Do Weeds Hinder the Establishment of Native Plants on a Reclaimed North American Boreal Mine Site?
Abstract
1. Introduction
2. Materials and Methods
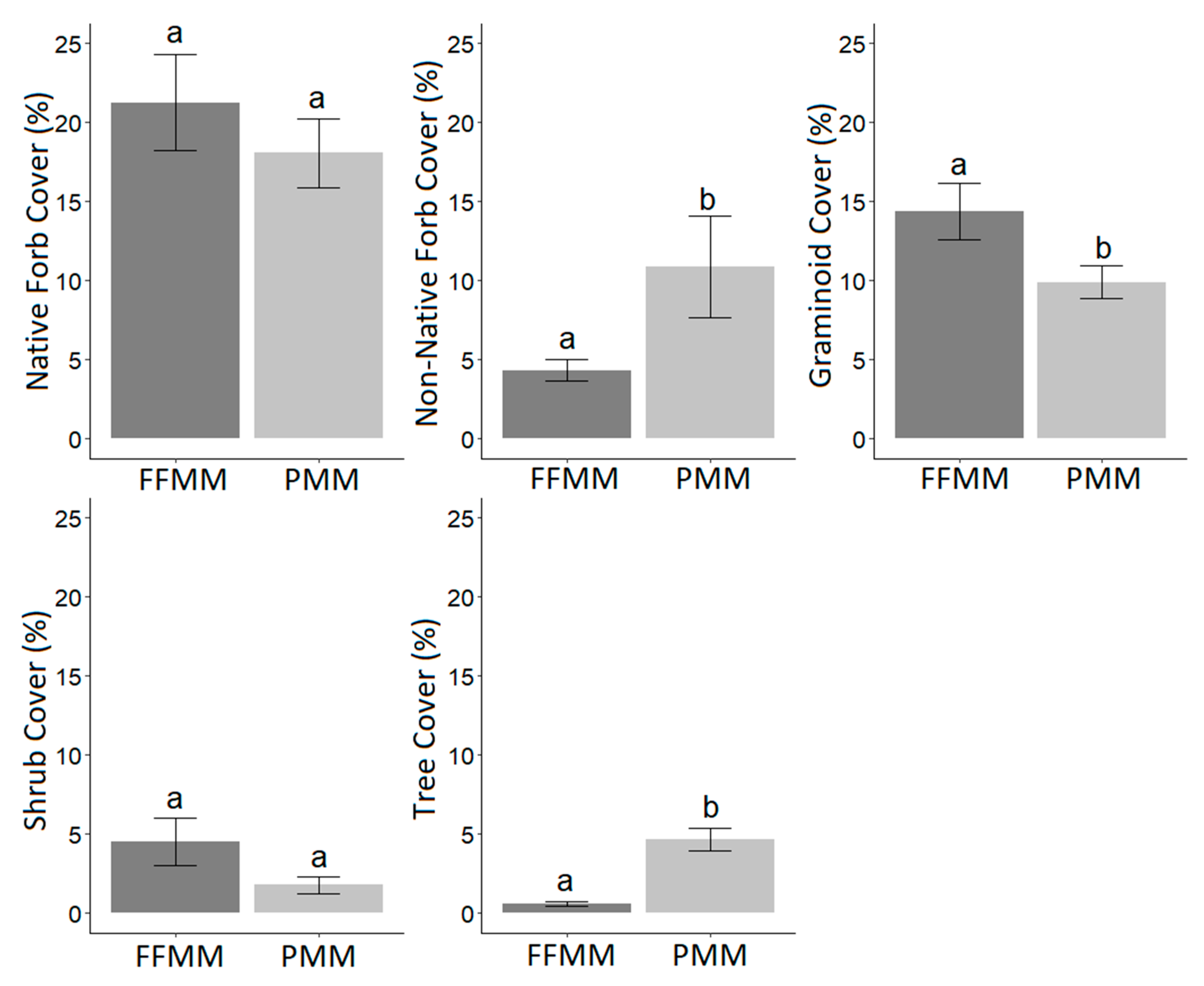
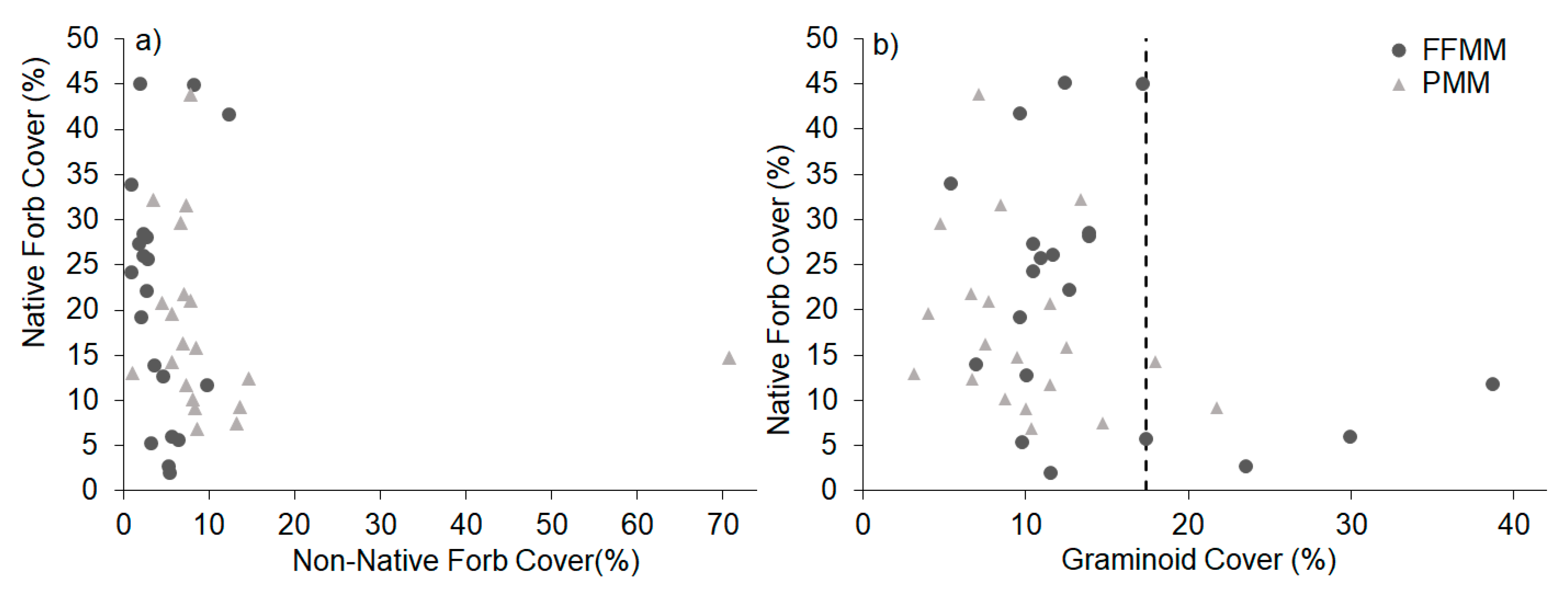
3. Results
4. Discussion
5. Management Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cole, D.E.; King, J.R.; Oyarzun, D.A.; Dietzler, T.H.; McClay, A. Experiences with invasive plant management and ecology in Alberta. Can. J. Plant Sci. 2007, 87, 1013–1022. [Google Scholar] [CrossRef]
- Small, C.; Degenhardt, D.; Drozdowski, B.; Thacker, S.; Powter, C.; Schoonmaker, A.; Schreiber, S. Optimizing Weed Control for Progressive Reclamation: Literature Review; Canada’s Oil Sands Innovation Alliance: Calgary, AB, Canada, 2018; 48p. [Google Scholar]
- Luck, L.; Bellairs, S.M.; Rossiter-Rachor, N.A. Residual herbicide treatments reduce Andropogon gayanus (Gamba Grass) recruitment for mine site restoration in northern Australia. Ecol. Manag. Restor. 2019, 20, 214–221. [Google Scholar] [CrossRef]
- Franke, M.E.; Zipper, C.; Barney, J.N. Native hardwood tree seedling establishment following invasive autumn-olive (elaeagnus umbellata) removal on a reclaimed coal mine. Invasive Plant Sci. Manag. 2018, 11, 155–161. [Google Scholar] [CrossRef]
- Holmes, P.M. Shrubland restoration following woody alien invasion and mining: Effects of topsoil depth, seed source, and fertilizer addition. Restor. Ecol. 2001, 9, 71–84. [Google Scholar] [CrossRef]
- González-Muñoz, N.; Costa-Tenorio, M.; Espigares, T. Invasion of alien Acacia dealbata on Spanish Quercus robur forests: Impact on soils and vegetation. For. Ecol. Manag. 2012, 269, 214–221. [Google Scholar] [CrossRef]
- Alberta Environment. Guidelines for Reclamation to Forest Vegetation in the Athabasca Oil Sands Region, 2nd ed.; Terrestrial Subgroup of the Reclamation Working Group of the Cumulative Environmental Management Association: Fort McMurray, AB, Canada, 2010. [Google Scholar]
- Baker, H.G. The evolution of weeds. Annu. Rev. Ecol. Syst. 1974, 5, 1–24. [Google Scholar] [CrossRef]
- Espeland, E.; Perkins, L. Weed establishment and persistence after water pipeline installation and reclamation in the mixed grass prairie of Western North Dakota. Ecol. Restor. 2017, 35, 303–310. [Google Scholar] [CrossRef]
- Errington, R.C.; Pinno, B.D. Early successional plant community dynamics on a reclaimed oil sands mine in comparison with natural boreal forest communities. Ecoscience 2015, 22, 133–144. [Google Scholar] [CrossRef]
- Mackenzie, D.D.; Naeth, M.A. The role of the forest soil propagule bank in assisted natural recovery after oil sands mining. Restor. Ecol. 2009, 18, 418–427. [Google Scholar] [CrossRef]
- Gingras-Hill, T.; Nwaishi, F.C.; Macrae, M.; Price, J.S.; Petrone, R.M. Ecohydrological functioning of an upland undergoing reclamation on post-mining landscape of the Athabasca oil sands region, Canada. Ecohydrology 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Cavieres, L.; Arroyo, M.T.K.; Peñaloza, A.; Molina-Montenegro, M.; Torres, C. Nurse effect of Bolax gummifera cushion plants in the alpine vegetation of the Chilean Patagonian Andes. J. Veg. Sci. 2002, 13, 547–554. [Google Scholar] [CrossRef]
- Bruno, J.F.; Stachowicz, J.J.; Bertness, M.D. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 2003, 18, 119–125. [Google Scholar] [CrossRef]
- Jordan, N.; Larson, D.L.; Huerd, S.C. Soil modification by invasive plants: Effects on native and invasive species of mixed-grass prairies. Biol. Invasions 2007, 10, 177–190. [Google Scholar] [CrossRef]
- Maestre, F.T.; Bautista, S.; Cortina, J.; Bellot, J. Potential for using facilitation by grasses to establish shrubs on a semiarid degraded steppe. Ecol. Appl. 2001, 11, 1641–1655. [Google Scholar] [CrossRef]
- Alberta Environment and Water. Best Management Practices for Conservation of Reclamation Materials in the Mineable Oil Sands Region of Alberta; Province of Alberta: Edmonton, AB, Canada, 2012; pp. 1–161. [Google Scholar]
- Natural Regions Committee. Natural Regions and Subregions of Alberta; Natural Regions Committee: Edmonton, AB, Canada, 2006.
- Canadian Climate Normals 1981–2010 Station Data. Available online: https://climate.weather.gc.ca/climate_normals/results_1981_2010_e.html?searchType=stnProv&lstProvince=AB&txtCentralLatMin=0&txtCentralLatSec=0&txtCentralLongMin=0&txtCentralLongSec=0&stnID=2704&dispBack=0 (accessed on 22 April 2020).
- Pinno, B.D.; Errington, R.C. Maximizing natural trembling aspen seedling establishment on a reclaimed boreal oil sands site. Ecol. Restor. 2015, 33, 43–50. [Google Scholar] [CrossRef]
- Pinno, B.D.; Hawkes, V.C. Temporal trends of ecosystem development on different site types in reclaimed boreal forests. Forests 2015, 6, 2109–2124. [Google Scholar] [CrossRef]
- Tannas, K.E. Common Plants of the Western Rangelands—Volume 3: Forbs, 1st ed.; Alberta Agriculture, Food and Rural Development: Lethbridge, AB, Canada, 2004. [Google Scholar]
- Tannas, K.E. Common Plants of the Western Rangelands—Volume 1: Grasses and Grass-Like Species, 1st ed.; Alberta Agriculture, Food and Rural Development: Lethbridge, AB, Canada, 2001. [Google Scholar]
- Moss, E.H.; Packer, J. Flora of Alberta, 2nd ed.; University of Toronto Press: Toronto, ON, Canada, 1994. [Google Scholar]
- Tannas, K.E. Common Plants of the Western Rangelands—Volume 2: Trees and Shrubs, 1st ed.; Alberta Agriculture, Food and Rural Development: Lethbridge, AB, Canada, 2003. [Google Scholar]
- United States Department of Agriculture. Available online: https://plants.sc.egov.usda.gov/adv_search.html (accessed on 7 December 2019).
- de Bortoli, L.A.; Pinno, B.D.; Mackenzie, M.D.; Li, E.H. Plant community composition and tree seedling establishment in response to seeding and weeding treatments on different reclamation cover soils. Can. J. For. Res. 2019, 49, 836–843. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Snively, A.E.K.; Fair, J.M.; Landhausser, S.M. Early trajectories of forest understory development on reclamation sites: Influence of forest floor placement and a cover crop. Restor. Ecol. 2015, 23, 698–706. [Google Scholar] [CrossRef]
- Lieffers, V.J.; Macdonald, S.E.; Hogg, E.H. Ecology of and control strategies for Calamagrostis canadensis in boreal forest sites. Can. J. For. Res. 1993, 23, 2070–2077. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Lieffers, V.J. Rhizome plasticity and clonal foraging of Calamagrostis canadensis in response to habitat heterogeneity. J. Ecol. 1993, 81, 769–776. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Mulak, T.L.; Lieffers, V.J. The effect of roots and litter of Calamagrostis canadensis on root sucker regeneration of Populus tremuloides. Forestry 2007, 80, 481–488. [Google Scholar] [CrossRef]
- Landhausser, S.M.; Stadt, K.J.; Lieffers, V.J. Screening for control of a forest weed: Early competition between three replacement species and Calamagrostis canadensis of Picea glauca. J. Appl. Ecol. 1996, 33, 1517. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Fenniak, T.E. Understory plant communities of boreal mixedwood forests in western Canada: Natural patterns and response to variable-retention harvesting. For. Ecol. Manag. 2007, 242, 34–48. [Google Scholar] [CrossRef]
- Pinno, B.D.; Sherr, I.; Errington, R.C.; Shea, K. Islands – Soil Patches and Plant Community Dynamics on a New Oil Sands Reclamation Design. J. Am. Soc. Min. Reclam. 2016, 5, 28–44. [Google Scholar] [CrossRef]
- Package ‘lmperm’. Available online: https://cran.r-project.org/web/packages/lmPerm/lmPerm.pdf (accessed on 7 December 2019).
- Package ‘mvpart’. Available online: https://mran.microsoft.com/snapshot/2014-12-11/web/packages/mvpart/mvpart.pdf (accessed on 7 December 2019).
- Foster, B.L.; Tilman, D. Dynamic and static views of succession: Testing the descriptive power of the chronosequence approach. Plant Ecol. 2000, 146, 1–10. [Google Scholar] [CrossRef]
- Alday, J.G.; Pallavicini, Y.; Marrs, R.H.; Martínez-Ruiz, C. Functional groups and dispersal strategies as guides for predicting vegetation dynamics on reclaimed mines. Plant Ecol. 2011, 212, 1759–1775. [Google Scholar] [CrossRef]
- Spellman, K.V.; Schneller, L.C.; Mulder, C.P.H.; Carlson, M.L. Effects of non-native Melilotus albus on pollination and reproduction in two boreal shrubs. Oecologia 2015, 179, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Wedin, D. Dynamics of nitrogen competition between successional grasses. Ecology 1991, 72, 1038–1049. [Google Scholar] [CrossRef]
- MacDougall, A.S.; Turkington, R. Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 2005, 86, 42–55. [Google Scholar] [CrossRef]
- Dhar, A.; Comeau, P.G.; Karst, J.; Pinno, B.D.; Chang, S.X.; Naeth, A.M.; Vassov, R.; Bampfylde, C. Plant community development following reclamation of oil sands mine sites in the boreal forest: A review. Environ. Rev. 2018, 298, 1–13. [Google Scholar] [CrossRef]
- Plants Database: Sonchus arvensis, L. Field Sowthistle. Available online: https://plants.usda.gov/core/profile?symbol=SOAR2 (accessed on 7 December 2019).
- Turkington, R.A.; Cavers, P.B.; Rempel, E. The biology of Canadian weeds. 29. Melilotus alba Desr. and M. offtcinalis (L.) Lam. ROY. Can. J. Planr Sci. 1978, 58, 523–537. [Google Scholar] [CrossRef]
- Provincially Regulated Weeds. Available online: https://www.alberta.ca/provincially-regulated-weeds.aspx (accessed on 7 December 2019).
- Trepanier, K.E.; Pinno, B.D.; Errington, R.C. Dominant drivers of plant community assembly vary by soil type and time in reclaimed forests. Plant Ecol. 2020. [Google Scholar] [CrossRef]
- Brown, C.S.; Bugg, R.L. Effects of established perennial grasses on introduction of native forbs in California. Restor. Ecol. 2001, 9, 38–48. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Lieffers, V.J. Growth of Populus tremuloides in association with Calamagrostis canadensis. Can. J. For. Res. 1998, 28, 396–401. [Google Scholar] [CrossRef]
- Henkel-Johnson, D.; Macdonald, S.E.; Bork, E.W.; Thomas, B.R. Influence of weed composition, abundance, and spatial proximity on growth in young hybrid poplar plantations. For. Ecol. Manag. 2016, 362, 55–68. [Google Scholar] [CrossRef]
- Strong, W.L. Vegetation development on reclaimed lands in the Coal Valley Mine of western Alberta, Canada. Can. J. Bot. 2000, 78, 110–118. [Google Scholar] [CrossRef]
- Audet, P.; Pinno, B.D.; Thiffault, E. Reclamation of boreal forest after oil sands mining: Anticipating novel challenges in novel environments. Can. J. For. Res. 2015, 45, 364–371. [Google Scholar] [CrossRef]
- Hart, S.A.; Chen, H.Y.H. Understory vegetation dynamics of North American boreal forests. CRC. Crit. Rev. Plant Sci. 2006, 25, 381–397. [Google Scholar] [CrossRef]
- Cole, R.J.; Holl, K.D.; Zahawi, R.A. Seed rain under tree islands planted to restore degraded lands in a tropical agricultural landscape. Ecol. Appl. 2010, 20, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, A.; Jolliffe, P. Indices of plant competition. J. Ecol. 2003, 91, 707–720. [Google Scholar] [CrossRef]
| FFMM | PMM | p | ||
|---|---|---|---|---|
| Soil | Soil Temperature (°C) | 14.1 (2.2) | 15.4 (1.4) | 0.090 |
| VWC (%) | 16.5 (4.4) | 27.0 (6.4) | <0.001 | |
| pH | 6.9 (0.7) | 6.5 (0.9) | 0.176 | |
| Average Species Richness Per 1 m2 Quadrat | Tree | 1 | 2 | <0.001 |
| Native Forb | 10 | 9 | 0.118 | |
| Non-Native Forb | 3 | 4 | 0.270 | |
| Graminoid | 5 | 5 | 1 | |
| Shrub | 2 | 1 | 0.980 |
| FFMM | PMM | |||
|---|---|---|---|---|
| Functional Group | Species | Average Cover Per Plot (%) | Species | Average Cover Per Plot (%) |
| Non-Native Forbs | Sonchus arvensis | 2.6 | Melilotus alba | 4.4 |
| Melilotus alba | <1.0 | Sonchus arvensis | 3.7 | |
| Taraxacum officinale | <1.0 | Crepis tectorum | 1.5 | |
| Crepis tectorum | <1.0 | Taraxacum officinale | <1.0 | |
| Native Forbs | Chamerion angustifolium | 6.2 | Chamerion angustifolium | 6.5 |
| Equisetum arvense | 3.9 | Equisetum arvense | 5.8 | |
| Lathyrus ochroleucus | 1.9 | Achillea millefolium | 1.2 | |
| Fragaria virginiana | 1.7 | Lathyrus ochroleucus | <1.0 | |
| Graminoids | Calamagrostis canadensis | 6.8 | Calamagrostis canadensis | 5.3 |
| Poa pratensis | 2.7 | Agropyron trachycaulum | 1.0 | |
| Agropyron trachycaulum | 2.5 | Carex spp. | <1.0 | |
| Leymus innovatus | 1.3 | Poa pratensis | <1.0 | |
| Shrubs | Rubus idaeus | 3.7 | Salix spp. | 1.0 |
| Rosa acicularis | <1.0 | Rubus idaeus | <1.0 | |
| Cornus sericea | <1.0 | Rosa acicularis | <1.0 | |
| Ribes oxyacanthoides | <1.0 | Shepherdia canadensis | <1.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trepanier, K.E.; Burton, B.; Pinno, B.D. Do Weeds Hinder the Establishment of Native Plants on a Reclaimed North American Boreal Mine Site? Diversity 2021, 13, 76. https://doi.org/10.3390/d13020076
Trepanier KE, Burton B, Pinno BD. Do Weeds Hinder the Establishment of Native Plants on a Reclaimed North American Boreal Mine Site? Diversity. 2021; 13(2):76. https://doi.org/10.3390/d13020076
Chicago/Turabian StyleTrepanier, Kaitlyn E., Brea Burton, and Bradley D. Pinno. 2021. "Do Weeds Hinder the Establishment of Native Plants on a Reclaimed North American Boreal Mine Site?" Diversity 13, no. 2: 76. https://doi.org/10.3390/d13020076
APA StyleTrepanier, K. E., Burton, B., & Pinno, B. D. (2021). Do Weeds Hinder the Establishment of Native Plants on a Reclaimed North American Boreal Mine Site? Diversity, 13(2), 76. https://doi.org/10.3390/d13020076






