Chloro(η2,η2-cycloocta-1,5-diene){1-benzyl-3-[(S)-2-hydroxy-1-methylethyl]benzimidazol-2-ylidene}rhodium(I)
Abstract
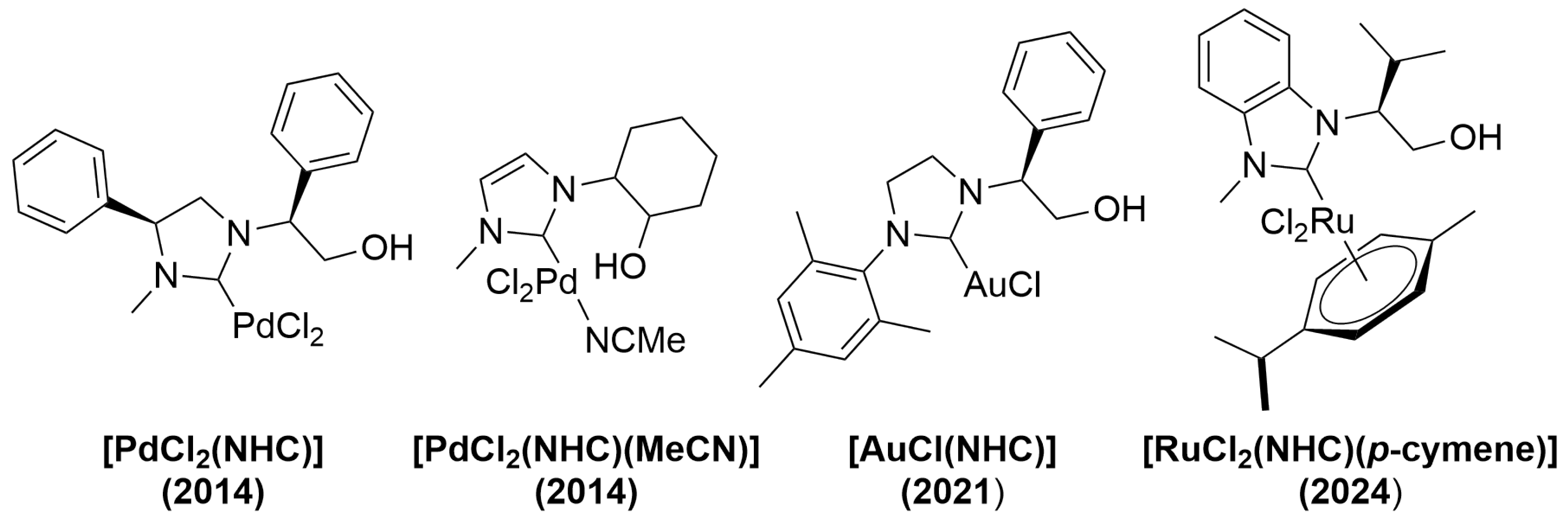
1. Introduction
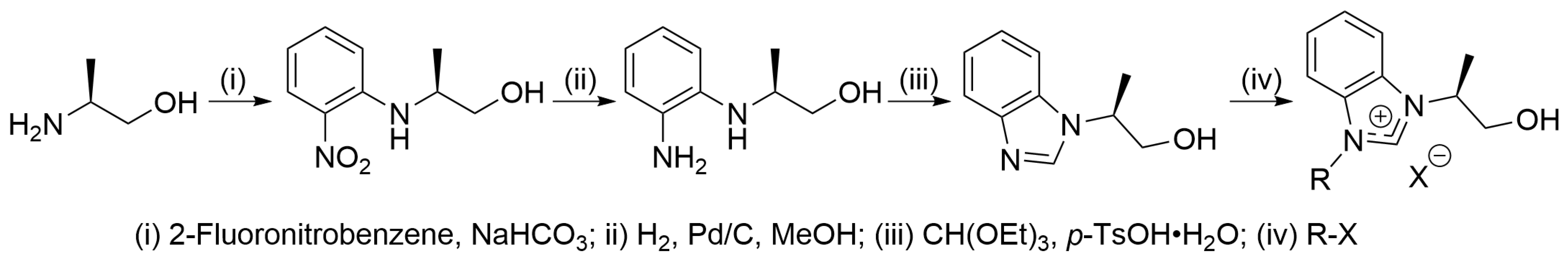
2. Results and Discussion
3. Materials and Methods
3.1. Chloro(η2,η2-cycloocta-1,5-diene){1-benzyl-3-[(S)-2-hydroxy-1-methylethyl]-benzimidazol-2-ylidene}rhodium(I) (1b)
3.2. 1-[(S)-2-hydroxy-1-methylethyl]-3-(diphenylmethyl)benzimidazolium Bromide (2a)
3.3. Bromo(η2,η2-cycloocta-1,5-diene){1-[(S)-2-hydroxy-1-methylethyl]-3-diphenylmethylbenzimidazol-2-ylidene}rhodium(I) (2b)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pape, F.; Teichert, J.F. Dealing at Arm’s Length: Catalysis with N-Heterocyclic Carbene Ligands Bearing Anionic Tethers. Eur. J. Org. Chem. 2017, 38, 4206–4229. [Google Scholar] [CrossRef]
- Fliedel, C.; Labande, A.; Manoury, E.; Rinaldo, P. Chiral N-heterocyclic carbene ligands with additional chelating group(s) applied to homogeneous metal-mediated asymmetric catalysis. Coord. Chem. Rev. 2019, 394, 65–103. [Google Scholar] [CrossRef]
- Wanga, F.; Liua, L.-j.; Wanga, W.; Li, S.; Shi, M. Chiral NHC–metal-based asymmetric catalysis. Coord. Chem. Rev. 2012, 256, 804–853. [Google Scholar] [CrossRef]
- Neshat, A.; Mastrorilli, P.; Mobarakeh, A.M. Recent Advances in Catalysis Involving Bidentate N-Heterocyclic Carbene Ligands. Molecules 2022, 27, 95. [Google Scholar] [CrossRef]
- Jayaraj, A.; Raveedran, A.V.; Latha, A.T.; Priyadarshini, D.; Swamy, P.C.A. Coordination Versatility of NHC-metal Topologies in Asymmetric Catalysis: Synthetic Insights and Recent Trends. Coord. Chem. Rev. 2023, 478, 214922. [Google Scholar] [CrossRef]
- Faraji, L.; Jadidi, K.; Notash, B. Synthesis of novel chiral bidentate hydroxyalkyl-N-heterocyclic carbene ligands and their application in palladium-catalyzed Mizoroki–Heck couplings and asymmetric addition of diethylzinc to benzaldehyde. Tetrahedron Lett. 2014, 55, 346–350. [Google Scholar] [CrossRef]
- Mariconda, A.; Grisi, F.; Costabile, C.; Falcone, S.; Bertolasi, V.; Longo, P. Synthesis, characterization and catalytic behaviour of a palladium complex bearing a hydroxy-functionalized N-heterocyclic carbene ligand. New J. Chem. 2014, 38, 762–769. [Google Scholar] [CrossRef]
- Jónsson, H.F.; Orthaber, A.; Fiksdahl, A. Studies on gold(I) and gold(III) alcohol functionalised NHC complexes. Dalton Trans. 2021, 50, 5128–5138. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.L.; Scarisbrick, A.C.; Blake, A.J.; Wilson, C. Chelating alkoxy-N-heterocyclic carbene complexes of silver and copper. Chem. Commun. 2001, 2340–2341. [Google Scholar] [CrossRef] [PubMed]
- Napoli, M.; Saturnino, C.; Cianciulli, E.I.; Varcamonti, M.; Zanfardino, A.; Tommonaro, G.; Longo, P.J. Silver(I) N-heterocyclic carbene complexes: Synthesis, characterization and antibacterial activity. J. Organomet. Chem. 2013, 725, 46–53. [Google Scholar] [CrossRef]
- Hameury, S.; de Fremont, P.; Breuil, P.-A.R.; Olivier-Bourbigou, H.; Braunstein, P. Synthesis and Characterization of Palladium(II) and Nickel(II)Alcoholate-Functionalized NHC Complexes and of Mixed Nickel(II)−Lithium(I) Complexes. Inorg. Chem. 2014, 53, 5189–5200. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Kawashima, H.; Kamisue, R.; Kashioka, Y.; Nakahigashi, Y.; Usui, R. Asymmetric Allylic Alkylation Reaction Catalyzed by Copper Species Bearing a Chiral Bidentate Hydroxyalkyl-N-Heterocyclic Carbene Ligand. Trends Org. Chem. 2024, in press. [Google Scholar]
- Matsuoka, Y.; Ishida, Y.; Sasaki, D.; Saigo, K. Cyclophane-Type Imidazolium Salts with Planar Chirality as a New Class of N-Heterocyclic Carbene Precursors. Chem. Eur. J. 2008, 14, 9215–9222. [Google Scholar] [CrossRef] [PubMed]
- Enders, D.; Gielen, H.; Runsink, J.; Breuer, K.; Brode, S.; Boehn, K. Diastereoselective Synthesis of Chiral (Triazolinylidene)rhodium Complexes Containing an Axis of Chirality. Eur. J. Inorg. Chem. 1998, 1998, 913–919. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakaguchi, S.; Matsuo, S. Chloro(η2,η2-cycloocta-1,5-diene){1-benzyl-3-[(S)-2-hydroxy-1-methylethyl]benzimidazol-2-ylidene}rhodium(I). Molbank 2024, 2024, M1811. https://doi.org/10.3390/M1811
Sakaguchi S, Matsuo S. Chloro(η2,η2-cycloocta-1,5-diene){1-benzyl-3-[(S)-2-hydroxy-1-methylethyl]benzimidazol-2-ylidene}rhodium(I). Molbank. 2024; 2024(2):M1811. https://doi.org/10.3390/M1811
Chicago/Turabian StyleSakaguchi, Satoshi, and Shogo Matsuo. 2024. "Chloro(η2,η2-cycloocta-1,5-diene){1-benzyl-3-[(S)-2-hydroxy-1-methylethyl]benzimidazol-2-ylidene}rhodium(I)" Molbank 2024, no. 2: M1811. https://doi.org/10.3390/M1811
APA StyleSakaguchi, S., & Matsuo, S. (2024). Chloro(η2,η2-cycloocta-1,5-diene){1-benzyl-3-[(S)-2-hydroxy-1-methylethyl]benzimidazol-2-ylidene}rhodium(I). Molbank, 2024(2), M1811. https://doi.org/10.3390/M1811





