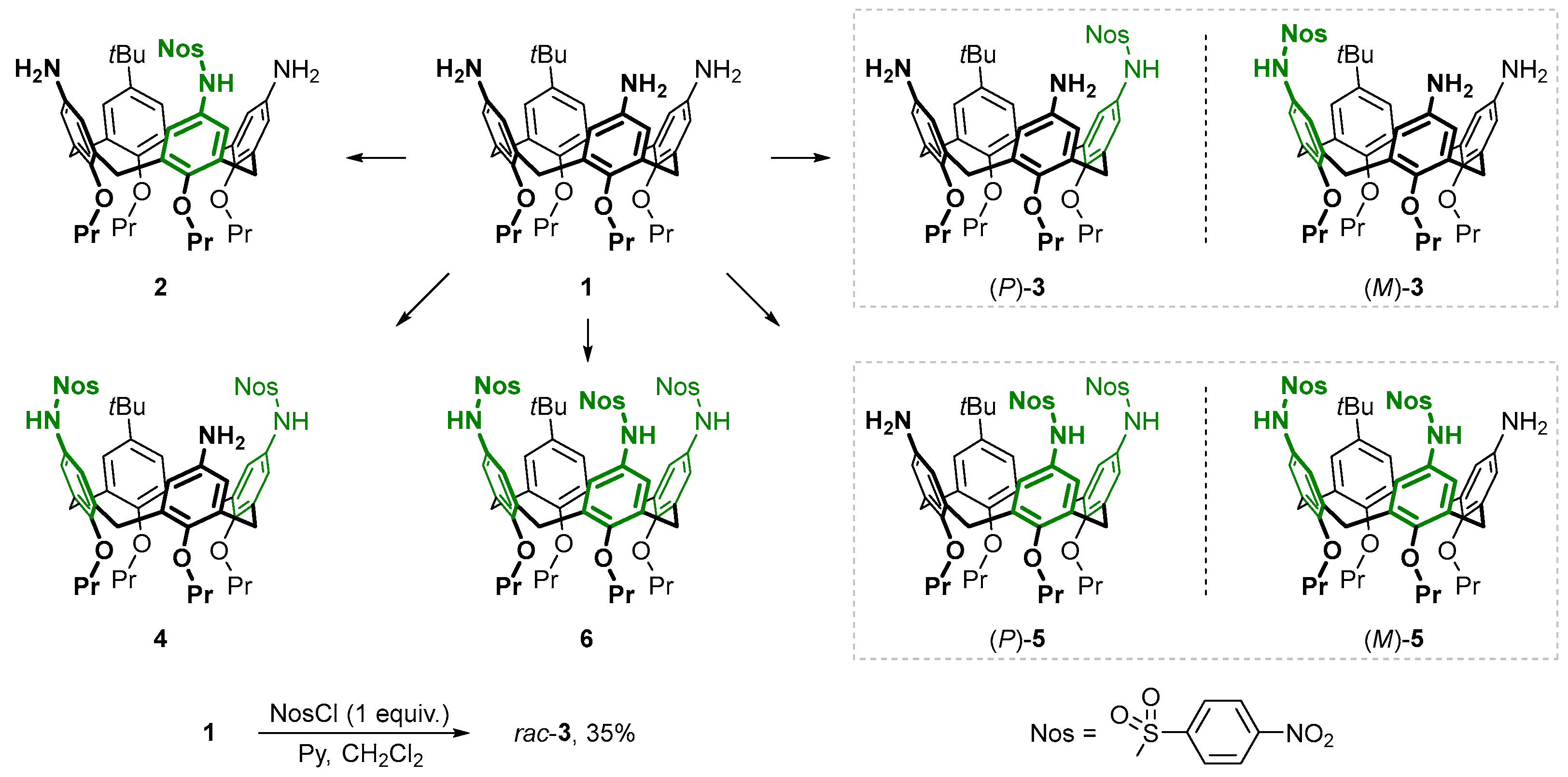2. Results and Discussion
The cone-shaped calix[4]arene
1 [
19], containing three amino groups and one
tert-butyl group at the wide rim, accompanied by four
n-propyl groups at the narrow rim to prevent conformational inversion of the macrocycle, was used as the starting compound. Treatment of this triamine with 4-nitrobenzenesulfonyl chloride (NosCl) can yield five different products, in which one (compounds
2 and
3), two (compounds
4 and
5), and all three amino groups (compound
6) of the macrocycle are sulfonylated (
Scheme 1). Of them, calixarenes
2,
4, and
6 possess the same
Cs symmetry as triamine
1, having, respectively, one Nos group located in the distal aromatic unit of the calixarene relative to the
tert-butyl one, two Nos groups located proximally to the
tert-butyl group, or three Nos groups in the molecule. Another two potential sulfonylation products containing one Nos group near the
tert-butyl group (compound
3) or two adjacent Nos groups (compound
5) are asymmetric, and thus they should form as racemic mixtures of the enantiomers (
P)-
3/(
M)-
3 and (
P)-
5/(
M)-
5, respectively, due to the inherent chirality phenomenon.
These features facilitate the interpretation of the 1H NMR spectra of the reaction mixtures, since Cs symmetric and asymmetric reaction products can be easily differentiated by simply counting the signals from the calixarene aromatic protons (two singlets and two doublets from Cs symmetric products and eight doublets from asymmetric products), while integration of the signals provides the necessary information on the number of Nos groups attached to the calixarene core in each case.
To reveal whether calixarene triamine
1 could be selectively sulfonylated, it was first reacted with 2 equivalents of NosCl, yielding a mixture containing all five reaction products as well as some unreacted calixarene
1. Notably, symmetrical bis- and tris-sulfonylated calixarenes
4 and
6 clearly dominated among the reaction products, and the third most populated product was the asymmetric sulfonamide
3 having a single modified amino group, although these compounds could not be isolated in pure form from the mixture. This may indicate a preferential sulfonylation of the amino groups located proximally rather than distally to the
tert-butylated aromatic unit of the calixarene, which is also statistically expected for the chemical transformation of one of the three amino groups in calixarene
1 (since two amino groups are located proximally, and only one amino group is located distally to the
tert-butylated aromatic unit of the macrocycle). To confirm this, the reaction was carried out using 1 equivalent of NosCl to achieve higher selectivity by suppressing the formation of bis- and tris-sulfonylated products. Under these conditions, a conversion of the starting calixarene of ~80% was achieved, yielding a mixture containing up to 55 mol. % of asymmetric monosulfonylated calix[4]arene
3. From this mixture, calixarene
3 (designated
rac-
3 because it is a racemic mixture of calixarenes (
P)-
3 and (
M)-
3) was successfully isolated by column chromatography, providing an overall yield in the synthesis of 35% (
Scheme 1). As noted above, the asymmetric calixarene
3 can be easily distinguished from the symmetric isomer
2 by its
1H NMR spectrum, which contains eight doublets of aromatic protons of the calixarene and four pairs of doublets from four different ArCH
2Ar groups of the calixarene, clearly indicating the asymmetry of the cone-shaped macrocycle. For the NMR spectra of compound
3, see the Materials and Methods section and
Figures S1 and S2.
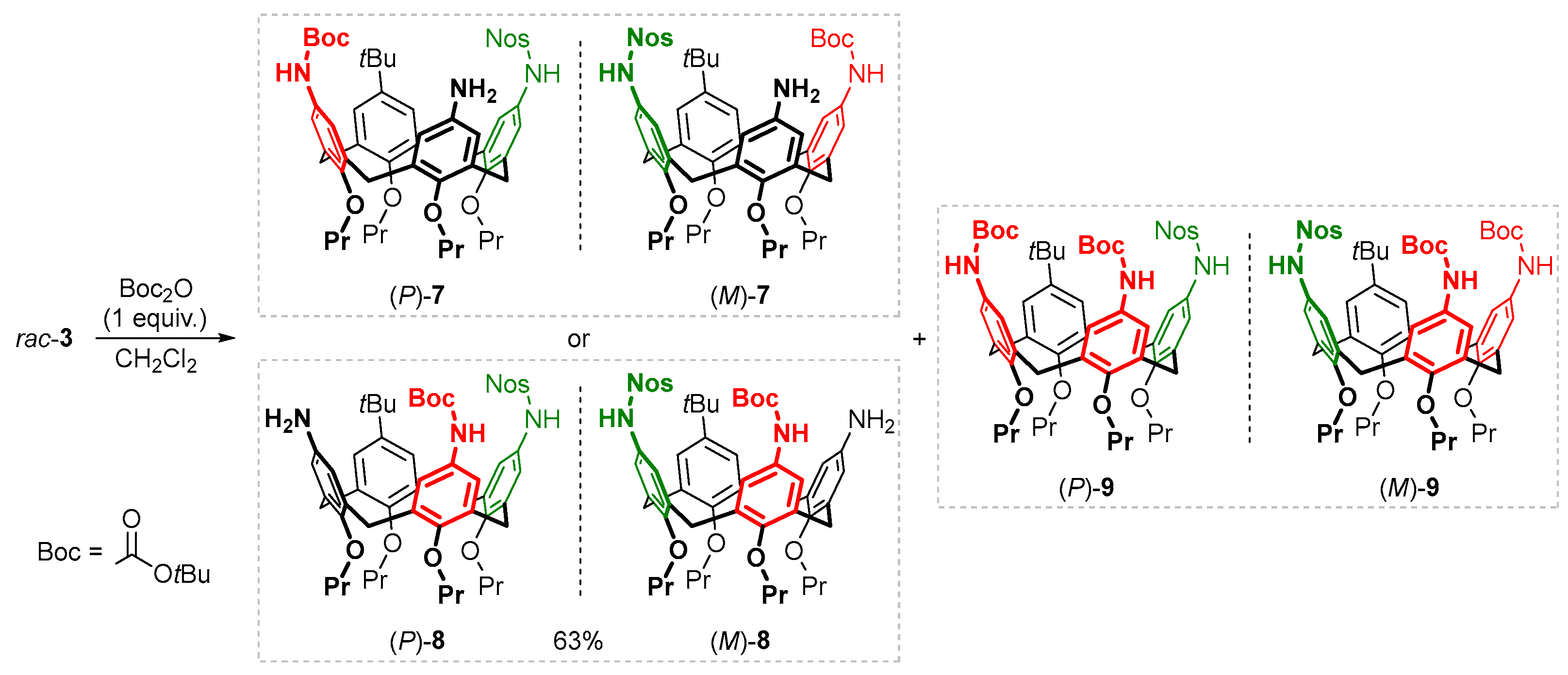
In addition to the sulfide-cleavable Nos group, a
tert-butoxycarbonyl (Boc) protection (which can be removed orthogonally with trifluoroacetic acid) was introduced into calix[4]arene
3, still having two free amino groups in its structure. For this purpose, compound
3 was treated with 1 equivalent of Boc
2O, resulting in a mixture containing ~10 mol % of unreacted calixarene
3, ~10 mol % of exhaustively acylated product
9, and a single monoacylated product, which clearly predominated (
Scheme 2). Notably, in the
1H NMR spectrum of the reaction mixture, only trace signals were detected of what could be an isomeric monoacylated calixarene. From this mixture, the major product having two of the three amino groups protected with different moieties was isolated in 63% yield. Both NMR spectra and ESI-MS data clearly showed that the obtained compound was asymmetric and contained one Nos and one Boc fragment, leaving one of the amino groups unreacted. However, the exact structure of the compound could not be determined from these data, since they corresponded to one of two possible acylation products having a Boc group attached to an amino group located near the
tert-butylated (compound
7) or Nos-containing (compound
8) aromatic unit of the macrocycle.
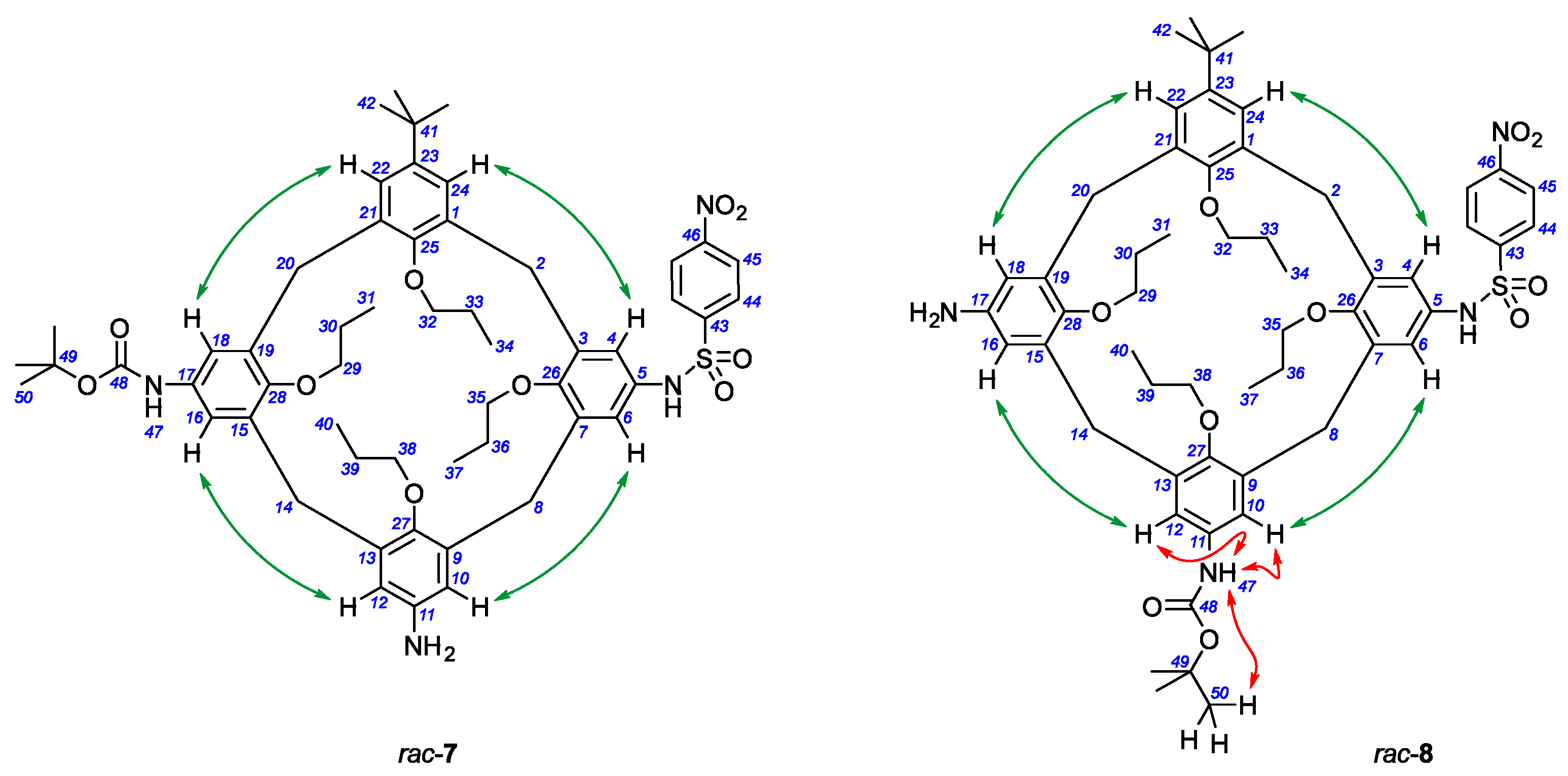
To overcome this and establish the exact structure of the monoacylation product, a set of 2D NMR spectra of the obtained compound was acquired, including
1H,
13C-heteronuclear correlations HSQC (Heteronuclear Single Quantum Coherence) and HMBC (Heteronuclear Multiple Bond Correlation), as well as
1H,
1H-homonuclear correlations COSY (COrrelation SpectroscopY) and NOESY (Nuclear Overhauser Effect Spectroscopy). Using these correlations, all signals in the
1H and
13C NMR spectra were assigned to the calixarene core and substituents (the data are provided in the Materials and Methods section, see
Figure 1 for the atom numbering). Key information needed to determine the exact structure of the monoacylated product was obtained from NOESY data. Thus, the intensive NOEs between the pairs of aromatic protons of calixarene H
4–H
24, H
6–H
10, H
12–H
16 and H
18–H
22 identified the sequence of adjacent aromatic units of the calixarene core, the NOEs between the protons of the
tert-butoxy group H
50 and the amide proton H
47 allowed to clearly distinguish the signal of the latter from the signal of the sulfonamide proton (which appeared to be extremely broadened and had no identifiable signal in the
1H NMR spectrum), and, finally, the strong NOEs between the amide proton H
47 and the aromatic protons of calixarene H
10 and H
12 indicated the exact position of the Boc groups in the calixarene core (
Figure 1). The obtained data clearly confirmed that the monoacylated product has the structure of calixarene
8, in which the Nos and Boc groups are located in two adjacent aromatic units of the calixarene.
The obtained data show that the reaction between Nos-protected calixarene
3 and 1 equivalent of Boc
2O proceeds selectively and leads mainly to monoacylated calixarene
8, rather than to its isomer
7, and thus the amino group of compound
3 located near the Nos-containing aromatic unit of calixarene is involved in the reaction much more readily than the other one. This difference between the amino groups in calixarene
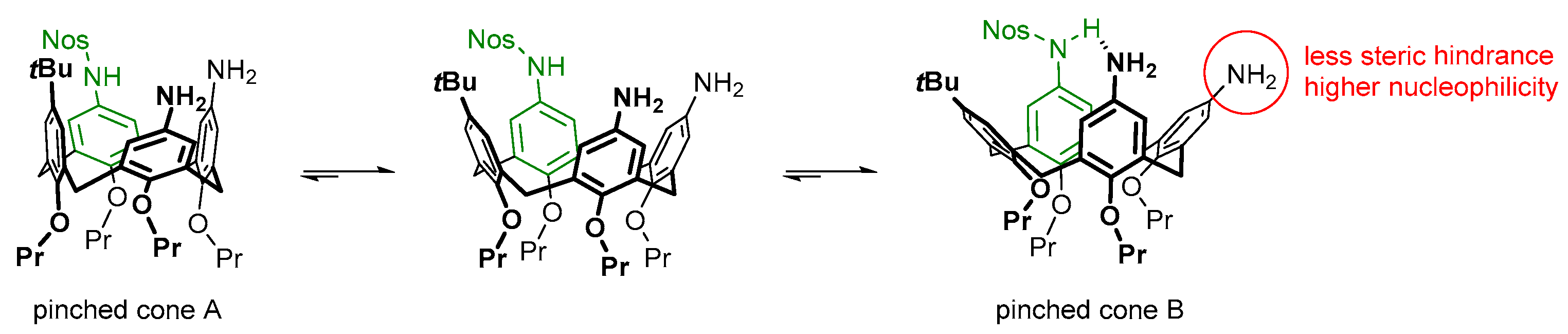
3 can be explained by their different steric accessibility and nucleophilicity due to the non-equivalence of the two pinched cone structures arising from residual conformational motions of the calixarene core in compound
3 (
Figure 2). Indeed, the sulfonamide group NH is acidic. Therefore, it can form a hydrogen bond with one of the amino groups of the same molecule if such an interaction is structurally possible. Of the two free amino groups in calixarene
3, the one located proximally to the sulfonylated aromatic unit of the macrocycle can not be reached by the above NH group. In contrast, the second amino group, located in the distal aromatic unit of the macrocycle, can come into close contact with the sulfonamide group due to interconversions of the calixarene core between the two pinched cone conformers A and B (
Figure 2). As a result, the distal amino group may accept a hydrogen bond and has reduced nucleophilicity. In the pinched cone conformer B, thus stabilized, the proximal amino group appears pushed out of the calixarene cavity and turns out to be more accessible for interactions with the bulky Boc
2O molecule. These conclusions are not in contradiction with the sulfonylation selectivity observed in the first transformation step, where the reaction between the parent calixarene triamine
1 and 2 equivalents of NosCl yielded the symmetrical bis(sulfonamide)
4 among the major reaction products, suggesting that its formation occurs via distal sulfonylation of the intermediate calixarene
3 (see
Scheme 1). In this case, a much more electrophilic reagent (NosCl) was used, which exhibits high activity in reactions with both activated and deactivated amino groups of calixarene
3, while having a smaller size compared to Boc
2O, allowing the reaction to proceed statistically rather than selectively.
Thus, the asymmetric calix[4]arene
8 contains a sequence of a free amino group and amino groups protected by Boc- and Nos-moieties, arranged at the wide rim of a cone-shaped macrocycle. This compound appears to be a good precursor for the preparation of multifunctional structures possessing inherent chirality by threefold modification with three different electrophiles, alternating with two amino group deprotection steps. However, calixarene
8 was actually obtained as a racemic mixture of the enantiomers (
P)-
8 and (
M)-
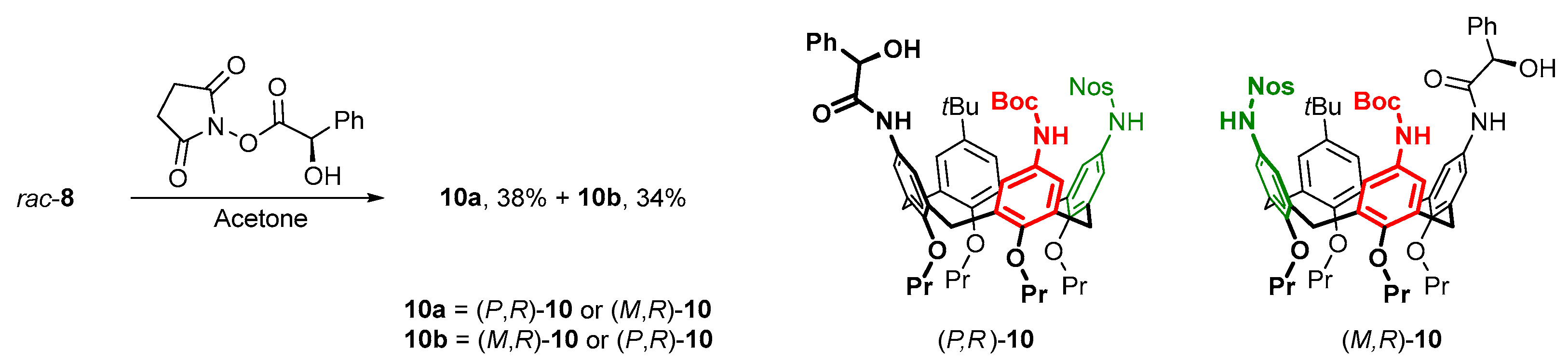
8, which must be separated during further transformations if optically pure products are required. To reveal whether such separation of the inherently chiral (
P)- and (
M)-calixarene cores could be achieved using chiral auxiliaries, an (
R)-mandelic acid residue was introduced into the free amino group of calixarene
rac-
8. To avoid self-condensation of mandelic acid, its in situ prepared succinimide ester was used for acylation, resulting in a mixture of two diastereomers (
P,
R)-
10 and (
M,
R)-
10. The mixture was successfully separated using conventional column chromatography on silica to obtain two pure compounds, denoted as
10a and
10b, in 38% and 34% yield, respectively (
Scheme 3).
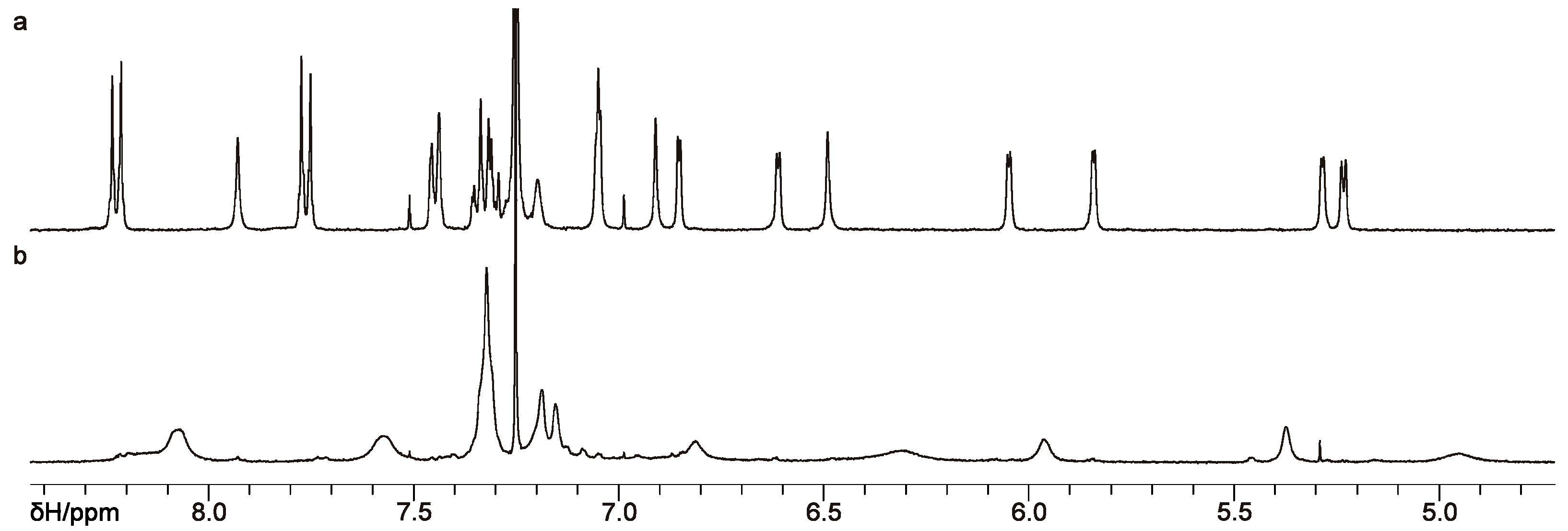
The difference in the NMR spectra of the obtained compounds turned out to be significantly greater than expected for two diastereomers. Indeed, the
1H NMR spectrum of compound
10a obtained from its solution in CDCl
3 was clear and contained the expected set of sharp signals from the asymmetric calix[4]arene substituted at the wide rim with
tert-butyl groups, a Nos-protected amino group, a Boc-protected amino group, and amino groups acylated with mandelic acid (
Figure 3a, for the full spectrum see
Figure S9). In contrast, the
1H NMR spectrum of diastereomer
10b measured in this solvent was extremely broadened (
Figure 3b, for the full spectrum see
Figure S11) and could be only partially interpreted. When polar DMSO-
d6 was used as the solvent, the
1H NMR spectrum of compound
10b became much better resolved and interpretable, although some residual broadening was still present, and, in particular, the doublets from the aromatic protons of the calixarene core appeared as broadened signals (see
Figure S12). Broadening of the signals was also observed in the
13C NMR spectrum of compound
10b dissolved in DMSO-
d6, which prevented the interpretation of the spectrum (see
Figure S13 for the
13C NMR spectrum of compound
10b).
The drastic difference in the spectral patterns observed for solutions of calixarenes 10a and 10b in CDCl3 indicates a slow conformational exchange and/or the coexistence of several multicalixarene aggregates due to intra- and intermolecular hydrogen bonds broken by DMSO-d6, which is characteristic of compound 10b, but not of 10a, which has exactly the same set of functional groups arranged differently at the wide rim of the macrocycle. Due to the above broadening of the spectral pattern, no NMR experiments based on the nuclear Overhauser effect (NOESY or ROESY) can be performed to access the conformational/aggregation behavior of calixarene 10b in more detail. More importantly, due to the broadening, NMR methods failed to determine the stereoconfiguration of the inherently chiral calixarene core in compounds 10a and 10b relative to the (R)-mandelic acid moieties in the absence of fully assigned 1H and 13C spectra and NOESY/ROESY data for both diastereomers obtained under similar conditions.
Attempts to grow single crystals of compounds 10a and 10b using X-ray crystallography to determine their exact structure also failed. Therefore, it was not possible to correlate the structures of these calixarenes with those of the diastereomers (P,R)-10 and (M,R)-10. Nevertheless, the successful separation of diastereomers 10a and 10b clearly demonstrates that the inherently chiral calixarene cores containing protected amino groups at the wide rim can be resolved using chiral auxiliaries. Thus, this approach can be implemented for the further preparation of enantiopure multifunctional calixarenes starting from orthogonally protected calixarene triamine 8.
3. Materials and Methods
Column chromatography was performed on silica gel 60 (0.063–0.200 mm). Commercial reagents were used as received. Calixarene
1 [
19] was prepared by a catalytic reduction of the available tris-nitrated calixarene precursor [
6] similar to that described for the lower-rim
n-pentylated analogue [
20].
1H and 13C NMR spectra were acquired on Bruker Avance 400 and Avance 600 spectrometers (Bruker, Billerica, MA, USA) at room temperature. High-resolution ESI mass spectra were obtained from a Sciex TripleTOF 5600+ spectrometer (AB Sciex, Singapore).
3.1. Nos-Protected Calix[4]arene rac-3
To a solution of calixarene 1 (1.24 g, 1.79 mmol) in dry dichloromethane (15 mL), pyridine (0.14 mL, 1.79 mmol) and 4-nitrobenzenesulfonyl chloride (0.40 g, 1.79 mmol) were added, and the mixture was stirred at room temperature for 24 h. Saturated aqueous NaHCO3 was added, the organic phase was separated, washed with water and brine, dried, and the solvent was evaporated under reduced pressure. The residue was purified by column chromatography (silica, gradient from n-hexane/ethyl acetate 5:1 to n-hexane/ethyl acetate 5:2). Yield 0.54 g (35%), pale yellow solid. M.p. 158–160 °C. 1H NMR (400 MHz, CDCl3): δ = 9.72 (bs, 1H; SO2NH), 8.31–8.26 (m, 2H; ArHNos), 7.82–7.78 (m, 2H; ArHNos), 7.03 (d, 1H, 4JHH = 2.4 Hz; ArH), 6.92 (d, 1H, 4JHH = 2.4 Hz; ArH), 6.45 (d, 1H, 4JHH = 2.7 Hz; ArH), 6.29 (d, 1H, 4JHH = 2.7 Hz; ArH), 5.63 (d, 1H, 4JHH = 2.5 Hz; ArH), 5.60 (d, 1H, 4JHH = 2.8 Hz; ArH), 5.46 (d, 1H, 4JHH = 2.5 Hz; ArH), 5.42 (d, 1H, 4JHH = 2.8 Hz; ArH), 4.34 (d, 1H, 2JHH = 13.7 Hz; ArCH2Ar), 4.33 (d, 1H, 2JHH = 13.5 Hz; ArCH2Ar), 4.26 (d, 2H, 2JHH = 13.6 Hz; ArCH2Ar), 3.94–3.71 (m, 4H; OCH2), 3.65–3.59 (m, 2H; OCH2), 3.57–3.51 (m, 2H; OCH2), 2.99 (d, 1H, 2JHH = 13.9 Hz; ArCH2Ar), 2.98 (d, 1H, 2JHH = 13.5 Hz; ArCH2Ar), 2.88 (d, 1H, 2JHH = 13.6 Hz; ArCH2Ar), 2.87 (d, 1H, 2JHH = 13.7 Hz; ArCH2Ar), 1.89–1.73 (m, 8H; OCH2CH2), 1.31 (s, 9H; C(CH3)3), 1.08 (t, 3H, 3JHH = 7.4 Hz; CH3), 1.05 (t, 3H, 3JHH = 7.4 Hz; CH3), 0.80 (t, 3H, 3JHH = 7.4 Hz; CH3), 0.79 (t, 3H, 3JHH = 7.4 Hz; CH3) ppm. 13C NMR (100 MHz, CDCl3): δ = 155.70, 155.31, 150.77, 149.94, 149.49, 147.80, 144.40, 140.41, 137.68, 137.57, 137.47, 136.03, 136.00, 134.96, 134.82, 134.59, 134.36, 128.48 (CAr), 128.21, 128.14, 128.10, 125.79, 125.70, 123.86, 115.88, 115.82, 115.51, 115.39 (CHAr), 76.78, 76.45, 76.33, 76.16 (OCH2CH2), 33.95 (C(CH3)3), 31.61 (C(CH3)3), 31.23, 30.98, 30.97 (ArCH2Ar), 23.43, 23.41, 22.84, 22.65 (CH2CH3), 10.84, 10.80, 9.68, 9.62 (CH3) ppm. HRMS ESI-MS: m/z: 879.4359 [M + H]+ for C50H63N4O8S (879.4361).
3.2. Nos-Boc-Protected Calix[4]arene rac-8
To a solution of calixarene 3 (0.54 g, 0.61 mmol) in dry dichloromethane (10 mL), di-tert-butyl dicarbonate (0.13 g, 0.61 mmol) was added, and the mixture was stirred at room temperature for 24 h. The solvent was evaporated under reduced pressure, and the residue was purified by column chromatography (silica, n-hexane/ethyl acetate 5:1). Yield 0.37 g (63%), pale yellow solid. M.p. 157–159 °C. 1H NMR (600 MHz, CDCl3): δ = 8.27–8.23 (m, 2H; ArHNos45), 7.80–7.77 (m, 2H; ArHNos44), 7.29 (bs, 1H; ArH12), 7.03 (d, 1H, 4JHH = 2.4 Hz; ArH22), 6.90 (d, 1H, 4JHH = 2.4 Hz; ArH24), 6.81 (d, 1H, 4JHH = 2.6 Hz; ArH10), 6.44 (s, 1H; CONH47), 5.65 (d, 1H, 4JHH = 2.3 Hz; ArH6), 5.57 (d, 1H, 4JHH = 2.8 Hz; ArH16), 5.43 (d, 1H, 4JHH = 2.3 Hz; ArH4), 5.41 (d, 1H, 4JHH = 2.8 Hz; ArH18), 4.35 (d, 1H, 2JHH = 13.7 Hz; ArCH2Ar2ax), 4.33 (d, 1H, 2JHH = 13.5 Hz; ArCH2Ar20ax), 4.32 (d, 1H, 2JHH = 13.7 Hz; ArCH2Ar8ax), 4.31 (d, 1H, 2JHH = 13.7 Hz; ArCH2Ar14ax), 3.93–3.84 (m, 4H; OCH232), 3.88–3.80 (m, 2H; OCH238), 3.65–3.81 (m, 2H; OCH235), 3.57–3.53 (m, 2H; OCH229), 3.00 (d, 1H, 2JHH = 13.7 Hz; ArCH2Ar14eq), 2.99 (d, 1H, 2JHH = 13.5 Hz; ArCH2Ar20eq), 2.98 (d, 1H, 2JHH = 13.7 Hz; ArCH2Ar8eq), 2.98 (d, 1H, 2JHH = 13.7 Hz; ArCH2Ar2eq), 1.87–1.75 (m, 8H; OCH2CH230, 33, 36, 39), 1.57 (s, 9H; OC(CH3)350), 1.30 (s, 9H; C(CH3)342), 1.08 (t, 3H, 3JHH = 7.4 Hz; CH331), 1.06 (t, 3H, 3JHH = 7.4 Hz; CH337), 0.81 (t, 3H, 3JHH = 7.4 Hz; CH334), 0.80 (t, 3H, 3JHH = 7.4 Hz; CH340) ppm. 13C NMR (150 MHz, CDCl3): δ = 155.71 (25), 155.40 (26), 153.83 (27), 153.05 (48), 150.00 (28), 149.55 (43), 147.79 (46), 144.52 (23), 137.77 (17), 137.36 (9), 137.30 (13), 136.05 (1), 135.98 (21), 135.01 (3), 134.67 (7), 134.63 (15), 134.24 (19), 132.16 (11), 128.59 (6), 128.56 (5), 128.17 (44), 128.12 (4), 125.81 (24), 125.78 (22), 123.78 (45), 119.22 (12), 119.16 (10), 115.62 (18), 115.55 (16), 80.28 (49), 76.85 (29), 76.56 (35), 76.34 (38), 76.25 (32), 33.96 (41), 31.61 (42), 31.27 (20), 31.26 (2), 31.06 (14), 31.04 (8), 28.44 (50), 23.45 (36), 23.43 (30), 22.89 (33), 22.73 (39), 10.83 (37), 10.79 (31), 9.69 (40), 9.66 (34) ppm. HRMS ESI-MS: m/z: 979.4880 [M + H]+ for C55H71N4O10S (979.4885).
3.3. Diastereomers (P,R)-10 and (M,R)-10
To a cooled (0–5 °C) solution of (R)-mandelic acid (0.15 g, 0.98 mmol) and N-hydroxysuccinimide (0.11 g, 0.98 mmol) in dry acetone (4 mL), a solution of N,N′-dicyclohexylcarbodiimide (0.20 g, 0.98 mmol) in dry acetone (4 mL) was added dropwise while stirring. The mixture was stirred while cooling for 2 h, and then a solution of calixarene rac-8 (0.32 g, 0.33 mmol) in dry acetone (4 mL) was added. The mixture was stirred at room temperature for 24 h. The precipitate formed was separated by filtration, washed with acetone, and discarded. The combined filtrates were evaporated under reduced pressure, and the residue was dissolved in dichloromethane. The solution was washed with water and brine, dried, and the solvent was evaporated. The residue was subjected to column chromatography (silica, n-hexane/ethyl acetate 8:1) and fractions containing calixarenes 10a and 10b were eluted successively, from which pure compounds were obtained upon recrystallization from n-hexane. Compound 10a: Yield 0.138 g (38%), pale yellow solid. M.p. 165–167 °C. 1H NMR (400 MHz, CDCl3): δ = 8.25–8.20 (m, 2H; ArHNos), 7.93 (s, 1H; SO2NH), 7.79–7.73 (m, 2H; ArHNos), 7.47–7.42 (m, 2H; ArHPh), 7.37–7.29 (m, 3H; ArHPh), 7.20 (bs, 1H; ArH), 7.05 (d, 1H, 4JHH = 2.3 Hz; ArH), 7.04 (d, 1H, 4JHH = 2.3 Hz; ArH), 6.91 (s, 1H; CONH), 6.85 (d, 1H, 4JHH = 2.3 Hz; ArH), 6.61 (d, 1H, 4JHH = 2.5 Hz; ArH), 6.49 (s, 1H; CONH), 6.05 (d, 1H, 4JHH = 2.5 Hz; ArH), 5.84 (d, 1H, 4JHH = 2.5 Hz; ArH), 5.28 (d, 1H, 4JHH = 2.4 Hz; ArH), 5.23 (d, 1H, 3JHH = 4.1 Hz; CH(OH)), 4.35 (d, 2H, 2JHH = 13.1 Hz; ArCH2Ar), 4.34 (d, 1H, 2JHH = 13.6 Hz; ArCH2Ar), 4.33 (d, 1H, 2JHH = 13.5 Hz; ArCH2Ar), 3.98–3.81 (m, 4H; OCH2), 3.67 (d, 1H, 3JHH = 4.1 Hz; CH(OH)), 3.64–3.58 (m, 2H; OCH2), 3.57–3.51 (m, 2H; OCH2), 3.05 (bd, 2H; ArCH2Ar), 3.02 (d, 1H, 2JHH = 13.1 Hz; ArCH2Ar), 2.91 (d, 1H, 2JHH = 13.6 Hz, ArCH2Ar), 1.93–1.75 (m, 8H; OCH2CH2), 1.56 (s, 9H; OC(CH3)3), 1.30 (s, 9H; C(CH3)3), 1.07 (t, 3H, 3JHH = 7.4 Hz; CH3), 1.04 (t, 3H, 3JHH = 7.4 Hz; CH3), 0.83 (t, 3H, 3JHH = 7.4 Hz; CH3), 0.81 (t, 3H, 3JHH = 7.4 Hz; CH3) ppm. 13C NMR (100 MHz, CDCl3): δ = 171.47 (C=O), 155.28, 154.35, 153.51 (CAr), 153.26 (C=O), 152.80, 149.73, 145.52, 144.90, 138.72, 137.13, 136.84, 135.50, 135.41, 134.77, 134.76, 134.01, 133.75, 132.25, 130.43, 128.63 (CAr), 128.49, 128.31, 128.16, 126.30, 126.06, 125.82, 124.45, 123.90, 123.26, 120.69, 119.88 (CHAr), 77.12 (OC(CH3)3), 77.00, 76.73, 76.43, 76.34 (OCH2), 74.53 (CH(OH)), 34.03 (C(CH3)3), 31.57 (C(CH3)3), 31.15, 31.07, 30.99 (ArCH2Ar), 28.43 (OC(CH3)3), 23.41, 23.38, 22.94, 22.67 (CH2CH3), 10.77, 10.73, 9.73, 9.70 (CH3) ppm. HRMS ESI-MS: m/z: 1135.5075 [M + Na]+ for C63H76N4NaO12S (1135.5073). Compound 10b: Yield 0.123 g (34%), pale yellow solid. M.p. 152–154 °C. 1H NMR (400 MHz, CDCl3): δ = 8.29–8.01 (bs, 3H; NH + ArHNos), 7.57 (bs, 2H; ArHNos), 7.36–7.28 (bs, 6H; ArHPh + ArH), 7.23–7.13 (bs, 4H; NH + ArH), 6.82 (bs, 1H; ArH), 6.31 (bs, 1H; ArH), 6.43 (bs, 1H; ArH), 5.96 (bs, 1H; ArH), 5.37 (bs, 1H; ArH), 4.95 (bs, 1H; CH(OH)), 4.48–4.27 (m, 5H; ArCH2Ar + CH(OH)), 4.02–3.80 (m, 4H; OCH2), 3.68–3.51 (m, 4H; OCH2), 3.18 (d, 1H, 2JHH = 13.6 Hz; ArCH2Ar), 3.11 (d, 1H, 2JHH = 13.6 Hz; ArCH2Ar), 3.00 (d, 1H, 2JHH = 13.9 Hz; ArCH2Ar), 2.98 (d, 1H, 2JHH = 13.3 Hz; ArCH2Ar), 1.97–1.76 (m, 8H; OCH2CH2), 1.43 (s, 9H; OC(CH3)3), 1.41 (bs, 9H; C(CH3)3), 1.09 (t, 3H, 3JHH = 7.4 Hz; CH3), 1.05 (t, 3H, 3JHH = 7.4 Hz; CH3), 0.85 (t, 3H, 3JHH = 7.4 Hz; CH3), 0.80 (t, 3H; 3JHH = 7.4 Hz; CH3) ppm. 1H NMR (400 MHz, DMSO-d6): δ = 10.13 (s, 1H; CONH), 9.54 (s, 1H; CONH), 8.68 (bs, 1H; SO2NH); 8.37–8.32 (m, 2H; ArHNos), 8.00–7.94 (m, 2H; ArHNos), 7.48–7.43 (m, 2H; ArHPh), 7.34–7.23 (m, 4H; ArH + ArHPh), 7.13 (bs, 1H; ArH), 6.73 (bs, 1H; ArH), 6.68 (bs, 1H; ArH), 6.56 (bd, 1H; ArH), 6.40 (bs, 1H; ArH), 6.27 (d, 1H, 3JHH = 5.0 Hz; CH(OH)), 6.18 (bs, 1H; ArH), 5.04 (d, 1H, 3JHH = 5.0 Hz; CH(OH)), 4.28 (d, 1H, 2JHH = 12.7 Hz; ArCH2Ar), 4.25 (d, 1H, 2JHH = 12.9 Hz; ArCH2Ar), 4.23 (d, 1H, 2JHH = 12.9 Hz; ArCH2Ar), 4.22 (d, 1H, 2JHH = 13.1 Hz; ArCH2Ar), 3.88–3.73 (m, 4H; OCH2), 3.71–3.54 (m, 4H; OCH2), 3.05 (d, 1H, 2JHH = 13.1 Hz; ArCH2Ar), 2.99 (d, 2H, 2JHH = 12.9 Hz; ArCH2Ar), 2.98 (d, 1H, 2JHH = 12.9 Hz; ArCH2Ar), 1.91–1.75 (m, 8H; OCH2CH2), 1.40 (s, 9H; OC(CH3)3), 1.00 (t, 3H, 3JHH = 7.5 Hz; CH3), 0.97 (t, 3H, 3JHH = 7.5 Hz; CH3), 0.88 (t, 3H, 3JHH = 7.5 Hz; CH3), 0.87 (t, 3H, 3JHH = 7.5 Hz; CH3), 0.73 (bs, 9H; C(CH3)3) ppm. HRMS ESI-MS: m/z: 1135.5072 [M + Na]+ for C63H76N4NaO12S (1135.5073).