Abstract
Substituted thiourea derivatives represent a structurally diverse group of compounds with high biological activity. In this paper, we present the synthesis of N-(9-ethyl-9H-carbazol-2-yl)-N′-(1-phenylethyl)thiourea by condensation reaction. The structure of the title compound was confirmed by 1H and 13C nuclear magnetic resonance (NMR), Fourier-transform infrared spectroscopy (FT-IR), and high-resolution mass spectrometry (HRMS).
1. Introduction
The 1,3-disubstituted thiourea group is a useful structure for the construction of various compounds with a wide spectrum of biological properties, such as cytotoxic [1], antibacterial [2], antiviral [3], anti-inflammatory [4], and antioxidant [5]. The bioactivity of thioureas has also been demonstrated by their binding activity to serotonergic receptors and their effect on the central nervous system in mice [6].
Tricyclic carbazole ring is a versatile structural element of many chemotherapeutic agents (Figure 1), including anticancer agents (ellipticine, alectinib), antihypertensives (carvedilol, carazolol), and nonsteroidal anti-inflammatory drugs (carprofen) [7]. The broad anticancer properties of carbazole-based molecules cover their cytotoxic action against leukemia, human lung [8], colon, and breast [9] cancer cell lines, as well as normal cells [8]. Carbazole derivatives have also been reported for the treatment of neurological disorders such as multiple sclerosis, Parkinson’s disease, and Alzheimer’s disease. These compounds are capable of inhibiting the formation and aggregation of beta-amyloid peptides [10] and exhibit neuroprotective [11] or antioxidant [12] effects.
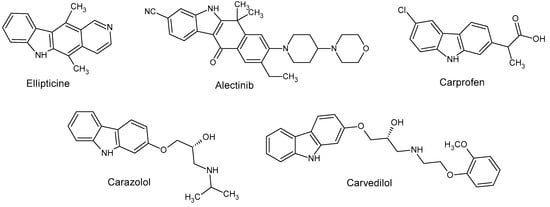
Figure 1.
Structures of carbazole-based drugs.
Our research continues to explore the potential for developing new drugs combining two pharmacophores: a small, polar thiourea branch and a bulky hydrophobic carbazole motif. Carbazole–thiourea hybrids are a promising group of compounds that could be effective in the treatment of various diseases and potentially useful in clinical therapies.
2. Results and Discussion
A complex aromatic derivative, N-(9-ethyl-9H-carbazol-2-yl)-N′-(1-phenylethyl)thiourea, was synthesized by condensation of an appropriate carbazole-based amine with 1-phenylethylisothiocyanate, according to the one-step reaction procedure described in our previous paper (Scheme 1) [1]. The weakly polar 9-ethyl-9H-carbazole core, reported to be cytotoxic, combined with a small alkylphenylthiourea motif, responsible for diverse bioactivities, offers the possibility of finding a biologically active molecule. The identity of the product was confirmed by structural characterization techniques, namely 1H NMR, 13C NMR, HRMS, and FT-IR (see Supplementary Materials).
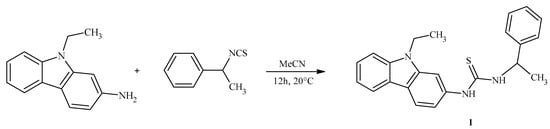
Scheme 1.
Synthesis of N-(9-ethyl-9H-carbazol-2-yl)-N′-(1-phenylethyl)thiourea.
The two protons of the thiourea branch attached to the nitrogen atoms are characterized by variable chemical environments; one is bound to an aromatic carbon and the other to an aliphatic carbon, and they therefore differ in their lability. These protons are considered exchangeable and often appear as broad signals with variable chemical shifts depending on concentration, solvent, and temperature. Because they are lability-dependent and participate in hydrogen bonding or acid–base equilibrium, they can readily exchange with deuterium in the deuterated solvent (DMSO). In this case, the corresponding signal from the proton of the -NH group disappears. In the 1H NMR spectrum of compound 1, a singlet peak was observed at δ 9.45 ppm, integrating one proton, indicating the presence of one of the -NH- groups in the thiourea branch. The 13C NMR spectrum peak at δ 180.7 ppm confirmed the presence of a C=S group in the compound’s structure. The hydrogen of the -CH- group appeared as a quartet at δ 5.65 ppm, while the associated methyl group was shown as a doublet at δ 1.48 ppm. The N-CH2- group of the carbazole ring appeared as a quartet at 4.42 ppm, and its methyl group appeared as a doublet of doublets at 1.31 ppm. The IR spectrum showed characteristic absorption bands corresponding to N–H stretching vibrations at ν = 3360 cm−1. The C=S (thiocarbonyl) stretching vibration was found at 1224 cm−1.
3. Materials and Methods
3.1. General Information
9-Ethyl-9H-carbazol-3-amine and 1-phenylethylisothiocyanate were supplied from Sigma Aldrich. Acetonitrile and ethanol were supplied from POCh (Polskie Odczynniki Chemiczne, Gliwice, Poland). All chemicals were of analytical grade and were used without any further purification. Prior to usage, acetonitrile was kept in crown cap bottles over anhydrous phosphorus pentoxide (Carl Roth, Karlsruhe, Germany). The NMR spectra were recorded on a Bruker AVANCE DMX400 spectrometer (Billerica, MA, USA), operating at 300 MHz (1H NMR) and 75.5 MHz (13C NMR). The spectra were measured in DMSO and are given as δ values (in ppm) relative to TMS. Mass spectral ESI measurements were carried out on LCT Micromass TOF HiRes apparatus (Milford, MA, USA). Fourier-transform infrared spectroscopy (FT-IR) studies were performed using a PerkinElmer Spectrum 1000 (Waltham, MA, USA) spectrometer. Transmission spectra were acquired in the 3800–500 cm−1 range at a spectral resolution of 2 cm−1 from KBr pellets using 30 scans. The obtained spectra were processed using GRAMS/AI 8.0 (Thermo Fisher Scientific, Waltham, MA, USA) and KaleidaGraph 3.5 (Synergy Software, Reading, PA, USA) software. All the ATR–FTIR spectroscopic measurements were taken at 2 cm−1 spectral resolution with 60 co-added scans (range 4000–500 cm−1). Shimadzu IRAffinity–1SATR correction was performed. Analytical TLC was carried out on silica gel F254 (Merck, Darmstadt, Germany) plates (0.25 mm thickness).
3.2. Preparation of N-(9-ethyl-9H-carbazol-2-yl)-N′-(1-phenylethyl)thiourea (1)
A solution of commercially available 9-ethyl-9H-carbazol-3-amine (0.00107 mol) in anhydrous acetonitrile (5 mL) was treated with (1-isothiocyanatoethyl)benzene (1-phenylethylisothiocyanate) (0.00107 mol). The mixture was stirred at room temperature for 12 h. Then, the solvent was evaporated, and the solid residue was crystallized from anhydrous ethanol.
Yield 56% (0.4 g), yellowish powder, m.p. 173–175 °C. 1H NMR (300 MHz, DMSO) δ (ppm): 9.45 (s, 1H, NH), 8.12–8.10 (m, 2H, Harom), 7.98–7.95 (m, 1H, Harom), 7.59–7.54 (m, 2H, Harom), 7.48–7.34 (m, 5H, Harom), 7.29–7.23 (m, 1H, Harom), 7.22–7.16 (m, 1H, Harom), 5.65 (q, 1H, CH(CH3)), 4.42 (q, 2H, CH2CH3, J = 7.0 Hz), 1.48 (d, 3H, CH(CH3), J = 7.2 Hz), 1.31 (dd, 3H, CH2CH3, J1 = 6.9 Hz, J2 = 7.2 Hz). 13C NMR (75.5 MHz, DMSO) δ (ppm): 180.7, 144.1, 139.9, 137.3, 130.5, 128.3, 126.7, 126.3, 125.8, 124.0, 122.0, 120.4, 118.7, 117.1, 109.1, 108.9, 52.8, 37.0, 21.9, 13.7. HRMS (ESI) calc. for C23H23N3S [M + H]+: 374.1686, found: [M + H]+ = 374.1691 m/z. FT-IR (KBr, cm−1): 3360 (N–H amide stretching); 3168, 3010 (=C–H aromatic vibrations); 2965 (υ(C–H) asym. CH3), 2937 (υ(C–H) asym. CH2); 2874 (υ(C–H) sym. CH3/CH2); 1581 (C=C aromatic ring skeletal stretching/δ(N–H)/ν(C–N) thiourea); 1536 (C–H aromatic band/δ(N–H) coupled with C–N (thiourea)); 1481 (CH3/CH2 (δ) deformation/C–H aromatic vibrations); 1316 (C–N aromatic amine vibrations/δ(CH)); 1224 (υ(C=S)); 1142 (ν(C–N)amine/C=S stretching); 1010 (υ(C–N) or C–H aromatic vibrations); 940 (out-of-plane C–H aromatic vibrations); 878 (out-of-plane C–H aromatic vibrations); 811 (out-of-plane C–H aromatic); 738 (rocking CH2/out-of-plane C–H aromatic); 693 (out-of-plane C–H aromatic).
4. Conclusions
In summary, N-(9-ethyl-9H-carbazol-2-yl)-N′-(1-phenylethyl)thiourea (1) was synthesized by a simple condensation reaction under mild conditions in satisfactory yield. The pure compound was obtained as a light-yellow powder. Its structure was confirmed by HRMS, as well as 1H NMR, 13C NMR, and FT-IR spectroscopy. Further research is ongoing into the antiproliferative properties of this derivative and its ability to bind to serotonergic receptors.
Supplementary Materials
The following supporting information for the characterization of 1 can be downloaded online: Figure S1: Molfile of Compound 1; Figure S2: HRMS of compound 1; Figure S3: 1H NMR spectrum of compound 1 (300 MHz in DMSO); Figure S4: 13C NMR spectrum of compound 1 (75 MHz in DMSO); Figure S5: FT-IR spectrum of compound 1; Figure S6: The superimposed ATR–FTIR spectra of compound 1 (C) and its substrates: 9-ethyl-9H-carbazol-3-amine (A) and 1-phenylethylisothiocyanate (B).
Author Contributions
Conceptualization, A.B.; methodology, M.B.; software, R.M.; validation, A.M.-K., M.B. and R.M.; formal analysis, A.M.-K.; investigation, A.M.-K.; resources, D.S.-L.; data curation, R.S.; writing—original draft preparation, M.P.-T. and A.B. writing—review and editing, A.B.; visualization, R.S.; supervision, D.S.-L.; project administration, R.M.; funding acquisition, R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Strzyga-Łach, P.; Chrzanowska, A.; Kiernozek-Kalińska, E.; Żyżyńska-Granica, B.; Podsadni, K.; Podsadni, P.; Bielenica, A. Proapoptotic effects of halogenated bis-phenylthiourea derivatives in cancer cells. Arch. Pharm. 2023, 356, e2300105. [Google Scholar] [CrossRef] [PubMed]
- Rana, P.; Parupalli, R.; Akhir, A.; Saxena, D.; Maitra, R.; Imran, M.; Malik, P.; Mahammad Ghouse, S.; Joshi, S.V.; Srikanth, D.; et al. Synthesis and biological evaluation of new naphthalimide-thiourea derivatives as potent antimicrobial agents active against multidrug-resistant Staphylococcus aureus and Mycobacterium tuberculosis. RSC Med. Chem. 2024, 15, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Koshizuka, T.; Majima, R.; Takahashi, K.; Ishioka, K.; Suzutani, T.; Inoue, N. Characterization of a thiourea derivative that targets viral transactivators of cytomegalovirus and herpes simplex virus type 1. Antivir. Res. 2021, 196, 105207. [Google Scholar] [CrossRef] [PubMed]
- Wagdy, R.A.; Chen, P.J.; Hamed, M.M.; Darwish, S.S.; Chen, S.H.; Abadi, A.H.; Abdel-Halim, M.; Hwang, T.L.; Engel, M. From EGFR kinase inhibitors to anti-inflammatory drugs: Optimization and biological evaluation of (4-(phenylamino)quinazolinyl)-phenylthiourea derivatives as novel NF-κB inhibitors. Bioorg. Chem. 2022, 127, 105977. [Google Scholar] [CrossRef] [PubMed]
- Kollu, U.; Avula, V.K.R.; Vallela, S.; Pasupuleti, V.R.; Zyryanov, G.V.; Neelam, Y.S.; Chamarthi, N.R. Synthesis, antioxidant activity and bioinformatics studies of L-3-hydroxytyrosine templated N-alkyl/aryl substituted urea/thioureas. Bioorg. Chem. 2021, 111, 104837. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.; Ali Shah, S.W.; Khan, A.U.; Badshah, H.; Darwish, H.W.; Aschner, M.; Alam, W.; Khan, H. Preclinical and in silico studies of 3-benzothioyl-1-(3-hydroxy-3-phenyl-3-propyl)-1-methylthiourea: A promising agent for depression and anxiety. Eur. J. Pharmacol. 2025, 989, 177226. [Google Scholar] [CrossRef] [PubMed]
- Głuszynska, A. Biological potential of carbazole derivatives. Eur. J. Med. Chem. 2015, 94, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Kuo, I.-C.; Lin, J.-J.; Lu, Y.-C.; Chen, C.-T.; Back, H.-T.; Lou, P.-J.; Chang, T.-C. A novel carbazole derivative, BMVC: A potential antitumor agent and fluorescence marker of cancer cells. Chem. Biodivers. 2004, 1, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-J.; Chao, Y.; Chang, Y.-H.; Ho, F.-M.; Huang, L.-J.; Huang, Y.-L.; Luh, T.-Y.; Chen, C.-P.; Lin, W.-W. Cell apoptosis induced by a synthetic carbazole compound LCY-2-CHO is mediated through activation of caspase and mitochondrial pathways. Biochem. Pharmacol. 2005, 70, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wong, Y.; Ng, O.T.W.; Bai, L.-P.; Kwong, D.W.J.; Ke, Y.; Jiang, Z.-H.; Li, H.-W.; Yung, K.K.L.; Wong, M.S. Inhibition of beta-amyloid peptide aggregation by multifunctional carbazole-based fluorophores. Angew. Chem. Int. Ed. 2012, 51, 1804–1810. [Google Scholar]
- Tasset, I.; Espínola, C.; Medina, F.J.; Feijoo, M.; Ruiz, C.; Moreno, E.; Gomez, M.M.; Collado, J.A.; Munoz, C.; Muntane, J.; et al. Neuroprotective effect of carvedilol and melatonin on 3-nitropropionic acid-induced neurotoxicity in neuroblastoma. J. Physiol. Biochem. 2009, 65, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Chen, M.; Li, M.; Luo, B.; Zhao, Y.; Huang, P.; Xue, F.; Rapposelli, S.; Pi, R.; Wen, S. Discovery of novel N-substituted carbazoles as neuroprotective agents with potent anti-oxidative activity. Eur. J. Med. Chem. 2013, 68, 81–88. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).