1. Introduction
Endemic species, particularly if distributed across a very small area, pose a tough set of problems in conservation biology, and their protection is complex and challenging for several reasons (see [
1]). Since they are distributed in a small, restricted area, each threat occurring there is usually affecting the whole species or a great portion of its individuals. Moreover, there is no or only a limited possibility for population augmentation or reintroduction. Finally, it is often considered that such range-restricted and small population-size species, distributed in unique areas, have small genetic diversity (lowered by genetic drift [
2,
3,
4]) and low effective population sizes, reducing their evolutionary potential and increasing their extinction risk [
5]. In small populations, the importance of genetic drift relative to selection is increased, and beneficial alleles are usually thought to be lost [
6]. On the other hand, deleterious alleles are more likely to reach high frequencies in comparison with the situation in larger populations [
6]. Thereafter, small populations are usually considered to have lowered fitness and are more prone to extinction. The reduced genetic diversity, often thought to be characteristic for small populations, makes them less able to respond to environmental changes than larger populations [
6].
The genus
Telestes Bonaparte, 1837 (family Leuciscidae, order Cypriniformes, class Actinopteri) is primarily comprised of freshwater fish distributed mostly in the Mediterranean area. Ecological conditions in their habitats include moderately cold, flowing or stagnant, well-oxygenated, clean water [
7]. Recent investigation of the evolutionary history of the genus [
8] revealed that the origin of
Telestes occurred in southern Europe, where the most ancient
Telestes species are still present. Moreover, an especially high diversity of
Telestes at species and intraspecific levels was revealed in the Adriatic watershed in Croatia and Bosnia and Herzegovina, as a consequence of a complex geological history of the region leading to triple colonization of that area by distinct
Telestes lineages. The isolation of rivers in this karstic area facilitated allopatric speciation, resulting in a high number of
Telestes species and great portion of endemic species in freshwater sources of Croatia and Bosnia and Herzegovina. Out of 14 currently recognized
Telestes species, as many as 10 are distributed in the mentioned area [
8], 9 of which are endemics, with very restricted distribution ranges.
Telestes croaticus (Steindachner, 1866) inhabits only four small karstic rivers (Jadova, Suvaja, Ričica and Obsenica), located in the Lika karstic region in Croatia;
T. tursky (Heckel, 1843) has a distribution range restricted to the small Čikola River, a tributary of the Krka River;
T. ukliva (Heckel, 1843) is restricted to the Cetina River basin, also in Croatia.
Telestes miloradi Bogutskaya, Zupančič, Bogut and Naseka, 2012 is found only in small springs and streams in the Konavosko karstic field in Croatia. Recent investigation [
8] disputed the distinctiveness of
T. dabar Bogutskaya, Zupančič, Bogut and Naseka, 2012, described from the Dabar karstic field in Bosnia and Herzegovina. Nevertheless,
T. metohiensis (Steindachner, 1901) is distributed in the watersheds of the karstic fields in southern Bosnia and Herzegovina. All the mentioned species are endemic species inhabiting small distribution areas in the Adriatic watershed. Three more endemic species have extremely small distribution ranges located at the border between the Adriatic and Black Sea watersheds in Croatia but belonging to the Black Sea watershed due to the underground connections. Those three species are:
T. fontinalis (Karaman, 1972) from the Krbavsko karstic field,
T. polylepis (Steindachner, 1866) known from a single locality—Šmit lake, and
T. karsticus Marčić and Mrakovčić, 2011, inhabiting few small streams in mountain region in Croatia.
The exceptionally high diversity of Telestes in freshwater systems of middle and southern Croatia and Bosnia and Herzegovina is a result of several phenomena:
Origin of the genus most probably took place in the mentioned region and colonization started from there [
8]
Complex geological history led to triple colonization by genetically distinct
Telestes lineages [
8]
Isolation of karstic rivers and lakes is promoting allopatric speciation (e.g., [
9,
10])
Undisturbed evolutionary development of populations in rivers that were not affected by glaciations, resulting in their high genetic diversities (e.g., [
9]).
Most of the endemic
Telestes species have very small distribution areas, inhabiting a single river or few watersheds in a single karstic field [
8,
11], making them extremely vulnerable to all anthropogenic changes affecting their limited habitats. Furthermore, it is possible that their effective population sizes are low due to the small habitat size but also due to particular environmental conditions, which include dry seasons, lowering of the water level, and droughts in part of or the whole water stream. There are indications that some, if not all,
Telestes species migrate to underground shelters during the dry season (personal observation) and maybe even use underground water channels for migrations, similarly to the genus
Delminichthys [
12], which also inhabits karstic watershed in the same area. Unfortunately, habitats and populations of these endemic species were not spared from significant anthropogenic threats, and in some localities, there are plans for even greater impact in spite of the invaluable importance of these unique biodiversity components. Previous investigation already revealed that, out of six endemic
Telestes species further investigated in this research, only
T. karsticus turned out to have lower intraspecific genetic diversity, whereas the remaining five species comprised high levels of genetic diversity [
8]. In this investigation, we analyzed intrapopulational genetic diversities, as well as relationships among populations in species comprising more than a single population. Furthermore, in order to contribute to practical conservation of the endemic
Telestes species, particularly to the design of conservational measures that are likely to be the most effective in ensuring the future viability and undisturbed evolutionary course of those species, we have investigated their population genetic structure and estimated their viabilities. Population viability analyses were carried out based on the current status of populations and habitats, as well as currently recognized and potential threats. Thereafter, after the first investigation in which we analyzed the phylogenetic structure and evolutionary history of the genus
Telestes [
8], in this investigation, we have made a further step towards understanding the genetic structure of the endemic
Telestes species as well as the causes and consequences of the observed genetic diversities, but also put strong emphasis on their conservation.
3. Results
A previous investigation [
8] already revealed the high genetic diversity of the majority of
Telestes species distributed in the karstic watersheds in Croatia, explained by a long-term evolutionary history in favorable conditions and a lack of bottlenecks during longer geologic periods. This investigation revealed intrapopulational diversities and differences between them. The intrapopulational diversities of all populations comprised under
T. croaticus and
T. ukliva are very high (
Table 2). Populations of
T. karsticus contain the lowest levels of genetic diversity, with all samples from Studenac expressing the same haplotype (no genetic diversity). The remaining populations of this species also express much lower genetic diversities than those found in populations of the remaining species. Of note,
T. fontinalis and
T. tursky, even though each species has an extremely small distribution range, comprise just a single population, and have small effective population sizes (1185 and 1685, respectively; see
Table 3), they express high levels of genetic diversity (
Table 2).
Populations of T. croaticus, T. karsticus, and T. ukliva express a high level of isolation, and only a very restricted gene flow has been noticed. The T. croaticus gene flow from Obsenica to Jadova (M = 3650, Nm = 29) as well as from Suvaja to Ričica (M = 1563, Nm = 13) has been estimated. The Sušik population of T. karsticus seems to be receiving immigrants from Studenac and Jasenak populations (for both population pairs M = 980, Nm = 11). Very small bi-directional migrations have been noticed between Cetina and Vinelić populations of T. ukliva (Nm = 5 in both directions). It is interesting that migrations were noticed among populations that are not connected to ground watercourses. Nevertheless, all estimated migration events are very small and cannot be considered as contributing significantly to the size or diversity of any of the populations. For T. croaticus, particularly high levels of nucleotide diversity (0.01074 vs. 0.00506, which is the maximum value observed in the remaining species) and the total number of nucleotide differences (12.225 vs. max. 5.758 in the remaining species) were observed. Among T. croaticus populations, those parameters were the highest in the Obsenica population, much higher than in any of the remaining populations and species.
Effective population sizes varied greatly among populations (
Table 3); however, for the majority of populations, they seem appropriately high regarding the carrying capacities of geographically restricted karstic habitats. Expectedly, the lowest effective population size was estimated for the
T. karsticus population from the Studenac stream, and estimates for the remaining
T. karsticus populations were lower than for populations belonging to the remaining species. For
T. karsticus populations, a low effective population size was observed inside the Obsenica population of
T. croaticus, whereas the remaining
T. croaticus populations have higher effective population sizes, particularly the population from the Ričica River.
Genetic differentiation tests revealed that populations of T. karsticus and T. ukliva are genetically uniform, whereas there is genetic distinctiveness between populations of T. croaticus. The original hypothesis that there is no genetic distinctiveness between populations was rejected only in the case of T. croaticus, where a permutation test revealed estimates obtained by all genetic differentiation tests to be statistically significant (p < 0.05).
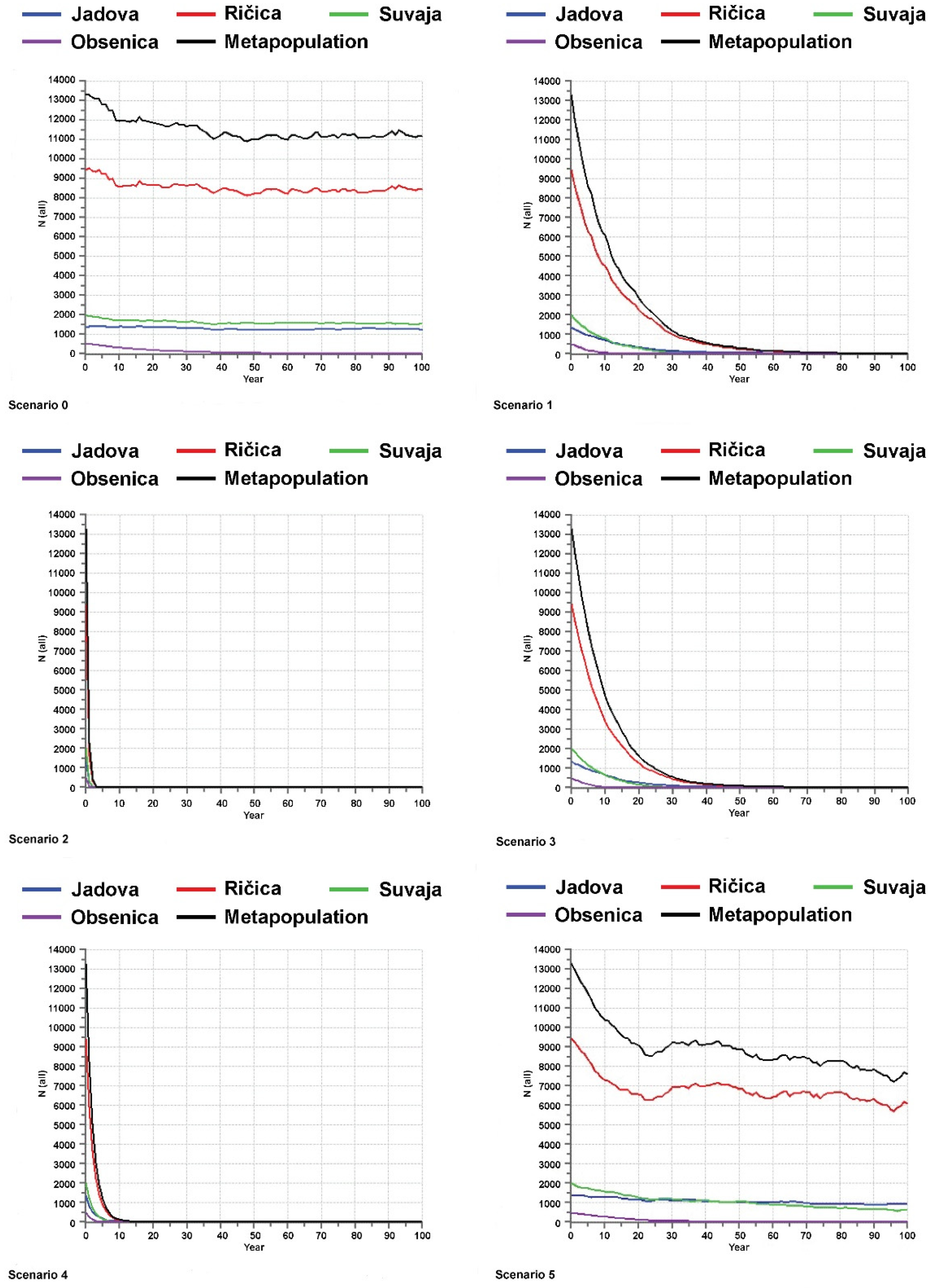
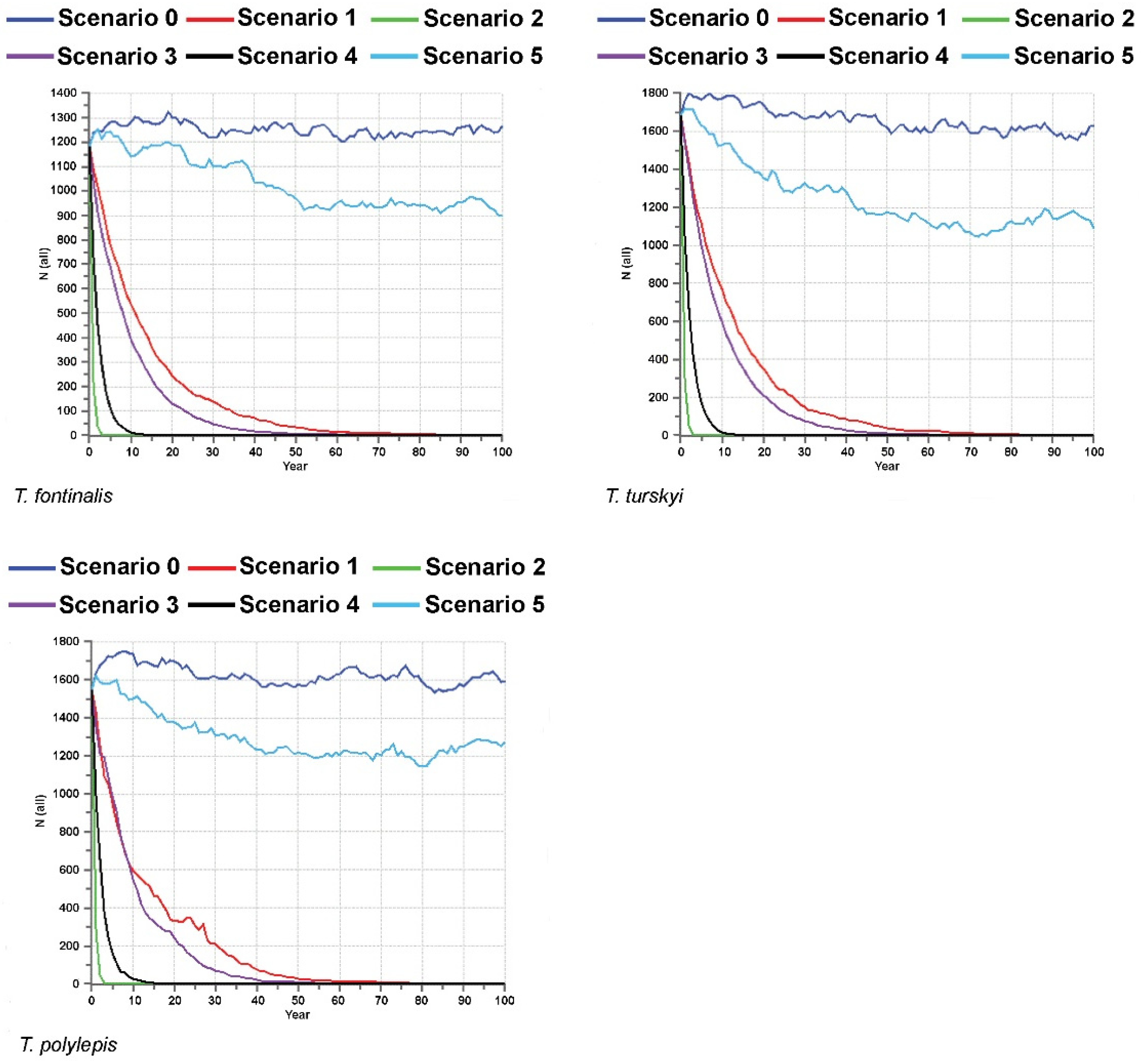
Interesting and important results were yielded by PVA analyses conducted on the endemic
Telestes species and based on a total of six different scenarios (Scenario 0 encompassing the current situation, without additional threats, and Scenarios 1–5 modelling the viability of the investigated
Telestes populations and species in cases of possible future threats and catastrophes) (
Figure 2,
Figure 3,
Figure 4 and
Figure 5). The viability of all populations and species is predicted under the current conditions (the probability of extinction in the next 100 years being 0). Moreover, Scenario 0 anticipates the stability of the majority of populations. Exceptions include
T. karsticus populations from the Studenac stream and Jasenak field for which a reduction in the effective population sizes is anticipated. Consequently, it is likely that the effective population size of
T. karsticus as a species will be reduced in the next 100 years if the current conditions remain. All four scenarios that comprise threats on habitats of the investigating species occurring and/or being more severe and intense (Scenarios 1–4) yielded a strong prediction of all populations experiencing extinction within the next 100 years. Scenarios 2 and 4 seem to be particularly problematic, which predict the very soon extinction of all endemic
Telestes species (within the next 15 years). Scenario 5, encompassing catastrophic events of strong negative effects on populations, is not likely to provoke their extinction, although fluctuations and reductions in the effective population sizes will probably occur under such conditions.
4. Discussion
Small, isolated populations inhabiting restricted areas are often considered to lose genetic diversity due to genetic drift [
2,
3,
6,
22]. Many other problems described by conservation genetics are also thought to be characteristic for small populations, such as the Allee effect, accumulation of deleterious mutations due to mutational meltdown, decrease in genetic diversity due to selection (the Bulmer effect), inbreeding depression, etc. [
6,
23]. There is evidence that in very small populations, natural selection is ineffective, contrary to significant genetic drift [
22], and the importance of random genetic drift is increased, acting towards the loss of beneficial alleles [
6]. Small populations are, thereafter, usually considered particularly endangered, and their future survival questionable. However, the results of this investigation on the endemic
Telestes species clearly show that those species are not necessarily endangered just because they have small populations and are distributed in restricted areas, nor do they express genetic problems usually reported for small populations.
Almost all investigated
Telestes species, despite their extremely restricted distribution ranges and mostly small effective population sizes, express moderate-to-high genetic diversities. During a long-term evolutionary history in their unique environments [
8], they have developed adequate adaptations and also accumulated high amounts of genetic diversity, which is probable the reason for their future viability. This hypothesis is corroborated by our results because Scenario 0 did not result in the extinction of any of the species, not even the ones comprised of a single population. When discussing the obtained results, it is necessary to have in mind the low sample number for
T. polylepis and several populations belonging to other species. Undoubtedly, a higher sample number would yield more precise and reliable results. Nevertheless, patterns that we have observed and present in this paper on the higher genetic diversity and effective population sizes corresponding with habitat carrying capacities have been observed in almost all species, and most of them are well represented with a satisfactory sample number, which makes our conclusions reliable.
Out of the investigated species, only T. karsticus has lower genetic diversity. From a conservational perspective, the situation in the Studenac stream is particularly worrying, where no genetic diversity has been recorded and all samples possess the same haplotype. Since all the remaining investigated populations express much higher levels of genetic polymorphism, the situation observed in the Studenac stream, but to a somewhat lesser extent also found in the Jasenak field population, are probable consequences of the local unfavorable conditions resulting in a bottleneck effect in the evolutionary history of the mentioned T. karsticus populations. Even though such results are not in accordance with the pattern more widely observed in this investigation (high genetic diversity present in small populations), this knowledge is very important from a conservational perspective. Without this knowledge, we might consider T. karsticus less endangered—because of its larger area of occupancy and extent of occurrence, as well as the inclusion of more than a single population—than some other endemic Telestes species, for example, T. polylepis, T. fontinalis, and T. ukliva. However, regardless of its somewhat larger distribution area and higher effective size, it has a reduced evolutionary potential (because of the reduced genetic diversity), and its viability is lower than that of the remaining investigated species, corroborated also by PVA analyses. These findings also point out the necessity of obtaining data from all populations before making conservational conclusions. Moreover, since the gene flow among T. karsticus populations is almost absent, each population depends solely on its own viability and effective size, which underlines the necessity for immediate, adequate conservation of those populations. Based on the obtained results, the conservation plan for T. karsticus should certainly include population augmentation, to prevent any additional reduction in population sizes that are predicted to occur under current conditions.
On the other hand, genetic diversities observed in the remaining populations reveal that small genetic polymorphisms are not necessary characters of small and isolated populations. Interestingly, contrary to the opinion that natural selection is ineffective in small-sized populations due to rapid genetic drift, some theoretical models [
24] suggest moderate or strong balancing selection on small populations, opposing random drift and maintaining polymorphism over thousands of generations. Moreover, it was recently proposed that small populations do not continuously decline in fitness due to the fixation of slightly deleterious mutations, but only until drift-selection balance is reached and the fixation of beneficial mutations counteracts the fixation of slightly deleterious mutations [
6,
25]. For the lack of small-effect deleterious mutations in small populations, their adaptation to drift-robust fitness peaks has been proposed as an explanation [
26]; the results of [
26] suggested that small populations evolve to alternative areas of the fitness landscape by maintaining small-effect beneficial mutations. Although the authors propose that the theory of drift robustness in small populations might be true for bacterial endosymbionts and RNA viruses, their hypothesis is worth investigation in higher organisms that naturally live in small populations, in harsh conditions of karstic watersheds and, based on our results, comprise much higher genetic diversities than expected in the presence of the significant genetic drift. Furthermore, there are literature data on other mechanisms opposing a reduction in genetic diversity and loss of fitness in small populations. The authors of Ref. [
27] suggested that reverse mutations (acting to return deleterious mutant alleles to the fit original forms) can substantially slow the loss of fitness. Several interesting reports (reviewed in [
6]) pointed to the possibility that the rate of beneficial mutations may even increase as the mean fitness of a small population drops.
Some reports [
6,
28] have proposed an effective population size of a few hundred individuals to be a critical borderline below which it is likely that the population will decline in fitness but above which beneficial mutations allow the population to persist. Populations above a critical threshold size seem to be able to persist as a result of the balancing effects of the fixation of beneficial alleles [
6]. Effective population sizes estimated for
Telestes populations are mostly in the range of a few hundred to two thousand (exceptions are both
T. ukliva populations and
T. croaticus in the Ričica River, which have even higher effective population sizes but also inhabit the two largest rivers among the investigated water bodies, which are, moreover, permanent). Thereafter, the effective population sizes of
Telestes species do not seem to be too small to enable population viability based on the conclusions made in [
6]. For most populations, it is not likely that carrying capacities in their small, karstic environments are much higher. Exceptionally high nucleotide diversity and the number of nucleotide differences noticed in
T. croaticus, particularly in the Obsenica River, corroborates pronounced genetic structuring of this species, as already revealed in a previous investigation [
8]. Both lineages are present in the Obsenica River, and their taxonomic status should be investigated further.
Based on the obtained results, the investigated populations mostly seem to be isolated, and the gene flow estimates indicate a very low or non-existing gene flow among populations. Nevertheless, estimation of the gene flow between populations inhabiting rivers that currently have no surface connections, together with their existence in streams with extreme fluctuations in the surface water level, indicates the possibility that
Telestes species exploit underground water systems as possible shelters during dry seasons and/or as migration routes between localities that have no aboveground connections. For example, the gene flow between
T. croaticus populations from the Obsenica and Jadova Rivers has been noticed, and those two rivers have no surface connections. Similarly, two populations of
T. karsticus (from Studenac and Jasenak localities) have no surface connections, but a small gene flow between them has been estimated. On the other hand, Suvaja and Ričica (inhabited by two
T. croaticus populations that also exchange migrants) and Cetina and Vinalić (inhabited by two populations of
T. ukliva) do have surface connections. A conclusion for migrations through underground water passages has already been reported for the genus
Delminichthys, which also inhabits karstic water systems in the same area [
12]. Thereafter, the possibility that fish of the genus
Telestes have similar adaptations deserves further investigation.
In addition to the important conclusion that small endemic populations are not doomed to extinction and seem to express significant evolutionary potential and viability under current conditions, PVA results clearly pinpointed the most dangerous threats for the investigated species. Since Scenarios 2 and 4 predicted the rapid extinction of all the investigated populations and species, their presumptions seem to be the most problematic for the endemic
Telestes species. Scenario 2 encompasses negative effects of invasive species, whereas Scenario 4 predicts simultaneous effects of different threats. Expectedly, simultaneous occurrence of several threats on the endemic
Telestes species would result in their joint effects reducing the population sizes and provoking species extinctions extremely quickly. Unfortunately, this scenario is not unlikely and cases of extinction vortex, wherein different environmental and genetic problems act at the same time, enlarging negative effects on the populations, present one of the biggest threats for biodiversity conservation [
29]. On the other hand, Scenario 2 reveals that invasive species can be pinpointed as the most dangerous individual threat for the endemic
Telestes species. Field observations corroborate this conclusion, particularly the disappearance of
T. karsticus from localities where trout species were introduced (personal observation). Invasive species are already present in habitats of several endemic
Telestes species [
30]. In some of the areas, the number of recorded non-native species even dominates the native ones [
31]. The effective removal of already present non-native species, as well as the design and implementation of a strategy for continuous monitoring, early discovery, and quick response in order to prevent non-native species from forming stabile populations in watersheds inhabited by native
Telestes species should be a top priority in Croatian practical nature conservation. The results of Scenario 3 evince that although skewed sex ratios have been recorded in various
Telestes species [
7,
19,
20,
21], if this phenomenon were to occur more often due to climate change (since the water temperature is possibly a factor influencing the sex ratio at birth), it would also result in the extinction of all populations within the next 100 years. Of note, in Scenario 3, we have calculated a constant change in the sex ratio and no mitigation effects that might occur as an adaptation of the investigated populations to climate change. Thereafter, it is likely that the effects of water temperature increment will not be as harsh as predicted by Scenario 4. As a conservational measure to prevent the negative effects of a skewed sex ratio on the population viability, we propose monitoring the sex ratio in
Telestes populations and augmentation with females from ex situ breeding programs in cases in which the sex ratio would be significantly in favor of males over multiple consecutive years. Interestingly, Scenario 5, comprising very intense effects of catastrophic events occurring twice in 100 years, did not result in the extinction of any of the endemic
Telestes species. Since karstic environments are unfavorable and experience pronounced fluctuations, it is probable that adaptations for such environments are more likely to enable survival after catastrophic events, if they do not occur too often. Thereafter, our results clearly evince that small endemic species are by no means doomed to extinction, nor do they necessarily comprise small levels of genetic diversity. On the contrary, during their evolution to specific environments, they seem to have accumulated beneficial mutations that make their fitness high and viability pronounced, and high genetic diversity is surely a reservoir for coping with environmental changes and ensuring the future evolutionary course of the endemic
Telestes species. Conservational measures should be primarily focused on the control and prevention of invasive species spreading, since they turned out to be the most dangerous threat for these unique biodiversity components.