Abstract
A novel spiro-pyrrolothiazole derivative bearing a 2-oxindole substituent was synthesized and characterized. The compound was prepared via a catalyst-free reaction under mild conditions and isolated using a straightforward workup procedure. The structure of the synthesized title compound was confirmed with 1H, 13C NMR spectra and X-Ray diffraction data.
1. Introduction
The oxindole scaffold is pharmacologically privileged, exhibiting a wide range of medicinally relevant properties. Its structural simplicity and prevalence in plant-based alkaloids have further cemented its status in modern drug discovery. Derivatives of oxindole are pharmacophoric fragments of synthetic drugs with a broad spectrum of action and are also actively used as agricultural chemicals [1,2,3,4,5,6,7]. Indolidan is used for the treatment of heart failure [8]; Ropinirole acts as a dopamine receptor agonist for Parkinson’s disease [9]. Beyond human medicine, the oxindole derivative AHO is known to induce systemic acquired resistance in plants [10,11]. The oxindole alkaloid Convolutamydine A and its structural analogs have anti-inflammatory properties [12] (Figure 1).
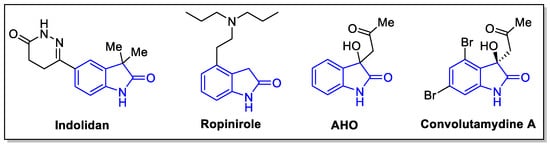
Figure 1.
Bioactive natural products with oxindole core.
We previously investigated the reaction of polyelectrophilic pyrrolobenzoxazinetriones with aldehyde thiosemicarbazones, resulting in the formation of spiro-pyrrolothiazoles with antinociceptive and antimicrobial activity [13,14]. Continuing our work, we introduced isatin-β-thiosemicarbazone into the reaction to obtain a promising derivative with an oxindole motif.
Herein, we report the synthesis and structural characterization of the new (Z)-8-hydroxy-6-(2-hydroxyphenyl)-9-(4-methylbenzoyl)-2-(((E)-2-oxoindolin-3-ylidene)hydrazineylidene)-1-thia-3,6-diazaspiro[4.4]non-8-ene-4,7-dione.
2. Results and Discussion
The initial benzoxazin-2-one 2 was prepared by reacting the methyl ester of p-toluoylpyruvic acid 1 with o-aminophenol. Next, compound 2 and oxalyl chloride in anhydrous chloroform were heated under reflux to give pyrrolobenzoxazinetrione 3 following the established general procedure [15] (Scheme 1).
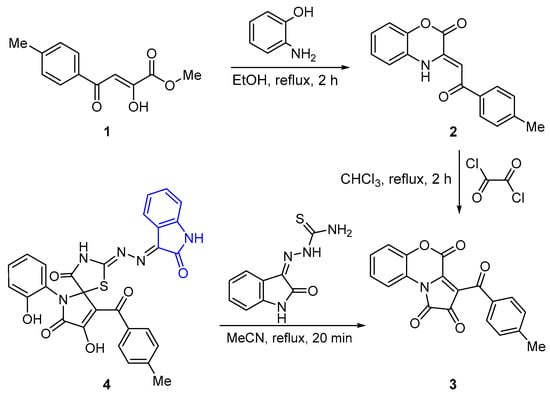
Scheme 1.
Synthesis of title compound 4.
Synthesis of the target oxindole-containing spiro-pyrrolothiazole 4 was achieved through the reaction of pyrrolobenzoxazinetrione 3 with isatin-β-thiosemicarbazone in 90% yield. The reaction proceeded optimally under reflux in anhydrous acetonitrile for 20 min (Scheme 1).
The formation of compound 4 is proposed to commence with nucleophilic addition of the thione sulfur from isatin-β-thiosemicarbazone to the electrophilic C3a position of pyrrolobenzoxazinetrione 3. This is followed by cyclization of the thiazole ring through an intramolecular attack by the N4H2 group on the lactone carbonyl of the benzoxazinone ring, resulting in ring opening at the C4–O5 bond (Scheme 2).
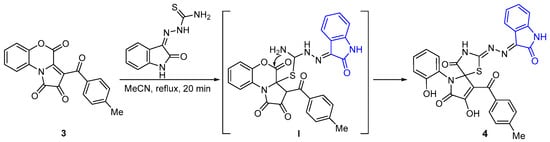
Scheme 2.
Possible scheme for the formation of compound 4.
The structure of compound 4 was confirmed based on 1H, 13C NMR spectroscopy (see Supplementary Materials). The 1H NMR spectrum of 4 showed signals of aromatic protons. Comparing with the 1H NMR spectra of products with aldehyde thiosemicarbazones [13], we identified a singlet of the amide NH group of oxindole fragment at 10.67 ppm. In addition, a singlet of the methyl group at 2.39 ppm, a singlet at 9.75 ppm and very broadened singlets at 12.96 and 4.18 ppm were detected.
In the 13C NMR spectrum of compound 4, three carbon signals (at 77.7, 158.9, and 145.6 ppm) were broadened, and two expected signals were not observed. The ambiguity of the spectral data, indicative of tautomeric equilibria (keto-enol and/or ring-chain tautomerism involving carbon–sulfur bond dissociation), necessitated the growth of single crystals for structural elucidation.
The structure of compound 4 was unambiguously confirmed by single-crystal X-ray diffraction analysis (CCDC 2504136) (Figure 2). The compound 4, solvated with disordered DMSO and H2O, crystallizes in the centrosymmetric space group of P–1 as a racemate.
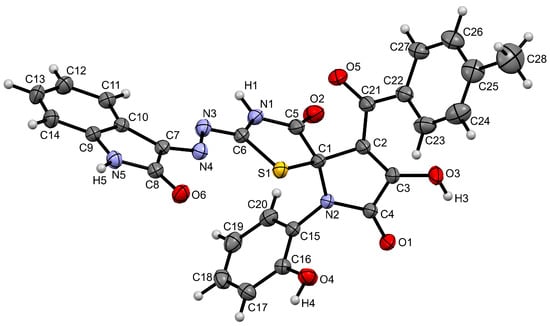
Figure 2.
Structure of compound 4 showing 30% probability amplitude displacement ellipsoids. DMSO and H2O solvate molecules are omitted for clarity.
Overall, the molecular geometry of compound 4 is similar to that of related spiro compounds derived from aldehyde thiosemicarbazones (CSD Refcodes: XOKLAN and XOKLER) [13]. Similarly to the o-hydroxybenzaldehyde thiosemicarbazone derivative (XOKLER), compound 4 features nearly coplanar thiazole, azine, and isatin moieties, indicating favorable conjugation, which contrasts with the bulkier o-nitrobenzaldehyde derivative (XOKLAN).
The packing patterns of all three related spiro compounds involve the formation of centrosymmetric dimers through hydrogen bonds between the hydroxypyrrolone moieties (Table 1, Figure 3). In the structure of 4, these dimers are further connected into a two-dimensional network via hydrogen bonds involving DMSO and water molecules.

Table 1.
Hydrogen bond geometry.
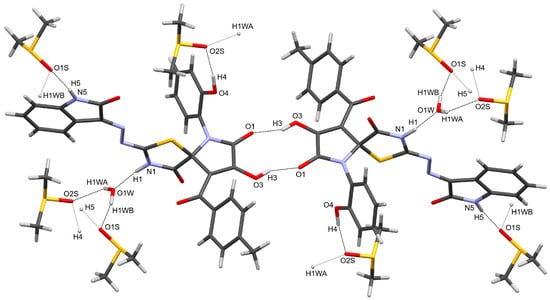
Figure 3.
Centrosymmetric dimer formation in compound 4. Minor disordered components of DMSO molecules are omitted for clarity.
3. Materials and Methods
3.1. General Experimental Information
Acetonitrile was dried over activated 4Å molecular sieves. Synthesis of isatin-β-thiosemicarbazone was performed by reaction of thiosemicarbazide with isatine in ethanol as described in [16]. Pyrrolobenzoxazinetrione 3 was synthesized as described in [15]. Melting points were measured with Mettler Toledo MP70 Melting Point apparatus (Schwerzenbach, Switzerland). 1H and 13C NMR spectra were recorded on a Bruker Avance III HD spectrometer (Faellanden, Switzerland) (at 400, 101 MHz, respectively) at 40 °C (313K) or 30 °C (303K) in DMSO-d6 using the residual solvent peak (DMSO-d6: δH = 2.50 ppm, δC = 39.52 ppm) as internal standards. Elemental analysis was performed on an analyzer vario Micro cube (Langenselbold, Germany).
3.2. Preparation of the Title Compound
A 25 mL round-bottom flask was charged with 3-(4-methylbenzoyl)-1H-benzo[b]pyrrolo[1,2-d][1,4]oxazine-1,2,4-trione (167 mg, 0.5 mmol, 1 equiv.), 2-(2-oxoindolin-3-ylidene)hydrazine-1-carbothioamide (110 mg, 0.5 mmol, 1 equiv.) and anhydrous acetonitrile (10.0 mL). After 20 min, having achieved complete conversion of compound 3 as judged by TLC analysis (the violet suspension of starting substrate 3 discolored within a few minutes); the mixture was cooled to rt. The resulting precipitate was filtered and washed with hexane to give (Z)-8-hydroxy-6-(2-hydroxyphenyl)-9-(4-methylbenzoyl)-2-(((E)-2-oxoindolin-3-ylidene)hydrazineylidene)-1-thia-3,6-diazaspiro[4.4]non-8-ene-4,7-dione (249 mg, 90% yield).
Orange solid. m.p. 241–243 °C.
1H NMR (400 MHz, DMSO-d6) δ 12.96 (br.s, 1H), 10.67 (s, 1H, NHoxindole), 9.75 (s, 1H), 7.75–7.69 (m, 2H), 7.40–7.26 (m, 5H), 7.04–6.92 (m, 3H), 6.87 (td, J = 7.6, 1.4 Hz, 1H), 6.81 (d, J = 7.8 Hz, 1H), 2.39 (s, 3H, Me). One of the protons was presumably located in the broadened peak at 4.18 ppm for 1H NMR. 13C NMR (101 MHz, DMSO-d6) δ 187.8 (C=Oketone), 164.9 (C=O), 158.9, 154.8, 153.0, 145.6, 143.42, 143.37, 134.8, 132.6, 130.8, 129.0 (2C), 128.9, 128.7 (2C), 121.9, 121.4, 120.2, 120.1, 119.4, 117.09, 117.05, 110.4, 77.7 (Cspiro), 21.1 (Me). Two carbons were extremely broad and invisible for 13C NMR. Anal. Calcd For C28H19N5O6S: C, 60.75; H, 3.46; N, 12.65; S, 5.79. Found: C, 60.98; H, 3.44; N, 12.68; S, 5.52.
3.3. X-Ray Crystallography
The single-crystal X-ray analysis of compound 4 was performed on an Xcalibur Ruby diffractometer (Agilent Technologies, Wroclaw, Poland) using Mo X-ray source (MoKα 0.71073 Å), scanning at 295(2) K. An empirical absorption correction was introduced via the multi-scan method using the SCALE3 ABSPACK algorithm [17]. Using OLEX2 [18], the structure was solved with the Superflip [19] program and refined by a full-matrix least-squares minimization in the anisotropic approximation of all non-hydrogen atoms with the SHELXL [20] program. Hydrogen atoms bound to carbon were positioned geometrically and refined using a riding model. Hydrogen atoms of NH and OH groups were refined independently with isotropic displacement parameters. Crystal structure of compound 4 was deposited at the Cambridge Crystallographic Data Centre with the deposition number CCDC 2504136. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures (accessed on 20 December 2025).
Crystal Data of compound 4. C28H19N5O6S∙2(C2H6OS)∙H2O, M = 727.81, triclinic, space group P–1, a = 8.4804(10) Å, b = 12.1040(14) Å, c = 17.6848(17) Å, α = 87.773(9) °, β = 84.772(9) °, γ = 75.345(10) °, V = 1748.7(3) Å3, T = 295(2) K, Z = 2, μ(Mo Kα) = 0.272 mm−1. The final refinement parameters: R1 = 0.0622 (for observed 5074 reflections with I > 2σ(I)); wR2 = 0.1729 (for all independent 8148 reflections, Rint = 0.0518), S = 1.023.
4. Conclusions
In summary, we developed the efficient synthesis of a novel spiro-pyrrolothiazole derivative. The target molecule, (Z)-8-hydroxy-6-(2-hydroxyphenyl)-9-(4-methylbenzoyl)-2-(((E)-2-oxoindolin-3-ylidene)hydrazineylidene)-1-thia-3,6-diazaspiro[4.4]non-8-ene-4,7-dione, was successfully obtained. Its structure was unambiguously confirmed by 1H NMR, 13C NMR spectra and X-ray data. This scaffold holds significant promise as a functional core structure for the development of novel biological agents and may also find important applications in agricultural chemistry.
Supplementary Materials
The following supporting information can be downloaded: 1H NMR and 13C NMR spectra of compound 4.
Author Contributions
Conceptualization, I.V.M.; methodology, D.N.B.; validation, D.N.B. and M.V.D.; formal analysis, M.V.D.; investigation, D.N.B. (synthesis), M.V.D. (X-ray analysis); data curation, I.V.M.; writing—original draft preparation, D.N.B.; writing—review and editing, D.N.B. and M.V.D.; visualization, D.N.B.; supervision, I.V.M.; project administration, I.V.M.; funding acquisition, M.V.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was performed with financial support of the Ministry of Science and Higher Education of the Russian Federation (FSNF-2025-0013).
Data Availability Statement
The data are contained within this article and the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Khetmalis, Y.M.; Shivani, M.; Murugesan, S.; Chandra Sekhar, K.V.G. Oxindole and Its Derivatives: A Review on Recent Progress in Biological Activities. Biomed. Pharmacother. 2021, 141, 111842. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Monga, Y.; Gupta, A.; Singh, S. 2-Oxindole and Related Heterocycles: Synthetic Methodologies for Their Natural Products and Related Derivatives. RSC Adv. 2023, 13, 14249–14267. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, M.A.; Hayallah, A.M.; Bukhari, S.N.A.; Musa, A.; Elmowafy, M.; Abdel-Rahman, H.M.; Abd El-Gaber, M.K. Design, Synthesis, Molecular Modeling, and Anticancer Evaluation of New VEGFR-2 Inhibitors Based on the Indolin-2-One Scaffold. Pharmaceuticals 2022, 15, 1416. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, M.; Hashemi, M.; Eskandarpour, V.; Zarghi, A.; Hadizadeh, F.; Ghodsi, R. New Indolin-2-Ones, Possessing Sunitinib Scaffold as HDAC Inhibitors and Anti-Cancer Agents with Potential VEGFR Inhibition Activity; Design, Synthesis and Biological Evaluation. Bioorg. Chem. 2025, 156, 108231. [Google Scholar] [CrossRef] [PubMed]
- Jhagta, C.; Singh, M. Oxindole Derivatives as Multi-Target Agents for Alzheimer’s Disease: A Promising Therapeutic Strategy. ChemistrySelect 2025, 10, e00743. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, S.; Chen, W.; Du, T.; Wang, D.; Wang, Z.; Chen, J. Recent Advances and Prospects of Indole Derivatives in the Discovery of New Agricultural Chemicals. J. Agric. Food Chem. 2025, 73, 22979–22993. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Yang, X.; Shi, S.; Bai, L.; Hu, D.; Song, R.; Song, B. 3-Hydroxy-2-Oxindole Derivatives Containing Sulfonamide Motif: Synthesis, Antiviral Activity, and Modes of Action. J. Agric. Food Chem. 2023, 71, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.W.; Krushinski, J.H.; Beedle, E.E.; Wyss, V.; Pollock, G.D.; Wilson, H.; Kauffman, R.F.; Hayes, J.S. Dihydropyridazinone Cardiotonics. The Discovery and Inotropic Activity of 1,3-Dihydro-3,3-Dimethyl-5-(1,4,5,6-Tetrahydro-6-Oxo-3-Pyridazinyl)-2H-Indol-2-One. J. Med. Chem. 1986, 29, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.H.; Sethi, K.D.; Hauser, R.A.; Davis, T.L.; Hammerstad, J.P.; Bertoni, J.; Taylor, R.L.; Sanchez-Ramos, J.; O’Brien, C.F.; Ropinirole Study Group. Ropinirole for the Treatment of Early Parkinson’s Disease. Neurology 1997, 49, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Jia, Y.; Shen, Y.; He, H.; Fang, R.; Chen, X.; Hao, X. 3-Acetonyl-3-hydroxyoxindole: A New Inducer of Systemic Acquired Resistance in Plants. Plant Biotechnol. J. 2008, 6, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Hao, X. Natural Product Sciences: An Integrative Approach to the Innovations of Plant Natural Products. Sci. China Life Sci. 2020, 63, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.D.; Zardo, R.S.; Figueiredo, G.S.M.; Silva, B.V.; Pinto, A.C. Anti-Inflammatory Properties of Convolutamydine A and Two Structural Analogues. Life Sci. 2014, 116, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Lukmanova, D.N.; Prikhod’ko, Y.I.; Dmitriev, M.V.; Mashevskaya, I.V.; Maslivets, A.N. Synthesis of Spiro[Pyrrole-2,5′-Thiazoles] by Heterocyclization of Pyrrolobenzoxazinetriones with Aromatic Aldehyde Thiosemicarbazones. Russ. J. Org. Chem. 2019, 55, 108–114. [Google Scholar] [CrossRef]
- Lukmanova, D.N.; Balandina, S.Y.; Makhmudov, R.R.; Mashevskaya, I.V. Antinociceptive and Antimicrobial Activity of Products from Reactions of Pyrrolobenzoxazinetriones with Thiosemicarbazones of Aromatic and Heteroaromatic Aldehydes. Pharm. Chem. J. 2020, 54, 236–240. [Google Scholar] [CrossRef]
- Maslivets, A.N.; Mashevskaya, I.V.; Krasnykh, O.P.; Shurov, S.N.; Andreichikov, Y.S. 5-membered 2, 3-dioxoheterocycles. 33. Synthesis of 3-aroyl-1,2-dihydro-4H-pyrrolo-[5.1-c][1,4]benzoxazine-1,2,4-triones and their interaction with water and alcohols. Zhurn. Org. Khim 1992, 28, 2545–2553. [Google Scholar]
- Hernándeza, W.; Paz, J.; Vaisberg, A.; Spodine, E.; Richter, R.; Beyer, L. Synthesis, Characterization, and In Vitro Cytotoxic Activities of Benzaldehyde Thiosemicarbazone Derivatives and Their Palladium (II) and Platinum (II) Complexes against Various Human Tumor Cell Lines. Bioinorg. Chem. Appl. 2008, 2008, 690952. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro, Version 1.171.37.33; Agilent Technologies: Wroclaw, Poland, 2014. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 16 November 2025).
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. Superflip—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.