Abstract
We restore the genus Paraunisaccoides (Haploporidae), synonymised earlier with the genus Skrjabinolecithum. Adult worms, detected in Vietnamese mullet fish, were highly similar to trematodes described as P. lobolecithum via digestive and genital system structures and relative organ arrangement. Differences are expressed as absence and presence of pads on the hermaphrodite duct, respectively, and the disjunction of some metric parameter values, namely body, ovary and eggs. Ribosomal DNA sequences, based on the phylogenetic analysis of Haploporidae, indicates that new worms represent a sister clade to Unisaccus tonkini. Genetic divergence between new worms and Skrjabinolecithum species can be interpreted as intergeneric. Based on morphological and molecular data, we recognise Paraunisaccoides as a valid genus within Waretrematinae and worms from Vietnam as a new species of this genus, P. elegans n. sp. Other worms detected in Vietnamese mugilids are morphologically similar to representatives of Paraunisaccoides и Skrjabinolecithum. However, molecular-based phylogenetic analysis showed that these trematodes are closely related to Unisaccus tonkini; the genetic divergence between them is at the interspecific level, despite considerable differences in vitellarium structure as intergeneric character. Accepting the priority of molecular results, we include these new worms into the genus Unisaccus as new species, Unisaccus halongi n. sp.
1. Introduction
Species of Haploporidae Nicoll, 1914, as well as other Haploporoidea, parasitising a wide range of definitive hosts, marine, freshwater, and eurihaline fish species, are cosmopolitan. Species of different subfamilies (except the cosmopolitan Megasoleninae Manter, 1935) are somehow related to certain geographical territories, infecting mainly fish species of Mugilidae Jarocki, 1822 and, rarely, Cyprinidae Rafinesque, 1815. Representatives of three subfamilies, Haploporinae, Waretrematinae Srivastava, 1937 and Pseudohaploporinae Atopkin, Besprozvannykh, Ha, Nguyen, Bguyen & Chalenko, 2018, of the eight known at present, have mainly been detected in the Indo-West Pacific region [1,2,3,4,5,6,7,8,9,10,11]. Most of the trematodes have been extracted from Mugilidae fish species.
We obtained specimens of two hypothetic species of Haploporidae in mullet fish from coastal waters of Halong Bay, Vietnam. In the present study, we performed morphological and molecular analyses to validate these species and to reconstruct the phylogenetic relationships of the family Haploporidae with new data.
2. Materials and Methods
2.1. Collection of Trematodes
Adult worms were collected from intestines of mullet fish (Mugilidae) in coastal waters near Cat Ba Island, Ha Long Bay, Vietnam (20°84′ N, 106°59′ E). Worms were rinsed in saline, previously defined under a microscope using temporal slides preparation technique, killed in hot distilled water and preserved in 70% ethanol. After fixation, they were replaced in 96% ethanol. Whole mounts were made by staining specimens with alum carmine, dehydrating them in graded ethanol series and clearing in clove oil, followed by mounting the specimens in Canada balsam under a coverslip on a glass slide. All measurements are given in micrometres.
2.2. DNA Extraction, Amplification and Sequencing
Three and four adult specimens of Unisaccus halongi n. sp. and Paraunisaccoides elegans n. sp., respectively, from 96% ethanol were used for molecular analysis (Table 1). Total DNA was extracted from flukes using a “hot shot” technique [12].

Table 1.
List of taxa used for molecular analysis.
28S ribosomal DNA (rDNA) 1200 base pairs (bp) in lenght was amplified by a polymerase chain reaction (PCR) method using the Q5 HF polymerase (New England Biolabs, Ipswich, MA, USA) and the primers 28S-A (5′-TCGATTCGAGCGTGAWTACCCGC-3′) [13] and 1500R (5′-GCTATCCTGAGGGAAACTTCG-3′) [14] with an annealing temperature of 55 °C. The ribosomal ITS1-5.8S-ITS2 fragment 1500 bp in lenght was amplified with the primers ITSF (5′-CGCCCGTCGCTACTACCGATTG-3′) [3] and S4R (5′-TATGCTTAAATTCAGCGGGT-3′) [15] with an annealing temperature of 54 °C. Negative and positive controls using both primer pairs were included. PCR parameters began with a 1 min denaturation at 98 °C, followed by 35 cycles of 10 s at 98 °C, 5 s at 54/55 °C and 30 s at 72 °C, and concluded with a 2 min extension at 72 °C.
PCR products were directly sequenced using an ABI Big Dye Terminator v.3.1 Cycle Sequencing Kit (Applied Biosystems, Waltham, MA, USA) as recommended by the manufacturer. The internal sequencing primers for 28S rDNA are described in [14], for ITS they are described in [16]. PCR product sequences were analysed using an ABI 3500 genetic analyser at the FSC of Biodiversity FEB RAS. Sequences were submitted to the GenBank database (Table 1).
2.3. Alignments and Phylogenetic Analysis
Ribosomal DNA sequences were assembled using the SeqScape v. 2.6 software provided by Applied Biosystems. Alignments and estimations of the number of variable sites and sequence differences were performed using the MEGA v. 7.1 software [17]. The values for genetic p-distances were calculated for the 28S ribosomal DNA fragment. Phylogenetic relationships were obtained using a concatenated data set of partial sequences of the 28S rRNA gene and ITS1-5.8S-ITS2 rDNA. Phylogenetic analysis was performed using the Bayesian algorithm in the MrBayes v. 3.2.6 software [18]. The best nucleotide substitution model, a transversional model with estimates of invariant sites and gamma-distributed among-site variation TVM + G+I [19], was estimated using the jModeltest v. 2.1.5 software [20] for partial 28S rDNA and combined ITS1-5.8S-ITS2-28S rDNA sequence data sets. Bayesian analysis was performed using 10,000,000 generations with two independent runs. Summary parameters and the phylogenetic tree were calculated with a burn-in of 3,000,000 generations. The significance of phylogenetic relationships was estimated using posterior probabilities [18]. Sequences of ITS1-5.8S-ITS2 rDNA and 28S rDNA of Brachycladium goliath (van Beneden, 1858) from GenBank were used as the outgroup. The authors of these and other sequences from GenBank [3,4,5,6,7,8,9,10,11,21,22,23,24,25,26,27,28,29,30,31] and their accession numbers are given in Table 1.
3. Results
3.1. Diagnosis of the Genus Paraunisaccoides
Family. Haploporidae Nicoll, 1914; Subfamily.
Waretrematinae Srivastava, 1937; Genus.
Paraunisaccoides Martin, 1973.
Body oval, fusiform or elongate. Eye-spot pigment dispersed. Oral sucker subterminal. Ventral sucker larger than oral sucker in anterior half of body. Prepharynx long, reach level of ventral sucker or posterior to ventral sucker. Pharynx round or transversely oval. Oesophagus short poorly defined. Caecum saccular. Testis single, in posterior end of body. Hermaphroditic sac oval with muscular sphincter at anterior end. External seminal vesicle sac-shaped or another form depending of fullness of sexual products. Internal seminal vesicle elongated. Hermaphroditic ducts with or without pads. Genital pore anterior to ventral sucker. Ovary round or transversal oval, immediately anterior to testis. Uterus short, between anterior margin of testis and hermaphroditic sac. Metraterm short, thin-walled. Eggs unnumerous, unembryonated, operculated. Mehlis’gland sinistrally to ovary. Vitellarium, consists from elongate thin of follicles. Vitelline fields can reach level of posterior edge of ventral sucker and to posterior end of body. Excretory bladder I-shaped with or without muscular sphincter. Type species: Paraunisaccoides lobolecithum Martin, 1973.
3.2. Paraunisaccoides elegans n. sp.
3.2.1. Taxonomic Summary
Host. Planiliza subviridis (Valenciennes, 1836).
Locality. Coastal water of Cat Ba Island, Ha Long Bay, northern Vietnam (20°84′ N, 106°59′ E).
Site. Intestine.
Prevalence. 3 of 5 specimens infected.
Intensity. 1–12 worms.
Type-deposition: Type No. 182-Tr, paratype No. 183-187-Tr. This material is held in the collection of the Zoological Museum (Institute of Biology and Soil Sciences, Far East Branch of the Russian Academy of Sciences, Vladivostok, Russia); e-mail: petrova@ibss.dvo.ru. Deposited: 29 July 2020.
Etymology: species name associated with trematode body form.
3.2.2. Morphology
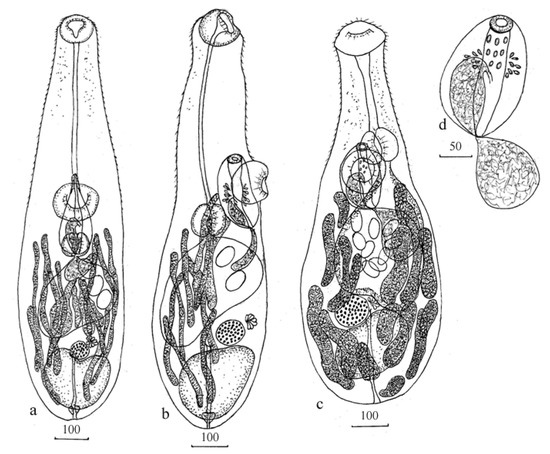
Figure 1.
Paraunisaccoides elegans n. sp.: (a) adult worm ventral, (b) adult worm lateral; Unisaccus halongi n. sp.: (c) adult worms, (d) cirrus sac. Measurements are given in µm.

Table 2.
Measurements (µm) of adult worms of new species Paraunisaccus elegans n. sp. and Unisaccus halongi n. sp. and known species Skrjabinolecithum lobolecithum and S. indicum.
Body elongate, with narrow forebody, tegument with needle-shaped spines. Eyes-spot pigmentation dispersed in anterior half of forebody. Oral sucker subterminal. Prepharynx long, reach level of ventral sucker or slightly posterior to ventral sucker. Pharynx round or transversely oval. Oesophagus short poorly defined. Caecum saccular, in middle of posterior half of body. Ventral sucker larger than oral sucker, at beginning of middle third of body. Testis single, in posterior end of body, irregular, with anterior recess. Hermaphroditic sac oval, at level ventral sucker and pharynx. Anterior end of hermaphroditic sac with muscular sphincter. External seminal vesicle sac-shaped or another form depending of fullness of sexual products. Internal seminal vesicle elongated. Prostatic cells unnumerous. Pads at hermaphroditic duct not identified. Genital pore opening on midline of body immediately before ventral sucker. Ovary round or transversal oval, adjacent to anterior margin of testis on midline of body. Uterus short, between anterior margin of testis and hermaphroditic sac. Metraterm short, thin-walled. Eggs few, light yellow, unembryonated, operculated. Mehlis’gland sinistrally to ovary. Vitellarium, consists from elongate thin of follicles. Vitelline fields can reach level of posterior edge of ventral sucker and to posterior end of body, merge at median line of body and cover of ovary and testis. Excretory bladder I-shaped with muscular sphincter, pore terminal.
3.2.3. Molecular Data
For four specimens of P. elegans n. sp. totals of 1291 and 1604 alignable characters with three (0.23%) and nineteen (1.18%) variable sites were generated for analysis in the 28S rRNA gene and ITS1-5.8S—ITS2 rDNA fragment datasets, respectively.
3.3. Unisaccus halongi n. sp.
3.3.1. Taxonomic Summary
Host. Crenimugil seheli (Fabricius, 1775).
Locality. Coastal water of Cat Ba Island, Ha Long Bay, northern Vietnam (20°84ʹN, 106°59ʹE).
Site. Intestine.
Prevalence. 5 of 37 specimens infected.
Intensity of infection. 1–5 worms.
Type-deposition: Type No. 188-Tr, paratype No. 189-193-Tr. This material is held in the collection of the Zoological Museum (Institute of Biology and Soil Sciences, Far East Branch of the Russian Academy of Sciences, Vladivostok, Russia); e-mail: petrova@ibss.dvo.ru. Deposited: 29 July 2020.
Etymology: species name is associated with location, where definitive host of these worms has been caught.
3.3.2. Morphology
Body elongate, with narrow forebody, tegument with needle-shaped spines. Eyes-spot pigmentation dispersed in forebody. Oral sucker subterminal, transversaly oval. Prepharynx long, slightly does not reach of ventral sucker or reach level of anterior half of ventral sucker. Pharynx at level ventral sucker, spherical, large, slightly smaller of oral sucker. Oesophagus short, poorly defined. Caecum single, saccular, thick-walled, in middle and partly in posterior third of body. Ventral sucker larger than oral sucker, at the border of anterior and posterior third of body. Testis single, in posterior end of body, irregular. Hermaphroditic sac oval, mostly at level ventral sucker. Anterior end of hermaphroditic sac with muscular sphincter. External seminal vesicle sac-shaped. Internal seminal vesicle oval. Size of seminal vesicles depends on fullness of sexual products. Prostatic cells unnumerous. Hermaphroditic duct with pads. Genital pore opening on midline of body immediately before ventral sucker. Ovary round, adjacent to anterior margin of testis. Uterus short, between anterior margin of testis and hermaphroditic sac. Metraterm equal to length hermaphroditic duct. Eggs few, light yellow, unembryonated, operculated. Mehlis’gland sinistrally to ovary. Vitellarium, consists from large elongate, wide of follicles, in close contact with each other. Vitelline fields between ventral sucker to posterior end of body, merge at median line of body and cover of ovary and testis. Excretory bladder I-shaped, pore terminal.
3.3.3. Molecular Data
For three specimens of S. indicum totals of 1240 and 972 alignable characters were generated for analysis in the 28S rRNA gene and ITS1-5.8S—ITS2 rDNA fragment datasets, respectively. Sequences within both datasets were identical to each other.
4. Discussion
On the basis of the last known classification of Haploporidae, provided by Overstreet and Curran [1], Vietnamese worms belong to this family by morphological characteristics, including organ topology, single testis, identical hermaphrodite sac structure, and single caecum. The latter characteristic is representative for worms of the genera Paraunisaccoides within Haploporidae, as well as Unisaccus and Unisaccoides (Unisaccinae Martin, 1973, Haploporidae), validated by Martin [33,34]. In 2005, Paraunisaccoides and Unisaccoides were synonymised with Skrjabinolecithum Belous, 1954 (Waretrematinae), and the type genus Unisaccus of Unisaccinae was transferred to Haploporinae [1]. This has led to recognising Unisaccinae as a synonym of Haploporinae [1].
Among representatives of Haploporidae, worms ex Planiliza subviridis from Vietnam are morphologically most similar with representatives of Skrjabinolecithum that can possess double for type species S. spasskii Belous, 1954, and also S. indicum (Zukov, 1972), S. puriforme Besprozvannykh et al., 2017 and S. spinosum Besprozvannykh et al., 2018 or single for S. lobolecitum (Martin, 1973), S. vetillosum (Martin, 1973) caecum. Vietnamese flukes and S. vetillosum characterized by hiatus in metric parameters for body, ventral sucker, hermaphrodite sac etc. (Table 2). Worms from our study show highest similarity with trematodes, detected in Mugil cephalus Linnaeus, 1758 in Australia [33], denoted as Paraunisaccoides (=Skrjabinolecithum) lobolecithum. These worms differ by the presence pads on the hermaphrodite sac for worms from Martin’s study [33] and, according to the figure provided, the presence of a large space between the ventral sucker and anterior end of the vitellarium (description of vitellarium arrangement absent in [33]). Alongside this, there is a disjunction of metric parameter values, namely body length and sizes of ovary and eggs (Table 2), between worms from [33] and those from our study. Although trematode specimens from both studies were detected in mugilids from relatively close geographical regions and with similar morphology, a number of differences, reported above, and the absence of molecular data for specimens from Australia do not allow to conclude that these trematodes belong to the same species. In our opinion, it is rational to currently consider Vietnamese worms as a new species of Haploporidae. A final conclusion of the validity or conspecificity of these species can be made after obtaining molecular data for the Australian worms, reported in [33]. Phylogenetic analyses performed on the basis of 28S rDNA partial sequences 1050 bp in length and concatenated 28S rDNA and 5.8S + ITS2 rDNA (1460 bp overall length) fragments for Haploporidae confirmed the membership of considered here Vietnamese worms to Waretrematinae (Figure 2 and Figure 3). However, within this subfamily, Vietnamese worms formed a common clade with Unisaccus tonkini instead of Skrjabinolecithum species. At the same time, the genetic distance values between Vietnamese worms, Unisaccus and Skrjabinolecithum, represent an intergeneric divergence level by both markers: 7.05 ± 0.78%–12.05 ± 1.0% by 28S rDNA and 8.05 ± 0.97%–12.10 ± 1.14% by ITS2 rDNA (alignment length 379 bp). These values are compatible with those between different genera within each subfamily: 3.62 ± 0.56%–13.93 ± 1.03% and 4.21 ± 0.73%–16.38 ± 1.35% for Waretrematinae, 5.9 ± 0.62% and 8.33 ± 0.91% for Pseudohaploporinae, 6.13 ± 0.68% for 10.11 ± 1.08% for Forticulcitinae Blasco-Costa, Balbuena, Kostadinova & Olson, 2009 by 28S rDNA and ITS2 rDNA, respectively [8,23,24]. Based on this, accepting the morphological similarity of Vietnamese worms and Australian described by Martin as Paraunisaccoides lobolecithum, we conclude that synonymisation of Paraunisaccoides with Skrjabinolecithum is unreasonable. In the light of this, we restore the genus Paraunisaccoides with the type species Paraunisaccoides lobolecithum Martin, 1973 with the inclusion of the new species Paraunisaccoides elegans n. sp. from the present study.
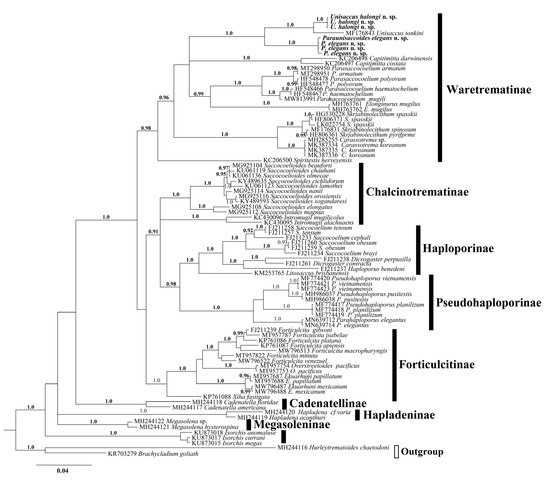
Figure 2.
Phylogenetic relationships of the family Haploporidae obtained with Bayesian algorithm based on partial 28S rRNA gene (alignment length 1050 bp). Nodal numbers—posterior probabilities that indicate statistical support of phylogenetic relationships, only significant values (0.9–1.0) are showed.
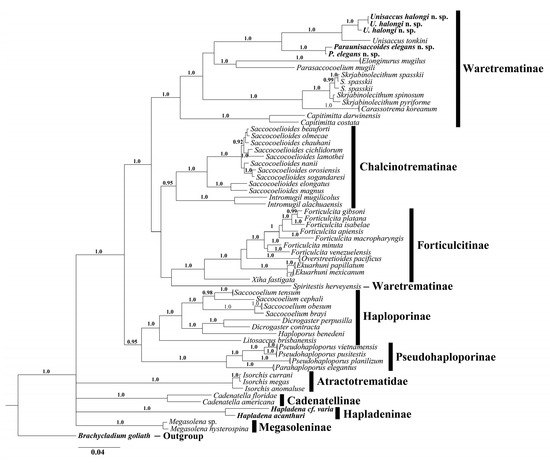
Figure 3.
Phylogenetic relationships of the family Haploporidae obtained with Bayesian algorithm based on concatenated ribosomal ITS2 and partial 28S rRNA gene (alignment length 1460 bp). Nodal numbers—posterior probabilities that indicate statistical support of phylogenetic relationships, only significant values (0.9–1.0) are showed.
New trematodes ex Crenimugil seheli from Vietnam, share many morphometrical characteristics with Paraunisaccoides, and also with Skrjabinolecithum. Alongside this, Vietnamese worms differ from Paraunisaccoides lobolecithum by lesser body length along with similar body width, higher ventral sucker width, lesser prepharynx and oesophagus length and lesser hermaphroditic sac length along with its higher width (Table 2). New worms differ from Paraunisaccoides elegans n. sp., described above, by presence of pads on hermaphroditic duct, and different structure of vitellarium follicules, and also by metric parameters of body width relative to body length, forebody length and forebody lengh/overall body length ratio and by a number of other characteristics (Table 2). Among Skrjabinolecithum, S. vitellosum and S. indicum, which were firstly detected in Planiliza subviridis and Etroplus surutensis (Bloch, 1790) from Australia coastal waters and the Arabian Sea, respectively [1,35], are most similar to new trematodes ex Vietnamese Crenimugil seheli from our study. However, new worms differ from S. vitellosum by presence of pads on hermaphroditic duct, different structure of vitellarium follicules and by most of metric parameters (Table 2). New worms are similar to S. indicum by most of metric parameters, except prepharynx length (Table 2). The main morphological difference between these worms is the presence of single and double caeca, and presence and absence of pads on hermaphroditic duct, respectively. Another difference for these trematodes can be found in the vitellarium structure. The vitellarium of S. indicum represents two poorly developed follicular fields [35], whereas for Vietnamese worms, the vitellarium is well developed and represents large elongate follicules. On the basis of stated above morphometrical differences between trematodes, we consider that worms ex Crenimugil seheli from Vietnam belong to neither S. vitellosum nor S. indicum. Additional molecular data on S. vitellosum and S. indicum from type locations are needed to clarify taxonomical and phylogenetic questions of these two species and new Vietnamese trematodes.
Molecular results support the membership trematodes ex Crenimugil seheli to Waretrematinae and indicate close relationships with Unisaccus tonkini (Figure 2 and Figure 3). Alongside this, worms from our material possess both common and uncommon morphological characteristics for Unisaccus. The similarity of these worms appears in the structure of genital and digestive systems. Differences of Unisaccus spp. and worms from our study observed in following characteristics: body pyriform to fusiform vs. body elongate; eggs embryonated vs. unembryonated; subspherical follicles vs. elongate follicles. Trematodes from our material are similar in terms of the listed characteristics to Paraunisaccoides elegans n. sp., which appears as sister clade relative to the Unisaccus clade on the Bayesian tree (Figure 2 and Figure 3). Genetic differentiation between these two clades ranged from 6.53 ± 0.76%–7.05 ± 0.5% by 28S rDNA sequence data and 7.94 ± 0.95%–8.05 ± 0.67% by ITS2 rDNA sequence data. These results indicate that trematodes from the two clades represent different genera.
Within clade, the p-distance values between worms ex Crenimugil seheli and U. tonkini were 2.59 ± 0.52% and 3.54 ± 0.67% by 28S rDNA and ITS2 rDNA, respectively, which is compatible with the interspecific values for other haploporid genera by these molecular markers: 0.43 ± 0.19% to 4.85 ± 0.65% by 28S rDNA and 2.15 ± 0.56% to 5.86 ± 0.87% by 5.8S + ITS2 rDNA [4,8,23,24,36].
These data provide evidence that worms from our material represent distinct valid species. Despite the existence of morphological differences between worms ex Vietnamese Crenimugil seheli and representatives of Unisaccus, we prefer the molecular results and recognise Vietnamese worms as a new species within Unisaccus, U. halongi n. sp. However, as we said above, additional morphological and molecular data on S. vitellosum and S. indicum from type locations are needed to clarify the taxonomical status of these two species and new trematodes from our study.
Author Contributions
Conceptualization, D.M.A. and V.V.B.; methodology, D.M.A., V.V.B. and A.Y.B.; software, D.M.A., A.Y.B.; validation, V.V.B., H.V.N., N.D.H. and T.V.N.; formal analysis, D.M.A., A.Y.B. and V.V.B.; investigation, H.V.N., V.V.B. and D.M.A.; resources, N.D.H., H.V.N. and T.V.N.; data curation, V.V.B. and N.D.H.; writing—original draft preparation, V.V.B. and D.M.A.; writing—review and editing, V.V.B., H.V.N. and D.M.A.; visualization, V.V.B. and D.M.A.; supervision, V.V.B. and N.D.H.; project administration, V.V.B. and N.D.H.; funding acquisition, V.V.B. and N.D.H. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the federal budget of the Russian Academy of Sciences, project No 0228-2019-0002.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Overstreet, R.M.; Curran, S.S. Family Haploporidae Nicoll, 1914. In Keys to the Trematoda; Jones, A., Bray, R.A., Gibson, D.I., Eds.; CABI: Cambridge, MA, USA, 2005; Volume 2, pp. 129–165. [Google Scholar]
- Pulis, E.E.; Overstreet, R.M. Review of haploporid (Trematoda) genera with ornate muscularization in the region of the oral sucker, including four new species and a new genus. Syst. Parasitol. 2013, 84, 167–191. [Google Scholar] [CrossRef]
- Andres, M.J.; Pulis, E.E.; Cribb, T.H.; Overstreet, R.M. Erection of the haploporid genus Litosaccus n.g. and its phylogenetic relationship within the Haploporidae Nicoll, 1914. Syst. Parasitol. 2014, 89, 185–194. [Google Scholar] [CrossRef]
- Besprozvannykh, V.V.; Atopkin, D.M.; Ermolenko, A.V.; Nikitenko, A.Y. Restoration of the genus Parasaccocoelium Zhukov, 1971 (Digenea: Haploporidae) and a description of two new species from mugilid fish in the Far East of Russia. J. Helminthol. 2015, 89, 565–576. [Google Scholar] [CrossRef][Green Version]
- Besprozvannykh, V.V.; Atopkin, D.M.; Ermolenko, A.V.; Nikitenko, A.Y. Morphometric and molecular analyses for a new species Skrjabinolecithum pyriforme n. sp. (Digenea: Haploporidae) in mullet fish from the Primorsky Region, Russia. J. Helminthol. 2017, 91, 625–632. [Google Scholar] [CrossRef][Green Version]
- Besprozvannykh, V.V.; Atopkin, D.M.; Ngo, H.D.; Ha, N.V.; Tang, N.V.; Beloded, A.Y. Morphometric and molecular analyses of two digenean species from the mullet: Skrjabinolecithum spinosum n. sp. from the Russian southern Far East and Unisaccus tonkini n. sp. from Vietnam. J. Helminthol. 2018, 92, 713–724. [Google Scholar] [CrossRef]
- Andres, M.J.; Pulis, E.E.; Overstreet, R.M. Description of three species of Isorchis (Digenea: Atractotrematidae) from Australia. Acta Parasitol. 2016, 61, 590–601. [Google Scholar] [CrossRef]
- Atopkin, D.M.; Besprozvannykh, V.V.; Ha, D.N.; Nguyen, V.H.; Nguyen, V.T.; Chalenko, K.P. A new subfamily, Pseudohaploporinae subfam. n. (Digenea: Haploporidae), with morphometric and molecular analyses of two new species: Pseudohaploporus vietnamensis n. g., sp. n. and Pseudohaploporus planilizum n. g., sp. n. from Vietnamese mullet. Parasitol. Int. 2018, 69, 17–24. [Google Scholar] [CrossRef]
- Atopkin, D.M.; Besprozvannykh, V.V.; Ha, D.N.; Nguyen, V.H.; Khamatova, A.Y.; Vainutis, K.S. Morphometric and molecular analyses of Carassotrema koreanum Park 1938 and Elonginurus mugilus Lu, 1995 (Digenea: Haploporidae) Srivastava, 1937 from the Russian Far East and Vietnam. Parasitol. Res. 2019, 118, 2129–2137. [Google Scholar] [CrossRef]
- Atopkin, D.M.; Besprozvannykh, V.V.; Ha, D.N.; Nguyen, V.H.; Nguyen, V.T. New species and new genus of Pseudohaploporinae (Digenea): Pseudohaploporus pusitestis sp. n. and Parahaploporus elegantus n. g., sp. n. (Digenea: Pseudohaploporinae) from Vietnamese mullet fish. Parasitol. Int. 2020, 75, 102023. [Google Scholar] [CrossRef]
- Atopkin, D.M.; Besprozvannykh, V.V.; Ha, D.N.; Nguyen, V.H.; Nguyen, V.T. New species of Parasaccocoelium (Haploporidae) and new genus Pseudohaplosplanchnus (Haplosplanchnidae) from mullet fish in the Far East of Russia and Vietnam: Morphological and molecular data. J. Helminthol. 2020, 94, e154. [Google Scholar] [CrossRef]
- Truett, G.E. Preparation of genomic DNA from animal tissues. In The DNA Book: Protocols and Procedures for the Modern Molecular Biology; Kieleczawa, J., Ed.; Jones & Bartlett Publisher: Burlington, MA, USA, 2006; pp. 33–46. [Google Scholar]
- Matejusova, I.; Cunningham, C.O. The first complete monogenean ribosomal RNA gene operon: Sequence and secondary structure of the Gyrodactylus salaris Malmberg, 1957, large subunit ribosomal RNA gene. J. Parasitol. 2004, 90, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Tkach, V.V.; Littlewood, D.T.J.; Olson, P.D.; Kinsella, J.M.; Swiderski, Z. Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Syst. Parasitol. 2003, 56, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Besprozvannykh, V.V.; Tatonova, Y.V.; Shumenko, P.G. Life cycle, morphology of developmental stages of Metorchis ussuriensis sp. nov. (Trematoda: Opisthorchiidae), and phylogenetic relationships of other opisthorchiids. J. Zool. Syst. Evol. Res. 2019, 57, 24–40. [Google Scholar] [CrossRef]
- Luton, K.; Walker, D.; Blair, D. Comparisons of ribosomal internal transcribed spacers from two congeneric species of flukes (Platyhelminthes: Trematoda: Digenea). Mol. Biochem. Parasitol. 1992, 56, 323–327. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F.; Nielsen, R.; Bollback, J.P. Bayesian inference of phylogeny and its impact on evolutionary biology. Science 2001, 294, 2310–2314. [Google Scholar] [CrossRef]
- Posada, D. Using MODELTEST and PAUP* to select a model of nucleotide substitution. Curr. Protoc. Bioinform. 2003, 6, 6.5.1–6.5.14. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModeltest2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Atopkin, D.M.; Beloded, A.Y.; Ngo, H.D. Molecular genetic characterization of the far eastern trematode Skrjabinolecithum spasskii Belous, 1954, (Digenea, Haploporidae), a parasite of mullets. Russ. J. Mol. Biol. 2015, 49, 422–429. [Google Scholar] [CrossRef]
- Atopkin, D.M.; Semenchenko, A.A.; Solodovnik, D.A.; Ivashko, Y.I.; Vinnikov, K.A. First next-generation sequencing data for Haploporidae (Digenea: Haploporata): Characterization of complete mitochondrial genome and ribosomal operon for Parasaccocoelium mugili Zhukov, 1971. Parasitol. Res. 2021, 120, 2037–2046. [Google Scholar] [CrossRef]
- Blasco-Costa, I.; Balbuena, J.A.; Kostadinova, A.; Olson, P.D. Interrelationships of the Haploporinae (Digenea: Haploporidae): A molecular test of the taxonomic framework based on morphology. Parasitol. Int. 2009, 58, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Gómez, L.; García-Valera, M. Unexpected morphological and molecular diversity of trematode (Haploporidae: Forticulcitinae) parasites of mullets from the ocean Pacific coasts in Middle America. Parasitol. Res. 2021, 120, 55–72. [Google Scholar] [CrossRef]
- Andrade-Gómez, L.; González-García, M.T.; García-Valera, M. Phylogenetic affinities of Forticulcitinae (Haploporidae) parasites of mullet from the Americas, with the description of three new species and notes on the genera and key species. Syst. Parasitol. 2021, 98, 455–476. [Google Scholar] [CrossRef]
- Andres, M.J.; Curran, S.S.; Fayton, T.J.; Pulis, E.E.; Overstreet, R.M. An additional genus and two additional species of Forticulcitinae (Digenea: Haploporidae). Folia Parasitol. 2015, 62, 025. [Google Scholar] [CrossRef] [PubMed]
- Curran, S.S.; Tkach, V.V.; Overstreet, R.M. A review of Polylekithum Arnold, 1934 and its familial affinities using morphological and molecular data, with description of Polylekithum catahoulensis sp. nov. Acta Parasitologica 2006, 51, 238–248. [Google Scholar] [CrossRef]
- Curran, S.S.; Pulis, E.E.; Andres, M.J.; Overstreet, R.M. Two new species of Saccocoelioides (Digenea: Haploporidae) with phylogenetic analysis of the family, including species of Saccocoelioides from North, Middle and south America. J. Parasitol. 2018, 104, 221–239. [Google Scholar] [CrossRef]
- Pulis, E.; Fayton, T.; Curran, S.; Overstreet, R. A new species of Intromugil (Digenea: Haploporidae) and redescription of Intromugil mugilicolus. J. Parasitol. 2013, 99, 501–508. [Google Scholar] [CrossRef]
- Andres, M.J.; Pulis, E.E.; Curran, S.S.; Overstreet, R.M. On the systematics of some marine haploporids (Trematoda) with the description of a new species of Megasolena Linton, 1910. Parasitol. Int. 2018, 67, 805–815. [Google Scholar] [CrossRef]
- Huston, D.C.; Cutmore, S.C.; Cribb, T.H. Isorchis cannoni n. sp. (Digenea: Atroctotrematidae) from Great Barrier Reef rabbitfishes and the molecular elucidation of its life cycle. J. Helmointhology 2018, 92, 604–611. [Google Scholar] [CrossRef]
- Briscoe, A.G.; Bray, R.A.; Brabec, J.; Littlewood, D.T. The mitochondrial genome and ribosomal operon of Brachycladium goliath (Digenea: Brachycladiidae) recovered from a stranded minke whale. Parasitol. Int. 2016, 65, 271–275. [Google Scholar] [CrossRef]
- Martin, W.E. A new genus and species of haploporid trematode (Haploporidae: Trematoda) from Australian mullet. Bull. South. Calif. Acad. Sci. 1973, 72, 166–168. [Google Scholar]
- Martin, W.E. A new subfamily, two new genera, and three new species of haploporid trematodes. Proc. Helminthol. Soc. 1973, 40, 112–117. [Google Scholar]
- Zhukov, E.V. New genera of trematodes from marine fishes of India. Parazitologya 1972, 6, 346–350. [Google Scholar]
- Andrade-Gómez, L.; Pinacho-Pinacho, C.D.; Hernández-Orts, J.S.; Sereno-Uribe, A.L.; García-Valera, M. Morphological and molecular analyses of a new species of Saccocoelioidea Szidat, 1954 (Haploporidae Nicoll, 1914) in the fat sleeper Dominator maculates (Blosh) (Perciformes: Eleotridae) from the Gulf of Mexico. J. Helminthol. 2017, 91, 504–516. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).