Abstract
Although the extent of near-shore and coastal habitats around the Antarctic Continent is limited, they host an abundant and diversified fish fauna dominated by notothenioids. Nevertheless, the spatial distribution of fishes at small scales and their relationships with the surrounding habitat are still poorly known. The purpose of this study is to provide new insights on the inshore fish community of Terra Nova Bay, Ross Sea, which is now part of the largest marine protected area established so far in the Southern Ocean. As a low-impact and effective methodology of investigation, an underwater photographic survey was conducted through two remotely operated vehicle (ROV) transects set down to 300 m depth. The faunistic inventory consisted of twelve species of notothenioids, which complements previous data obtained by conventional samplings. The most abundant species exhibited wide depth distribution ranges, and they were generally associated with areas with a rich benthic macrofauna composed of alcyonaceans, sponges, bryozoans, polychaetes, and echinoderms. Nesting behavior was documented in two species, Trematomus bernacchii and Pagetopsis macropterus. The present data provide further evidence of the importance of inshore waters for the local fish community, representing a proper habitat for settling, foraging, and spawning activities.
1. Introduction
The fish fauna inhabiting the continental shelf and slope of the Ross Sea is overwhelmingly dominated by the notothenioids, a single taxonomic group composed of four endemic, cold-adapted families: Artedidraconidae, Bathydraconidae, Channichthyidae and Nototheniidae [1]. Most members of this group are primarily benthic as adults, sharing a common ancestral benthic origin [2]. Although all notothenioids lack a swim bladder, the reduction of skeletal mineralization and lipid deposition in tissues allowed some of them to colonize the water column, an evolutionary process referred to as pelagization [3] and considered to be the hallmark of notothenioid radiation [4]. Such a diversification in buoyancy enabled notothenioids to fill a wide array of habitats other than the ancestral benthic, including the semipelagic, epibenthic, cryopelagic, and pelagic environments [2]. The acquisition of antifreeze glycopeptides represented a key innovation in the evolutionary path of notothenioids [5,6], which has totally replaced the previous non-notothenioid fish fauna since the late Eocene [4].
Because of the remoteness and presence of seasonal pack ice for most of the year, the knowledge of the fish communities living on the continental shelf of the Ross Sea remains limited and based on a few ship-based trawl surveys carried out in offshore waters during the last two decades [6]. Overall, four benthic trawl surveys were conducted in the western Ross Sea [7,8] and a single midwater and benthic trawl survey was conducted in the eastern Ross Sea [9]. The demersal fish assemblages recorded in the two areas were very similar, yielding a total of 50 species of notothenioids within the families Artedidraconidae (15), Bathydraconidae (10), Channichthyidae (10), and Nototheniidae (15). Compared to non-notothenioid fishes, consisting mainly of rajids and zoarcids, notothenioids accounted for 91.5% and 77.0% in abundance and biomass in the western Ross Sea [6] and 98.7% and 94.2% in the eastern Ross Sea, respectively [9].
Interestingly, the taxonomic composition of the fish fauna collected offshore in the Ross Sea by bottom trawling was rather different from that collected in inshore waters by passive fishing gears, such as trammel and gill nets, longlines, and traps [7]. Shore-based fishing surveys were carried out primarily in the coastal waters of Terra Nova Bay, in close proximity to the Italian Antarctic Station Mario Zucchelli [10,11]. Overall, the coastal fish fauna consisted of 27 species of notothenioids subdivided in Artedidraconidae (5), Bathydraconidae (3), Channichthyidae (6), and Nototheniidae (13) [12,13]. The bulk of catches were dominated by Trematomus bernacchii and Chionodraco hamatus, which were sampled predominantly within 200 m depth. The vertical pattern of distribution of the species provided evidence of spatial niche partitioning, the less frequent species being found either in shallow or deep waters, whereas the most abundant species were generally eurybathic [12,13].
Species distributions at finer spatial scales and their relationship with the surrounding habitat are still poorly known. A recent powerful tool for investigating Antarctic benthic communities is represented by the use of still and video cameras mounted on remotely operated vehicles (ROV) that enable documenting deeper habitats inaccessible to scuba divers. Compared to more conventional methodologies (i.e., benthic trawling), seafloor imagery is a non-destructive and effective survey method providing a fine-scale description of benthic assemblages while minimizing the impacts of direct sampling. The use of underwater photography is particularly advisable in marine protected areas (MPAs), which currently occupy about 12% of the Southern Ocean [14]. The Ross Sea region MPA was established under the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) jurisdiction only recently and, encompassing a surface of about 1.55 million km2, represents the world’s largest marine protected area [15].
With the aim to provide insights on the fine-scale distribution and species composition of the local fish community in relation to depth and benthic habitats, a photographic survey was conducted in the coastal waters of Terra Nova Bay, located within the no-take General Protection Zone (GPZ) of the Ross Sea region MPA.
2. Materials and Methods
The sampling sites were located within the Antarctic Specially Protected Area of Terra Nova Bay (ASPA 161), comprising an area of approximately 29.4 km2 between Adélie Cove and Tethys Bay (Figure 1).
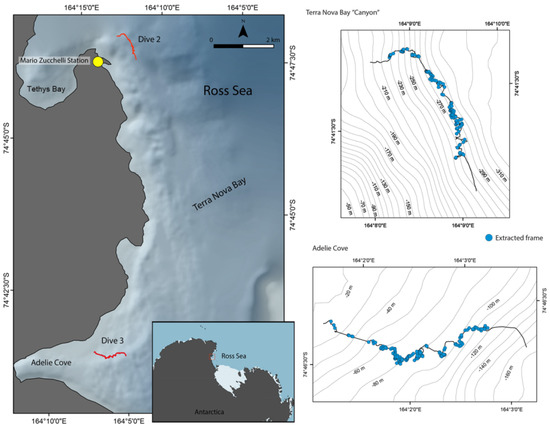
Figure 1.
Map of the study area, showing the location (in red on the left) and pathway of the ROV transects (on the right). The figure was partially redrawn from [16], with the kind permission of the authors.
The two transects were surveyed by a remotely operated vehicle (ROV, Pollux III), equipped with an underwater acoustic tracking system (USBL, Linkquest, TrackLink 1500 MA, San Diego, CA, USA) and connected to a Trimble dual-antenna system providing position and depth every 1 s. The photographic systems included a digital camera (Canon EOS 550, Canon EF-S 10–22 mm f/3.5–4.5 USM lens with double Speedlite 270EX flash, Canon, Tokyo, Japan) and a high-definition video camera (SONY HDR-HD7, Sony, Tokyo, Japan). A reference scale in photographs was represented by three laser beams spaced 10 cm apart. The ROV was deployed from two small motor vessels, “Malippo” (14 m, ~45 t, engine 1100 CV) and “Skua” (15 m, 40 t), during favorable weather conditions. Sampling data for each transect are summarized in Table 1.

Table 1.
Data of the ROV benthic survey carried out in coastal waters of Terra Nova Bay (TNB).
Each photograph, representing roughly an area of 6 m2, was analyzed for the taxonomic composition of biological communities, including benthos (macro- and megafauna) [16] and fishes. The bottom type and percentage of encrusting algae (Rhodophyta) on hard substrates were also recorded. In the study area, the seafloor was primarily characterized by granitic rock, interspersed with soft substrates composed of coarse sands, muddy sediments, or gravels. All fishes were identified to the species level, taking into account the morphological diagnostic characters reported in the literature [17,18]. The total number of species (S), the numerical percentage of each species (Pi), and the total number of individuals (n) were used to calculate indices of species diversity (Shannon, H′ = −ΣPi loge(Pi)), species richness (Margalef, d = (S − 1)(loge n)−1), and evenness (Pielou, J′ = H′(loge S)−1) for each transect.
3. Results
3.1. Terra Nova Bay “Canyon”
A total of 169 images were taken along this transect located in proximity of the Italian Station between 227 m and 300 m depth (Figure 1), mainly composed of hard substrate covered by a thin layer of soft sediment and sparsely of mobile substrate with patches of organic matter in degradation. The presence of fishes was recorded in 25 images (~15% of total), most of them showing a single individual. Overall, 31 individuals belonging to nine species of notothenioids were recorded (Table 2).

Table 2.
Species list, number of individuals, and biodiversity indices calculated for each ROV transect.
Among nototheniid, T. bernacchii overwhelmingly dominated the fish community along the transect, with 15 individuals spread over a wide depth range (Figure 2). They were frequently seen camouflaged amongst the benthic macrofauna or hiding inside the sponges guarding eggs batches (Figure 3A). The typical habitat of this species consisted of hard substrates with organic matter patches, populated by a rich benthic macrofauna, including sponges, alcyonaceans, bryozoans, polychaetes, crinoids, ophiuroids, holothurians, asteroids, and echinoids. The second most abundant species was Prionodraco evansii, a gregarious fish distributed over a relatively narrow depth range (Figure 2). It was found resting on soft substrates with sparse alcyonaceans, in association with high densities of shrimps (Chorismus antarcticus) (Pfeffer, 1887) (Figure 3B). Two individuals of Artedidraco skottsbergi, Trematomus borchgrevinki, and C. hamatus were recorded at depth between 265 and 300 m (Figure 2), on rocky bottoms densely populated by alcyonaceans and sponges. All other fish species were represented by single individuals with varying depth distribution along the transect, preferably on soft sediment with organic matter patches (Racovitzia glacialis and Trematomus pennellii), or on hard bottoms with a rich benthic macrofauna (Artedidraco loennbergi and Pagetopsis macropterus) (Figure 3C–F).
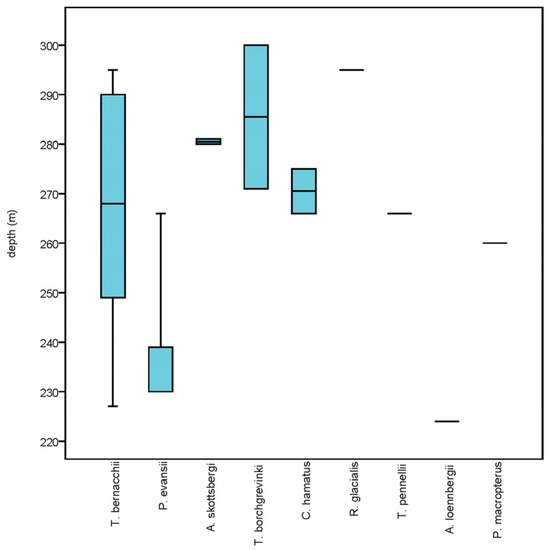
Figure 2.
Box plot showing median, 25–75 percentiles, and maximum-minimum depth distribution of species along the transect Terra Nova Bay “Canyon”.
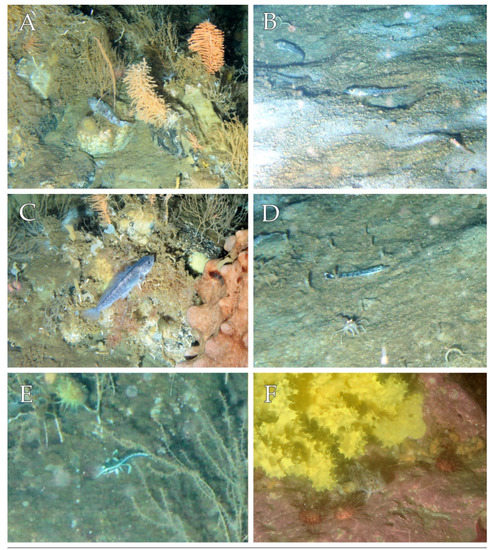
Figure 3.
Underwater photographic pictures of the notothenioid fishes recorded during the ROV survey in inshore waters of Terra Nova Bay. (A) Trematomus bernacchii; (B) Prionodraco evansii; (C) Trematomus borchgrevinki; (D) Racovitzia glacialis; (E) Artedidraco loennbergi; (F) Trematomus pennellii.
3.2. Adélie Cove
A total of 148 images were collected across this transect, which was located in front of Adélie Cove between 25 m and 120 m depth (Figure 1). The bottom consisted of hard substrate with encrusting coralline algae (Rhodophyta), partially replaced by mobile sediments below 100 m depth. Fish were recorded in 52 images (~35% of total photographs), each generally with one or two individuals. A total of 85 individuals belonging to nine species were recorded in this transect (Table 2). The most abundant species was P. evansii, with 35 individuals distributed between 70 m and 125 m (Figure 4). They were frequently seen grouped (up to ten individuals in a single image), resting or partially buried in soft sediments amongst dense aggregations of echinoids (Sterechinus neumayeri) (Meissner, 1900) and sparse alcyonaceans. Among nototheniids, T. bernacchii and T. pennellii were the second most abundant species, with a rather different distribution and habitat. Trematomus bernacchii was recorded on a relatively narrow depth range (70–100 m), almost exclusively on hard bottoms densely covered by alcyonaceans, sponges, bryozoans, polychaetes, ophiuroids, holothurians, and echinoids. Trematomus pennellii was spread over a wide depth range (Figure 4), inhabiting both hard and mobile substrates with a diversified and rich benthic macrofauna. Following in order of abundance, A. skottsbergi and C. hamatus exhibited an overlapped depth distribution (Figure 4), both preferring hard substrates with encrusting coralline algae alternated to soft sediments hosting a rich invertebrate macrofauna (Figure 5A,B). A single female of C. hamatus was gravid, suggesting that this species is likely a summer spawner. Two P. macropterus were recorded at different depths (Figure 4), either hiding behind holothurians or dwelling over a nest with a single layer of eggs amongst densely encrusted boulders (Figure 5C). Single specimens of Cryodraco atkinsoni and Histiodraco velifer were documented on soft sediments at about 100 m, perching on their long pelvic fins or resting on the bottom with a pronounced mental barbel, respectively (Figure 5D,E). Finally, the rare nototheniid Trematomus nicolai was encountered among large and bare hard blocks populated by clams (Adamussium colbecki) (Smith, 1902) and echinoids (S. neumayeri) (Figure 5F).
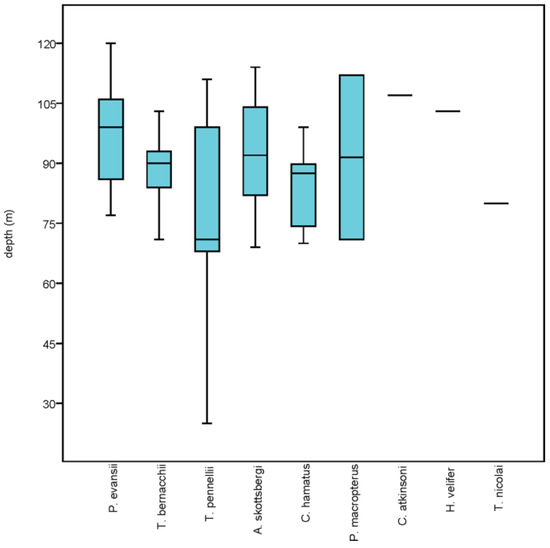
Figure 4.
Box plot showing median, 25–75 percentiles, and maximum-minimum depth distribution of species along the transect Adélie Cove.

Figure 5.
Underwater photographic pictures of the notothenioid fishes recorded during the ROV survey in inshore waters of Terra Nova Bay. (A) Artedidraco skottsbergi; (B) Chionodraco hamatus; (C) Pagetopsis macropterus; (D) Cryodraco atkinsoni; (E) Histiodraco velifer; (F) Trematomus nicolai.
3.3. Fish Biodiversity
The fish community documented during the ROV benthic survey accounted for 12 species of notothenioids, with a total of 116 individuals identified (Table 2). Both evenness and diversity were slightly higher in the shallower transect (i.e., Adélie Cove), whereas the species richness was lower than in the Terra Nova Bay “Canyon” transect due to the higher number of individuals and the same number of species.
4. Discussion
Compared to temperate and tropical marine environments, the extent of near-shore and coastal habitats around the Antarctic continent is currently reduced and highly fragmented, as a consequence of the isostatic depression of the continental shelf produced by the progressive extension of the ice sheet in the late Eocene–early Oligocene [19]. In addition, the near-shore environments are permanently covered by anchor ice and heavily impacted by iceberg scouring, affecting the species composition and population dynamics of local fish assemblages [20]. Despite these environmental constraints, the fish fauna inhabiting inshore waters of the Southern Ocean is extremely diversified and specific for each area in terms of species composition and relative abundance. In several sites located in east Antarctica, the inshore fish fauna is overwhelmingly dominated by the species of the genus Trematomus, and primarily by T. bernacchii [21,22,23,24]. Conversely, the fish fauna inhabiting the coastal waters off the west Antarctica consist mainly of species of the genus Notothenia Richardson, 1844 [25,26].
Remotely operated vehicles (ROV) equipped with still and video cameras to investigate the habitat and behavior of the benthic fish communities have been primarily employed in the Weddell Sea and Lazarev Sea [27,28,29]. On the other hand, ROV surveys have seldom been carried out in the western Ross Sea in proximity of the Italian Base of Terra Nova Bay [30], present study. As a complementary approach to the more conventional sampling methodologies, a ROV survey was conducted down to a depth of 120 m at Tethys Bay, yielding a total of 11 species of notothenioids. In particular, spawning aggregations of mature females of Trematomus eulepidotus Regan, 1914 were observed in shallow waters in spring, whereas a huge number of P. evansii was encountered on muddy sediment in waters deeper than 100 m [30]. Present results complemented previous ROV data [30], providing an overall inventory of 16 species.
The use of photographic equipment mounted on ROV provided new insights into the depth distribution and behavior of some species, also enabling the documentation of species otherwise difficult to be detected with conventional sampling gears. Nevertheless, species exhibiting negative phototaxis behavior or with benthopelagic habits might be underrepresented in this kind of surveying. Compared with the depth range reported for C. atkinsoni (300–800 m) and H. velifer (210–910 m) [31], the observation of both species at depth of ~100 m in Terra Nova Bay considerably extends their vertical distribution. Similarly, a cryopelagic species like T. borchgrevinki commonly associated with the undersurface of the ice down to 70 m [3,31] was documented swimming above the seafloor at 300 m depth. Yet, the record of a gravid female and a sexually mature male of C. hamatus with high first dorsal fin and flashy knobs on the tip of anal fin rays used in nest building [32] clearly indicates the closeness of the spawning season in this species. As reported by previous authors [33,34] and confirmed here, T. bernacchii and P. macropterus laid and guarded their eggs inside the large cup-shaped sponges or on flat stones, respectively. Finally, underwater photography allowed the documentation of a large number of small-sized fishes such as A. skottsbergi and P. evansii, whose catches using passive gears (i.e., nets) were prevented by their sedentary habits and streamlined body morphology, respectively [11,12]. Most of the species encountered during the ROV survey were associated with a rich benthic invertebrate macrofauna, likely representing a refuge from predators and a proper habitat for fish settling, foraging, and spawning activities. Finally, half of the species showed a wide vertical distribution, having been recorded in both transects.
In conclusion, the use of ROV has proven to be a reliable methodology for investigating composition, spatial distribution, and habitat preferences of benthic fish, especially in marine protected areas where the fishing activities are totally forbidden or strictly regulated. It is therefore advisable in the near future to extend the underwater photographic surveys to additional sites and/or depths of Terra Nova Bay, with the aim to provide a more exhaustive picture of the local inshore fish community. It would be highly desirable to encourage the development of similar surveys also in the framework of SCAR ANTOS (Antarctic Near-Shore And Terrestrial Observation System; https://www.scar.org/science/antos/about/ accessed on 20 February 2022) in order to acquire similar data in a variety of sites at the Antarctic continental scale.
Author Contributions
Conceptualization, M.L.M. and S.S.; methodology, S.C.; investigation, S.C., S.S. and P.M.; resources, S.S. and P.M.; data curation, M.L.M.; writing—original draft preparation, M.L.M.; writing—review and editing, S.C., P.M., and S.S.; funding acquisition, P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the GEOSMART (grant No. PNRA2013/AZ2.06, 29 May 2014–29 May 2017) and GRACEFUL (grant No. PNRA16_00069, 11 October 2017–10 October 2020) projects and funded by the Italian National Antarctic Research Program. Authors are grateful to the Italian National Antarctic Scientific Commission (CSNA) and the Italian National Antarctic program (PNRA) for the endorsement of the Special Issue initiative and to the Italian National Antarctic Museum (MNA) for the financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the captain and helmsman of the motor vessel “Malippo” and “Skua” for their expert assistance during the ROV activities. This paper is an Italian contribution to the Commission for the Conservation of Antarctic Marine Living Resources CONSERVATION MEASURE 91-05 (2016) for the Ross Sea region Marine Protected Area, specifically, addressing the priorities of Annex 91-05/C.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- La Mesa, M.; Eastman, J.T.; Vacchi, M. The role of notothenioid fish in the food web of the Ross Sea shelf waters: A review. Polar Biol. 2004, 27, 321–338. [Google Scholar] [CrossRef]
- Eastman, J.T. Antarctic Fish Biology: Evolution in an Unique Environment; Academic Press: San Diego, CA, USA, 1993. [Google Scholar]
- Klingenberg, C.P.; Ekau, W. A combined morphometric and phylogenetic analysis of an ecomorphological trend: Pelagization in Antarctic fishes (Perciformes: Nototheniidae). Biol. J. Linn. Soc. 1996, 59, 143–177. [Google Scholar] [CrossRef]
- Eastman, J.T. The nature of the diversity of Antarctic fishes. Polar Biol. 2005, 28, 93–107. [Google Scholar] [CrossRef]
- DeVries, A.L. The role of antifreeze glycopeptides and peptides in the freezing avoidance of Antarctic fishes. Comp. Biochem. Physiol. B 1988, 90, 611–621. [Google Scholar] [CrossRef]
- Wöhrmann, A.P.A. Freezing resistance in Antarctic fish. In Antarctic Communities: Species, Structure and Survival; Battaglia, B., Valencia, J., Walton, D.W.H., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 209–216. [Google Scholar]
- Eastman, J.T.; Hubold, G. The fish fauna of the Ross Sea, Antarctica. Antarct. Sci. 1999, 11, 293–304. [Google Scholar] [CrossRef]
- Clark, M.R.; Dunn, M.R.; McMillan, P.J.; Pinkerton, M.H.; Stewart, A.; Hanchet, S.M. Latitudinal variation of demersal fish assemblages in the western Ross Sea. Antarct. Sci. 2010, 22, 782–792. [Google Scholar] [CrossRef]
- Donnelly, J.; Torres, J.J.; Sutton, T.T.; Simoniello, C. Fishes of the eastern Ross Sea, Antarctica. Polar Biol. 2004, 27, 637–650. [Google Scholar] [CrossRef]
- Vacchi, M.; Greco, S.; La Mesa, M. Ichthyological survey by fixed gears in Terra Nova Bay (Antarctica). Fish list and first results. Mem. Biol. Mar. Oceanogr. 1991, 19, 197–202. [Google Scholar]
- Vacchi, M.; La Mesa, M.; Tarulli, E. Investigation by fixed gears on ichthyofauna of Terra Nova Bay (Ross Sea, Antarctica). In Atti del 9° Congresso dell’Associazione Italiana di Oceanologia e Limnologia, Santa Margerita Ligure, Italy, 20–23 November 1990; Albertelli, G., Ambrosetti, W., Picazzo, M., Ruffoni Riva, T., Eds.; Lang: Genova, Italy, 1990; pp. 659–664. [Google Scholar]
- Vacchi, M.; La Mesa, M.; Greco, S. The Coastal Fish Fauna of Terra Nova Bay, Ross Sea, Antarctica. In Ross Sea Ecology: Italian Antarctic Expeditions (1987–1995); Faranda, F.M., Guglielmo, L., Ianora, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 457–468. [Google Scholar]
- La Mesa, M.; Cattaneo-Vietti, R.; Vacchi, M. Species composition and distribution of the Antarctic plunderfishes (Pisces, Artedidraconidae) from the Ross Sea off Victoria Land. Deep Sea Res. II 2006, 53, 1061–1070. [Google Scholar] [CrossRef]
- Brooks, C.; Chown, S.L.; Douglass, L.L.; Raymond, B.P.; Shaw, J.D.; Sylvester, Z.T.; Torrens, C.L. Progress towards a representative network of Southern Ocean protected areas. PLoS ONE 2020, 15, e0231361. [Google Scholar] [CrossRef] [Green Version]
- CCAMLR Conservation Measure 91-05: Ross Sea Region Marine Protected Area. 2016. Available online: https://www.ccamlr.org/en/measure-91-05-2016 (accessed on 20 February 2022).
- Castellan, G.; Angeletti, L.; Canese, S.; Mazzoli, C.; Montagna, P.; Schiaparelli, S.; Taviani, M. Visual imaging of benthic carbonate-mixed factories in the Ross Sea Region Marine Protected Area, Antarctica. Minerals 2021, 11, 833. [Google Scholar] [CrossRef]
- Fischer, W.; Hureau, J.C. FAO Species Identification Sheet for Fishery Purposes. Southern Ocean (Fishing Areas 48, 58 and 88) (CCAMLR Convention Area); FAO: Rome, Italy, 1985. [Google Scholar]
- Gon, O.; Heemstra, P.C. Fishes of the Southern Ocean; JLB Smith Institute of Ichthyology: Grahamstown, South Africa, 1990. [Google Scholar]
- Clark, G.F.; Raymond, B.; Riddle, M.J.; Stark, J.S.; Johnston, M.L. Vulnerability of Antarctic shallow invertebrate-dominated ecosystems. Austral Ecol. 2015, 40, 482–491. [Google Scholar] [CrossRef]
- Brenner, M.; Buck, B.H.; Cordes, S.; Dietrich, L.; Jacob, U.; Mintenbeck, K.; Schröder, A.; Brey, T.; Knust, R. The role of iceberg scours in niche separation within the Antarctic fish genus Trematomus. Polar Biol. 2001, 24, 502–507. [Google Scholar]
- Williams, R. The inshore marine fishes of the Vestfold Hills region, Antarctica. Hydrobiologia 1988, 165, 161–167. [Google Scholar] [CrossRef]
- Eastman, J.T.; De Vries, A.L. Buoyancy studies of notothenioid fishes in McMurdo Sound, Antarctica. Copeia 1982, 1982, 385–393. [Google Scholar] [CrossRef]
- Hureau, J.C. Poissons antarctiques récoltés au cours de la onzième expédition française en Terre Adélie (1960–1962). Bull. Mus. Hist. Nat. Paris 1962, 34, 228–238. [Google Scholar]
- Naito, Y.; Iwami, T. Fish fauna in the northeastern parts of Lutzow-Holm Bay with some notes on the stomach contents. Mem. Natn. Inst. Polar Res. Tokyo Spec. Issue 1982, 23, 64–72. [Google Scholar]
- Casaux, R.; Barrera-Oro, E.; Baroni, A.; Ramon, A. Ecology of inshore notothenioid fish from the Danco Coast, Antarctic Peninsula. Polar Biol. 2003, 26, 157–165. [Google Scholar] [CrossRef]
- Daniels, R.A.; Lipps, J.H. Distribution and ecology of fishes of the Antarctic Peninsula. J. Biogeogr. 1982, 9, 1–9. [Google Scholar] [CrossRef]
- Ekau, W.; Gutt, J. Notothenioid fishes from the Weddell Sea and their habitat, observed by underwater photography and television. Proc. NIPR Symp. Polar. Biol. 1991, 4, 36–49. [Google Scholar]
- Gutt, J.; Ekau, W. Habitat partitioning of dominant high Antarctic demersal fish in the Weddell Sea and Lazarev Sea. J. Exp. Mar. Biol. Ecol. 1996, 206, 25–37. [Google Scholar] [CrossRef]
- Gutt, J.; Ekau, W.; Gorny, M. New results on the fish and shrimp fauna of the Weddell Sea and Lazarev Sea (Antarctic). Proc. NIPR Symp. Polar Biol. 1994, 7, 91–102. [Google Scholar]
- Vannini, G.; Bottaro, M.; Bono, R.; Modena, M.; Vacchi, M. First observations on the coastal fish assemblage at Terra Nova Bay (Ross Sea, Antarctica), during the austral springtime. Biol. Mar. Mediterr. 2009, 16, 372–373. [Google Scholar]
- Eastman, J.T. Bathymetric distributions of notothenioid fishes. Polar Biol. 2017, 40, 2077–2095. [Google Scholar] [CrossRef]
- Ferrando, S.; Castellano, L.; Gallus, L.; Ghigliotti, L.; Masini, M.A.; Pisano, E.; Vacchi, M. A demonstration of nesting in two Antarctic icefish (genus Chionodraco) using a fin dimorphism analysis and ex situ videos. PLoS ONE 2014, 9, e90512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, C.A. Observations on food and reproduction in Trematomus bernacchii (Pisces: Nototheniidae) from the Palmer Archipelago, Antarctica. Copeia 1980, 1980, 171–173. [Google Scholar] [CrossRef]
- La Mesa, M.; Piepenburg, D.; Pineda-Metz, S.; Riginella, E.; Eastman, J.T. Spatial distribution and habitat preferences of demersal fish assemblages in the southeastern Weddell Sea (Southern Ocean). Polar Biol. 2019, 42, 1025–1040. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).