(Z)-N-Carbamothioyl-4-hydroxy-2-oxo-4-(p-tolyl)but-3-enamide
Abstract
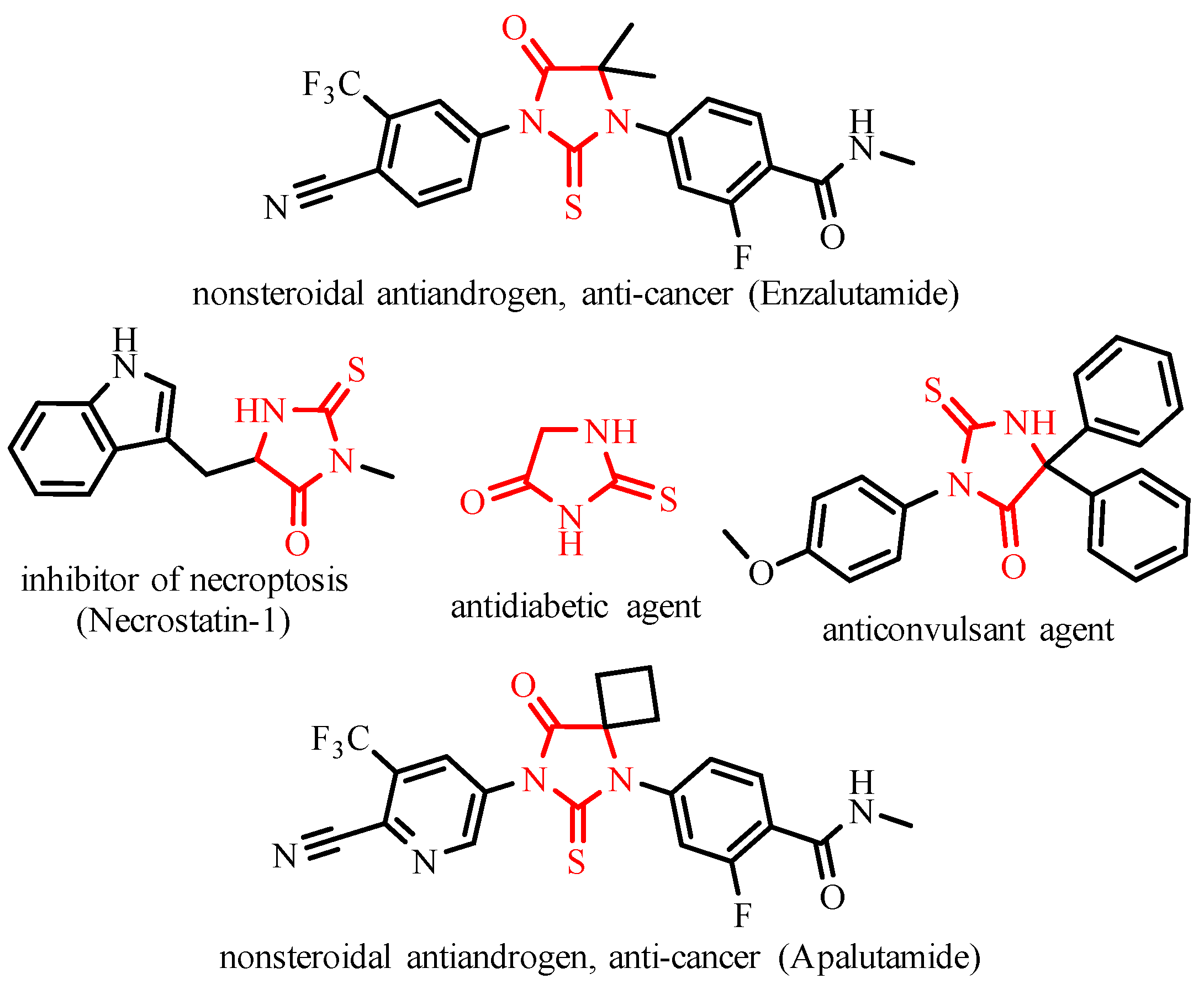
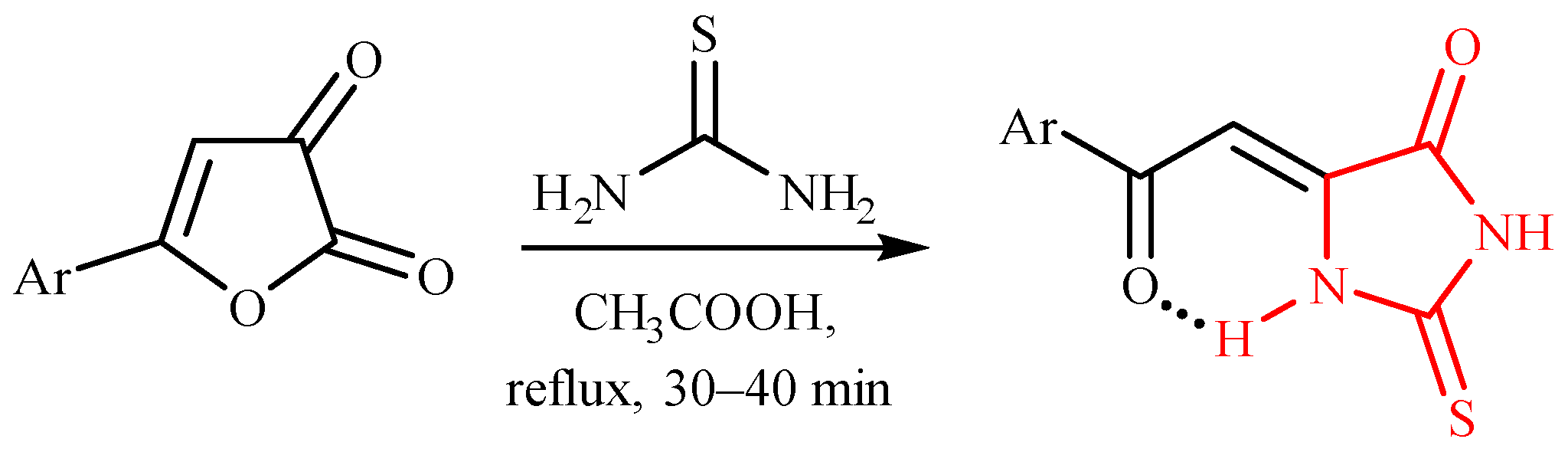
1. Introduction
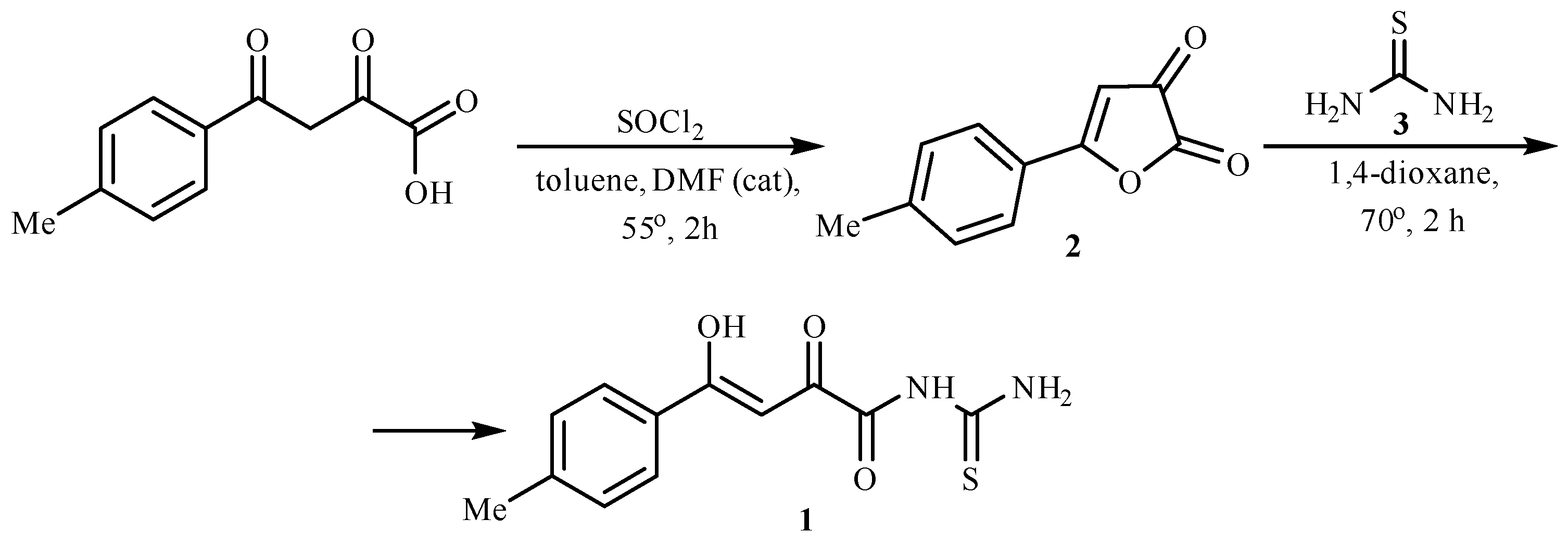
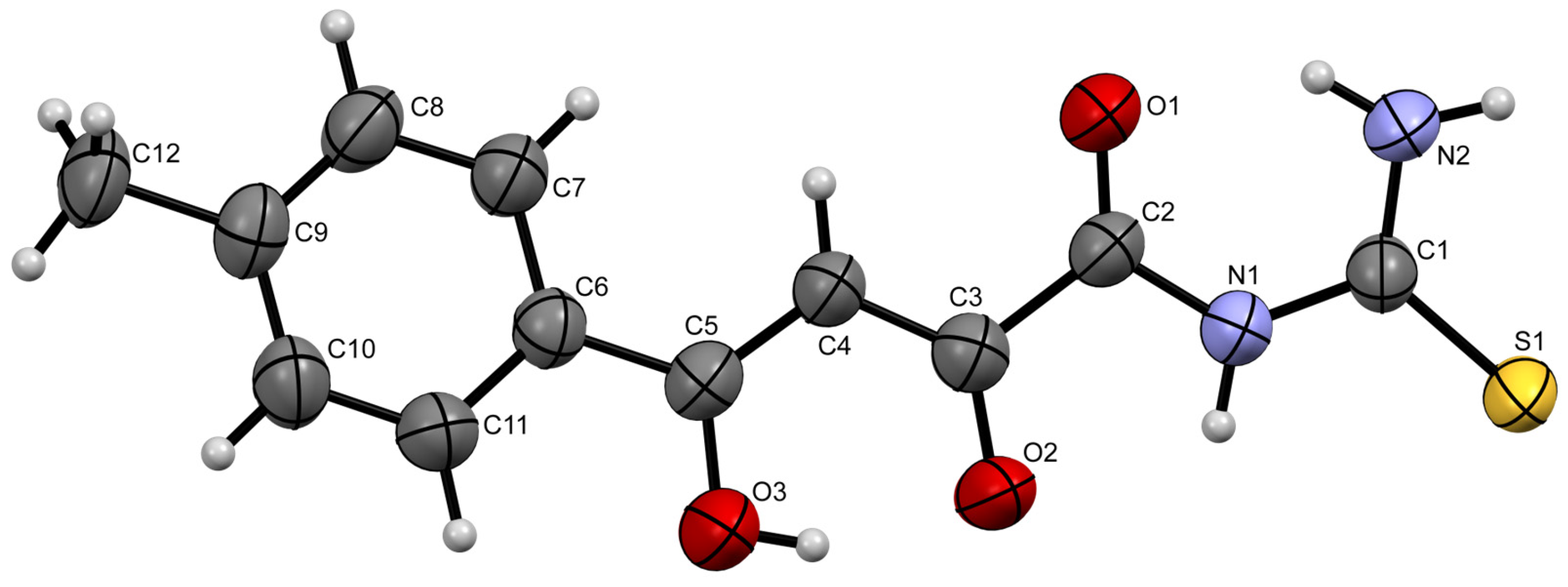
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. (Z)-N-Carbamothioyl-4-hydroxy-2-oxo-4-(p-tolyl)but-3-enamide 1
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khirallah, S.M.; Ramadan, H.M.M.; Shawky, A.; Qahl, S.H.; Baty, R.S.; Alqadri, N.; Alsuhaibani, A.M.; Jaremko, M.; Emwas, A.-H.; Saied, E.M. Development of Novel 1,3-Disubstituted-2-Thiohydantoin Analogues with Potent Anti-Inflammatory Activity; In Vitro and In Silico Assessments. Molecules 2022, 27, 6271. [Google Scholar] [CrossRef] [PubMed]
- Elhady, H.A.; El Desoky, S.; Al-Shareef, H.; El-Mekawy, R. Synthesis, reactions, and applications of 2-thiohydantoin derivatives. Acta Pol. Pharm. 2019, 76, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Uma, S.; Devika, P. In Vitro studies on the antidiabetic activity of 2-thiohydantoin using α-amylase and α-glucosidase. Asian J. Pharm. Clin. Res. 2019, 12, 155–157. [Google Scholar] [CrossRef]
- Eunsun, P. CAplus and MEDLINE. ACS Med. Chem. Lett. 2022, 13, 1459–1467. [Google Scholar]
- Eberli, D.; Kranzbühler, B.; Mortezavi, A.; Sulser, T.; Salemi, S. Apalutamide in combination with autophagy inhibitors improves treatment effects in prostate cancer cells. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 683.e19–683.e26. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-Q.; Wang, S.-Z.; Lei, H.-B.; Liu, X. Necrostatin-1: A promising compound for neurological disorders. Front. Cell. Neurosci. 2024, 18, 1408364. [Google Scholar] [CrossRef] [PubMed]
- Andreichikov, Y.S.; Nekrasov, D.D.; Rudenko, M.A.; Nalimova, Y.A. Reaction of 5-aryl-2,3-dihydrofuran-2,3-diones with N-substituted ureas and their thio and seleno analogs. Chem. Heterocycl. Compd. 1988, 10, 1411–1413. [Google Scholar]
- Derevnina, A.O.; Andreeva, A.A.; Maslivets, A.N. (Z)-N-Carbamoyl-4-hydroxy-4-(4-methoxyphenyl)-2-oxobut-3-enamide. Molbank 2024, 2024, M1844. [Google Scholar] [CrossRef]
- Stepanova, E.E.; Lukmanova, D.N.; Kasatkina, S.O.; Dmitriev, M.V.; Maslivets, A.N. Facile Synthesis of Regioisomeric N-Alkyl Substituted 3- Methylene-3,4-dihydroquinoxalin-2(1H)-ones. ChemistrySelect 2019, 4, 12774–12778. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.42.74a; Rigaku Oxford Diffraction: Wroclaw, Poland, 2022. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 5 April 2024).
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Andreychikov, Y.S. Guidelines for Student Research Work: Methods of Synthesis of Biologically Active Heterocyclic Compounds; Perm University: Perm, Russia, 1988; p. 9. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derevnina, A.O.; Shklyaev, Y.V.; Maslivets, A.N. (Z)-N-Carbamothioyl-4-hydroxy-2-oxo-4-(p-tolyl)but-3-enamide. Molbank 2025, 2025, M2099. https://doi.org/10.3390/M2099
Derevnina AO, Shklyaev YV, Maslivets AN. (Z)-N-Carbamothioyl-4-hydroxy-2-oxo-4-(p-tolyl)but-3-enamide. Molbank. 2025; 2025(4):M2099. https://doi.org/10.3390/M2099
Chicago/Turabian StyleDerevnina, Alexandra O., Yurii V. Shklyaev, and Andrey N. Maslivets. 2025. "(Z)-N-Carbamothioyl-4-hydroxy-2-oxo-4-(p-tolyl)but-3-enamide" Molbank 2025, no. 4: M2099. https://doi.org/10.3390/M2099
APA StyleDerevnina, A. O., Shklyaev, Y. V., & Maslivets, A. N. (2025). (Z)-N-Carbamothioyl-4-hydroxy-2-oxo-4-(p-tolyl)but-3-enamide. Molbank, 2025(4), M2099. https://doi.org/10.3390/M2099






