Abstract
Previously, we demonstrated the synthesis of a well-defined hydroxyalkyl-functionalized N-heterocyclic carbene (NHC)/Ru(II) complex through the transmetalation reaction between [RuCl2(p-cymene)]2 and the corresponding NHC/Ag(I) complex derived from a chiral benzimidazolium salt using the Ag2O method. In this study, we successfully synthesized [RhX(cod)(NHC)] complexes through a one-pot deprotonation route. The hydroxyalkyl-substituted benzimidazolium salt reacted with [Rh(OH)(cod)]2 in THF at room temperature, affording the corresponding monodentate NHC/Rh(I) complex in nearly quantitative yield. The rhodium complex was characterized using NMR, HRMS measurement, and elemental analysis.
1. Introduction
Recently, there has been considerable interest in the transition metal complex having an N-heterocyclic carbene (NHC) ligand with hydroxyalkyl sidearms, owing to the numerous expected applications of complexes incorporating chiral NHC ligands in asymmetric catalysis [1,2,3,4,5]. Scheme 1 showcases representative monodentate NHC/metal complexes, the structural formulas of which have been elucidated through various analyses. In 2014, a [PdCl2(NHC)] complex was synthesized by reacting an imidazolinium salt bearing a hydroxyalkyl sidearm with PdCl2 in the presence of KOtBu [6]. Additionally, Grisi and coworkers synthesized a [PdCl2(NHC)(MeCN)] complex through transmetalation from the corresponding bis(NHC)/Ag complex [7]. Structural characterization of the NHC/Pd complex was performed using X-ray diffraction studies. Fiksdahl and coworkers reported the synthesis of an [AuCl(NHC)] complex by allowing a hydroxyalkyl-substituted imidazolinium salt derived from (S)-2-phenylglycinol to react with [AuCl(Me2S)] in the presence of K2CO3 [8]. Furthermore, several research groups have successfully synthesized well-defined anionic alkoxide–NHC/metal (Cu, Ag, Ni, and Pd) complexes [9,10,11].
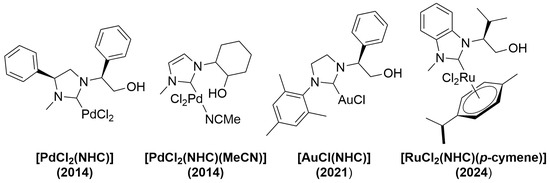
Scheme 1.
List of monodentate hydroxyalkyl-functionalized NHC/metal complexes.
Previously, we showed a highly enantioselective allylic alkylation reaction catalyzed by copper species bearing a chiral hydroxyalkyl-functionalized NHC ligand derived from naturally occurring α-amino acids [12]. During this investigation, we successfully synthesized a novel [RuCl2(NHC)(p-cymene)] complex from the hydroxyalkyl-substituted benzimidazolium salt using the Ag2O method (Scheme 1). The monodentate NHC/Ru(II) complex was obtained utilizing the following experimental procedure: specifically, an azolium salt derived from (S)-valinol was reacted with Ag2O at room temperature for 2 h. Subsequently, [RuCl2(p-cymene)]2 was introduced into the reaction vessel. After stirring the resultant reaction mixture at room temperature for 16 h, the corresponding [RuCl2(NHC)(p-cymene)] complex was obtained in a 62% yield (Scheme 1). Here, we present the synthesis and characterization of an NHC/Rh(I) complex featuring a hydroxyalkyl-functionalized NHC ligand. The desired [RhX(cod)(NHC)] complexes were synthesized via a one-pot deprotonation route by reacting benzimidazolium salt with [Rh(OH)(cod)]2.
2. Results and Discussion
Hydroxyalkyl-functionalized benzimidazolium salts were synthesized from commercially available chiral β-amino alcohols using Ishida and Saigo’s synthetic route (Scheme 2) [13]. The synthesis started with a nucleophilic substitution reaction between 2-fluoronitrobenzene and (S)-alaninol, yielding (S)-2-[N-(2-nitrophenyl)amino]propan-1-ol. Subsequent reduction of the nitro compound produced 2-[N-(2-aminophenyl)amino]propan-1-ol. Then, the condensation reaction between the resulting diamino compound and triethyl orthoformate afforded 2-(benzimidazol-1-yl)propan-1-ol. Finally, coupling the azole with an alkyl halide (R-X) produced the corresponding hydroxyalkyl-substituted benzimidazolium salt. In this study, we synthesized two azolium salts, 1a and 2a, derived from benzyl chloride and benzhydryl bromide, respectively. Further details are provided in Section 3.
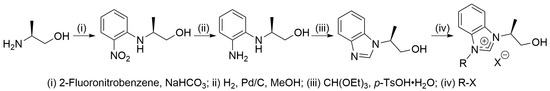
Scheme 2.
Synthetic route to hydroxyalkyl-substituted benzimidazolium salt.
In previous investigations, the synthesis of NHC/metal (Pd, Au, and Ru) complexes involved treating a hydroxyalkyl-substituted azolium salt with appropriate bases such as KOtBu, K2CO3, and Ag2O, as outlined in the introductory section (Section 1). In this study, we showed a synthetic approach for the NHC/Rh complex using the following one-pot deprotonation route: treatment of azolium salt 1a with [Rh(OH)(cod)]2 in THF at room temperature afforded monodentate [RhCl(cod)(NHC)] complex 1b in nearly quantitative yield (Scheme 3). Complex 1b, isolated as an air-stable yellow solid, was characterized by analyzing the acquired analytical and spectroscopic data (further details are provided in Section 3). In the HRMS (ESI-TOF) measurement, the [M−Cl]+ ion (M = C25H30ClN2ORh) was clearly observed. In the 13C{1H} NMR spectrum, a doublet signal at δ = 196.3 ppm with a typical C–Rh coupling constant of 50.8 Hz was observed for the carbene carbon atom. Complex 1b was found to exist as a 75:25 mixture of two NHC/Rh complexes. Enders et al. observed the hindered rotation of the carbene-metal bond in an NHC/Rh complex, which contained a bulky cyclooctadiene ligand [14]. The four doublet signals at δ = 100.7 ppm (J = 6.7 Hz), 99.9 ppm (J = 6.7 Hz), 70.0 ppm (J = 14.4 Hz), and 68.5 ppm (J = 14.4 Hz) were attributed to the four non-equivalent sp2 carbons of the cod ligand on complex 1b in the 13C{1H} NMR spectrum. Additionally, four singlet signals for the sp3 carbons of cod at δ = 33.8, 31.5, 29.5, and 27.6 ppm were observed. In the 1H NMR spectrum, the doublet signal assigned to the methyl group adjacent to the chiral carbon center of 1b appeared at δ = 1.78 ppm with a coupling constant of 7.6 Hz. Furthermore, the hydroxy group at the NHC sidearm was observed at δ = 3.27 ppm. This strongly suggests that [RhCl(cod)(NHC)] complex 1b possesses an N-hydroxyalkyl functional group, indicating that an anionic alkoxide-tethered NHC/Rh(I) complex is not formed under these reaction conditions.
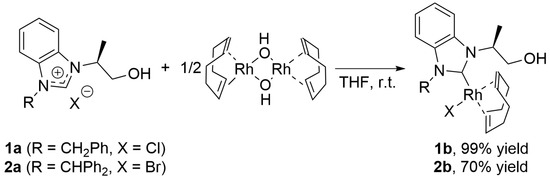
Scheme 3.
Synthesis of [RhX(cod)(NHC)] complexes 1b and 2b.
Similarly, [RhBr(cod)(NHC)] complex 2b was synthesized by reacting 2a featuring an N-CHPh2 wingtip with [Rh(OH)(cod)]2 in THF. Notably, following the reaction, a substantial amount of yellow precipitate formed. Subsequent simple filtration and washing with ether yielded the desired NHC/Rh(I) complex 2b in its pure form. This differs from the preparation of complex 1b. 1H NMR, HRMS measurements, and elemental analysis strongly supported the chemical structure of 2b (details are provided in Section 3). However, attempts to measure 13C{1H} NMR in CDCl3, (CD3)2SO, CD3OD, or (CD3)2CO failed owing to the poor solubility of 2b.
3. Materials and Methods
All reagents, including [Rh(OH)(cod)]2 and solvents were purchased from chemical suppliers and utilized without additional purification. 1H (400 MHz) and 13C{1H} (100 MHz) NMR spectra were acquired on a JEOL ECS-400 spectrometer (JEOL, Tokyo, Japan). Chemical shifts are reported downfield from TMS (δ = 0 ppm) for 1H NMR. For 13C{1H} NMR, chemical shifts are reported relative to CDCl3 as an internal reference. High-resolution mass spectrometry was conducted on a Bruker microTOF II instrument (Bruker Daltonik Gmbh, Bremen, Germany) employing electrospray ionization (ESI). Elemental analyses were carried out at Osaka University. An azolium salt, specifically 1-benzyl-3-[(S)-2-hydroxy-1-methylethyl]benzimidazolium bromide (1a), was synthesized following our previously reported procedure [12].
3.1. Chloro(η2,η2-cycloocta-1,5-diene){1-benzyl-3-[(S)-2-hydroxy-1-methylethyl]-benzimidazol-2-ylidene}rhodium(I) (1b)
1-Benzyl-3-[(S)-2-hydroxy-1-methylethyl]-benzimidazolium chloride (1a) (0.21 mmol, 64 mg) and [Rh(OH)(cod)]2 (0.1 mmol, 46 mg) were stirred in THF (2 mL) at room temperature for 16 h under Ar. After passing through a short silica gel column using THF as a solvent, the filtrate was evaporated using a rotary evaporator, yielding [RhCl(cod)(NHC)] complex 1b as a yellow solid (103 mg, 99% yield): 1H NMR (400 MHz, CDCl3): Major isomer: δ = 7.47 (d, J = 8.8 Hz, 1H), 7.34–7.27 (m, 5H), 7.14 (td, J = 0.8 and 10.0 Hz, 1H), 7.05 (t, J = 7.6 Hz, 1H), 6.98 (d, J = 8.0 Hz, 1H), 6.63–6.54 (m, 1H, CH2OH), 6.33 (d, J = 15.6 Hz, 1H, CH2Ph), 6.03 (d, J = 15.6 Hz, 1H, CH2Ph), 5.18–5.16 (m, 1H, CHcod), 5.12–5.07 (m, 1H, CH2OH), 4.22–4.12 (m, 1H, CHN), 4.11–4.09 (m, 1H, CHcod), 3.68–3.65 (m, 1H, CHcod), 3.52–3.26 (m, 1H, CHcod), 3.27–3.26 (m, 1H, OH), 2.59–2.43 (m, 2H, CH2cod), 2.37–2.28 (m, 1H, CH2cod), 2.19–2.06 (m, 3H, CH2cod), 1.92–1.83 (m, 2H, CH2cod), 1.78 (d, J = 7.6 Hz, 3H, CH3) ppm. Minor isomer: δ = 7.39 (d, J = 8.8 Hz, 1H), 7.18–7.12 (m, 1H), 6.93 (d, J = 8.0 Hz, 1H), 6.48 (d, J = 15.6 Hz, 1H, CH2Ph), 6.37 (d, J = 15.6 Hz, 1H, CH2Ph), 4.08–4.06 (m, 1H, CHcod), 3.57 (br, 1H, CHcod), 1.66 (d, J = 7.6 Hz, 3H, CH3) ppm. 13C{1H} NMR (100 MHz, CDCl3): Major isomer: δ = 196.3 (d, JC-Rh = 50.8 Hz, Ccarbene), 135.6, 135.5, 128.7, 127.7, 126.9, 122.3, 122.3, 111.4 (C of of NHC ring), 111.3 (C of of NHC ring), 100.7 (d, JC-Rh = 6.7 Hz, CHcod), 99.9 (d, JC-Rh = 6.7 Hz, CHcod), 70.0 (d, JC-Rh = 14.4 Hz, CHcod), 68.5 (d, JC-Rh = 14.4 Hz, CHcod), 63.7 (NCH), 59.4 (CH2O), 52.5 (CH2Ph), 33.8 (CH2cod), 31.5 (CH2cod), 29.5 (CH2cod), 27.6 (CH2cod), 16.3 (CH3) ppm. Minor isomer: δ = 196.4 (d, JC-Rh = 50.8 Hz, Ccarbene), 135.9, 134.7, 128.7, 127.6, 126.8, 122.4, 111.1 (C of the NHC ring), 99.3 (d, JC-Rh = 6.7 Hz, CHcod), 98.5 (d, JC-Rh = 6.7 Hz, CHcod), 63.8 (NCH), 58.4 (CH2O), 32.9 (CH2cod), 30.7 (CH2cod), 28.9 (CH2cod), 27.9 (CH2cod), 17.0 (CH3) ppm. HRMS (ESI-TOF), m/z: calculated for C25H30N2ORh [M-Cl]+, 477.1408, found, 477.1385. The NMR spectrum can be found in the Supplementary Materials.
3.2. 1-[(S)-2-hydroxy-1-methylethyl]-3-(diphenylmethyl)benzimidazolium Bromide (2a)
A mixture of (S)-2-benzimidazol-1-yl-propan-1-ol (4.2 mmol, 740 mg) and benzhydryl bromide (4.0 mmol, 989 mg) in toluene (18 mL) was stirred at 110 °C for 72 h. The solvent was evaporated under reduced pressure upon completion of the reaction, resulting in a brown liquid. The product obtained from the residue was isolated via column chromatography on silica gel (CH2Cl2/CH3OH = 95/5). The desired azolium salt 2a was further purified by reprecipitating in CHCl3 and CH3CO2C2H5, yielding a white solid (620 mg, 37% yield): 1H NMR (400 MHz, CDCl3): δ = 9.86 (s, 1H, NCHN+), 7.93 (d, J = 8.5 Hz, 1H), 7.55 (t, J = 7.9 Hz, 1H), 7.48–7.38 (m, 12H), 7.24 (d, J = 8.5 Hz, 1H), 5.20–5.16 (m, 1H, CH2O), 5.09 (t, J = 6.5 Hz, 1H, OH), 4.05–3.95 (m, 2H, NCH and CH2O), 1.64 (d, J = 6.7 Hz, 3H, CH3) ppm. 13C{1H} NMR (100 MHz, CDCl3): δ = 140.9 (NCHN+), 135.2, 131.9, 131.3, 129.5, 129.4, 129.4, 129.3, 128.6, 128.4, 127.0, 126.9, 114.9 (C of azolium), 114.3 (C of azolium), 66.5 (NCH), 62.9 (OCH2), 57.4 (NCH2), 16.7 (CH3) ppm. Elemental analysis calculated (%) for C23H23BrN2O: C 65.25, H 5.48, N 6.62; found: C 65.15, H 5.44, N 6.56. The NMR spectrum can be found in the Supplementary Materials.
3.3. Bromo(η2,η2-cycloocta-1,5-diene){1-[(S)-2-hydroxy-1-methylethyl]-3-diphenylmethylbenzimidazol-2-ylidene}rhodium(I) (2b)
Azolium bromide 2a (0.137 mmol, 58 mg) and [Rh(OH)(cod)]2 (0.065 mmol, 30 mg) were stirred in THF (2 mL) at room temperature for 16 h under Ar. After the reaction, a yellow precipitate formed. The resulting yellow solid was collected by filtration and washed with ether. The desired [RhBr(cod)(NHC)] complex 2b was obtained as a yellow solid (58 mg, 70% yield): 1H NMR (400 MHz, CDCl3): Major isomer: δ = 8.75 (s, 1H, Ph2CH), 7.47 (d, J = 8.2 Hz, 1H), 7.41–7.39 (m, 5H), 7.37–7.35 (m, 2H), 7.25–7.23 (m, 3H), 7.09 (t, J = 7.7 Hz, 1H), 6.89 (t, J = 7.9 Hz, 1H), 6.74 (d, J = 8.2 Hz, 1H), 6.56–6.50 (m, 1H, CH2OH), 5.31 (br, 1H, CHcod), 5.12–5.09 (m, 1H, CH2OH), 4.27–4.19 (m, 1H, CHN), 4.15–4.10 (m, 1H, CHcod), 3.50 (br, 1H, CHcod), 3.27 (dd, J = 2.9 and 9.3 Hz, 1H, OH), 2.78 (br, 1H, CHcod), 2.54–2.42 (m, 2H, CH2cod), 2.23–2.15 (m, 1H, CH2cod), 2.09 (br, 1H, CH2cod), 1.99 (br, 1H, CH2cod), 1.85–1.64 (m, 2H, CH2cod), 1.44–1.43 (m, 1H, CH2cod), 1.78 (d, J = 7.2 Hz, 3H, CH3) ppm. Minor isomer: δ = 3.74 (br, 1H, CHcod), 2.15 (br, 1H, CH2cod), 1.73 (d, J = 7.2 Hz, 3H, CH3) ppm. Elemental analysis calculated (%) for C31H34BrN2ORh▪H2O: C 57.16, H 5.57, N 4.30; found: C 57.54, H 5.31, N 4.45. HRMS (ESI-TOF), m/z: calculated for C31H34N2Rh [M-Br]+, 553.1721, found, 553.1723. The NMR spectrum can be found in the Supplementary Materials.
4. Conclusions
In summary, two rhodium complexes featuring hydroxyalkyl-functionalized NHC ligands were successfully synthesized by treating the corresponding benzimidazolium salts with [Rh(OH)(cod)]2 via a one-pot deprotonation route. This is a new addition to hydroxyalkyl-functionalized NHC/metal complexes. Further investigations are underway to explore the preparation of conformationally stable anionic alkoxide–NHC/metal complexes and their potential applications in enantioselective transformation reactions.
Supplementary Materials
The following supporting information information is available online. Figures S1 and S2: 1H NMR and 13C{1H} NMR of compound 1b; Figures S3 and S4: 1H NMR and 13C{1H} NMR of compound 2a; and Figure S5: 1H NMR and HMRS of compound 2b.
Author Contributions
Conceptualization, Methodology, and Writing—original draft preparation, S.S.; Investigation—experiments and analyses, S.M. and S.S.; Writing—review and editing, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pape, F.; Teichert, J.F. Dealing at Arm’s Length: Catalysis with N-Heterocyclic Carbene Ligands Bearing Anionic Tethers. Eur. J. Org. Chem. 2017, 38, 4206–4229. [Google Scholar] [CrossRef]
- Fliedel, C.; Labande, A.; Manoury, E.; Rinaldo, P. Chiral N-heterocyclic carbene ligands with additional chelating group(s) applied to homogeneous metal-mediated asymmetric catalysis. Coord. Chem. Rev. 2019, 394, 65–103. [Google Scholar] [CrossRef]
- Wanga, F.; Liua, L.-j.; Wanga, W.; Li, S.; Shi, M. Chiral NHC–metal-based asymmetric catalysis. Coord. Chem. Rev. 2012, 256, 804–853. [Google Scholar] [CrossRef]
- Neshat, A.; Mastrorilli, P.; Mobarakeh, A.M. Recent Advances in Catalysis Involving Bidentate N-Heterocyclic Carbene Ligands. Molecules 2022, 27, 95. [Google Scholar] [CrossRef]
- Jayaraj, A.; Raveedran, A.V.; Latha, A.T.; Priyadarshini, D.; Swamy, P.C.A. Coordination Versatility of NHC-metal Topologies in Asymmetric Catalysis: Synthetic Insights and Recent Trends. Coord. Chem. Rev. 2023, 478, 214922. [Google Scholar] [CrossRef]
- Faraji, L.; Jadidi, K.; Notash, B. Synthesis of novel chiral bidentate hydroxyalkyl-N-heterocyclic carbene ligands and their application in palladium-catalyzed Mizoroki–Heck couplings and asymmetric addition of diethylzinc to benzaldehyde. Tetrahedron Lett. 2014, 55, 346–350. [Google Scholar] [CrossRef]
- Mariconda, A.; Grisi, F.; Costabile, C.; Falcone, S.; Bertolasi, V.; Longo, P. Synthesis, characterization and catalytic behaviour of a palladium complex bearing a hydroxy-functionalized N-heterocyclic carbene ligand. New J. Chem. 2014, 38, 762–769. [Google Scholar] [CrossRef]
- Jónsson, H.F.; Orthaber, A.; Fiksdahl, A. Studies on gold(I) and gold(III) alcohol functionalised NHC complexes. Dalton Trans. 2021, 50, 5128–5138. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.L.; Scarisbrick, A.C.; Blake, A.J.; Wilson, C. Chelating alkoxy-N-heterocyclic carbene complexes of silver and copper. Chem. Commun. 2001, 2340–2341. [Google Scholar] [CrossRef] [PubMed]
- Napoli, M.; Saturnino, C.; Cianciulli, E.I.; Varcamonti, M.; Zanfardino, A.; Tommonaro, G.; Longo, P.J. Silver(I) N-heterocyclic carbene complexes: Synthesis, characterization and antibacterial activity. J. Organomet. Chem. 2013, 725, 46–53. [Google Scholar] [CrossRef]
- Hameury, S.; de Fremont, P.; Breuil, P.-A.R.; Olivier-Bourbigou, H.; Braunstein, P. Synthesis and Characterization of Palladium(II) and Nickel(II)Alcoholate-Functionalized NHC Complexes and of Mixed Nickel(II)−Lithium(I) Complexes. Inorg. Chem. 2014, 53, 5189–5200. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Kawashima, H.; Kamisue, R.; Kashioka, Y.; Nakahigashi, Y.; Usui, R. Asymmetric Allylic Alkylation Reaction Catalyzed by Copper Species Bearing a Chiral Bidentate Hydroxyalkyl-N-Heterocyclic Carbene Ligand. Trends Org. Chem. 2024, in press. [Google Scholar]
- Matsuoka, Y.; Ishida, Y.; Sasaki, D.; Saigo, K. Cyclophane-Type Imidazolium Salts with Planar Chirality as a New Class of N-Heterocyclic Carbene Precursors. Chem. Eur. J. 2008, 14, 9215–9222. [Google Scholar] [CrossRef] [PubMed]
- Enders, D.; Gielen, H.; Runsink, J.; Breuer, K.; Brode, S.; Boehn, K. Diastereoselective Synthesis of Chiral (Triazolinylidene)rhodium Complexes Containing an Axis of Chirality. Eur. J. Inorg. Chem. 1998, 1998, 913–919. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).