Abstract
Structural optimization integrating triphenylamine and quaternary ammonium salt-based antimicrobial peptide mimics has yielded novel theranostic hybrid molecules. These compounds exhibit fluorescence imaging, photodynamic antibacterial activity, and membrane-disruption capabilities, demonstrating broad-spectrum efficacy with low resistance induction. In this study, we successfully developed a novel triphenylamine-quaternary ammonium derivative (TPQ), ultimately obtaining the target compound (E)-8-(4-(4-(diphenylamino)styryl)phenoxy)-N,N,N-trimethyloctan-1-aminium bromide (Compound 5). This achievement provides a potent strategy against growing bacterial drug resistance.
1. Introduction
In 2025, the World Health Organization issued two reports that sounded a global alarm: the progress in antibiotic development is failing to keep pace with the urgent need to combat drug-resistant infections [1]. The current research advancements are critically insufficient, facing both a shortage of viable new drug candidates and a severe lack of innovative approaches to effectively counter resistant bacteria.
Antimicrobial peptides have played a role in antibacterial therapy for many years. Generally, bacteria remain susceptible to these peptides, and human antimicrobial peptides are central to innate immunity. However, the clinical application of antimicrobial peptides is hampered by cost and stability issues. Consequently, developing non-peptidic mimics of antimicrobial peptides is increasingly becoming a key research direction for novel antibacterial agents [2,3].
Quaternary Ammonium Compounds (QACs) are cationic organic chemicals used as preservatives and disinfectants [4]. Their antimicrobial action owes to the electrostatic interaction between their positively charged nitrogen atoms and the negatively charged phospholipid head groups in bacterial membranes [5]. Structurally, QACs comprise one or more positively charged quaternary ammonium groups and at least one hydrophobic alkyl chain, with the cationic charge typically balanced by halogen counterions (Figure 1I,II) [6,7].
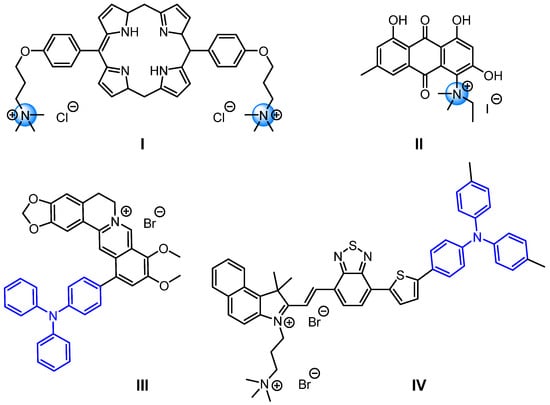
Figure 1.
Structures of representative quaternary ammonium compounds and triphenylamine derivatives.
Triphenylamine (TPA) is an important organic compound whose molecular structure consists of three benzene rings connected to a central nitrogen atom, forming a “propeller” or “shamrock”-like configuration. While TPA itself does not exhibit significant antibacterial activity, the lone pair of electrons on its nitrogen atom can delocalize into the benzene rings [8]. This excellent characteristic makes it a strong electron donor, thus serving as an ideal pharmacophore or structural scaffold for antimicrobial drug development (Figure 1III,IV) [9,10]. Additionally, TPA can act as a photosensitizer. Upon exposure to light of a specific wavelength, it absorbs photon energy and transitions from the ground state to an excited state. The excited TPA then transfers energy to surrounding molecular oxygen, generating highly reactive oxygen species (ROS) such as singlet oxygen (1O2) [11]. ROS irreversibly oxidizes and damages bacterial cell membranes, proteins, lipids, and DNA, leading to bacterial death. Moreover, since ROS attacks multiple cellular targets simultaneously, bacteria struggle to develop resistance through single mutations [12].
In this study, we successfully designed and synthesized triphenylamine quaternary ammonium salt derivatives, promising a new direction for developing novel antibacterial agents.
2. Results and Discussion
For the synthesis of triphenylamine quaternary ammonium salt derivatives, we first conducted synthetic exploration using commercially available p-cresol as the starting material (Scheme 1). The specific procedure involved reacting the phenolic hydroxyl group with a bromoalkane via nucleophilic substitution in N,N-dimethylformamide (DMF) using ammonium carbonate as a base at room temperature overnight, yielding intermediate 3. This was followed by reaction with trimethylamine at 90 °C for 12 h, leading to the formation of quaternary ammonium salt 4 through nucleophilic substitution. However, despite testing various catalysts (such as piperidine, p-toluenesulfonic acid, and sodium ethoxide), we were unable to successfully introduce the triphenylamine group into compound 4 (Scheme 1).
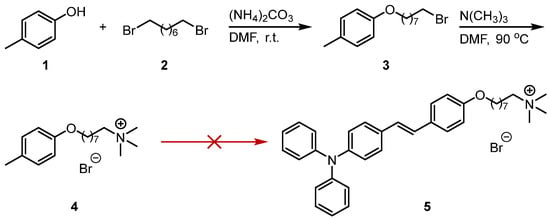
Scheme 1.
Attempts on the synthetic route of (E)-8-(4-(4-(diphenylamino)styryl)phenoxy)-N,N,N-trimethyloctan-1-aminium bromide (5).
Regarding the issue that the initially attempted p-cresol quaternization route failed to introduce the triphenylamine group, we speculate that the reason lies in the low reaction activity of the benzene ring system in Compound 4 (Scheme 1). Inspired by relevant literature research and the previous work of our group, we adjusted the synthetic strategy: we envision using the pre-prepared triphenylamine derivative (Compound 6) as the starting material [13], which directly reacts with trimethylamine through a nucleophilic substitution reaction at its terminal bromine atom to achieve the one-step synthesis of the triphenylamine quaternary ammonium salt derivative (Compound 5) (Scheme 2).
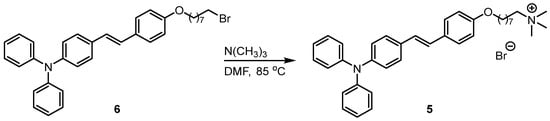
Scheme 2.
Synthetic Route of (E)-8-(4-(4-(diphenylamino)styryl)phenoxy)-N,N,N-trimethyloctan-1-aminium bromide (5).
Finally, using (E)-4-((4-(8-bromooctyl)oxy)styryl)-N,N-diphenylaniline (Compound 6) as the starting material, we synthesized the target product via a quaternization reaction with trimethylamine (Scheme 2). The reaction was carried out in DMF at 85 °C overnight, and its mechanism involves the nucleophilic attack of the nitrogen atom of trimethylamine on the terminal bromine atom of Compound 6, followed by the departure of the bromide ion as a leaving group, ultimately resulting in the successful preparation of Compound 5 (Supplementary Materials). This synthetic route not only effectively introduces the positively charged quaternary ammonium cation but also completely retains the eight-carbon alkyl chain, successfully constructing the hydrophilic-hydrophobic structural units required for the target product. This strategy not only solves the technical difficulty of modifying the benzene ring with insufficient reactivity encountered previously but also achieves the efficient and reliable synthesis of triphenylamine quaternary ammonium salt derivatives.
3. Materials and Methods
3.1. Chemicals and Instrumentations
Unless otherwise specified, all reagents used were of analytical grade. The key reagents included: Ammonium carbonate, N,N-Dimethylformamide, p-Cresol, 1,8-Dibromooctane, Trimethylamine, Piperidine, p-Toluenesulfonic acid, Sodium ethoxide. The main instruments were as follows: Thermostatic heating magnetic stirrer (Model DF-101S) (Shanghai, China), manufactured by Shanghai Yukang Science and Education Instrument Equipment Co., Ltd., Rotary evaporator (Model N-1100V), obtained from EYELA (Tokyo Rika Kikai Co., Ltd., Tokyo, Japan); Chemical diaphragm pump (Model MZ 2C NT), product of Vacuubrand GmbH, Wertheim, Germany; Vacuum drying oven (Model DZF-6020), supplied by Gongyi Jinghua Instrument Co., Ltd. (Gongyi, China); Circulating water vacuum pump (Model SHZ-D(III)), produced by Zhengzhou Yuxiang Instrument Equipment Co., Ltd. (Zhengzhou, China); Electronic balance (Model ME203E), brand of Mettler Toledo, Zurich, Switzerland; Triple-purpose UV analyzer (Model ZF-7), manufactured by Shanghai Kanghua Biochemical Instrument Co., Ltd. (Shanghai, China); Ultrasonic cleaner (Model KQ5200), obtained from Kunshan Ultrasonic Instrument Co., Ltd. (Kunshan, China); Automatic image melting point apparatus (Model SGW®-650), produced by Shanghai Yidian Physical Optics Instrument Co., Ltd. (Shanghai, China); High-resolution quadrupole-time-of-flight mass spectrometer (Model Q-TofMicro), product of Waters-Micromass Co., Ltd., Milford, MA, USA; Superconducting nuclear magnetic resonance spectrometer (Model DPX-400), manufactured by Bruker Corporation, Ettlingen, Germany.
3.2. (E)-8-(4-(4-(Diphenylamino)styryl)phenoxy)-N,N,N-trimethyloctan-1-aminium Bromide (5)
In a round-bottomed flask (25 mL), compound 6 (0.2 g, 0.41 mmol, 1.00 eq.) and trimethylamine (0.78 mL, 2.06 mmol, 5 eq.) were dissolved in N,N-dimethylformamide (DMF, 8 mL). The reaction mixture was heated to 85 °C and stirred for 12 h. After the reaction was completed and cooled to room temperature, the solvent was removed by rotary evaporation. The product was precipitated with petroleum ether (100 mL), followed by filtration, yielding the final product 5 (0.1 g) as a yellow solid with a yield of 54% and a melting range of 279.6–281.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.53–7.43 (m, 4H), 7.30 (t, J = 7.7 Hz, 4H), 7.09–6.88 (m, 12H), 3.97 (t, J = 6.5 Hz, 2H), 3.34–3.28 (m, 2H), 3.08 (s, 9H), 1.76–1.61 (m, 4H), 1.35 (t, J = 6.6 Hz, 8H). 13C NMR (101 MHz, DMSO-d6) δ 158.7, 147.7, 147.0, 132.1, 130.4, 129.4, 127.6, 127.2, 126.9, 126.1, 124.4, 123.9, 123.0, 114.8, 68.1, 34.1, 32.9, 29.3, 29.3, 28.8, 28.2, 26.1. HRMS (ESI) Calculated for C37H45N2O+: 533.3526, found: 533.3531.
4. Conclusions
In this study, two distinct synthetic routes were explored for the preparation of the target amphiphilic molecule. Ultimately, a more direct strategy was adopted, which utilized a preconstructed skeleton containing both the triphenylamine moiety and the alkyl chain from previous work. This was followed by efficient quaternization at the terminal position, successfully affording the target compound, (E)-8-(4-(4-(diphenylamino)styryl)phenoxy)-N,N,N-trimethyloctan-1-aminium bromide, in a single step.
Supplementary Materials
The following supporting information can be downloaded online: 1H NMR spectrum, 13C NMR spectrum and HRMS spectrum of compound 5.
Author Contributions
Conceptualization, S.Y.; software, C.D.K.A.; investigation, Y.-N.W.; formal analysis, M.A.-W.; data curation, Y.M. and R.L.; writing—original draft preparation, S.Y.; writing—review and editing, E.Z. and Y.-H.Z.; supervision, E.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Department of Science and Technology of Henan Province (No. 252102311218) and the Open Grant from the Pingyuan Laboratory (2023PY-OP-0103).
Data Availability Statement
The data are contained within this article and the Supplementary Materials.
Acknowledgments
We gratefully acknowledge Yiqiong Sun and Kaiwei Zhu for valuable discussions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Katie, K. The rise of ‘nightmare bacteria’: Antimicrobial resistance in five charts. Nature 2025, 646, 526–527. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.-Z.; Feng, Y.; Jacob Pollard Judy, N.C.; Michael, J.R.; Robert, B.; Raquel, F.E.; Richard, M.E.; Paul, B.S. Ceragenins Cholic Acid Based Mimics of Antimicrobial Peptides. Acc. Chem. Res. 2007, 10, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Nan, G.; Sun, J.; Li, X.; Yao, Y.; Hu, Y.; Zhao, J.; Shan, A.; Wang, J. Overcoming Delivery Challenges of Antimicrobial Peptides for Clinical Translation: From Nanocarriers to Molecular Modifications. Drug Resist. Updates 2025, 83, 101289. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Qi, S.; Wang, W.; Lu, H. Synergistic Effects of Quaternary Ammonium Compounds and Antibiotics on the Evolution of Antibiotic Resistance. Water Res. 2025, 275, 123206. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Anna, K.M.; William, A.A.; Christopher, W.M.; Patrick, J.M. Quaternary Ammonia Compounds in Disinfectant Products: Evaluating the Potential for Promoting Antibiotic Resistance and Disrupting Wastewater Treatment Plant Performance. Environ. Sci. Adv. 2024, 3, 208–226. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Song, Z.; Tan, Z.; Cheng, J. Recent Advances in Design of Antimicrobial Peptides and Polypeptides toward Clinical Translation. Adv. Drug Deliv. Rev. 2021, 170, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Chalothorn, T.; Vatcharin, R.; Souwalak, P.; Sakawrat, P.; Chittreeya, T. Synthesis and Antibacterial Activity of Emodin and Its Derivatives against Methicillin-Resistant Staphylococcus Aureus. Tetrahedron Lett. 2019, 60, 151004. [Google Scholar] [CrossRef]
- Li, R.; Sun, M.; Li, Z.-H.; Qu, Y.; Li, Y.; Maxwell, A.-W.; Li, D.; Kong, H.; Wu, Y.; Adib, A.H.; et al. Important Role of Triphenylamine in Modulating the Antibacterial Performance Relationships of Antimicrobial Peptide Mimics by Alkyl Chain Engineering. J. Med. Chem. 2025, 68, 10299–10313. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Li, D.; Li, Y.; Li, R.; Kong, H.; Qu, Y.; Wu, Y.; Liu, J.; Qin, S.; Zhang, E.; et al. Dual AIE Combination Strategy: An Efficient Bioaie Active Photosensitizer Derived from Berberine for Bacterial Elimination. Chem. Eng. J. 2025, 519, 165603. [Google Scholar] [CrossRef]
- Gu, H.; Liu, W.; Sun, W.; Du, J.; Fan, J.; Peng, X. Single-Molecule Photosensitizers for Nir-Ii Fluorescence and Photoacoustic Imaging Guided Precise Anticancer Phototherapy. Chem. Sci. 2022, 13, 9719–9726. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, W.; Shang, W.; Du, X.; Liu, H.; Tang, B.Z.; Qiu, D.; Yu, R.; Su, H. A “Simple” Phototheranostic Agent for High-Performance Type I Photodynamic and Photothermal Synergistic Cancer Therapy. J. Colloid Interface Sci. 2026, 703, 139117. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, F.; De Melo, W.C.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial Strategies Centered around Reactive Oxygen Species—Bactericidal Antibiotics, Photodynamic Therapy, and Beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Q.; Wang, Y.-N.; Zhu, K.-W.; Li, R.; Ampomah-Wireko, M.; Amengor, C.D.K.; Zhang, E.; Zhao, Y.-H. (E)-4-(4-((8-Bromooctyl)oxy)styryl)-N,N-diphenylaniline. Molbank 2025, 2025, M2087. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).