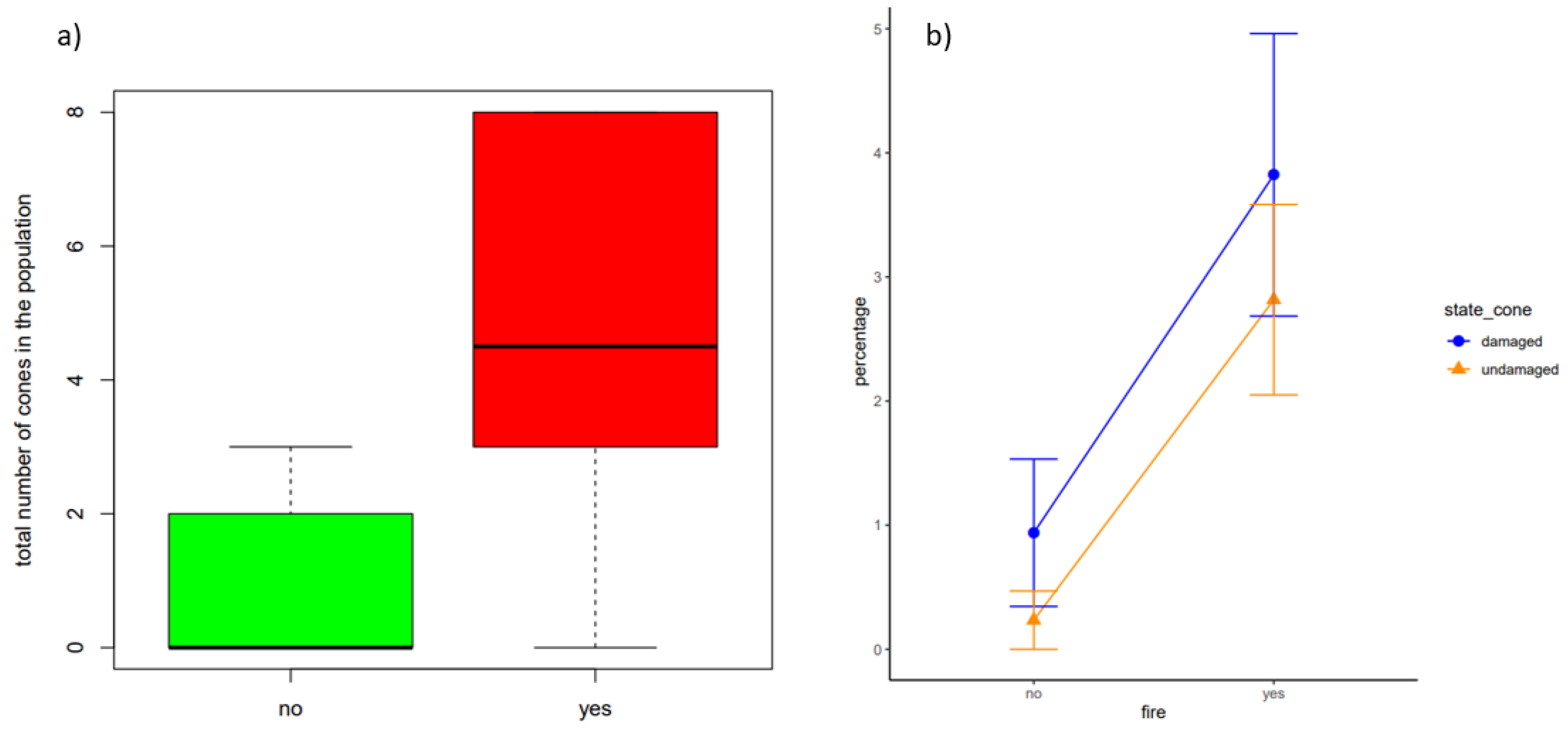
3.2. Effects of Fire on Population Structure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagalingum, N.S.; Marshall, C.R.; Quental, T.B.; Rai, H.S.; Little, D.P.; Mathews, S. Recent synchronous radiation of a living fossil. Science 2011, 334, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Yessoufou, K.; Bamigboye, S.O.; Daru, B.H.; Bank, M. Evidence of constant diversification punctuated by a mass extinction in the African cycads. Ecol. Evol. 2014, 4, 50–58. [Google Scholar] [CrossRef]
- Condamine, F.L.; Nagalingum, N.S.; Marshall, C.R.; Morlon, H. Origin and diversification of living cycads: A cautionary tale on the impact of the branching process prior in Bayesian molecular dating. BMC Evol. Biol. 2015, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Norstog, K.J.; Nicholls, T.J. The Biology of the Cycads; Cornell University Press: Ithaca, NY, USA, 1997. [Google Scholar]
- Brenner, E.D.; Stevenson, D.W.; Twigg, R.W. Cycads: Evolutionary innovations and the role of plant-derived neurotoxins. Trends Plant Sci. 2003, 8, 446–452. [Google Scholar] [CrossRef]
- Hermsen, E.J.; Taylor, E.L.; Taylor, T.N. Morphology and ecology of the Antarcticycas plant. Rev. Palaeobot. Palynol. 2009, 153, 108–123. [Google Scholar] [CrossRef]
- Mankga, L.T.; Yessoufou, K. Factors driving the global decline of cycad diversity. AoB PLANTS 2017, 9, plx022. [Google Scholar] [CrossRef] [PubMed]
- Yessoufou, K.; Daru, B.H.; Tafirei, R.; Elansary, H.O.; Rampedi, I. Integrating biogeography, threat and evolutionary data to explore extinction crisis in the taxonomic group of cycads. Ecol. Evol. 2017, 7, 2735–2746. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Koh, L.P.; Peh, K.S.-H.; Tan, H.T.W.; Chazdon, R.L.; Corlett, R.T.; Lee, T.M.; Colwell, R.K.; Brook, B.W.; Sekercioglu, C.H.; et al. Correlates of extinction proneness in tropical angiosperms. Divers. Distrib. 2008, 14, 1–10. [Google Scholar] [CrossRef]
- Yessoufou, K.; Daru, B.H.; Davies, T.J. Phylogenetic patterns of extinction risk in the Eastern arc ecosystem an African bio-diversity hotspot. PLoS ONE 2012, 7, e47082. [Google Scholar] [CrossRef]
- Davies, T.J.; Smith, G.F.; Bellstedt, D.U.; Boatwright, J.S.; Bytebier, B.; Cowling, R.M.; Forest, F.; Harmon, L.J.; Muasya, A.M.; Schrire, B.D.; et al. Extinction risk and diversification are linked in a plant biodiversity hostspot. PLoS Biol. 2011, 9, e1000620. [Google Scholar] [CrossRef]
- Daru, B.H.; Yessoufou, K.; Mankga, L.T.; Davies, T.J. A global trend towards the loss of evolutionary unique species in Mangrove ecosystem. PLoS ONE 2013, 8, e66686. [Google Scholar] [CrossRef]
- Bazzaz, F.A. Characteristics of populations in relation to disturbance innaturalandman-modified ecosystems. In Disturbance and Ecosystems; Mooney, H.A., Godron, M., Eds.; Springer: New York, NY, USA, 1984; pp. 259–277. [Google Scholar]
- Ahlgren, C.E.; Kozlowsila, T.T. Fire and Ecosystems; Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Whelan, R.J. The Ecology of Fire; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Pausas, J.G.; Lamont, B.B.; Paula, S.; Appezzato-da-Glória, B.; Fidelis, A. Unearthing belowground bud banks in fire-prone eco-systems. New Phytol. 2018, 217, 1435–1448. [Google Scholar] [CrossRef]
- Pausas, J.G. Bark thickness and fire regime: Another twist. New Phytol. 2017, 213, 13–15. [Google Scholar] [CrossRef]
- Pausas, J.G.; Pratt, R.B.; Keeley, J.E.; Jacobsen, A.L.; Ramirez, A.R.; Vilagrosa, A.; Paula, S.; Kaneakua-Pia, I.N.; Davis, S.D. Towards understanding resprouting at the global scale. New Phytol. 2016, 209, 945–954. [Google Scholar] [CrossRef]
- Auld, T.D.; Bradstock, R.A. Soil temperatures after the passage of a fire: Do they influence the germination of buried seeds? Aust. J. Ecol. 1996, 21, 106–109. [Google Scholar] [CrossRef]
- Gill, A.M. Fire and the Australian flora: A review. Aust. For. 1975, 38, 4–25. [Google Scholar] [CrossRef]
- Harnett, D.C.; Richardson, D.R. Population biology of Bonamia grandiflora (Convolvulaceae): Effects offire on plant and seedbank dynamics. Am. J. Bot. 1989, 76, 361–369. [Google Scholar] [CrossRef]
- Gill, A.M. Fire adaptive traits of vascular plants. In Fire Regimes and Ecosystem Properties: Proceedings of the Conference, 11–15 December 1978, Honolulu, Hawaii; Mooney, H.A., Bonnicksen, J.M., Christensen, N.L., Reiners, W.F., Eds.; USDA Forest Service General Technical Report, WO-26; USDA Forest Service: Washington, DC, USA, 1981; pp. 208–230. [Google Scholar]
- Brewer, J.S.; Platt, W.J. Effects of fire season and herbivory on reproductive success in a clonal forb, Pityopsis graminifolia. J. Ecol. 1994, 82, 665–675. [Google Scholar] [CrossRef]
- Spier, L.P.; Snyder, J.R. Effects of wet-and dry-season fire son Jacquemontia curtisii, a south Florid apine forest endemic. Nat. Areas J. 1998, 18, 350–357. [Google Scholar]
- Christensen, N.L. Fire and soil-plant nutrient relations in a pine- wiregrass savanna on the coastal plain of North Carolina. Oecologia 1977, 31, 27–44. [Google Scholar] [CrossRef]
- Menges, E. Factors limiting fecundity and germination in small populations of Silene regia (Caryophyllaceae), a rare hummingbird- pollinated prairie forb. Am. Midl. Nat. 1995, 133, 242–255. [Google Scholar] [CrossRef]
- Baird, A.M. Regeneration after fire in King’s Park, Western Australia. J. R. Soc. W. Aust. 1977, 60, 1–22. [Google Scholar]
- Dolva, J.M.; Scott, J.K. The association between the mealybug, Pseudococcus macrozamiae, ants and the cycad Macrozamia riedlei in a fire-prone environment. J. R. Soc. W. Aust. 1982, 65, 33–36. [Google Scholar]
- Ornduff, R. Size classes, reproductive behavior, and insect associates of Cycas media (Cycadaceae) in Australia. Bot. Gaz. 1991, 152, 203–207. [Google Scholar] [CrossRef]
- Beaton, J.M. Fire and water: Aspects of Australian aboriginal management. Arch. Ocean. 1982, 17, 51–58. [Google Scholar] [CrossRef]
- Pate, J.S. Biology of the S.W. Australian cycad Macrozamia riedlei (Fisch. Ex Gaudich). In The Biology, Structure, and Systematics of the Cycadales; Stevenson, D.W., Norstog, K.J., Eds.; Palm and Cycad Societies of Australia: Milton, QL, USA, 1993; pp. 125–130. [Google Scholar]
- Grobbelaar, N.J.; Meyer, J.M.; Burchmore, J. Coning and sex ratio of Encephalartos transvenosus at the Modjadji Nature Reserve. S. Afr. J. Bot. 1989, 55, 79–82. [Google Scholar] [CrossRef]
- Watkinson, A.R.; Powell, J.C. The life history and population structure of Cycas armstrongii in monsoonal northern Australia. Oecologia 1997, 111, 341–349. [Google Scholar] [CrossRef]
- Grove, T.; O’Connell, A.; Malajczuk, N. Effects of Fire on the Growth, Nutrient Content and Rate of Nitrogen Fixation of the Cycad Macrozamia riedlei. Aust. J. Bot. 1980, 28, 271–281. [Google Scholar] [CrossRef]
- Tang, W. Reproduction in the Cycad Zamia pumila in a fire-climax habitat: An eight-year study. Bull. Torrey Bot. Club 1990, 117, 368. [Google Scholar] [CrossRef]
- Griffiths, A.D.; Schult, H.J.; Gorman, J. Wild harvest of Cycas arnhemica (Cycadaceae): Impact on survival, recruitment and growth in Arnhem Land, northern Australia. Aust. J. Bot. 2005, 53, 771–779. [Google Scholar] [CrossRef]
- Preece, L.D.; Duguid, A.W.; Albrecht, D.E. Environmental determinants of a restricted cycad in central Australia, Macrozamia macdonnellii. Aust. J. Bot. 2007, 55, 601–607. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef]
- Swart, C.; Rowswell, R.; Donaldson, J.; Barker, N. Population structure and survival of the critically endangered cycad Encephalartos latifrons in South Africa. S. Afr. J. Bot. 2019, 127, 80–90. [Google Scholar] [CrossRef]
- Liddle, D.T. The Ecology of Cycas Armstrongii and Management of Fire in Australia’s Tropical Savannas. Ph.D. Thesis, Charles Darwin University, Darwin, Australia, 2004. [Google Scholar]
- Fawcett, P.K.S.; Norstog, K.J. Zamia pumila in South Florida: A preliminary report on its pollinators R. slossoni, a snout weevil, and P. zamiae, a clavicorn beetle. In The Biology, Structure, and Systematics of the Cycadales; Stevenson, D.W., Knorstog, K.J., Eds.; Palm and Cycad Societies of Australia: Milton, QL, USA, 1993; pp. 109–120. [Google Scholar]
- Negrón-Ortiz, V.; Gorchov, D.L. Effects of fire season and postfire herbivory on the cycad Zamia pumila (Zamiaceae) in slash pine savanna, Everglades National Park, Florida. Int. J. Plant Sci. 2000, 161, 659–669. [Google Scholar] [CrossRef][Green Version]
- Giddy, C. Cycads of South Africa, 2nd ed.; C. Struik (Pty) Ltd. Publishers: Cape Town, South Africa, 1984. [Google Scholar]
- Goode, D. Cycads of Africa; Struik Winchester: Cape Town, South Africa, 1989. [Google Scholar]
- Osborne, R. The world cycad census and a proposed revision of the threatened species status for cycad taxa. Biol. Conserv. 1995, 71, 1–12. [Google Scholar] [CrossRef]
- Donaldson, J.S. Regional overview: Africa. In Status Survey and Conservation Action Plan, Cycad; Donaldson, J.S., Ed.; IUCN/SSC Cycad Specialist Group, The World Conservation Union: Gland, Switzerland; Cambridge, UK, 2003. [Google Scholar]
- Osborne, R.; Calonje, M.A.; Hill, K.D.; Stanberg, L.; Stevenson, D.W. The world list of cycads. In Proceedings of the 8th International Conference on Cycad Biology, CYCAD 2012, Panama City, Panama, 13 – 15 January 2008. [Google Scholar]
- Hall, J.A.; Walter, G.H. Seed dispersal of the Australian cycad Macrozamia miquelii (Zamiaceae): Are cycads megafauna dispersed “grove forming” plants? Am. J. Bot. 2013, 100, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [PubMed]
- Vovides, A.P. Spatial distribution, survival, and fecundity of Dioon edule (Zamiaceae) in a tropical deciduous forest in Veracruz, Mexico, with notes on its habitat. Am. J. Bot. 1990, 77, 1532–1543. [Google Scholar] [CrossRef]
- Hough, A.F. Some diameter distributions in forest stands of northwestern Pennsylvania. J. For. 1932, 30, 933–943. [Google Scholar]
- Meyer, H.A.; Stevenson, D.D. The structure and growth of virgin beech, birch, maple, hemlock forests in northern Pennsylvania. J. Agric. Res. 1943, 67, 465–478. [Google Scholar]
- Meyer, H.A. Structure, growth and drain in balanced uneven-aged forests. J. For. 1952, 50, 85–92. [Google Scholar]
- Lorimer, C.G. Age structure and disturbance history of a Southern Appalachian virgin forest. Ecology 1980, 61, 1169–1184. [Google Scholar] [CrossRef]
- Kimmins, J.P. Forest Ecology; Macmillan: New York, NY, USA, 1987. [Google Scholar]
- Leak, W.A. Long-term structural change in uneven-aged northern hardwoods. For. Sci. 1996, 42, 160–165. [Google Scholar]
- Cancino, J.; Gadow, K.V. Stem number guide curves for uneven-aged forests, development and limitations. In Continuous Cover Forestry; Gadow, K.V., Nagel, J., Saborowski, J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 163–174. [Google Scholar]
- Veríssimo, A.; Barreto, P.; Mattos, M.; Tarifa, R.; Uhl, C. Logging impacts and prospects for sustainable forest management in an old Amazonian frontier: The case of Paragominas. For. Ecol. Manag. 1992, 55, 169–199. [Google Scholar] [CrossRef]
- Dauber, E.; Fredericksen, T.S.; Pena, M. Sustainability of timber harvesting in Bolivian tropical forests. For. Ecol. Manag. 2005, 214, 294–304. [Google Scholar] [CrossRef]
- Zimmerman, B.L.; Kormos, C.F. Prospects for sustainable logging in tropical forests. BioScience 2012, 62, 479–487. [Google Scholar]
- FAO. Mangrove Forests Management Guidelines; Technical Report FAO Forestry Paper 117; FAO: Rome, Italy, 1994; pp. 169–191. [Google Scholar]
- Ashton, E.; Macintosh, D. Preliminary assessment of the plant diversity and community ecology of the Sematan mangrove forest, Sarawak, Malaysia. For. Ecol. Manag. 2002, 166, 111–129. [Google Scholar] [CrossRef]
- Bosire, J.O.; Kairo, J.G.; Kazungu, J.; Koedam, N.; Guebas, F. Spatial and temporal regeneration dynamics in Ceriops tagal (Perr.) C.B. Rob. (Rhizophoraceae) mangrove forests in Kenya West. Indian Ocean J. Mar. Sci. 2008, 7, 69–80. [Google Scholar]
- Silvertown, J.; Franco, M.; Pisanty, I.; Mendoza, A. Comparative plant demography-relative importance of life-cycle components to the finite rate of increase in woody and herbaceous perennials. J. Ecol. 1993, 81, 465–476. [Google Scholar] [CrossRef]
- Gaoue, O.G.; Yessoufou, K. Strong seedling recruitment does not limit mangrove vulnerability to harvest Environ. Res. Lett. 2019, 14, 064019. [Google Scholar] [CrossRef]
- Morris, W.F.; Pfister, C.A.; Tuljapurkar, S.; Haridas, C.V.; Boggs, C.L.; Boyce, M.S.; Bruna, E.M.; Church, D.R.; Coulson, T.; Doak, D.F.; et al. Longevity can buffer plant and animal populations against changing climatic variability. Ecology 2008, 89, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, I.; Mandle, L.; Ticktin, T.; Gaoue, O. What do matrix population models reveal about the sustainability of nontimber forest product harvest? J. Appl. Ecol. 2011, 48, 815–826. [Google Scholar] [CrossRef]
- Adler, P.B.; Salguero-Gómez, R.; Compagnoni, A.; Hsu, J.S.; Ray-Mukherjee, J.; Mbeau-Ache, C.; Franco, M. Functional traits explain variation in plant life history strategies. Proc. Natl. Acad. Sci. USA 2014, 111, 740–745. [Google Scholar] [CrossRef]
- Gaoue, O.G. Transient dynamics reveal the importance of early life survival to the response of a tropical tree to harvest. J. Appl. Ecol. 2016, 53, 112–119. [Google Scholar] [CrossRef]
- Gaoue, O.G.; Gado, C.; Natta, A.K.; Kouagou, M. Recurrent fruit harvesting reduces seedling density but increases the frequency of clonal reproduction in a tropical tree. Biotropica 2018, 50, 69–73. [Google Scholar] [CrossRef]
- Eby, S.L.; Anderson, T.M.; Mayemba, E.P.; Ritchie, M.E. The effect of fire on habitat selection of mammalian herbivores: The role of body size and vegetation characteristics. J. Anim. Ecol. 2014, 83, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Coe, M.A.; Gaoue, O.G. Increased clonal growth in heavily harvested ecosystems failed to rescue ayahuasca lianas from decline in the Peruvian Amazon rainforest. J. Appl. Ecol. 2023, 60, 2105–2117. [Google Scholar] [CrossRef]
- Donaldson, J.S. Encephalartos lanatus Stapf & Burtt Davy. National Assessment: Red List of South African Plants Version 2020.1. 2009. Available online: http://redlist.sanbi.org/species.php?species=823-25 (accessed on 8 April 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigasa, M.N.; Yessoufou, K.; Magadlela, A.; Otang-Mbeng, W.; Suinyuy, T.N. Population Structure of an African Cycad: Fire May Stimulate the Coning Phenology of Encephalartos lanatus (Zamiaceae) and Also Predispose Its Cones to Damage. Diversity 2023, 15, 1075. https://doi.org/10.3390/d15101075
Sigasa MN, Yessoufou K, Magadlela A, Otang-Mbeng W, Suinyuy TN. Population Structure of an African Cycad: Fire May Stimulate the Coning Phenology of Encephalartos lanatus (Zamiaceae) and Also Predispose Its Cones to Damage. Diversity. 2023; 15(10):1075. https://doi.org/10.3390/d15101075
Chicago/Turabian StyleSigasa, Memory N., Kowiyou Yessoufou, Anathi Magadlela, Wilfred Otang-Mbeng, and Terence N. Suinyuy. 2023. "Population Structure of an African Cycad: Fire May Stimulate the Coning Phenology of Encephalartos lanatus (Zamiaceae) and Also Predispose Its Cones to Damage" Diversity 15, no. 10: 1075. https://doi.org/10.3390/d15101075
APA StyleSigasa, M. N., Yessoufou, K., Magadlela, A., Otang-Mbeng, W., & Suinyuy, T. N. (2023). Population Structure of an African Cycad: Fire May Stimulate the Coning Phenology of Encephalartos lanatus (Zamiaceae) and Also Predispose Its Cones to Damage. Diversity, 15(10), 1075. https://doi.org/10.3390/d15101075






