Abstract
Southern Thailand represents a region of unique freshwater biodiversity with many endemic taxa, including a number of freshwater mussel species (Bivalvia: Unionidae). In this study, we recognize 13 taxa in the tribes Contradentini, Rectidentini, Pseudodontini (subfamily Gonideinae), and Indochinellini (subfamily Parreysiinae) that inhabit different localities in the Songkhla Lake, Tapi River, and Tha Taphao River basins. Based on the results of morphological and phylogenetic analyses, we discovered among these mussels six taxa new to science, including one genus, three species, and two subspecies. New taxonomic names are introduced here as follows: Songkhlanaia gen. nov.; S. tamodienica gen. & sp. nov.; Sundadontina plugpomenica sp. nov.; Monodontina vondembuschiana tapienica ssp. nov.; M. vondembuschiana thasaenica ssp. nov. (Pseudodontini); and Trapezoideus thachiadensis sp. nov. (Contradentini). These new taxa confirm the high conservation priority of the Southern Thai freshwater mussel fauna.
1. Introduction
Southern Thailand is part of the mega-diverse Southeast Asian region with a plethora of freshwater mussel species [1,2,3,4]. This area is located on the Thai-Malay Peninsula between two biogeographic barriers, the Kra Isthmus and the Songkhla (Van Steenis’s Alor Setar-Singora) Line. The Kra Isthmus represents the lowest and narrowest part of the Thai-Malay Peninsula [5]. The Songkhla Line approximately stretches from Alor Setar in the south to Singora city in the north [6]. The isthmus serves as a significant biogeographic barrier between the freshwater mussel faunas of Sundaland and Western Indochina, and the Songkhla Line separates Southern Thailand from Peninsular Malaysia [1,6]. The diverse freshwater system of Southern Thailand includes larger drainages such as the Tapi River basin [7] and a huge area of the Songkhla Lake basin extending through three administrative provinces (Phattalung, Songkhla, and Nakhon Si Thammarat) [8], as well as smaller rivers such as the Tha Taphao, Patthani, Saiburi, and others.
A comprehensive revision of Thai freshwater mussels carried out by Brandt [1] has demonstrated that the fauna of Southern Thailand is most closely related to that of western Malaysia. Representatives of the tribes Indochinellini (Scabies), Contradentini (Physunio and Lens), and Rectidentini (Hyriopsis and Ensidens), as well as different taxa of the tribe Pseudodontini (e.g., Pilsbryoconcha), were recognized there by earlier scholars [1,2,4]. Nevertheless, the mussel fauna in this part of Thailand is less well studied than those of Central Thailand or Peninsular Malaysia, particularly with regards to its molecular features [2,4,9,10,11,12,13,14,15,16].
This study aims to revise the freshwater mussel fauna of Southern Thailand and to describe the new taxa discovered based on morphological characters and phylogenetic data.
2. Materials and Methods
2.1. Data Sampling
Freshwater mussel specimens (Unionidae) were collected by Dr. Vachira Lheknim from different localities in Southern Thailand, including Chumphon, Surat Thani, Nakhon Si Thammarat, Phatthalung, and Songkhla provinces. Three main basins, i.e., the Tapi River, Songkhla Lake, and Tha Taphao River basins, were examined (Figure 1).
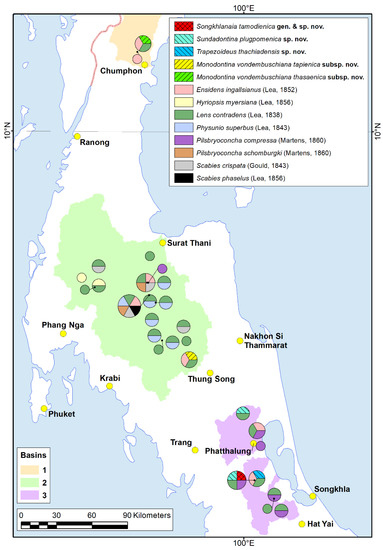
Figure 1.
Distribution ranges of Unionidae from Southern Thailand, including the new taxa. The new taxa are marked by corresponding shading and color. The river basins are highlighted in color: 1—Tha Taphao River basin; 2—Tapi River basin; 3—Songkhla Lake basin. The map was created using ESRI ArcGIS 10 software (www.esri.com/arcgis, accessed on 19 October 2022).
Small tissue snips were preserved in 96% ethanol for DNA analyses. The shell vouchers and tissue snips of collected specimens (including the holotypes and paratypes of the new taxa) are deposited in the Russian Museum of Biodiversity Hotspots (RMBH thereafter), N. Laverov Federal Center for Integrated Arctic Research of the Ural Branch of the Russian Academy of Sciences (Arkhangelsk, Russia). Selected paratypes are deposited in the collection of the Natural History Museum, Prince of Songkla University, in Hat Yai, Songkhla, Thailand.
2.2. Morphological Studies
Comparative morphological analyses of freshwater mussel species were conducted using standard diagnostic features such as the shell outlines, structure of pseudocardinal and lateral teeth, muscle attachment scars, and the shape and position of the umbo [17,18]. All specimens were compared to the original descriptions and images of nominal taxa, described from Southeast Asia [1,2,4,9,10,11,12,13,14,15,16]. For the type series of the new taxa, shell length (L), shell height (H), and shell width (W) were measured with electronic calipers (Table 1). All measurements were taken at maximum diameter. The shells in the figures are oriented according to the lowest points of two adductor muscle scars, which are placed on a horizontal line.

Table 1.
Shell measurements and GenBank sequence accession numbers for the holotypes and selected paratypes of the new freshwater mussel taxa (Unionidae) from Southern Thailand.
2.3. Molecular and Phylogenetic Analyses, Species Delimitation
Sequences of three molecular markers, i.e., the mitochondrial cytochrome c oxidase subunit I (COI) and 16S ribosomal RNA (16S rRNA) and the nuclear 28S ribosomal RNA (28S rRNA) genes, were generated for newly collected specimens from Southern Thailand (Table 1 and Table S1). DNA extraction, PCR amplification, and sequencing were carried out as described in our previous work [18,19].
Molecular diagnoses for the new species were prepared based on available COI, 16S rRNA, and 28S rRNA gene sequence alignments for each genus using the toggle conserved sites tool in MEGA11 at the 50% level [3,20,21]. Uncorrected genetic p-distances between the new species and their congeners were calculated through MEGA11 [21]. The alignment of each gene sequence set was prepared using the MUSCLE algorithm of MEGA11 [21].
The five-partition phylogeny (3 codons of COI + 16S rRNA + 28S rRNA) was based on 111 haplotypes of the Unionidae (Table S2). Several species from the subfamily Gonideinae, i.e., Gonidea angulata (Lea, 1838), Leguminaia wheatleyi (Lea, 1862), Lamprotula leaii (Gray in Griffith & Pidgeon, 1833), and Potomida littoralis (Cuvier, 1798), were used as outgroups. Maximum likelihood (ML) phylogenetic analysis was carried out through the online server of IQ-TREE v1.6.12 (W-IQ-TREE) with automatic identification of evolutionary models [22] and ultrafast bootstrap analysis (UFBoot) with 5000 bootstrap alignments [23]. Models of sequence evolution for each partition calculated using Model Finder [24] and the Bayesian Information Criterion (BIC) were as follows: 1st codon of COI: F81 + I + G; 2nd codon of COI: GTR + G; 3rd codon of COI: TN + I + G; 16S rRNA: TIM2 + I + G; and 28S rRNA: TN + G. The Bayesian Inference (BI) phylogenetic analysis was performed in MrBayes v3.2.7 [25] at the San Diego Supercomputer Center through the CIPRES Science Gateway [26]. The same evolutionary models were implemented in the input file. We used the following parameters: two runs with four Markov chains (three heated and one cold, temperature = 0.2), 15,000,000 generations, and tree sampling every 1000th generation, 15% of the trees were discarded as burn-in. The majority rule consensus tree was calculated from the remaining trees. The convergence of the MCMC chains to a stationary distribution was checked visually using an MCMC trace analysis tool (Tracer v1.7; [27]).
We used three species delimitation methods applied to the COI dataset for distinguishing the Pseudodontini and Trapezoideus taxa separately. The ML analysis was conducted based on an alignment of 140 COI haplotype sequences of the Pseudodontini (Figure S1) and 42 COI haplotype sequences of Trapezoideus using the online server of IQ-TREE v1.6.12 (W-IQ-TREE). The analysis was conducted using an automatic identification of evolutionary models applied for each partition (three codons of COI) [22] and an ultrafast bootstrap (UFBoot) with 5000 bootstrap alignments [23]. Gibbosula laosensis (NCBI acc. No. JX497731) was used to root the tree. We used an implementation of the bPTP model through an online bPTP server (http://species.h-its.org/ptp, accessed on 20 October 2022) with 500,000 MCMC cycles for the Pseudodontini dataset and 100,000 MCMC cycles for the Trapezoideus dataset and 10% burn-in [28]. Additionally, we applied the mPTP model on an mPTP server (https://mptp.h-its.org/#/tree, accessed on 20 October 2022 [29] and one distance-based method (ASAP, Assemble Species by Automatic Partitioning) with the K2P model option through an online server (https://bioinfo.mnhn.fr/abi/public/asap/, accessed on 20 October 2022) [30]. In all cases, the outgroup taxon was removed from the input tree.
2.4. Nomenclatural Acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature (ICZN), and hence the new names contained herein are available under that Code from the electronic edition of this article. This article and the nomenclatural acts it contains have been registered in ZooBank (http://zoobank.org), the online registration system for the ICZN. The Life Science Identifier (LSID) for this publication is as follows: https://zoobank.org/urn:lsid:zoobank.org:pub:9937CAD2-1BBA-4294-B671-60860153C68A.
3. Results
3.1. Morphological Data
Comparative morphological analyses initially allowed recognizing and assigning all studied specimens to nine genera, i.e., Ensidens, Hyriopsis, Lens, Physunio, Scabies, Pilsbryoconcha, Trapezoideus, Monodontina, and Sundadontina (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7). The one specimen, which was subsequently described as a new genus and species, was assigned to Monodontina due to rather thin shell, wide posterior margins, and weak teeth and marked as Monodontina sp. (Figure 2). Several species, such as Ensidens ingallsianus (Lea, 1852), Hyriopsis myersiana (Lea, 1856), Lens contradens (Lea, 1838), Physunio superbus (Lea, 1843), and Pilsbryoconcha compressa (Martens, 1860), were rather well distinguishable by diagnostic traits. Extremely thin and flat specimens of Pilsbryoconcha were assigned to P. schomburgki (Martens, 1860) (Figure 2B). Although samples of Lens contradens were highly variable in the shell shape and sculpture, these specimens revealed a characteristic lamellar tooth structure, which is typical for the species (Figure 4A–H).
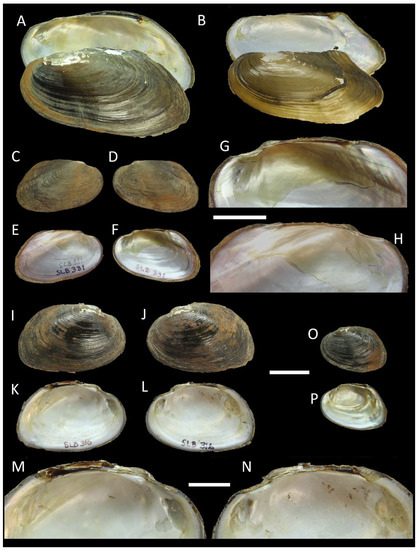
Figure 2.
Shells of Pilsbryoconcha, Songkhlanaia, and Sundadontina from Southern Thailand. (A) P. compressa (Martens, 1860), RMBH biv 1310/1, Klong Nong Thung Tong, Surat Thani Province; (B) Pilsbryoconcha schomburgki (Martens, 1860) stat. rev., specimen RMBH biv 1284/1, Old main Tapi River, Surat Thani Province; (C–H) Songkhlanaia tamodienica gen. & sp. nov., holotype RMBH biv 1332, Klong Plug Pom, Phatthalung Province: right (C) and left (D) valve view, inside view of left (E) and right (F) valve, interior view zoom fragment of right (G) and left (H) valve; (I–N) Sundadontina plugpomenica sp. nov., holotype RMBH biv 1324/1, Klong Pa-Payom, Phatthalung Province: right (I) and left (J) valve view, inside view of left (K) and right (L) valve, interior view zoom fragment of left (M) and right (N) valve; (O,P) S. plugpomenica sp. nov., specimen RMBH biv 1331, Klong Plug Pom, Phatthalung Province: (O) left valve view, (P) inside view of right valve. Scale bar = 20 mm (A–F,I–L,O,P); scale bar = 10 mm (G,H,M,N). Photos: Ekaterina S. Konopleva.
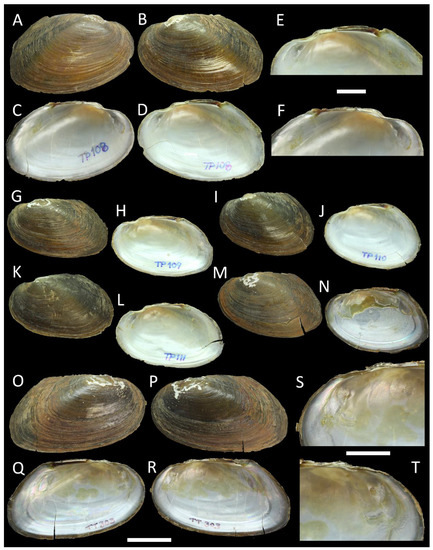
Figure 3.
Shells of Monodontina from Southern Thailand. (A–F) Monodontina vondembuschiana tapienica ssp. nov., holotype RMBH biv 1272/1, Main Klong Min, Nakhon Si Thammarat Province: right (A) and left (B) valve view, inside view of left (C) and right (D) valve, interior view zoom fragment of right (E) and left (F) valve; (G,H) M. v. tapienica ssp. nov., paratype RMBH biv 1272/2, the same locality: (G) left valve view, (H) inside view of right valve; (I,J) M. v. tapienica ssp. nov., paratype RMBH biv 1272/3, the same locality: (I) left valve view, (J) inside view of right valve; (K,L) M. v. tapienica ssp. nov., paratype RMBH biv 1272/4, the same locality: (K) left valve view, (L) inside view of right valve; (M,N) M. v. thasaenica ssp. nov., paratype RMBH biv 1321/2, Main Klong Thasae, Chumphon Province: (M) left valve view, (N) inside view of right valve; (O–T) M. v. thasaenica ssp. nov., holotype RMBH biv 1321/1, the same locality: right (O) and left (P) valve view, inside view of left (Q) and right (R) valve, anterior fragment of right (S) and left (T) valve. Scale bar = 20 mm (A–D,G–R); scale bar = 10 mm (E,F,S,T). Photos: Ekaterina S. Konopleva.
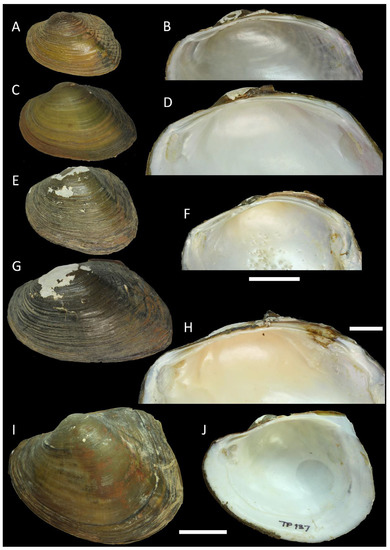
Figure 4.
Shells of Lens and Physunio (Contradentini) members from Southern Thailand. (A,B) Lens contradens (Lea, 1838), specimen RMBH biv 1274/10, Main Klong Min River, Nakhon Si Thammarat Province: (A) left valve view, (B) zoom inside view of right valve; (C,D) L. contradens, specimen RMBH biv 1275/1, Main Klong Sok River, Surat Thani Province: (C) left valve view, (D) zoom inside view of right valve; (E,F) L. contradens, specimen RMBH biv 1280, Main Tapi River, Nakhon Si Thammarat Province: (E) left valve view, (F) zoom inside view of right valve; (G,H) L. contradens, specimen RMBH biv 1278, Main Tapi River, Surat Thani Province: (G) left valve view, (H) zoom inside view of right valve; (I,J) Physunio superbus (Lea, 1843), specimen RMBH biv 1277/1, Main Tapi River, Surat Thani Province: (I) left valve view, (J) inside view of right valve. Scale bar = 20 mm (A,C,E,G,I,J); scale bar = 10 mm (B,D,F,H). Photos: Ekaterina S. Konopleva.
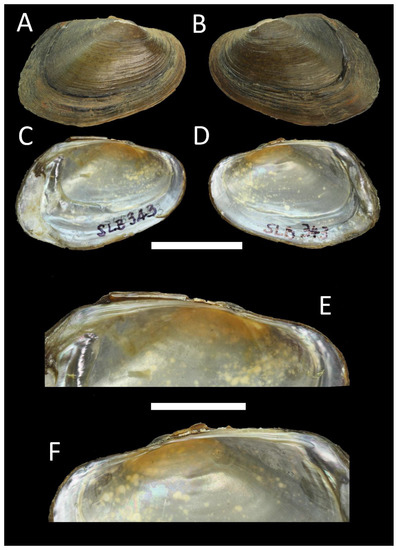
Figure 5.
Shell of Trapezoideus thachiadensis sp. nov. (Contradentini) from Southern Thailand, holotype RMBH biv 1340, Klong Tha Chiad, Phatthalung Province: right (A) and left (B) valve views, inside view of left (C) and right (D) valves, interior view zoom fragment of left € and right (F) valves. Scale bar = 20 mm (A–D); scale bar = 10 mm (E,F). Photos: Ekaterina S. Konopleva.
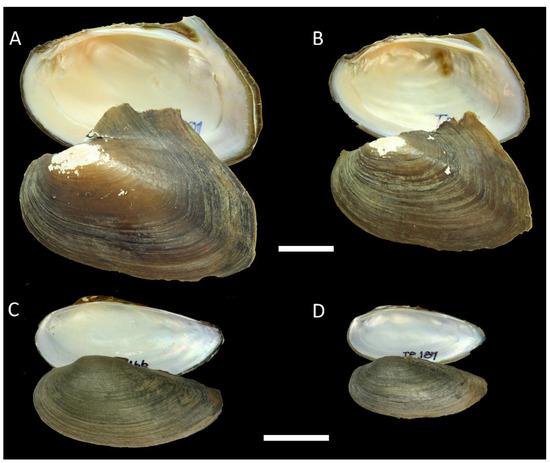
Figure 6.
Shells of Hyriopsis and Ensidens (Rectidentini) members from Southern Thailand. (A) Hyriopsis myersiana (Lea, 1856), specimen RMBH biv 1270/1, Main Klong Sok River, Surat Thani Province; (B) H. myersiana, specimen RMBH biv 1271/1, the same river; (C) Ensidens ingallsianus (Lea, 1852), specimen RMBH biv 1285/1, Old Main Tapi River, Surat Thani Province; (D) E. ingallsianus, specimen RMBH biv 1292/2, Klong Bang Kian Sa River, Surat Thani Province. Scale bars = 20 mm. Photos: Ekaterina S. Konopleva.
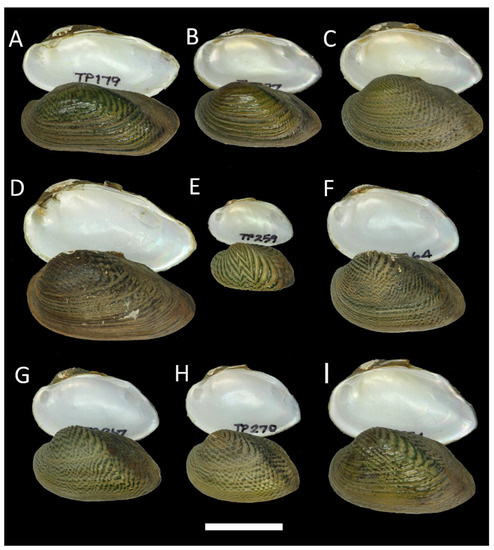
Figure 7.
Shells of Scabies (Indochinellini) members from Southern Thailand. (A) Scabies phaselus (Lea, 1856), specimen RMBH biv 1288/1, Old main Tapi River, Surat Thani Province; (B) S. phaselus, specimen RMBH biv 1318/1, the same river; (C) S. phaselus, specimen RMBH biv 1318/9, the same river; (D) S. crispata (Gould, 1843), specimen RMBH biv 1287/1, Old main Tapi River, Surat Thani Province; (E) S. crispata, specimen RMBH biv 1312/1, Forest Park Lake, Surat Thani Province; (F) S. crispata (Gould, 1843), specimen RMBH biv 1313/1, the same lake; (G) S. crispata (Gould, 1843), specimen RMBH biv 1314, the same lake; (H) S. crispata (Gould, 1843), specimen RMBH biv 1315/1, the same lake; (I) S. crispata, specimen RMBH biv 1319/1, Old main Tapi River, Surat Thani Province. Scale bar = 20 mm. Photos: Ekaterina S. Konopleva.
Studied specimens of the genus Monodontina were initially separated into two morphospecies, one with a pronounced smooth tubercle-like pseudocardinal tooth on the right valve and the other with an almost reduced pseudocardinal tooth. These specimens seemed to be closely related to M. vondembuschiana (Lea, 1840), possessing a thin, winged shell and rather small tubercle-like pseudocardinal teeth (Figure 3). Specimens of Sundadontina with an ovoid, squab shell, knob-like pseudocardinal teeth, and deep adductor and anterior retractor muscle scars were defined as Sundadontina sp. Generally, the outlines of the shell were similar to those of Sundadontina moreleti (Crosse & Fischer, 1876), which was described from the Mekong basin in Cambodia (Figure 2I–N). Trapezoideus specimens had an ovoid, very thin shell, wide and biangular posteriorly, slender pseudocardinal teeth, blade-like lateral teeth, and shallow adductor muscle scars. The shell is somewhat similar to T. foliaceus (Gould, 1843), but is broader posteriorly (Figure 5).
Representatives of the genus Scabies were highly divergent by shell characteristics (e.g., shell sculpture and teeth structure) (Figure 7). In the first step of morphological analyses, we recognized three Scabies species, i.e., S. crispata (Gould, 1843), S. phaselus (Lea, 1856), and S. scobinatus (Lea, 1856). Several specimens (RMBH biv 1313, e.g., Figure 7F) with raised, strongly indented pseudocardinal teeth in the right valve and very deep adductor muscle scars were distinguished as Scabies sp. Specimens with strong, elongated, and convex-shelled, ridge-like pseudocardinal teeth (e.g., Figure 7A) were assigned to S. scobinatus. S. crispata and S. phaselus were generally assigned correct. Specimens with a cuneiform and oval-elongated shell, rostrate posteriorly with fractured pseudocardinal teeth, and deep anterior adductor muscle scars (e.g., Figure 7D,H) were distinguished as S. crispata. Shells with triangular pointed crest pseudocardinal teeth and less developed anterior adductor muscle scars (e.g., Figure 7B) were accepted as S. phaselus.
3.2. Molecular Phylogeny and Species Delimitation
Our five-partition phylogeny revealed eight already described species for the freshwater mussel fauna of Southern Thailand, i.e., Ensidens ingallsianus (Lea, 1852), Lens contradens (Lea, 1838), Physunio superbus (Lea, 1843), Scabies crispata (Gould, 1843), S. phaselus (Lea, 1856), H. myersiana (Lea, 1856), Pilsbryoconcha compressa (Martens, 1860), and P. schomburgki (Martens, 1860). Any other expected species of Scabies were not revealed. Furthermore, we discovered five undescribed lineages belonging to the Pseudodontini and Contradentini clades. Pilsbryoconcha schomburgki is distinguished based on conchological and molecular data and removed from the synonymy of P. compressa. Undescribed lineages represent highly supported clades in both ML and BI phylogenetic analyses (Figure 8). Molecular diagnoses and mean uncorrected COI p-distances from the nearest neighbors of the undescribed lineages are listed in Table 2 and Tables S3–S5. Diagnostic substitutions in 16S rRNA and/or 28S rRNA (if available) are fixed for all undescribed lineages (Table 2).
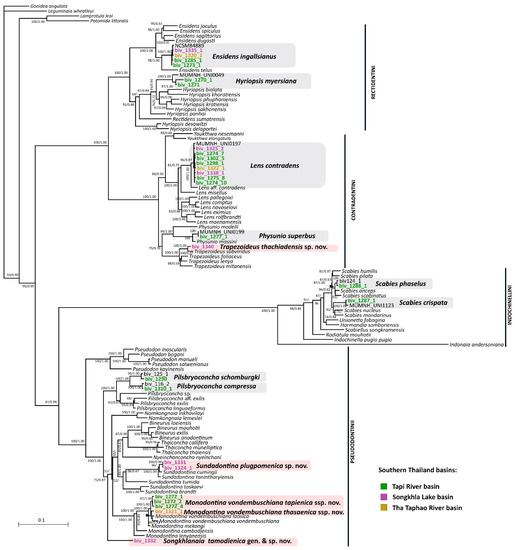
Figure 8.
Maximum likelihood phylogeny of the complete data set of mitochondrial and nuclear sequences (five partitions: three codons of COI + 16S rRNA + 28S rRNA) of the Unionidae from Southeast Asia, including available taxa from Southern Thailand (highlighted by grey rectangles). New taxa from Southern Thailand are marked by pink rectangles. The scale bar indicates the branch lengths. Black numbers near nodes or arrowed are ML ultrafast bootstrap support values (BS)/Bayesian posterior probabilities (BPP).

Table 2.
Molecular diagnoses of the new freshwater mussel species and subspecies (Unionidae) from Southern Thailand.
Output parameters of the bPTP model under the highest Bayesian solution for the Pseudodontini clade were as follows: estimated number of species = 29–60; mean value = 40.51; acceptance rate = 0.40. For the Trapezoideus clade, these values were as follows: estimated number of species = 5–36; mean value = 20.92; acceptance rate = 0.71. According to the highest Bayesian supported solution, one representative of Trapezoideus, Sundadontina, and a new Pseudodontini genus from Southern Thailand were recognized as distinct species (Dataset S1). In the mPTP analyses, only specimens of Trapezoideus and a new Pseudodontini genus were distinguished as separate species. Available Trapezoideus specimens were joined with T. subviridus Pfeiffer et al., 2021 [4], to form a single molecular operational taxonomic unit (MOTU) in the ASAP analysis. Monodontina representatives were not supported as separate taxa and were combined into one species with M. vondembuschiana (Lea, 1840), M. laosica Bolotov et al., 2020, and M. mekongi Bolotov et al., 2020 in all delimitation analyses (Dataset S1).
Two undescribed lineages distinguished as well-supported species-level clades in the phylogenetic and partly in the species delimitation analyses and also having multiple fixed diagnostic substitutions are described here as Trapezoideus thachiadensis sp. nov. (Contradentini) and Sundadontina plugpomenica sp. nov. (Pseudodontini). One of the undescribed lineages, representing a phylogenetically distant and morphologically peculiar clade among other genera of the Pseudodontini, is designated herein as a new genus and species, Songkhlanaia tamodienica gen. & sp. nov. Concerning morphological, phylogenetic, and species delimitation results for the Southern Thai members of the genus Monodontina, we decided to describe these mussels as two subspecies of the widespread M. vondembuschiana, i.e., M. v. tapienica ssp. nov. and M. v. thasaenica ssp. nov. Our expanded dataset indicates that M. laosica should also be considered a subspecies of M. vondembuschiana, while M. mekongi represents a separate species because of much larger mtDNA distances and the presence of several fixed substitutions in the nuclear 28S rRNA gene fragment [3].
3.3. Distribution Patterns
Our new data on Southern Thai freshwater mussel distribution revealed four of the most widespread species, i.e., Lens contradens, which was registered in almost all studied localities, as well as Phusunio superbus, Ensidens ingallsianus, and Pilsbryoconcha compressa (Figure 1 and Figure S2). Each new taxon was registered from a single locality, except for Sundadontina plugpomenica sp. nov., which was found in two rivers. Trapezoideus thachiadensis sp. nov., Sundadontina plugpomenica sp. nov., and Songkhlanaia tamodienica gen. & sp. nov. were collected from the Songkhla Lake basin. Monodontina vondembuschiana tapienica ssp. nov. inhabits the Tapi River basin, while M. v. thasaenica ssp. nov. is known to occur in the Tha Taphao River basin (Figure 1).
3.4. Taxonomy
Family Unionidae Rafinesque, 1820
Subfamily Gonideinae Ortmann, 1916
Tribe Pseudodontini Frierson, 1927
Genus Pilsbryoconcha Simpson, 1900
Pilsbryoconcha compressa (Martens, 1860)
=Spatha compressa Martens, 1860 (by original designation)
Type: Syntype NHMUK 1859.8.1.21 labelled “Khao-Kho, NE of Pakpriau, Siam”.
Type locality: Khao-kho, North-East of Pakpriau in Siam [Khao Kho District, approx. 16.633° N, 100.998° E, Phetchabun Province, Northern Thailand].
Material examined: 10 specimens from Southern Thailand (Table S1).
Distribution: Widespread throughout Thailand, Cambodia, and Laos [1]; recorded from the Tapi River basin and Songkhla Lake basin in Southern Thailand.
Comments: The species is one of the most widespread and common freshwater mussels in Thailand.
Pilsbryoconcha schomburgki (Martens, 1860)
=Anodonta (Lamproscapha) schomburgki Martens, 1860
Type: Holotype NHMUK 1859.5.23.8 labelled “Siam”.
Type locality: Siam [Thailand].
Material examined: Six specimens from Southern Thailand (Table S1).
Distribution: Based on DNA sequences, this species occurs in the Chi River drainage [19], Mae Klong River basin, the headwaters of Mun River and Tonle Sap basin, Khlong Phraphut stream [31] in Thailand; the Tha Taphao and Tapi River basins [31] in Southern Thailand.
Diagnosis: Shells linguiform, with broad, biangular posterior margin, sometimes pointed; smooth periostracum with strong concentric growth lines; umbo not projected; hinge without dentition; anterior adductor muscle scars drop-like, posterior adductor muscle scars indiscernible. This species is similar to P. compressa, but some specimens differ from the latter taxon by having extremely thin and compressed shells, wider and biangular posteriorly.
Comments: This taxon was reinstated from synonymy with Pilsbryoconcha compressa based on morphological features and phylogenetic data collected during our study and recently by Jeratthitikul et al. [31]. Earlier sequenced specimens of P. schomburgki from the Chi River were marked as Pilsbryoconcha aff. compressa sp.1 [18,32].
Genus Songkhlanaia Konopleva, Lheknim, Sriwoon, Kondakov, Vikhrev & Bolotov gen. nov.
Type species:Songkhlanaia tamodienicagen. & sp. nov.
Etymology: The new genus is named after the Songkhla Lake basin, where it was discovered.
Diagnosis: The shell assigned to the new genus is rectangular-shaped and compressed, constricted anteriorly, with a straight upward dorsal margin and distinct prominent folds on the posterior slope. One pseudocardinal tubercule-like tooth on each valve; lateral teeth obsolete.
Distribution: Songkhla Lake basin in Southern Thailand.
Comments: The new genus represents a distinct phylogenetic lineage that is distant from other members of the tribe Pseudodontini (Figure 8).
Songkhlanaia tamodienica Konopleva, Lheknim, Sriwoon, Kondakov, Vikhrev & Bolotov gen. & sp. nov.
Figure 2C–H
Holotype: RMBH biv 1332. Southern Thailand: Klong Plug Pom, middle reach of Klong Tamod, SLB, Ban Kok Sai, Tambon Mae Kree, Tamod District, Phatthalung Province, 7.3324° N, 100.0917° E, 27.v.2021, Lheknim leg. Sequence numbers of the holotype: OP729832 (COI), OP735386 (16S rRNA), and OP735369 (28S rRNA). Shell measurements of the holotype: SL = 44.2 mm, SH = 25.3 mm, and SW = 13.4 mm (Table 1).
Etymology: The name of the new species is related to the Tamod District of Thailand, where its type locality is situated.
Diagnosis: The shell of the new species is rectangular and compressed, with a constricted anterior margin and a sculptured posterior slope with two fine ridges, one smooth tubercular or triangular pseudocardinal tooth in the right valve, and a smooth and somewhat lingula-shaped tooth in the left valve.
Description: Shell rectangular, rather compressed, not thick, anterior margin rounded, rather constricted, ventral margin almost straight, dorsal margin straight upward, posterior slope sculptured with two fine folds, the lower of which is more prominent, posterior margin oblique, rounded in the lower part. Umbo is tiny, slightly elevated, and eroded. Periostracum is rusty-brown with visible growth lines that are closely-spaced. Nacre is blue-whitish with a yellow tint around the umbo cavity, which is not deep. One tubercular pseudocardinal tooth on the right valve, maybe somewhat pointed (like a triangle), smooth. Pseudocardinal tooth on the left valve tubercule (lingula-shaped and smooth). Lateral teeth are obsolete. Anterior adductor muscle scars are somewhat drop-like, shallow, and contiguous with anterior retractor and anterior protractor scars; posterior adductor muscle scars are somewhat rounded and shallow. The pallial line is well-marked and continuous.
Distribution: Songkhla Lake basin in Southern Thailand.
Comments: The species is described based on a single available specimen, the holotype. Lens contradens, Pilsbryoconcha compressa, and Sundadontina plugpomenica sp. nov. were found living together with the new species.
Genus Sundadontina Bolotov et al., 2020
Sundadontina plugpomenica Konopleva, Lheknim, Sriwoon, Kondakov, Vikhrev & Bolotov sp. nov.
Figure 2I–P
Holotype: RMBH biv 1324/1. Southern Thailand: Klong Pa-Payom, SLB, Ban Teng, Tambon Laem Tanod, Khuan Kanun District, Phatthalung Province, 7.8456° N, 99.9933° E, 26.v.2021, Lheknim leg. Sequence numbers of the holotype: OP729828 (COI), OP735383 (16S rRNA), and OP735367 (28S rRNA). Shell measurements of the holotype: SL = 54.3 mm, SH = 35.0 mm, and SW = 21.1 mm (Table 1).
Paratypes: the same locality, date, and collectors, two specimens (RMBH biv 1324, including biv 1324/2 and biv 1324/3 sequenced); Southern Thailand: Klong Plug Pom, middle reach of Klong Tamod, SLB, Ban Kok Sai, Tambon Mae Kree, Tamod District, Phatthalung Province, 7.3324° N, 100.0917° E, one specimen (RMBH biv 1331, sequenced), 27.v.2021, Lheknim leg.
Etymology: The name of the new species is related to the Klong Plug Pom, from which it was collected.
Diagnosis: The shells of this species are ovoid and thick, with massive knob-like pseudocardinal teeth and deep anterior adductor and retractor muscle scars. The new species also differs from its congeners by fixed nucleotide substitutions in the COI and 16S rRNA gene fragments (Table 2).
Description: Shell is ovoid, thick, moderately inflated, rounded anteriorly, posteriorly truncated or somewhat rounded, ventral margin slightly curved, dorsal margin elevated, and sculptured with hardly visible ridges. Umbo is rather small, slightly elevated and eroded. Periostracum is dark-brown with a blackish and rusty tint, while nacre is whitish or yellowish. Umbo cavity small. Right valve with one massive, developed knob-like, slightly pointed pseudocardinal tooth, smooth; left valve with one thick, smooth, knob-like, or rectangular pseudocardinal tooth shifted to the anterior margin. Lateral teeth are obsolete. Anterior adductor muscle scars deep, oval-shaped, contiguous with retractor and anterior protractor scars, anterior retractor scars are very deep; posterior adductor muscle scars are somewhat rounded. The pallial line is distinct and continuous.
Distribution: Songkhla Lake basin in Southern Thailand.
Comments: The new species is conchologically very similar to Sundadontina moreleti (https://science.mnhn.fr/institution/mnhn/collection/im/item/2000-34623?listIndex=6andlistCount=68). Lens contradens and Pilsbryoconcha compressa live in sympatry with Sundadontina plugpomenica sp. nov.
Genus Monodontina Conrad, 1853
Monodontina vondembuschiana(Lea, 1840)
=Margaritana vondembuschiana Lea, 1840
Comments: Based on our new phylogenetic data, this species contains four subspecies: the nominate (Malaysia and the Greater Sunda Islands); M. v. laosica Bolotov et al., 2020 stat. rev. (Mekong River basin, Laos); M. v. tapienica ssp. nov. (Tapi River basin, Southern Thailand); and M. v. thasaenica ssp. nov. (Tha Taphao River basin, Southern Thailand).
Monodontina vondembuschiana tapienica Konopleva, Lheknim, Sriwoon, Kondakov, Vikhrev & Bolotov ssp. nov.
Figure 3A–L
Holotype: RMBH biv 1272/1. Southern Thailand: Main Klong Min, Tapi River basin, Tambon Kaew San, Nabon District, Nakhon Si Thammarat Province, 8.2997° N, 99.5580° E, 14.v.2021, Lheknim leg. Sequence numbers of the holotype: OP729818 (COI) and OP735377 (16S rRNA). Shell measurements of the holotype: SL = 58.1 mm, SH = 36.2 mm, and SW = 20.3 mm (Table 1).
Paratypes: same locality, date, and collectors; three specimens (RMBH biv 1272, including biv 1272/2, biv 1272/3, and biv 1272/4 sequenced).
Etymology: The name of the new subspecies is dedicated to the Tapi River basin, where it was collected.
Diagnosis: The shells of this subspecies are ovoid or oval-elongated, rather inflated, with an upward dorsal margin, two distinct folds slanting downward on the posterior slope, with a small but prominent hill-like or rectangular pseudocardinal tooth on the right valve. The subspecies is morphologically similar to M. mekongi but more thick and elongated. The new subspecies also differs from its congeners and other subspecies by fixed nucleotide substitutions in the COI, 16S rRNA, and 28S rRNA gene fragments (Table 2).
Description: Shell is medium sized, ovoid or oval-elongated, moderately inflated and thick, anteriorly rounded, posteriorly somewhat rounded or truncated, ventral margin curved, dorsal margin curved in passing from the posterior margin, mainly for young individuals. Posterior slope sculptured with two distinct prominent folds strayed from umbo. Umbo rather pronounced and eroded. Periostracum is brown with well-visible growth lines and nacre that is blue-whitish. One prominent smooth, hill-like, or rectangular pseudocardinal tooth on the right valve, maybe slightly pointed. The pseudocardinal tooth on the left valve is less developed, tubercule-like, or almost reduced. Lateral teeth absent or obsolete, as faintly visible rudimentary lamellae. Umbo cavity is moderately deep with light-yellow tint. Anterior adductor muscle scars are drop-shaped, marked, and contiguous with retractor and anterior protractor scars; posterior adductor muscle scars are more rounded and rather shallow. The pallial line is not deep.
Distribution: Tapi River basin in Southern Thailand.
Comments:Lens contradens and Ensidens ingallsianus live in sympatry with M. vondembuschiana tapienica ssp. nov.
Monodontina vondembuschiana thasaenica Konopleva, Lheknim, Sriwoon, Kondakov, Vikhrev & Bolotov ssp. nov.
Figure 3M–T
Holotype: RMBH biv 1321/1. Southern Thailand: Main Klong Thasae, Tha Taphao River Basin, nearby Wat Na Srang, Thasae District, Chumphon Province, 10.6753° N, 99.1737° E, 11.iv.2021, Lheknim leg. Sequence number of the holotype: OP729822 (COI), OP735379 (16S rRNA) and OP735364 (28S rRNA). Shell measurements of the holotype: SL = 62.8 mm, SH = 35.4 mm, and SW = 17.7 mm (Table 2).
Paratypes: the same locality, date, and collectors; one specimen (RMBH biv 1321/2, sequenced).
Etymology: The name of the new subspecies is related to its type locality in the Main Klong Thasae River.
Diagnosis: The shells of the subspecies are rhomboidal and compressed with a tiny umbo and almost reduced pseudocardinal teeth on the right valve, and smooth, low, hill-like, or also reduced pseudocardinal teeth on the left valve. The new subspecies also differs from its congeners and other subspecies by fixed nucleotide substitutions in the COI and 16S rRNA gene fragments (Table 2).
Description: Shell is rhomboidal, elongated, moderately thick, rather compressed, anterior margin rounded, posterior margin truncated, ventral margin somewhat curved, and dorsal margin slightly elevated. Posterior slope sculptured with two slightly visible folds strayed from umbo. Umbo tiny and eroded. Periostracum is brown with rusty tint, nacre that is blue-whitish with yellow spots around the umbo cavity, and an umbo cavity that is small. One very shallow, hill-like, or almost reduced pseudocardinal tooth on the right valve; one small tubercular (hill-like) pseudocardinal tooth on the left valve; smooth or almost reduced. Lateral teeth are obsolete. Anterior adductor muscle scars are visible, drop-like or oval-shaped, and contiguous with retractor and anterior protractor scars; posterior adductor muscle scars are more rounded. The pallial line is distinct.
Distribution: Tha Taphao River Basin in Southern Thailand.
Comments:Lens contradens and Ensidens ingallsianus live in sympatry with M. vondembuschiana thasaenica ssp. nov.
Tribe Contradentini Modell, 1942
Genus Lens Simpson, 1900
Lens contradens (Lea, 1838)
Figure 4A–H
=Unio contradens Lea, 1838 (by original designation)
Type: Holotype USNM 85185 labelled “Java”.
Type locality: Java.
Material examined: 114 specimens from Southern Thailand (Table S1).
Distribution: Tapi and Tha Taphao River basins, Songkhla Lake basin in Southern Thailand; Chao Phraya and Mae Khlong River basins in Central and Western Thailand, coastal drainages of Southeastern Thailand, the Malay Peninsula, and the Greater Sunda Islands [4,12]. A few local populations are known to occur in the Mekong (Kok, Ing, and Loei rivers) and Salween (Moei River) drainages [4].
Comments:Lens contradens is the most widespread species in Thailand, characterizing by a high degree of variability in the shell shape (Figure 4A–H).
Genus Physunio Simpson, 1900
Figure 4I,J
Physunio superbus (Lea, 1843)
=Unio superbus Lea, 1843 (by original designation)
Type: Lectotype USNM 83934 [4].
Type locality: New Holland [Australia, erroneous] [4].
Material examined: 17 specimens from Southern Thailand (Table S1).
Distribution: Tapi River basin in Southern Thailand; Meklong, Chao Phraya, and Bang Pakong drainages in Eastern and Central Thailand; Malaysia, Sumatra, and Java [4].
Comments:Lens contradens, Ensidens ingallsianus, Pilsbryoconcha schomburgki, Scabies crispata, and S. phaselus were registered together with Physunio superbus.
Genus Trapezoideus Simpson, 1900
Trapezoideus thachiadensis Konopleva, Lheknim, Sriwoon, Kondakov, Vikhrev & Bolotov sp. nov.
Holotype: RMBH biv 1340. Southern Thailand: Klong Tha Chiad, middle reach of Klong Tha Madua, Songkhla Lake basin, Tambon Mae Kree, Tamod District, Phatthalung Province, 7.3452° N, 100.1064° E, 27.v.2021, Lheknim leg. Sequence numbers of the holotype: OP729805 (COI), OP735372 (16S rRNA), and OP735357 (28S rRNA). Shell measurements of the holotype: SL = 37.5 mm, SH = 24.4 mm, and SW = 12.0 mm (Table 1).
Etymology: This species is named after its type locality, the Klong Tha Chiad, belonging to the Songkhla Lake watershed in Southern Thailand.
Diagnosis: The shells of the species are obovate, extremely thin, posteriorly broad, and biangular, with slender lamellar pseudocardinal teeth, elongate and slightly curved laterals, and shallow adductor muscle scars. Phylogenetically, it is closer to T. subviridus, but it is conchologically more similar to T. foliaceus, although it could be distinguished from the latter species by having a much broader posterior margin. The new species also differs from its congeners by fixed nucleotide substitutions in the COI, 16S rRNA, and 28S rRNA gene fragments (Table 2).
Description: Shell small, obovate, very thin, rather compressed, with an anterior margin rounded, a posterior margin broad and biangular, a ventral margin convex, and a dorsal margin curving towards the posterior side. Periostracum that is olive-brown with tiny brown spots; nacre that is blue-gray with an orange tint around the umbo. Umbo is very small and eroded, just slightly elevated above the hinge line. Tiny wrinkles are hardly visible along dorsal margin behind the umbo. Two lamellar, sharp, parallel to each other pseudocardinal teeth on the right valve, posterior tooth almost reduced. One lamellar, sharp pseudocardinal tooth on the left valve. Lateral teeth are blade-like, emerging directly behind pseudocardinals, one in the right valve and two in the left valve. In left valve upper posterior lateral tooth almost parallel to dorsal margin, anterior lateral tooth slightly downward from the anterior tooth; in right valve lateral tooth slightly curved. Anterior adductor muscle scars are oval-shaped, shallow, and contiguous with anterior retractor muscle scar; anterior protractor muscle scars are somewhat trapezoidal or rhomboid, hardly visible; and posterior adductor muscle scars are rounded, shallow.
Distribution: Songkhla Lake basin, Southern Thailand.
Comments: The new species was described based on one available specimen (the holotype). Lens contradens was also found living together with T. thachiadensis sp. nov.
Tribe Rectidentini Modell, 1942
Genus Hyriopsis Conrad, 1853
Hyriopsis myersiana (Lea, 1856)
Figure 6A,B
=Unio myersianus Lea, 1856 (by original designation)
Type: Lectotype USNM 83950.
Type locality: Siam [Thailand].
Material examined: Seven specimens from Southern Thailand (Table S1).
Distribution: Tapi River basin in Southern Thailand; Meklong, Chao Phraya, and Bang Pakong drainages in Central and Western Thailand [4,14].
Comments:Lens contradens was found living together with H. myersiana.
Genus Ensidens Frierson, 1911
Ensidens ingallsianus (Lea, 1852)
Figure 6C,D
=Unio ingallsianus Lea, 1852 (by original designation)
Type: Holotype USNM 85933.
Type locality: Siam [Thailand].
Material examined: 26 specimens from Southern Thailand (Table S1).
Distribution: Tapi and Tha Taphao River basins, and Songkhla Lake basin in Southern Thailand; Meklong, Chao Phraya, and Bang Pakong River basins in Western and Central Thailand, Peninsular Malaysia [4,14].
Comments:Ensidens ingallsianus was collected from sites, also inhabited by Scabies crispata, S. phaselus, Lens contradens, Pilsbryoconcha schomburgki, P. compressa, Monodontina vondembuschiana tapienica ssp. nov., and Trapezoideus thachiadensis sp. nov.
Subfamily Parreysiinae Henderson, 1935
Tribe Indochinellini Bolotov, Pfeiffer, Vikhrev and Konopleva, 2018
Genus Scabies Haas, 1911
Scabies phaselus (Lea, 1856)
Figure 7A–C
=Unio phaselus Lea, 1856 (by original designation)
Type: Lectotype USNM_84143 labelled “Siam” (USNM).
Type locality: Siam. S.R. House, M. D.
Material examined: 14 specimens from Southern Thailand (Table S1).
Distribution: Tapi River basin in Southern Thailand; Mekong, Meklong, and Chao Phraya basins [11,13].
Comments:Scabies phaselus was found only in one locality in Southern Thailand (Old Main Tapi River, 8.7520° N, 99.2315° E). Scabies crispata, Ensidens ingallsianus, Pilsbryoconcha schomburgki, and Lens contradens were registered as living together with S. phaselus.
Scabies crispata (Gould, 1843)
Figure 7D–I
=Unio crispata Gould, 1843 (by original designation)
Type: Lectotype MCZ_169099 labelled “Tavoy, British Burmah. F. Mason” (MCZ).
Type locality: Tavoy, British Burmah [Dawei River, Myanmar; not rediscovered by us in the type locality].
Material examined: 39 specimens from Southern Thailand (Table S1).
Distribution: Tapi River basin in Southern Thailand; Mekong and Chao Phraya basins; rivers draining into the Gulf of Thailand [11,13]; probably also the upstream part of the Dawei River basin, Myanmar [1].
Comments:Lens contradens, Ensidens ingallsianus, Pilsbryoconcha schomburgki, and Scabies phaselus were registered living together with S. crispata.
4. Discussion
4.1. Taxonomic Issues
As a result of morphological and phylogenetic analyses of the Unionidae from Southern Thailand, 13 taxa from the tribes Contradentini, Rectidentini, Pseudodontini, and Indochinellini, including five species-group taxa new to science (three species and two subspecies), were distinguished. Among the new taxa, there are representatives of well-known genera such as Trapezoideus (T. thachiadensis sp. nov.), Sundadontina (S. plugpomenica sp. nov.), and Monodontina (M. vondembuschiana tapienica ssp. nov. and M. v. thasaenica ssp. nov.). In addition, a distant genus-level lineage of the Pseudodontini was discovered and is described as the new genus Songkhlanaia gen. nov. (S. tamodienica gen. & sp. nov.).
The majority of the unionid species in the study area are rather well-distinguishable using only morphological features and distributional data, except for a few representatives of the genus Scabies, which are characterized by rather variable conchological traits [11,13] (Figure 7). Our phylogenetic analyses supported the new species and genus-level clades well, whereas the species delimitation modeling returned controversial results for Trapezoideus thachiadensis sp. nov., Sundadontina plugpomenica sp. nov., and Monodontina vondembuschiana subspecies. Despite a rather low level of COI divergence between Trapezoideus thachiadensis sp. nov. and T. subviridus (uncorrected pairwise p-distance = 2.4%), the new species has substantial conchological differences (Figure 5) and multiple fixed nucleotide substitutions (Table 2). The minimum level of COI genetic divergence of 2.8% for Sundadontina plugpomenica sp. nov. is also appropriate for species delimitation [32,33,34,35]. Interestingly, this species is conchologically very similar to S. moreleti, which was described from the Lower Mekong in Cambodia. However, given the rather restricted distribution ranges of most Sundadontina species [3], the lineage from Southern Thailand most likely represents a separate taxon. A significant level of COI genetic divergence of around 2% for both new Monodontina lineages, morphological differences between them and other congeners, and relatively low COI genetic variation within the vondembuschiana-group call for a revision of these taxa, with the separation of four allopatric subspecies for the widespread M. vondembuschiana. Isolated populations of this species on the Greater Sunda Islands may also represent separate subspecies, but this hypothesis needs to be tested based on an expanded DNA sequence dataset.
4.2. Distributional Patterns and Conservation Priorities
All studied taxa belong to the Sundaland freshwater mussel fauna. The most widespread species is Lens contradens, as has also been revealed in other studies [4,36,37]. Such a wide distribution and broad ecological range are expressed in high disparity with regard to shell shape and sculpture between local populations (Figure 4A–H), which may reflect a high adaptive capacity. In contrast, the new taxa have narrow geographic ranges and could be endemic to their localities. Numerous new vertebrate and invertebrate taxa were recently described from Southern Thailand, including toads [38], lizards [39], frogs [40], and pseudoscorpions [41]. The location of Southern Thailand between two biogeographic barriers, the Isthmus of Kra and the Songkhla Isthmus, probably corresponding to seaways in the past [3], may allude to the existence of other divergent species- and genus-level lineages.
Our discovery of the new freshwater mussel taxon confirms the high diversity of the aquatic fauna in Southern Thailand and increases its significance for conservation. Data about major threats for freshwater mussels in Thailand is very restricted, but these threats may include climate change, pollution, and sediment accumulation [2]. It is also known that Southern Thailand is exposed to the conversion of lowland forest to rubber and commercial oil palm plantations, leading to habitat degradation and a significant reduction in biodiversity [42]. Freshwater mussels, as one of the most threatened animal groups in the world [2,10,43,44,45,46], require intense monitoring and should be a high priority taxon for conservation. In particular, this applies to the potentially endemic species described from Southern Thailand in this study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15010010/s1, Figure S1: Maximum likelihood phylogeny based on 3 codons of COI for Pseudodontini (W-IQ-TREE). New taxa from Southern Thailand are marked by blue and green rectangles. Scale bar indicates the branch lengths. Black numbers near nodes are ML ultrafast bootstrap support values (BS); Figure S2: Number of collecting localities of freshwater mussels (Unionidae) discovered in Southern Thailand; Table S1: List of specimens collected from Southern Thailand and used in this study, including species names, voucher numbers, tissue codes, localities, coordinates, dates, collector and GenBank sequence accession numbers; Table S2: List of sequences used in the phylogenetic reconstructions (five partitions: three codons of COI + 16S rRNA + 28S rRNA); Table S3: Genetic divergences (mean pairwise uncorrected p-distances ± standard error estimate, %) between Trapezoideus thachiadensis sp. nov. and its congeneric species based on the mitochondrial COI gene sequences; Table S4: Genetic divergences (mean pairwise uncorrected p-distances ± standard error estimate, %) between Sundadontina plugpomenica sp. nov. and its congeneric species based on the mitochondrial COI gene sequences; Table S5: Genetic divergences (mean pairwise uncorrected p-distances ± standard error estimate, %) between Monodontina taxa based on the mitochondrial COI gene sequences. Distances between four subspecies of M. vondembuschiana are highlighted by blue; Dataset S1: Species delimitation results.
Author Contributions
Conceptualization, E.S.K. and V.L.; Methodology, A.V.K., A.A.T. and E.S.K.; Software, E.S.K.; Validation, E.S.K., I.N.B. and A.V.K.; Formal Analysis, E.S.K.; Investigation, E.S.K., V.L. and R.S.; Data Curation, E.S.K. and I.N.B.; Writing—Original Draft Preparation, E.S.K.; Writing—Review and Editing, E.S.K., I.N.B., V.L. and I.V.V.; Visualization, E.S.K. and M.Y.G.; Funding Acquisition, I.N.B., M.Y.G., V.L. and R.S. All authors discussed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Molecular analyses and mapping were supported by the Russian Ministry of Science and Higher Education (project No. FUUW-2022-0056 to I.N.B. and M.Y.G.), morphological and phylogenetic studies were supported by the Russian Science Foundation (grant No. 21-17-00126 to I.N.B.), field sampling was supported by the Prince of Songkla University (research project No. SCI6401005S “Distribution and abundance of freshwater mussels in Plant Genetic Protection Area of RSPG, Rajjaprabha Reservoir, EGAT, and nearby areas Surat Thani Province” to V.L. and R.S.).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data used in the current study are available from the Supplementary Material or corresponding authors on request. Sequences obtained during this study were deposited in GenBank (http://www.ncbi.nlm.nih.gov/) under the accession numbers mentioned in the text.
Acknowledgments
We are very grateful to Simon Schneider and two anonymous reviewers for their valuable comments which greatly improved the earlier versions of this paper.
Conflicts of Interest
No potential conflict of interest was reported by the authors.
References
- Brandt, R.A.M. The non-marine aquatic Mollusca of Thailand. Arch. Molluskenkd. 1974, 105, 1–423. [Google Scholar]
- Zieritz, A.; Bogan, A.E.; Froufe, E.; Klishko, O.; Kondo, T.; Kovitvadhi, U.; Kovitvadhi, S.; Lee, J.H.; Lopes-Lima, M.; Pfeiffer, J.M.; et al. Diversity, biogeography and conservation of freshwater mussels (Bivalvia: Unionida) in East and Southeast Asia. Hydrobiologia 2018, 810, 29–44. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Konopleva, E.S.; Vikhrev, I.V.; Gofarov, M.Y.; Lopes-Lima, M.; Bogan, A.E.; Lunn, Z.; Chan, N.; Win, T.; Aksenova, O.V.; et al. New freshwater mussel taxa discoveries clarify biogeographic division of Southeast Asia. Sci. Rep. 2020, 10, 6616. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, J.M.; Graf, D.L.; Cummings, K.S.; Page, L.M. Taxonomic revision of a radiation of Southeast Asian freshwater mussels (Unionidae: Gonideinae: Contradentini+Rectidentini). Invertebr. Syst. 2021, 35, 394–470. [Google Scholar] [CrossRef]
- Liao, P.-C.; Chiang, Y.-C.; Huang, S.; Wang, J.-C. Gene flow of Ceriops tagal (Rhizophoraceae) across the Kra Isthmus in the Thai Malay Peninsula. Bot. Stud. 2009, 50, 193–204. [Google Scholar]
- Parnell, J. The biogeography of the Isthmus of Kra region: A review. Nord. J. Bot. 2013, 31, 1–15. [Google Scholar] [CrossRef]
- Lheknim, V. Annotated Checklist for a Collection of Fishes from Tapi River Basin, south Thailand. Nat. Hist. J. Chulalongkorn Univ. 2004, 4, 83–98. [Google Scholar]
- Iwasaki, S.; Shaw, R. Integrated Lagoon Fisheries Management: Resource Dynamics and Adaptation; Emerald Publisher: Bingley, UK, 2010. [Google Scholar]
- Pfeiffer, J.M.; Graf, D.L. Evolution of bilaterally asymmetrical larvae in freshwater mussels (Bivalvia: Unionoida: Unionidae). Zool. J. Linn. Soc. 2015, 175, 307–318. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Froufe, E.; Ghamizi, M.; Mock, K.E.; Kebapçı, Ü.; Klishko, O.; Kovitvadhi, S.; Kovitvadhi, U.; Paulo, O.S.; Pfeiffer, J.M.; et al. Phylogeny of the most species-rich freshwater bivalve family (Bivalvia: Unionida: Unionidae): Defining modern subfamilies and tribes. Mol. Phylogenet. Evol. 2017, 10, 174–191. [Google Scholar] [CrossRef]
- Pfeiffer, J.M.; Graf, D.L.; Cummings, K.S.; Page, L.M. Molecular phylogeny and taxonomic revision of two enigmatic freshwater mussel genera (Bivalvia: Unionidae incertae sedis: Harmandia and Unionetta) reveals a diverse clade of Southeast Asian Parreysiinae. J. Molluscan Stud. 2018, 84, 404–416. [Google Scholar] [CrossRef]
- Jeratthitikul, E.; Phuangphong, S.; Sutcharit, C.; Prasankok, P.; Kongim, B.; Panha, S. Integrative taxonomy reveals phenotypic plasticity in the freshwater mussel Contradens contradens (Bivalvia: Unionidae) in Thailand, with a description of a new species. Syst. Biodivers. 2019, 17, 134–147. [Google Scholar] [CrossRef]
- Jeratthitikul, E.; Sucharit, C.; Prasankok, P. Molecular phylogeny of the Indochinese freshwater mussel genus Scabies Haas, 1911 (Bivalvia: Unionidae). Trop. Nat. Hist. 2019, 19, 21–36. Available online: https://li01.tci-thaijo.org/index.php/tnh/article/view/181195 (accessed on 21 July 2022).
- Jeratthitikul, E.; Paphatmethin, S.; Zieritz, A.; Lopes-Lima, M.; Ngor, P.B. Hyriopsis panhai, a new species of freshwater mussel from Thailand (Bivalvia: Unionidae). Raffles Bull. Zool. 2021, 69, 124–136. [Google Scholar] [CrossRef]
- Muanta, S.; Jeratthitikul, E.; Panha, S.; Prasankok, P. Phylogeography of the freshwater bivalve genus Ensidens (Unionidae) in Thailand. J. Molluscan Stud. 2019, 85, 224–231. [Google Scholar] [CrossRef]
- Konopleva, E.S.; Bolotov, I.N.; Pfeiffer, J.M.; Vikhrev, I.V.; Kondakov, A.V.; Gofarov, M.Y.; Tomilova, A.A.; Tanmuangpak, K.; Tumpeesuwan, S. New freshwater mussels from two Southeast Asian genera Bineurus and Thaiconcha (Pseudodontini, Gonideinae, Unionidae). Sci. Rep. 2021, 11, 8244. [Google Scholar] [CrossRef]
- Konopleva, E.S.; Bolotov, I.N.; Vikhrev, I.V.; Gofarov, M.Y.; Kondakov, A.V. An integrative approach underscores the taxonomic status of Lamellidens exolescens, a freshwater mussel from the Oriental tropics (Bivalvia: Unionidae). Syst. Biodivers. 2017, 15, 204–217. [Google Scholar] [CrossRef]
- Konopleva, E.S.; Pfeiffer, J.M.; Vikhrev, I.V.; Kondakov, A.V.; Gofarov, M.Y.; Aksenova, O.V.; Lunn, Z.; Chan, N.; Bolotov, I.N. A new genus and two new species of freshwater mussels (Unionidae) from western Indochina. Sci. Rep. 2019, 9, 4106. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Kondakov, A.V.; Vikhrev, I.V.; Aksenova, O.V.; Bespalaya, Y.V.; Gofarov, M.Y.; Kolosova, Y.S.; Konopleva, E.S.; Spitsyn, V.M.; Tanmuangpak, K.; et al. Ancient river inference explains exceptional Oriental freshwater mussel radiations. Sci. Rep. 2017, 7, 2135. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Vikhrev, I.V.; Kondakov, A.V.; Konopleva, E.S.; Gofarov, M.Y.; Aksenova, O.V.; Tumpeesuwan, S. New taxa of freshwater mussels (Unionidae) from a species-rich but overlooked evolutionary hotspot in Southeast Asia. Sci. Rep. 2017, 7, 11573. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Chernomor, O.; Von Haeseler, A.; Minh, B.Q. Terrace aware data structure for phylogenomic inference from supermatrices. Syst. Biol. 2016, 65, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2017, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef]
- Kapli, P.; Lutteropp, S.; Zhang, J.; Kobert, K.; Pavlidis, P.; Stamatakis, A.; Flouri, T. Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics 2017, 33, 1630–1638. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef]
- Jeratthitikul, E.; Paphatmethin, S.; Sutcharit, C.; Ngor, P.B.; Inkhavilay, K.; Prasankok, P. Phylogeny and biogeography of Indochinese freshwater mussels in the genus Pilsbryoconcha Simpson, 1900 (Bivalvia: Unionidae) with descriptions of four new species. Sci. Rep. 2022, 12, 20458. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Hattori, A.; Kondo, T.; Lee, J.H.; Kim, S.K.; Shirai, A.; Hayashi, H.; Usui, T.; Sakuma, K.; Toriya, T.; et al. Freshwater mussels (Bivalvia: Unionidae) from the rising sun (Far East Asia): Phylogeny, systematics, and distribution. Mol. Phylogenet. Evol. 2020, 146, 1–27. [Google Scholar] [CrossRef]
- Pieri, A.M.; Inoue, K.; Johnson, N.A.; Smith, C.H.; Harris, J.L.; Robertson, C.; Randklev, C.R. Molecular and morphometric analyses reveal cryptic diversity with freshwater mussels (Bivalvia: Unionidae) of the western Gulf coastal drainages of the USA. Biol. J. Linn. Soc. 2018, 124, 261–277. [Google Scholar] [CrossRef]
- Smith, C.H.; Johnson, N.A.; Inoue, K.; Doyle, R.D.; Randklev, C.R. Integrative taxonomy reveals a new species of freshwater mussel, Potamilus streckersoni sp. nov. (Bivalvia: Unionidae): Implications for conservation and management. Syst. Biodivers. 2019, 17, 331–348. [Google Scholar] [CrossRef]
- Klunzinger, M.W.; Whisson, C.; Zieritz, A.; Benson, J.A.; Stewart, B.A.; Kirkendale, L. Integrated taxonomy reveals new threatened freshwater mussels (Bivalvia: Hyriidae: Westralunio) from southwestern Australia. Sci. Rep. 2022, 12, 20385. [Google Scholar] [CrossRef] [PubMed]
- Zieritz, A.; Lopes-Lima, M.; Bogan, A.E.; Sousa, R.; Walton, S.; Rahim, K.A.A.; Wilson, J.J.; Ng, P.Y.; Froufe, E.; McGowan, S. Factors driving changes in freshwater mussel (Bivalvia, Unionida) diversity and distribution in Peninsular Malaysia. Sci. Total Environ. 2016, 571, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Zieritz, A.; Lopes-Lima, M. Handbook and National Red-List of the Freshwater Mussels of Malaysia; Zieritz & Lopes-Lima: Kuala Lumpur, Malaysia, 2018. [Google Scholar]
- Suwannapoom, C.; Grismer, L.L.; Pawangkhanant, P.; Poyarkov, N.A. A new species of stream toad of the genus Ansonia Stoliczka, 1870 (Anura: Bufonidae) from Nakhon Si Thammarat Range in southern Thailand. Zootaxa 2022, 5168, 119–136. [Google Scholar] [CrossRef]
- Trivalairat, P.; Sumontha, M.; Kunya, K.; Chiangkul, K. Acanthosaura meridiona sp. nov. (Squamata: Agamidae), a new short-horned lizard from southern Thailand. Herpetol. J. 2022, 32, 34–50. [Google Scholar] [CrossRef]
- Yodthong, S.; Rujirawan, A.; Stuart, B.L.; Aowphol, A. A new Limnonectes (Anura:Dicroglossidae) From Southern Thailand. Animals 2021, 11, 566. [Google Scholar] [CrossRef]
- Harvey, M.S. A new species of Garypus (Pseudoscorpiones: Garypidae) from southern Thailand. Rev. Suisse Zool. 2021, 128, 221–225. [Google Scholar] [CrossRef]
- Aratrakorn, S.; Thunhikorn, S.; Donald, P.F. Changes in bird communities following conversion of lowland forest to oil palm and rubber plantations in southern Thailand. Bird Conserv. Int. 2006, 16, 71–82. [Google Scholar] [CrossRef]
- Sousa, R.; Halabowski, D.; Labecka, A.M.; Douda, K.; Aksenova, O.; Bespalaya, Y.; Bolotov, I.; Geist, J.; Jones, H.A.; Konopleva, E.; et al. The role of anthropogenic habitats in freshwater mussel conservation. Glob. Chang. Biol. 2021, 27, 2298–2314. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Konopleva, E.S.; Vikhrev, I.V.; Win, T.; Lunn, Z.; Chan, N.; Gofarov, M.Y.; Kondakov, A.V.; Tomilova, A.A.; Pasupuleti, R.; et al. Follow the Footsteps of Leonardo Fea: An Example of an Integrative Revision of Freshwater Mussel Taxa Described from the Former British Burma (Myanmar). J. Zool. Syst. Evol. Res. 2022, 2022, 6600359. [Google Scholar] [CrossRef]
- Jeratthitikul, E.; Sutcharit, C.; Ngor, P.B.; Prasankok, P. Molecular phylogeny reveals a new genus of freshwater mussels from the Mekong River Basin (Bivalvia: Unionidae). Eur. J. Taxon. 2021, 775, 119–142. [Google Scholar] [CrossRef]
- Pfeiffer, J.M.; Graf, D.L. Re-analysis confirms the polyphyly of Lamprotula Simpson, 1900 (Bivalvia: Unionidae). J. Molluscan Stud. 2013, 79, 249–256. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).