Abstract
Coral reefs of Vietnam are highly threatened by a combination of anthropogenic impacts and natural disturbances. As a result, preservation of the remaining reefs is a major governmental concern. Con Dao Islands, located in the coastal area of southern Vietnam in the South China Sea, still possess diverse and healthy coral communities. Coral surveys conducted in 2017–2020 on six sites within the marine protected area of Con Dao National Park revealed extensive coral cover (62.8–95.5%) and diversity (168 stony coral species). Coral communities were mostly dominated by Acroporidae followed by Poritidae and Fungiidae. Temporal dynamics over a 3-year period exhibited no significant decrease in the cover of dominant coral taxa, despite the severe thermal anomaly in 2019 and subsequent moderate coral bleaching, suggesting that the local corals may be successfully acclimating to the current level of thermal stress, although further study of coral adaptation in this region is warranted. High diversity and coral cover, together with the potential of resistance and resilience to repeated thermal stress in coral communities of the Con Dao Archipelago, highlights the need for authorities to pay special attention to this area and to expand conservational efforts to preserve this unique natural site.
1. Introduction
Coral reefs are one of the most diverse and productive ecosystems in the world. Currently, coral reefs face a wide variety of global and local threats, including warming of the sea surface temperature (SST), ocean acidification, coral diseases, outbreaks of coral predators, coastal development, agricultural runoff, pollution, overfishing, destructive fishing and coral mining [1,2,3]. The global coverage of living coral has declined by half since the 1950s [3,4], and the present rate and scale is such that coral loss may be total by 2050 [5,6,7]. The coral reefs of Vietnam are highly threatened, with only 1% being considered healthy, and coral cover ranging from 50% to 75% [2,3,8]. A few fringing coral reefs in the coastal provinces of south-central Vietnam have remained relatively healthy, such as those in Nha Trang Bay in Khanh Hoa Province [9] and in the coastal area of Nui Chua National Park in Ninh Thuan Province [10]. Chronic anthropogenic disturbances and a severe outbreak of corallivorous crown-of-thorns starfish Acanthaster sp. (COTS) led the reefs in Nha Trang Bay to collapse in 2019 [11], while a long-term COTS outbreak and thermally induced coral bleaching in 2019 resulted in a dramatic coral decline in Nui Chua National Park [12]. Thus, only remote island areas, such as the Con Dao National Park established on Con Dao Islands (southern Vietnam) and some reefs within the Spratly Archipelago in the southern South China Sea still harbor healthy coral communities with high biodiversity [13,14,15].
The Con Dao Islands are located in the South China Sea, 185 km southeast from the city of Vung Tau of Ba Ria-Vung Tau Province in southern Vietnam. The Con Dao National Park (CDNP) was designated in 1993 by the Ba Ria-Vung Tau Provincial People’s Committee, comprising an overall area of 150 km2 with 60 km2 dedicated to special use forest and a 90 km2 marine area [16]. The CDNP was declared by Ramsar Wetland to support high biodiversity due to its shallow marine waters and wetland areas, 18 km2 of coral reefs, 10.4 km2 of seagrass beds and 0.4 km2 of mangrove forests [17]. The CDNP’s coral reefs are highly diverse with over 370 coral species and over 200 coral fish species [17,18]. In addition, the waters of the CDNP provide nursing and nesting sites for about 90% of Vietnam’s sea turtle population, and its sea grass meadows support a small population of the critically endangered marine mammal dugong Dugong dugon, numbering around 9–12 individuals [19]. The surveys conducted by Nha Trang Institute of Oceanography have shown the presence of 1323 species of marine fauna and flora, including 44 species listed in the Red Data Book of Vietnam [20]. Thus, the Con Dao Archipelago can be considered a biodiversity hotspot of South East Asia with close similarity of Con Dao’s coral composition to the Spratly Islands area, whereas coral species richness (~400 species) is comparable with Brunei, Malaysia and Palawan, i.e., areas neighboring or included in the Coral Triangle [21]. However, scant data have been published on the ecological status of coral reefs within CDNP and on their resistance and resilience under current global and local threats.
Extensive coral bleaching and large-scale mortality result from heat shocks induced by SST anomalies closely related to El Niño Southern Oscillation (ENSO) events [22,23]. In the coastal waters of Vietnam, the ENSO strongly impacts fluctuations of SST and primary production of phytoplankton, although these fluctuations are significantly varied due to the annual development of regional Vietnamese upwelling in the narrow offshore shelf of south-central Vietnam [24,25,26]. Moreover, the intensity of ENSO events is expected to increase in the seas of South East Asia, including Vietnam, and NOAA Optimum Interpolation SST records testify that during ENSO periods the SST in this region is higher than in other areas [27,28]. Finally, there has been a global fourfold increase in the frequency of extreme ENSO events, from one event occurring every 60 years in the last century until the late 1980s, to one event every 15 years in the present day [29], which has inevitably aggravated the worldwide coral reef decline.
To date, only four publications have highlighted coral bleaching events related to the ENSO in the CDNP in 1998 and 2005 [30,31] and in 2019 during one of the most severe thermal anomalies [32,33]. Results of the coral surveys conducted by CDNP staff yearly at permanent locations using the rapid ReefCheck protocol remain inaccessible to the public. Some data on two coral bleaching events in 2010 and 2016 are presented in local media [34,35]. Nevertheless, the surveys carried out by CDNP staff showed that, up to 2015, both coral and seagrass coverage were stable. Densities of top predators (groupers and spiny lobsters) decreased, whereas moray eels and humphead wrasses had disappeared by 2015 due to fishing activity [19]. Our first coral survey conducted within the CDNP in 2017 confirmed high coral cover in local coral communities mostly dominated by acroporids [14]. Repeated surveys were performed in 2019 and 2020 on the selected target sites. The aim of this study was to estimate temporal dynamics in coral communities of the CDNP over a 3-year period and to track the possible response of local reef-building corals to the severe thermal stress that occurred in 2019.
2. Material and Methods
2.1. Study Area and Environmental Features
Con Dao Archipelago consists of 16 islands situated in the South China Sea on the wide shelf of southern Vietnam (Figure 1). The marine protected area of the CDNP comprises 14,000 ha and includes 1800 ha of coral reefs [20]. There are no permanent sources of fresh water on any of the islands in the Con Dao Archipelago. Thus, the impact of terrestrial run-off on marine ecosystems is negligible.
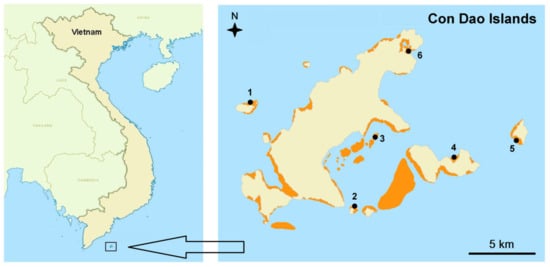
Figure 1.
Location of Con Dao Islands and study sites (numbers). Coral reefs are shown in orange.
The CDNP is located in a tropical cyclone passage area in the Western Pacific; most cyclones arrive at the Vietnamese coast from northeast to southeast of the Western Pacific and move westward across the South China Sea [36]. Typhoon Linda in October 1997 was the strongest cyclone to hit southern Vietnam in the past 100 years, bringing unexpectedly significant damage to this area including Con Dao Archipelago [37]. However, according to long-term weather observations (http://www.weather.unisys.com/hurricane; accessed on 1 September 2022), there have been no cyclones and tropical storms with an intensity higher than a seven on the Beaufort scale (moderate gale) in the archipelago.
For the analysis of sea surface temperature (SST) dynamics during the study period (2017–2020), the data were derived from SST and degree heating weeks (DHWs) charts archived by the NOAA Coral Reef Watch [38] (https://coralreefwatch.noaa.gov/product/5km/index.php; accessed on 7 June 2022). In addition, two HOBO® temperature loggers (U22-001 model, Onset, Bourne, MA, USA) were deployed in April 2019 at 3 and 12 m of depth at target site 1 (Figure 1) at Hon Tre Lon Island, and the temperature was recorded four times a day for 11 months to track variations in near-bottom temperature over one year.
2.2. Sampling Design
The initial coral survey was performed in May 2017 on 6 sites within the marine protected area of the CDNP (Figure 1). Repeated surveys were conducted on the three selected target sites 1, 3 and 6, in April 2019 and February 2020. These target sites were selected because of their coral community composition dominated by Acroporidae, which is typical for an archipelago [13]. The base of fringing reefs at sites 1 and 6 were located at 12 and 10 m of depth, respectively. These reefs were surveyed in two horizons of the reef slope: 3–5 m and 9–12 m of depth. The inner semi-closed area of site 4 represented shallow coral shoals with a base at 4–6 m of depth, which were surrounded by a sandy plain. This site was surveyed at 2–4 m.
The phototransect method was used to estimate the cover of stony corals, macroalgae, dead coral and rubble. At each site, four phototransects, each 25 m long, were deployed in a line within the same depth horizon and separated by 10 m intervals. Each phototransect consisted of 20 random photoquadrats with each photoquadrat covering 0.25 m2 of sampling area. These same transects were used to estimate the abundance of COTS within a belt width of 4 m (with a total of four 4 × 25 m belt transects per site).
2.3. Data Analysis
The percentage cover of living stony corals (on a genus level with subdivision on the species level for the dominant genera), dead coral framework, coral rubble and macroalgae were estimated using CPCe software [39] with 25 randomly spaced points within each photoquadrat (with a total of 500 points per transect). The dead coral framework herein represents dead, unbroken coral colonies that retained their typical structure. The size (maximum diameter) of the largest adult colonies of table Acropora (mostly A. hyacinthus) and massive Porites (mostly P. lobata and P. lutea) was measured at each site for the assumptions on coral resistance.
The identification of coral taxa was based on the works of Veron [40] and Latypov [41] and verified with World Register of Marine Species (https://www.marinespecies.org/aphia.php?p=taxdetails&id=1363; accessed on 10 September 2022).
The susceptibility of coral taxa to bleaching associated with SST anomalies was identified following the generally accepted hierarchy of thermal susceptibility of Indo-Pacific reef-building corals [42,43,44,45,46,47]. To assess temporal variations in total coral cover and dominant coral taxa cover at three target sites (Table 1), one-way analysis of variance (ANOVA) was used. Prior to analysis, data were tested for homogeneity of variance (Cochran C-test) and log-transformed [log (x + 1)]. Calculations were carried out using the software STATISTICA® 8.0 for Windows (StatSoft Inc. 2007).

Table 1.
Subdivision of recorded stony coral taxa according to their thermal susceptibility. The occurrence of dominant genera in study sites is shown. Thermal susceptibility of coral genera is based on data from [42,43,44,45,46,47].
3. Results
3.1. Sea Water Temperature Dynamics
Four-year remote sensing SST data for southern Vietnam (Figure 2) revealed that the coral bleaching threshold (CBT) was surpassed all four years of the study period (2017–2020), although only the episodes in 2019 and 2020 were sufficiently strong to result in severe coral bleaching and mortality, as the DHWs exceeded NOAA’s Second Bleaching Alert Level (13 °C-week in 2019 and 10 °C-week in 2020, Figure 2). Coral bleaching in June 2019 varied between 20% and 50% of coral cover (CDNP staff personal communication). This temperature elevation event was also recorded by two temperature loggers installed at site 1 at 3 m and 12 m of depth. The temperature exceeded the CBT from the middle of April till the beginning of June 2019 and remained between 30.5 and 31.7 °C (Figure 3). The difference between the temperature at the two depths was insignificant (t-test, t = 0.45; P = 0.65) and remained within 0.5 °C (Figure 3). The total DHWs over a 12-week window reached 6 °C-week, which is half that of NOAA’s remotely obtained DHWs values for the larger area of southern Vietnam (Figure 2), as no values exceeding the CBT at Con Dao Islands was recorded in June. Nevertheless, 6 °C-week was adequate for the development of significant coral bleaching (NOAA’s First Bleaching Alert Level; 4 ≤ DHW < 8 °C-week). The coral survey in 2020 was performed in February prior to the onset of the increase in SST; therefore, the impact of this anomaly was not considered for the analysis.
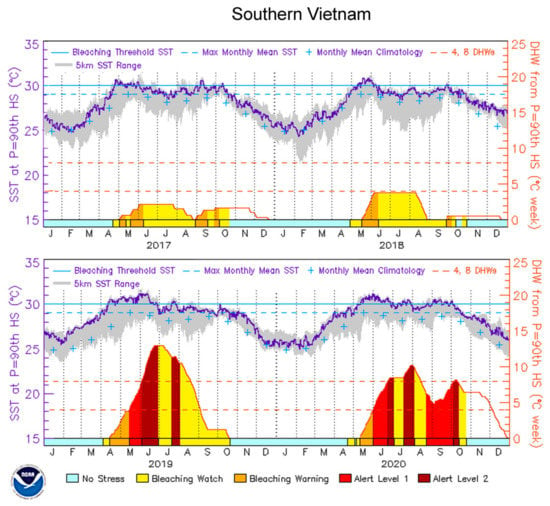
Figure 2.
Sea surface temperature (SST) dynamics (°C) and degree heating weeks (DHWs, °C a week) offshore southern Vietnam (from 8°38′ N to 13°45′ N) in the study period (2017–2020) according to NOAA Coral Reef Watch time series data (https://coralreefwatch.noaa.gov/product/vs/timeseries/east_asia.php#southern_vietnam), last accessed 20 November 2022).
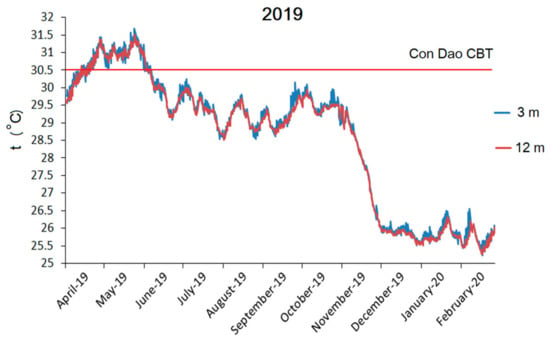
Figure 3.
Temperature dynamics over 11 months at 3 m and 12 m of depth obtained by HOBO® u22-001 temperature loggers. The coral bleaching threshold (CBT) is shown by the red line. The CBT used here is based on NOAA Coral Reef Watch time series data for southern Vietnam and leveled to sea surface temperature (SST) 1 °C higher than the maximum monthly mean SST for this area. The year is pointed out next to the month: 19 mean 2019; 20 mean 2020.
3.2. Coral Communities
The initial surveys conducted in 2017 revealed thriving coral communities mostly dominated by Acroporidae with coral cover range from high to continuous (62.8–95.5%, mean 82.5 ± 11.1%, Figure 4). In total, 168 species of reef-building corals from 54 genera, including non-scleractinian octocoral Heliopora coerulea and hydrocorals Millepora dichotoma and M. platyphylla, were recorded at six study sites (Table 1). The highest relative proportion of dead stony corals (30.6%) was recorded at site 3 in the semi-closed inner bay of Con Son Island, testifying to the moderate coral decline in this reef in the recent past. Both site 3 and site 2 revealed >5% coral rubble (Figure 5). Site 6 was the only site with an abundant macroalgae (9.2%) dominated by Sargassum sp. in the shallow zone (3–5 m) in the inner part of the bay. Among the seven selected dominant coral genera, the largest contributor in terms of cover and species richness was Acropora (Table 1).

Figure 4.
Dominant coral communities in the water area of Con Dao National Park. (A): multi-tiered stands of table Acropora (mostly A. hyacinthus and A. cytherea) in the upper reef slopes (2–5 m depth, sites 1–6); (B): branching Acropora (mostly A. intermedia, A. muricata, A. grandis and A. robusta) in the upper and middle reef slopes (3–7 m depth, sites 1–6); (C): assemblages of foliaceous Montipora (mostly M. aequituberculata and M. hispida) together with branching Acropora in the middle reef slopes (4–8 m depth, sites 1, 2, 4 and 6); (D): large heads of massive Porites (mostly P. lobata and P. lutea) in the lower reef slopes (7–12 m depth, sites 1, 4 and 6). (Photos taken by K.S. Tkachenko).
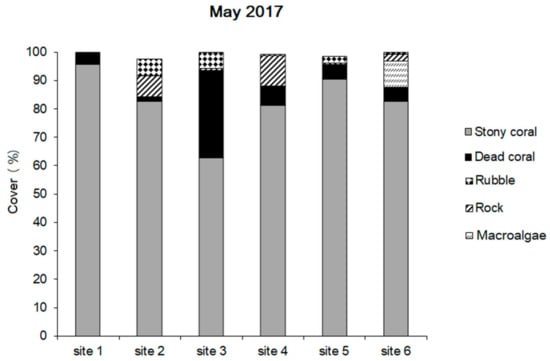
Figure 5.
Distribution of the major benthic categories at 6 sites surveyed in May 2017.
Temporal dynamics in total stony coral cover, dead coral cover and the dominant coral genera cover at three target sites revealed no significant changes (ANOVA, p >0.05 for all cases except for Acropora and stony coral cover mostly represented by Acropora in the shallow zone of site 6; Figure 6A–C, Table 2) and signs of coral decline following the severe thermal anomaly in 2019 (no significant increase in the cover of dead coral was recorded). The shallowest site 3, located in the semi-closed bay of Con Son Island, showed a decrease in stony coral cover (mostly represented by Acropora) (Figure 6B). In contrast, site 6 revealed a significant increase in Acropora and total stony coral cover in the shallow zone (ANOVA, Figure 6C, Table 2), whereas the abundance of Sargassum sp. significantly decreased between 2017 and 2019 (ANOVA, Figure 6C, Table 2). Table Acropora was mostly dominated by adult colonies in the size range of 150–250 cm in the largest diameter of a colony at all three target sites, with the size of the largest colonies exceeding 300 cm. The largest colonies of massive Porites varied between 200 and 400 cm in diameter. Only one COTS individual was recorded in 2019 at site 3.
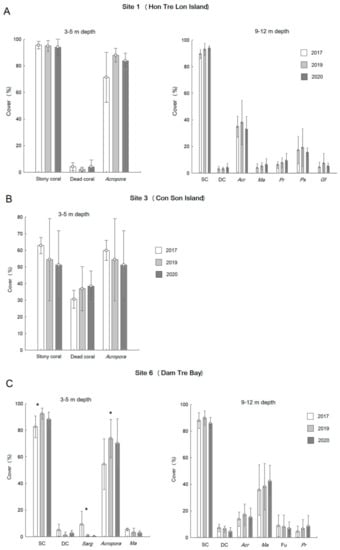
Figure 6.
Temporal variations over the 3-year period in covers (±SD) of stony coral, dead coral and dominant coral taxa (by cover > 5% in at least one of 3 years of observations) at three target sites ((A–C), sites 1, 3 and 6). SC: stony coral; DC: dead coral; Acr: Acropora spp.; Sarg: Sargassum sp.; Ma: Montipora aequituberculata; Pr: Porites rus; Ps: Pachyseris speciosa; Gf: Galaxea fascicularis; Fu: Fungiidae. Significant differences (ANOVA, p < 0.05) are marked by asterisks.

Table 2.
Results of one-way ANOVA for temporal differences in major categories and dominant taxa at three study sites.
4. Discussion
The present results show signs of coral acclimation to thermal stress in the coral communities of Con Dao Islands. An assessment of a coral bleaching event in the CDNP in June 2019 at five study sites using the ReefCheck protocol revealed high cover of live stony corals (mean 65.6 ± 18%), of which only one-quarter (25 ± 11.1%) were bleached to a different extent [32]. Moreover, out of all the dominant coral taxa in the CDNP, thermally susceptible Acropora and Montipora exhibited only 2.6% and 11% bleached coverage, respectively, whereas thermally resistant massive Porites showed 50% bleached coverage [32,33]. The previous severe thermal anomaly in 2010 was equivalent to that in 2019 (the DHWs of 13 °C-week in 2010 reached NOAA’s Second Bleaching Alert Level), and coral bleaching in the CDNP was more pronounced (mean cover of bleached coral was 43.3 ± 21.1%), although the pattern of coral bleaching was similar to that in 2019 with the largest and smallest proportions of bleached corals being Poritidae and Acroporidae, respectively [34]. The first recorded coral bleaching event at the Con Dao Islands in October 1998 resulted in 37.8% of the stony coral colonies being bleached [30]. The subsequent ENSO-related thermal anomalies in this area in 2005, 2010 and 2016 demonstrated a similar reverse hierarchy of coral bleaching; thermally susceptible Acroporidae were bleached much less severely than thermally resistant Poritidae [31,34,35]. Published data for 1998 and 2019 [30,32] coral bleaching events allowed us to graphically show this continued pattern of decreased bleaching in 2019 for some dominant coral genera (Figure 7).
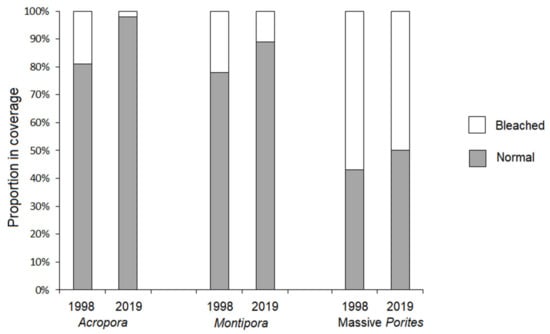
Figure 7.
Variations in proportion of bleached vs. normal coral colonies of the dominant coral taxa of the CDNP during 1998 and 2019 ENSO-related thermal anomalies (based on data presented in [30,32]).
Our study revealed no significant post-bleaching response of the dominant Acroporidae and Poritidae species to thermal stress in 2019 and a stable, healthy state of coral communities in general. In addition, the size of the largest table Acropora colonies observed at target sites ‘indicates’ the resistance to thermal stress events. Given that colonies of table Acropora larger than 15 cm stabilize their radial extension at a nearly constant rate of ~10 cm·year−1 [48,49], the largest colonies (>300 cm in diameter) observed in the present study had been developing for ~30 years and had survived all significant thermal anomalies in this area over the last three decades. In addition, the largest colonies of thermally resistant massive Porites (200–400 cm in diameter) at sites 1 and 6 exhibited high rates of survivorship during heat stresses for at least the last two centuries given their radial extension rate of ~1 cm·year−1 [50] and respective age of 200+ years. Differences in the proportions of dead coral (mostly Acroporidae) and coral rubble between the shallow and semi-closed site 3 and more open and deeper study sites could be due to the shallowness and higher irradiance (and UV radiation) of site 3 and its lower rate of water exchange, which contributes to higher coral bleaching and mortality.
Studies have reported that the thermal acclimation of corals in environments with naturally higher thermal fluctuations may lead to a higher tolerance of corals and to significant differences in the bleaching response among different locations during thermal anomalies [43,51,52,53]. Pre-exposure to high sublethal temperatures may increase the thermal tolerance of reef-building corals, and thus, corals in the outer reefs and deeper waters as well as corals in high-latitude reefs may become more susceptible to bleaching relative to their conspecifics from inner and shallower reefs and lagoon habitats [43,54]. Depending both on the location and the severity of the past sublethal coral bleaching events, such thermal acclimation may totally reverse the “susceptibility” of coral taxa and cause a shift in the widely accepted hierarchy of coral bleaching responses. Consequently, after exposure to rises in SST, the thermally resistant poritids become less resistant to bleaching than thermally susceptible acroporids, as was shown for Malaysian reefs [53]. A similar shift may have occurred in the coral communities off the Con Dao Islands. Nevertheless, adaptations to heat stress are location-dependent, and prior thermal exposures do not guarantee acclimation, as was demonstrated by the coral communities of the Great Barrier Reef, where prior thermal stress exposures in 1998 and 2002 did not diminish the severity of coral bleaching in 2016 [55]. Even stress-resistant corals from thermally extreme reefs in northwest Australia were unable to increase their bleaching threshold after 6 months of acclimation to +1 °C warming [56]. In addition, downscaling linear models of seasonal and inter-annual SST variability in the tropics showed that climate change will overwhelm thermal refugia for reef-building corals, stipulated by oceanographic features such as upwelling, strong ocean currents, etc., from 84% of coral reef pixels under the present-day climate to 0.2% at 1.5 °C, and 0% at 2 °C of global warming [57]. Coral reefs located in environments with high temporal SST variability and high historical thermal exposure such as those of Con Dao Archipelago are used to identify coral reef refugia as these reefs have been able to acclimate/adapt to thermal stress [58,59]. In the light of current trends in climate change, reefs exposed to highly variable temperature environment may be better able to facilitate the recovery of the low variability thermal refugia once they become exposed to thermal stress by supplying more thermally resistant larval recruits [57]. Increasing the vulnerability of corals to ocean warming provides a rationale for human-assisted larval translocation to restore degraded reefs in cooler areas (higher latitude or upwelling areas) with corals from thermally extreme reefs [56].
Thermal tolerance and reductions in bleaching response occur due to several physiological and morphological features of corals. The following allochtonic and hereditary characteristics are shown to contribute to coral survival and acclimation the most: (1) the dominance of heat-resistant genotypes of symbiotic algae (dinoflagellates) in coral tissue; (2) a higher density of symbiotic algae in coral tissue; (3) the possession of massive growth forms with larger corallites and the ability to retract coral polyps deeper into a corallite during thermal stress; (4) a higher coral tissue biomass and thickness; (5) the ability for alternative heterotrophic nutrition during the bleaching period; and (6) the ability to produce protective proteins, amino acids, and antioxidants mitigating the impact of thermal shock and UV radiation [60,61,62,63,64,65,66]. The density of symbiotic dinoflagellates, the Chl a content and the tissue biomass in the five dominant scleractinian genera of the South China Sea (Acropora, Montipora, Pavona, Porites, and Dipsastraea) are shown to significantly vary along the latitudinal gradient from north to south within the South China Sea, and the lowest values of these parameters were attributed to its southern part (the area of Spratly Islands) [67]. Qin et al. [67] believed that such a difference is likely due to the peculiarities of the long-term thermal history in the region and possibly higher UV radiation induced by higher water transparency in this remote area of the South China Sea. Further research into the biological underpinnings of coral survivorship in the Con Dao Islands is recommended to better understand the way these communities are responding to climate change.
In the light of presented data, the CDNP represents an oasis of healthy coral reefs with high biodiversity and potential for adaptation and acclimation to the globally changing environment. The government of Vietnam and authority of Ba Ria-Vung Tau Province should pay special attention to this unique area and contribute to the development of its sustainable management. Effective preservation is only possible under the condition that fishing activity in the water area of the CDNP is totally banned. In addition, only carefully verified ecological and educational tourism in this area should be permitted, without the massive construction of touristic resorts and hotels and concomitant aquaculture development that was seen in Nha Trang Bay, which resulted in significant coral degradation [9]. In the face of escalating cumulative local and global threats, the fate of the regionally significant CDNP coral reef ecosystems depends on responsible and sustainable management.
Author Contributions
Conceptualization, K.S.T.; Methodology, K.S.T.; Software, K.S.T.; Validation, K.S.T., V.V.D. and V.T.H.; Formal Analysis, K.S.T.; Investigation, K.S.T.; Resources, V.V.D. and V.T.H.; Data Curation, K.S.T.; Writing—Original Draft Preparation, K.S.T.; Writing—Review and Editing, K.S.T.; Visualization, K.S.T.; Supervision, K.S.T.; Project Administration, K.S.T., V.V.D. and V.T.H.; Funding Acquisition, V.V.D. and V.T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Joint Vietnam—Russia Tropical Science and Technology Research Center, Ecolan E-3.1, Task 4.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We are grateful to the staff of CDNP for their assistance in a field work and to the three anonymous reviewers for their constructive comments, which enable the improvement of the manuscript quality.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kleypas, J.A.; Eakin, C.M. Scientists’ perceptions of the threats to coral reefs: Results of a survey of coral reef researchers. Mar. Poll. Bull. 2007, 80, 419–436. [Google Scholar]
- Wilkinson, C. Status of Coral Reefs of the World: 2008; Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre: Townsville, QLD, Australia, 2008. [Google Scholar]
- Burke, L.; Reytar, K.; Spalding, M.; Perry, A. Reef at Risk. Revisited; World Resource Institute: Washington, DC, USA, 2011. [Google Scholar]
- Eddy, T.D.; Lam, V.W.Y.; Reygondeau, G.; Cisneros-Montemayor, A.M.; Greer, K.; Palomares, M.L.D.; Bruno, J.F.; Ota, Y.; Cheung, W.W.L. Global decline in capacity of coral reefs to provide ecosystem services. One Earth 2021, 4, 1278–1285. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Sale, P.F. Management of coral reefs: Where we have gone wrong and what we can do about it. Mar. Poll. Bull. 2008, 56, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Veron, J.E.N. Ocean acidification and coral reefs: An emerging big picture. Diversity 2011, 3, 262–274. [Google Scholar] [CrossRef]
- Dung, L.D. The status of coral reefs in central Vietnam’s coastal water under climate change. Aquat. Ecosyst. Health Manag. 2020, 23, 323–331. [Google Scholar] [CrossRef]
- Tkachenko, K.S.; Britayev, T.A.; Huan, N.; Pereladov, M.V.; Latypov, Y. Influence of anthropogenic pressure and seasonal upwelling on coral reefs in Nha Trang Bay (Central Vietnam). Mar. Ecol. 2016, 37, 1131–1146. [Google Scholar] [CrossRef]
- Vo, S.T.; de Vantier, L.; Tuyen, H.T.; Hoang, P.K. Ninh Hai waters (south Vietnam): A hotspot of reef corals in the western South China Sea. Raffles Bull. Zool. 2014, 62, 513–520. [Google Scholar]
- Tkachenko, K.S.; Huan, N.H.; Thanh, N.H.; Britayev, T.A. Extensive coral reef decline in Nha Trang Bay, Vietnam: Acanthaster planci outbreak: The final event in a sequence of chronic disturbances. Mar. Freshw. Res. 2020, 72, 186–199. [Google Scholar] [CrossRef]
- Tkachenko, K.S.; Dung, V.V.; Ha, V.T.; Huan, N.H. Coral reef collapse in South-Central Vietnam: A consequence of multiple negative effects. Aquat. Ecol. 2022, 1–9. [Google Scholar] [CrossRef]
- Latypov, Y.Y.; Selin, N.I. Current status of coral reefs of islands in the Gulf of Siam and southern Vietnam. Russ. J. Mar. Biol. 2011, 37, 255. [Google Scholar] [CrossRef]
- Tkachenko, K.S. The status of coral communities in three marine national parks of Vietnam. Sci. Rec. RSHMU 2018, 52, 110–119. (In Russian) [Google Scholar]
- Tkachenko, K.S.; Hoang, D.T.; Dang, H.N. Ecological status of coral reefs in the Spratly Islands, South China Sea (East Sea) and its relation to thermal anomalies. Estuar. Coast. Shelf Sci. 2020, 238, 106722. [Google Scholar] [CrossRef]
- WWF. Assessment of Legal Documents and Policies Relating to Management of Special-Use Forest in Vietnam; WWF: Hanoi, Vietnam, 2001. [Google Scholar]
- CDNP Authority. Ecotourism Development Project for Con Dao National Park Period 2016–2020; CDNP Authority: Con Dao, Vietnam, 2016. (In Vietnamese)
- DeVantier, L.M. Reef-Building Corals and Coral Communities of Con Dao Islands, Vietnam: Rapid Ecological Assessment of Biodiversity; Worldwide Fund for Nature Indo-China Program: Hanoi, Vietnam, 2002. [Google Scholar]
- Khuu, D.T.; Jones, P.J.S.; Ekins, P. A governance analysis of Con Dao National Park, Vietnam. Mar. Policy 2021, 127, 103986. [Google Scholar] [CrossRef]
- Hue, T.D. Proposal for Inclusion of Con Dao National Park, Viet Nam into IOSEA Site Network. In Proceedings of the 8th Meeting of the Signatory States, Da Nang, Vietnam, 21–25 October 2019; Available online: https://www.cms.int/iosea-turtles/en/document/proposal-inclusion-con-dao-national-park-viet-nam-iosea-site-network (accessed on 22 April 2022).
- Huang, D.; Licuanan, W.Y.; Hoeksema, B.W.; Chen, C.A.; Ang, P.O.; Huang, H.; Lane, D.J.W.; Vo, S.T.; Waheed, Z.; Affendi, Y.A.; et al. Extraordinary diversity of reef corals in the South China Sea. Mar. Biodivers. 2015, 45, 157–168. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Baird, A.H.; Connolly, S.R.; Dietzel, A.; Eakin, C.M.; Heron, S.F.; Hoey, A.S.; Hoogenboom, M.O.; Liu, G.; et al. Global warming transforms coral reef assemblages. Nature 2018, 556, 492–496. [Google Scholar] [CrossRef]
- Lough, J.M.; Anderson, K.D.; Hughes, T.P. Increasing thermal stress for tropical coral reefs: 1871–2017. Sci. Rep. 2018, 8, 6079. [Google Scholar] [CrossRef]
- Duong, P.X.; Du, H.T.; Linh, V.T.T.; Thai, T.D.; Thu, P.M. The effect of ENSO on hydrological structure and environment in the South Central Coast-Vietnam. J. Mar. Sci. 2020, 2, 10–16. [Google Scholar] [CrossRef]
- Tac, V.V.; Huan, N.H.; Son, T.P.H.; Tien, N.M.; Khang, N.H.T.; Quang, P.; Chung, T.V. Sea surface temperature anomaly in the coastal waters of Vietnam related to ENSO phenomenon. Vietnam J. Mar. Sci. Technol. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Hieu, N.T.D.; Huan, N.H.; Van, T.T.; Lien, N.P. Assessing the distribution and variation characteristics of marine primary productivity in the coastal marine area of Vietnam South Centre. IOP Conf. Ser. Earth Environ. Sci. 2022, 964, 012011. [Google Scholar] [CrossRef]
- Wang, C.; Wang, W.; Wang, D.; Wang, Q. Interannual variability of the Southeast Asia Sea associated with El Niño. J. Geophys. Res. Oceans 2006, 111, 1–19. [Google Scholar] [CrossRef]
- Dao, H.N.; Vu, H.T.; Kay, S.; Sailley, S. Impact of seawater temperature on coral reefs in the context of climate change. A case study of Cu Lao Cham—Hoi An Biosphere Reserve. Front. Mar. Sci. 2021, 8, 704682. [Google Scholar] [CrossRef]
- Cai, W.; Borlace, S.; Lengaigne, M.; van Rensch, P.; Collins, M.; Vecchi, G.; Timmermann, A.; Santoso, A.; McPhaden, M.J.; Wu, L.; et al. Increasing frequency of extreme El Niño events due to greenhouse warming. Nat. Clim. Change 2014, 4, 111–116. [Google Scholar] [CrossRef]
- Vo, S.T. The corals at Con Dao Archipelago (South Vietnam): Before, during and after the bleaching event in 1998. In Proceedings of the 9th International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2002; Bali Convention Center: Bali, Indonesia, 2002; Volume 2, pp. 895–899. [Google Scholar]
- Ben, H.X.; Vo, S.T.; Hoang, P.K. Mass mortality of corals and reef living features at Con Dao archipelago (Vietnam) in October 2005. Vietnam J. Mar. Sci. Technol. 2008, 8, 59–70. (In Vietnamese) [Google Scholar]
- Hoang, P.K.; Tuan, V.S.; Quang, T.M.; Hoc, D.T.; Tuyen, H.T. Bleaching of corals in Nha Trang, Ninh Thuan, Con Dao and Phu Quoc islands in June–July 2019. Vietnam J. Mar. Sci. Technol. 2020, 20, 55–60. (In Vietnamese) [Google Scholar] [CrossRef]
- Vo, S.T.; Phan, K.H.; Hua, T.T.; Thai, M.Q.; Hoang, X.B. Genus-specific bleaching at Con Dao Islands, Southern Vietnam, June 2019. Galaxea J. Coral Reef Stud. 2020, 22, 27–28. [Google Scholar] [CrossRef]
- Giang, N.T. Short Report on Coral Bleaching Event in Con Dao 2010; CDNP Authority: Con Dao, Vietnam, 2010. (In Vietnamese)
- Giang, N.T. Mass Coral Bleaching in Con Dao 2016; CDNP Authority: Con Dao, Vietnam, 2016. Available online: https://nld.com.vn/thoi-su-trong-nuoc/san-ho-tai-con-dao-bi-tay-trang-hang-loat-20160616172609116.htm (accessed on 4 May 2022).
- Wang, Y. Composite of typhoon-induced sea surface temperature and chlorophyll—A responses in the South China Sea. J. Geophys. Res. Oceans 2020, 125, e2020JC016243. [Google Scholar] [CrossRef]
- Anh, L.T.; Takagi, H.; Thao, N.D. Storm surge and high waves due to 1997 typhoon Linda: Uninvestigated worst storm event in Southern Vietnam. J. Jpn. Soc. Civ. Eng. Ser. B3 2019, 75, I73–I78. [Google Scholar] [CrossRef]
- Skirving, W.; Marsh, B.; De La Cour, J.; Liu, G.; Harris, A.; Maturi, E.; Geiger, E.; Eakin, C.M. CoralTemp and the Coral Reef Watch Coral Bleaching Heat Stress Product Suite Version 3.1. Remote Sens. 2020, 12, 3856. [Google Scholar] [CrossRef]
- Kohler, K.E.; Gill, S.M. Coral Point Count with Excel extension (CPCe): A visual basic program for determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 2006, 32, 1259–1269. [Google Scholar] [CrossRef]
- Veron, J.E.N. Corals of the World [Three Volumes]; Australian Institute of Marine Science: Townsville, QLD, Australia, 2000.
- Latypov, Y.Y. Scleractinian Corals of Vietnam; Science Publishing Group: New York, NY, USA, 2014. [Google Scholar]
- Baird, A.H.; Marshall, P.A. Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2002, 237, 133–141. [Google Scholar] [CrossRef]
- Marshall, P.A.; Baird, A.H. Bleaching of corals on the Great Barrier Reef: Differential susceptibility among taxa. Coral Reefs 2000, 19, 155–163. [Google Scholar] [CrossRef]
- Kayanne, H.; Harii, S.; Ide, Y.; Akimoto, F. Recovery of coral populations after 1998 bleaching of Shiraho Reef, in the southern Ruykyus, NW Pacific. Mar. Ecol. Prog. Ser. 2002, 239, 93–103. [Google Scholar] [CrossRef]
- McClanahan, T.R. The relationship between bleaching and mortality of common corals. Mar. Biol. 2004, 144, 1239–1245. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Baird, A.H.; Marshall, P.A.; Toscano, M.A. Comparing bleaching and mortality responses of hard corals between southern Kenya and the Great Barrier Reef, Australia. Mar. Poll. Bull. 2004, 48, 327–335. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Ateweberhan, M.; Graham, N.A.J.; Wilson, S.K.; Ruiz Sebastian, C.; Guillaume, M.M.M.; Bruggemann, J.H. Western Indian Ocean coral communities: Bleaching responses and susceptibility to extinction. Mar. Ecol. Progr. Ser. 2007, 337, 1–13. [Google Scholar] [CrossRef]
- Sheppard, C.R.C.; Harris, A.; Sheppard, A.L.S. Archipelago-wide coral recovery patterns since 1998 in the Chagos Archipelago, central Indian Ocean. Mar. Ecol. Prog. Ser. 2008, 362, 109–117. [Google Scholar] [CrossRef]
- Stimpson, J. The effect of shading by the table coral Acropora hyacinthus on understory corals. Ecology 1985, 66, 40–53. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Anderson, K.D.; Hoogenboom, M.O.; Widman, E.; Baird, A.H.; Pandolfi, J.M.; Edmunds, P.J.; Lough, J.M. Spatial, temporal and taxonomic variation in coral growth–implications for the structure and function of coral reef ecosystems. Oceanogr. Mar. Biol. 2015, 53, 215–295. [Google Scholar]
- Goreau, T.J. Bleaching and reef community change in Jamaica: 1951–1991. Am. Zool. 1992, 32, 683–695. [Google Scholar] [CrossRef]
- Oliver, T.A.; Palumbi, S.R. Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 2011, 30, 429–440. [Google Scholar] [CrossRef]
- Guest, J.R.; Baird, A.H.; Maynard, J.A.; Muttaqin, E.; Edwards, A.J.; Campbell, S.J.; Yewdall, K.; Affendi, Y.A.; Chou, L.M. Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS ONE 2012, 7, e33353. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, K.S.; Soong, K. Dongsha Atoll: A potential thermal refuge for reef-building corals in the South China Sea. Mar. Environ. Res. 2017, 127, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kerry, J.T.; Alvarez-Noriega, M.; Alvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Schoepf, V.; Carrion, S.A.; Pfeifer, S.M.; Naugle, M.; Dugal, L.; Bruyn, J.; McCulloch, M.T. Stress-resistant corals may not acclimatize to ocean warming but maintain heat tolerance under cooler temperatures. Nat. Commun. 2019, 10, 4031. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.M.; Forster, P.M.; Heron, S.F.; Stoner, A.M.K.; Beger, M. Future loss of local-scale thermal refugia in coral reef ecosystems. PLoS Clim. 2022, 1, e0000004. [Google Scholar] [CrossRef]
- Mumby, P.J.; Elliott, I.A.; Eakin, C.M.; Skirving, W.J.; Paris, C.B.; Edwards, H.J.; Enriquez, S.; Prieto, R.I.; Cherubin, L.M.; Stevens, J.R. Reserve design for uncertain responses of coral reefs to climate change. Ecol. Lett. 2011, 14, 132–140. [Google Scholar] [CrossRef]
- Magris, R.A.; Heron, S.F.; Pressey, R.L. Conservation planning for coral reefs accounting for climate warming disturbances. PLoS ONE 2015, 10, e0140828. [Google Scholar] [CrossRef]
- Baker, A.C.; Starger, C.J.; McClanahan, T.R.; Glynn, P.W. Coral’s adaptive response to climate change. Nature 2004, 430, 741. [Google Scholar] [CrossRef]
- Ladner, J.T.; Barshis, D.J.; Palumbi, S.R. Protein evolution in two co-occurring types of Symbiodinium: An exploration into the genetic basis of thermal tolerance in Symbiodinium clade D. BMC Evol. Biol. 2012, 12, 217. [Google Scholar] [CrossRef]
- Grottoli, A.G.; Rodrigues, L.J.; Palardy, J.E. Heterotrophic plasticity and resilience in bleached corals. Nature 2006, 440, 1186. [Google Scholar] [CrossRef]
- Wooldridge, S.A. Differential thermal bleaching susceptibilities amongst coral taxa: Re-posing the role of the host. Coral Reefs 2014, 33, 15–27. [Google Scholar] [CrossRef]
- Brown, B.E. Coral bleaching: Causes and consequences. Coral Reefs 1997, 16, S129–S138. [Google Scholar] [CrossRef]
- Baird, A.H.; Bhagooli, R.; Ralph, P.J.; Takahashi, S. Coral bleaching: The role of the host. Trends Ecol. Evol. 2009, 24, 16–20. [Google Scholar] [CrossRef]
- Tkachenko, K.S. Coral reefs in the face of ecological threats of the 21st century. Biol. Bull. Rev. 2017, 7, 64–83. [Google Scholar] [CrossRef]
- Qin, Z.; Yu, K.; Wang, Y.; Xu, L.; Huang, X.; Chen, B.; Li, Y.; Wang, W.; Pan, Z. Spatial and intergeneric variation in physiological in physiological indicators of corals in the South China Sea: Insights into their current state and their adaptability to environmental stress. J. Geophys. Res. Ocean. 2019, 124, 3317–3332. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).