Abstract
Seaweed communities perform a variety of ecological services, including primary productivity supply, biological habitat construction, water purification, and acting as marine carbon sinks. The abundance of seaweed is the basis for the assessment of ecological services in communities. The Ma’an Archipelago, adjacent to the Yangtze River estuary in China, is an important and typical island group. In this study, the abundance of seaweed in the typical coastal islands of the Ma’an Archipelago, Zhejiang Province, was evaluated by means of sonar detection and scuba diving sampling methods. The organic carbon content of six dominant seaweed species was measured to estimate the carbon sequestration capacity of the dominant species in the Ma’an Archipelago. The results show that 27 species of Rhodophyta, 10 species of Ochrophyta, and two species of Chlorophyta were found in the Ma’an Archipelago. Seaweed was distributed in the coastal areas of the islands, with a distribution width of 2–60 m. Gouqi Island had the longest shoreline, and there, the distribution depth of the seaweed reached 15 m and the area of the seaweed community was the largest. The slope of the rocks in the Sanheng survey area was large and the width of the seaweed community was small. The distribution area of seaweed in the Ma’an Archipelago was 6.51–13.43 km2 and the organic carbon content of the seaweed was 33.16 ± 3.26%. The biomass of Ochrophyta in the Ma’an Archipelago was the largest, followed by Chlorophyta and Rhodophyta. Among the six dominant species, the carbon sequestration of Sargassum thunbergii was the largest, at 277.91–848.74 t per year, and that of Undaria pinnatifida was the smallest. This study provides scientific guidance for the assessment of the primary productivity supply, carbon sink, and conservation capacity of seaweeds in China.
1. Introduction
In recent years, due to the construction of offshore engineering projects, increased water pollution, and inappropriate human harvesting, the abundance of seaweed in the Ma’an Archipelago has decreased dramatically, and the diversity of seaweed has also decreased [1]. The existing seaweed abundance has become the focus of many researchers [2]. Seaweed can absorb dissolved inorganic carbon in water through photosynthesis and transform it into organic carbon, performing an important ecological service as an oceanic carbon sink [3]. Although there are differences in nutrients, temperature, and light conditions in different areas, there is no significant difference in the proportion of organic carbon content in one species [4]; however, there are specific differences in the organic carbon content of different seaweed species. It is generally believed that the inorganic carbon utilization capacity of different phyla of seaweed is highest in Chlorophyta, and lower in Ochrophyta and Rhodophyta [5]. This is related to the distribution of seaweeds, as seaweeds distributed in intertidal zones are more adaptable to strong light than those in subtidal zones. The net primary productivity of global seaweed is 2.36 times the total of global seagrass, mangroves, and marshes. Seaweed can transport more than 80% of its own organic carbon to sediments for storage [6]. In response to climate change, the Chinese government has set a national strategic goal: “strive to reach the peak of carbon dioxide emissions by 2030, and strive to achieve carbon neutrality by 2060” [7]. The traditional survey areas of seaweed abundance are mostly in intertidal zones, while research on biodiversity is rare in subtidal zones with larger abundances of seaweed [8]. Seaweed samples are often collected using quadrats, which require a large amount of manpower and material resources [9]. The application of underwater acoustic technology makes it possible to investigate the diversity and assess the distribution scale of seaweed in subtidal zones.
In this study, the biodiversity and biomass of the offshore seaweed in the Ma’an Archipelago were investigated using acoustic scanning combined with diving sampling technology, and the carbon sequestration of the seaweed was evaluated by determining the organic carbon content of the dominant species. This study is a reference for the rational utilization of seaweed abundance and the protection of biodiversity in the Ma’an Archipelago, and it provides scientific evidence and technical support for the evaluation of carbon sequestration values of seaweed and the protection of coastal ecosystems.
2. Materials and Methods
2.1. Investigation Area
The Ma’an Archipelago is located north of Zhoushan, Zhejiang Province, and includes many islands such as Luhua Island and Huaniao Island, as well as several reefs. Because of the perennial impact of the confluence of the Yangtze Diluted Water and the Taiwan Warm Current, there are many species of marine life in the archipelago. It has formed a marine ecosystem dominated by rich marine life, unique island landforms, and intertidal zones, which are of great value for development, research, and conservation [10]. The thriving seaweeds contribute to the high primary productivity of the coastal waters and provide an excellent habitat and breeding place for marine organisms [11]. Seaweed beds, formed by dense collections of seaweed, are not only important for the conservation of fish, the eutrophication of the sea, and the improvement of the ecological environment in the coastal waters of the Ma’an Archipelago, but they are also sensitive to climate change and are an indispensable part of the blue carbon sink in coastal zones [12]. The north of the Ma’an Archipelago is mostly composed of argillaceous sediments, and the south mostly comprises scattered islands and reefs. This area is the boundary between the temperate warm water type and the subtropical warm water type of seaweed bed [13]. Luhua Island, in the Ma’an Archipelago, is the northern boundary of Sargassum vachellianum distribution along the coast of China [14]. The sea area of the Ma’an Archipelago is a typical representative area for studying the distribution of seaweed along the coast of China (Figure 1).
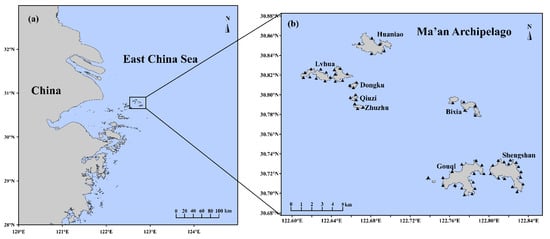
Figure 1.
Investigation area. (a) Ma’an Archipelago is located in the Yangtze delta, China. (b) The eight representative islands in this survey. The triangles indicate the diving sampling stations.
2.2. Investigation Method
In order to analyze the biodiversity and assess the carbon sequestration of seaweed, our field survey was conducted in May 2021, which is the most prolific season for seaweed in the Ma’an Archipelago. Based on historical documents, expert consultations, and mass visits, we surveyed the distribution characteristics of seaweed in eight islands. We investigated the nearshore area with a rock shoreline length of ≥ 1 km.
We used the ship-borne BioSonics MX echo detector (BioSonics Inc., Seattle, WA, USA) to detect the outer and inner boundaries of the distribution of seaweed in the nearshore waters, and then carried out “S”-type route surveys within the inner and outer boundaries (Figure 2). According to the feedback information from the sonar detector, we selected representative sites for diving sampling. At each station, the sampling area was divided into shallow (0–3 m), medium (3–5 m), and deep (5–10 m) layers according to the depth, terrain, and distribution of seaweed. Three parallel samples were collected for each water layer, with a quadrat size of 30 cm × 30 cm. In this survey, there were 60 diving stations, and 540 quadrats were collected. The seaweed samples were classified, numbered, and stored according to the station and depth. After being brought to the laboratory, the samples were identified for species, the attached organisms on the seaweed surface were cleaned with deionized water, the surface water was absorbed with absorbent paper, and then the biological parameters such as height and wet weight were determined. We took seaweed samples, freeze-dried them at −80 °C for 24 h to a constant weight, measured the dry weight, and then calculated the dry ratio. After the freeze-dried samples were ground with agate, the organic carbon content in the seaweed was determined using a total organic carbon analyzer (TOC-VCSH, Shimadzu, Shimadzu Inc., Tokyo, Japan).
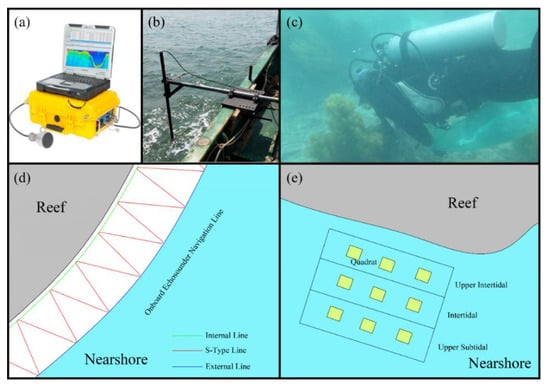
Figure 2.
Equipment and methods. (a) BioSonics MX echo detector; (b) special support for detection; (c) scuba diving; (d) sonar scanning track line; (e) quadrat layout for seaweed sampling.
3. Data Analysis
We preprocessed the acoustic data through the built-in Visual Habitat software of BioSonics MX. The rising edge threshold was −40 dB, and the plant detection threshold was −75 dB. Original 8.0 (Origin Lab, Northampton, MA, USA) software was used for data postprocessing, and histograms were drawn. Surfer 12.0 (Golden Software, Golden, CO, USA) software was used to draw the distribution map of the height and coverage of seaweed near the island. We selected the dominant species with biomass ≥100 g/m2 (wet weight) for the determination of organic carbon content. In this study, six species of seaweed were selected: Ulva pertusa, Sargassum fusiforme, Sargassum horneri, Undaria pinnatifida, Sargassum thunbergii, and Sargassum vachellianum. Biomass was calculated using wet weight and organic carbon content, and carbon sequestration was calculated using dry weight.
4. Results
4.1. Species Diversity
Our results show that the coastal shallow waters of the Ma’an Archipelago were rich in species diversity and seaweed biomass. The water temperature in the Ma’an Archipelago was 24.1–25.6 °C, the pH was 8.34–8.39, and the salinity was 29.7–30.7‰. In total, we counted 39 species from Ochrophyta, Rhodophyta, and Chlorophyta. There were 27 species of Rhodophyta, e.g., Pterocladiella capillacea, Graateloupia livida, Chondria crassicaulis, and Calliarthron yessoense; 10 species of Ochrophyta, e.g., Sargassum thunbergii, Sargassum fusiforme, Sargassum vachellianum, and Sargassum horneri; and two species of Chlorophyta, i.e., Ulva pertusa and Cladophora albida (Table 1).

Table 1.
Species diversity of the seaweeds in the Ma’an Archipelago.
Sargassum thunbergii, Sargassum fusiforme, and Ulva pertusa were distributed near the shore of the six survey areas. No Sargassum vachellianum was found in the HN area. Sargassum horneri was only found in the survey areas of GQ and SS. Ishige okamurai, Gelidium kintaroi, and Grateloupia filicina were only found in HN. Cladophora albida only appeared in the GQ area. A total of 28 species of seaweed were found in the GQ area, 16 in the SS area, and 7 in the HN area.
4.2. Distribution Characteristics
According to the scuba diving sampling and acoustic scanning analysis, seaweeds were distributed near the coast of each island. The distribution of seaweed in the shallow water of the coastal zone was banded, and the distribution was patchy and discontinuous on the small scale. The average biomass of Ochrophyta in the Ma’an Archipelago was the largest at 4311.19 g/m2, while the values for Chlorophyta and Rhodophyta were 192.22 g/m2 and 169.30 g/m2, respectively. In the nearshore areas of the six survey areas, SS had the largest biomass of Ochrophyta, with an average of 6252.88 g/m2. For Rhodophyta, the average biomass was largest in the in GQ area, with 465.92 g/m2. The average biomass of Chlorophyta was largest in the LH area, at 461.70 g/m2 (Table 2).

Table 2.
Distribution characteristics of seaweed.
From the scanning data of the BioSonics MX echo detector, we found that the distribution widths of the seaweed communities in the Ma’an Archipelago differed due to the slope of the rock, with a width range of 2–35 m. The nearshore terrain of the GQ area was flat, and the width of its seaweed community was 15–35 m; the terrain of the BX area was steeper than that of GQ, and its seaweed distribution width was 4–8 m. In addition, the sonar data showed us the distribution depth of the seaweed in the Ma’an Archipelago. During the high-tide period, seaweed was distributed below a 1 m water depth, and the deepest area was in GQ, which reached up to 15 m in depth. Based on acoustic data, we then estimated the distribution area of the offshore seaweed in different areas. The area of the seaweed community in the GQ area was about 3.17–7.40 km2, and that in SS was about 2.30–3.10 km2. The area of the seaweed community in LH was the smallest, at about 0.17–0.51 km2.
According to the detection data from the BioSonics MX echo detector, we drew height and coverage distribution maps of the offshore seaweed in the six survey areas. The survey area of SH included three islands, DK (Dongku), QZ (Qiuzi), and ZZ (Zhuzhu) (Figure 3 and Figure 4).
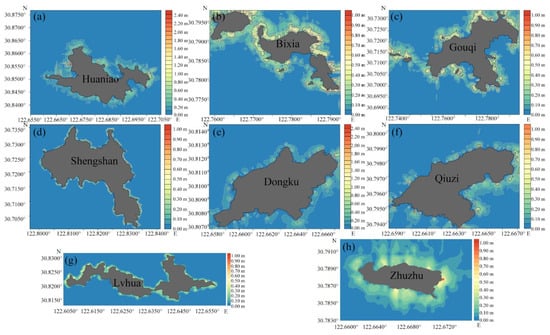
Figure 3.
Height distributions of seaweed in the Ma’an Archipelago. (a) HN (Huaniao), (b) BX (Bixia), (c) GQ (Gouqi), (d) SS (Shengshan), (e) DK (Dongku), (f) QZ (Qiuzi), (g) LH (Lvhua), and (h) ZZ (Zhuzhu).
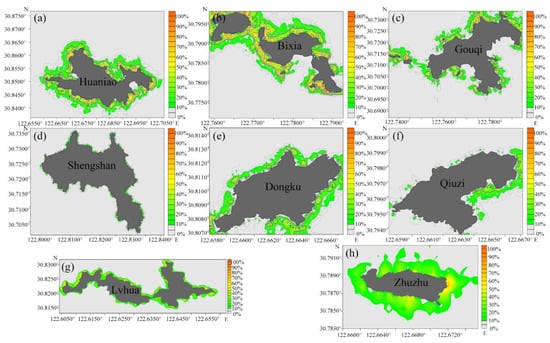
Figure 4.
Coverage distributions of seaweed in the Ma’an Archipelago. (a) HN (Huaniao), (b) BX (Bixia), (c) GQ (Gouqi), (d) SS (Shengshan), (e) DK (Dongku), (f) QZ (Qiuzi), (g) LH (Lvhua), and (h) ZZ (Zhuzhu).
Among the 39 species of seaweed, the average height of Ochrophyta was higher than that of Rhodophyta and Chlorophyta. Among them, the height of Sargassum horneri in Ochrophyta was the largest and reached 137.0 cm in the SS area. The dense clumps of Sargassum horneri had formed a spectacular “underwater forest” in SS and GQ. The height of the seaweed near the coast of each island was mostly 20–70 cm, and the coverage was generally 20–50%. The seaweed in HN was 10–60 cm tall, and the coverage of the community was about 20–45%, reaching 70% in the mouth of the bay. The height of the seaweed in BX was 30–60 cm and the coverage was more than 20%. The seaweed height in GQ was 10–90 m and the coverage was 10–50%.
It can be seen from the distribution map that seaweeds were often distributed in discontinuous patch-like clusters, and the coverage gradually increased from shallow to deep areas; then, with the depth continuing to increase, the light intensity of the seaweed weakened and the coverage gradually decreased. In addition, combined with the survey, we found that there was a large distribution of seaweed at the mouth of the bay and in areas with rapid water flow. For example, the coverage of the seaweed community reached more than 90% at the local protruding cliff corner of GQ and BX. Furthermore, islands (such as LH, DK, QZ, and ZZ) that are less affected by human fishery activities had large biomasses of seaweed near the shore. There was almost no seaweed in the sandy bottom area of GQ.
4.3. Carbon Sequestration Capacity
We selected six representative species of seaweed (widely distributed or with large biomass) in the Ma’an Archipelago to analyze their organic carbon content: Ulva pertusa, Sargassum fusiforme, Sargassum horneri, Undaria pinnatifida, Sargassum thunbergii, and Sargassum vachellianum (Figure 5).

Figure 5.
Six representative species in the Ma’an Archipelago. (a) Ulva pertusa, (b) Sargassum fusiforme, (c) Sargassum horneri, (d) Undaria pinnatifida, (e) Sargassum thunbergii, and (f) Sargassum vachellianum.
We determined the biological characteristics and analyzed the data of six representative seaweed species from the Ma’an Archipelago; Figure 6 represents their height and biomass. Sargassum thunbergii was distributed in all investigated areas. The height of Sargassum thunbergii in SS was the highest at 54 ± 8.11 cm. The biomass of this species in BX was the lowest at 1237.24 ± 159.91 g/m2. The height of Undaria pinnatifida in SS was the highest, but its biomass was lower than that in LH and GQ. Ulva pertusa was distributed in all regions, but its biomass in LH was 461.70 ± 183.33 g/m2, much higher than in other survey areas. We did not find Sargassum vachellianum in the coastal waters of HN, and Sargassum horneri only appeared in the coastal waters of GQ and SS. Sargassum fusiforme was distributed in all the islands, and its height was highest in SS at 55.87 ± 7.20 cm. The biomass of Sargassum fusiforme was lowest in GQ at 119.73 ± 34.71 g/m2.
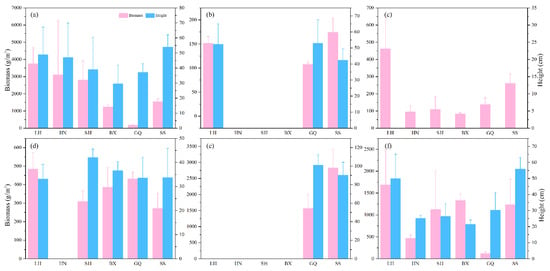
Figure 6.
Height and biomass of seaweed species in the Ma’an Archipelago. (a) Sargassum thunbergii, (b) Undaria pinnatifida, (c) Ulva pertusa, (d) Sargassum vachellianum, (e) Sargassum horneri, and (f) Sargassum fusiforme. Abbreviations: LH (Lvhua), HN (Huaniao), SH (Sanheng), BX (Bixia), GQ (Gouqi), and SS (Shengshan). Ulva pertusa was membranous and its height was not counted.
We then measured the organic carbon content of six representative seaweed species from the Ma’an Archipelago (Table 3). The total average organic carbon content of the seaweeds was 33.12 ± 3.22%; the carbon content of Undaria pinnatifida was the highest at 36.84 ± 3.33%, followed by Sargassum horneri, and the organic carbon content of Ulva pertusa was the lowest (29.11 ± 2.10%).

Table 3.
The organic carbon content of seaweed species in the Ma’an Archipelago.
Then, we analyzed the carbon sequestration of the six representative seaweed species from each survey area in the Ma’an Archipelago. The carbon sequestration of Sargassum thunbergii was more than that of the other five species of seaweed. Ulva pertusa, Sargassum thunbergii, and Sargassum fusiforme were distributed in all survey areas. Undaria pinnatifida was found in LH, GQ, and SS. Sargassum horneri appeared in GQ and SS; its biomass was 161.66–344.25 t in GQ, and 173.24–437.85 in SS. The carbon sequestration of Ulva pertusa in BX was 1.49–2.98 t, and that of Sargassum thunbergii was 23.01–46.03 t, lower than that in other islands. The amount of carbon sequestration of Sargassum fusiforme and Sargassum vachellianum in SS was lower than in other areas (Table 4).

Table 4.
Carbon sequestration of seaweed in the Ma’an Archipelago (t).
It can be seen from Table 4, due to the long rocky shorelines of GQ and SS, the carbon sequestration values of the six representative seaweeds were significantly higher than in other areas. The carbon sequestration of Sargassum horneri in SS was the largest at 173.24–437.85 t, and in GQ at 161.66–344.25 t. The carbon sequestration of Ulva pertusa in BX was the lowest at 1.49–2.98 t. The carbon sequestration values of Ulva pertusa, Sargassum thunbergii, Sargassum fusiforme, Undaria pinnatifida, and Sargassum horneri in SS were greater than those in other islands; the island with the largest amount of carbon sequestration from Sargassum vachellianum was GQ, 94.41–220.40 t. In the six survey areas, the total carbon sequestration amount for Sargassum thunbergii was about 277.91–848.74 t; Undaria pinnatifida had the lowest carbon fixation at 56.48–103.97 t.
5. Discussion
5.1. Distribution Characteristics
The growth and distribution of seaweed are affected by many environmental factors, such as water temperature, light intensity, and depth. Each environmental factor affects the others and they jointly determine the growth of seaweed [15]. Among them, light intensity is one of the main factors affecting the distribution and community development of seaweed, as light intensity and the composition of light quality affect the photosynthesis of seaweed. Different species have different demands on various environmental factors, such as different requirements for waves and light. For example, the distribution of seaweed in coastal zones from coastal shallow water to deep areas goes from Chlorophyta, to Ochrophyta, to Rhodophyta [16].
The slope of coastal zones affects the current near the shore. We found that seaweed such as Sargassum horneri and Sargassum vachellianum thrive in the protruding capes of GQ and SS, while Ulva pertusa of Chlorophyta is widely distributed in sunken bays. Zhang (2018) studied the relationship between waves and seaweed distribution and suggested that the spores of Sargassum horneri and Sargassum vachellianum have strong adhesion abilities and that mature seaweed has a high tolerance to wave impact [17]. Furthermore, where the water flow is rapid, the nutrient exchange is frequent, and the height of seaweed is higher than in places where the current is slow [18,19,20]. These conclusions are consistent with the distribution characteristics of seaweed in our study, such as Sargassum horneri and Ulva pertusa. Of the Ochrophyta species found in Ma’an Archipelago, Sargassum horneri, Sargassum vachellianum, and Undaria pinnatifida are tall; Undaria pinnatifida had an average height of 47.67 ± 7.45 cm. The seaweeds of Rhodophyta and Chlorophyta were mostly low to the ground, which resulted in the biomass of Ochrophyta being greater than that of Rhodophyta and Chlorophyta.
Human fishery activities affect the environment and thus affect the growth and distribution of seaweed [21]. Although the abundance of seaweed in the Ma’an Archipelago was high, Sargassum fusiforme and Undaria pinnatifida growing in intertidal zones are often trampled and harvested by fishermen for food or sale. There are no specific and effective fishery management systems in these areas. There is an endless stream of tourists year round in these areas, especially in GQ. When fishing, tourists indiscriminately walk on rocks, causing the death of seaweed seedlings. Local fishermen also often collect seaweed as a specialty for tourists to taste. Moreover, fishermen often use the low tide to cut a large number of mature Sargassum thunbergii and Sargassum fusiforme samples to sell. This collection of mature seaweed greatly reduces the number of spores released in the next year. Thus, due to these activities, although the rocky shoreline of GQ was the longest, the biomass of Sargassum fusiforme was the smallest, at only 119.73 ± 34.71 g/m2. The rocky shoreline of SS is 15.38 km long. The biomass of Undaria pinnatifida reached 174.19 ± 29.75 g/m2, and the biomass of SS was less than that of LH and GQ, as the latter areas were less affected by fishing activities. In order to protect the natural abundance of Undaria pinnatifida, SS has been designated as a protection zone for Undaria pinnatifida in China.
In the nearshore region of each island, Sargassum thunbergii, Ulva pertusa, and Sargassum fusiforme were investigated. Because these three species of seaweed are distributed in shallow depths or grow in intertidal zones, the required lighting intensity was sufficient. Sargassum horneri and Sargassum vachellianum often grow at depths of 3–7 m; Sargassum horneri demands more light intensity than Sargassum vachellianum. The water quality of the Ma’an Archipelago is turbid due to the impact of the Yangtze River flushing water all year round. GQ and SS are located at the southernmost point, far from the mouth of the Yangtze River, with high water transparency and small amounts of sediments from the Yangtze River, and thus Sargassum horneri can grow. To the north of the Ma’an Archipelago, the seabed is mainly composed of sediment. Without rocks, seaweed cannot grow firmly. According to Zhang (2019), LH is located in the northernmost zone of the Ma’an Archipelago and is the northernmost distribution area of Sargassum vachellianum along the coast of China [14].
5.2. Carbon Sequestration Capacity
Seaweed transforms carbon dioxide and inorganic salt in the water into organic nutrients for its growth by photosynthesis, and thus seaweed can act as a carbon sink [22]. Some seaweed enters the food chain through sea urchins, some organic molecules are transported and buried in the seabed in the form of debris, and some fragments are decomposed and dissolved in water [23].
Duarte has suggested that the amount of carbon absorbed by marine plants is up to 120~329 Mt every year [24]. Seaweed can absorb 20–35% of the total CO2 from anthropogenic emissions, effectively reducing the concentration of CO2 in the atmosphere and potentially slowing down the rate of global climate change [5,25,26]. Studies have shown that the carbon sequestration potential of seaweed in the global continental shelf area could be 0.7 Gt per year, accounting for 35% of the total net carbon sequestration in the global oceans [27]. From 1999 to 2012, the carbon sequestration of seaweeds cultivated in China was 41.85 × 104 t/a, and the ecological value of fixed CO2 was 429 million yuan per year [28].
The carbon sink capacity of seaweed is significantly affected by light intensity, and its carbon sequestration capacity increases with the increase in light intensity. However, when the light intensity increases to the light saturation point, the carbon sink capacity of seaweed does not increase [29]. Seaweed grows in intertidal and subtidal zones, and, with the rise and fall of the tide, the light intensity it receives fluctuates. Therefore, the carbon sequestration capacity of seaweed is affected by the change in solar radiation intensity and the rise and fall of the tide [30].
Our results, looking at six species of seaweed, show that the organic carbon content of Ulva pertusa is less than 30%, which is smaller than that of the other five Ochrophyta species. In the Ma’an Archipelago, the biomass of Ochrophyta is greater than Rhodophyta and Chlorophyta because of their tall structure. Ochrophyta exhibit a special blue light effect, which means they have a higher pH compensation point under blue light than under red light, i.e., Ochrophyta has a stronger ability to use inorganic carbon under blue light [6]. Rhodophyta can absorb blue light and can conduct photosynthesis even in deep subtidal zones, and they have the most species. Ochrophyta has the most biomass, indicating that this community tends to reach maturity [31]. This proves that the seaweed communities in the Ma’an Archipelago are in a stable state. Because seaweed needs to be fixed on a rocky surface, the length of the rocky shoreline affects the amount of seaweed biomass. Among the six areas in our survey, the rocky shorelines of the BX and SH areas were 7.80 and 8 km long, respectively, which were shorter than the other areas. Therefore, the carbon sequestration of the seaweed in these two areas was smaller than in GQ and SS. In addition, SS and GQ were far from the estuary of the Yangtze River. Thus, the growth of the seaweeds was less affected by flushing water, with higher transparency, which further led to the carbon sequestration of seaweed in GQ and SS being higher than in other areas.
6. Conclusions
The Ma’an Archipelago is rich in seaweed species. We found 39 species of seaweed, including 27 species of Rhodophyta, 10 species of Ochrophyta, and two species of Chlorophyta. The distribution depth of the seaweed was within 15 m. The biomass of Ochrophyta in the Ma’an Archipelago was the largest at 4311.19 g/m2. The height of the seaweed near the shore of each island was 20–70 cm, the coverage was 20–50%, and the distribution area was 6.51–13.43 km2.
The average organic carbon content of the six representative species in the Ma’an Archipelago was 33.12 ± 3.22%, with specific differences in seaweed species. Because Ulva pertusa of Chlorophyta is distributed in intertidal zones, its organic carbon content was the smallest. Sargassum horneri only appeared in GQ and SS and its carbon fixation was 334.90–782.10 t. The shoreline of SH and BX is short, and the carbon sequestration here was small. The carbon sequestration of Sargassum thunbergii in the Ma’an Archipelago was the largest at 277.91–848.74 t, and that of Undaria pinnatifida was the smallest. SS is located in the southeast of the Ma’an Archipelago and is less affected by the flushing water of the Yangtze River. The biomass of Sargassum horneri and Sargassum thunbergii were large and thus they had the largest carbon sequestration values.
Author Contributions
Conceptualization, Z.W. and K.W.; methodology, X.L., X.Z., H.Y. and Y.G.; software, X.L. and J.L.; validation, S.Z., J.C. and X.L.; formal analysis, X.L. and X.Z.; investigation, X.L. and J.C.; resources, Z.W.; data curation, X.L. and J.C.; writing—original draft preparation, X.L. and X.Z.; writing—review and editing, H.Y., Y.G., J.L. and S.Z.; visualization, J.L., X.L. and S.Z.; supervision, S.Z.; project administration, Z.W. and K.W.; funding acquisition, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fund of the Key Laboratory of Marine Ranching, Ministry of Agriculture and Rural Affairs, China (KLMR-2022-04); the Fund of the East China Sea Bureau of the Ministry of Natural Resources, China (202201); and the Fund of the National Natural Science Foundation of China (41876191).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We appreciate the support of our funding agencies, the Ministry of Agriculture and Rural Affairs, China, the East China Sea Bureau of the Ministry of Natural Resources, China, and the National Natural Science Foundation of China. We also thank the editor and the anonymous reviewers, whose comments have significantly improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luo, S.H.; Shen, M.; Lin, J.; Wu, X.C.; Liu, H.S. Sediment characteristics and bearing capacity in an artificial reef area of Ma’an Archipelago. J. Fish. China 2019, 43, 441–453. [Google Scholar]
- Wu, Z.L.; Cui, X.S.; Tang, F.H.; Xiong, M.S. Research on genecology of benthic macroalgae. Fish. Inf. Strategy 2018, 33, 36–44. [Google Scholar]
- Pangestuti, M.B.; Suhartini, S.; Hidayat, N. Life cycle assessment of bioenergy production from macroalgae: A review. IOP Conf. Ser. Earth Environ. Sci. 2021, 924, 012070. [Google Scholar] [CrossRef]
- Yan, Q.W.; Huang, H.J.; Chen, J.T.; Yang, X.G. Estimation of Carbon Sink Capacity of Algal Mariculture in the Coastal Areas of China. Adv. Mar. Sci. 2011, 29, 537–545. [Google Scholar]
- Ou, G.Y.; Wang, X.J.; Yang, A.Q.; Ke, A.Y.; Guan, W.C. Interspecific differences in carbon sink capacity of macroalgae. J. Zhejiang Agric. Sci. 2017, 58, 1436–1439. [Google Scholar]
- He, P.M.; Liu, Y.Y.; Zhang, J.W.; Wu, H.L.; Yu, K.F.; Huo, Y.Z.; Zhang, J.H. Research progress on the effects of macroalgae on carbon sink. J. Fish. Sci. China 2015, 22, 588–595. [Google Scholar]
- Yang, Y.F.; Luo, H.T.; Wang, Q.; He, Z.L.; You, A.M. Large-scale cultivation of seaweed is effective approach to increase marine carbon sequestration and solve coastal environmental problems. Bull. Chin. Acad. Sci. 2021, 36, 259–269. [Google Scholar]
- Aurélie, B.; Charles, F.B.; Marc, V.; Thierry, T. The ups and downs of a canopy-forming seaweed over a span of more than one century. Sci. Rep. 2019, 9, 5250. [Google Scholar]
- Miss, L.J.; Hoare, A.H.; Hughes, H. Antimicrobial Properties of Fucus Vesiculosus and Porphyra Dioica Collected from the Irish Coast. Sure-J. Sci. Undergrad. Res. J. 2019, 1, 5. [Google Scholar]
- Li, X.M.; Wang, K.; Chen, J.Q.; Zhang, S.Y. Allometric Growth of Sargassum fusiforme (Ochrophyta, Fucales) Organs in the Maturation Period Based on Biomass Analysis of Samples from Gouqi Island. J. Mar. Sci. Eng. 2021, 9, 1320. [Google Scholar] [CrossRef]
- Chen, J.Q.; Li, X.M.; Wang, K.; Zhang, S.Y.; Li, J. Estimation of Seaweed Biomass Based on Multispectral UAV in the Intertidal Zone of Gouqi Island. Remote Sens. 2022, 14, 2143. [Google Scholar] [CrossRef]
- Hynes, S.; Chen, W.; Vondolia, K.; Armstrong, C.; O’Connor, E. Valuing the ecosystem service benefits from kelp forest restoration: A choice experiment from Norway. Ecol. Econ. 2021, 179, 106833. [Google Scholar] [CrossRef]
- Li, X.M.; Wang, K.; Zhang, S.Y.; Feng, M.P. Distribution and Flora of Seaweed Beds in the Coastal Waters of China. Sustainability 2021, 13, 3009. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Bi, Y.X.; Wang, W.D.; Sui, Y.Z.; Lu, K.E.; Feng, M.P.; Liang, J.; Zhou, D.D. Spatial distribution patterns of Sargassum vachellianum in coastal waters of northern zhejiang typical islands. Acta Hydrobiol. Sin. 2019, 43, 1114–1121. [Google Scholar]
- Liu, Q.Q.; Yang, F.; Ma, M.J.; Zhou, Y.L.; Li, Y.H.; Yang, Y. The effects of temperature on the absorption efficiency of nitrogen and phosphorus and photosynthetic physiological charateristics in four macroalgae species. Acta Hydrobiol. Sin. 2018, 42, 1050–1056. [Google Scholar]
- Lalegerie, F.; Gager, L.; Stiger-Pouvreau, V.; Connan, S. The stressful life of red and brown seaweeds on the temperate intertidal zone: Effect of abiotic and biotic parameters on the physiology of macroalgae and content variability of particular metabolites. Adv. Bot. Res. 2020, 95, 247–287. [Google Scholar]
- Zhang, S.Y.; Xiang, C.; Zhou, X.J.; Liu, S.R.; Cheng, X.P.; Wang, K. Photosynthetic fluorescence characteristics of six macroalgae species in seaweed beds of Gouqi Island, Zhejiang, China. Chin. J. Appl. Ecol. 2018, 29, 3441–3448. [Google Scholar]
- Bi, Y.X.; Miao, H.; Wang, H.J.; Yang, Q.F. Study on ecological restoration technology of macroalgae based on spore adhesion function. Acta Hydrobiol. Sin. 2022, 46, 160–167. [Google Scholar]
- Koehl, M.A.R.; Silk, W.K.; Liang, H.; Mahadevan, L. How kelp produce blade shapes suited to different flow regimes: A new wrinkle. Integr. Comp. Biol. 2008, 48, 834–851. [Google Scholar] [CrossRef]
- Hurd, C.L. Water motion, marine macroalgal physiology, and production. J. Phycol. 2000, 36, 453–472. [Google Scholar] [CrossRef]
- Sanghvi, D.; Chaudhury, N.R.; Jain, B.K. Macroalgae as indicator species for shore platform zones of Dwarka, Gujarat, India. Indian J. Mar. Sci. 2019, 4, 48. [Google Scholar]
- Cabrera, R.; Díaz-Larrea, J.; Umanzor, S. New Records of Marine Macroalgae on the Caribbean on Coast of Costa Rica. Am. J. Plant Sci. 2019, 10, 1708–1728. [Google Scholar] [CrossRef][Green Version]
- An, X.L.; Li, X.M.; Wang, K.; Liu, H.Y. Species Analysis and Seasonal Succession of Marine Macroalgae in the Intertidal Zone of Qinhuangdao. J. Ocean Technol. 2019, 38, 70–76. [Google Scholar]
- Duarte, C.M.; Chiscano, C.L. Seagrass biomass and production: A reassessment. Aquat. Bot. 1999, 65, 159–174. [Google Scholar] [CrossRef]
- Khatiwala, S.; Primeau, F.; Hall, T. Reconstruction of the history of anthropogenic CO2 concentrations in the ocean. Nature 2009, 462, 346–349. [Google Scholar] [CrossRef]
- Laffoley, D.; Grimsditch, G. The Management of Natural Coastal Carbon Sinks; IUCN: Gland, Switzerland, 2009; p. 53. [Google Scholar]
- Alpert, S.B.; Spencer, D.F.; Hidy, G. Biospheric options for mitigating atmospheric carbon dioxide levels. Energy Convers. Manag. 1992, 33, 729–736. [Google Scholar] [CrossRef]
- Quan, W.; Ying, M.M.; Kang, H.J.; Xu, C.L.; Zhou, Q.H.; Liang, W.J.; Lin, Z.S.; Cai, J.B. Marine algae culture and the estimation of carbon sink capacity in the coastal areas of China. J. Fish. China 2014, 38, 509–514. [Google Scholar]
- Liu, Y.Q.; Zhang, C.X.; Sun, X.L.; Sun, J.; Yang, G.H. Carbon Sequestration Potential Research of Macroalage in the Intertidal Rocky Zone in Naozhou Island. J. Guangdong Ocean Univ. 2019, 39, 78–84. [Google Scholar]
- Han, B.P.; Han, Z.G.; Fu, X. Algae Photosynthetic Mechanism and Models; Science Press: Beijing, China, 2003; pp. 48–53. [Google Scholar]
- Sousa, W.P. Experimental investigations of disturbance and ecological succession in a rocky intertidal algal community. Ecol. Monogr. 1979, 49, 227–254. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).